Simple Summary
The family Equidae enjoys an iconic evolutionary record, especially the genus Equus which is actively investigated by both paleontologists and molecular biologists. Nevertheless, a comprehensive evolutionary framework for Equus across its geographic range, including North, Central and South America, Eurasia and Africa, is long overdue. Herein, we provide an updated taxonomic framework so as to develop its biochronologic and biogeographic frameworks that lead to well-resolved paleoecologic, paleoclimatic and phylogenetic interpretations. We present Equus’ evolutionary framework in direct comparison to more archaic lineages of Equidae that coexisted but progressively declined over time alongside evolving Equus species. We show the varying correlations between body size, and we use paleoclimatic map reconstructions to show the environmental changes accompanying taxonomic distribution across Equus geographic and chronologic ranges. We present the two most recent phylogenetic hypotheses on the evolution of the genus Equus using osteological characters and address parallel molecular studies.
Abstract
Studies of horse evolution arose during the middle of the 19th century, and several hypotheses have been proposed for their taxonomy, paleobiogeography, paleoecology and evolution. The present contribution represents a collaboration of 19 multinational experts with the goal of providing an updated summary of Pliocene and Pleistocene North, Central and South American, Eurasian and African horses. At the present time, we recognize 114 valid species across these continents, plus 4 North African species in need of further investigation. Our biochronology and biogeography sections integrate Equinae taxonomic records with their chronologic and geographic ranges recognizing regional biochronologic frameworks. The paleoecology section provides insights into paleobotany and diet utilizing both the mesowear and light microscopic methods, along with calculation of body masses. We provide a temporal sequence of maps that render paleoclimatic conditions across these continents integrated with Equinae occurrences. These records reveal a succession of extinctions of primitive lineages and the rise and diversification of more modern taxa. Two recent morphological-based cladistic analyses are presented here as competing hypotheses, with reference to molecular-based phylogenies. Our contribution represents a state-of-the art understanding of Plio-Pleistocene Equus evolution, their biochronologic and biogeographic background and paleoecological and paleoclimatic contexts.
Keywords:
Equidae; Equinae; hipparionini; protohippini; equini; paleoecology; paleoclimatology; biochronology; phylogeny; evolution 1. Introduction
Studies on the evolution of the family Equidae started in the middle of the 19th century following the opening of the western interior of the United States. Marsh [1] produced an early orthogenetic scheme of Cenozoic horse evolution detailing changes in the limb skeleton and cheek teeth. Gidley [2] challenged the purported orthogonal evolution of horses with his own interpretation of equid evolution. Osborn [3] chose not to openly debate the phylogeny of Equidae but rather displayed Cenozoic horse diversity in his 1918 treatise on the unparalleled American Museum of Natural History’s collection of fossil equids. Matthew [4], however, did produce a phylogeny detailing 10 stages, actual morphological grades, ascending from Eohippus to Equus. Stirton [5] provided a widely accepted augmentation of Matthew’s earlier work with his revised orthogonal scheme of North American Cenozoic Equidae. Simpson [6] published his book on horses, which was the most authoritative account up to that time. His scheme was vertical rather than horizontal in its view of North American equid evolution, with limited attention paid to the extension of North American taxa into Eurasia and Africa. MacFadden [7] updated in a significant way Simpson’s [6] book, depicting the phylogeny, geographic distribution, diet and body sizes for the family Equidae.
This work principally focused on the evolution of Equus and its close relatives in North and South America, Eurasia and Africa during the Plio-Pleistocene (5.3 Ma–10 ka). We documented several American lineages that overlap Equus in this time range, whereas in Eurasia and Africa, only hipparionine horses co-occurred with Equus beginning at ca. 2.6 Ma, which we included in this work for their biogeographical and paleoecological significances. These taxa were reviewed by MacFadden [7] and Bernor et al. [8] including the references therein.
In the present manuscript, we aimed to revise and discuss the most recent knowledge on the Equinae fossil record, with the following goals:
- (1)
- Provide a systematic revision of tridactyl and monodactyl horses from 5.3 Ma to 10 ka. These taxa are discussed in chronological order, from the oldest to the youngest occurrence in each region starting from North and Central America, South America, Eastern Asia (i.e., Central Asia, China, Mongolia and Russia), Indian Subcontinent, Europe and Africa, with their temporal ranges, geographic distributions, time of origins and extinctions. Part of this information is also reported in Table S1;
- (2)
- Integrate the distribution of the fossil record with paleoclimate and paleoecological data in order to provide new insights into the evolution of Equinae and their associated paleoenvironments;
- (3)
- Compare and discuss the latest morphological and genetic-based phylogenetic hypotheses on the emergence of the genus Equus. Recently, Barrón–Ortiz et al. [9] and Cirilli et al. [10] provided phylogenies of Equus, including fossil and extant species, with different resulting hypotheses. We compare and discuss the results of these two competing hypotheses here;
- (4)
- Provide a summary synthesis of the major patterns in the evolution, adaptation and extinction of Equidae 5.3 Ma–10 ka.
2. Materials and Methods
We provide a revised taxonomy of all Plio-Pleistocene Equus across the Americas, Eurasia and Africa summarizing previously published research, as a group of 19 researchers from Europe and North and South America. We provide an updated chronology and geographic distribution for these species. We followed the international guidelines for fossil and extant horse measurements published by Eisenmann et al. [11] and Bernor et al. [12]. Over the last 30 years, these methods have been applied to several case studies in Equinae samples from North and South America, Eurasia and Africa, which led to the identification of new species as well as to the clarification of the taxonomic fossil record. More recently, Cirilli et al. [13] and Bernor et al. [14] provided a combination of analyses to analyze fossil and extant samples including univariate, bivariate and multivariate analyses on cranial and postcranial elements using boxplots, bivariate plots, Log10 ratio diagrams and PCA. We found that robust, overlapping statistical and analytical methods led to a finer resolution of the taxonomy and, ultimately, biogeographical and paleoecological studies. We provide essential information on those species we recognized in the record under consideration.
The taxonomic revision of the 5.3 Ma to 10 ka equid genera and species is given below and includes the authorship, chronological and paleobiogeographic ranges and some historical and evolutionary considerations on the taxon. A reduced emended diagnosis of the species is reported in the Supplementary Materials in order to offer the most relevant anatomical features to identify the taxon. Additional information is reported in Table S1.
We compiled a global Neogene dataset of Equinae body mass estimates and paleodietary information for extensive palaeoecological analyses. These data were collected during several museum visits and complemented with data from publications and databases. Paleodietary information included results from traditional mesowear [15], low-magnification microwear [16] and isotopic analyses of equids from American, Eurasian and African localities (Tables S2 and S3). Net primary productivity (NPP) estimates were calculated for equine localities from the mean hypsodonty and mean longitudinal loph counts of large herbivorous mammal communities using the equation of Oksanen et al. [17]. The equation was as follows: NPP = 2601 − 144HYP − 935 LOP, where HYP is the mean ordinated hypsodonty, and LOP is the mean longitudinal loph count.
For the selected localities, body mass estimates based on metapodial measurements of equine paleopopulations [18,19], univariate mesowear scores calculated using the method of Saarinen et al. [20], and NPP estimates [17] were included to test whether the diet and productivity of the paleoenvironments were connected with body size patterns in equine horses. For this purpose, we used ordinary least squares linear models with body mass estimates as the dependent variable and the mean mesowear scores and NPP estimates as the explaining variables. These analyses were based on Eurasian and African Equus because of the large amount of data available and high variation in the ecology and environments of that genus during the Pleistocene, particularly in Europe but also, to some extent, in Asia and Africa. Because of slight methodological differences concerning the North American equine data [21] (Section 6.2 in the Supplementary Materials), we discuss them separately from Eurasian and African Equus in the context of the patterns revealed by the Eurasian and African Equus models. We also discuss the paleoenvironmental and habitat properties of key equine species based on what is known regarding the vegetation type and climate in their environments/paleoenvironments. Furthermore, we compared the patterns in the equine body size evolution, dietary ecologies and paleoenvironments between the continents and discuss how differential changes in biome distribution on the different continents could explain the observed differences in the body size patterns between continents.
We assembled data on the large herbivorous mammals (i.e., Artiodactyla, Perissodactyla, Proboscidea and Primates) from the NOW database [22] and calculated the mean ordinated crown height for each locality (Table S4) following Fortelius et al. [23] for lists with at least two species with a hypsodonty value. All NOW localities between 7 Ma to recent from North and South America, Eurasia and Africa were included in the study and divided into four different age groups: 7–4 Ma; 4–2.5 Ma; 2.5–1.5 Ma; 1.5 to the recent. Mean ordinated crown height is a robust proxy for humidity and productivity at the regional scale [23,24,25,26]. We plotted the results onto present-day maps and interpolated between the localities using Quantum GIS 3.14.16 Pi. For the interpolations, thematic mapping and grid interpolation were used with the following settings: 20 km grid size; 800 km search radius; 800 grid borders. The interpolation method employed an inverse distance-weighted algorithm (IDW).
Finally, we discuss the most recent phylogenetic outcomes on the origin of the genus Equus. We compared the morphological-based cladistic results of Barrón-Ortiz et al. [9] and Cirilli et al. [10] with the genomic-based analyses of Orlando et al. [27], Jónsson et al. [28] and Heintzman et al. [29] in order to identify similarities between the two different cladistic approaches.
3. Systematics of the Equinae since 5.3 Ma in North, Central and South America
3.1. North and Central America
Horses have been commonly found in numerous terrestrial North and Central American vertebrate faunas. From the Middle Miocene through the Early Pleistocene, the diversity of horses encompassed the genera Astrohippus, Boreohippidion, Calippus, Cormohipparion, Dinohippus, Nannipus, Neohipparion, Pliohippus, Protohippus, Pseudohipparion and an Hipparionini of indeterminate genus or species. The genus Equus is commonly interpreted to have first appeared during the Blancan North American Land Mammal Age (NALMA), though recent analyses propose an earlier origin of crown-group Equus that extends into the Middle to Late Pliocene [29]. Nevertheless, the genus peaked in diversity and widespread geographic distribution during the Pleistocene. In the particular case of North and Central America, we use Equus in the broad sense (i.e., sensu lato), as the generic taxonomy of this group of equids has not been resolved and is an area of ongoing study (e.g., [9,10,29]).
What follows is a summary of the species of equids present in North and Central America from the Late Miocene to the Late Pleistocene (Hemphillian to Rancholabrean NALMAs) based on a review of the literature. In the particular case of Equus sensu lato, we recognized potentially valid species based on a meta-analysis of relevant studies (published between 1901 and 2021; n = 68) discussing fossil specimens of this group of equids; details of this analysis are provided in the Supplementary Materials.
- 1. Calippus elachistus, Hulbert, 1988 [30] (Right Mandibular Fragment with m2–m3, UF342139). This species seems to be restricted to Florida (USA) from the Late Miocene to the Late Hemphillian. The occlusal dimensions of its cheeck teeth are much smaller than any other species of Calippus, except Ca. regulus, with slightly smaller occlusal dimensions in the early to middle wear stages and significantly smaller basal crown lengths than Ca. regulus.
- 2. Calippus hondurensis, Olson and McGrew, 1941 [31] (Partial Skull Containing Left P2–M3 and Right P2–4, WM 1769). This species has been reported in Puntarenas (Costa Rica), Gracias (Honduras), Mexico (Guanajuato, Hidalgo, Jalisco and Zacatecas) and in the USA (Florida). It may be distinguished by its small size and relatively small protocone.
- 3. Dinohippus leardi, Drescher, 1941 [32] (M1, CIT 2645). This species has been recorded from the Late Miocene in California (USA). The size of the molars is similar to that of Pliohippus nobilis or larger in unworn teeth.
- 4. Dinohippus spectans, Cope, 1880 [33] (Left M2 with Associated or Referred P2, AMNH 8183). This species has been recorded in Oregon, Nevada, California, Texas and Idaho (USA) dating to the Late Miocene, with molar teeth of larger size than those of any of the extinct American horses, except Equus excelsus, approximately equal to those of Hippidion principale.
- 5. Astrohippus ansae, Matthew and Stirton, 1930 [34] (Partial Left Maxilla with P2-M3, UC30225). This species is a Hemphilian–Blancan species recorded in Zacatecas (Mexico), New Mexico, Oklahoma and Texas (USA).
- 6. Astrohippus stockii, Lance, 1950 [35] (Palate with P2-M2, Front Portion of M3 on the Right Maxilla and P2-M2 on the Left One, CIT3576). This species has been recorded in Chihuahua, Guanajuato and Jalisco (Mexico) and Florida, New Mexico and Texas (USA), from the latest Hemphilian to Blancan NALMAs. Astrohippus stockii is smaller than A. ansae, but it possesses higher-crowned cheek teeth [35]. In recent phylogenetic analyses, A. stockii was recovered as the sister group to the clade composed of “Dinohippus” mexicanus plus Equus sensu lato [8,36] or the sister group to the clade composed of successive species of “Dinohippus” (i.e., “Dinohippus” leardi, “Dinohippus” interpolatus, Dinohippus leidyanus and “Dinohippus” mexicanus) plus Equus sensu lato [37].
- 7. Dinohippus interpolatus, Cope, 1893 [38] (First and Second Upper Molars, Plate XII, Figures 3 and 4). This is a late Hemphillian-Blancan species that has been recorded in Hidalgo and Zacatecas (Mexico) and in California, Kansas, New Mexico and Texas (USA).
- 8. Dinohippus leiydianus, Osborn, 1918 [3] (Skull, Jaws, Vertebrae, Fore and Hind Limbs, Considerable Portions of the Ribs and Other Parts of the Skeleton of One Individual, AMNH 17224). This species comes from the late Hemphillian–Blancan with records in Alberta (Canada) and in Arizona, California, Kansas, Nebraska and Oklahoma (USA).
- 9. Dinohippus mexicanus, Lance, 1950 [35] (Partial Left Maxilla with P2-M3 and Part of the Zygomatic Arch, LACM-CIT 3697). This species has been found in Chihuahua, Guanajuato, Jalisco, Hidalgo, Nayarit and Zacatecas (Mexico) and in California, Florida, New Mexico and Texas (USA) from Hemphillian to Blancan NALMAs. It is a medium-sized monodactyl equine horse.
- 10. Cormohipparion occidentale, Leidy, 1856 [39] (Four Left and One Right Upper Cheek Teeth, ANSP 11287). This species is a Hemphillian–Blancan species recorded in California, Florida, Nebraska, New Mexico and Oklahoma (USA). It is a large and hypsodont North American hipparion.
- 11. Nannippus aztecus, Mooser, 1968 [40] (Fragmented Right Maxillary with P3–M3, FO 873). This species has been recorded in Mexico (i.e., Chihuahua, Guanajuato and Jalisco) and in the USA (i.e., Alabama, Florida, Louisiana, Mississippi, Oklahoma and Texas) from the latest Hemphillian to Blancan NALMAs. It is a small-sized horse.
- 12. Nannippus lenticularis, Cope, 1893 [3] (Two Upper Cheek Teeth). This species has been recorded from Hemphillian to Blancan NALMAs in Alberta (Canada) and in Alabama, Kansas, Nebraska, Oklahoma and Texas (USA).
- 13. Nannippus peninsulatus, Cope, 1885 [41] (M2, AMNH8345). This is a Hemphillian–Blancan species with records in Guanajuato, Hidalgo, Jalisco and Michoacan (Mexico) and in Arizona, Florida, Kansas, Nebraska, New Mexico and Texas (USA). Nannippus peninsulatus was a highly cursorial equid that appears to have been functionally monodactyl [42,43]. It had an estimated body mass of 59.6 kg [44].
- 14. Neohipparion eurystyle, Cope, 1893 [38] (TMM 40289-1). This species has been recorded in Alberta (Canada); Guanajuato, Hidalgo, Jalisco and Zacatecas (Mexico); Alabama, California, Florida, Kansas, Nebraska, Oklahoma and Texas (USA) from the Hemphillian to Blancan NALMAs. It is a very hypsodont medium-sized hipparion.
- 15. Neohipparion leptode, Merriam, 1915 [45] (Lower Molar, UCMP 19414). This species is a Hemphillian–Blancan species recorded in California, Kansas, Nebraska, Nevada, Oklahoma and Oregon (USA). It is a large hipparion.
- 16. Hipparionini genus and Species Indeterminate. Hulbert and Harington [46] reported a remarkable specimen of a Hipparionini equid from the Canadian Arctic, which represents the northernmost fossil record of an equid reported to date. It was found in an Early Pliocene deposit (~3.5–4 Ma) from the Strathcona Fiord, Beaver Pond locality, Ellesmere Island, Canada [46]. The specimen consists of associated maxillae and premaxillae with the right dI1 and dP2–dP4 and the left dP1–dP4 of a young foal (approximately 6–10 months of age) [46]. It is a relatively large hipparionine equid (estimated adult tooth row length of 150 mm), with deciduous premolars that have low crowns; complex enamel plications; oval, isolated protocones; a facial region that shows a reduced preorbital fossa located posterior to the infraorbital foramen [46]. This combination of traits is not known in any contemporaneous North American hipparionines, but it is found in some Asiatic hipparionines, particularly Plesiohipparion, indicating possible affinities with this group and suggesting a previously unrecognized dispersal event from Asia into North America [8,46]. Alternatively, the Ellesmere Island hipparionine could represent a previously unknown autochthonous lineage of high-latitude North American hipparionines that potentially evolved from the mid-Miocene North American Cormohipparion [46].
- 17. Neohipparion gidley, Merriam, 1915 [45] (Left M3, UCMP 21382). This species is a Hemphillian species recorded in California and Oklahoma (USA). It is the largest of the North American hipparions.
- 18. Boreohippidion galushai, MacFadden and Skinner, 1979 [47] (Partial Skull with Well-Preserved Dentition, AMNH 100077). This is a late Hemphillian horse from Arizona (USA).
- 19. Cormohipparion emsliei, Hulbert, 1987 [48] (partial skull with most of the right maxilla including dP1, P2-M3; right and left premaxillae with I1–I3; edentulous fragment of the left maxilla with alveoli for dP1 and P2). UF 94700. All elements possibly belong to the same individual as they present similar stages of tooth wear and preservation. It is a species recorded in the latest Hemphillian to Blancan NALMAs in Alabama, Florida and Louisiana (USA). It is a medium-sized species of Cormohipparion.
- 20. Pseudohipparion simpsoni, Webb and Hulbert, 1986 [49] (Associated P3-M1, UF 12943). This is a latest Hemphillian species recorded in Florida, Kansas, Oklahoma and Texas (USA).
- 21. Pliohippus coalingensis, Merriam, 1914 [50] (UCMVP 21341). This species is a Pliocene horse from California (USA).
- 22. Nannippus beckensis, Dalquest and Donovan, 1973 [51] (Partial Skull with Right and Left P2–M3, TMM41452-1). This is a Blancan species from Texas (USA). This species is a medium-sized and moderately hypsodont hipparion.
- 23. Equus simplicidens, Cope, 1892 [52] (Left M1, TMM 40282-6). This species is interpreted to have been a medium- to large-sized equid with primitive dentition [53], recorded in Baja California (Mexico) and Arizona, California, Idaho, Kansas, Nebraska and Texas (USA) from Blancan to Irvingtonian. The species was initially based upon fragmented molars, with sizes comparable to E. occidentalis and E. caballus [52]. According to Skinner [54], E. simplicidens shows great similarities in the skull and dentition with the modern Equus grevyi, and the differences in the skull are small and expected in temporal and geographic separation. Gidley [55] described the Hagerman horses based upon characters common to all zebrine horses, with taxonomically significant differences from non-zebrines, but the characters used to distinguish it from other zebrines are of doubtful validity [56]. Comparing E. simplicidens with the East African Grevy’s zebra, E. grevyi, Skinner [54] included both in the subgenus Dolichohippus [57]. This proposal was questioned by Forsten and Eisenmann [57], as the cranial similarities found by Skinner [54] might be allometrically related to the large skull size [57]. Furthermore, Skinner [54] did not compare the basicranium, missing the comparison of Franck’s Index (i.e., the distance from the staphylioin to the hormion and from the hormion to the basion). The index was considered phylogenetically important, as the lengthening of the hormion to the basion distance seems to have led to a decrease in the index during Equus evolution, with a high index being related to a more primitive character than a lower derived index [57]. In Forsten and Eisenmann’s [57] analysis, both E. simplicidens and Pliohippus (Dinohippus), considered the generic ancestor of Equus, presented a high index, while E. grevyi and the other extant species presented lower indices. Following Matthew [4], Forsten and Eisenmann [57] also suggested E. simplicidens should be included in the subgenus Plesippus [4,58]. Equus simplicidens has long been considered the earliest common ancestor of Equus [57], but a recent analysis of the genus Equus suggested that Plesippus and Allohippus should be elevated to a generic rank, indicating Allohippus stenonis as the sister taxon to Equus and Plesippus simplicidens and P. idahoensis as the sister taxa to the Allohippus plus Equus clades [9]. On the other hand, recent cladistic analyses combined with morphological and morphometrical comparisons of skulls suggest E. simplicidens as the ancestor of Equus, not endorsing Plesippus and Allohippus at the genus or subgenus level [10].
- 24. Equus idahoensis, Merriam, 1918 [59] (Upper Left Premolar, UCMP 22348). This is a Blancan and early Irvingtonian species recorded in Arizona, California, Idaho and Nevada (USA). The type locality is Locality 3036C, in the beds of the Idaho locality, near Froman Ferry on the Snake River, 8 mi SW Caldwell, Idaho. According to Winans [56], none of the traits from the original description of E. idahoensis are unique to this species. However, large samples of specimens (e.g., Grandview, Idaho; 111 Ranch, Arizona) have been referred to as E. idahoensis, which have distinctive morphological features [9,60] that indicate that this is a potentially valid species. The cheek teeth are large and heavily cemented.
- 25. Equus enormis, Downs and Miller, 1994 [61] (The holotype, IVCM 32, is a partial skull and right and left mandibles, with the right distal humerus, right radius-ulna, MCIII, unciform, magnum, trapezoid and MCIII, phalanges 1, 2, and 3 of the manus; partial pelvis, right femur, MTIII with MTII and MTIV and phalanx 3 of the pes from Vallecito Creek, Anza-Borrego Desert State Park, San Diego County, California, USA). This species is known primarily from the late Blancan – Irvingtonian of California (USA). Equus enormis is a large-sized monodactyl horse with an estimated height at the withers of 1.5 m.
- 26. Equus cumminsii, Cope, 1893 [38] (Fragmentary Upper Molar, TMM 40287-14). This small species has been recorded in Kansas and Texas (USA) from Blancan to early Irvingtonian (NALMAs). Although it is poorly represented by fossils and the type of specimen is a single damaged tooth, some authors consider this species as an early ass based on dental morphology [60,62,63].
- 27. Equus calobatus, Troxell, 1915 [64] (Left MTIII, YPM 13470). This species is a large stilt-legged horse reported from the late Blancan to early Rancholabrean NALMAs, with records in Alberta (Canada); Aguascalientes (Mexico); Colorado, Kansas, Nebraska, New Mexico, Oklahoma and Texas (USA). The original discovery consisted of “unusually long and slender” limb bones [64] from Rock Creek, Texas, but no single holotype specimen was designated. Hibbard [65], therefore, selected YPM 13460 as the lectotype. Because the lectotype and cotypes are limb bones with no distinctive characters other than the large size and relative slenderness of the metapodials, there are few morphological criteria available for evaluating this species. Multiple studies [29,56] have synonymized E. calobatus with Equus (or Haringtonhippus) francisci, but other studies consider it a valid species [66,67].
- 28. Equus scotti, Gidley, 1900 [68] (Associated Skeleton with Skull, Mandible, Complete Feet and Forelimb Bones, One Complete Foot and Hindlimb and All the Cervical, Several Dorsal and Lumbar Vertebrae, AMNH 10606). This species is recorded from the late Blancan to Rancholabrean NALMAs in Alberta, Ontario, Saskatchewan and Yukon (Canada) and in California, Florida, Idaho, Kansas, Nebraska, New Mexico, Oklahoma and Texas (USA). Winans [54] also interpreted E. scotti to be on average slightly smaller than E. simplicidens, but the measurements provided in that study actually indicate the opposite, and subsequent review confirms that E. scotti was of a larger form than E. simplicidens.
- 29. Equus stenonis anguinus, Azzaroli and Voorhies, 1993 [69] (Complete Skull and Jaw, USNM 23903). This is a late Blancan species recorded in Arizona and Idaho (USA). It is described as similar to E. stenonis from the Early Pleistocene of Italy, with skull dimensions falling within the size range of this latter species [69]; however, the limb bones are, on average, more elongated. Equus stenonis anguinus possesses a preorbital pit and a deep narial notch as do the European E. stenonis samples.
- 30. Equus conversidens, Owen, 1869 [70] (Fragmentary Right and Left Maxilla with All Cheek Teeth, IGM4008, Old Catalog Number MNM-403). This is a widespread species reported to have ranged from the Irvingtonian to Rancholabrean with a geographic distribution encompassing North and Central America: Alberta (Canada); Aguacaliente de Cartago (Costa Rica); Apopa Municipality (El Salvador); Aguascalientes, Chiapas, Hidalgo, Jalisco, Michoacán, Nuevo Leon, Puebla, Oaxaca, San Luis Potosi, Sonora, Estado de Mexico, Tlaxcala, Yucatán and Zacatecas (Mexico); Azuero Peninsula and El Hatillo (Panama); Arizona, California, Florida, Kansas, Nebraska, New Mexico, Oklahoma, Texas and Wyoming (USA). The holotype specimen from the Tepeyac Mountain was described by Owen [70] based upon photos [71]. Owen considered the species to be almost identical to E. curvidens (South American E. neogeus) but with cheek tooth rows converging towards their anterior ends. Cope [72] interpreted the anterior convergence of the cheek tooth rows to be an artifact of the restoration compounded by the photography and assigned the specimen to E. tau, albeit without any stated justification. Gidley [69] interpreted the two sides of the maxilla to be from different individuals, since they were found separately and with missing broken edges. Hibbard [65] provided a reconsideration of the specimen and confirmed that Cope [68] was correct regarding the distortion of the palate and that Gidley [73] was incorrect regarding the two sides deriving from different individuals. Azzaroli [74] described a fragmentary skull (LACM 308/123900) from Barranca del Muerto near Tequixquiac, Mexico, in which the “two tooth rows converge rostrally, giving evidence that the palate of the holotype (of E. conversidens) was correctly mounted and that Owen’s name is after all appropriate”.
- 31. Equus lambei, Hay 1915 [75] (~200 ka–~10 ka) (nearly complete skull from a female, USNM8426, collected from Gold Run Creek in the Klondike Region, Yukon Territory, Canada). This species inhabited the steppe–tundra grasslands of Beringia, with remains having been recovered from Siberia, Alaska, and the Yukon (extending slightly into the adjacent Northwest Territories). Recent genomic evidence suggests that E. lambei and E. ferus may represent a single species [29,76,77,78], although further research is required before this phylogeny can be resolved (see Supplementary Materials, Section 3.4 supplementary text for a discussion on E. ferus in North America).
- 32. Equus (or Haringtonhippus) francisci, Hay, 1915 [76] (Complete Cranium, Mandible and MTIII, TMM 34–2518). This species has been recorded from Irvingtonian to Rancholabrean localities in the Yukon (Canada); Aguascalientes, Estado de Mexico, Jalisco, Puebla, Sonora, and Zacatecas (Mexico); Alaska, Arizona, Florida, Kansas, Nebraska, New Mexico, Texas and Wyoming (USA). It is the oldest name assigned to the stilt-legged group and was first described as being similar to E. tau but with different P3-M1 proportions, which is expected in teeth at different stages of wear and, therefore, probably not a significant taxonomical difference (56). Eisenmann et al. [67] reassigned E. francisci to the genus Amerhippus, as Amerhippus francisci. Heintzman et al. [29] assigned stilt-legged, non-caballine specimens from Gypsum Cave, Natural Trap Cave, the Yukon and elsewhere to their new genus Haringtonhippus under the species Ha. francisci, based upon complete mitochondrial and partial nuclear genomes as well as morphological data and a crown group definition of the genus Equus. Barrón-Ortiz et al. [9] considered Haringtonhippus to be a synonym of Equus and regarded both E. francisci and E. conversidens as distinct taxa based upon morphological criteria.
- 33. Equus fraternus, Leidy, 1860 [79] (Upper Left P2, AMNH 9200). This species has been recorded in Alberta (Canada) and in Florida, Illinois, Mississippi, Nebraska, Pennsylvania, South Carolina and Texas (USA) from Irvintonian to Rancholabrean. Winans [56] considered the dental characteristics used to identify the species to vary with wear and that the specimens used in its diagnosis represented more than one individual, making it uncertain whether to attribute it to one species. Azzaroli [74,80] referred several complete skulls, mandibles and other bones from the southeastern USA to this species.
- 34. Equus pseudaltidens, Hulbert, 1995 [67] (right maxillary with worn DP2, DP3, DP4, M1 and M2 and unerupted P2, P3 and P4 (BEG 31186-35); right and left mandibles with worn i1, di2, dp2, dp3, dp4, m1 and m2 and unerupted p2, p3 and p4 (BEG 31186-36); cranium lacking occiput (BEG 31186-37); right third metacarpal (BEG 31186-3); right and left femora (BEG 31186-2, 34); right and left tibiae (BEG 31186-1, 10); right and left third metatarsals (BEG 31186-4, 7); first phalanx (BEG 31186-24), all thought to belong to the same individual and estimated to have been approximately 3 years old [81]). This species was originally described as Onager altidens by Quinn [81]. The use of Onager instead of Hemionus by Quinn [81] was invalid [67]. Referral of this species to the genus Equus makes it a homonym of Equus altidens, von Reichenau, 1915 [53,67,82]. Therefore, Hulbert [67] proposed the replacement name E. pseudaltidens for E. altidens (Quinn). Also referred to this species are a pair of maxillae (BEG 31186-23) and a right mandible (BEG 31186-22) of an animal approximately 1 year old, and 24 deciduous and permanent upper and lower teeth recovered from the type locality [81]. Equus pseudaltidens is known from the Irvingtonian–Rancholabrean and it has been reported from the Gulf Coastal Plain of Texas [67,81] and possibly from Coleman, Florida [67]. It is a stilt-legged equid with metapodial dimensions that are similar to extant hemionines [67,76]. Compared to other stilt-legged equids discussed here, Equus pseudaltidens is smaller than E. calobatus but larger than both E. francisci and E. cedralensis [67,76,83]. Kurtén and Anderson [84] synonymized E. (Hemionus) pseudaltidens with Equus (Hemionus) hemionus. Winans [85] assigned it to her E. francisci species group. Hulbert [67] considered that E. pseudaltidens and E. francisci were distinct species and hypothesized that they are sister taxa. Azzaroli [74] synonymized E. pseudaltidens (as Onager altidens) with E. semiplicatus. Eisenmann et al. [67] considered E. pseudaltidens distinct from E. semiplicatus and E. francisci and assigned it along with the latter species to Amerhippus. Heintzman et al. [29] considered E. altidens (=E. pseudaltidens) a junior synonym of Haringtonhippus francisci.
- 35. Equus verae, Sher, 1971 [86] (Holotype Mandible with a Full Row of Teeth, GIN 835-123/21, River Bolshaja Chukochya Exp. 21, Kolyma Lowland, Northeast Yakutia). This species is a large-bodied, stout-legged Early Pleistocene (Olyorian)–Rancholabrean species recorded in Northeastern Siberia (Russia) and the Yukon (Canada). E. verae is much larger than the E. stenonis species and similarities in the teeth and the size of limb bones with E. suessenbornensis, suggesting a subspecies position for E. verae as well as for E. coliemensis (see below). Eisenmann [87,88] suggested that E. verae may belong to the subgenus Sussemionus, but this has not been substantiated by other authors.
- 36. Equus occidentalis, Leidy, 1865 [89] (Lectotype Left P3, VPM 9129). It is a Rancholabrean NALMA horse with records in Mexico (i.e., Baja California and Sonora) and the USA (i.e., Arizona, California, Nevada, New Mexico and Oregon). Leidy [89] named E. occidentalis from two upper premolars and one lower molar from two widely separated geographic localities but did not designate a holotype. Gidley [73] selected a left P3 from Tuolumne County, California, as the lectotype. Merriam [90] referred to E. occidentalis thousands of bones of a large and stout-limbed equid recovered from Rancho La Brea. Savage [91] and Miller [92] believed that the equid from Rancho La Brea, identified as E. occidentalis, did not conform to the lectotype designated by Gidley [73], but neither of these authors proposed a new name. Azzaroli [74] decided to retain the name E. occidentalis sensu Merriam [90] and selected the skull figured by Merriam [90] as his lectotype. However, since Gidley [73] had already designated a lectotype for the species, according to ICZN Article 74, no subsequent lectotype designations can be made. Brown et al. [93] concluded that some of Leidy’s original fossils of E. occidentalis (exclusive of the Tuolumne County tooth) most likely come from the McKittrick asphalt deposits; this locality was not named by Leidy [89], because the town of McKittrick, California, was not named until 1900. These authors also confirmed that many specimens from the original type series of E. occidentalis closely resemble the large Pleistocene horses from McKittrick and Rancho La Brea [93]. Barrón-Ortiz et al. [94] recognized the presence of this species outside of the North American Western Interior during the Late Pleistocene. Barrón-Ortiz et al. [9] recognized it as a valid species closely related to E. neogeus.
- 37. Equus cedralensis, Alberdi et al., 2014 [83] (fragment of a mandibular ramus formed by two specimens: one p2-m3 right row (DP-2675 I-2 15) and a second fragment of the symphysis with the anterior dentition (DP-2674 I-2 8), articulated together from Rancho La Amapola, Cedral, San Luis Potosí, Mexico, and stored at the Paleontological Collection (DP-INAH) of the Laboratorio de Arqueozoología “M. en C. Ticul Álvarez Solórzano” Subdirección de Laboratorios y Apoyo Académico, INAH in Mexico City). This species is primarily known from the Rancholabrean of Mexico (i.e., Aguascalientes, Chihuahua, Estado de Mexico, Michoacán, Puebla and San Luis Potosí). Equus cedralensis is an equid with a small body mass (estimated mean mass of 138 kg) [83,95]. Equus cedralensis was diagnosed as stout-legged, but a recent analysis placed it within stilt-legged horses and with its dental morphology being similar to Ha. francisci. Jimenez-Hidalgo and Diaz-Sibaja [96] considered it a junior synonym of Ha. francisci. Equus cedralensis differs from the holotype of Ha. francisci, as it is smaller in size and the lower first and second incisors possess enamel cups.
- 38. Equus mexicanus, Hibbard, 1955 [65] (cranium lacking the LM3 (No. 48 (HV-3)) from Tajo de Tequixquiac, Estado de México, Mexico, and stored at the Museo Nacional de Historia Natural; the specimen is cataloged as IGM4009). This species is known from the Rancholabrean of Mexico (i.e., Aguascalientes, Chiapas, Estado de Mexico, Jalisco, Michoacán, Oaxaca, Puebla, San Luis Potosi and Zacatecas) and the USA (i.e., California, Oregon and Texas). Equus mexicanus is a large body sized species (estimated mean mass of 458 kg) [83,95]. Winans [85] placed E. mexicanus in her Equus laurentius species group, but as with other species groups in this study, this was not a strict synonymy. Azzaroli [74] recognized E. mexicanus as a valid taxon, noting that previous investigations had proposed synonymy with and tentatively identified as E. pacificus but rejecting this, since the latter species was initially based upon a single tooth. Barrón-Ortiz [97] assigned specimens identified as E. mexicanus to E. ferus scotti. Barrón-Ortiz et al. [94] assigned specimens identified as E. mexicanus to E. ferus. Barrón-Ortiz et al. [9] recognized E. mexicanus as a valid taxon distinct from E. ferus.
3.2. South America
Two genera, Equus and Hippidion, inhabited the South American continent with records from the Late Pliocene to the Late Pleistocene [98,99,100]. Hippidion is an endemic genus of South American horses characterized mostly by the retraction of the nasal notch, a particular tooth morphology (considered more primitive than Equus and comparable with Pliohippus) and the robustness and shortness of its limb bones [98]. The genus is, at present, represented by three species: Hippidion saldiasi, Hippidion devillei and Hippidion principale [98]. On the other hand, the South American Equus is represented by a single species, E. neogeus, with caballine affinities and metapodial variation corresponding to an intraspecific characteristic representing a smooth cline [9,99,100].
- 1. Equus neogeus, Lund, 1840 [101] (MTIII, 866 Zoologisk Museum). This is a Middle–Late Pleistocene species (Ensenandan and Lujanian SALMA) and the only representative of the genus in the South American continent [9,98,99,100]. Most records are from the Late Pleistocene, but its earliest appearance is recorded in the Middle Pleistocene in Tarija, Bolivia, dated at approximately 1.0–0.8 Ma [102,103]. It has a wide geographic range distribution, encompassing all of South America, except for the Amazon basin and latitudes below 40° [100]. The species probably became extinct sometime during the Late Pleistocene–Holocene transition as suggested by the youngest direct radiocarbon date of 11,700 BP (Río Quequén Salado, Argentina [104]).
- 2. Hippidion saldiasi, Roth, 1899 [105] (p2, Museo Nacional de La Plata). This is a Late Pleistocene species, dated between 12,000 and 10,000 years BP, mostly known from Argentinian and Chilean Patagonia, with records in Central Chile and the Atacama Desert [98,106]. The last records for the species were radiocarbon dated between 12,110 and 9870 BP in Southern Patagonia (Cerro Bombero, Argentina [107]) and Cueva Lago Sofía, Chile [108].
- 3. Hippidion principale, Lund, 1846 [109] (M2, Peter W. Lund Collection, ZMK). This is a Late Pleistocene species (Lujanian SALMA), with records in Argentina, Bolivia, Brazil and Uruguay [98]. This species represents the largest Hippidion. There are few radiocarbon-dated records for this species, with the youngest situated at approximately 16,130 BP (Arroyo La Carolina, Argentina) [104]; however, remains found in archaeological contexts dating close to 13,200 BP in Tagua, Central Chile [110], suggest a later presence for this taxon.
- 4. Hippidion devillei, Gervais, 1855 [111] (P2–P3 Row and Fragmented Astragalus, IPMNHN). This species has been reported in Uquia (Argentina, Late Pliocene–Early Pleistocene), Tarija (Bolivia) and Buenos Aires (Argentina) from the Middle Pleistocene (Ensenadan SALMA) to the Late Pleistocene (Lujanian SALMA) and in Brazil [98]. This taxon has been directly radiocarbon dated only from cave contexts in the high Andes of Peru, with the youngest record of 12,860 BP [112,113].
4. Systematics of the Equinae since 5.3 Ma in Eurasia and Africa
4.1. Eastern and Central Asia (China, Mongolia, Russia, Uzbekistan, Kazakhstan and Tajikistan)
The fossil record of the three-toed horses from Eastern Asia includes four different genera (i.e., Plesiohipparion, Cremohipparion, Proboscidipparion and Baryhipparion) with seven identified species. As for the Indian Subcontinent, Europe and Africa (see below), the Miocene–Pliocene boundary marks the extinction of the genera Hippotherium, Hipparion s.s., Sivalhippus and Shanxihippus [8]. On the other hand, Sun and Deng [114] argued that the Equus Datum in China is represented by the simultaneous appearance of five stenonine Equus species: E. eisenmannae, E. sanmeniensis, E. huanghoensis, E. qyingingensis and E. yunnanensis. Subsequently, Sun et al. [115] indicated the E. qyingingensis FAD at 2.1 Ma.
- 1. Plesiohipparion houfenense, Teilhard de Chardin and Young, 1931 [116] (MN13–MN15; 6–3.55Ma). The lectotype RV 31031 includes the right p3–m3 from Jingle, Shanxi. The earliest P. houfenense first occurs in the Late Miocene Khunuk Formation, Kholobolchi Nor, Mongolia [8,117,118,119] and the Late Miocene/Early Pliocene Goazhuan Formation of the Yushe Basin (5.8–4.2 Ma) [8,120]. It also occurs into the Pliocene of China.
- 2. Proboscidipparion pater, Matsumoto, 1927 [121] (MN14–MN15; 5–3.5 Ma). The lectotype THP14321 is a skull with a mandible estimated to be 4–3.55 Ma [122]. This species is reported from the Yushe Basin (China), and it may be the original source for the evolution of the Pliocene European species Proboscidipparion crassum and Proboscidipparion heintzi.
- 3. Plesiohipparion huangheense, Qiu et al., 1987 [122] (MN15; 5.0–3.55). The lectotype THP 10097 is a lower jaw fragment, including the cheek teeth, from the Yushe Basin [122]. It is a Chinese species reported from Inner Mongolia at 3.9 Ma [7,119,122] and more broadly from the MN15 of China and India [8]. Ultimately, Pl. aff. huangheense has been reported from the Early Pleistocene in Gulyazi, Turkey [123].
- 4. Cremohipparion licenti, Qiu et al., 1987 [122] (MN15, circa 4.0 Ma). The holotype is THP20764, an incomplete cranium from the Yushe Basin [122]. This distinctly Chinese species is the latest occurring member of the genus Cremohipparion and is reported from the Yushe Basin [8].
- 5. Baryhipparion insperatum, Qiu et al., 1987 [122] (MN16–MNQ17; 3.55–1.8 Ma). The holotype is THP19009, an incomplete cranium with the mandible from the Yushe Basin [122]. It is reported that this species is from the Pliocene in China.
- 6. Plesiohipparion shanxiense, Bernor et al., 2015 [124] (MNQ17; 2.5–1.8 Ma). The holotype is F:AM111820, a complete skull with the mandible [124]. This species, previously recognized as Plesiohipparion cf. P. houfenense [117], is the largest and, at the same, the time youngest member of the genus in Eastern Eurasia. It is believed to be 2.0 Ma in age [124]. It may represent the last evolutionary stage of the genus Plesiohipparion in China. The absence of the POF suggest an evolutionary relationship with Pl. houfenense.
- 7. Proboscidipparion sinense, Sefve, 1927 [125] (MN17-MQ1; 2.5–1.0 Ma). The holotype is PMU M3925, a complete cranium from Henan Province, China. Proboscidipparion sinense occurs later in the record and is approximately one-seventh larger than P. pater. Proboscidipparion sinense is the latest occurring hipparion in China extending its range up to 1.0 Ma [126].
- 8. Equus eisenmannae, Qiu et al., 2004 [127] (2.55–1.86 Ma). The holotype is IVPP V13552, a complete cranium with and the mandible from Longdan [127]. It is a large-sized horse, mostly known from the Early Pleistocene locality of Longdan (China), similar in size to E. livenzovensis (see below), and the primitive features of the cranial morphology suggest a close evolutionary relationship with E. simplicidens [10,13]. At the present time, its evolutionary linkage with other Chinese Equus is not known.
- 9. Equus sanmeniensis, Teilhard de Chardin and Piveteau, 1930 [128] (2.5–0.8 Ma). The lectotype is NIH 002 (Paris), a complete cranium with the mandible from Nihewan, Hebei Province. It is a large-sized, Early Pleistocene species from North and northwest China, Siberia (Aldan River, Bajakal Lake area), Kazakhstan and Tajikistan [114,129]. Sun and Deng [114] suggested a morphological similarity between E. sanmeniensis and E. simplicidens, although diversified from E. stenonis. This evolutionary hypothesis has also been supported by Cirilli et al. [10,13] through morphometric studies on crania. Equus sanmeniensis has been reported from the Early to Middle Pleistocene [114,130].
- 10. Equus huanghoensis, Chow and Liu, 1959 [131] (2.5–1.7 Ma). The holotype is IVPP V2385–2389, with three upper premolars and two molars from Huanghe, Shanxi (Sun and Deng, 2019). It is a large-sized, Early Pleistocene species from the localities of Nihewan (Hebei), Linyi (Shanxi), Sanmenxia Pinglu (Shanxi), Xunyi (Shaanxi) and Nanjing (Jiangsu). Sun and Deng [114] supported the hypothesis provided by Deng and Xue [130] that E. huanghoensis is a stenonid horse, considering the Nihewan sample as one of the Equus species with the largest palatal length with E. eisenmannae. The morphometric analyses of Cirilli et al. [10,13] show a primitive morphology of the cranium, similar to E. simplicidens and distinct from E. stenonis. Ao et al. [132] indicated the age of 1.7 Ma as the youngest record of this species in China.
- 11. Equus yunnanensis, Colbert, 1940 [133] (2.5–0.01 Ma). The lectotype is IVPP V 4250.1, an almost complete but deformed cranium. It is a medium-sized, Early Pleistocene species from the Chinese localities of Yuanmou (Yunnan), Liucheng (Guangxi), Jianshi and Enshi (Hubei), Hanzhong (Shaanxi) and Huili (Sichuan) and from Irrawaddy in Myanmar. The species was initially described by Colbert [133] on isolated cheek teeth, while better knowledge of this species came with the new discoveries from the Yuanmou locality. Deng and Xue [130] proposed a close evolutionary relationship with E. wangi, whereas Sun and Deng [114] suggested a close evolutionary relationship with E. teilhardi, suggesting that these species are distinct from all other Chinese stenonid horses [114].
- 12. Equus teilhardi, Eisenmann, 1975 [134] (2.0–1.0 Ma). The holotype is NIH001, an incomplete mandible. It is a medium-sized, Early Pleistocene species from northwestern and North China. Sun et al. [135] proposed a close evolutionary relationship between E. teilhardi and E. yunnanensis, later supported by the cladistic analysis of Sun and Deng [114].
- 13. Equus qyingyangensis, Deng and Xue, 1999 [130] (2.1–1.2 Ma). The holotype is NWUV 1128, an incomplete cranium. It is a medium-sized, Early Pleistocene species from northwestern and North China. Eisenmann and Deng [136] recognized some close anatomical features between E. simplicidens and E. qyingyangensis, suggesting a close evolutionary relationship between these two species. The latest phylogenetic results of Sun and Deng [114] and Cirilli et al. [10] support this last hypothesis. A new E. qyingyangensis sample has recently been described from Jinyuan Cave [115], with an FAD of 2.1 Ma.
- 14. Equus wangi, Deng and Xue, 1999 [130] (2.0–ca. 1.0 Ma). The holotype is NWUV 1170, a complete upper and lower cheek teeth rows from Gansu Province, Early Pleistocene [130]. Sun and Deng [114] reported a large size for E. wangi, similar to E. eisenmanne, E. sanmeniensis and E. huanghoensis. The phylogenetic position of E. wangi is not well defined, although Sun and Deng [114] highlighted a possible closer relationship with E. eisenmannae than any other stenonine Equus.
- 15. Equus pamirensis, Sharapov, 1986 [137] (Early Pleistocene). The holotype is IZIP 1-438 (Institute of Zoology and Parasitology, Uzbekistan), a complete upper tooth row from Kuruksai [137], approximately 2 Ma (MN17), possibly earlier. It is a large species of stenonine horse described from the Kuruksai, 18 km NE of Baldzuan, Tajikistan, in the Kuruksai River valley in the Afghan–Tajik Depression [138]. The site has been correlated to the middle Villafranchian. The taxonomy of this horse is contentious [138], with it being referred to variously as Equus (Allohippus) aff. sivalensis [137] and E. stenonis bactrianus [139]. We currently regard this as a distinct species.
- 16. Central Asian Small Equus sp. from Kuruksai [138] (Early Pleistocene). This is a small species of horse that co-occurs with the larger E. pamirensis at Kuruksai. Metrically, the metapodials fall within the range of variation of E. stehlini [138]. Similar small horse remains have also been found in the Early–Middle Pleistocene Lakhuti 1 locality in the Afghan–Tajik Depression [138].
- 17. Equus (Hemionus) nalaikhaensis, Kuznetsova and Zhegallo, 1996 [140]. This species was found in the late Early Pleistocene and early Middle Pleistocene (approximately 1 my, Jaramillo paleomagnetic episode) in Mongolia. The lectotype PIN 3747/500 is represented by the incomplete skull of an old male from Nalajkha [141].
- 18. Equus coliemensis, Lazarev, 1980 [142]. The holotype is a skull with very worn teeth (col. IA 1741). The type locality is the river Bolshaja Chukochya, Kolyma lowland, northeast Yakutia, Siberia, Russia. It is reported from the late Early Pleistocene in northeastern Siberia (Russia). Recently, Eisenmann [87] included E. coliemensis in the subgenus Sussemonius.
- 19. Equus lenensis, Rusanov, 1968 [143]. The holotype skull comes from the Lena River delta (GIN Yakutia col. 33). The type locality is the river Bolshaja Chukochya, Kolyma lowland, northeast Yakutia, Siberia (Russia). It is reported from the Middle Pleistocene from northeastern Siberia (Russia). Lazarev [144] considered E. lenensis to be close to the North American E. lambei, even larger and more heavily built than the latter. It is also known from the Middle Pleistocene in Yakutia Equus orientalis (Equus caballus/ferus orientalis) and Equus nordostensis (Equus ferus/caballus nordostensis) [143,144,145]. Equus nordostensis is characterized by a large size based on the skull, with low plication of the “marks” of the upper teeth and a long protocone [144]. According to Kuzmina [129], it is junior synonym of E. mosbachensis. Equus orientalis has a large skull and a long snout with an elongated teeth row, flat protocone and rare plication of the upper teeth [144].
- 20. Equus beijingensis, Liu, 1963 [146] (late Middle Pleistocene). The holotype is V2573-2574, a palate and jaw from Zhoukoudian, China [146,147]. It is a Chinese caballine horse recovered mostly form locality 21 of Zhoukoudian. Unfortunately, the species is not well represented, although Forsten [147] indicated a similar size with E. sanmeniensis. Liu [146] also indicated E. sanmeniensis as the possible ancestor for E. beijingensis; nevertheless, this hypothesis was discarded by Fortsen [147] and Deng and Xue [130], who identified E. beijingensis as a caballine horse, characterized by a U-shaped linguaflexid. Its evolutionary position is not well defined, although Forsten [147] and Deng and Xue [130] proposed that E. beijingensis is a relative of European E. ferus (their E. mosbachensis) or of a North Americam caballine horse [130].
- 21. Equus valeriani, Gromova, 1946 [148] (Late Middle–Late Pleistocene). The hypodigm includes upper and lower cheek teeth, figured in Eisenmann et al. [149]. It is an enigmatic taxon, described by Gromova [148], from Samarkand, Uzbekistan. According to Gromova [148] and Eisenmann et al. [149], it shows a stenonine metaconid-metastylid in the lower cheek teeth but with a long protocone in the upper cheek teeth. Its possible occurrence has also been proposed in Syrie (Kéberien Géométrique d’Umm el Tlel), although this identification still remains uncertain [149].
- 22. Equus dalianensis, Zhow et al., 1985 [150] (Late Pleistocene). The holotype is V821966, an incomplete mandible preserving two lower cheek teeth rows. It is a Chinese caballine horse described from Gulongshan Cave, Liaoning. Forsten [147] demonstrated close morphological and morphometrical similarities with E. ferus gemanicus, E. ferus orientalis and E. ferus chosaricus, suggesting that they all represent individual populations of a single widespread species, E. ferus. Deng and Xue [130] suggested a common origin for E. dalianensis and E. przewalskii, with no ancestor-descendant relationships between them. Nevertheless, a recent genomic analysis by Yuan et al. [151] revealed that E. dalianensis is a separate clade of caballine horses, distinct from E. przewalskii.
- 23. Equus ovodovi, Eisenmann and Vasiliev, 2011 [152] (0.04–0.01 Ma). The holotype is IAES 21, a fragmentary palate from Proskuriakova Cave [152]. It was described in the Late Pleistocene site of Proskuriakova Cave (Khakassia, southwestern Siberia, Russia). It was first considered a species related to E. hydruntinus and modern hemiones, although the genomic analyses by Orlando et al. [153] suggest a relationship with wild asses, representing a new separated fossil clade with no extant relatives [150]. For this reason, Orlando et al. [153] and Eisenmann and Vasiliev [152] included this species in the subgenus Sussemionus. Molecular studies suggest that E. ovodovi is a sister to extant zebras and is nested within the clade that includes both extant zebra and asses [154]. Equus ovodovi has been recognized in the Late Pleistocene of southern and eastern Russia and more recently in China [152,154,155].
- 24. Equus hemionus, Pallas, 1774 [156] (0.0 Ma). Pallas [156] did not refer to any holotype or lectotype but gave a detailed description of the anatomical features of the species, associated with an illustration of an animal located near Lake Torej-Nur, Transbaikal area [156] (V.19, pp. 394–417, Pl. VII). Equus hemionus is known as the Asiatic wild ass, distributed in China, India, Iran, Mongolia and Turkmenistan. Historically, it has also been reported in Afghanistan, Armenia, Azerbaijan, Georgia, Iraq, Jordan, Kuwait, Kyrgyzstan, Russia, Saudi Arabia, Syria, Tajikistan, Turkey and Ukraine. Four different subspecies are identified, mostly describing the present areal distribution: E. hemionus hemionus (Mongolia), E. hemionus khur (India), E. hemionus kulan (Turkmenistan) and E. hemionus onager (Iran). Another extinct subspecies was recognized in Syria, E. hemionus hemippus. The fossil record is not well studied, but crania and mandibles of the species have been reported from Narmada Valley in Central India [157], the Indian state of Gujarat [158], and the Son Valley in Northern India [159]. This species is distinguished from E. namadicus by its smaller size and smaller protocones on the premolars. Radiocarbon dates from these deposits suggest that this species entered South Asia during the last glacial period, most likely from West Asia [160,161]. The most recent genetic analyses suggest that the onager and kiang populations diverged evolutionarily ca. 0.4–0.2 Ma [28].
- 25. Equus kiang, Moorcroft, 1841 [162] (0.0 Ma). Moorcroft [162] did not designate any holotype or lectotype but provided a general description of the species [162]. Equus kiang is known as the Tibetan ass, with a distribution in China, Pakistan, India, Nepal and, possibly, Bhutan. Three subspecies have been identified, E. kiang kiang, E. kiang holdereri and E. kiang polyodont, with different authors pointing out their subspecific statuses [162,163,164,165]. At the present time, no paleontological information is available for E. kiang. Jonsson et al. [28] distinguished E. kiang from E. hemionus as a valid taxon. The most recent morphological cladistic analysis found E. kiang and E. hemionus to be stenonine horses [10].
- 26. Equus przewalskii, Poliakov, 1881 [166] (~0.1–0.0 Ma). The holotype, a skull (number 512) and skin (number 1523), are found in the collection of the Laboratory of Evolutionary Morphology, Moscow (museum exposition), originally obtained by N. M. Polyakov in Central Asia, southern Dzhungaria, in 1878 [129]. For a complete description of the species see Groves [167] and Grubb and Groves [168]. It is an extant species of caballine horse that is found in small geographic areas of Central Asia, although, historically, it once ranged from Eastern Europe to eastern Russia [169]. In China, E. przewalskii is common in the Late Pleistocene (~0.1–0.012 Ma) sites in the northern and central regions of the country, but it is absent in the Holocene, except in northwestern China [170]. It shows close morphological similarities with Equus ferus (see below), and both are members of caballine horses [10,28,153]. While genomic analyses have shown that the Przewalski’s horses are the descendants of the first domesticated horses from the Botai culture in Central Asia (Kazakhstan) around 5.5 ka [171,172], subsequent morphological studies have shown that Botai horses are not domestic horses but harvested wild Prezwalski’s horses [173].
4.2. Indian Subcontinent
The Siwalik Group and co-eval sediments of the Himalayan Foreland Basin preserve an exceptional record of hipparionine and equine horses. The earliest lineage, Cormohipparion, appears in the record at approximately 10.8 Ma. The diverse indigenous Sivalhippus lineage ranges from 10.4 to 6.8 Ma, “Hipparion” from 10 to 9.6 Ma and Cremohipparion from 8.8 to 7.2 Ma [174]. Across the Mio–Pliocene boundary, a turnover in hipparion taxa seems to have taken place, with the older lineages being replaced in the Late Pliocene by Plesiohipparion and Eurygnathohippus along with a distinct but poorly known species “Hippotherium” antelopinum [119,175]. These Late Pliocene hipparionines are replaced by stenonine equids represented by Equus sivalensis and a small species of ass-like Equus in the Early Pleistocene [176]. By the Middle Pleistocene, a third species of large stenonine horse, Equus namadicus, is common in peninsular India deposits; this species went extinct in the Late Pleistocene [177]. Equus hemionus doesn’t appear in the record until ~0.03 Ma [177], and Equus caballus is found in Holocene archaeological sites [178].
- 1. “Hippotherium” antelopinum, Falconer and Cautley, 1849 [179] (3.6–2.6 Ma). The lectotype is NHMUK PV M.2647, a subadult right maxilla fragment with P2-M3. It is a species of hipparionine horse from the late Pliocene age deposits between the rivers Yamuna and Sutlej in India. This taxon has been the subject of much nomenclatural confusion. Lydekker named the lectotype and along with the hypodigm, placed it within the genus Hipparion. Later authors [180,181,182] have referred Miocene hipparionine material collected on the Potwar Plateau in Pakistan to this táxon and reassigned the species to the genus Cremohipparion. However, given that the hypodigm of “Hippotherium” antelopinum comes from the Late Pliocene and does not preserve any apomorphies of Cremohipparion; we refer to the late Pliocene specimens as “Hippotherium” antelopinum, separate from the Potwar Plateau specimens from the Dhok Pathan Formation, which can still be taxonomically referred to as Cremohipparion, but a formal description of the species with a new type of specimen is required. A more comprehensive study currently in preparation will attempt to resolve this issue
- 2. Plesiohipparion huangheense, Qiu et al., 1987 [122] (3.6–2.6 Ma). Jukar, et al. [119] identified NHMUK PV OR 15790, a mandibular fragment with p4-m1, originally classified as “Hippotherium” antelopinum, as P. huangheense from the Late Pliocene of the Siwalik Hills.
- 3. Eurygnathohippus sp. (3.6–2.6 Ma). Four mandibular cheek teeth from the Late Pliocene of the Siwaliks from the Potwar Plateau in Pakistan and the Siwalik Hills in India were identified by Jukar et al. [175] as Eurygnathohippus sp. These specimens all bear the characteristic single pli-caballinid and ectostylid.
- 4. Equus sivalensis, Falconer and Cautley, 1849 [179] (2.6–0.6 Ma). The lectotype is NHMUK PV M.16160, an incomplete cranium from the Siwaliks [183]. It is a large species of stenonine horse found in the Siwaliks of the Indian Subcontinent, ranging from the Potwar Plateau in the west to the Nepal Siwaliks in the east. The exact temporal distribution is unknown; however, based on paleomagnetic dating of the Pinjor Formation where the species was found, it likely ranges in age from 2.6 to 0.6 Ma [176,184]. However, some potentially older occurrences from just below the Gauss–Matuyama boundary (>2.6 Ma) have also been reported [185,186].
- 5. Equus sp. (~2.2–1.2 Ma). This is small species of Equus with smaller slender metapodials has been reported from the Pinjor Formation of the Upper Siwaliks. This species has been referred to as Equus sivalensis minor [187], Equus sp. A [188] or Equus cf. E. sivalensis [189]. A set of postcranial remains, including metapodials, astragali and phalanges, which were formerly tentatively referred to as “Hippotherium” antelopinum, are now believed to belong to this small species of Equus [138,184]. Based on specimens collected from the Mangla–Samwal Anticline and the Pabbi Hills in Pakistan, this species likely ranges in age from ~2.2 to 1.2 Ma [176]. Geographically, it ranges from the Pabbi Hills to the river Yamuna in the east.
- 6. Equus namadicus, Falconer and Cautley, 1849 [179] (~0.5–0.015 Ma). The lectotype is NHMUK PV M.2683, an incomplete cranium from the Siwaliks [183]. It is a large-sized stenonine horse from the Middle and Late Pleistocene of the Indian Subcontinent. The stratigraphic range includes the Middle Pleistocene Surajkund Formation in Central India [190] and Late Pleistocene deposits throughout peninsular India [177]. However, Lydekker [191] reported some specimens from the uppermost Upper Siwaliks, which might suggest that the species extends back to the early Middle Pleistocene.
4.3. Europe
As in Eastern and Central Asia, the Mio–Pliocene boundary represents a relevant turnover of three-toed horses in Europe, with the extinction of the genera Hippotherium and Hipparion s.s. The Pliocene and Pleistocene are characterized by the persistence of Cremohipparion, and the dispersion of Proboscidipparion and Plesiohipparion [7], represented by five species. The Equus Datum is represented by the oldest species, Equus livenzovensis, at ca. 2.6 Ma in Russia, Italy, France and Spain [176], which led to the Equus stenonis’ evolution and to the radiation of the African fossil species [10,13,184,192]. At the present time, we recognize 13 species in the genus Equus during the Pleistocene.
- 1. Plesiohipparion longipes, Gromova, 1952 [193] (7–3.0 Ma). The holotype is PIN2413/5030, a complete mt3 from Pavlodar [193]. It has been identified from Pavlodar (Kazhakstan), Akkasdagi and Calta (Turkey) [194] and Baynunah (UAE) [8,195]. In all cases, Pl. longipes was recognized by its extreme length dimensions of mc3s and mt3s. None of these attributions of Plesiohipparion display the characteristics of extremely angled and pointed metaconids and metastylids of Pl. houfenense, Pl. huangheense, Pl. rocinantis or Pl. shanxiense. If all these taxa are referable to Plesiohipparion, the chronologic range would be 7 Ma to the Early Pleistocene and the geographic range being from China to Spain.
- 2. “Cremohipparion” fissurae, Crusafont and Sondaar, 1971 [196] (MN14-15). The holotype is an mt3 from Layna, Spain, figured in Crusafont and Sondaar [196] (Pl. 1). It was originally described as “Hipparion” fissurae. The species is recorded from the MN15 Pliocene localities in Spain [197] and more recently from the MN14 of Puerto de la Cadena [198]. The most recent analyses provided by Cirilli et al. [192] suggest an attribution to the genus Cremohipparion, yielding this species as the possible last representative of the genus in Europe. Its evolutionary framework is not yet defined.
- 3. Proboscidipparion crassum, Gervais, 1859 [199] (ca. 4.0–2.7 Ma; MN14-16). Deperet [200] described and figured the sample from Roussillon, France, without assigning a holotype or lectotype. The sample figured by Deperet [200] (Pl. V, Figures 6–10; Pl. VI) represents the hypodigm for the species. The sample is a small-sized equid with remarkable similarities with Pr. heintzi [192,194]. No complete crania are known from this species, whereas it is well documented by isolated upper and lower cheek teeth and postcranial elements. Bernor and Sen [194] showed that Pr. crassum also has a very short mc3, whereas Cirilli et al. [192] gave substantial indications in cranial and postcranial elements for its attribution to the genus Proboscidipparion. This species is mostly known from the Pliocene of France (Perpignan, Montpellier) but also from Dorkovo (Bulgaria) and Reg Crag (England) [197,201,202].
- 4. Proboscidipparion heintzi, Eisenmann and Sondaar, 1998 [203] (MN15). The holotype is MNHN.F.ACA49A, a complete mc3 from Calta, Turkey [203]. It was originally identified and described “Hipparion” heintzi [203], whereas Bernor and Sen [194] restudied and allocated this taxon to Pr. heintzi, recognizing the similarity of the Calta juvenile skull MNHN.F.ACA336 to Chinese Pr. pater in the retracted and anteriorly broadly open nasal aperture accompanied by very elongate anterostyle of dP2 [190]. It has only been reported from the locality of Calta.
- 5. Plesiohipparion rocinantis, Hernández-Pacheco, 1921 [204] (3.0–2.58 Ma). The lectotype is a p3/4 figured in Alberdi [205] (Pl. 6, Figure 4). It is the largest three-toed horse from Europe. Qiu et al. [122], followed by Bernor et al. [124,206] and Bernor and Sun [207], recognized this species as being a member of the Plesiohipparion clade by cranial and postcranial morphological features. Plesiohipparion rocinantis is reported between 3.0 and 2.6 Ma [208,209] from La Puebla de Almoradier, Las Higuerelas and Villaroya (Spain); Roca-Neyra (France); Red Crag (England); Kvabebi (Georgia). This species may include “Hipparion” moriturum from Ercsi (Hungary) and the sample from Sèsklo (Greece) previously ascribed to Plesiohipparion cf. Pl. shanxiense [210]. It represents the last occurrence of Plesiohipparion in Europe at the Plio–Pleistocene transition [8,192].
Rook et al. [211] reported the occurrence of “Hipparion” sp. from the Early Pleistocene locality of Montopoli, Italy (2.6 Ma). The “Hipparion” sp. from Montopoli is represented by a single incomplete upper cheek tooth that, however, shows some morphological features distinct from the first Equus, E. livenzovensis occurring in the same locality [211]. Together with Villarroya, Roca-Neyra, Sèsklo, Guliazy and Kvabebi, the Montopoli specimen represents one of the last occurrences of three-toed horses in Europe and may, in fact, be a small species of Cremohipparion.
- 6. Equus livenzovensis, Bajgusheva, 1978 [212] (2.6–2.0 Ma). The holotype is POMK L-4, a fragmentary skull from Liventsovka [212] in the Early Pleistocene. The species represents the Equus Datum in Western Eurasia, Early Pleistocene localities dated at the Plio–Pleistocene boundary (2.58 Ma) such as Liventsovka (Russia), Montopoli (Italy) and El Rincón–1 (Spain) [184,212,213,214,215,216,217,218,219]. Recent research [214,216,220] suggests the occurrence of E. livenzovensis in Eastern European localities, dated between 2.58 and 2.0 Ma. The species shows the typical stenonine morphology, even though it is a large-sized horse.
- 7. Equus major, Delafond and Depéret, 1893 [221] (?2.6–1.9 Ma). The hypodigm is represented by a P2-M1 and a 1ph3 figured by Delafond and Depéret [221] from Chagny, France. It is poorly represented in the Early Pleistocene of Europe, being represented by few remains and localities. It was described by an incomplete upper cheek tooth row and postcranial elements from the Early Pleistocene locality of Chagny (Central France). Following the ICZN guidelines, Alberdi et al. [216] established that E. major has priority over Equus robustus Pomel, 1853 [222]; Equus stenonis race major Boule, 1891 [223]; Equus bressanus Viret, 1954 [224]; Equus major euxinicus Samson, 1975 [225]. Equus major has been reported from the European sites of Senèze, Chagny, Pardines and Le Coupet (France); Tegelen (the Netherlands); East Reunion and Norfolk (England) [216], and, as reported by Forsten [214], it may also be present at Liventsovka (Russia). It is a large-sized monodactyl horse, the largest species of the European Early Pleistocene.
- 8. Equus stenonis, Cocchi, 1867 [226] (2.45–ca. 1.6 Ma). The holotype is IGF560, a complete cranium from the Upper Valdarno Basin, Italy [13]. It was the most widespread Equus species during the Early Pleistocene (MNQ17 and MNQ18). In the last century, E. stenonis samples were identified under several subspecies as E. stenonis vireti, E. stenonis senezensis, E. stenonis stenonis, E. stenonis granatensis, E. stenonis guthi, E. stenonis mygdoniensis, E. stenonis anguinus, E. stenonis pueblensis and E. stenonis olivolanus. Recently, Cirilli et al. [13] reevaluated these subspecies, considering most of them to be ecomorphotypes of the same species, resulting in recognizing that E. stenonis is a monotypic, polymorphic species. At the present time, the species is reported as having occurred from Georgia to Spain in the circum-Mediterranean area as well as the Levant.
- 9. Equus senezensis, Prat, 1964 [227] (2.2–2.0 Ma). The lectotype includes a left P2-M3 and two mc3s, figured in Prat [227] (Pl.1 Figure C; Pl.2 Figure D,E). It is a medium-sized horse, distributed in France and Italy. Earlier referred to as E. stenonis senezensis [227], it has been recognized as a different species by Alberdi et al. [216] and Cirilli et al. [13]. It is morphologically similar to the European E. stenonis but with a reduced size. It is reported from the type locality of Senèze and possibly from Italy between 2.2 and 2.1 [228,229].
- 10. Equus stehlini, Azzaroli, 1964 [230] (1.9–1.78 Ma). The holotype is IGF563, an incomplete cranium from the Upper Valdarno Basin. It is the smallest Early Pleistocene Equus species. Over the last decades, it was considered both either a species or a subspecies of E. senezensis Azzaroli [216,230]. Cirilli [229] found that it can be considered a different Early Pleistocene species that probably evolved from E. senezensis. At the present time, E. stehlini is known only from Italy [228,229].
- 11. Equus altidens, von Reichenau, 1915 [82] (1.8–0.78 Ma). The lectotype is a right p2 figured in von Reichenau [82] (Pl. 6, Figure 17) from Sussenborn, Germany. It is a medium-sized horse, intermediate in size between E. stehlini and E. stenonis, occurring in Western Eurasia in the late Early to early Middle Pleistocene. Originally described from the Middle Pleistocene in Süssenborn [82], over the last decades its chronologic range was extended to the late Early Pleistocene, representing the most widespread species after 1.8 Ma until the Middle Pleistocene [231,232]. Recently, Bernor et al. [14] reported its first occurrence in Dmanisi (Georgia, 1.85–1.76 Ma), supporting the hypothesis of a dispersion of this species from east to west, being part of a faunal turnover that included several other mammalian species during this time frame [14,233,234]. It was also identified in Moldova (Tiraspol, layer 5) [129]. Several populations of E. altidens have been identified as different subspecies (i.e., E. altidens altidens and E. altidens granatensis). Nevertheless, within the nomen, E. altidens may also be included in other European taxa such as Equus marxi von Reichenau, 1915 [82]; Equus hipparionoides Vekua, 1962 [235]; E. stenonis mygdoniensis Koufos, 1992 [236]; Equus granatensis Eisenmann, 1995 [13,216,232,233,237,238]. Its origin remains controversial. Guerrero Alba and Palmqvist [239] proposed a possible African origin, claiming it to be part of the E. numidicus–E. tabeti evolutionary lineage. This latter hypothesis was also reported by Belmaker [240]. Eisenmann [87] included E. altidens in the new subgenus Sussemionus, with other Early and Middle Pleistocene species. More recently, Bernor et al. [14] proposed a new evolutionary hypothesis, considering that E. altidens originated in Western Asia and is potentially related to living E. hemionus and E. grevyi.
- 12. Equus suessenbornensis, Wüst, 1901 [241] (1.5–0.6 Ma). The lectotype is IQW1964/1177, a P2-M3 from Süssenborn, Germany. It is a large horse, larger than E. stenonis and E. livenzovensis but smaller than E. major. As for E. altidens, the species has been described from the Middle Pleistocene in Süssenborn [241], even if its best-known sample comes from the Georgian locality of Akhalkalaki (ca. 1.0 Ma) [242]. In addition to Akhalkalaki and Süssenborn, it has been reported in Central European localities such as Stránská Skála (Czech Republic) and Ceyssaguet and Solilhac (France) [216,243,244]. Over the last decades, its biochronologic range was extended to the late Early Pleistocene, with its earliest occurrence in the Italian localities of Farneta and Pirro Nord [216,231] and in the Spanish sites of Barranco León 5 and Fuente Nueva 3 [245].
- 13. Equus apolloniensis, Koufos et al., 1997 [246] (1.2–0.9 Ma). The holotype is LGPUT-APL-148, a nearly complete cranium from Apollonia, Greece [246]. It is a peculiar species of the late Early Pleistocene, mostly recorded from the locality of Apollonia–1 (Mygdonia Basin, Greece) and, possibly, from other localities of the Balkans and Anatolia [244,246,247]. As reported by Gkeme et al. [247], this species differs from E. stenonis and other European Early Pleistocene Equus, with a distinct cranial morphology, and its size is intermediate between E. stenonis and E. suessenbornensis. Koufos et al. [246] interpreted E. apolloniensis as an intermediate species between E. stenonis and E. suessenbornensis, whereas Eisenmann and Boulbes [248] considered E. apolloniensis as “a step within the lineage of asses”.
- 14. Equus wuesti, Musil, 2001 [249] (1.1–0.9 Ma). The holotypes are IQW1980/17067 and IQW1981/17619, two fragmentary mandibles with p2-m3 from Untermassfeld, Germany [249]. It has been established from the Epivillafranchian locality of Untermassfeld (Germany). Musil [249] reported isolated teeth, mandibles and long bones with primitive and derivate characteristics, and a larger size when compared with the widespread E. altidens. This evidence was also reported by Palombo and Alberdi [232], highlighting a more robust morphology of the postcranial elements. However, scholars disagree on its possible origin. Forsten [250] considered E. wuesti close to and derived from E. altidens, whereas others [249,251] recognized E. wuesti as the possible source for E. altidens. Nevertheless, considering the latest E. altidens discoveries [14,231,232,238], this last hypothesis seems to not be well supported. Palombo and Alberdi [232] suggest also that it can represent an ecomorphotype of E. altidens.
- 15. Equus petralonensis, Tsoukala, 1989 [252] (ca. 0.4 Ma). The holotype is PEC-500, an mc3 from Petralona Cave, Greece [252]. It is a slender and gracile horse from Greece (Petralona Cave). However, the taxonomic status of this species has been actively debated. Forsten [250] considered E. petralonensis as being a member of the E. altidens group, together with the Early Pleistocene equids from Libakos, Krimini and Gerakaou. Eisenmann et al. [67] synonymized E. petralonensis with Equus hydruntinus, within the subspecies E. hydruntinus petralonensis. Despite the taxonomic controversy surrounding this species, E. petralonensis may be considered a stenonine horse because of its mandibular cheek tooth morphology [246].
- 16. Equus graziosii, Azzaroli, 1969 [253] (MIS 6). No access number is available for the holotype, which is figured in Azzaroli [254] (Pl. XLV, Figure 1a,b) as a complete cranium from Val di Chiana, Arezzo. It is an enigmatic species from the late Middle–Late Pleistocene. It was described as a different species by Azzaroli [254] based on a partial cranium, mandibles and postcranial elements. According to Azzaroli [254], the cranium and maxillary and mandibular cheek teeth have typical asinine features, although some other authors have highlighted the morphological similarities with E. hydruntinus [244,255,256]. The evolutionary and phylogenetic position of E. graziosii is still questionable.
- 17. Equus hydruntinus, Regalia, 1907 [257] (0.6–0.01 Ma). There is no holotype. The hypodigm includes isolated upper and lower molars and the fragments of radius and tibia from Grotta Castello (Sicily, Italy). It is known also as the European wild ass, which is a small-sized and slender horse from the Middle and Late Pleistocene in Europe. Its geographic range spans across Europe and is documented in numerous localities [244]. Apparently, the first specimens of this species were also found in Central Asia in several Uzbekistan Late Weichselian localities associated with the Late Paleolithic [129,258]. Its evolutionary history has been debated by many authors, who have proposed different scenarios such as a direct origin from E. altidens [150,259,260], Equus tabeti [237,239] or, more recently, as a new taxon arrival from Asia [228,238]. Nevertheless, the most recent DNA analyses relate E. hydruntinus as a morphotype of the modern E. hemionus, proposing the subspecies E. hemionus hydruntinus [261]. However, several morphological features distinguish E. hydruntinus from E. hemionus (for a detailed discussion, see [244]), allowing for the consideration of E. hydruntinus as a still valid name for fossil identification. The oldest remains of E. hydruntinus were reported from the Middle Pleistocene levels of Vallparadìs, ca. 0.6 Ma [262,263], although the species has been documented in Western Eurasia until the Holocene [244]. Different subspecies have been suggested including E. hydruntinus minor (Lunel Viel, France), E. hydruntinus danubiensis (Romania), E. hydruntinus petralonensis (Petralona Cave, Greece) and E. hydruntinus davidi (Saint-Agneau, France). As reported by Boulbed and Van Asperen [244], E. hydruntinus adapted to semiarid, steppe conditions with a preference for temperate climates, although it could tolerate limited cold conditions.
- 18. Equus ferus, Boddaert, 1758 [264] (ca. 0.7–0.6 Ma). Boddaert [264] did not refer to any holotype or lectotype but gave a detailed description of the anatomical features of the species [264] (p. 159). It first appears in Western Eurasia in the early Middle Pleistocene, although a precise age is not available at the present time. The species has been questioned mostly from a taxonomic viewpoint, wherein many subspecies or different species have been erected to identify the Middle and Late Pleistocene fossil samples of the caballine horses as Equus mosbachensis, E. mosbachensis tautavelensis, E. mosbachensis campdepeyri, E. mosbachensis micoquii, E. mosbachensis palustris, Equus steinheimensis, Equus torralbae, Equus achenheimensis, E. ferus taubachensis, E. ferus piveteaui, E. ferus germanicus, E. ferus antunesi, E. ferus gallicus, E. ferus latipes, E. ferus arcelini and Equus caballus. These taxa are based on the size or morphological differences among the different fossil samples, representing an interesting case of morphological variability within the same lineage. These different subspecies have been considered to be chrono species by Eisenmann and Kuznetsova [265]. Nevertheless, van Asperen [266] noted that differences in the size and morphology of Middle and Late Pleistocene caballine horses can be observed, although they are not more variable than modern ponies or highly homogeneous groups such as Arabian horses or E. przewalskii [244]. Moreover, no unidirectional or evolutionary trend in size and shape can be identified, whereas morphology and size fluctuate over time [19,266]. For this reason, the proposal of Boulbes and van Asperen [244] to consider these species/subspecies as ecomorphological variants of the same species, E. ferus, seems the most parsimonious position. Moreover, as reported by van Asperen [267] following the ICZN, the correct species to indicate the wild caballine horses should be E. ferus and not E. caballus, which refers to domesticated forms. In addition, the genomic studies of Weinstock et al. [77] and Orlando et al. [153] support the genetic variation of the Middle and Late Pleistocene caballine horses.
4.4. Africa
The 5.3 Ma to 10 ka record of Equidae in Africa include two groups of Equinae: Hipparionini and Equini. Churcher and Richardson [268] provided a comprehensive review of African Equidae that was updated by Bernor et al. [269] for its taxonomic content, biogeography and paleoecology with some consideration of the molecular evolution. Churcher and Richardson’s review [268] of the literature that led to their revision was extensive, and the reader is referred to their article for a complete rendering of the record. We documented 10 (+4 not well defined) species of hipparions with three genera Cremohipparion, Sivalhippus and Eurygnathohippus, and 13 species of Equus in the African record, but there certainly could be more or less, although significant synonymies were cited by Bernor et al. [269] and pending new studies, especially on the Equus samples. The Equus Datum in Africa has been a matter of debate over the last years. Bernor et al. [269] and Rook et al. [176] reported the first known occurrence of the genus Equus in in East Africa at 2.33 Ma in the Omo Shungura Formation, member G (Equus sp.). Materials from these earliest occurring Equus are not well represented across the skull, mandible, dentition and postcranial elements. Nevertheless, recent research in the North African sequence of Oued Boucherit (Algeria) have recalibrated the localities of Aïn Boucherit, El Kherba and Aïn Hanech. The lowermost stratigraphic level of Ain Boucherit has been dated at 2.44 Ma [270], where Equus numidicus, Equus cf. E. numidicus and Equus tabeti have been reported [270,271]. Therefore, this new North African age for the Equus Datum anticipates the earliest occurrence of Equus in the north rather than in East Africa.
- 1. Cremohipparion periafricanum, Villalta and Crusafont, 1957 [272] (6.8–4.0 Ma). The lectotype is a P2-M3 figured in Alberdi [205] (Pl.3, Figure 3) from Vadecebro II, Spain. It is a small (dwarf) hipparion that is a close relative (or senior synonym) of Cr. nikosi from the Quarry 5 levels of Samos, Greece, dated 6.8 Ma [206]. Fragmentary remains of Cr. aff. periafricanum have been reported from Tizi N’Tadderth, Morocco [273], and Sahabi, Libya [274].
- 2. Eurygnathohippus feibeli, Bernor and Harris, 2003 [275] (6.8–4.0 Ma). The holotype is KNM-LT139, a partial right forelimb including a fragmentary radius, mt3, a1ph3, a2ph3, partial mc2, a1ph2, a2ph2, a2ph3 and a partial mc4 [275]. There were additional dental and postcranial elements from Lower and Upper Nawata that were referred to this species. Bernor and Harris [275] suggested that the Ekora 4 cranium, ca. 4.0 Ma, was a late surviving member of Eu. feibeli. Whereas Churcher and Richardson [268] recognized “Hipparion” sitifense in the North African Late Miocene–Pliocene horizons, Bernor and Harris [275] and Bernor and Scott [276] noted that the type material described by Pomel [277] could not be located and could potentially be confused either with Cr. periafricanum or Eu. feibeli.
- 3. Sivalhippus turkanensis, Hooijer and Maglio, 1973 [278] (6.5–4.0 Ma). The holotype is KNM-LT136, an adult female cranium. Bernor and Harris [275] assigned this as being a species of Eurygnathohippus, Eu. turkanense. Subsequent studies of the Sivalhippus clade [174] and by Sun et al. [279] demonstrate the close identity of cranial, dental and, in particular, postcranial anatomy of Si. turkanensis and Si. perimensis and the extension of this genus into China and Africa in the Late Miocene.
- 4. Eurygnathohippus hooijeri, Bernor and Kaiser, 2006 [280] (5.0 Ma). The holotype is SAMPQ-L22187, a complete adult female skull with associated dentition, mandible and dentition and postcranial characteristics from the earliest Pliocene Langebaanweg E Quarry, South Africa [280]. Hooijer [281] originally described the specimen under the nomen “Hipparion” cf. H. baardi.
- 5. Eurygnathohippus woldegabrieli, Bernor et al., 2013 [282] (4.4–4.2 Ma). The holotype is ARA-VP-3/21, an incomplete mandible [282]. The hypodigm include also 156 dental and postcranial specimens from 14 localities at Aramis (Middle Awash), Ethiopia. The type specimen is ARA-VP-3/21 a mandible including symphysis, right partial ramus with p2 and p3, left ramus with p2 and 3 preserved and pr-m3 poorly preserved. Mandibular incisor teeth and canines, if originally present are lacking.
- 6. Eurygnathohippus afarense Eisenmann, 1976 [283] (KH3, Hadar, ca. 3.0 Ma). The holotype is AL363-18, a partial cranium from the Kada Hadar Member [283]. “Hipparion” afarense was nominated for skeletal material originating from the Kada Hadar 3 horizon, Hadar, Ethiopia. Eisenmann [283] also referred a mandible, AL177-2, to E. afarense.
- 7. Eurygnathohippus hasumense, Eisenmann, 1983 [284] (3.8–3.2 Ma). The holotype is KNM-ER 2776, a p4-m2 from zones B and C of the Kubi Algi Formation [284]. She included cheek teeth of common morphology from the Chemeron Formation, Kenya and the Denen Dora Member of the Hadar Formation, Ethiopia. A cranium with associated mandible was included in this hypodigm, AL340-8 [269] (Figure 13 in Bernor et al. [269]), and a partial skeleton including cheek teeth and complete postcranial elements, AL155-6 from DD2, ca. 3.2 Ma. Bernor et al. [281,285] analyzed a series of Ethiopian Eurygnathohippus establishing a phyletic relationship that currently includes Eu. feibeli, Eu. woldegabrieli, Eu. “afarense”, Eu. hasumense and Eu. cornelianus.
- 8. Eurygnathohippus pomeli Eisenmann and Geraads, 2006, [286] (ca. 2.5 Ma). The holotype is AaO-3647, an almost complete, but transversely crushed skull [286]. It was originally described as “Hipparion” pomeli. Reference [286] reported a well-preserved assemblage of hipparionini Ahl al Oughlam near Casablanca. Eisenmann and Geraads [286] argued that the sample is homogeneous and biochronologically correlative with eastern African faunas that are ca. 2.5 Ma, roughly contemporaneous with Omo Shungura D. Bernor and Harris [275] recognized this as a species of Eurygnathohippus. The northward extension of Plio–Pleistocene Eurygnathohippus into North Africa is remarkable as was its extension into India at this time [175].
- 9. Eurygnathohippus cornelianus, van Hoepen, 1930 [287] (ca. 2.6–1.0 Ma). The hypodigm includes a mandibular dentition from Cornelia, Orange Free State, with hypertrophied i1s and i2s and atrophied i3s placed immediately posterior to the i2s [287] (plates 20–22). Leakey [288] (pl. 20, 4 figures) reported the occurrence of “Stylohipparion albertense” (=Eu. cornelianus) from Bed II, Olduvai Gorge, Tanzania based on premaxillae and mandibular symphyses with identical incisor morphology. Hooijer [281] reported an adult skull from Olduvai BKII which he referred to Hipparion cf. H. ethiopicum which is likely a member of the Eu. cornelianus lineage. Eisenmann [284] did not recognize the existence of Eu. cornelianus at Olduvai, but referred an immature cranium, KNM-ER3539 to Eu. cornelianus. Armour-Chelu et al. [289] and Armour-Chelu and Bernor [290] argued that the first evidence of this clade may be from the Upper Ndolanya Beds, Tanzania, circa 2.6 Ma. It is also likely present in the Omo Shungura F, dated 2.36. Bernor et al. [8,269] advanced the hypothesis that Eu. cornelianus is a member of an evolving lineage that occurred in East and South Africa between ca. 2.4 and less than 1 Ma. The specimen/locality content of Eurygnathohippus is currently under investigation.
- 10. Hipparion (Eurygnathohippus) steytleri, van Hoepen, 1930 [287]. Van der Made et al. [291] argued that the author named Hipparion steytleri based on a right M1/1, left M3 and left m1-2 that formed a type series. Van Hoepen [287] also named Eu. cornelianus on the basis of a mandibular symphysis. Van der Made et al. [291] considered the nomen H. stytleri to have priority over Eu. cornelianus, but the cheek teeth of H. stytleri are of insufficient diagnostic value in themselves to define a valid species as stipulated by Article 75.5 of the ICZN. On the other hand, Van Hoepen [287] exercised considerable foresight in recognizing the highly derived state of the type specimen mandible of Eu. cornelianus because of its very wide mandibular symphysis with hugely hypertrophied i1 and i2 with very reduced, peg-like i3 situated immediately posterior along the mid-line of i2. Moreover, Leakey [288] illustrated a series of Eu. cornelianus mandibular symphyses and premaxillae from Olduvai Bed 1. Bernor et al. [292] described a juvenile skull of Eu. cornelianus (RMNH67) from Olduvai Gorge [293] which has a long preorbital bar, faint preorbital fossa and a dP2 with an extended anterostyle. Eurygnathohippus cornelianus sensu strictu has a known chronologic range of 2–1 Ma, but the lineage apparent extends lower in time.
- 11. Hipparion (Eurygnathohippus) libycum Pomel, 1897 [273]. The hypodigm includes a left p3/4 figured by Pomel ([273], pl. 1, Figures 5–7), two lower cheek teeth from “carriers des gres ouvertes a la campagne Brunie” in Oran (pl. 1, Figures 1–7) and a distal epiphysis of a third metatarsal from “carriers de grès du quartier”, St-Pierre, Oran. Hopwood [289] assigned the left p3/4 figured by Pomel ([273], pl. 1, Figures 5–7) as a lectotype [287]. It shows gracile metapodials. Van der Made et al. [287] reported that the original type specimens from Oran are in the Central Faculty of Algiers (MGFCA) but have provided no accession numbers for the holotype.
- 12. Hipparion (Eurygnathohippus) ambiguum, Pomel, 1897 [277]. The hypodigm includes a right P2 from Beni Fouda (Ain Boucherit) [277] (pl. 2, Figures 2–4). Previously the repository was unknown. Van der Made et al. [291] (Figures 2a,b and 3), redrafted an image by Pomel [277] and reported that the specimen is currently maintained in the Central Faculty of Algiers (MGFCA) but offered no photographic images or measurements of this specimen. Van der Made et al. [291] have provided no accession number for the holotype. The type locality of Aïn Boucherit has a magnetostratigraphic date of 2.44 [291] whereas the East African localities referred to by Van der Made et al. [291] have an age range of 3.8–1.2 Ma [269].
- 13. Hipparion (Eurygnatohippus) massoesylium, Pomel [277]. The hypodigm includes five teeth from “puits Carouby” and “aux portes d’Oran” (also Puits Karoubi), left P4, M1-3 and right M3 (pl. 1, Figures 8–10). Van der Made et al. [291] considered these specimens to be the holotype of the species. Van der Made et al. [291] have reported that the holotype is kept in the Central Faculty of Algiers (MGFCA) but have provided no accession numbers for the associated cheek teeth.
- 14. “Hipparion” sitifense, Pomel, 1897 [277]. The hypodigm includes four specimens including two teeth from St. Arnaud, a calcaneum from a nearby locality and a tooth figured by Thomas [294]. Pomel figured an M1/2 (Pl. 1, Figures 13–16), a right P4 (Pl. 1, Figures 11 and 12) and a calcaneum (Pl.2, Figures 9 and 10). The M1/2 figured by Pomel represents the lectotype [291]. The latter authors re-figured the original illustrations from Pomel [277], but this figure does not have a scale bar, and the specimens do not have formal institutional accession numbers. Eisenmann [295] stated that it is not known where the “type specimens” from Saint Arnaud et al. are, whereas Van der Made et al. [291] report that the original material is in the Central Faculty of Algiers (MGFCA; p. 44). Van der Made et al. [291] nominated a lectotype citing a specimen figured by Pomel [277] (pl. 1, Figures 13–15) without specifically designating a specimen accession number, institution, element and providing a redrafted original figure of the specimen without the benefit of a scale. It cannot be known if “H. sitifense” is referable to the genus Hipparion or other small hipparionins Cremohipparion or Eurygnathohippus (for which mandibular cheek teeth are needed). Arambourg [296,297] reported a specimen “from the type locality (for which we cannot be certain)” described and figured a mandibular specimen “with rounded metaconid-metastylid and no ectostylid”. It should be noted that this material is not a legitimate sample of “the original type series of Pomel, 1897”. Arambourg [296,297] assigned material from Ain el Hadj Baba, Mascara, Saint Donat and Aïn el Bey to “H. sitifense”. These are all small sized.
The Equus Datum in Africa has been a matter of debate over the last years. Bernor et al. [269] and Rook et al. [176] reported the first known occurrence of the genus Equus in East Africa at 2.33 Ma in the Omo Shungura Formation, member G (Equus sp.). Material of these earliest occurring Equus are not well represented across the skull, mandible, dentition and postcranial elements. During this interval of time, the most common European species is E. stenonis and possibly some late surviving populations of E. livenzovensis [13], E. eisenmannae, and E. sanmeniensis in China. Nevertheless, recent research in the North African sequence of Oued Boucherit (Algeria) have recalibrated the localities of Aïn Boucherit, El Kherba and Aïn Hanech. The lowermost stratigraphic level of Aïn Boucherit has been dated at 2.44 Ma [270], where Equus numidicus, Equus cf. E. numidicus and Equus tabeti have been reported [270,271]. Therefore, this new North African age for the Equus Datum anticipates the earliest occurrence of Equus in the North rather than in East Africa.
- 15. Equus numidicus, Pomel, 1897 [277] (2.44–1.2 Ma). The hypodigm includes a right P2 [273] (Pl. 2, Figure 2). Arambourg reported cranial and postcranial elements from Aïn Boucherit and Aïn Jourdel (Pl. 18, Figures 6 and 7; Pl. 19–20; Figures 58 and 62). It is a medium-sized horse approximately the size of a large zebra that originated from Aïn Boucherit and Aïn Hanech, Algeria, ca. 2.44–2.0 Ma [270,271,295]. No complete crania are known, although incomplete cranial remains associated with postcranial elements have been reported [296]. The evolutionary relationships of E. numidicus are not well defined. Azzaroli [298] pointed out a possible relationship to E. stenonis, although it was no longer investigated. However, some anatomical features of the upper and lower cheek teeth and postcranial elements resemble those of E. stenonis [297,298].
- 16. Equus tabeti, Arambourg, 1970 [297] (2.44–1.2 Ma). The holotype is a partial palate (1949.2:773) figured in Arambourg [297] (Pl. 21, Figure 3). It is a medium-small sized species of Equus with “asinine” maxillary cheek teeth, stenonine mandibular cheek teeth and slender third metapodials and phalanges [284,297]. No complete crania are known, even if some incomplete crania and mandibles have been reported by Arambourg [297]. The type material originates from Aïn Hanech, Algeria [297]. Geraads [299] estimated the age of Aïn Hanech to be 1.2 Ma. Eisenmann [284] reported the possible presence of Equus cf. tabeti at Koobi Fora, Kenya believing that it is a primitive ass and may have been derived from E. numidicus. The recent analyses of Duval et al. [270] suggest an earlier occurrence of E. tabeti and E. numidicus at 2.44 Ma in the lowermost levels of Aïn Boucherit. Beside North Africa, E. tabeti is reported from Early Pleistocene sites of “Ubeidiya”, Bizat Ruhama and Qafzed (Levantine corridor [300,301,302]) and from East Africa [183].
- 17. Equus koobiforensis, Eisenmann, 1983 [284] (2.1–1.0 Ma). The holotype specimen is KNM-ER 1484, a complete skull recovered from the Notochoerus scotti Zone, Area 130, just below the KBS Tuff, ca. 1.9 Ma. Eisenmann [284] reported a number of close dental similarities shared by E. koobiforensis and European E. stenonis but did not suggest a direct phylogenetic relationship between these taxa. Azzaroli [258] stated that E. koobiforensis was essentially a Grevy’s zebra. Bernor et al. [183] cited the likely evolutionary relationship between North American Pliocene E. simplicidens, European E. stenonis and E. koobiforensis. Cirilli et al. [10] demonstrated the explicit cladistic relationships between the E. simplicidens -E. stenonis-E. koobiforensis-E. grevyi clade, whereas Cirilli et al. [13] reinforced this result on robust statistical grounds. No precise age is available for its last occurrence in the fossil record.
- 18. Equus oldowayensis, Hopwood, 1937 [293] (1.9–1.0 Ma). The holotype is a lower jaw from an animal approximately 2 years old [293] (Figures 1 and 2; Catalogue Number VIII, 353, in the Bayerische Paläontologische Staatssammlung, Munich). Hopwood [293] also designated a lower incisive region with the left incisors and right first incisor (BMNH M14199) as the paratype. The original Olduvai collection deposited in Munich, which included the type of E. oldowayensis, was destroyed together with its catalogue, during WW II (K. Heissig personal communication with Churcher and Hooijer; [269]). Equus oldowayensis is usually reported from the type locality of Olduvai (1.8 Ma, Tanzania), but no precise ages are available for its chronologic range. Recently, Bernor et al. [183] reported an incomplete cranium from Olorgesailie (ca. 1.0 Ma, Kenya).
- 19. Equus capensis, Broom, 1909 [303] (ca. 2.0?–? Ma). It was a large-bodied horse estimated to be 150 cm at the withers, with a body mass of approximately 400 kg [304]. It originated from South Africa. Churcher [305] synonymized E. helmei, E. cawoodi, E. kubmi, E. zietsmani and specimens of E. harrisi and E. plicatus into E. capensis. Equus capensis was widely distributed in the Plio–Pleistocene of South Africa, although no information is known about its first and last occurrence in the fossil record.
- 20. Equus mauritanicus, Pomel, 1897 [277] (1.0 Ma). The hypodigm include isolated teeth and postcranial elements, figured in Pomel [277] (Pl.3–8). It is reported from a large sample from Tighenif (Algeria). Churcher and Richardson [268] referred E. mauritanicus to a subspecies of E. burchellii (=E. quagga mauritanicus). Eisenmann [284,295] recognized E. mauritanicus as a distinct species of Hippotigris and claimed similarities to E. stenonis in the dentition. Eisenmann [284] further introduced the notion of cross-breeding between E. mauritanicus and Quaggas. Churcher and Richardson [268] reported an extensive distribution of E. (Hippotigris) “burchelli” (=quagga) from North, East and South Africa. The possible relationships of E. mauritanicus with plain zebras have been suggested by Eisenmann [295] and more recently from Bernor et al. [14] by morphological analyses. Beside the type locality of Tighenif, E. mauritanicus is reported also in Oumm Qatafa (Juden Desert, Egypt, Middle Pleistocene [306]).
- 21. Equus melkiensis Bagtache et al. 1984 [307] (Late Pleistocene). The holotype is a short mc3 (I.P.H. Allo. 61–1314) recovered from Allobroges, Algeria and of latest Pleistocene age. Eisenmann [236] reported E. melkiensis also from Morocco. This species has been identified also at Gesher Benot Ya’akov and Nahal Hesi (Israel; [87,301]) and Oumm Qatafa (Egypt, Middle Pleistocene, [306]).
- 22. Equus algericus, Bagtache et al. 1984 [307] (Late Pleistocene). The holotype is IPH61-103, a m2 from Allobroges, Algeria [307] (Figure 1). It is reported to be a caballine species with a withers height of approximately 1.44 m. Equus algericus was also reported from Morocco [308], which are purported to have the characteristic caballine metaconid-metastylid (=double knot) morphology.
- 23. Equus grevyi, Oustalet, 1882 [309] (0.5–0.0 Ma). Oustalet [309] (v.10, pp. 12–14) described the anatomical features of the species, with an associated illustration (Figures 1 and 2). He also reported that the living animal was donated to the Museum National d’Histoire Naturelle in Paris, where the holotype should be kept. Equus grevyi is the largest living wild equid with a withers height of 140–160 cm. Azzaroli [259], Bernor et al. [14,183] and Cirilli et al. [10,13] all have cited the close relationship between European E. stenonis, E. koobiforensis and E. grevyi. Equus grevyi is currently distributed in the arid regions of Ethiopia and Northern Kenya and has recently vanished from Somalia, Djibouti and Eritrea [269]. Eisenmann [284] recognized Equus cf. E. grevyi from the Metridiochoerus compactus zone, the Guomde Formation and Galana Boi beds of Kenya based on both cheek tooth and postcranial remains. Bernor et al. [183] and Cirilli et al. [10,13] have recognized E. grevyi as a terminal member of the E. simplicidens–E. stenonis–E. koobiforensis–E. grevyi clade. Recently, O’Brien et al. [310] reported an incomplete cranium ascribed to E. grevyi from the Kapthurin Formation (Kenya) dated between 547 and 392.6 ka. This age represents the best dated E. grevyi FAD, at the present time.
- 24. Equus quagga, Boddaert, 1785 [264] (?1.0–0.0 Ma). Boddaert [264] did not identify a holotype or lectotype for E. quagga but gave a description of the anatomical features of the species (p. 160). It has a shoulder height ranging from a mean of 128 cm in males and 123 cm in females. Equus quagga is one of the most widely distributed African ungulates ranging from southern Sudan and southern Ethiopia to northern Nambia and northern South Africa. Several subspecies have been recognized including E. quagga crawshaii, E. quagga borensis, E. quagga boehmi, E. quagga chapmani, E. quagga burchellii and E. quagga quagga. Fossil remains have been reported from North to South Africa. Leonard et al. [311] suggest that the various subspecies of E. quagga differentiated between 120 and 290 ka. Fossil remains of quagga have been reported from South African Plio–Pleistocene karst deposits, but their certain identity in North and East Africa are somewhat elusive [269]. Pedersen et al. [312] have identified a South African region as the likely source for the origin of the plain zebras from which all extant populations expanded from at approximately 370 ka. Moreover, the genetic analyses of Pedersen et al. [312] have reported a remarkable gene flow in the extant E. quagga subspecies, highlighting the challenge of identifying the subspecific designation only by morphology, identifying at least four genetic clusters for E. quagga boehmi and E. quagga crawshayi.
- 25. Equus zebra, Linnaeus, 1758 [313] (?0.5–0.0 Ma). Linnaeus [313] does not refer any holotype or lectotype but gives a description of the anatomical features of the species (p. 101). It is a medium-sized, long-legged zebra with a mean shoulder height ranging from 124–127 cm. There are two recognized sub-species, E. zebra zebra (Cape Mountain Zebra) and E. zebra hartmannae (Hartmann’s Mountain Zebra). Churcher and Richardson [264] report a relatively small sample from the Middle Pleistocene to recent fossil remains of E. zebra in South Africa.
- 26. Equus (Asinus) africanus, Heuglin and Fitzinger, 1866 [314]. The lectotype is designated as a skull of an adult female collected by von Heuglin near Atbarah River, Sudan, and in Stuttgart (SMNS32026). Equus (Asinus) africanus are equines of small size and stocky build. Churcher [315] reported the earliest occurrence of this taxon from the middle of Bed II, Olduvai Gorge (>1.2 Ma). This identification was based on a single third metatarsal, which was short (231 mm) and slender, although not to the extent of E. tabeti. Two subspecies are recognized, E. (A.) africanus africanus von Heuglin and Fitzinger, 1866 [314] (Nubian wild ass) and E. (A.) africanus somaliensis Noak, 1884 [316] (Somali wild ass) [317,318,319]. The African wild ass E. africanus is widely believed to have been the ancestor of the domestic donkey [269] and, more recently, believed to be the descendant of the Early Pleistocene species E. tabeti [320]. Nevertheless, this last evidence has only been suggested and not yet proven.
5. Biochronology and Biogeography
Rook et al. [176] recently provided a comprehensive biochronology and biogeography for latest Neogene–Pleistocene Equus-bearing horizons of Eurasia, Africa and North and South America. Following this recent summary, we report here a synthesis of major Equinae evolutionary events for the last 5.3 million years across these continents. Berggren and Van Couvering [321] suggest the application of the term biochron for units of geologic time that are based on paleontological data without reference to lithostratigraphy or rock units. Mammal biochronologic scales have been developed for Europe (ELMA), Asia (ALMA), North America (NALMA) and South America (SALMA) and, most recently, for Africa (AFLMA). These timescales are variously expressed in terms of conventional mammal biostratigraphic zones or as land mammal ages (LMAs). Each timescale based on land mammals in different continental landmasses has its own history of development reflecting the uniqueness of the records and the extent to which faunal succession has been resolved.
5.1. NALMA Timescale and Equinae Evolution
The Hemphillian NALMA ranges from approximately 8 Ma to about 4.9 Ma, with four faunal stages (Hh1-Hh4), and correlates with the Late Miocene to Early Pliocene in age, the most recent age (Hh4) extending over the Mio–Pliocene boundary (5.3–4.9 to 4.6 Ma). Equids recorded from this interval include the genera Dinohippus (i.e., D. leidyanus, D. interpolatus, D. leardi, D. spectans and D. mexicanus,), the hipparions Cormohipparion (i.e., Co. occidentale and Co. emsliei), Nannippus (i.e., Na. aztecus, Na. beckensis, Na. lenticularis and Na. peninsulatus), Neohipparion (i.e., Ne. eurystyle, Ne. gidley and Ne. leptode), Calippus (i.e., Ca. elachistus, Ca. hondurensis), Astrohippus (i.e., A. stocki and A. ansae) and Boreohippidion (i.e., B. galushai). These last are taxa that hold-over from the Late Miocene.
Hemphillian Hh4 ranges into the Early Pliocene until 4.6 Ma, and a range of 4.9–4.6 Ma for the earliest Blancan has been proposed [322]. In fact, the succeeding Blancan NALMA has been defined by the first appearance in North America of arvicoline rodents circa 4.8 Ma. The Blancan has recently been subdivided into five intervals: Blancan I (4.9–4.62 Ma.), Blancan II (4.62–4.1 Ma.), Blancan III (4.1–3.0 Ma.), Blancan IV (3.0–2.5 Ma.) and Blancan V (2.5–1.9 Ma.) [322]. The equid Dinohippus is known to persist into Blancan I and II intervals, while E. simplicidens, E. idahoensis and E. cumminsii are known from the Blancan III and later Blancan assemblages. The diminutive hipparionine horse Na. peninsulatus is reported from the Blancan V interval but does not survive into the Irvingtonian. The Blancan IV/V boundary corresponds closely to the base of the Pleistocene.
The Irvingtonian NALMA is subdivided into three units: Irvingtonian I (~1.9–0.85 Ma), Irvingtonian II (0.85–0.4 Ma) and Irvingtonian III (0.4–0.195 Ma). Early Irvingtonian assemblages includes the equids E. scotti, E. conversidens [60], and Equus (or Haringtonhippus) francisci (possibly including E. calobatus).
Finally, the Rancholabrean NALMA extends from 0.195 to about 0.11 Ma with the onset of the Holocene. Common Rancholabrean equid species include E. scotti, E. conversidens and E. (or Haringtonhippus) francisci. Equus occidentalis is also abundant in the American southwest during this period. Fossils resembling E. ferus (e.g., E. lambei) have also been documented from Rancholabrean faunas.
5.2. SALMA Timescale and Equinae Evolution
Two lineages of Equidae occurred in South America during the Pleistocene, Hippidion and Equus. Although there are no records of Hippidion in Central or North America, most evidence suggests that both lineages originated in Holarctica and then migrated independently to South America during the important biogeographical event known as the Great American Biotic Interchange (GABI) [103,323,324]. However, there is no record for Equidae in South America until the beginning of Pleistocene, following the formation of the Isthmus of Panama from the early Pliocene onward (approximately 3 Ma) [103,324]. The earliest record of Equidae in South America is Hippidion principale from Early Pleistocene deposits (Uquian) of Argentina [323,324]. However, the age of the first record of Equus in South America is controversial. Traditionally, its earliest record is from middle Pleistocene (Ensenadan SALMA) of Tarija outcrops in Southern Bolivia, based on a biostratigraphic sequence at Tolomosa Formation and independently calibrated to occur between ~0.99 and ˂0.76 Ma [103]. Nevertheless, there is no consensus regarding the age of these deposits and some researchers consider the deposition in Tarija to have occurred only during the Late Pleistocene [325] (and references therein). Recently, it was proposed that only one species of Equus lived in South America during the Pleistocene, E. neogeus [99]. This species is considered a fossil-index for deposits of Lujanian SALMA (late Pleistocene-earliest Holocene; 0.8 to 0.011 Ma). Although E. neogeus was widely distributed in South America, only few localities are calibrated by independent chronostratigraphic data, indicating a Lujanian SALMA [98]. Therefore, the dispersal of Equus into South America occurred during the GABI, but if it is considered that Equus’ earliest record is in the Late Pleistocene, it thus followed the fourth and latest phase of the GABI or Equus migrated to South America during GABI 3, considering its early record to be in the Middle Pleistocene [103]. All equids that occurred in South America during the Pleistocene (Hippidion and Equus) became extinct in the early Holocene [99,324].
5.3. ALMA Timescale and Equinae evolution
Equids first appear in Asia in the Miocene, between 11.4 and 11.1 Ma with the dispersal of the three-toed horse Cormohipparion [8,274,326]. Thereafter, a diverse assemblage of hipparionines is seen between ~10.2 and 6.0 Ma. Hipparionines are found across the Mio-Pliocene boundary which lies in the Dhok Pathan Formation (ca. 5.3 Ma) on the Potwar Plateau in Pakistan [327]. Hipparionines are rare in South Asia between 6.0 and 2.6 Ma, but recently, three taxa have been reported between 3.6 Ma and 2.6 Ma: “Hippotherium”, Plesiohipparion, and Eurygnathohippus [119,174]. The youngest indeterminate hipparionine records are dated paleomagnetically ~2.6–2.5 Ma, around the same time the first Equus occurs in South Asia [328,329]. Equus makes its appearance just above the Gauss-Matuyama boundary, which coincides with the Plio-Pleistocene transition.
In China E. eisenmannae, a large yet primitive stenonine horse from the Longdan loessic section in Linxia Basin, Gansu Province, was magnetically dated to 2.55 Ma for its lower fossil-producing horizon, which is the earliest record of Equus in China. Although represented by poorly preserved fossils, Equus sp. from Zanda Basin in southern Tibet [118] was dated to 2.48 Ma, suggesting fast dispersion of Equus even in higher elevations.
In the Siwaliks, remains of Equus have been found in sediments ranging from 2.6 to 0.6 Ma, a period termed the Pinjor Faunal Zone [328]. As noted in Bernor et al. [182], two morphotypes of Equus have been recorded—a large taxon called Equus sivalensis and a smaller taxon sometimes called Equus sivalensis minor for specimens from the the Upper Pinjor Formation near the town of Mirzapur [330], Equus cf. E. sivalensis from the Pabbi Hills [192] and Equus sp. (small) for specimens from the Mangla–Samwal anticline [190]. Equus sivalensis has been recorded from the entire temporal range of the Pinjor Faunal Zone [329]; however, the temporal range of the smaller horse appears to be restricted to ~2.2–1.2 Ma.
Equus sanmeniensis is magnetically dated 1.7–1.6 Ma from the Shangshazui stone artifact site in the classical Nihewan Basin and to 1.66 Ma from the nearby Majuangou III hominin tool site. This species was also recorded from the Homo erectus site at Gongwangling, Lantian, Shaanxi Province, and magnetically dated to a slightly younger ~1.54–1.65 Ma. Equus yunnanensis from the Homo erectus site at Niujianbao in Yuanmou Basin was magnetically dated to 1.7 Ma. [331] In the Late Pleistocene, Equus namadicus and Equus hemionus are known from the Indian peninsula [332].
5.4. ELMA Timescale and Equinae Evolution
The Miocene fossil record (Vallesian and Turiolian Land Mammal Ages) as well as the Pliocene one (Ruscinian and early Villafranchian Land Mammal Ages) in Europe do not have monodactyl horses. These times are characterized by different hipparionine horses’ evolutionary lines and, during the Pliocene, three lineages of hipparions persisted in Europe: Cremohipparion (C. fissurae), Plesiohipparion (Pl. longipes, Pl. rocinantis) and Proboscidipparion (Pr. heintzi, Pr. crassum) [8,206,270]. It is during the early to middle Villafranchian transition, that E. livenzovensis first occurs in Southwest Russia and Italy at around 2.6 Ma (beginning of middle Villafranchian; Early Pleistocene) and constitutes the regional Equus First Appearance Datum [10,13,184,192]. In Europe, the earliest representatives of the genus Equus co-existed with the last hipparionin horses (the genera Plesiohipparion, Proboscidipparion and Cremohipparion) in the Early Pleistocene [10], although at the present time the effective co-existance of Equus and hipparion is found in the localities of Montopoli (Italy) and Roca-Neyra (France).
Equus livenzovensis appears to be at the base of the radiation of the later lineage of fossils horses, the European Pleistocene Equus stenonis group (=stenonine horses). The European stenonine horses have been recently revised [10,13]. In addition to E. livenzovensis, the species (and their chronological ranges) included in this group are E. stenonis (end of middle Villafranchian to early late Villafranchian; Early Pleistocene, 2.4–1.7 Ma; [13]), E. stehlini (late Villafranchian; Early Pleistocene, 1.8–1.6 Ma; [229]), E. altidens, and E. suessenbornensis (end of the late Villafranchian to Early Galerian, Early Pleistocene to Early Middle Pleistocene, 1.6–0.6 Ma; [184]). The most relevant turnover for the Equus species occurs in the Middle Pleistocene (ca 0.6 Ma) with the first occurrence of E. ferus (or E. ferus mosbachensis) in Mosbach (Germany) and E. hydruntinus from Vallparadìs (Spain) [240]. The arrival of these two species marks the extinction of the Early and early Middle Pleistocene Equus species.
5.5. AFLMA Timescale and Equinae Evolution
The Miocene to Pleistocene mammal record of Africa is overall less complete than the fossil record on other continents with no established land mammal age scheme for Africa at a continental scale was established until very recent times [333].
The completeness of the mammal fossil record across the continent is extremely variable with regions in which the Neogene record is totally missing and others (such as Kenya or Ethiopia) with a relatively continuous and well documented record [334].
Without an established continental-scale biochronology, Africa’s “biochronology” is based on stratigraphic ordering in different sedimentary basins and is largely dependent on radiochronology with limited use of magnetostratigraphy. As an example, Pickford [335] subdivided Miocene faunas from Kenyan sites into Faunal Sets I to VII; suggesting age spans for late Miocene sets are 12.0–10.5 Ma (V), 10.5–7.5 (VI), and 7.5–5.5 (VII).
The Late Miocene–Early Pliocene boundary (Sugutan and Baringian Land Mammal Ages) is poorly represented in Africa. Hipparionine horses are first found in North and East Africa circa 10.5 Ma [8] the richest locality being the Algerian site of Bou Hanifia and the Ethiopian site of Chorora [269]. At the end of Late Miocene (Baringian), diversification of the hipparionine genus Eurygnathohippus (exhibiting evolutionary relationships to Siwalik hipparionines; [175]) was well underway, as was significant the branching by endemic elephants and marked successes by new bovid tribes and suines arriving from Eurasia.
The Plio–Pleistocene time period has been rigorously studied biochronologically in Africa by temporal distributions of elephants and suids, often in conjunction with the dating of hominin finds. Equus first occurs during the Early Pleistocene, even if this occurrence event in Africa is delayed relative to Eurasia, where it is at ca 2.6 Ma. Indeed, the most recent results identify the presence of the genus Equus in Nord Africa at ca. 2.44 Ma (E. numidicus, [269]) and in East Africa in lower Member G of the Omo Shungura Formation ca. 2.33 Ma (Shunguran Land Mammal Age; [176]). The first occurring African Equus is apparently related to European E. stenonis [10,13]. Representatives of the genus Equus are two times as abundant as Eurygnathohippus during the Early Pleistocene (Natronian Land Mammal Age) like in the Ethiopian locality of Daka [336], with Eurygnathohippus sharply declining in its numbers in East and South Africa after 1 Ma (Naivashan Land Mammal Age). Unfortunately, little is known of the first occurrence of the living species. Recently, a report by O’Brien et al. [310] of an incomplete E. grevyi cranium from the Kapthurin Formation (Kenya, 547–392.6 ka) represents the best dated E. grevyi FAD.
5.6. General Remarks about Equinae Biochronology
Unlike high-resolution biostratigraphic tools available in the marine realm, mammalian biochronology is not permissive of recognizing strictly synchronous events at global scale. Nevertheless, a review of the currently available evidence of the Land Mammal Ages, defined and calibrated across different continents (either in North and South America, Eurasia and Africa), allows us to recognize major faunal change (corresponding to the limit between subsequent Land Mammal Ages) always correlatable within the magnetochronostratigraphic scale and to place in this framework the main evolutionary events occurring around the world along the Equinae evolutionary history.
6. Paleoecology
In this chapter, we present some new paleoecological insights in diet and body size on the subfamily equinae, with special remarks for the genus Equus.
6.1. Relationships of Diet, Habitats and Body Size in Equines, with Pleistocene Equus from Eurasia and Africa as a Particular Example
In general, equines of the genus Equus tend to have mesowear values indicating grazing diets, but there is considerable variation in diets and body size (Table S2), which likely reflect differences in habitat preference and social strategies, as well as the effect of available vegetation in different paleoenvironments. Our new analyses based on the most extensive compilation of body mass and palaeodiet data of particularly in Eurasian equines largely confirm previous hypotheses of the relationship between body size, diet, behavior and environments of equines during the Neogene, and during the Quaternary in particular. A few main hypotheses have been presented for the main factors that affected body size evolution and body size variation (also within species) of equine horses during the Neogene and the Quaternary. First, resource availability and quality (mainly regulated by annual primary productivity as well as nutritional properties and chemical defenses of available plants) have been suggested to be a limiting factor for some of the small-sized equine species or populations [13,19,244,267]. Conversely, the positive effect of seasonally high productivity and high resource quality due to low plant defense mechanisms has been suggested to explain the particularly large body size of Pleistocene herbivorous mammals in general [337,338,339,340]. Third, the effects of differential habitat heterogeneity, social structures and population density (intraspecific competition) on limiting the body size of some Equus species/paleopopulations, especially purely grazing ones that were abundant in open environments, has been discussed [19,20,340]. Observations of modern and Pleistocene Equus populations indicate that large-sized species tend to have smaller group sizes and population densities and more mixed or browse-dominated diets than small-sized species which are graze dedicated [19].
Ordinary least squares multiple regression models indicate that both diet and productivity of environments (estimated NPP values) are related to the variation in body size, both between and within the species of Equus during the Pleistocene-present (Figure 1). The “All Equus” model includes a wide range of extant and Pleistocene Equus populations from Eurasia and Africa, and it shows a significant negative effect of mesowear score and a significant positive effect of NPP on Equus body mass (although there is remarkable scatter especially in the residuals of the NPP estimate effect on body mass). The pattern is similar in the most abundant Pleistocene species/lineages of European Equus, including E. stenonis, E. altidens and the Middle–Late Pleistocene caballines (E. ferus + E. mosbachensis), although the patterns were statistically less robust (but note the small sample size especially in the case of E. stenonis and E. altidens). The connection between diet and body size was particularly robust, especially for the all-Equus model, indicating that very large species of Equus had more browse-dominated diets than small and medium-sized species/populations (Figure 1). Furthermore, the association of large size and more browse-dominated diets is shown for Africa as well as Eurasia (Figure 2).
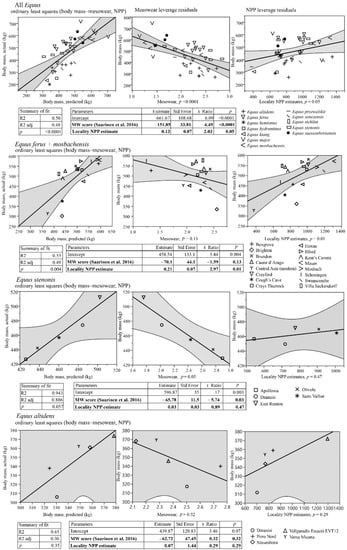
Figure 1.
Ordinary least squares models of the effect of the mesowear score and the estimated NPP of the localities on the body mass estimates of the genus Equus from the Pleistocene of Eurasia.
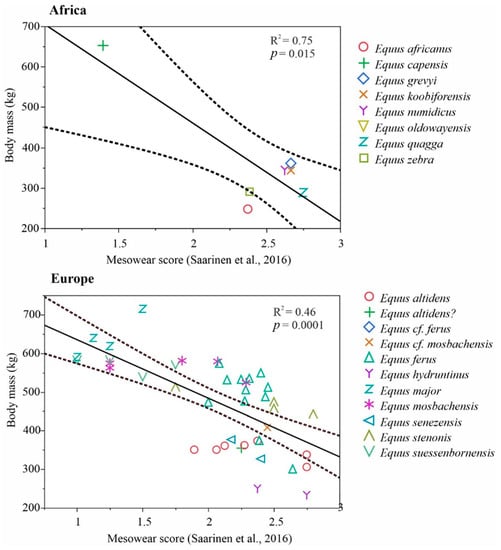
Figure 2.
Linear regressions between body mass and the mesowear of Equus in Africa and Europe during the Quaternary. On both continents, the body mass of Equus was significantly negatively related to the mesowear score, although in Africa, this pattern was entirely driven by one very large-sized species, E. capensis. Adapted from [20].
These results indicate that both primary production and differences in the dietary niche had an effect on the body size of Equus. Large size in Equus is associated with more browse-dominated diets and more productive paleoenvironments, while small and medium-sized horses typically occupied less productive environments and had more purely grazing diets. The association of diet and body size appears stronger than the association of estimated NPP and diet, except perhaps in E. ferus/mosbachensis. A possible explanation for this is that the diet includes a signal of niche partitioning between sympatric species of Equus. As Saarinen et al. [19] discuss, when a small and a large species of Equus occurred sympatrically, the larger species was typically less abundant in the fossil assemblage and (in all such cases, including the new ones added in the present study) had more browse-dominated diet. Thus, the effect of larger group sizes of grazing, gregarious populations of Equus on limiting their body size via the effect of larger population densities and more intense intraspecific competition appears to have been a significant mechanism limiting their body size, although there was also a more general positive effect of primary production on body size.
This model of the relationship of body size with diet (affected by available vegetation and dietary niche partitioning), population density (associated with dietary preferences and social strategies of different Equus species) and habitat (vegetation openness, heterogeneity and resource availability) based on data from Eurasian and African Equus is by and large supported by more general observations from earlier equines and from Equus from North America. In Eurasian and African hipparionines, large body size is typically associated with browse-dominated diets, such as in Hippotherium from the early late Miocene of Europe, while smallest body sizes tend to occur in grazing taxa, such as the small species of Cremohipparion from the circum-Mediterranean environments during the late late Miocene (Table S2) [8].
Tracking the abrasion incurred on molars in deep time (hypsodonty index), the level of abrasion incurred by individuals cumulatively in their lifetimes (mesowear) and the short-term acquisition of microwear scars from exogenous grit and/or food items has shed light on dietary and environmental shifts through time for North American Equini. By using three dietary proxies with different temporal resolution capabilities, the amounts of different levels of dietary abrasion as well as the possible causes of this abrasion was elucidated. Highly hypsodont members of the subfamily Equinae appear in North America about 17.5 Ma (late early Miocene-Hemingfordian) [341]. It is at this time that high degrees of large pitting are found in their dental enamel, and they begin to show scratch textures regardless of dietary classification indicating heavy exposure to exogenous grit. Also, highly hypsodont equines first appeared in the late Middle Miocene (ca. 14 million years ago), well after the projected availability of pervasive open grasslands (earliest Miocene) [342,343] or even the latest Oligocene [344]. Thus, the appearance of hypsodonty in Miocene equines was not synchronous with the appearance of grasslands in North America, and exogenous grit appears to have been a contributing factor to increased exposure to abrasion and subsequent increases in crown height in fossil horses rather than grazing alone [21,345,346]. Mesowear patterns closely mirror hypsodonty trends (i.e., higher mesowear scores when hypsodonty increases.) The species of Equus shown in Table S3 have abrasive mesowear consistent with grazing. Thus, grass was an important dietary item as it is today. Also, as these taxa, while grazing, were feeding low to the ground, they would have encountered grit encroaching on their food items which would have contributed further to their abrasive mesowear. Even so, microwear has shown that roughly 80% of these taxa (Table S3) seasonally or regionally engaged in mixed feeding, or in one case, browsing. This demonstrates that hypsodonty, although advantageous for consuming grass, does not preclude consuming leaves or other browse at times. The other and earlier Equini shown in Table S3 (i.e., Cormohipparion, Pseudhipparion, Calippus, Hipparion, Dinohippus, Neohipparion and Nannipus) exhibited a less grazing type mesowear and/or microwear indicative of a tendency to either consume grass or a mixture of grass and browse. These results are consistent with the fact that even after open grasslands became pervasive in North America, forest vegetation was apparently also available until the late Miocene [21,342,347].
Analyses of body mass and mesowear of Pleistocene Equus from Mexico and the USA (Alaska) show that the body sizes of most of the species were relatively small compared to the larger species from the Pleistocene of Europe, and all of these species had grass-dominated diets, although some variation occurred at a smaller scale than in the Pleistocene of Europe (Table S2). The relatively small body size and lesser variation in size and diet in North American Equus paleopopulations compared to Europe could reflect less productive paleoenvironments in general, with the estimated NPP values for nearly all of the North American and Mexican localities being comparatively low (between ca. 299 and 730 g(C)/m2/a) (Table S2). The highest estimated NPP (of the sites analyzed here) is at Rancho la Brea (ca. 839 g(C)/m2/a), which is also the only one of those localities that has a very large-sized species, E. occidentalis. Pérez-Crespo et al. [348] noted that the sympatric E. conversidens and Equus (or Haringtonhippus) francisci from Valsequillo, Mexico, had differences in diet and body size that corresponded with the “Eurasian and African Equus model”, with the larger species E. conversidens having more browse-based and the smaller Equus (or Haringtonhippus) francisci having more grazing dietary signal. The observation that equines in general tend to have heavily grazing diets in North America since the late Miocene makes sense (and more so than in the Eurasia and Africa, where more browsing and mixed-feeding forms occurred, even among Pleistocene Equus), as it explains the evolution of the prominent grazing adaptations of this group in North America. Interestingly, it seems that body sizes of North American equines were on average smaller than European equines, mostly lacking the very largest body size category (above ca. 550 kg in Equus), with E. occidentalis being the only exception. Species of this largest size category such as E. major, E. suessenbornensis and E. mosbachensis in Eurasia and E. capensis in Africa, were the ones with most browse-dominated diets during the Pleistocene. It is possible that the evolution of these giant species of Equus is associated with changes in the dietary niche and population densities in the more wooded, comparatively high-productivity paleoenvironments of Pleistocene Europe in particular, while the more open-adapted, grazing horses in North America and much of Africa and Central Asia attained more modest sizes due either to less productive environments or grazing, gregarious ecological strategies that limited individual body size.
6.2. North America
The major evolution and diversification of equids occurred in North America even though a number of successive dispersals took place to Eurasia. Equidae apparently evolved in isolation from Eurasia in North America from the middle Eocene to the late Oligocene [44]. During the Tertiary, equids were very widespread in North America. In fact, at most fossil localities, they are the most common medium- to large-sized mammals recovered [44]. Equids achieved their maximum diversity in the late Miocene [44,349] resulting in the evolution of the subfamily Equinae [350].
The Miocene was also a time of craniodental reorganization of the equid skull. [351,352]. These trends began in parahippine and merychippine equids and eventually led to the genus Equus, which is thought to have evolved from Dinohippus in the Pliocene [346,353]. The dramatic changes in equid skulls, teeth and limbs have long been thought to reflect evolutionary adaptations to a changing environment, often thought of as evidence that grasslands expanded during this time [354,355,356,357,358,359,360,361,362]. Gross changes in dental morphology reveal a true shift toward more abrasive diets including grass in the derived Equinae from the middle Miocene onward reflecting their adaptation to grazing in more open environments [346,363]. While grazing was clearly an important long-term dietary strategy for derived Equinae once they appear in North America, some also engaged periodically in short-term mixed feeding regionally or seasonally.
Interestingly, it appears that there was less variation in the dietary ecology of North American derived equines than the species that encountered a wide range of environments from wooded to open in Eurasia. Middle Miocene equines such as Co. quinni and Co. goorisi were small sized and had mixed to grazing diets (Table S2). The species of Equus from the Pleistocene of Mexico and North America mostly have relatively small body sizes and mostly grazing mesowear (Table S2). The relatively small body sizes could be related to the grazing, gregarious lifestyle or the relatively low-productivity, open paleoenvironments of the North American paleopopulations summarized in this study. In Mexico and Southern USA, the small species E. cedralensis and E. conversidens had grass-dominated mesowear signals and occupied low-productivity, sometimes nearly desert-like environments as suggested by low estimated NPP values of their localities (Table S2). Equus mexicanus and E. scotti were large (mean body mass between 450 and 480 kg), but not as large as the “very large”, predominantly mixed-feeding or browse-dominated “woodland” species in the Eurasia, such as E. major and E. suessenbornensis, both with average body masses around 550–600 kg (Table S2). The only species in the “very large” size category from the North American sites is E. occidentalis. This species occupied a relatively high-productivity paleoenvironment in Rancho la Brea (Table S2), where its mesowear signal indicates predominantly grazing diet [363]. However, the proportion of sharp cusps is also relatively high in the Rancho la Brea population of E. occidentalis, and microwear texture analyses indicate that it consumed a significant proportion of woody browse during the pre-LGM cool stages at Rancho la Brea, so at least periodically significant inclusion of browse is indicated for the diet of also this very large equine [364]. In Alaska, the small to medium-sized E. lambei occupied a cold mammoth steppe paleoenvironment with relatively modest estimated NPP, and its mesowear signal indicates a grazing or at least heavily grass-dominated diet (Table S2).
6.3. Eurasia
The earliest hipparionines that dispersed from North America to Eurasia at the beginning of the late Miocene were medium-sized (around 160 kg in body mass) species of the genus Cormohipparion, such as Co. sinapensis from Sinap, Turkey. These were relatively slender and modest-sized and probably occupied relatively open environments from East Asia to Turkey [8]. Early on, however, the larger, more robust hipparionines of the genus Hippotherium emerged and were widespread across Eurasia. Hippotherium primigenium was a relatively large species (body mass 200–250 kg) with predominantly browsing diets that occupied primarily forest and woodland environments in Central Europe during the early late Miocene [8]. Considerable dietary variation occurred in Hi. primigenium, being purely browsing in the forested paleoenvironment of Höwenegg and more grass-dominated in the locally more open floodplain environment in Eppelsheim, in Germany [365,366]. Two species of Hippotherium, H. primigenium and H. kammerschmittae, from the later late Miocene had browse-dominated diets in Dorn Dürkheim, Germany [366]. During the later late Miocene (Turolian), hipparionines diversified in Eurasia, and included several species and lineages of different body size and dietary ecology. In the Mediterranean realm and Western Asia for example, medium-sized (ca. 100–200 kg) species of Hipparion and Cremohipparion species were mixed-feeders, whereas larger species of the genus Hippotherium (with body masses more than 200 kg) retained browse-dominated or mixed diets, while the small species of Cremohipparion (body mass less than 100 kg) had grazing diets (Tables S2 and S3) [8]. Hipparionines thus seem to by and large reflect the model of larger sizes being related to more browse-dominated diets and smaller sizes to grazing diets, as in the example of Equus during the Pleistocene in Eurasia. Similarly high diversity of hipparionines occurred in East Asia during the latest Miocene.
During the Pliocene, the diversity of hipparionines in Eurasia dropped drastically and there was a turnover in the species composition, with a few, in general large-sized (200–350 kg) species in the genera Plesiohipparion, Baryhipparion and Proboscidipparion surviving [8]. All these genera had mostly mixed-feeding diets, with Plesiohipparion and Proboscidipparion having wide geographic ranges from East Asia to Europe [8]. Considerable ecological flexibility seems typical, especially for Proboscidipparion. While Pr. sinense occupied relatively open environments in East Asia and had a mixed but relatively abrasion-rich mesowear signal [8], Proboscidipparion sp. from Red Crag, England (latest Pliocene, ca. 2.7 Ma) had a browse-dominated diet [8,367] and lived in a warm-temperate forest environment [368] (Tables S2 and S3).
The earliest species of Equus to disperse from North America to Eurasia were relatively large sized but ecologically quite generalized, grazing, open-adapted species such as E. eisenmannae in East Asia and E. livenzovensis in Western Asia and Europe. These taxa had average body masses around 500 kg and at least E. eisenmannae had mesowear values indicating typical grazing diet for the genus (Tables S2 and S3). At the beginning of the Pleistocene, the first of the specialized, very large-sized and robust woodland horses with mixed and even browse-dominated diets, E. major, emerged in Western Europe. This species typically occurs in Early Pleistocene sites in Europe where palaeoenvironmental proxies such as pollen records and large mammal ecometrics indicate relatively wooded and productive paleoenvironments such as in Red Crag (UK) and Tegelen (Netherlands) [19,369]. Equus major was one of the largest species of equid, with mean body mass around 600 kg, and maximum body mass of ca. 800 kg.
The common Early Pleistocene species of European Equus, E. stenonis, occurred in a wide range of localities suggesting broad tolerance of environmental conditions. It was a medium-large species of Equus, with mean body mass between 400 and 500 kg (Table S2). Available paleodietary evidence indicates mostly grazing diets for E. stenonis [370,371] (Tables S2 and S3), but the small sample from East Runton, UK, had a more mixed dietary signal (Table S2). Analysis of body mass, mesowear and the NPP of E. stenonis paleopopulations indicates a strong inverse relationship of the amount of grass in diet and body size (Figure 1). There was also a geographic pattern of body size in E. stenonis, with the Western European populations associated with more high-productivity environments having on average larger body sizes than Eastern European populations, which occurred in less productive environments [13]. Equus senezensis was a smaller species with mean body mass around 350 kg and a grazing diet and occupied mostly open landscape [372,373] (Tables S2 and S3).
Later during the Early Pleistocene, the large-sized E. suessenbornensis and the small-sized E. altidens became prominent species in Eurasia, being the dominant species there during the late Early and early Middle Pleistocene. Equus suessenbornensis was a very large-sized and robust species, comparable in size to the earlier Pleistocene Western European Equus major (with mean body mass within paleopopulations ranging from over 500 kg to slightly over 600 kg). Similar to other very large species of Equus, E. suessenbornensis typically had mixed to even browsing diets [19,374] (Tables S2 and S3), and although being widespread in Europe, it was typically less abundant than the small E. altidens, also where these two co-occurred. Equus altidens was the earliest identified hemionine and it shares interesting similarities in mesowear signal to extant hemionines. As in modern hemionines, most paleopopulations of E. altidens show heavily grazing mesowear signals [375] (Table S3), but also relatively abundant association of low occlusal relief and sharp cusps in some localities [375]. This kind of mesowear signal suggests diet based mostly on grasses but also including a significant component of dry, open environment browse such as aridity-resistant shrubs [15]. Some populations also display microwear patterns compatible with a mixed diet suggesting a certain degree of dietary plasticity for this species [369,375] (Table S3). Mean body mass estimates of E. altidens vary around 350 kg (Table S2). In general, E. altidens tends to be associated with paleoenvironments where dental ecometrics of large herbivorous mammal communities indicate relatively modest primary production estimates (between ca. 700 and 900). In Guadix-Baza Basin, Andalucia, Spain, the paleoenvironments of E. altidens have been suggested to have been similar to present Mediterranean woodlands and forest-steppes [374,376,377]. The diet of E. altidens reflects differences in paleoenvironments, being purely grazing in Venta Micena and Vallparadìs (EVT12 layer; MIS 31) but more mixed in Barranco León and Fuente Nueva 3 in Guadix-Baza Basin, Andalucia, where the paleoenvironment was more Mediterranean forest or woodland type and in layer EVT7 (MIS 21) of Vallparadìs where environmental conditions became more humid, and seasonality might have increased following the “0.9 Ma event” [374,375]. In Süssenborn, Germany, this species occurred in a paleoenvironment which has been interpreted periodically cool and relatively open, but not periglacial, based on the faunal association [378].
The Middle Pleistocene marks the arrival of caballine horses in Eurasia, a significant turnover event. Equus mosbachensis (=E. ferus mosbachensis/E. ferus), the typical caballine during the early Middle Pleistocene in Europe, was a very large and robust form (mean body mass from over 500 kg to nearly 600 kg), and it displayed more diverse dietary adaptations including grazing, mixed or even browse-dominated diets [19,373,379] (Tables S2 and S3). Large, browse-dominated forms of this taxon are associated with relatively wooded paleoenvironments such as Boxgrove in the UK and Schöningen in Germany [19,380,381]. Even grazing populations can be found in habitats dominated by wooded landscapes (e.g., open woodlands), feeding also in closed environments such as in Fontana Ranuccio (0.4 Ma) [374,382] (Table S3). The cold-stage paleopopulation of E. mosbachensis from Caune d’Arago, France, had somewhat smaller average body size and more grazing diet (Tables S2 and S3). The wild horse (E. ferus) was abundant and widespread in Eurasia during the late Middle and Late Pleistocene, with small-sized forms having more grazing dietary signals and occurring in sites with smaller estimated NPP than larger forms of the species (Figure 1, Table S2). The smallest forms of E. ferus with most grazing dietary signals come from sites where associated paleobotanical evidence indicates very open and grass-dominated “mammoth steppe” environments, such as Brighton (MIS 6 glacial) and Gough’s Cave (MIS2 glacial) in UK (Table S2) [383,384]. Conversely, large forms of E. ferus typically occurred in more wooded paleoenvironments, such as Grays Thurrock (MIS 9 interglacial) and Brundon and Ilford (MIS 7 interglacial) in the UK, and Taubach (last interglacial, MIS 5) in Germany, and had less purely grazing diets (Table S2) [385,386]. Further east, a medium-large sized form of E. ferus (“E. ferus latipes”) had a grass-dominated diet at the locality of Kostenki 14 in western Russia (Table S2), where it occupied a cool and arid steppe environment [387]. The northernmost populations from Taimyr and Yakutia, Northern Siberia, “E. lenensis”, mostly lived in cold, low-productivity steppe-tundra environments, although the northern edge of boreal forest advanced in these areas during warmer stages [388]. They are characterized by small average body size and grass-dominated mesowear signal, although some individuals show sharper and more high-relief cusps indicating inclusion of browse or non-grass herbaceous vegetation in their diet (Table S2). Equus hydruntinus had a more limited range in Eurasia during the late Pleistocene, and similarly to other hemionines, it seems to have been associated with relatively open habitats and it consistently had grass-dominated diets (Tables S2 and S3) [389].
The extant equids in Eurasia are currently limited to the Central Asian hemionines (E. hemionus and E. kiang) and the Przewalski horse (E. przewalski). These are all relatively small-sized members of the genus, they all have grazing diets, and they occupy the steppe environments of Central Asia (Tables S2 and S3). Similar to E. altidens, the hemionines today have a comparatively high proportion of low and sharp mesowear among Equus, indicating some inclusion of “dry browse” or non-grass herbs in diet, in addition to grass (Table S2).
6.4. Africa
The earliest hipparionine with palaeodietary evidence from Africa is Cormohipparion sp. from the late Miocene of Chorora, Ethiopia (ca. 8.5 Ma), which has a browse-dominated mesowear signal and medium body size (ca. 160 kg) (Tables S2 and S3) [8,274,390,391,392,393,394]. Since this earliest record, most of the equines in Africa show mesowear and other paleodietary evidence suggesting grass-dominated to grazing diets. The hipparionines of the genus Eurygnathohippus were relatively large in size (over 200 kg in mean body mass) and yet they had remarkably grass-dominated diets (Tables S1 and S2), unlike the large-sized hipparionines in Eurasia, which tend to have more mixed or browse-dominated diets. This could reflect adaptation of the African derived hipparionines of the genus Eurygnathohippus to graze in relatively productive, but grass-dominated savanna environments.
After the arrival of Equus in Africa in the Pleistocene, most of the African equine species had grazing diets and were of small body size compared to a much wider range of sizes and diets in Eurasia (Figure 2), probably reflecting similarity in their adaptations to grazing in grass-dominated African savanna environments. The only clear exception to this pattern is the very large-sized South African species E. capensis, which had a more mixed or even browse-dominated dietary signal, paralleling the relationship between diet and body size observed for the Pleistocene of Europe (Figure 2; Table S1).
The extant African zebras (E. quagga, E. grevyi and E. zebra) all have relatively small body size compared with the large Pleistocene species of Equus (particularly in Eurasia) and they typically have some of the most purely grazing diets among the equids (Table S2). The Grevy’s zebra (E. grevyi) is the largest of these species, and the largest extant species of wild Equus, but it has a relatively tall and slender morphology, with elongate metapodials compared to the Quagga, and mean body mass estimates are relatively modest (around 360 kg on average) compared to many of the Pleistocene taxa, resembling however those of E. koobiforensis from the Pleistocene of East Africa. Our mesowear data suggest that the proportion of sharp but low-relief cusps is higher in E. grevyi than the rest of extant zebras, indicating perhaps a somewhat higher proportion of dry browse such as dry-adapted shrubs in its diet (Table S2). The Africa wild ass (E. africanus) also has a relatively high proportion of low and sharp mesowear, despite mostly grazing dietary signal, which could also reflect inclusion of dry browse in the arid environments of this species (Table S2) [395].
7. Climate and Evolution
Figure 3 provides the distribution of Equinae in North America, 7–4 Ma. As with the succeeding climate and evolution maps, the numbers on these maps are tied to Table S4. During this time frame, 10 genera are recognized from North America (Calippus, Dinohippus, Cormohipparion, Nannippus, Neohipparion, Astrohippus, Boreohippidion, Pliohippus, Pseudohipparion and Equus), 3 from Eurasia (Plesiohipparion, Proboscidipparion and Cremohipparion) and 3 from Africa (Cremohipparion, Eurygnathohippus and Sivalhippus). The Equinae in North America include Ca. elaschistus, Ca. hondurensis, D. interpolatus, D. leardi, D. leydianus, D. spectans, Co. occidentale, Na. aztecus, Na. lenticularis, Na. peninsulatus, Ne. leptode, A. ansae, A. lenticularis, Bo. galushai, D. mexicanus, Co. emsliei, Plio. coalingensis, Ne. eurystyle, Ne. gidleyi, Ps. simpsoni, Na. beckensis, Equus/Plesippus simplicidens, E. cumminsi, E. enormis and Equus/Plesippus idahoensis. The Eurasian record includes Pl. longipes, Pl. houfenense, Pr. pater, Pr. crassum, Cr. fissurae and Pl. huangheense, wheres Africa has Cr. periafricanum, Eu. feibeli, S. turkanensis, Eu. hooijeri and Eu. woldegabrieli (taxa 1–36, Table S4). Calippus, Dinohippus, Cormohipparion, Nannippus, Neohipparion and Astrohippus have records extending back to 10 Ma and amongst these Cormohipparion, Nannippus, and Neohipparion are hipparionine horses. Dinohippus has a chronology beginning at 10.3 Ma and D. mexicanus (5.3–4.6 Ma) is demonstrably related to Equus. Four species of Equus, E. cumminsi, E. enormis, E. idahoensis and E. simplicidens first occur in the Blancan (since 4.9 Ma) and in particular, E. simplicidens would appear to be related to first occurring Eurasian Equus [10,190]. Large mammal Mean HYP in North America ranges from 2.0–2.5, whereas Europe has the lowest mean HYP ranging from 1.5–2, with higher values (2.0–2.5) in Turkey, Greece and Spain. Africa has mostly 2.0 values with slightly lower values in the horn of Africa, whereas Asia shows mostly 2.0 values with localized areas ranging between 2.0 and slightly above 2.5 in China, Mongolia, Kazakhstan and Iran.
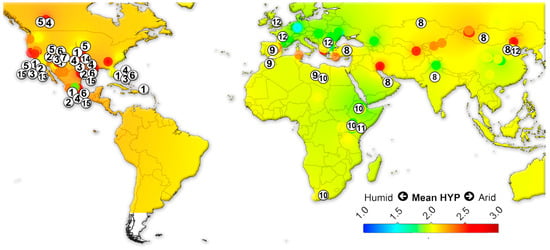
Figure 3.
Spatial distribution of the large herbivorous genera mean ordinated crown height through time ranges 7 to 4 Ma in North, Central and South America, Eurasia and Africa. The mean ordinated hypsodonty map represents the paleoclimatological conditions grading from most humid (blue) to most arid (red). Numbers in white circles show coded number of each taxa given in Table S4. The mean ordinated hypsodonty values are represented by the color-coded circles indicate the spatial position of the localities that mean hypsodonty scores calculated (Table S5). IDW interpolation algorithm hypothetically interpolates no data (no locality) area based on the actual data. These areas should be ignored.
Figure 4 presents the distribution of taxa between 4 and 2.6 Ma. During this time frame, 4 genera are recognized from North America (Pliohippus, Nannippus, Equus and Plesiohipparion), 6 from Eurasia (Plesiohipparion, Proboscidipparion, Cremohipparion, “Hippotherium”, Eurygnathohippus, Baryhipparion) and 2 from Africa (Eurygnathohippus, Cremohipparion). North American taxa carrying over into this interval include Plio. coalingensis, Na. beckensis, Equus/Plesippus simplicidens, E. cumminsi and Equus/Plesippus idahoensis. The persisting Eurasia taxa include Pl. longipes, Pl. houfenense, Pr. pater, Pr. crassum, Cr. fissurae and Pl. huangheense, whereas most African species disappeared, with the only survival of the hipparion genera Eurygnathohippus and Cremohipparion. First occurring taxa include Plesiohipparion sp. in Ellesmere Island (N. America), Ba. insperatum, Cr. licenti, “Hippotherium” antelopinum, Eurygnathohippus sp, Pr. heintzi, Pl. rocinantis, E. afarensis, Eu. hasumense and Eu. cornelianus (Africa). The African clade Eurygnatohippus is found there during this interval and is represented by a lower cheek tooth from India at the end of this temporal interval. “Hippotherium” antelopinum is a medium sized hipparionine whose type locality is in India. North America records the immigration of Plesiohipparion into Greenland. North America and South America have mostly Mean HYP between 2.0 and 2.5, with some areas in the west recording values of around 2.5 and others between 1.5–2.0.
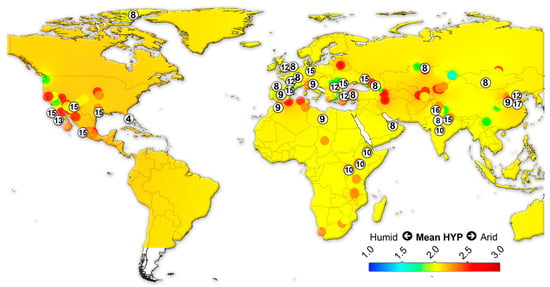
Figure 4.
Spatial distribution of the large herbivorous genera mean ordinated crown height through time ranges 4 to 2.6 Ma in North, Central and South America, Eurasia and Africa. The mean ordinated hypsodonty map represents the paleoclimatological conditions grading from most humid (blue) to most arid (red). Numbers in white circles show coded number of each taxa given in Table S4. The mean ordinated hypsodonty values are represented by the color-coded circles indicate the spatial position of the localities that mean hypsodonty scores calculated (Table S5). IDW interpolation algorithm hypothetically interpolate no data (no locality) area based on the actual data. These areas should be ignored.
Figure 5 presents the distribution of taxa between 2.58 and 1.5 Ma, including the Equus Datum in Eurasia at the beginning of the Pleistocene. During this time frame, 2 genera are recognized from North America (Nannippus and Equus), 4 from Eurasia (Plesiohipparion, Proboscidipparion, Baryhipparion and Equus) and 2 from Africa (Eurygnathohippus and Equus). North America records E. calobatus, E. scotti, E. stenonis anguinus, E. conversidens, E. ferus/lambei, E. francisci, E. fraternus and E. pseudaltidens during this interval, in addiction to Na. beckensis, Equus/Plesippus simplicidens, E. cumminsi and Equus/Plesippus idahoensis. Europe records several species of Equus in this interval, including: E. livenzovensis, E. major, E. stenonis, E. senezensis, E. stehlini and E. altidens. The Indian subcontinent has E. sivalensis and Equus sp. in India. China records several Equus species during this interval including E. eisenmannae, E. sanmeniensis E. huanghoensis, E. yunnanensis, E. qingyangensis, E. teihardi and E. wangi, with Ba. insperatum, Pl. shanxiense and Pr. sinense. Central Asia includes E. pamirensis, whereas Africa includes the Equus species in North Africa (E. numidicus) and Equus sp. in East Africa. Overall, the African record includes E. tabeti in North Africa with Eu. pomeli, E. koobiforensis, E. oldowayensis in East Africa and E. capensis and E. zebra in South Africa. The earliest species of Equus are found in some localities at ca. 2.6 Ma in Europe, Siwalik Hills and China, which shows values between 2.0–2.5. These values are more diffused in Eurasia compared with Figure 4, although are still present values between 1.5–2.0 in China, Russia, Caucasus and Europe. Africa overall has values between 2.0 and 2.5 through most of the continent, with isolated areas between 2.5–3.0 in North and East Africa. During this interval, most continental mammal records are dry with mean hypsodonty mostly being 2.0 or higher with several higher incidences of 2.5 or higher.
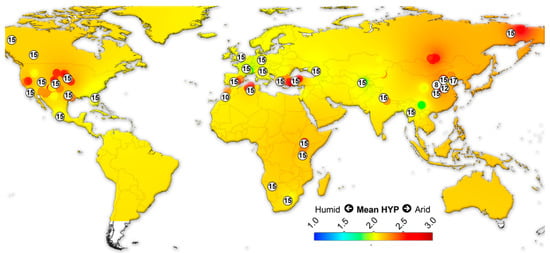
Figure 5.
Spatial distribution of the large herbivorous genera mean ordinated crown height through time ranges 2.58 to 1.5 Ma in North, Central and South America, Eurasia and Africa. The mean ordinated hypsodonty map represents the paleoclimatological conditions grading from most humid (blue) to most arid (red). Numbers in white circles show coded number of each taxa given in Table S4. The mean ordinated hypsodonty values are represented by the color-coded circles indicate the spatial position of the localities that mean hypsodonty scores calculated (Table S5). IDW interpolation algorithm hypothetically interpolate no data (no locality) area based on the actual data. These areas should be ignored.
Figure 6 presents the distribution of taxa between 1.5 Ma to recent. During this time frame, 2 genera are potentially recognized from North America (Equus and Haringtonhippus), 2 from South America (Equus and Hippidion), 2 from Eurasia (Equus and Proboscidipparion) 2 from Africa (Equus and Eurygnathohippus). This time frame includes a number of taxa that carry over from the 2.58–1.5 Ma interval including Equus/Plesippus simplicidens, E. cumminsi, Equus/Plesippus idahoensis, E. calobatus, E. scotti, E. conversidens, E. ferus/lambei, E. francisci, E. fraternus and E. pseudaltidens (North America); E. sameniensis, E. yunnanensis, E. qingyangensis, E. teilhardi, E. wangi (China); E. sivalensis (India); E. numidicus, E. tabeti (North Africa); E. koobiforensis, E. oldowayensis (East Africa); E. capensis, E. zebra (South Africa). Taxa first occurring between 1.5 Ma to recent are E. verae, E. cedralensis, E. mexicanus and E. occidentalis for North America; E. neogeus, Hippidion devillei, Hippidion saldiasi, Hippidion principale for South America; E. beijingensis, E. dalianensis, E. hemionus (also India), E. kiang (also Nepal) and E. przewalskii in China; E. nalaikensis and E. colimensis, E. lenensis and E. ovodovi in Central and North Asia; E. suessenbornensis, E. apolloniensis, E. wuesti, E. hipparionoides, E. marxi, E. ferus, E. hydruntinus, E. petraolnensis and E. graziosi in Europe; E. mauritanicus, E. melkiensis and E. algericus in North Africa and E. africanus, E. grevyi and E. quagga in East and South Africa. This time frame record also records the last occurrence of the hipparionini horses in Asia with Proboscidipparion and Africa with Eurygnathohippus [8]. Mean HYP shows North and South America having more moderate climates around values of 2.0 and 2.5, with values lower than 2.0 in local areas in the East and the West. Europe likewise has Mean HYP values around 2.0 with values lower than 2.0 in Central and Eastern Europe, whereas Asia, the Indian Subcontinent and Africa have higher values hovering around Mean HYP of 2.5. Mean HYP values between 1.5–2.0 are also found in China.
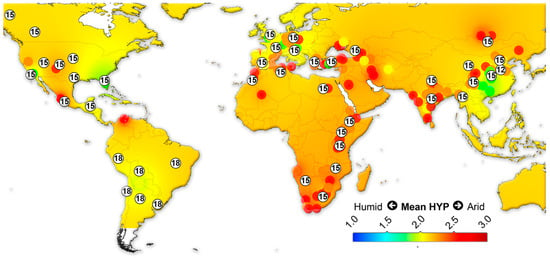
Figure 6.
Spatial distribution of the large herbivorous genera mean ordinated crown height through time ranges 1.5 Ma to recent in North, Central and South America, Eurasia and Africa. The mean ordinated hypsodonty map represents the paleoclimatological conditions grading from most humid (blue) to most arid (red). Numbers in white circles show the coded numbers of each taxon given in Table S4. The mean ordinated hypsodonty values are represented by the color-coded circles indicate the spatial position of the localities that mean hypsodonty scores calculated (Table S5). IDW interpolation algorithm hypothetically interpolate no data (no locality) area based on the actual data. These areas should be ignored.
The mean hypsodonty map patterns indicate that while most of Eurasia and Africa occupied by mild or humid values, arid environmental conditions were prominent in North America between 7 and 4 Ma (Figure 3). At the late Miocene–Early Pliocene transition, moist conditions occurred in Europe with an arid belt extending eastward into Asia and Africa. By the end of the Pliocene arid conditions remained in North America and aridity began to increase on the mid-latitudes of Eurasia and along the Rift Valley of East Africa and the western corners of North Africa (Figure 4). Mean ordinated crown height patterns of the large herbivorous mammal communities indicate that while Southeast Asia, Central and Western Europe, and Florida and California in North America occupied by semi-humid or humid values, arid environmental conditions persisted or increased drastically rest of the World and in particular in East and South Africa, 1.5 Ma to recent (Figure 6).
Overall, these maps exhibit a general trend of increased drying over time. Most occurrences of Equus are with Mean HYP values of 2.0–2.5. Very few archaic Equinae taxa continue across the Pliocene–Quaternary boundary. North America retained the more primitive lineages of Pliohippus and Nannippus up into the 2.58–1.5 Ma interval. Hipparionines persisted up into the Pleistocene of Europe, as late as 1.0 Ma in China and slightly later than 1.0 in Africa. The extinction of older North American and Eurasian-African lineages would appear to be associated with the expansion of more open country dry conditions.
8. Phylogeny
As reported in the introduction, recently two morphological-based cladistic analyses have re-evaluated the origin of the genus Equus. Herein, we present the state of art of these cladistic hypotheses, with a separate section on the contribution from the molecular phylogenies.
Barrón-Ortiz et al. [9] undertook a phylogenetic analysis using a matrix of 32 characters (22 cranial, 6 mandibular, 3 autopodial, and estimated body size). The authors included in the matrix 21 Equini species, two of which were considered as outgroups, Acritohippus stylodontus, and Pliohippus pernix. Barrón-Ortiz et al. [9] undertook the analysis using TNT 1.1 [396] with the implicit enumeration option (exhaustive search), using equal weighting for the characters, and without a collapsing rule. They treated all characters as unordered.
Cirilli et al. [10] have combined a new matrix including 30 Operational Taxonomic Units (OTUs) and 129 characters (72 cranial, 40 mandibular and 17 on autopodia), 68 of which were new and the other extrapolated from the recent published matrices on perissodactyl phylogeny [9,114,397,398,399,400]. The characters were mainly coded by direct observations. The ingroup included a comprehensive sample of 26 equid species and the outgroup was represented by the Brazilian tapir Tapirus terrestris, the rhinocerotoid Hyrachyus eximius, the Rhinocerotidae Trigonias osborni and the early-diverging equid Merychippus insignis. The analysis was performed in PAUP 4.0b10, with Heuristic search, TBR and 1000 replications with additional random sequence, and gaps treated as missing. In this analysis, 24 characters have been ordered and 105 characters unordered. All characters were equally weighted.
8.1. What Is Equus? Paradigms, Phylogeny, and Taxonomy
The primary objectives of the study conducted by Barrón-Ortiz et al. [9] were to review and discuss different paradigms for understanding generic-level taxonomy, particularly in regards to the delimitation of mammalian genera, and to evaluate how those different paradigms impact the concept and contents of Equus in a given phylogenetic tree. Barrón-Ortiz et al. [9] established a new phylogenetic tree of derived Equini for that analysis. The tree served as a model for the evaluation of how distinct paradigms impact our placement of generic names on any given tree.
8.1.1. What Is a Genus?
Although several studies have discussed limitations of and provided alternatives to the Linnaean taxonomic system [401,402,403,404,405,406], Linnaean taxonomy continues to be widely used to study and communicate about past and present biodiversity [407]. This is especially true when it comes to the binomial nomen (genus and species). Because of the widespread use of binomial nomenclature within and outside of the life sciences, the genus is perhaps the most important higher-level taxonomic rank. Therefore, the question about how we define and delimit genera is not a trivial one as it affects how we view, study, and communicate about biological organisms. Equus is a model taxon for such discussions because of its complex generic and species-level history. At the core of the delimitation of Equus within any phylogenetic tree lie the philosophical and practical issues regarding the definition of genera and how best to reconcile taxonomy with evolutionary history.
Different paradigms exist for understanding and delimiting genera. In the case of mammals, Barrón-Ortiz et al. [9] identified four that are commonly used in combination with monophyly to delimit genera: phylogenetic gaps, uniqueness of adaptive zone, crown group definition, and divergence time [407,408,409,410,411]. One of the primary distinctions between the paradigms is the way genera are conceived. At one extreme, the uniqueness of adaptive zone paradigm conceives genera as having some level of biological reality beyond monophyly (i.e., a genus occupies a unique adaptive zone). An adaptive zone corresponds to a particular mode of life or a unique ecological situation [6,409,412,413]. At the other extreme, under the divergence time and crown group paradigms, genera are arbitrarily defined [168,410,411,414] and are not conceived as having biological reality other than monophyly. The phylogenetic gaps paradigm occupies an intermediate position. Under this paradigm, genera are not necessarily conceived as having some level of biological reality, but the gaps between monophyletic groups of species used to delimit genera arise from biological processes such as speciation, extinction, evolutionary and adaptive radiations, and unequal rates of evolution [409]. Because genera may be conceived under different paradigms, it is important for researchers to explicitly state their operational paradigm when considering questions of generic-level taxonomy.
8.1.2. Morphological Phylogenetic Analysis of Derived Equini
For the second study objective, Barrón-Ortiz et al. [9] conducted a morphological phylogenetic analysis of derived Equini. The phylogenetic analysis produced three equally most parsimonious trees of 85 steps with consistency (CI) and retention (RI) indices of 0.57 and 0.80, respectively [9] Figure 1 for the strict consensus tree.
Using the strict consensus tree obtained in their analysis, Barrón-Ortiz et al. [9] evaluated how the four paradigms commonly used to delimit mammalian genera impacted the concept and contents of Equus, although we emphasize that the same could be applied to any phylogenetic hypothesis. The results of this evaluation and taxonomic implications are summarized below.
8.1.3. Phylogentic Gaps and the Concept of Equus
Under the phylogenetic gaps paradigm, a genus is comprised of a single species or a monophyletic group of species, separated from other single species or monophyletic groups of species of the same rank by a decided gap [409]. In the context of a phylogenetic analysis, the gaps between single species or monophyletic groups of species can be measured by the number of synapomorphic traits. Application of this paradigm to the strict consensus tree of Barrón-Ortiz et al. [9], suggested that Equus should be delimited to clade 6, as this clade shows the most synapomorphic traits, including the species E. neogeus, E. occidentalis, E. ferus, E. mexicanus, E. hemionus, E. quagga, E. conversidens and E. francisci. The Early Pleistocene E. stenonis and the North American E. simplicidens and E. idahoensis are not included in this clade.
8.1.4. Uniqueness of Adaptive Zone and the Concept of Equus
In the uniqueness of adaptive zone paradigm, a genus is comprised of a single species or a monophyletic group of species that occupies a different adaptive zone (i.e., a unique mode of life) from the one occupied by species of another genus [409,413]. Application of this criterion in the context of a phylogenetic analysis requires: (1) the identification of traits (i.e., character states) that allow or potentially allow a single species or a monophyletic group of species to occupy a unique adaptive zone and (2) identifying where those traits occur in the tree.
The unique mode of life of Equus could potentially be defined as “ungulate mammals that are adapted to live in generally open, arid habitats and that can thrive on low-quality, high-fiber foods such as grasses and other coarse and tough vegetation” [9,19]. Potential morphological adaptations for this mode of life include modifications to the locomotory [415] and digestive systems, particularly the dentition [9]. Based on the position of the majority of purported, adaptive zone-related traits, Equus is assigned to clade 6, or possibly clade 7, in the strict consensus tree of Barrón-Ortiz et al. [9], under the uniqueness of adaptive zone paradigm. The identification to the clade 7 would include E. stenonis in the genus Equus, but not E. simplicidens and E. idahoensis.
8.1.5. Crown Group and the Concept of Equus
This paradigm follows a nominalist perspective to the definition of taxa. The nominalist perspective assumes that the limits of named taxa are arbitrary conventions, and then proceeds to spell out those conventions [414]. Under the crown group paradigm, a genus is defined as the clade that includes the most recent common ancestor of all extant species assigned to that genus, and all descendants of that ancestor. Therefore, under this paradigm, Equus is defined as the clade that includes the most recent common ancestor of all extant species assigned to Equus, and all descendants of that ancestor. Equus is constrained to clade 6 in the strict consensus tree of Barrón-Ortiz et al. [9] based on the crown group paradigm, which include the same species obtained under the phylogenetic gaps paradigm.
8.1.6. Divergence Time and the Concept of Equus
The divergence time paradigm states that a species or a monophyletic group of species should be regarded as a distinct genus if it diverged well-before the Miocene-Pliocene boundary (4–7 Ma) [168,410,411]. Application of this paradigm in the context of a phylogenetic analysis requires the creation of a time-calibrated phylogeny. Based on the time-calibrated phylogeny of Equini of Barrón-Ortiz et al. [9], Equus is delimited to clade 9 under the divergence time paradigm. Here, E. stenonis and the North American E. simplicidens and E. idahoensis should be included in the Equus clade.
8.1.7. Taxonomic Implications
Barrón-Ortiz et al. [9] concluded that Equus should be delimited to clade 6 in their phylogenetic analysis, based on the fact that three out of the four paradigms used to define mammalian genera identified clade 6 as the most suitable position for Equus. This taxonomic arrangement excludes E. stenonis, E. idahoensis, E. simplicidens, and “Dinohippus” mexicanus from the genus Equus and it implies that Haringtonhippus is a junior synonym of Equus. Some researchers have assigned E. simplicidens and E. idahoensis to Plesippus at the generic or subgeneric rank [4,57,59,416,417], with Plesippus simplicidens selected as the type species [4,416]. Likewise, E. stenonis has been referred to Allohippus at the generic or subgeneric rank [416]. Based on the results of their analysis, Barrón-Ortiz et al. [9] suggested that Plesippus and Allohippus should be elevated to generic rank, “Dinohippus” mexicanus should be assigned to a new genus, and Haringtonhippus should be synonymized with Equus.
8.2. Cirilli et al. [10] Phylogeny: Equus Modeled as a Sigle Monophyletic Clade
The results from Cirilli et al. [10] differ from the previous phylogeny of Barròn-Ortiz et al. [9]. Cirilli et al. [10] obtained a single most parsimonious tree from the matrix used, and the genus Equus is modeled as a single clade with node 52 being supported by 18 unambiguous synapomorphies, and 13 of these have a CI ≥ 0.500 [10], (Figure 2), not allowing the endorsement of Plesippus or Allohippus at generic or subgeneic level. In particular, the genus is defined by a linear lateral outline of the skull, the absence or reduction of the buccinator fossa, the presence of a shallow depression on the lingual margin of the protocone, the squared shape of the protocone on P2, the presence of an elongated pli caballine on P3 and P4, the squared shape of the protocone on P3 and P4, a V-shaped morphology of the linguaflexid, part of the metaconid-metastylid complex, a squared morphology of the lingual side of the metastylid, a strong and broad 3rd phalanx, a reduced lateral second and fourth metapodials. Moreover, additional analyses as the bootstrap tree supports the Equus clade with 99/100 replications [10], (Supplemental Materials). According to Brochu and Sumrall [418], clades within a cladogram are named if two criteria are met: the clade is stable and unlikely to collapse, and there is a need to discuss the group. In addition, Bryant [419], Cantino et al. [420], and Schulte et al. [421] provide some guidelines for the establishment of clade names, including the application of methods for measuring nodal support, careful consideration of those taxa that are likely to move around in different analyses, and use of multiple basal taxa as specifiers for node-based groups. A recently proposed phylogenetic nomenclatural system [422,423,424] specified that all supraspecific taxonomic nomina be explicitly defined on the basis of common ancestry. In the work by Cirilli et al. [10], E. simplicidens is considered as the common ancestor of all the Equus species, and place at the base of their radiation, separated from the genus Dinohippus. This view is supported by recent molecular analyses of the group, where all the extant equid taxa are grouped into a single genus, Equus [27,28,29,425]. A large Equus clade, including some fossil taxa, is also identified by Heintzman et al. [29], where a new clade composed by representatives of Haringtonhippus is supposed to diverge from Equus during the early Pliocene. It would appear that Haringtonhippus is convergent in cranial and postcranial features with Asian E. hemionus and perhaps Pleistocene E. altidens.
8.2.1. Phylogenetic Gaps, Crown Group, Adaptive Zone, and Divergence Time Applied to the Phylogeny of Cirilli et al. [10]
The phylogenetic gaps criterion identify a genus as a taxonomic category containing a single species, or a monophyletic group of species, which is separated from other taxa of the same rank by a relevant gap. The results from Cirilli et al. [10] support the definition of Equus as being a single monophyletic clade since E. simplicidens, grouped separately from the species included in the genus Dinohippus.
As reported above, the concept of the adaptive zone implies that ecological factors contribute to the speciation process. In this regard, for the genus Equus may be taken in consideration the progressive shift to a diet mostly based on C4 grasses. The palaeoecological studies based on the North American record provide some insights between the last representative of the genus Dinohippus, D. mexicanus, and the first forms of Equus. As reported by MacFadden et al. [426] and Semprebon et al. [427], fossil and extant species of Equus have been almost grazers or mixed feeders (except for some large species) [19], whereas some populations of the late Hemphillian D. mexicanus from Florida show a browsing signal. However, other late Hemphillian Dinohippus samples were identified as mostly grazer, suggesting that the dietary transition from browser to mixed feeders and grazer may already have occurred in North America. Nevertheless, the presence of some individual with δ13C values of 24.7 and 21.5 per mil in the D. mexicanus sample studied by MacFadden et al. [426] suggests that some individuals of this sample were feeding on C4 grasses. This evidence indicates that some populations of Dinohippus shifted to a more grazing diet, which may have led to speciation process to new forms adapted to new environments. This would have affected not only the diet, but also the increase of the body mass, from ca. 300 kg in D. mexicanus to 300 and 400 kg in E. simplicidens [10,426]. Moreover, MacFadden et al. [426] reported that the dietary shift from browser to widespread grazing in Equus may have occurred during the early Pliocene in North America between, 4.8 and 4.5 Ma, a time frame coherent with the first occurrences of E. simplicidens.
The crown group as defined as being a collection of species composed of the living representatives of the collection, the most recent common ancestor of the collection, and all descendants of the most recent common ancestor. In this regard, the results from Cirilli et al. [10] include zebras, asses and the caballine horses in a single clade with the most recent common ancestor identified as being E. simplicidens.
Moreover, the concept of the most recent common ancestor is directly linked also to divergence time, and to the estimations based on the genomic analyses from Orlando et al. [27], which estimated a time frame of 4.5–4.0 Ma for the origin of the most recent common ancestor for Equus. This estimated ages confirm some one of the oldest discoveries of E. simplicidens in North America [428,429,430] and therefore supporting the hypothesis of E. simplicidens as the first representative of the genus Equus.
To summarize, phylogenetic gaps, crown group, adaptive zone and divergence time are congruent for identify of the Equus clade at node 52 of the phylogeny from Cirilli et al. [9], in agreement with the MPT, Bootstrap and UPGMA tree.
8.2.2. Living and Fossil Equids
The results by Cirilli et al. [10] support the taxonomic division of caballines (domestic horse and Przewalski’s wild horse) and noncaballines (zebras and African and Asiatic asses) proposed by morphological data [62] and other molecular and combined studies [431,432,433,434,435] ([8] UPGMA analyses, supplemental information). Similarly to previous studies, Cirilli et al. [10] reported a paraphyletic origin of the extant zebra species as proposed in the literature using cranial morphology [397], palaeogenetics [27,436,437], and nuclear data [438,439,440], but with a low levels of support of the nodes. Other molecular analyses instead suggested a monophyly of the zebra species with the mountain zebra placed as the sister taxon of Burchell’s and Grevy’s zebras [432,440,441,442]; a result affected, anyway, by the absence of fossil representatives of this group in the analyses.
8.3. The Contribution from the Molecular Phylogeny
In the last two decades, new perspectives on the evolution of the genus Equus have been reported with the contribution from the molecular phylogeny. Orlando et al. [27] coded the genome of a fossil horse dated ca. 780–560 ka, identifying that the most recent common ancestor for the genus Equus emerged at ca. 4.5–4.0 Ma in North America, which is now in agreement with oldest findings and occurrences of the North American E. simplicidens. Analogous results were obtained also by Vilstrup et al. [425], which however highlighted the distinction of the North American stilt legged horses from the living asses, supporting a different evolution which led to a similar morphology. Moreover, Vilstrup et al. [425] identified zebras and asses as distinct clades, proposing an estimated age for origin of the plain zebras at ca. 0.7 ± 0.1 Ma, and divergence from the Grevy’s zebas at ca. 1.5 Ma.
More inputs came from Jónsson et al. [28], which identified all the living equids belonging to a single genus, Equus. Moreover, Jónsson et al. [28] estimated that living zebras and asses cluster into a single monophyletic clade originating at ca. 2 Ma, that the African and Asiatic asses diverged slightly later, at ca. 1.8 Ma, and that the living zebras already diverged at ca. 1 Ma. These estimated ages are in agreement with the first occurrence of fossil species related to living zebras (E. koobiforensis, E. mauritanicus) or asses (E. altidens, E. tabeti). Equus hemionus and E. kiang diverged later, between 356–233 ka. Jónsson et al. [28] estimated also that the gene flow between caballine and stenonine horses ceased between 3.4 and 2.1 Ma, which is in agreement with the dispersal of the stenonine horses in Eurasia at the beginning of the Pleistocene.
Heintzmann et al. [29] used the crown group definition for the genus Equus and focused their study on the North American species, especially on the stilt legged species, which were previously identified close to Asian asses [56,62,69]. Nevertheless, the genetic analyses of Orlando et al. [153] and Vilstrup et al. [425] separated these species from the Asian asses and placed them close to the caballine horses. The new phylogeny from Heintzmann et al. [29] has identified the North American stilt horses as a distinct branch from the living and fossil Equus, diverging between 5.7–4.1 Ma, during the late Hemphillian or early Blancan. This separation anticipates the origin of the most recent common ancestor identified by Orlando et al. [27]. Heintzmann et al. [29] proposed a new genus, Harringtonhippus, for the stilt legged horses from North America, represented by the species Ha. Francisci. However, this taxonomy is not accepted by other authors on philosophical grounds [9].
Vershinina et al. [78] identified two dispersal event for the caballine horses. The first occurred between 0.95–0.45 Ma, in east to west direction, consistent with the oldest findings of the caballine horses in Eurasia. The second occurred at 0.2–0.05 Ma, bidirectional but predominantly west to east, due the identification of metapopulations of Eurasian Late Pleistocene horses in Alaska and Northern Yukon, which provided the opportunity for a gene flow between the North American and Eurasia horses during the Late Pleistocene.
Another interesting perspective comes from the subgenus Sussemionus. This subgenus was proposed by Eisenmann [256] as an informal group of species from the Early and Middle Pleistocene of Eurasia. Later, Eisenmann [87] formalized the subgenus, characterized by a combination of some dental features [87]. Eisenmann [87] included in this subgenus the species E. coliemensis, E. suessenbornensis, E. verae, E. granatensis, E. hipparionoides and E. altidens. Following the description of the author, the genus includes some anatomical features observed in the Süssenborn sample and in the modern Asian asses. Nevertheless, recent molecular studies [29,151,153,427,443] identified this subgenus separated from the living species even included in the genus Equus, surviving until the late Holocene with the species E. ovodovi, a Late Pleistocene species from Siberia. However, it should be noted that this subgenus has never been tested with a morphological based cladistic analysis, which is needed to address its taxonomic status.
9. Conclusions
Nineteen collaborating international scientists provide herein a detailed review and synthesis of fossil Equinae occurrences from the Plio-Plesitocene and recent of North, Central and South America, Eurasia and Africa including fossil and living species. At the present time, our review has identified valid 114 (+4) species of Equinae from 5.3 Ma to recent including 38 from North America, 4 from South America, 26 from East Asia, 6 from the Indian Subcontinent, 18 from Europe and 26 from Africa. In all continents other than South America, more primitive equine clades persisted after Equus appeared, and extinction of these more archaic clades were diachronous at the continental to inter-continental scale. While actively researched over the last several decades, Equinae taxonomy is not wholly settled and there are challenges to unifying them at the genus and higher taxonomic levels. That being said, the taxonomy of Equinae reviewed herein has allowed us to provide well resolved biochronology and biogeography, paleoecology and paleoclimatic context of the 5.3 Ma–recent Equinae records.
The paleoclimatic maps from 7 Ma to recent have shown a more suitable environment for the evolution of the modern Equini in North America rather Eurasia and Africa during the Pliocene, with more arid conditions which favored speciation of Equus and its dispersal into Eurasia and Africa at the beginning of the Pleistocene. This result is congruent with the hypothesis of several previous morphological, paleoecological and molecular studies cited herein which support the origin of Equus during the Pliocene in North America.
Finally, we presented the most recent cladistic morphological based hypotheses on the origin of the genus Equus, combined with the results from the molecular phylogenies. Phylogenetic evidence suggests that the genus Equus is closely related with Dinohippus, from which evolved. The North American E. simplicidens represents the ancestral species for the origin of the stenonine horses in Eurasia and Africa, culminated with the evolution of modern zebras and asses. This last point is supported also by the molecular analyses, which have hypothesized that North American stilt legged horses diverged from the living asses and are not phylogenetically linked. However, more studies are needed to shed light on the evolution of the caballine horses, which at the present time remains unresolved. Lastly, we acknowledge the different interpretations of morphological and molecular based cladistic analyses and the need to better integrate these studies going forward.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11091258/s1, Table S1: taxonomy; Table S2: body mass and diet; Table S3: Equini paleoecology_summary table; Table S4: Taxa coded on the MeanHYP maps; Table S5: Mean Hyposodonty data; Table S6: North and Central American Equus sensu lato.
Author Contributions
Conceptualization, R.L.B. and O.C.; methodology, O.C., H.M., C.I.B.-O., J.S., G.S., F.K. and R.L.B.; North and Central America, H.M., C.I.B.-O., Z.L., A.H.M.-L., J.A.-C., E.D. and E.S.; South America, H.M. and N.A.V.; eastern Asia, O.C., R.L.B., A.M.J. and D.P.; Indian Subcontinent, A.M.J., R.L.B., O.C.; Europe, O.C., R.L.B. and D.P.; Africa, R.L.B. and O.C.; Biochronology and Biogeography, L.R., L.P., R.L.B., O.C. and F.K.; Paleoecology, J.S., F.S., G.S. (with the contribution of C.I.B.-O., A.H.M.-L., D.P., Z.L., O.C and R.L.B.); Climate and Evolution, F.K., O.C. and R.L.B.; Phylogeny: 8.1, C.I.B.-O., C.N.J. and H.M.; 8.2, O.C., L.P. and R.L.B.; 8.3, O.C.; Conclusions, R.L.B. and O.C. Simple Summary, R.L.B. and O.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation (DBI:ABI 1759882 and 1759821) to R.L. Bernor and E. Davis, respectively, under the aegis of the FuTRES Equid working group for which they, O. Cirilli and H. Machado have been funded. F.K. has been funded by Finnish Cultural Foundation (project nr. 00220063). Z.L. acknowledge the Admission Scholarship from University of Ottawa, and a Canadian Graduate Scholarship-Doctoral Program from Natural Sciences and Engineering Research Council of Canada. A.M.J. thanks the Yale Institute for Biospheric Studies for funding. L.P. thanks the European Commission’s Research Infrastructure Action, EU-SYNTHESYS projects AT-TAF-2550, DE-TAF-3049, GB-TAF-2825, HU-TAF-3593, HU-TAF-5477, ES-TAF-2997, the SYNTHESYS Project http://www.synthesys.info/ (access on 5 August 2022) which is financed by European Community Research Infrastructure Action under the FP7 “Capacities” Program, and the research project “Ecomorphology of fossil and extant Hippopotamids and Rhinocerotids” granted to L.P. by the University of Florence (“Progetto Giovani Ricercatori Protagonisti” initiative). J.S. wishes to acknowledge the Academy of Finland (AoF. project nr. 340775/346292, “NEPA-Non-analogue ecosystems in the past”). F.S. is supported by Sapienza “5 per mille” funds (ref. SPC: 2021-0070-1350-175998).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated by this study are available in this manuscript and the accompanying Supplementary Materials (Supplementary Materials Tables S1–S6).
Acknowledgments
We acknowledge the curators of the museums that allowed us to study the collections of fossil and extant species cited herein, the scientists who have made their original data available to the scientific community. We also acknowledge Vera Eisenmann for her generosity publicly sharing her data on fossil horses on her website (http://vera-eisenman.com; access on 5 August 2022). C.I.B.O. would like to thank Marisol Montellano-Ballesteros, Christopher N. Jass, Leonardo S. Avilla, William T. Taylor, Alwynne Beaudoin and Duncan Ross Parliament for discussions on horse taxonomy. We thank three anonymous reviewers for their comments which have improved the quality of this manuscript. This is the FuTRES publication no. 32.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marsh, C.O. Polydactyle Horses, Recent and Extinct. Am. J. Sci. 1879, 17, 499–505. [Google Scholar] [CrossRef]
- Gidley, J.W. Revision of the Miocene and Pliocene Equidae in North America. Bull. Am. Mus. Nat. Hist. 1907, 23, 865–934. [Google Scholar]
- Osborn, H.F. Equidae of the Oligocene, Miocene, and Pliocene of North America. Iconographic type revision. Mem. Am. Mus. Nat. Hist. New Ser. 1918, 2, 1–326. [Google Scholar]
- Matthew, W.D. A new link in the ancestry of the horse. Am. Mus. Novit. 1924, 131, 130–131. [Google Scholar]
- Stirton, R.A. Phylogeny of North American Equidae. Univ. Calif. Publ. Geol. Sci. 1940, 25, 165–198. [Google Scholar]
- Simpson, G.G. Horses: The Story of the Horse Family in the Modern World and through Sixty Million Years of History; Oxford University Press: New York, NY, USA, 1951; pp. 1–247. [Google Scholar]
- MacFadden, B.J. Fossil Horses—Evidence for Evolution. Science 2005, 307, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Bernor, R.L.; Kaya, F.; Kaakinen, A.; Saarinen, J.; Fortelius, M. Old World hipparion evolution, biogeography, climatology and ecology. Earth Sci. Rev. 2021, 221, 103748. [Google Scholar] [CrossRef]
- Barrón-Ortiz, C.I.; Avilla, L.S.; Jass, C.N.; Bravo-Cuevas, V.M.; Machado, H.; Mothé, D. What is Equus? Reconciling taxonomy and phylogenetic analyses. Front. Ecol. Evol. 2019, 7, 343. [Google Scholar] [CrossRef]
- Cirilli, O.; Pandolfi, L.; Rook, L.; Bernor, R.L. Evolution of Old World Equus and origin of the zebra-ass clade. Sci. Rep. 2021, 11, 10156. [Google Scholar] [CrossRef]
- Eisenmann, V.; Alberdi, M.T.; De Giuli, C.; Staesche, U. Methodology. In Studying Fossil Horses; Woodburne, M., Sondaar, P.Y., Eds.; E.J. Brill Press: Leiden, The Netherlands, 1988; pp. 1–71. [Google Scholar]
- Bernor, R.L.; Tobien, H.; Hayek, L.A.C.; Mittmann, H.W. Hippotherium primigenium (Equidae, Mammalia) from the late Miocene of Höwenegg (Hegau, Germany). Andrias 1997, 10, 1–230. [Google Scholar]
- Cirilli, O.; Saarinen, J.; Pandolfi, L.; Rook, L.; Bernor, R.L. An updated review on Equus stenonis (Mammalia, Equidae): New implications for the European Early Pleistocene Equus taxonomy and paleoecology, and remarks on the Old World Equus evolution. Quat. Sci. Rev. 2021, 269, 107155. [Google Scholar] [CrossRef]
- Bernor, R.L.; Cirilli, O.; Buskianidze, M.; Lordkipanidze, D.; Rook, L. The Dmanisi Equus: Systematics, biogeography and paleoecology. J. Hum. Evol. 2021, 158, 103051. [Google Scholar] [CrossRef] [PubMed]
- Fortelius, M.; Solounias, N. Functional characterization of ungulate molars using the abrasion-attrition wear gradient: A new method for reconstructing paleodiets. Am. Mus. Novit. 2000, 3301, 1–36. [Google Scholar] [CrossRef]
- Solounias, N.; Semprebon, G. Advances in the reconstruction of ungulate ecomorphology with application to early fossil equids. Am. Mus. Novit. 2002, 3366, 1–49. [Google Scholar] [CrossRef]
- Oksanen, O.; Žliobaitė, I.; Saarinen, J.; Lawing, A.M.; Fortelius, M. A Humboldtian approach to life and climate of the geological past: Estimating palaeotemperature from dental traits of mammalian communities. J. Biogeogr. 2019, 46, 1760–1776. [Google Scholar] [CrossRef]
- Scott, K.M. Postcranial dimensions of ungulates as predictors of body mass. In Body Size in Mammalian Palaeobiology; Damuth, J., MacFadden, B.J., Eds.; Cambridge University Press: New York, NY, USA, 1990; pp. 301–355. [Google Scholar]
- Saarinen, J.; Cirilli, O.; Strani, F.; Meshida, K.; Bernor, R.L. Testing equid body mass estimate equations on modern zebras—with implications to understanding the relationship of body size, diet and habitats of Equus in the Pleistocene of Europe. Front. Ecol. Evol. 2021, 9, 622412. [Google Scholar] [CrossRef]
- Saarinen, J.; Eronen, J.; Fortelius, M.; Seppä, H.; Lister, A.M. Patterns of diet and body mass of large ungulates from the Pleistocene of Western Europe, and their relation to vegetation. Palaeontol. Electron. 2016, 19, 1–58. [Google Scholar] [CrossRef]
- Mihlbachler, M.C.; Rivals, F.; Solounias, N.; Semprebon, G.M. Dietary change and evolution of horses in North America. Science 2011, 331, 1178–1181. [Google Scholar] [CrossRef]
- The NOW Community. New and Old World Database of Fossil Mammals (NOW). Licensed under CC BY 4.0. 2022. Available online: https://nowdatabase.org/now/database/ (accessed on 4 April 2022).
- Fortelius, M.; Eronen, J.T.; Jernvall, J.; Liu, L.; Pushkina, D.; Rinne, J.; Tesakov, A.; Vislobokova, I.A.; Zhang, Z.; Zhou, L. Fossil mammals resolve regional patterns ofEurasian climate change during 20 million years. Evol. Ecol. Res. 2002, 4, 1005–1016. [Google Scholar]
- Eronen, J.T.; Evans, A.S.; Fortelius, M.; Jernvall, J. The impact of regional climate on the evolution of mammals: A case study using fossil horses. Evolution 2010, 64, 398–408. [Google Scholar] [CrossRef]
- Eronen, J.T.; Polly, P.D.; Fred, M.; Damuth, J.; Frank, D.C.; Mosbrugger, V.; Scheidegger, C.; Stenseth, N.C.; Fortelius, M. Ecometrics: The grains that bind the past and present together. Integr. Zool. 2010, 5, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Puolamäki, K.; Eronen, J.T.; Mirzaie Ataabadi, M.; Hernesniemi, E.; Fortelius, M. Dentalfunctional traits of mammals resolve productivity in terrestrial ecosystemspast and present. Proc. R. Soc. Lond. Biol. Sci. 2012, 279, 2793–2799. [Google Scholar]
- Orlando, L.; Vilstrup, L.; Ginolhac, A.; Zhang, G.; Froese, D.; Albrechtsen, A.; Stiller, M.; Schubert, M.; Cappellini, E.; Petersen, B.; et al. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 2013, 499, 74–80. [Google Scholar] [CrossRef]
- Jónsson, H.; Schubert, M.; Seguin-Orlando, A.; Ginolhac, A.; Petersen, L.; Fumagalli, M.; Albrechtsen, A.; Petersen, B.; Korneliussen, T.S.; Vilstrup, J.T.; et al. Speciation with gene flow in equids despite extensive chromosomal plasticity. Proc. Natl. Acad. Sci. USA 2014, 111, 18655–18660. [Google Scholar] [CrossRef] [PubMed]
- Heintzman, P.D.; Zazula, G.D.; MacPhee, R.D.; Scott, E.; Cahill, J.A.; McHorse, B.K.; Kapp, J.D.; Stiller, M.; Wooller, M.J.; Orlando, L.; et al. A new genus of horse from Pleistocene North America. eLife 2017, 6, e29944. [Google Scholar] [CrossRef]
- Hulbert, R.C. Calippus and Protohippus (Mammalia, Perissodactyla, Equidae) from the Miocene (Barstovian-early Hemphillian) of the Gulf Coastal Plain. Bull. Fla. State Mus. Biol. Sci. 1988, 32, 221–340. [Google Scholar]
- Olson, E.C.; McGrew, P.O. Mammalian fauna from the Pliocene of Honduras. Bullettin Geol. Soc. Am. 1941, 52, 1219–1244. [Google Scholar] [CrossRef]
- Drescher, A.B. Later Tertiary Equidae from the Tejon Hills, California. Carneg. Inst. Wash. Pub. 1941, 530, 1–23. [Google Scholar]
- Cope, E.D. A new Hippidium. Am. Nat. 1880, 14, 223. [Google Scholar]
- Matthew, W.D.; Stirton, R.A. Equidae from the Pliocene of Texas. Bullettin 1930, 19, 349–396. [Google Scholar]
- Lance, J.F. Palaeontología y estratigrafía del Pliocene de Yepómera, estado de Chihuahua 1a Parte: Equidos, excepto Neohipparion. Inst. Geol. Univ. Nac. Autónoma México 1950, 54, 83–94. [Google Scholar]
- Prado, J.E.; Alberdi, M.T. A cladistic analysis of the horses of the tribe Equini. J. Paleontol. 1996, 39, 663–680. [Google Scholar]
- Kelly, T.S. New Middle Miocene equid crania from California and their implications for the phylogeny of the Equini. Nat. Hist. Mus. Los Angel. Cty. Contrib. Sci. 1998, 473, 1–43. [Google Scholar] [CrossRef]
- Cope, E.D. A Preliminary Report on the Vertebrate Paleontology of the Llano Estacado; Geological Survey of Texas: Austin, TX, USA, 1893; pp. 1–136. [Google Scholar]
- Leidy, J. Notices of some remains of extinct Mammalia, recently discovered by Dr. F.V. Hayden, in the badlands of Nebraska. Proc. Acad. Nat. Sci. Phila. 1856, 8, 59. [Google Scholar]
- Mooser, O. Fossil Equidae from the middle Pliocene of the Central Plateau of Mexico. Southwest. Nat. 1968, 13, 1–12. [Google Scholar] [CrossRef]
- Cope, E.D. Pliocene horses of southwestern Texas. Am. Nat. 1885, 19, 1208–1209. [Google Scholar]
- Sondaar, P.Y. The osteology ofthe manus of fossil and Recent Equidae. Ned. Akad. Wettenschappen 1968, 25, 1–76. [Google Scholar]
- MacFadden, B.J. Systematics and phylogeny of Hipparion, Neohipparion, Nannippus, and Cormohipparion (Mammalia, Equidae), from the Miocene and Pliocene of the New World. Bull. Am. Mus. Nat. Hist. 1984, 179, 196. [Google Scholar]
- MacFadden, B.J. Fossil Horses: Systematics, Paleobiology, and Evolution of the Family Equidae; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Merriam, J.C. New Species of the Hipparion Group from the Pacific Coast and Great Basin Provinces of North America; University of California Press: Oakland, CA, USA, 1915; Volume Voume 9, pp. 1–8. [Google Scholar]
- Hulbert, R.C.; Harrington, R. An early Pliocene hipparionine horse from the Canadian Arctic. Paleontology 1999, 42, 1017–1025. [Google Scholar] [CrossRef]
- MacFadden, B.J.; Skinner, M. Diversification and biogeography of the one-toed horses Onohippidium and Hippidion. Yale Peabody Mus. Nat. Hist. Postilla 1979, 175, 1–9. [Google Scholar]
- Hulbert, R.C. Late Neogene Neohipparion (Mammalia, Equidae) from the Gulf Coastal Plain of Florida and Texas. J. Paleontol. 1987, 61, 809–830. [Google Scholar] [CrossRef]
- Webb, S.D.; Hulbert, R.C. Systematics and evolution of Pseudhipparion (Mammalia, Equidae) from the late Neogene of the Gulf Coastal Plain and the Great Plains. In Vertebrates, Phylogeny, and Philosophy; Flanagan, K.M., Lillegraven, J.A., Eds.; University of Wyoming: Laramie, WY, USA, 1986; pp. 237–272. [Google Scholar]
- Merriam, J.C. Correlation between the Tertiary of the Great Basin and That of the Marginal Marine Province in California. Science 1914, 40, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Dalquest, W.W.; Donovan, T.J. A new three-toed horse (Nannippus) from the late Pliocene of Scurry County, Texas. J. Paleont. 1973, 47, 34–45. [Google Scholar]
- Cope, E.D. A contribution to the vertebrate paleontology of Texas. Proc. Am. Philos. Soc. 1892, 30, 123–131. [Google Scholar]
- Dalquest, W.W.; Schultz, G.E. Ice Age Mammals of Northwestern Texas; Midwestern State University Press: Wichita Falls, TX, USA, 1992. [Google Scholar]
- Orlando, M.F. Order Perissodactyla. In Early Pleistocene Preglacial and Glacial Rocks and Faunas of North-Central Nebraska; Skinner, M.F., Hibbard, C.W., Eds.; American Museum of Natural History: New York, NY, USA, 1972; Volume 148, pp. 117–125. [Google Scholar]
- Gidley, J.W. A new Pliocene horse from Idaho. J. Mammal. 1930, 16, 52–60. [Google Scholar] [CrossRef]
- Winans, M.C. Revision of North American fossil species of the genus Equus (Mammalia: Perissodactyla: Equidae). Ph.D. Thesis, University of Texas, Austin, TX, USA, 1985; p. 264. [Google Scholar]
- Forsten, A.; Eisenmann, V. Equus (Plesippus) simplicidens (Cope), not Dolichohippus. Mammalia 1995, 59, 85–89. [Google Scholar] [CrossRef]
- Gazin, C.L. A study of the fossil horses remains from the Upper Pliocene of Idaho. Proc. U.S. Natl. Mus. 1936, 83, 281–320. [Google Scholar] [CrossRef]
- Merriam, J.C. New Mammalia from the Idaho Formation. Bull. Dep. Geol. 1918, 10, 523–530. [Google Scholar]
- Scott, E. Equus idahoensis from the Pliocene of Arizona, and Its Role in Plesippine Evolution in the American Southwest. 2005. Available online: http://www.sbcounty.gov/museum/discover/divisions/geo/pdf/SVP_2005b_EquusIdahoensis.pdf (accessed on 10 March 2022).
- Downs, T.; Miller, G.J. Late Cenozoic equids from the Anza-Borrego Desert of California. Nat. Hist. Mus. Los Angel. Co. Contrib. Sci. 1994, 440, 1–90. [Google Scholar] [CrossRef]
- Forsten, A. Mitochondrial-DNA time-table and ethe evolution of Equus: Comparison of molecular and paleontological evidence. Ann. Zool. Fenn. 1992, 28, 301–309. [Google Scholar]
- Azzaroli, A. The genus Equus in Europe. In European Neogene Mammal Chronology; Lindsay, E.H., Fahlbusch, V., Mein, P., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 339–355. [Google Scholar]
- Troxell, E.L. The Vertebrate Fossils of Rock Creek, Texas. Am. J. Sci. 1915, 39, 613–638. [Google Scholar] [CrossRef]
- Hibbard, C.W. Pleistocene vertebrates from the Upper Becerra (Becerra Superior) formation, Valley of Tequixquiac, Mexico, with notes on other Pleistocene forms. Contrib. Mus. Paleontol. Univ. Mich. 1955, 12, 47–96. [Google Scholar]
- Eisenmann, V.; Howe, J.; Pichardo, M. Old World Hemiones and New World slender species (Mammalia, Equidae). Palaeovertebrata 2008, 36, 159–233. [Google Scholar] [CrossRef]
- Hulbert, R.C. Equus from Leisey Shell Pit 1A and other Irvingtonian localities from Florida. Bull. Fla. Mus. Nat. Hist. 1995, 37, 553–602. [Google Scholar]
- Gidley, J.W. A new species of Pleistocene horse from the Staked Plains of Texas. Bull. Am. Mus. Nat. Hist. 1900, 13, 111–116. [Google Scholar]
- Azzaroli, A.; Voorhies, M. The genus Equus in North America: The Blancan species. Palaeontogr. Ital. 1993, 80, 175–198. [Google Scholar]
- Owen, R. On Fossil Remains of Equines from Centrai and South America. Philos. Trans. Lond. 1869, 159, 559–573. [Google Scholar]
- Mooser, O.; Dalquest, W.W. Pleistocene mammals from Aguascalientes, Central Mexico. J. Mammal. 1975, 56, 781–820. [Google Scholar] [CrossRef]
- Cope, E.D. Extinct Mammalia of the Valley of Mexico. Proc. Am. Philos. Soc. 1884, 22, 1–15. [Google Scholar]
- Gidley, J.W. Tooth characters and revision of the North American species of the genus. Bull. Am. Mus. Nat. Hist. 1901, 14, 91–142. [Google Scholar]
- Azzaroli, A. The genus Equus in North America—The Pleistocene species. Paleontogr. Ital. 1998, 85, 1–60. [Google Scholar]
- Hay, O.P. Description of a new species of Pleistocene horse, Equus lambei, from the Pleistocene of Yukon Territory. Proc. U. S. Natl. Mus. 1917, 53, 435–443. [Google Scholar] [CrossRef][Green Version]
- Hay, O.P. Contributions to the knowledge of the mammals of the Pleistocene of North America. Proc. U. S. Natl. Mus. 1915, 48, 515–575. [Google Scholar] [CrossRef]
- Weinstock, J.; Willerslev, E.; Sher, A.; Tong, W.; Ho, S.; Rubenstein, D.; Storer, J.; Burns, J.; Martin, L.; Bravi, C.; et al. Evolution, systematics, and phylogeography of Pleistocene horses in the New World: A molecular perspective. PLoS Biol. 2005, 3, e241. [Google Scholar] [CrossRef]
- Vershinina, A.O.; Heintzman, P.D.; Froese, D.G.; Zazula, G.; Cassatt-Johnstone, M.; Dalen, L.; Der Sarkissian, C.; Dunn, S.G.; Ermini, L.; Gamba, C.; et al. Ancient horse genomes reveal the timing and extent of dispersals across the Bering Land Bridge. Mol. Ecol. 2021, 23, 6144–6161. [Google Scholar] [CrossRef]
- Leidy, J. Description of Vertebrate Fossils. In Post-Pleiocene Fossils of South-Carolina; Holmes, F.S., Ed.; Russel and Jones: Charleston, SC, USA, 1860; pp. 99–122. [Google Scholar]
- Azzaroli, A. A Synopsis of the Quaternary species of Equus in North America. Boll. Soc. Paleont. Ital. 1995, 34, 205–221. [Google Scholar]
- Quinn, J.H. Pleistocene Equidae of Texas. Econ. Geol. Rep. Investig. 1957, 33, 1–51. [Google Scholar]
- von Reichenau, W. Beitrage zur naheren Kenntnis fossiler Pferde aus deutschem Pleistozan, insbesondere über die Entwicklung und die Abkaustadiem del Gebisses von Hochterrassenpferde (Equus mosbachensis v. R.). Abh. Gross. Hess. Geolog. Darmstadt 1915, 7, 1–55. [Google Scholar]
- Alberdi, M.T.; Arroyo-Cabrales, J.; Marin-Leyva, A.H.; Polaco, O.J. Study of Cedral Horses and their place in the Mexican Quaternary. Rev. Mex. Cienc. Geológicas 2014, 31, 221–237. [Google Scholar]
- Kurten, B.; Anderson, E. Pleistocene Mammals of North America; Columbia University Press: New York, NY, USA, 1980; pp. 1–442. [Google Scholar]
- Winans, M.C. A quantitative study of the North American fossil species of the genus Equus. Evol. Perissodactyls 1989, 72, 262–297. [Google Scholar]
- Sher, A.V. Mlekopitaiushchie i Stratigrafia Pleistotsena Krainego Severo-Vostoka SSSR i Severnoi Ameriki; Nauka: Moskva, Russia, 1971. [Google Scholar]
- Eisenmann, V. Sussemionus, a new subgenus of Equus (Perissodactyla, Mammalia). Comp. Rendus. Biol. 2010, 333, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, V. The equids from Liventsovka and other localities of the Khaprovskii Faunal Complex, Russia: A revision. Geobios 2022, 70, 17–33. [Google Scholar] [CrossRef]
- Leidy, J. Bones and teeth of horses from California and Oregon. Proc. Acad. Nat. Sci. Phila. 1865, 17, 94. [Google Scholar]
- Merriam, J.C. Preliminary report on the horses of Rancho La Brea. Bull. Dep. Geol. Sci. 1913, 7, 397–418. [Google Scholar]
- Savage, D.E. Late Cenozoic vertebrates of the San Francisco Bay Region. Bull. Dep. Geol. Sci. 1951, 28, 215–314. [Google Scholar]
- Miller, W.E. Pleistocene vertebrates of the Los Angeles Basin and vicinity (exclusive of Rancho La Brea). Bull. Los Angel. Cty. Mus. 1971, 10, 1–124. [Google Scholar]
- Brown, K.E.; Akersten, W.A.; Scott, E. Equus occidentalis Leidy from “Asphalto”, Kern County, California. In La Brea and Beyond: The Paleontology of Asphalt-Preserved Biotas; Harris, J.M., Ed.; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 2015; pp. 81–89. [Google Scholar]
- Barrón-Ortiz, C.I.; Rodrigues, A.T.; Theodor, J.M.; Kooyman, B.P.; Yang, D.Y.; Speller, C.F. Cheek tooth morphology and ancient mitochondrial DNA of late pleistocene horses from the western interior of North America: Implications for the taxonomy of North American Late Pleistocene. PLoS ONE 2017, 12, e0183045. [Google Scholar] [CrossRef]
- Marín-Leyva, A.H.; Arroyo-Cabrales, J.; García-Zepeda, M.L.; Ponce-Saavedra, J.; Schaaf, P.; Pérez-Crespo, V.P.; Pedro Morales-Puente, P.; Cienfuegos-Alvarado, E.; Alberdi, M.T. Feeding ecology and habitat of Late Pleistocene Equus horses fromwest-central Mexico using carbon and oxygen isotopes variation. Rev. Mex. Cienc. Geol. 2016, 33, 157–169. [Google Scholar]
- Jimenez-Hidalgo, E.; Diaz-Sibaja, R. Was Equus cedralensis a non-stilt legged horse? Taxonomical implication for the Mexican Pleistocene horses. Ameghiniana 2020, 57, 284–288. [Google Scholar] [CrossRef]
- Barrón-Ortiz, C. The Late Pleistocene Extinction in North America: An Investigation of Horse and Bison Fossil Material and Its Implication for Nutritional Extinction Models. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2016. [Google Scholar] [CrossRef]
- Prado, J.L.; Alberdi, M.T. Fossil Horses of South America; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–150. [Google Scholar]
- Machado, H.; Grillo, O.; Scott, E.; Avilla, L. Following the footsteps of the South American Equus: Are autopodia taxonomically informative? J. Mammal. Evol. 2018, 25, 397–405. [Google Scholar] [CrossRef]
- Machado, H.; Avilla, L. The Diversity of South American Equus. Did Size Really Matter? Front. Ecol. Evol. 2019, 7, 235. [Google Scholar] [CrossRef]
- Lund, P.W. Nouvelles Recherches sur la Faune fossile du Brésil. Ann. Sci. Nat. 1840, 13, 310–319. [Google Scholar]
- MacFadden, B.J.; Siles, O.; Zeitler, P.; Johnson, N.M.; Campbell, K.E. Magnetic polarity stratigraphy of the middle Pleistocene (Ensenadan) Tarija Formation of southern Bolivia. Quat. Res. 1983, 19, 172–187. [Google Scholar] [CrossRef]
- MacFadden, B.J.; Zeitler, P.K.; Anaya, F.; Cottle, J.M. Confirmation of the middle Pleistocene age of the sedimentary sequence from Tarija, Bolivia. Quat. Res. 2013, 79, 268–273. [Google Scholar] [CrossRef]
- Prado, J.L.; Martinez-Maza, C.; Alberdi, M.T. Megafauna extinction in South America: A new chronology for theArgentine Pampas. Palaeogeogr. Palaeoclim. Palaeoecol. 2015, 425, 41–49. [Google Scholar] [CrossRef]
- Roth, S. El mamífero misterioso de la Patagonia Grypotherium domesticum. II. Descripción de los restos encontrados en la caverna de Última Esperanza. Rev. Mus. Plata 1899, 9, 421–453. [Google Scholar]
- Labarca, R.; Caro, F.J.; Villavicencio, N.A.; Capriles, J.M.; Briones, E.; Latorre, C.; Santoro, C.M. A Partially Complete Skeleton of Hippidion saldiasi Roth, 1899 (Mammalia: Perissodactyla) from the Late Pleistocene of the High Andes in Northern Chile. J. Vertebr. Paleontol. 2021, 40, e1862132. [Google Scholar] [CrossRef]
- Paunero, R.S.; Rosales, G.; Prado, J.L.; Alberdi, M.T. Cerro bombero: Registro de hippidion saldiasi roth, 1899 (equidae, perissodactyla) en el holoceno temprano de Patagonia (Santa Cruz, Argentina). Estud. Geol. 2008, 64, 89–98. [Google Scholar] [CrossRef][Green Version]
- Massone, M.; Prieto, A. Evaluacion de la modalidad cultural Fell 1 en Magallanes. Chungara 2004, 1, 303–315. [Google Scholar] [CrossRef]
- Lund, P.W. Meddelelse af det ubbytte de i 1844 undersögte Knoglehuler have avgivet til Kundskaben om Brasiliens Dyreverden för sidste Jordomvaeltning. Ebenda 1846, 12, 1–94. [Google Scholar]
- Montané, J. Paleo-Indian remains from Lagunade Tagua Tagua, Central Chile. Science 1968, 161, 1137–1138. [Google Scholar] [CrossRef] [PubMed]
- Gervais, P. Recherches sur les Mammiferes Fossiles de l’Amerique Meridionale; Bertrand, P., Ed.; Librarie-Editeur: Paris, France, 1855; pp. 1–63. [Google Scholar]
- Shockey, B.J.; Salas-Gismondi, R.; Baby, P.; Guyot, J.-L.; Baltazar, M.C.; Huamán, L.; Clack, A.; Stucchi, M.; Pujos, F.; Emerson, J.M.; et al. New Pleistocene Cave Faunas of the Andes of Central Perú: Radiocarbon Ages and the Survival of Low Latitude, Pleistocene DNA. Palaeontol. Electron. 2009, 12, 15. [Google Scholar]
- Villavicencio, N.A.; Werdelin, L. The casa del diablo cave (puno, peru) and the late pleistocene demise of megafauna in the andean altiplano. Quat. Sci. Rev. 2018, 195, 21–31. [Google Scholar] [CrossRef]
- Sun, B.; Deng, T. The Equus Datum and the Early Radiation of Equus in China. Front. Ecol. Evol. 2019, 7, 429. [Google Scholar] [CrossRef]
- Sun, B.; Liu, W.; Liu, J.; Liu, L.; Jin, C. Equus qiyngyangensis in Jinyan Cave and its paleozoographic significance. Quat. Int. 2020, 591, 35–46. [Google Scholar] [CrossRef]
- Teilhard de Chardin, P.; Young, C.C. Fossil mammals from the late Cenozoic of northern China. Palaeontol. Sin. 1931, 9, 1–67. [Google Scholar]
- Flynn, L.J.; Bernor, R.L. Late Tertiary mammals from the Mongolian People’s Republic. Am. Mus. Novit. 1987, 2872, 1–16. [Google Scholar]
- Wang, X.; Li, Q.; Xie, G.; Saylor, J.E.; Tseng, Z.J.; Takeuchi, G.T.; Deng, T.; Wang, Y.; Hou, S.; Liu, J.; et al. Mio-Pleistocene Zanda Basin biostratigraphy and geochronology, pre-Ice Age fauna, and mammalian evolution in western Himalaya. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 374, 81–95. [Google Scholar] [CrossRef]
- Jukar, A.; Sun, B.; Bernor, R.L. The first occurrence of Plesiohipparion huangheense (Qiu, Huang & Guo, 1987) (Equidae, Hipparionini) from the late Pliocene of India. Boll. Soc. Paleo. Ital. 2018, 57, 125–132. [Google Scholar]
- Qiu, Z.X.; Qiu, Z.D.; Deng, T.; Li, C.K.; Zhang, Z.Q.; Wang, B.Y.; Wang, X. Neogene land mammal stages/ages of China. In Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology; Wang, X., Flynn, L.J., Fortelius, M., Eds.; Columbia University Press: New York, NY, USA, 2013; pp. 29–90. [Google Scholar]
- Matsumoto, H. On Hipparion richthofeni Koken. Sci. Rep. Tohoku Univ. 1927, 10, 59–75. [Google Scholar]
- Qiu, Z.; Huang, W.; Guo, Z. The Chinese hipparionine fossils. Palaeontol. Sin. New Ser. 1987, 175, 1–250. [Google Scholar]
- Bernor, R.L.; Lipscomb, D. The systematic position of “Plesiohipparion” aff. huangheense (Equidae, Hipparionini) from Gülyazi, Turkey. Mitt. Bayer. Staatssamml. Paläontol. Hist. Geol. 1991, 31, 107–123. [Google Scholar]
- Bernor, R.L.; Sun, B.; Chen, Y. Plesiohipparion shanxiense n. sp. from the Early Pleistocene (Nihowanian) of E Shanxi, China. Boll. Soc. Paleontol. Ital. 2015, 54, 197–210. [Google Scholar]
- Sefve, I. Die Hipparionen Nord-Chinas. Palaeo. Sin. Ser. 1927, 4, 1–54. [Google Scholar]
- Liu, P.; Lovlie, R. Magnetostratigraphic age of Pleistocene loess/paleosol sections at Kehe, Shanxi. J. Stratigr. 2007, 31, 240–246. [Google Scholar]
- Qiu, Z.; Deng, T.; Banyue, W. Early Pleistocene mammalian fauna from Longdan, Dongxiang, Gansu, China. Palaeontol. Sin. New Ser. 2004, 191, 1–245. [Google Scholar]
- Teilhard de Chardin, P.; Piveteau, J. Les mammifères fossiles de Nihowan (Chine). Ann. Paléont. 1930, 19, l–134. [Google Scholar]
- Kuzmina, I.E. Horses of North Eurasia from the Pliocene till the Present Time; Vereschagin, N.K., Ed.; Russian academy of Sciences: Saint-Petersburg, Russia, 1997; Volume 273, pp. 1–224. [Google Scholar]
- Deng, T.; Xue, X. Chinese Fossil Horses of Equus and Their Environment; China Ocean Press: Beijing, China, 1999. [Google Scholar]
- Chow, M.; Liu, H. Fossil equine teeth from Shansi. Vert. PalAsiat. 1959, 1, 133–136. [Google Scholar]
- Ao, H.; An, Z.; Dekkers, M.J.; Li, Y.; Xiao, G.; Zhao, H.; Qiang, X. Pleistocene magnetochronology of the fauna and Paleolithic sites in the Nihewan Basin: Significance for environmental and hominin evolution in North China. Quat. Geochronol. 2013, 18, 78–92. [Google Scholar] [CrossRef][Green Version]
- Colbert, E. Pleistocene mammals from the Ma Kai Valley of northern Yunnan, China. Am. Mus. Novit. 1940, 1099, 1–10. [Google Scholar]
- Eisenmann, V. Nouvelles interpretations des restes d’équidés (Mammalia, Perissodactyla) de Nihowan (Pléistocène inférieur de la Chine du Nord): Equus teilhardi nov. sp. Geobios 1975, 8, 125–134. [Google Scholar] [CrossRef]
- Sun, B.; Deng, T.; Liu, Y. Early Pleistocene Equus (Equidae, Perissodactyla) from Andersson Loc. 32 in Qixian, Shanxi, China. Hist. Biol. 2017, 31, 211–222. [Google Scholar] [CrossRef]
- Eisenmann, V.; Deng, T. Equus qingyangensis (Equidae, Perissodactyla) of the Upper Pliocene of Bajiazui, China: Evidence for the North American origin of an Old World lineage distinct from E. stenonis. Quaternaire 2005, 2, 113–122. [Google Scholar]
- Sharapov, S. Kuruksaiskii Kompleks Pozdnepliotsenovykh Mlekopitajushchikh Afgano-Adzhikistanskoi Depressii; Izdatelstvo Donish: Dushanbe, Tajikistan, 1986; p. 270. [Google Scholar]
- Forsten, A.; Sharapov, S. Fossil equids (Mammalia, Equidae) from the Neogene and Pleistocene of Tadzhikistan. Geodiversitas 2000, 22, 293–314. [Google Scholar]
- Vangengeim, E.A.; Sotnikova, M.V.; Alekseeva, L.I.; Vislobokova, I.A.; Zhegallo, V.I.; Zazhigin, V.S.; Shevyreva, N.S. Biostratigrafiya Pozdnego Pliotsena—Rannego Pleistotsena Adzhikistana; Nuaka: Moscow, Russia, 1988; p. 125. [Google Scholar]
- Kuznetsova, T.V.; Zhegallo, V.I. Taxonomic Diversity of Equids from the Nalaikha Locality (Mongolia). In Proceedings of the International Conference on the Conditions of the Theriofauna in Russia and Adjacent Countries, Moscow, Russia, 1–3 February 1995; pp. 174–178. [Google Scholar]
- Kuznetsova, T.V.; Zhegallo, V.I. Equus (Hemionus) nalaikhaensis from the Pleistocene of Mongolia, the Earliest Kulan of Central Asia. Paleontol. J. 2009, 43, 574–583. [Google Scholar] [CrossRef]
- Lazarev, P.A. Anthropogenic Horses of Yakutia; Nauka: Moscow, Russia, 1980; p. 190. (In Russian) [Google Scholar]
- Rusanov, B.S. Biostratigraphy of Cenoizoic Deposits of Southern Yakutia; Nauka: Moscow, Russia, 1968; p. 459. [Google Scholar]
- Lazarev, P.A. Large mammals of Anthropogene in Yakutia; Nauka: Moscow, Russia, 2008; p. 160. (In Russian) [Google Scholar]
- Spasskaya, N.; Pavlinov, I.; Sharko, F.; Boulygina, E.; Tsygankova, S.; Nedoluzhko, A.; Mashchenko, E. Morphometric and genetic analyses of diversity of the Lena horse (Equus lenensis Russanov, 1968; Mammalia: Equidae). Russ. J. Theriol. 2021, 20, 82–95. [Google Scholar] [CrossRef]
- Liu, H.Y. A new species of Equus from Locality 21 of Zhoukoudian. Vert PalAsiat 1963, 7, 318–322. [Google Scholar]
- Forsten, A. Chinese fossil horses of the genus Equus. Acta Zool. Fenn. 1986, 181, 1–40. [Google Scholar]
- Gromova, V.I. O novoj iskopaiemoj loshadi iz srednej Azii. Dokl. Akad. Nauk SSSR 1946, 54, 349–351. [Google Scholar]
- Eisenmann, V.; Helmer, D.; Segui, M.S. The big Equus from Geometric Kebaran of Umm el Tlel, Syria: Equus valeriani, Equus capensis or Equus caballus? Proc. Fifth Int. Symp. Archaeozoology Southwest. Asia Adjac. Areas 2002, 1, 62–73. [Google Scholar]
- Zhou, X.; Sun, Y.; Xu, Q.; Li, Y. Note on a new Late Pleistocene Equus from Dalian. Vertebr. Palasiat. 1985, 23, 69–76. [Google Scholar]
- Yuan, J.; Hou, X.; Barlow, A.; Preick, M.; Taron, U.H.; Alberti, F.; Basler, N.; Deng, T.; Lai, X.; Hofreiter, M.; et al. Molecular identification of Late and terminal Pleistocene Equus ovodovi from northeastern China. PLoS ONE 2019, 14, e0216883. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, V.; Vasiliev, S. Unexpected finding of a new Equus species (Mammalia, Perissodactyla) belonging to a supposedly extinct subgenus in Late Pleistocene deposits of Khakassia (southwestern Siberia). Geodiversitas 2011, 33, 519–530. [Google Scholar] [CrossRef]
- Orlando, L.; Metcalf, J.L.; Alberdi, M.T.; Telles-Antunes, M.; Bonjean, D.; Otte, M.; Martin, F.; Eisenmann, V.; Mashkour, M.; Morello, F.; et al. Revising the recent evolutionary history of equids using ancient DNA. Proc. Natl. Acad. Sci. USA 2009, 106, 21754–21759. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Sheng, G.; Preick, M.; Sun, B.; Hou, X.; Chen, S.; Taron, U.H.; Barlow, A.; Wang, L.; Hu, J.; et al. Mitochondrial genomes of Late Pleistocene caballines horses from China belong to a separate clade. Quat. Sci. Rev. 2020, 250, 106691. [Google Scholar] [CrossRef]
- Plasteeva, N.; Vasiliev, S.; Kosintsev, P. Equus (Sussemionus) ovodovi Eisenmann et Vasiliev, 2011 from the Laye Pleistocene of Western Siberia. Russ. J. Theriol. 2015, 14, 187–200. [Google Scholar] [CrossRef]
- Pallas, P.S. Equus hemionus. Novi. Comment. Acad. Sci. Imp. Petropol. 1774, 19, 394–417. [Google Scholar]
- Biswas, S.K. Regional Tectonic Framework, Structure and Evolution of the Western Marginal Basins of India. Tectonophysics 1987, 135, 302–327. [Google Scholar]
- Costa, A.G. A new Late Pleistocene fauna from arid coastal India: Implications for inundated coastal refugia and human dispersals. Quat. Int. 2017, 436, 253–269. [Google Scholar] [CrossRef]
- Dassarma, D.C.; Biswas, S. Newly discovered Late Quaternary vertebrates from the terraced alluvial fills of the Son Valley. Indian J. Earth Sci. 1977, 4, 122–136. [Google Scholar]
- Turvey, S.T.; Sathe, V.; Crees, J.J.; Jukar, A.M.; Chakraborty, P.; Lister, A.M. Late Quaternary megafaunal extinctions in India: How much do we know? Quat. Sci. Rev. 2021, 252, 106740. [Google Scholar] [CrossRef]
- Stewart, M.J.; Louys, G.J.; Price, N.A.; Drake, H.S.; Petraglia, M.D. Middle and Late Pleistocene mammal fossils of Arabia and surrounding regions: Implications for biogeography and hominin dispersals. Quat. Int. 2019, 515, 12–29. [Google Scholar] [CrossRef]
- Moorcroft, W. In Ladakh and Kashmir; in Peshawar, Kabul, Kunduz, and Bokhara. In Travels in the Provinces of Hindustan and the Panjab; John Murray: London, UK, 1841; p. 312. [Google Scholar]
- Shah, N. Status and action plan for the kiang (Equus kiang). In Equids: Zebras, Asses and Horses. Status Survey and Conservation Action Plan; Moehlman, P.D., Ed.; IUCN: Gland, Switzerland, 2002; pp. 72–81. [Google Scholar]
- Wang, Y.X. A Complete Check-List of Mammal Species and Subspecies in China: A Taxonomic and Geographic Reference; China Forestry Publishing House: Beijing, China, 2002. [Google Scholar]
- Schaller, G.B. Wildlife of the Tibetan Steppe; University of Chicago Press: Chicago, IL, USA, 1998. [Google Scholar]
- Poliakov, I.S. Przewalski horse (Equus Przevalskii n. sp.). In Izvestija Russkogo Geograficheskogo Obshchestva; Russian Academy of Sciences: Saint Petersburg, Russia, 1881; Volume 17, pp. 1–19. (In Russian) [Google Scholar]
- Groves, C. Morphology, habitat, and taxonomy. In Przewalski’s Horse: The History and Biology of an Endangered Species; Boyd, L., Houpt, K.A., Eds.; State University of New York Press: Albany, NY, USA, 1994; pp. 39–59. [Google Scholar]
- Groves, C.; Grubb, P. Ungulate Taxonomy; Johns Hopkins University Press: Baltimore, MD, USA, 2011. [Google Scholar]
- Heptner, V.; Nasimovich, A.A.; Bannikov, A.G. Mammals of the Soviet Union. In Artiodactyla and Perissodactyla; Vysshaya Shkola: Moscow, Russia, 1961; Volume 1. [Google Scholar]
- Deng, T. Ages of some Late Pleistocene faunas based on the presence of Equus przewalskii (Perissodactyla, Equidae). Vertebr. Palasiat. 1999, 23, 51–56. [Google Scholar]
- Gaunitz, C.; Fages, A.; Hanghøj, K.; Albrechtsen, A.; Khan, N.; Schubert, M.; Seguin-Orlando, A.; Owens, I.J.; Felkel, S.; Bignon-Lau, O.; et al. Ancient genomes revisit the ancestry of domestic and Przewalski’s horses. Science 2018, 360, 111–114. [Google Scholar] [CrossRef]
- Librado, P.; Orlando, L. Genomics and Evolutionary History of Equids. Annu. Rev. Anim. Biosci. 2020, 7, 16–41. [Google Scholar] [CrossRef]
- Taylor, W.T.T.; Barrón-Ortiz, C.I. Rethinking the evidence for early horse domestication at Botai. Sci. Rep. 2021, 11, 7440. [Google Scholar] [CrossRef]
- Wolf, D.; Bernor, R.L.; Hussain, S.T. A systematic, biostratigraphic, and paleobiogeographic reevaluation of the Siwalik hipparionine horse assemblage from the Potwar Plateau, Northern Pakistan. Palaeontogr. Abt. 2013, 300, 1–115. [Google Scholar] [CrossRef]
- Jukar, A.M.; Sun, B.; Nanda, A.C.; Bernor, R.L. The first occurrence of Eurygnathohippus (Mammalia, Perissodatyla, Equidae) outside Africa and its biogeographic significance. Boll. Soc. Paleo. Ital. 2019, 58, 171–179. [Google Scholar]
- Rook, L.; Bernor, R.L.; Avilla, L.D.; Cirilli, O.; Flynn, L.; Jukar, A.M.; Sanders, W.; Scott, E.; Wang, X. Mammal Biochronology (Land Mammal Ages) around the World from the Late Miocene to Middle Pleistocene and Major Events in Horse Evolutionary History. Front. Ecol. Evol. 2019, 7, 278. [Google Scholar] [CrossRef]
- Jukar, A.M.; Lyons, S.K.; Wagner, P.J.; Uhen, M.D. Late Quaternary extinctions in the Indian Subcontinent. Paleogeol. Paleoclim. Paleoecol. 2021, 562, 110137. [Google Scholar] [CrossRef]
- Thomas, P.K.; Joglekar, P. Holocene Faunal Studies. Man Environ. 1994, 19, 179–203. [Google Scholar] [CrossRef]
- Falconer, H.; Cautley, P.T. Illustrations-Part IX Equidae, Camelidae, Sivatherium. In Fauna Antiqua Sivalensis, Being the Fossil Zoology of the Sewalik Hills in the North of India; Smith, Elder and Co.: London, UK, 1849. [Google Scholar]
- Hussain, S.T. Revision of Hipparion (Equidae, Mammalia) from the Siwalik Hills of Pakistan and India. Geol. Mijnb. 1971, 147, 80–81. [Google Scholar]
- MacFadden, B.J.; Woodburne, M.O. Systematics of the Neogene Siwalik Hipparions (Mammalia, Equidae) based on cranial and dental morphology. J. Vert. Paleo 1982, 2, 185–218. [Google Scholar] [CrossRef]
- Bernor, R.L.; Hussain, S.T. An Assessment of the Systematic, Phylogenetic and Biogeographic Relationships of Siwalik Hipparionine horses. J. Vert. Paleo 1985, 5, 32–87. [Google Scholar] [CrossRef]
- Lydekker, R. Containing the order Ungulata, suborders Perissodactyla, Toxodontia, Condylartha, and Ambylopoda. In Catalogue of the Fossil Mammalia in the British Museum (Natural History) Part III; Taylor Francis: London, UK, 1886. [Google Scholar]
- Bernor, R.L.; Cirilli, O.; Jukar, A.M.; Potts, R.; Buskianidze, M.; Rook, L. Evolution of Early Equus in Italy, Georgia, the Indian Subcontinent, East Africa, and the Origins of African Zebras. Front. Ecol. Evol. 2019, 7, 166. [Google Scholar] [CrossRef]
- Patel, R.; Nainwal, H.C.; Sharma, T.; Rana, R.S. National Workshop on Indian Siwalik: Recent Advances and Future Research. In Equus cf. sivalensis from the Tatrot Formation (Upper Pliocene) of Jhil-Bankabara Area Sirmaur District, Himachal Pradesh, India; Geological Survey of India: Lucknow, India, 2017; pp. 51–53. [Google Scholar]
- Moigne, A.M.; Dambricourt Malassé, A.; Singh, M.; Kaur, A.; Gaillard, C.; Karir, B.; Pal, S.; Bhardwaj, V.; Abdessadok, S.; Chapon Sao, C.; et al. The faunal assemblage of the paleonto-archeological localities of the Late Pliocene Quranwala Zone, Mosol Formation, Siwalik Range, NW India. Palevol 2016, 15, 359–378. [Google Scholar] [CrossRef]
- Gaur, R.; Chopra, S.R.K. Taphonomy, fauna, environment and ecology of Upper Sivaliks (Plio-Pleistocene) near Chandigarh, India. Nature 1984, 308, 353–355. [Google Scholar] [CrossRef]
- Hussain, S.T.; van den Bergh, G.; Steensma, K.J.; de Visser, J.A.; de Vos, J.; Mohammad, A.; van Dam, J.; Sondaar, P.; Malik, S.B. Biostratigraphy of the Plio-Pleistocene continental sediments (Upper Siwaliks) of the Mangla-Samwal anticline, Azad Kashmir, Pakistan. Proc. K. Ned. Akad. Wet. 1992, 95, 65–80. [Google Scholar]
- Dennell, R.; Coard, R.; Turner, A. The biostratigraphy and magnetic polarity zonation of the Pabbi Hills, northern Pakistan: An Upper Siwalik (Pinjor Stage) Upper Pliocene–Lower Pleistocene fluvial sequence. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 234, 168–185. [Google Scholar] [CrossRef]
- Sonakia, A.; Biswas, S. Fossil mammals including early man from the Quaternary deposits of the Narmada and Son basins of Madhya Pradesh, India. Palaeontol. Indica 2011, 53, 1–84. [Google Scholar]
- Lydekker, R. Siwalik and Narbada Equidae. Mem. Geol. Soc. India Palaeontol. Indica Ser. 1882, 2, 67–98. [Google Scholar]
- Cirilli, O.; Bernor, R.L.; Rook, L. New insights on the Early Pleistocene equids from Roca-Neyra (France, central Europe): Implications for the Hipparion LAD and the Equus FAD in Europe. J. Paleontol. 2021, 95, 406–425. [Google Scholar] [CrossRef]
- Gromova, V. Le Genre Hipparion; Bureau de Recherches Geologiques et Minieres: Orléans, France, 1952; Volume 12, pp. 1–288. [Google Scholar]
- Bernor, R.L.; Sen, S. The Early Pliocene Plesiohipparion and Proboscidipparion (Equidae, Hipparionini) from Çalta, Turkey (Ruscinian Age, c. 4.0 Ma). Geodiversitas 2017, 39, 285–314. [Google Scholar] [CrossRef]
- Bernor, R.L.; Beech, M.; Bibi, F. Equidae from the Baynunah Formation. In Sands of Time; Springer: Berlin/Heidelberg, Germany, 2022; pp. 259–280. [Google Scholar]
- Crusafont, P.M.; Sondaar, P. Una nouvelle espece d’Hipparion du Pliocéne terminal d’Espagne. Palaeovertebrata 1971, 4, 59–66. [Google Scholar] [CrossRef][Green Version]
- Alberdi, M.T.; Alcalà, L. A study of the new samples of the Pliocene Hipparion (Equidae, Mammalia) from Spain and Bulgaria. Trans. R. Soc. Edinb. Earth Sci. 1999, 89, 167–186. [Google Scholar] [CrossRef]
- Piñero, P.; Agustí, J.; Oms, O.; Fierro, I.; Montoya, P.; Mansino, S.; Ruiz-Sánchez, F.; Alba, D.M.; Alberdi, M.T.; Blain, H.A.; et al. Early Pliocene continental vertebrate Fauna at Puerto de la Cadena (SE Spain) and its bearing on the marine-continental correlation of the Late Neogene of Eastern Betics. Paleogeogr. Palaeoclim. Palaeoecol. 2017, 479, 102–114. [Google Scholar] [CrossRef]
- Gervais, P. Zoologie et Paléontologie Française (Animaux Vertébrés): Deuxième Edition; Arthus Bertrand: Paris, France, 1859; pp. 1–544. [Google Scholar]
- Depéret, C. Les animaux pliocènes du Roussilon. Mem. Soc. Geol. Fr. 1890, 3, 1–198. [Google Scholar]
- Forsten, A. The hipparions (Mammalia, Equidae) of Suffolk, England. Trans. R. Soc. Edinb. Earth Sci. 2001, 92, 115–120. [Google Scholar] [CrossRef]
- Forsten, A. Latest Hipparion Cristol, 1832 in Europe. A review of the Pliocene Hipparion crassum Gervais Group and other finds (Mammalia, Equidae). Geodiversitas 2002, 24, 465–486. [Google Scholar]
- Eisenmann, V.; Sondaar, P. Pliocene vertebrate locality of Calta, Ankara, Turkey. Hipparion. Geodiversitas 1998, 20, 409–439. [Google Scholar]
- Hernández-Pacheco, E. La llanura manchega y sus mamíferos fósiles: Yacimiento de La Puebla de Almoradier. Com. Investig. Pleontol. Mem. 1921, 28, 1–41. [Google Scholar]
- Alberdi, M.T. El género Hipparion en España, revision e historia evolutive. Trab. Sobre Neógeno Cuatern. 1974, 1, 1–139. [Google Scholar]
- Bernor, R.L.; Koufos, G.D.; Woodburne, M.O.; Fortelius, M. The evolutionary history and biochronology of European and southwestern Asian late Miocene and Pliocene hipparionine horses. In The Evolution of Western Eurasian Neogene Mammal Faunas; Bernor, R.L., Fahlbusch, V., Mittman, H.W., Eds.; Columbia University Press: New York, NY, USA, 1996; pp. 307–338. [Google Scholar]
- Bernor, R.L.; Sun, B.Y. Morphology through ontogeny of Chinese Proboscidipparion and Plesiohipparion and observations on their Eurasian and African relatives. Vertebr. Palasiat. 2015, 53, 73–92. [Google Scholar]
- Agustì, J.; Oms, O. On the age of the last hipparionine faunas in western Europe. Comptes Rendus Acad. Sci. Paris Sci. 2001, 332, 291–297. [Google Scholar] [CrossRef]
- Azanza, B.; Morales, M.; Andrés, M.; Cerdeño, E.; Alberdi, M.T. Los macromamíferos y la datación del yacimiento clásico de Villarroya. In Villarroya, Yacimiento Clave de la Paleontologìa Riojana; Alberdi, M.T., Azanza, B., Cervante, E., Eds.; Instituto de Estudios Riojanos: La Rioja, Spain, 2016; pp. 267–280. [Google Scholar]
- Athanassiou, A. A Villafranchian Hipparion-bearing mammal fauna from Sésklo (E. Thessaly, Greece): Implications for the question of Hipparion-Equus sympatry in Europe. Quaternary 2018, 12, 12. [Google Scholar] [CrossRef]
- Rook, L.; Cirilli, O.; Bernor, R.L. A late occurring “Hipparion” from the middle Villafranchian of Montopoli, Italy (Early Pleistocene; MN16b; ca. 2.5 Ma). Boll. Soc. Paleontol. Ital. 2017, 56, 333–339. [Google Scholar]
- Bajgusheava, V.S. Krupnaja Loshad Khaprovskogo Kompleksa is alluvija Severo Vostochnoto Priazovija. Invesija Servo Kavk. Nauchnogo Zentra Vychei Shkoly 1978, 1, 98–102. [Google Scholar]
- Gromova, V.I. Istorija loshadej (roda Equus) v Starom Svete. Chast’ 1. Obzor i opisanie form. Tr. Paleontol. Inst. 1949, 17, 1–373. [Google Scholar]
- Forsten, A. The fossil horses (Equidae, Mammalia) from the Plio–Pleistocene of Liventsovka near Rostov–Don, Russia. Geobios 1997, 31, 645–657. [Google Scholar] [CrossRef]
- Alberdi, M.T.; Cerdeño, E.; López–Martínez, N.; Morales, J.; Soria, M.D. La Fauna Villafranquiense de El Rincon–1 (Albacete, Castilla—La Mancha). Estud. Geol. 1997, 53, 69–93. [Google Scholar] [CrossRef]
- Alberdi, M.T.; Ortiz–Jaureguizar, E.; Prado, J.L. A quantitative review of European stenonoid horses. J. Paleontol. 1998, 72, 371–387. [Google Scholar] [CrossRef]
- Azzaroli, A. On Equus livenzovensis Baigusheva 1978 and the “stenonid” lineage of Equids. Paleontogr. Ital. 2000, 87, 1–17. [Google Scholar]
- Bernor, R.L.; Cirilli, O.; Wang, S.Q.; Rook, L. Equus cf. livenzovenzis from Montopoli, Italy (early Pleistocene; MN16b; ca. 2.6 Ma). Boll. Soc. Paleontol. Ital. 2018, 57, 203–216. [Google Scholar]
- Cherin, M.; Cirilli, O.; Azzarà, B.; Bernor, R.L. Equus stenonis (Equidae, Mammalia) from the Early Pleistocene of Pantalla (Italy) and the dispersion of stenonines horses in Europe. Boll. Soc. Paleontol. Ital. 2021, 60, 1–18. [Google Scholar]
- Terhune, C.; Curran, S.; Croitor, R.; Drăguşin, V.; Gaudin, T.; Petrulescu, A.; Robinson, C.; Robu, M.; Werdelin, L. Early Pleistocene fauna of the Olteţ Valley of Romania: Biochronological and biogeographical implications. Quat. Int. 2020, 553, 14–33. [Google Scholar] [CrossRef]
- Delafond, F.; Depéret, C. Etude des Gites Minéraux de la France. In Les Terrains Tertiaires de la Bresse et Leurs Gites de Lignites et de Minerais de Fer; Imprimerie National: Paris, France, 1893; pp. 1–332. [Google Scholar]
- Pomel, A. Catalogue Méthodique et Descriptif des Vertebres Fossiles Découverts dans le Bassin Hydrographique Supérieur de la Loire et Surtout Dans la Vallée de Son Affluent Principal; L’Allirée Chez, J., Baillière, B., Eds.; Hachette: Paris, France, 1853; pp. 1–123. [Google Scholar]
- Boule, M. Bulletin de la Société Géologique de France; BSGF—Earth Sciences Bulletin: Vienne, France, 1891; Volume 18, p. 801. [Google Scholar]
- Viret, J. Le loess à bancs durcis de Saint Vallier (Drome) et sa faune de mammifères villagranchiens. Nouv. Arch. Mus. D’histoire Nat. Lyon 1954, 4, 1–200. [Google Scholar]
- Samson, P. Les équidés fossiles de Roumanie (Pliocène moyen—Pléistocène supérieur). Geol. Romana 1975, 14, 165–354. [Google Scholar]
- Cocchi, I. L’uomo fossile dell’Italia Centrale. Mem. Della Soc. Ital. Sci. Nat. 1867, 2, 1–180. [Google Scholar]
- Prat, F. Les equidés villafranchiens en France, genre Equus. Cah. Quat. 1980, 2, 1–290. [Google Scholar]
- Palombo, M.R.; Alberdi, M.T.; Bellucci, L.; Sardella, R. An intriguing middle–sized horse from Coste San Giacomo (Anagni Basin, central Italy). Quat. Res. 2017, 87, 347–362. [Google Scholar] [CrossRef]
- Cirilli, O. Equus stehlini Azzaroli, 1964 (Perissodactyla, Equidae). A revision of the most enigmatic horse from the Early Pleistocene of Europe, with new insights on the evolutionary history of European medium- and small-sized horses. Riv. Paleont. Strat. 2022, 128, 241–265. [Google Scholar] [CrossRef]
- Azzaroli, A. The two Villafranchian Horses of the Upper Valdarno. Palaeontogr. Ital. 1964, 59, 1–12. [Google Scholar]
- Alberdi, M.T.; Palombo, M.R. The late Early to early Middle Pleistocene stenonoid horses from Italy. Quat. Int. 2013, 288, 25–44. [Google Scholar] [CrossRef]
- Palombo, M.R.; Alberdi, M.T. Light and shadows in the evolution of South European stenonoid horses. Foss. Impr. 2017, 73, 115–140. [Google Scholar] [CrossRef]
- Rook, L.; Martínez–Navarro, B. Villafranchian: The long story of a Plio–Pleistocene European large mammal biochronologic unit. Quat. Int. 2010, 219, 134–144. [Google Scholar] [CrossRef]
- Bartolini-Lucenti, S.; Cirilli, O.; Pandolfi, L.; Bernor, R.L.; Bukhsianidze, M.; Carotenuto, F.; Lordkipanidze, D.; Tsikaridze, N.; Rook, L. Zoogeographic significance of Dmanisi large mammal assemblage. J. Hum. Evol. 2022, 163, 103125. [Google Scholar] [CrossRef]
- Vekua, A.K. The Lower Pleistocene Mammalian Fauna of Akhalkalaki. Tbilisi. 1962, pp. 1–191. (In Georgian). Available online: http://www.rhinoresourcecenter.com/index.php?s=1&act=pdfviewer&id=1435388432&folder=143 (accessed on 16 July 2022).
- Koufos, G.D. Early Pleistocene equids from Mygdonia basin (Macedonia, Greece). Palaeontogr. Ital. 1992, 79, 167–199. [Google Scholar]
- Eisenmann, V. Equus granatensis of Venta Micena and evidence for primitive non–stenonid horses in the Lower Pleistocene. In The Hominids and Their Environment during the Lower and Middle Pleistocene of Eurasia; Gilbert, J., Sanchez, F., Gilbert, L., Ribot, F., Eds.; Museo de Prehistoria y Paleontología: Orce, Spain, 1995; pp. 175–189. [Google Scholar]
- Koufos, G.D.; Vlachou, T.; Gkeme, A. The Fossil Record of Equids (Mammalia: Perissodactyla: Equidae) in Greece. In Fossil Vertebrates of Greece; Vlachos, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2, pp. 351–401. [Google Scholar]
- Guerrero-Alba, S.; Palmqvist, P. Estudio morfometrico del caballo de Venta Micena (Orce, Granada) y su comparacion con los equidos modernos y del Plio- Pleistoceno en el viejo mundo. Paleontol. Evol. 1997, 30, 93–148. [Google Scholar]
- Belmaker, M. Early Pleistocene faunal connections between Africa and Eurasia: An ecological perspective. In Out of Africa I: The First Hominin Colonization of Eurasia; Fleagle, J.G., Shea, J.J., Grine, F.E., Baden, A.L., Leakey, R.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 183–205. [Google Scholar]
- Wüst, E. Untersuchungen über das Pliozan und das Alteste Pleistozan Thüringens, nordlich vom Thüringer Walde und westlich von der Saale. Abh. Nat. Ges. Halle 1900, 23, 1–352. [Google Scholar]
- Vekua, A.K. The Lower Pleistocene mammalian fauna of Akhalkalaki (Southern Georgia, USSR). Palaeontogr. Ital. 1986, 74, 63–96. [Google Scholar]
- Musil, R. Die Pferdefunde der lokalität Stránská Skála. Anthropos 1972, 20, 185–192. [Google Scholar]
- Boulbes, N.; Van Asperen, E.N. Biostratigraphy and palaeoecology of European Equus. Front. Ecol. Evol. 2019, 7, 301. [Google Scholar] [CrossRef]
- Alberdi, M.T. Estudio de los caballos de los yacimientos de Fuente Nueva–3 y Barranco León–5 (Granada). In Ocupaciones Humanas en el Pleistoceno Inferior Y Medio de la Cuenca de Guadix–Baza; Toro, I., Martínez–Navarro, B., Agustí, J., Eds.; Arqueología Monografías, Junta de Andalucía, Consejería de Cultura: Andalucía, Spain, 2010; pp. 291–306. [Google Scholar]
- Koufos, G.D.; Kostopoulos, D.S.; Sylvestrou, I.A. Equus apolloniensis n. sp. (Mammalia, Equidae) from the latest Villafranchian locality of Apollonia, macedonia, Greece. Paleontol. Evol. 1997, 30, 49–76. [Google Scholar]
- Gkeme, A.G.; Koufos, G.D.; Kostopoulos, D.S. Reconsidering the Equids from the Early Pleistocene Fauna of Apollonia 1 (Mygdonia Basin, Greece). Quaternary 2021, 4, 12. [Google Scholar] [CrossRef]
- Eisenmann, V.; Boulbes, N. New results on equids from the Early Pleistocene site of Untermassfeld. In The Pleistocene of Untermassfeld Near Meiningen (Thütingen, Germany); Kahlke, R.D., Ed.; Romisch Germanischen Zentralmuseums: Weimar, Germany, 2020; pp. 1295–1321. [Google Scholar]
- Musil, R. Die Equiden–Reste aus dem Unterpleistozän von Untermassfeld. In Das Pleistozän von Untermassfeld bei Meiningen (Thüringen); Kahlke, R.D., Ed.; Monographien, Römisch–Germanisches Zentralmuseum, Forschungsinstitut für vor und Frühgeschichte: Liebniz, Germany, 2001; pp. 557–587. [Google Scholar]
- Forsten, A. A review of Equus stenonis Cocchi (Perissodactyla, Equidae) and related forms. Quat. Sci. Rev. 2000, 18, 1373–1408. [Google Scholar] [CrossRef]
- Lister, A.M.; Parfitt, S.A.; Owen, F.J.; Collinge, S.E.; Breda, M. Metric analysis of ungulate mammals in the early Middle Pleistocene of Britain, in relation to taxonomy and biostratigraphy: II: Cervidae, Equidae and Suidae. Quat. Int. 2010, 228, 157–179. [Google Scholar] [CrossRef]
- Tsoukala, E. Contribution to the Study of the Pleistocene Fauna of Large Mammals (Carnivora, Perissodactyla, Artiodactyla) from Petralona Cave Chalkidiki (N. Greece). Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Serres, 1989. [Google Scholar]
- Azzaroli, A. On a Late Pleistocene ass from Tuscany with notes on thehistory of ass. Palaeontol. Ital. 1979, 71, 27–47. [Google Scholar]
- Azzaroli, A. Pleistocene and living Horses of the old World. Palaeontogr. Ital. 1966, 61, 1–15. [Google Scholar]
- Caloi, L. Il genere Equus nell’Italia centrale. Studi Geol. Camerti 1995, 469–486. [Google Scholar]
- Eisenmann, V. Pliocene and Pleistocene Equids: Palaeontology versus molecular biology. Cour. Forsch. Senckenberg 2006, 256, 71–89. [Google Scholar]
- Regalia, E. Sull’Equus (Asinus) hydruntinus Regalia della grotta di Romanelli (Castro, Lecce). Arch. Antropol. Etnol. 1907, 37, 375–390. [Google Scholar]
- Vishnyatsky, L.B. The Palaeolithic of Central Asia. J. World Prehistory 1999, 13, 69–122. [Google Scholar] [CrossRef]
- Musil, R. Die Equiden–Reste aus dem Pleistozän von Süssenborn bei Weimar. Paläontologische Abh. Paläozool. 1969, 3, 617–666. [Google Scholar]
- Azzaroli, A. Ascent and decline of monodactyl equids: A case for prehistoric overkill. Ann. Zool. Fenn. 1992, 28, 151–163. [Google Scholar]
- Bennett, E.A.; Champlot, S.; Peters, J.; Arbuckle, B.S.; Guimaraes, S.; Pruvost, M.; Bar-David, S.; Davis, S.J.M.; Gautier, M.; Kaczensky, P.; et al. Taming the late Quaternary phylogeography of the Eurasiatic wild ass through ancient and modern DNA. PLoS ONE 2017, 12, e0174216. [Google Scholar]
- Aurell-Garrido, J.; Madurell-Malapeira, J.; Alba, D.M.; Moyà-Solà, S. The stenonian and caballoid equids from the Pleistocene section of Vallparadís (Terrassa, Catalonia, Spain). Cidaris 2010, 30, 61–66. [Google Scholar]
- Madurell-Malapeira, J.; Minwer-Barakat, R.; Alba, D.M.; Garcés, M.; Gómez, M.; Aurell-Garrido, J.; Ros-Montoya, S.; Moya-Sola, S.; Berastegui, X. The Vallparadís section (Terrassa, Iberian peninsula) and the latest Villafranchian faunas of Europe. Quat. Sci. Rev. 2010, 29, 2972–2982. [Google Scholar] [CrossRef]
- Boddaert, P. Elenchus Animalium, Volumen 1: Sistens Quadrupedia Huc Usque Nota, Erorumque Varietates; C. R. Hake: Rotterdam, The Netherlands, 1785; p. 174. [Google Scholar]
- Eisenmann, V.; Kuznetsova, T.V. Early Pleistocene equids (Mammalia, Perissodactyla) of Nalaikha (Mongolia) and the emergence of modern Equus Linnaeus, 1758. Geodiversitas 2004, 26, 535–561. [Google Scholar]
- van Asperen, E.N. Implications of age variation and sexual dimorphism in modern equids forMiddle Pleistocene equid taxonomy. Int. J. Osteoarchaeol. 2013, 23, 1–12. [Google Scholar] [CrossRef]
- van Asperen, E.N. Ecomorphological adaptations to climate and substrate in late middle Pleistocene caballoid horses. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 297, 584–596. [Google Scholar] [CrossRef]
- Churcher, C.S.; Richardson, M.L. Equidae. In Evolution of African Mammals; Maglio, V.J., Cooke, H.S.B., Eds.; Harvard University Press: Cambridge, MA, USA, 1978; pp. 379–422. [Google Scholar]
- Bernor, R.L.; Armour-Chelu, M.; Gilbert, H.; Kaiser, T.; Schulz, E. Equidae. In Cenozoic Mammals of Africa; Werdelin, L., Sanders, W., Eds.; University of California Press: Berkeley, CA, USA, 2010; pp. 685–721. [Google Scholar]
- Duval, M.; Sahnouni, M.; Parés, J.P.; van der Made, J.; Abdessadok, S.; Harichane, Z.; Cheheb, R.C.; Boulaghraif, K.; Pérez-Gonzalez, A. The Plio-Pleistocene sequesnce of Oued Boucherti (Algeria): A unique chronologically-constrained archeological and paleontological record in North Africa. Quat. Sci. Rev. 2021, 271, 107116. [Google Scholar] [CrossRef]
- Sahnouni, M.; Pares, J.M.; Duval, M.; Caceres, I.; Harichane, Z.; Van der Made, J.; Perez-Gonzalez, A.; Abdessadok, S.; Kandi, N.; Derradji, A.; et al. 1.9-million- and 2.4 million-year-old artifacts and stone toolcutmarked bones from Aïn Boucherit, Algeria. Science 2017, 362, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Villalta, J.F.; Crusafont, M. Les gisements de mammifères du Néogène espagnol. Compt. Rendus Soc. Geol. Fr. 1957, 1, 9–10. [Google Scholar]
- Cirilli, O.; Zouhri, S.; Boughabi, S.; Benvenuti, M.; Bernor, R.L.; Papini, M.; Rook, L. The hipparionine horses (Perissodactyla: Mammalia) from the Late Miocene of Tizi N’Tadderht (southern Ouarzazate basin; Central High Atlas; Morocco). Riv. Paleontol. Strat. 2020, 126, 1–12. [Google Scholar]
- Bernor, R.L.; Boaz, N.T.; Cirilli, O.; El-Shawaihdi, M.; Rook, L. Sahabi Eurygnathohippus feibeli; its systematic, stratigraphic, chronologic and biogeographic contexts. Riv. Paleont. Strat. 2020, 126, 561–581. [Google Scholar]
- Bernor, R.L.; Harris, J. Systematics and Evolutionary Biology of the Late Miocene and Early Pliocene Hipparionine Horses from Lothagam, Kenya. In Lothagam: The Dawn of Humanity in Eastern Africa; Leakey, M., Harris, J., Eds.; Columbia University Press: New York, NY, USA, 2003; pp. 387–438. [Google Scholar]
- Bernor, R.L.; Scott, R.S. New Interpretations of the Systematics, Biogeography and Paleoecology of the Sahabi Hipparions (latest Miocene), Libya. Geodiversitas 2003, 25, 297–319. [Google Scholar]
- Pomel, A. Les equidés: Carte géologie de l’Algérie. Monogr. Paléontol. 1897, 12, 1–44. [Google Scholar]
- Hooijer, D.A.; Maglio, V.J. The earliest Hipparion south of the Sahara, in the late Miocene of Kenya. Proc. K. Ned. Akad. Wet. 1973, 76, 311–315. [Google Scholar]
- Sun, B.; Zhang, X.; Liu, Y.; Bernor, R.L. Sivalhippus ptychodus and Sivalhippus platyodus (Perissodactyla, Mammalia) from the late Miocene of China. Riv. Ital. Paleo. Strat. 2018, 124, 1–22. [Google Scholar]
- Bernor, R.L.; Kaiser, T. Systematics and Paleoecology of the Earliest Pliocene Equid, Eurygnathohippus hooijeri n. sp. from Langebaanweg, South Africa. Mitt. Aus Hamburischen Zool. Mus. Inst. 2006, 103, 147–183. [Google Scholar]
- Hooijer, D.A. The late Pliocene Equidae of Langebaanweg, Cape Province, South Africa. Zool. Verh. 1976, 148, 3–39. [Google Scholar]
- Bernor, R.L.; Gilbert, H.; Semprebon, G.; Simpson, S.; Semaw, S. Eurygnathohippus woldegabrieli sp. nov. (Perissodactyla: Mammalia) from the Middle Pliocene of Aramis, Ethiopia (4.4 Ma.). J. Vert. Paleo. 2013, 33, 1472–1485. [Google Scholar] [CrossRef]
- Eisenmann, V. Le protostylide: Valeur systematique et signification phylétique chez les espèces actuelles et fossiles du genre Equus (Perissodactyla, Mammalia). Z. Saugetierkd. 1976, 41, 349–365. [Google Scholar]
- Eisenmann, V. Family Equidae. The Fossil Ungulates: Proboscidea, Perissodactyla, and Suidae. In Koobi Fora Research Project; Harris, J.M., Ed.; Clarendon Press: London, UK, 1983; Volume 2, pp. 156–214. [Google Scholar]
- Bernor, R.L.; Scott, R.S.; Haile-Selassie, Y. A Contribution to the Evolutionary History of Ethiopian Hipparionine Horses: Morphometric Evidence from the Postcranial Skeleton. Geodiversitas 2005, 27, 133–158. [Google Scholar]
- Eisenmann, V.; Geraads, D. Hipparion pomeli sp. nov. from the late Pliocene of Ahl al Oughlam, Morocco, and a revision of the relationships of Pliocene and Pleistocene African hipparions. Palaeo Afr. 2006, 42, 51–98. [Google Scholar]
- van Hoepen, E.C.N. Fossiele Pferde van Cornelia. Bloemfontein 1930, 2, 13–24. [Google Scholar]
- Leakey, L.S.B. A Preliminary Report on the Geology and Fauna. In Olduvai Gorge 1951–1961; Cambridge University Press: Cambridge, UK, 1965; Volume 1. [Google Scholar]
- Armour-Chelu, M.; Bernor, R.L.; Mittmann, H.-W. Hooijer’s hypodigm for “Hipparion” cf. ethiopicum (Equidae, Hipparioninae) from Olduvai, Tanzania and comparative material from the East African Plio-Pleistocene. Abh. Bayer. Akad. Wiss. 2006, 30, 15–24. [Google Scholar]
- Armour-Chelu, M.; Bernor, R.L. Equidae. In Geology and Paleontology of Laetoli; Harrison, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 295–326. [Google Scholar]
- van der Made, J.; Boulaghraif, K.; Chelli Cheheb, R.; Caceres, I.; Harichane, Z.; Sahnouni, M. The last North African hipparions —Hipparion decline and extinction follows a common pattern. N. Jb. Geol. Palaont. Abh. 2022, 303, 39–97. [Google Scholar] [CrossRef]
- Bernor, R.L.; Coillot, T.; Wolf, D. Phylogenetic Signatures in the Juvenile Skull and Dentition of Olduvai Gorge Eurygnathohippus cornelianus (Mammalia: Equidae). Riv. Ital. Paleontol. Stratigr. 2014, 120, 243–252. [Google Scholar]
- Hopwood, A.T. Die fossilen Pferde von Oldoway. Wiss. Ergeb. Oldoway Exped. 1937, 4, 112–136. [Google Scholar]
- Thomas, P. Recherches stratigraphiques et paleontologiques sur quelques formations d’eau douce de l’Algerie. Mem. Soc. Geol. Fr. 1884, 3, 1–50. [Google Scholar]
- Eisenmann, V. Les Chevaux (Equus Sensu Lato) Fossiles et Actuels: Crânes et Dents Jugales Supérieures; Editions du Centre National de la Recherche Scientifique: Paris, France, 1980. [Google Scholar]
- Arambourg, C. Vertébrés continentaux du Miocène supérieur de l’Afrique du Nord. Publ. Serv. Cart. Geol. L’algerie 1959, 4, 1–161. [Google Scholar]
- Arambourg, C. Les Vertébrés du Pléistocène de l’Afrique du Nord. Arch. Mus. Natl. D’histoiry Nat. 1970, 10, 1–127. [Google Scholar]
- Azzaroli, A. On Villafranchian Palaearctic Equids and their allies. Palaeontogr. Ital. 1982, 72, 74–97. [Google Scholar]
- Geraads, D.; Raynal, J.-P.; Eisenmann, V. The earliest human occupation of North Africa: A reply to Sahnouni et al. (2002). J. Hum. Evol. 2004, 46, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Tchernov, E. Memoires et Travaux du Centre de Recherche Francais de Jerusalem 5. In The Lower Pleistocene Mammals of ‘Ubeidiya (Jordan Valley); Association Paleorient: Paris, France, 1986. [Google Scholar]
- Yeshurun, R.; Zaidner, Y.; Eisenmann, V.; Martinez-Navarro, B.; Bar-Oz, G. Lower Paleolithic hominin ecology at the fringe of the desert: Faunal remains from Bizat Ruhama and Nahal Hesi, Northern Negev, Israel. J. Hum. Evol. 2011, 60, 492–507. [Google Scholar] [CrossRef]
- Tchernov, E. The Faunal Sequence of the Southwest Asian Middle Paleolithic in Relation to Hominid Dispersal Events. In Neandertals and Modern Humans in Western Asia; Akazawa, T., Aoki, K., Bar-Yosef, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 77–94. [Google Scholar]
- Broom, R. On evidence of a large horse recently extinct in South Africa. Ann. S. Afr. Mus. 1909, 7, 281–282. [Google Scholar]
- Eisenmann, V. Equus capensis (Mammalia, Perissodactyla) from Elandsfontein. Palaeontol. Afr. 2000, 36, 91–96. [Google Scholar]
- Churcher, C.S. The fossil equidae from the Krugersdorp Caves. Ann. Transvaal Mus. 1970, 26, 145–168. [Google Scholar]
- Morom, N.; Lazagabaster, I.A.; Shafir, R.; Natalio, F.; Eisenmann, V.; Kolsa Horwitz, L. The Middle Pleistocene mammalian fauna of Oumm Qatafa Cave, Judean Desert: Taxonomy, taphonomy and paleoenvironment. J. Quat. Sci. 2022, 37, 612–638. [Google Scholar] [CrossRef]
- Bagtache, B.; Hadjouis, D.; Eisenmann, V. Presence d’un Equus caballine (E. algericus n. sp.) et d’une autre espece nouvelle d’Equus (E. melkiensis n. sp.) dans l’Atérien des Allbroges, Algerie. Comptes Rendus Séances L’académie Sci. Paris 1984, 298, 609–612. [Google Scholar]
- Aouraghe, H.; Debenath, A. Les Equides du Pleistocene Superior de la grotte Zourah a el Harhoura, Maroc. Quaternarie 1999, 10, 283–292. [Google Scholar] [CrossRef]
- Oustalet, E. Une nouvelle espèce de zèbre, le zèbre de Grévy (Equus grevyi). Nature 1882, 10, 12–14. [Google Scholar]
- O’Brien, K.; Tryon, C.A.; Blegen, N.; Kimeu, B.; Rowan, J.; Faith, J.T. First appearance of Grévy’s zebra (Equus grevyi), from the Middle Pleistocene of the Kapthurin Formation, Kenya, sheds lights on the evolution and paleoecology of large zebras. Quat. Sci. Rev. 2021, 256, 106835. [Google Scholar] [CrossRef]
- Leonard, J.A.; Rohland, N.; Glaberman, S.; Fleischer, R.C.; Caccone, A.; Hofreiter, M. A rapid loss of stripes: The evolutionary history of the extinct quagga. Biol. Lett. 2005, 1, 291–295. [Google Scholar] [CrossRef]
- Pedersen, C.E.T.; Albrechtsen, A.; Etter, P.D.; Johnson, E.A.; Orlando, L.; Chikhi, L.; Siegismund, H.R.; Heller, R. A southern African origin and cryptic structure in highly mobile plains zebras. Nat. Ecol. Evol. 2018, 2, 491–498. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species cum Characteribus, Differentiis, Synonimis, Locis: Editio Decima, Reformata; Laurentii Salvii: Stockholm, Sweden, 1758; pp. 1–824. [Google Scholar]
- von Heuglin, T. Systematische Übersicht der Vögel Nord-Ost-Afrika’s: Mit Einschluss der Arabischen Küste des Rothen Meeres und der Nil-Quellen-Länder Südwärts bis zum IV. Grade Nördl. Breite; K. Akademie der Wissenschaften: Berlin, Germany, 1866. [Google Scholar]
- Churcher, C.S. Oldest ass recovered from Olduvai Gorge, Tanzania, and the origin of asses. J. Paleontol. 1982, 56, 1124–1132. [Google Scholar]
- Noack, N. Neues aus der Tierhandlung von Karl Hagenbeck, sowie aus dem Zoologischen Garten in Hamburg. Zool. Gart. 1884, 25, 100–115. [Google Scholar]
- Groves, C.P. The taxonomy, distribution, and adaptations of recent equids. In Equids in the Ancient World; Meadow, R.H., Uerpmann, H.-P., Eds.; Reichert: Wiesbaden, Germany, 1986; pp. 11–65. [Google Scholar]
- Denzau, G.; Denzau, H. (Eds.) Wildesel; Thorbecke: Stuttgart, Germany, 1999; pp. 1–221. [Google Scholar]
- Groves, C.P.; Smeenk, C. The nomenclature of the African wild ass. Zool. Meded. 2007, 81, 121–135. [Google Scholar]
- Sam, Y. African origins of modern asses as seen from paleontology and DNA: What about the Atlas wild ass? Geobios 2020, 58, 73–84. [Google Scholar] [CrossRef]
- Berggren, W.A.; Van Couvering, J.A. The late Neogene: Biostratigraphy, geochronology and paleoclimatology of the last 15 million years in marine and continental sequences. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1974, 16, 1–216. [Google Scholar]
- Bell, C.J.; Lundelius, E.L., Jr.; Barnosky, A.D.; Graham, R.W.; Lindsay, E.H.; Ruez, D.R., Jr.; Semken, H.A.; Webb, S.D.; Zakrzwski, R.J. The Blancan, Irvingtonian, and Rancholabrean Mammal Ages. In Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology; Woodburne, M.O., Ed.; Columbia University Press: New York, NY, USA, 2004; pp. 232–314. [Google Scholar]
- Woodburne, M.O. The great American biotic interchange: Dispersals, tectonics, climate, sea level and holding pens. J. Mammal. Evol. 2010, 17, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Avilla, L.S.; Bernardes, C.; Mothé, D. A new genus for Onohippidium galushai Macfadden and Skinner, 1979 (Mammalia, Equidae), from the late Hemphillian of North America. J. Vertebr. Paleontol. 2015, 35, e925909. [Google Scholar] [CrossRef]
- Coltorti, M.; Abbazzi, L.; Ferretti, M.P.; Iacumin, P.; Paredes Rios, F.; Pellegrini, M.; Pieruccini, P.; Rustioni, M.; Rook, L. Last Glacial mammals in South America: A new scenario from the Tarija Basin (Bolivia). Naturwissenschaften 2007, 94, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Bernor, R.L.; Golich, U.B.; Harzahauser, M.; Semprebon, G.M. The Pannonian C hipparions from the Vienna Basin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 476, 28–41. [Google Scholar] [CrossRef]
- Barry, J.C.; Behrensmeyer, A.K.; Badgley, C.E.; Flynn, L.J.; Peltonen, H.; Cheema, I.U.; Pilbeam, D.; Lindsay, E.H.; Raza, S.M.; Rajpar, A.R.; et al. The Neogene Siwaliks of the Potwar Plateau, Pakistan. In Fossil Mammals of Asia; Wang, X., Flynn, L.J., Fortelius, M., Eds.; Columbia University Press: New York, NY, USA, 2013; pp. 373–399. [Google Scholar]
- Opdyke, N.D.; Lindsay, E.; Johnson, G.D.; Johnson, N.; Tahirkheli, R.A.K.; Mirza, M.A. Magnetic polarity stratigraphy and vertebrate paleontology of the upper siwalik subgroup of northern Pakistan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1979, 27, 1–34. [Google Scholar] [CrossRef]
- Patnaik, R. Indian Neogene Siwalik mammalian biostratigraphy: An overview. In Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology; Wang, X., Flynn, L.J., Fortelius, M., Eds.; Columbia University Press: New York, NY, USA, 2013; pp. 423–444. [Google Scholar]
- Gaur, R.; Chopra, S.R.K. On a new subspecies of Equus from Pinjor Formation of Upper Sivaliks—With remarks on Sivalik Equus. J. Palaeontol. Soc. India 1984, 29, 19–25. [Google Scholar]
- Zhu, R.X.; Potts, R.; Pan, Y.X.; Yao, H.T.; Lü, L.Q.; Zhao, X. Early evidence of the genus Homo in East Asia. J. Hum. Evol. 2008, 55, 1075–1085. [Google Scholar] [CrossRef]
- Chauhan, P.R. Large mammal fossil occurrences and associated archaeological evidence in Pleistocene contexts of peninsular India and Sri Lanka. Quarter. Int. 2008, 192, 20–42. [Google Scholar] [CrossRef]
- Van Couvering, J.; Delson, E. African Land Mammal Ages. J. Vert. Pal. 2020, 40, 5. [Google Scholar] [CrossRef]
- Werdelin, L. Chronology of Neogene Mammal localities. In Cenozoic Mammals of Africa; Werdelin, L., Sanders, W.L., Eds.; University of California Press: Berkeley, CA, USA, 2010; pp. 27–43. [Google Scholar]
- Pickford, M. Preliminary Miocene mammalian biostratigraphy for Western Kenya. J. Hum. Evol. 1981, 10, 73–97. [Google Scholar] [CrossRef]
- Gilbert, H.; Bernor, R.L. Equidae. In Homo Erectus—Pleistocene Evidence from the Middle Awash, Ethiopia; Gilbert, H., Asfaw, B., Eds.; University of California Press: Berkeley, CA, USA, 2008; pp. 133–166. [Google Scholar]
- Geist, V. The relation of social evolution and dispersal in ungulates during the Pleistocene, with emphasis on Old World deer and the genus Bison. Quat. Res. 1971, 1, 285–315. [Google Scholar] [CrossRef]
- Guthrie, D.R. Frozen Fauna of the Mammoth Steppe—The Story of Blue Babe; University of Chicago Press: Chicago, IL, USA, 1990. [Google Scholar]
- McNab, B.K. Geographic and temporal correlations of mammalian size reconsidered: A resource rule. Oecologia 2010, 164, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, J. Ecometrics of Large Herbivorous Land Mammals in Relation to Climatic and Environmental Changes during the Pleistocene. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2014. [Google Scholar]
- Damuth, J.; Janis, C. On the relationships between hypsodonty and feeding ecology in ungulate mammals, and its utility in paleoecology. Biol. Rev. Camb. Philos. Soc. 2011, 86, 733–758. [Google Scholar] [CrossRef]
- Strömberg, C. Using phytolith assemblages to reconstruct the origin and spread of grass-dominated habitats in the great plains of North America during the late Eocene to early Miocene. Palaeogeo. Palaeoclim. Palaeoecol. 2004, 207, 239–275. [Google Scholar] [CrossRef]
- Strömberg, C. Decoupled taxonomic radiation and ecological expansion of open-habitat grasses in the Cenozoic of North America. Proc. Natl. Acad. Sci. USA 2005, 102, 11980–11984. [Google Scholar] [CrossRef]
- Strömberg, C. Evolution of Grasses and Grassland Ecosystems. Annu. Rev. Earth Planet. Sci. 2011, 39, 517–544. [Google Scholar] [CrossRef]
- Janis, C.; Sahney, S.; Benton, M. Grit not grass: Concordant patterns of early origin of hypsodonty in Great Plains ungulates and Glires. Palaeogeo. Palaeoclim. Palaeoecol. 2012, 366, 1–10. [Google Scholar]
- Semprebon, G.M.; Rivals, F.; Janis, C.M. The role of grass vs. exogenous abrasives in the paleodietary patterns of North American ungulates. Front. Ecol. Evol. 2019, 7, 65. [Google Scholar] [CrossRef]
- Strömberg, C.; McInerney, F. The Neogene transition from C3 to C4 grasslands in North America: Assemblage analysis of fossil phytoliths. Paleobiology 2011, 37, 50–71. [Google Scholar] [CrossRef]
- Pérez-Crespo, V.A.; Bravo-Cuevas, V.M.; Arroyo-Cabrales, J. Feeding habits of Equus conversidens and Haringtonhippus francisci from Valsequillo, Puebla, México. Hist. Biol. 2021, 34, 1252–1259. [Google Scholar] [CrossRef]
- Hulbert, R.C. Taxonomic evolution in North American Neogene horses (Subfamily Equinae): The rise and fall of an adaptive radiation. Paleobiology 1993, 19, 216–234. [Google Scholar] [CrossRef]
- Hulbert, R.C.; MacFadden, B.J. Morphological transformation and cladogenesis at the base of the adaptive radiation of Miocene hypsodont horses. Am. Mus. Novit. 1991, 3000, 1–61. [Google Scholar]
- Radinsky, L. Allometry and reorganization of horse skull proportions. Science 1983, 221, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Radinsky, L. Ontogeny and phylogeny in horse skull evolution. Int. J. Org. Evol. 1984, 38, 1–15. [Google Scholar] [CrossRef]
- Hulbert, R.C. Phylogenetic interrelationships and evolution of North American late Neogene Equidae. In The Evolution of Perissodactyls; Prothero, D.R., Schoch, R.M., Eds.; Clarendon Press: Oxford, UK, 1989; pp. 176–196. [Google Scholar]
- Osborn, H.F. The Age of Mammals; MacMillan Company: New York, NY, USA, 1910. [Google Scholar]
- Scott, W.B. A History of Land Mammals in the Western Hemisphere; MacMillian Company: New York, NY, USA, 1937. [Google Scholar]
- Simpson, G.G. Tempo and Mode in Evolution; Columbia University Press: New York, NY, USA, 1944. [Google Scholar]
- Stirton, R.A. Observation of evolutionary rates in hypsodonty. Evolution 1947, 1, 32–41. [Google Scholar] [CrossRef]
- Webb, S.D. A history of savanna vertebrates in the New World. Part I: North America. Annu. Rev. Ecol. Syst. 1977, 8, 355–380. [Google Scholar] [CrossRef]
- Webb, S.D. The rise and fall of the late Miocene ungulate fauna in North America. In Coevolution; Nitecki, M.H., Ed.; University of Chicago Press: Chicago, IL, USA, 1983; pp. 267–306. [Google Scholar]
- Janis, C.M. The significance of fossil ungulate communities as indicators of vegetation structure and climate. In Fossils and Climate; Brenchley, P.J., Ed.; John Wiley & Sons: New York, NY, USA, 1984; pp. 85–104. [Google Scholar]
- Janis, C.M. Tertiary mammal evolution in the context of changing climates, vegetation, and tectonic events. Annu. Rev. Ecol. Syst. 1993, 24, 467–500. [Google Scholar] [CrossRef]
- Webb, S.D.; Opdyke, N.D. Global climatic influence on Cenozoic land mammal faunas. National Research Council Panel on Effects of Past Global Change on Life. In Effects of Past Global Change on Life; National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Cohen, J.; DeSantis, L.R.G.; Lindsay, E.L.; Meachen, J.A.; O’Keefe, F.R.; Southon, J.R.; Binder, W.J. Dietary stability inferred from dental mesowear analysis in large ungulates from Racho La Brea and opportunistic feeding during the late Pleistocene. Palaeogeo. Palaeoclim. Palaeoecol. 2021, 570, 110360. [Google Scholar] [CrossRef]
- Jones, D.B.; DeSantis, L.R.G. Dietary ecology od ungulates from La Brea tar pits in southern California: A multi-proxy approach. Palaeogeo. Palaeoclim. Palaeoecol. 2017, 466, 110–117. [Google Scholar] [CrossRef]
- Kaiser, T.M.; Solounias, N.; Fortelius, M.; Bernor, R.L.; Schrenk, F. Tooth mesowear analysis on Hippotherium primigenium from the Vallesian Dinotheriensande (Germany). A blind test study. Carolinea 2000, 58, 103–114. [Google Scholar]
- Kaiser, T.M.; Bernor, R.L.; Scott, R.S.; Franzen, J.L.; Solounias, N. New Interpretations of the Systematics and Palaeoecology of the Dorn Dürkheim 1 Hipparions (Late Miocene, Turolian Age [MN11]) Rheinhessen, German. Senckenberg Lethea 2003, 83, 103. [Google Scholar] [CrossRef]
- Rivals, F.; Lister, A.M. Dietary flexibility and niche partitioning of large herbivores through the Pleistocene of Britain. Quat. Sci. Rev. 2016, 146, 116–133. [Google Scholar] [CrossRef]
- Head, M.J. Pollen and dinoflagellates from the Red Crag at Walton-on-the-Naze, Essex: Evidence for a mild climatic phase during the early Late Pliocene of eastern England. Geol. Mag. 1998, 135, 803–817. [Google Scholar] [CrossRef]
- Zalasiewicz, J.A.; Mathers, S.J.; Hughes, M.J.; Gibbard, P.L.; Peglar, S.M.; Harland, R.; Nicholson, R.A.; Boulton, G.S.; Cambridge, P.; Wealthall, G.P. Stratigraphy and Palaeoenvironments of the Red Crag and Norwich Crag Formations between Aldeburgh and Sizewell, Suffolk, England. Philos. Trans. R. Soc. Lond. 1988, 322, 221–272. [Google Scholar]
- Rivals, F.; Athanassiou, A. Dietary adaptations in an ungulate community from the late pliocene of greece. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 265, 134–139. [Google Scholar] [CrossRef]
- Strani, F.; DeMiguel, D.; Bellucci, L.; Sardella, R. Dietary response of early pleistocene ungulate communities to the climate oscillations of the Gelasian/Calabrian transition in central Italy. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 499, 102–111. [Google Scholar] [CrossRef]
- Strani, F.; DeMiguel, D.; Sardella, R.; Bellucci, L. Paleoenvironments and climatic changes in the italian peninsula during the early pleistocene: Evidence from dental wear patterns of the ungulate community of Coste San Giacomo. Quat. Sci. Rev. 2015, 121, 28–35. [Google Scholar] [CrossRef]
- Strani, F.; Pushkina, D.; Bocherens, H.; Bellucci, L.; Sardella, R.; DeMiguel, D. Dietary adaptations of early and middle pleistocene equids from the anagni basin (Frosinone, Central Italy). Front. Ecol. Evol. 2019, 7, 176. [Google Scholar] [CrossRef]
- Saarinen, J.; Oksanen, O.; Zliobaite, I.; Fortelius, M.; DeMiguel, D.; Azanza, B.; Bocherens, H.; Luzón, C.; Solano-Garcia, J.; Yravedra, J.; et al. Pliocene to Middle Pleistocene climate history in the Guadix-Baza Basin, and the environmental conditions of early Homo in Europe. Quat. Sci. Rev. 2021, 268, 107132. [Google Scholar] [CrossRef]
- Strani, F.; DeMiguel, D.; Alba, D.; Moyà-Solà, S.; Bellucci, L.; Sardella, R.; Madurell-Malapeira, J. The effects of the “0.9 Ma event” on the Mediterranean ecosystems during the Early-Middle Pleistocene transition as revealed by dental wear patterns of fossil ungulates. Quat. Sci. Rev. 2019, 210, 80–89. [Google Scholar] [CrossRef]
- Blain, H.-A.; Lozano-Fernández, I.; Agustí, J.; Bailon, S.; Menéndez, L.; Patrocinio, M.; Ros-Montoya, S.; Manuel, J.; Toro-Moyano, I.; Martínez-Navarro, B.; et al. Refining upon the climatic background of the early Pleistocene hominid settlement in western Europe: Barranco León and Fuente Nueva-3 (Guadix- Baza Basin, SE Spain). Quat. Sci. Rev. 2016, 144, 132–144. [Google Scholar] [CrossRef]
- Sánchez-Bandera, C.; Oms, O.; Blain, H.-A.; Lozano-Fernández, I.; Bispal-Chinesta, J.F.; Agustí, J.; Saarinen, J.; Fortelius, M.; Titton, S.; Serrano-Ramos, A.; et al. New stratigraphically constrained palaeoenvironmental reconstructions for the first human settlement in Western Europe: The Early Pleistocene herpetofaunal assemblages from Barranco León and Fuente Nueva 3 (Granada, SE Spain). Quat. Sci. Rev. 2020, 243, 106466. [Google Scholar] [CrossRef]
- Kahlke, R.D.; García, N.; Kostopoulos, D.S.; Lacombat, F.; Lister, A.M.; Mazza, P.P.; Spassov, N.; Titov, V.V. Western Palaearctic palaeoenvironmental conditions during the Early and early Middle Pleistocene inferred from large mammal communities, and implications for hominin dispersal in Europe. Quat. Sci. Rev. 2011, 30, 1368–1395. [Google Scholar] [CrossRef]
- Rivals, F.; Julien, M.-A.; Kuitems, M.; Van Kolfschoten, T.; Serangeli, J.; Drucker, D.G.; Bocherens, H.; Conard, N.J. Investigation of equid paleodiet from Schöningen 13 II-4 through dental wear and isotopic analyses: Archaeological implications. J. Hum. Evol. 2015, 89, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.B. Excavation of the Lower Paleolithic site at Amey’s Eartham Pit, Boxgrove, West Sussex: A preliminary report. Proc. Prehist. Soc. 1986, 52, 215–245. [Google Scholar] [CrossRef]
- Urban, B.; Bigga, G. Environmental reconstruction and biostratigraphy of late Middle Pleistocene lakeshore deposits at Schöningen. J. Hum. Evol. 2015, 89, 57–70. [Google Scholar] [CrossRef]
- Strani, F.; DeMiguel, D.; Bona, F.; Sardella, R.; Biddittu, I.; Bruni, L.; De Castro, A.; Guadagnoli, F.; Bellucci, L. Ungulate dietary adaptations and palaeoecology of the Middle Pleistocene site of Fontana Ranuccio (anagni, central italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 496, 238–247. [Google Scholar] [CrossRef]
- Leroi-Gourhan, A. Pollen analysis of sediment samples from Gough’s Cave, Cheddar. Proc. Univ. Bristol. Spelaeol. Soc. 1986, 17, 141–144. [Google Scholar]
- Bates, M.R.; Bates, C.R.; Gibbard, P.L.; Macphail, R.I.; Owen, F.J.; Parfitt, S.A.; Preece, R.C.; Roberts, M.B.; Robinson, J.E.; Whittaker, J.E.; et al. Late Middle Pleistocene deposits at Norton Farm on the West Sussex coastal plain, southern England. J. Quat. Sci. 2000, 15, 61–89. [Google Scholar] [CrossRef]
- West, R.G.; Lambert, C.A.; Sparks, B.W.; Dickson, J.H. Interglacial deposits at Ilford, Essex. Philos. Trans. R. Soc. 1964, 247, 185–212. [Google Scholar]
- Gibbard, P.L. Pleistocene History of the Lower Thames Valley; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Sedov, S.N.; Khokhlova, O.S.; Sinitsyn, A.A.; Korkka, A.V.; Ortega, B.; Solleiro, E.; Rozanova, M.S.; Kuznetsova, A.M. Kazdym Late Pleistocene paleosol sequences as an instrument for the local paleographic reconstruction of the Kostenki 14 key section (Voronezh oblast) as an example. Eurasian Soil Sc. 2010, 43, 876–892. [Google Scholar] [CrossRef]
- Ukraintseva, V.V. Mammoths and the Environment; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- van Asperen, E.N.; Stefaniak, K.; Proskurnyak, I.; Ridush, B. Equids from Emine-Bair-Khosar Cave (Crimea, Ukraine): Co-occurrence of the stenonid Equus hydruntinus and the caballoid E. ferus latipes based on skull and postcranial remains. Palaeontol. Electron. 2012, 15, 28. [Google Scholar] [CrossRef]
- Bernor, R.L.; Kaiser, T.M.; Nelson, S.V. The Oldest Ethiopian Hipparion (Equinae, Perissodactyla) from Chorora: Systematics, Paleodiet and Paleoclimate. Cour. Forsch. Inst. Senckenberg 2004, 246, 213–226. [Google Scholar]
- Bernor, R.L. The Latest Miocene Hipparionine (Equidae) from Lemundong’o, Kenya. Kirtlandia 2007, 56, 148–151. [Google Scholar]
- Bernor, R.L.; Kaiser, T.M.; Wolf, D. Revisiting Sahabi Equid Species Diversity, Biogeographic Patterns and Diet Preferences. Gharyounis Bull. 2008, 5, 159–167. [Google Scholar]
- Bernor, R.L.; Haile Selassie, Y. Equidae. In Ardipithecus Kadabba: Late Miocene Evidence from the Middle Awash, Ethiopia; Haile-Selassie, Y., Woldegabriel, G., Eds.; University of California Press: Berkeley, CA, USA, 2009; pp. 397–428. [Google Scholar]
- Bernor, R.L.; Boaz, N.T.; Rook, L. Eurygnathohippus feibeli (Perissodactyla: Mammalia) from the late Miocene of Sahabi (Libya) and its evolutionary and Biogeographic Significance. Bolletino Soc. Paleontol. Ital. 2012, 51, 39–48. [Google Scholar]
- Schulz, E.; Kaiser, T.M. Historical distribution, habitat requirements and feeding ecology of the genus Equus (Perissodactyla). Mammal. Rev. 2013, 43, 111–123. [Google Scholar] [CrossRef]
- Goloboff, P.; Farris, J.; Nixon, K. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Bennett, D.K. Stripes do not a zebra make, Part I: A cladistic analysis of Equus. Syst. Zool. 1980, 29, 272–287. [Google Scholar] [CrossRef]
- Antoine, P.O. Phylogénie et évolution des Elasmotheriina (Mammalia, Rhinocerotidae). Mémoir. Mus. Natl. Hist. 2002, 188, 1–359. [Google Scholar]
- Antoine, P.O.; Downing, K.F.; Crochet, J.Y.; Duranthon, F.; Flynn, L.J.; Marivaux, L.; Métais, G.; Rajpar, A.R.; Roohi, G. A revision of Aceratherium blanfordi Lydekker, 1884 (Mammalia: Rhinocerotidae) from the Early Miocene of Pakistan: Postcranials as a key. Zool. J. Linn. Soc. Lond. 2010, 160, 139–194. [Google Scholar] [CrossRef]
- Pandolfi, L. Persiatherium rodleri, gen. et sp. nov. (Mammalia, Rhinocerotidae), from the Late Miocene of Maragheh (Northwestern Iran). J. Vert Paleontol. 2015, 36, e1040118. [Google Scholar] [CrossRef]
- de Queiroz, K.; Gauthier, J. Phylogenetic taxonomy. Annu. Rev. Ecol. Syst. 1992, 23, 449–480. [Google Scholar] [CrossRef]
- Ereshefsky, M. The Poverty of the Linnaean hierarchy. A Philosophical Study of Biological Taxonomy; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Papavero, N.; Llorente-Bousquets, J.; Minoro Abel, J. Proposal of a new system of nomenclature for phylogenetic systematics. Arq. Zool. 2001, 36, 1–145. [Google Scholar] [CrossRef][Green Version]
- Béthoux, O. Propositions for a character-state-based biological taxonomy. Zool. Scr. 2007, 36, 409–416. [Google Scholar] [CrossRef]
- Cantino, P.D.; de Queiroz, K. PhyloCode: International Code of Phylogenetic Nomenclature (Version 4c). 2010. Available online: http://www.ohio.edu/phylocode (accessed on 5 June 2022).
- Zachos, F.E. Linnaean ranks, temporal banding, and time-clipping: Why not slaughter the sacred cow? Biol. J. Linn. Soc. 2011, 103, 732–734. [Google Scholar] [CrossRef]
- Vences, M.; Guayasamin, J.M.; Miralles, A.; de la Riva, I. To name or not to name: Criteria to promote economy of change in Linnaean classification schemes. Zootaxa 2013, 3636, 201–244. [Google Scholar] [CrossRef]
- Hennig, W. Phylogenetic Systematics; University Illinois Press: Champaign, IL, USA, 1966. [Google Scholar]
- Mayr, E. Principles of Systematic Zoology; McGraw-Hill: New York, NY, USA, 1969. [Google Scholar]
- Groves, C.P. Primate Taxonomy; Smithsonian Institution Press: Washington, DC, USA, 2001. [Google Scholar]
- Groves, C.P. The what, why, and how of primate taxonomy. Int. J. Primatol. 2004, 25, 1105–1126. [Google Scholar] [CrossRef]
- Simpson, G.G. The Major Features of Evolution; Columbia University Press: New York, NY, USA, 1953. [Google Scholar]
- Mayr, E. Taxonomic categories in fossil hominids. Cold Spring Harb. Symp. Quant. Biol. 1950, 15, 109–118. [Google Scholar] [CrossRef]
- de Queiroz, K. Replacement of an essentialistic perspective on taxonomic definitions as exemplified by the definition of “Mammalia”. Syst. Biol. 1994, 43, 497–510. [Google Scholar] [CrossRef]
- Janis, C.M.; Bernor, R.L. The Evolution of Equid Monodactyly: A Review Including a New Hypothesis. Front. Ecol. Evol 2019, 7, 119. [Google Scholar] [CrossRef]
- Schultz, J.R. Plesippus francescana (Frick) from the late Pliocene, Coso Mountains, California, with a review of the genus Plesippus. In Contributions to Palæontology. Publications of the Carnegie Institution of Washington; Carnegie institution of Washington: Washington, DC, USA, 1936; Volume 1, pp. 1–13. [Google Scholar]
- Eisenmann, V.; Baylac, M. Extant and fossil Equus (Mammalia, Perissodactyla) skulls: A morphometric definition of the subgenus Equus. Zool. Scr. 2000, 29, 89–100. [Google Scholar] [CrossRef]
- Brochu, A.B.; Sumrall, C.D. Phylogenetic Nomenclature and Paleontology. J. Paleontol. 2001, 75, 754–757. [Google Scholar] [CrossRef]
- Bryant, H.N. Explicitness, Stability, and Universality in the Phylogenetic Definition and Usage of Taxon Names: A Case Study of the Phylogenetic Taxonomy of the Carnivora (Mammalia). Syst. Biol. 1996, 45, 174–189. [Google Scholar] [CrossRef]
- Cantino, P.D.; Olmstead, R.G.; Wagstaff, S.J. A Comparison of Phylogenetic Nomenclature with the Current System: A Botanical Case Study. Syst. Biol. 1997, 46, 313–331. [Google Scholar] [CrossRef]
- Schulte, J.S.; Macey, J.R.; Larson, A.; Papenfuss, T.J. Molecular Tests of Phylogenetic Taxonomies: A General Procedure and Example Using Four Subfamilies of the Lizard Family Iguanidae. Mol. Phylogenetics Evol. 1998, 10, 367–376. [Google Scholar] [CrossRef]
- de Queiroz, K.; Gauthier, J. Phylogeny as a Central Principle in Taxonomy: Phylogenetic Definitions of Taxon Names. Syst. Biol. 1990, 39, 307–322. [Google Scholar] [CrossRef]
- de Queiroz, K.; Gauthier, J. Toward a phylogenetic system of biological nomenclature. Trends Ecol. Evol. 1994, 9, 27–31. [Google Scholar] [CrossRef]
- de Queiroz, K.; Cantino, P.D. Taxon Names, Not Taxa, Are Defined. Taxon 2001, 50, 821–826. [Google Scholar] [CrossRef]
- Vilstrup, J.T.; Seguin-Orlando, A.; Stiller, M.; Ginolhac, A.; Raghavan, M.; Nielsen, S.C.A.; Weinstock, J.; Froese, D.; Vasiliev, S.K.; Ovodov, N.D. Mitochondrial Phylogenomics of Modern and Ancient Equids. PLoS ONE 2013, 8, e55950. [Google Scholar]
- MacFadden, B.; Solounias, N.; Cerling, T.E. Ancient Diets, Ecology, and Extinction of 5-Million-Year-Old Horses from Florida. Science 1999, 283, 824–827. [Google Scholar] [CrossRef]
- Semprebon, G.M.; Rivals, F.; Solounias, N.; Hulbert, R.C. Paleodietary reconstruction of fossil horses from the Eocene through Pleistocene of North America. Palaeogeogr. Palaeoclim. Palaeoecol. 2016, 442, 110–127. [Google Scholar] [CrossRef]
- Miller, W.E. The Late Pliocene Las Tunas Local Fauna from Southernmost Baja California, Mexico. J. Paleontol. 1980, 54, 762–805. [Google Scholar]
- MacFadden, B.J.; Carranza-Castañeda, O. Cranium of Dinohippus mexicanus (Mammalia: Equidae) from the Early Pliocene (latest Hemphillian) of Central Mexico, and the origin of Equus. Bull. Fla. Mus. Nat. Hist. 2002, 43, 163–185. [Google Scholar]
- Carranza-Castañeda, O. Dinohippus mexicanus (Early—Late, Late, and Latest Hemphillian) and the transition to genus Equus, in Central Mexico faunas. Front. Earth Sci. 2019, 7, 89. [Google Scholar] [CrossRef]
- Lowenstein, J.M.; Rayder, O.A. Immunological systematics of the extinct quagga (Equidae). Experientia 1985, 41, 1192–1193. [Google Scholar] [CrossRef]
- George, M.; Rayder, O.A. Mitochondrial DNA evolution in the genus Equus. Mol. Biol. Evol. 1986, 3, 535–546. [Google Scholar]
- Oakefull, E.A.; Clegg, J.B. Phylogenetic Relationships within the Genus Equus and the Evolution of α and θ Globin Genes. J. Mol. Evol. 1998, 47, 772–783. [Google Scholar] [CrossRef]
- Kruger, K.; Gaillard, C.; Stranzinger, G.; Rieder, S. Phylogenetic analysis and species allocation of individual equids using microsatellite data. J. Anim. Breed. Genet. 2005, 122, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.C.; Ryder, O.A. Molecular phylogeny and evolution of the Perissodactyla. Zool. J. Linn. Soc. 2011, 163, 1289–1303. [Google Scholar] [CrossRef]
- Orlando, L.; Mashkour, M.; Burke, A.; Douady, C.J.; Eisenmann, V.; Hanni, C. Geographic distribution of an extinct equid (Equus hydruntinus: Mammalia, Equidae) revealed by morphological and genetical analyses of fossils. Mol. Ecol. 2006, 15, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Orlando, L.; Male, D.; Alberdi, M.T.; Prado, J.L.; Prieto, A.; Cooper, A.; Hanni, C. Ancient DNA Clarifies the Evolutionary History of American Late Pleistocene Equids. J. Mol. Evol. 2008, 66, 533–538. [Google Scholar] [CrossRef]
- Flint, J.; Ryder, O.A.; Clegg, J.B. Comparison of theα-globin gene cluster structure in Perissodactyla. J. Mol. Evol. 1990, 30, 36–42. [Google Scholar] [CrossRef]
- Wallner, B.; Brem, G.; Muller, M.; Achmann, R. Fixed nucleotide differences on the Y chromosome indicate clear divergence between Equus przewalskii and Equus caballus. Anim. Genet. 2003, 34, 453–456. [Google Scholar] [CrossRef]
- Kaminski, M. The biochemical evolution of the horse. Comp. Biochem. 1979, 63, 175–178. [Google Scholar] [CrossRef]
- Ishida, N.; Oyunsuren, T.; Mashima, S.; Mukoyama, H.; Naruya, S. Mitochondrial DNA sequences of various species of the genus Equus with special reference to the phylogenetic relationship between Przewalskii’s wild horse and domestic horse. J. Mol. Evol. 1995, 41, 180–188. [Google Scholar] [CrossRef]
- Oakenfull, E.A.; Lim, H.N.; Ryder, O.A. A survey of equid mitochondrial DNA: Implications for the evolution, genetic diversity and conservation of Equus. Conserv. Genet. 2000, 1, 341–355. [Google Scholar] [CrossRef]
- Cai, D.; Zhu, S.; Gong, M.; Zhang, N.; Wen, J.; Qiyao, L.; Sun, W.; Shao, X.; Guo, Y.; Cai, Y.; et al. Radiocarbon and genomic evidence for the survival of Equus Sussemionus until the late Holocene. eLife 2022, 11, e73346. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).