Simple Summary
Xylaria, a large, complex, and cosmopolitan genus of Ascomycota, are known as a source of bioactive secondary metabolites with antibacterial, antioxidative, anti-carcinogenic, and other properties. The species of this genus usually grow on decayed wood, fallen fruits or seeds, fallen leaves or petioles, and termite nests. The present paper describes species of Xylaria associated with fruits and seeds using morphological and multigene phylogenetic analyses based on specimens collected in Southwest China. There are few detailed reports on Xylaria taxonomy from China, especially on the species associated with fallen fruits and seeds. In this study, we describe four new species from the genus Xylaria with pale-colored ascospores on fallen fruits. They are described, illustrated, and compared with morphologically similar species, and their nucleotide sequences of ITS, RPB2, and β-tubulin were obtained and analysed. Our study reports new species of Xylaria with pale-colored ascospores associated with fallen fruits and seeds in China for the first time.
Abstract
Xylaria, a large and cosmopolitan genus of Ascomycota, plays an important ecological role in forest ecology as wood-decomposers, and serve as a source of bioactive secondary metabolites. The present work concerns a survey of Xylaria from Southwest China. Four new species of Xylaria with pale-colored ascospores associated with fallen fruits and seeds are described and illustrated based on morphological and phylogenetic evidences. The phylogeny inferred from a combined dataset of ITS-RPB2-β-tubulin sequences supports these four species as distinct species. The four new taxa, namely Xylaria rogersii, X. schimicola, X. theaceicola, and X. wallichii, are compared and contrasted against morphologically similar species. A dichotomous identification key to all the accepted species of Xylaria associated with fallen fruits and seeds is given.
1. Introduction
The genus Xylaria Hill ex Schrank is one of the most complex and difficult genera in the Xylariaceae. Stromata morphology of many species often vary greatly in color, size, and even in general shape with stages of development. The genus is widely distributed in tropical, subtropical, and temperate regions. More than 300 Xylaria species have been reported in the world [1], and more than 800 epithets are listed in Index Fungorum (http://www.indexfungorum.org/, accessed on 1 March 2022) [2]. Xylaria species are characterized by having upright, stipitate, woody to leathery stromata with perithecia entirely immersed [3,4].
Most Xylaria species inhabit decayed wood, while some grow on fallen fruits or seeds, leaves or petioles, and termite nests. The Xylaria species associated with fallen fruits/seeds or leaves/petioles are substrate-specific [5,6,7,8]. Examples include Xylaria magnoliae J.D. Rogers on Magnolia fruits, Xylaria xanthinovelutina Mont. on leguminous pods [6], X. carpophila (Pers.) Fr. on Fagus fruits, X. liquidambaris J.D. Rogers, Y.M. Ju & F. San Martín on Liquidambar fruit [9], X. guareae Læssøe et Lodge on Guarea guidonia, X. meliacearum Læssøe on fine litter of trees in the Meliaceae, and X. axifera Mont. on fallen petioles of Araliaceae [7]. However, some species are not restricted to fallen fruits or seeds, such as X. clusiae K.F. Rodrigues, J.D. Rogers & Samuels, X. duranii San Martín & Vanoye, and X. heloidea Penz. & Sacc., which can also be found on fallen leaves [5]. Therefore, it is ecologically interesting to study Xylaria species occurring on fruits and seeds. Certain Xylaria species on fruits and seeds have already been taxonomically studied [5,6,7,9,10,11,12]. Recently, Perera et al. [8] described two new species, X. fabacearum R.H. Perera, E.B.G. Jones & K.D. Hyde and X. fabaceicola R.H. Perera, E.B.G. Jones & K.D. Hyde, from Thailand, increasing the number of species on fruits and seeds to 29.
In China, about 70 species of Xylaria have been reported [13,14,15,16,17,18,19,20,21,22,23], but only four species are associated with fallen fruits and seeds. Teng [13] reported three species of Xylaria from fallen fruits and seeds, X. carpophila, X. xanthinovelutina, and X. warburgii Henn., whereas X. carpophila was a misidentified specimen. Huang et al. [24] described a new species, X. beilschmiediae G. Huang & L. Guo, on fallen fruit of Beilschmiedia percoriacea from Southern China. During investigations on the diversity of xylariaceous specimens in Southwest China, four undescribed Xylaria species associated with fallen fruits and seeds were collected and taxonomically characterized based on morphological criteria and phylogenetic analyses. The primary purpose of the present study is to use an integrative taxonomic approach for the delimitation and description of four new species of Xylaria from China, and to discuss the phylogeny of the genus Xylaria based on expanded sampling.
2. Materials and Methods
2.1. Sample Collection and Morphological Study
Field sampling trips in nature reserves and forest parks in tropical and subtropical regions of Southwest China were carried out by the authors. The photos of the materials were taken with a Canon camera G15 (Canon Corporation, Tokyo, Japan). Fresh specimens were dried with a portable drier (manufactured in China). Dried specimens were labeled and stored in an ultra-low freezer at −80 °C for 1 week to kill insects and their eggs, and then they were ready for morphological and molecular studies. Voucher specimens are deposited in the Fungarium of the Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences (FCATAS).
Microscopic features and measurements were made from slide preparations mounted in water, Melzer’s iodine reagent, 5% KOH, 1% SDS, and Indian ink. The average range of ascospore size refers to more than 95% of spores, and the extreme values are given in parentheses. In the text, the following abbreviations are used: L = mean ascospore length (arithmetical average of all ascospores); W = mean ascospore width (arithmetical average of all ascospores); M = L × W; Q = L/W ratio; n (a/b) = number of ascospores (a) measured from number of specimens (b). The photographs of asci, ascus apical apparatus, and ascospores were examined by differential interference microscopy (DIC) and bright field microscopy (BF) with a Zeiss Axio Scope A1 (Zeiss Corporation, Oberkochen, Germany) and a scanning electron microscope (SEM) (Hitachi Corporation, Tokyo, Japan), respectively. Stromatal surface and perithecia were observed and photographed using a VHX-600E microscope of the Keyence Corporation (Osaka, Japan). Color codes and names followed Rayner [25].
2.2. Molecular Procedures and Phylogenetic Analyses
Total DNA from herbarium specimens was extracted using a cetyltrimethylammonium bromide (CTAB) rapid extraction kit for plant genomes (Aidlab Biotechnologies, Beijing, China) according to the manufacturer’s instructions. Target regions of the ITS rDNA, RPB2, and β-tubulin, were amplified by polymerase chain reaction (PCR) using TaKaRa Taq (TaKaRa Bio, Kusatsu, Japan) and fungal specific primers. Approximately 500 base pairs of the ITS region were amplified with primers ITS5 and ITS4 [26], using the following procedure: initial denaturation at 98 °C for 5 min, followed by 30 cycles of 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 2 min, and a final extension of 72 °C for 10 min. For the RPB2 gene, about 1200 base pairs were amplified with primers fRPB2-5F and fRPB2-7cR [27], using the following procedure: initial denaturation at 95 °C for 5 min, followed by 30 cycles of 95 °C for 1 min, 55 °C for 2 min, and 72 °C for 2 min, and a final extension of 72 °C for 10 min. For the β-tubulin gene, about 1500 base pairs were amplified with primers T1 and T22 [28], using the following procedure: initial denaturation at 95 °C for 2 min, followed by 30 cycles of 95 °C for 1 min, 54–45 °C for 1.5 min, and 72 °C for 2 min, and a final extension of 72 °C for 10 min [29]. DNA sequencing was performed at BGI tech (Guangzhou, China), and all the newly generated sequences were submitted to GenBank (Table 1).
Two separate datasets, the concatenated ITS-RPB2-β-tubulin sequences of Xylaria and related genera in the family Xylariaceae, and ITS-only sequences of Xylaria from GenBank, were analyzed. Poronia pileiformis (Berk.) Fr. was selected as an outgroup [30]. The sequences of ITS, RPB2, and β-tubulin were aligned separately using the MAFFT V.7 online server (https://mafft.cbrc.jp/alignment/server/, accessed on 12 March 2022) [31] with the G-INS-i iterative refinement algorithm, and rechecked and improved manually using BioEdit v. 7.0.5.2 [32]. Phylogenetic analyses were carried out with maximum likelihood (ML) and Bayesian inference (BI) analysis, respectively. The ML analysis was performed using RaxML v.8.2.10 [33] with the bootstrap values obtained from 1000 replicates and the GTRGAMMA model of nucleotide evolution. The BI was performed using MrBayes 3.2.6 [34]. ITS sequences were inferred and used to confirm the Xylaria species identification carried out in the study. Phylogenetic trees were viewed in FigTree version 1.4.2 [35].

Table 1.
List of taxa used for the phylogenetic reconstruction. GenBank accession numbers, specimen numbers, origin, and reference studies are given. Holotype specimens are labeled with HT. Sequences from specimens highlighted in bold are derived from this study. N/A: not available.
Table 1.
List of taxa used for the phylogenetic reconstruction. GenBank accession numbers, specimen numbers, origin, and reference studies are given. Holotype specimens are labeled with HT. Sequences from specimens highlighted in bold are derived from this study. N/A: not available.
| Species | Specimen No. | Origin | Host | GenBank Accession Number | Reference | ||
|---|---|---|---|---|---|---|---|
| ITS | RPB2 | β-Tubulin | |||||
| Amphirosellinia fushanensis | HAST 91111209 (HT) | China | dead twigs | GU339496 | GQ848339 | GQ495950 | [36,37] |
| A. nigrospora | HAST 91092308 (HT) | China | dead twigs | GU322457 | GQ848340 | GQ49595 | [36,37] |
| Astrocystis mirabilis | HAST 94070803 | China | bamboo culms | GU322448 | GQ844835 | GQ49594 | [36] |
| As. sublimbata | HAST 89032207 | China | bamboo culms | GU322447 | GQ844834 | GQ495940 | [36] |
| Kretzschmaria guyanensis | HAST 89062903 | China | bark | GU300079 | GQ844792 | GQ478214 | [36] |
| K. sandvicensis | JDR 113 | USA | wood | GU300076 | GQ844786 | GQ478211 | [36] |
| Nemania abortiva | BiSH 467 (HT) | USA | decayed wood | GU292816 | GQ844768 | GQ470219 | [36] |
| N. diffusa | HAST 91020401 | China | bark | GU292817 | GQ844769 | GQ470220 | [36] |
| Podosordaria mexicana | WSP 176 | Mexico | horse dung | GU324762 | GQ853039 | GQ844840 | [36] |
| P. muli | WSP 167 (HT) | Mexico | mule dung | GU324761 | GQ853038 | GQ844839 | [36] |
| Poronia pileiformis | WSP 88113001 (ET) | China | cow dung | GU324760 | GQ853037 | GQ502720 | [36] |
| Rosellinia buxi | JDR 99 | France | Buxus sempervivens | GU300070 | GQ844780 | GQ470228 | [36] |
| R. necatrix | HAST 89062904 | China | root | EF026117 | GQ844779 | EF025603 | [36] |
| Xylaria adscendens | HAST 570 | Guadeloupe | wood | GU300101 | GQ844817 | GQ487708 | [36] |
| X. aethiopica | YMJ 1136 | Ethiopia | pods of Millettia ferruginea | MH790445 | MH785222 | MH785221 | [11] |
| X. allantoidea | HAST 94042903 | China | trunk | GU324743 | GQ848356 | GQ502692 | [36] |
| X. amphithele | HAST 529 | Guadeloupe | dead leaves | GU300083 | GQ844796 | GQ478218 | [36] |
| X. apoda | HAST 90080804 | China | bark | GU322437 | GQ844823 | GQ495930 | [36] |
| X. arbuscula | HAST 89041211 | China | bark | GU300090 | GQ844805 | GQ478226 | [36] |
| X. atrosphaerica | HAST 91111214 | China | bark | GU322459 | GQ848342 | GQ495953 | [36] |
| X. berteri | HAST 90112623 | China | wood | GU324749 | GQ848362 | AY951763 | [36] |
| X. betulicola | FCATAS750 (HT) | China | leaves of Betula | MF774332 | N/A | N/A | [22] |
| X. brunneovinosa | HAST 720 (HT) | China | ground of bamboo plantation | EU179862 | GQ853023 | GQ502706 | [36,38] |
| X. carpophila | CBS 453.72 | Netherlands | - | MH860527 | N/A | N/A | [39] |
| X. cirrata | HAST 664 (ET) | China | ground of vegetable farm | EU179863 | GQ853024 | GQ502707 | [36,38] |
| X. cranioides | HAST 226 | China | wood | GU300075 | GQ844785 | GQ478210 | [36] |
| X. crinalis | FCATAS751 (HT) | China | wood | MF774330 | N/A | N/A | [22] |
| X. cubensis | JDR 860 | USA | wood | GU991523 | GQ848365 | GQ502700 | [36] |
| X. culleniae | JDR 189 | Thailand | pod | GU322442 | GQ844829 | GQ495935 | [36] |
| X. curta | HAST 92092022 | China | bark | GU322443 | GQ844830 | GQ495936 | [36] |
| X. digitata | HAST 919 | Ukraine | wood | GU322456 | GQ848338 | GQ495949 | [36] |
| X. enterogena | HAST 785 | French Guiana | wood | GU324736 | GQ848349 | GQ502685 | [36] |
| X. fabacearum | MFLU 16-1061 (HT) | Thailand | seed pods of Fabaceae | NR171104 | MT212202 | MT212220 | [8] |
| X. fabaceicola | MFLU 16-1072 (HT) | Thailand | seed pods of Fabaceae | NR171103 | MT212201 | MT212219 | [8] |
| X. feejeensis | HAST 92092013 | China | bark | GU322454 | GQ848336 | GQ495947 | [36] |
| X. ficicola | HMJAU 22818 | China | leaves and petioles of Ficus auriculata | MZ351258 | N/A | N/A | [40] |
| X. filiformis | GUM 1052 | Iran | herbaceous stem | KP218907 | N/A | N/A | [41] |
| X. fimbriata | HAST 491 | Martinique | termite nest | GU324753 | GQ853022 | GQ502705 | [36] |
| X. fissilis | HAST 367 | Martinique | bark | GU300073 | GQ844783 | GQ470231 | [36] |
| X. frustulosa | HAST 92092010 | China | bark | GU322451 | GQ844838 | GQ495944 | [36] |
| X. cf. glebulosa | HAST 431 | Martinique | fruit | GU322462 | GQ848345 | GQ495956 | [36] |
| X. globosa | HAST 775 | Guadeloupe | bark | GU324735 | GQ848348 | GQ502684 | [36] |
| X. grammica | HAST 479 | China | wood | GU300097 | GQ844813 | GQ487704 | [36] |
| X. griseosepiacea | HAST 641 (HT) | China | ground of mango orchard | EU179865 | GQ853031 | GQ502714 | [36,38] |
| X. guareae | PR71 | Puerto Rico | - | AY909009 | N/A | N/A | [42] |
| X. haemorrhoidalis | HAST 89041207 | China | bark | GU322464 | GQ848347 | GQ502683 | [36] |
| X. hedyosmicola | FCATAS857 | China | leaves of Hedyosmum orientale | MZ227023 | MZ683407 | MZ221183 | [40] |
| X. hypoxylon | HAST 95082001 | China | wood | GU300095 | GQ844811 | GQ487703 | [36] |
| X. intracolorata | HAST 90080402 | China | bark | GU324741 | GQ848354 | GQ502690 | [36] |
| X. intraflava | HAST 725 (HT) | China | ground of bamboo plantation | EU179866 | GQ853035 | GQ502718 | [36] |
| X. juruensis | HAST 92042501 | China | Arenga engleri | GU322439 | GQ844825 | GQ495932 | [36] |
| X. karyophthora | DRH059 | Guyana | seeds of Chlorocardium sp. | KY564220 | KY564216 | N/A | [12] |
| X. laevis | HAST 95072910 | China | bark | GU324747 | GQ848360 | GQ502696 | [36] |
| X. lindericola | FCATAS852 | China | leaves of Lindera robusta | MZ005635 | MZ031982 | MZ031978 | [40] |
| X. liquidambaris | HAST 93090701 | China | fruits of Liquidambar formosana | GU300094 | GQ844810 | GQ487702 | [36] |
| X. meliacearum | JDR 148 | Puerto Rico | petioles and infructescence of Guarea guidonia | GU300084 | GQ844797 | GQ478219 | [36] |
| X. microceras | HAST 414 | Guadeloupe | wood | GU300086 | GQ844799 | GQ478221 | [36] |
| X. montagnei | HAST 495 | Martinique | wood | GU322455 | GQ848337 | GQ495948 | [36] |
| X. multiplex | JDR 259 | USA | wood | GU300099 | GQ844815 | GQ487706 | [36] |
| X. muscula | HAST 520 | Guadeloupe | dead branch | GU300087 | GQ844800 | GQ478222 | [36] |
| X. nigripes | HAST 653 | China | ground of mango orchard | GU324755 | GQ853027 | GQ502710 | [36] |
| X. ochraceostroma | HAST 401 (HT) | China | ground of mango orchard | EU179869 | GQ853034 | GQ502717 | [36,38] |
| X. oligotoma | HAST 784 | French Guiana | wood | GU300092 | GQ844808 | GQ487700 | [36] |
| X. ophiopoda | HAST 93082805 | China | bark | GU322461 | GQ848344 | GQ495955 | [36] |
| X. oxyacanthae | YMJ 1184 | Germany | seeds of Carpinus betulus | MF773430 | MF773434 | MF773438 | [5,36] |
| X. oxyacanthae | YMJ 1320 | Germany | fruits of Cornus sanguinea | MF773431 | MF773435 | MF773439 | [5,36] |
| X. palmicola | PDD 604 | New Zealand | fruits of palm | GU322436 | GQ844822 | GQ495929 | [36] |
| X. papulis | HAST 89021903 | China | wood | GU300100 | GQ844816 | GQ487707 | [36] |
| X. phyllocharis | HAST 528 | Guadeloupe | dead leaves | GU322445 | GQ844832 | GQ495938 | [36] |
| X. plebeja | HAST 91122401 | China | trunk of Machilus zuihoensis | GU324740 | GQ848353 | GQ502689 | [36] |
| X. polymorpha | JDR 1012 | USA | wood | GU322460 | GQ848343 | GQ495954 | [36] |
| X. polysporicola | FCATAS848 | China | leaves of Polyspora hainanensis | MZ005592 | MZ031980 | MZ031976 | [40] |
| X. reevesiae | HAST 90071609 (HT) | China | fruits of Reevesia formosana | GU322435 | GQ844821 | GQ495928 | [36] |
| X. regalis | HAST 920 | India | log of Ficus racemosa | GU324745 | GQ848358 | GQ502694 | [36] |
| X. rogersii | FCATAS913 | China | fruits of Magnolia sp. | MZ648825 | MZ707119 | MZ695799 | This study |
| X. rogersii | FCATAS914 | China | fruits of Magnolia sp. | MZ648826 | MZ707120 | N/A | This study |
| X. rogersii | FCATAS915 (HT) | China | fruits of Magnolia sp. | MZ648827 | MZ707121 | MZ695800 | This study |
| X. schimicola | FCATAS896 (HT) | China | fruits of Schima noronhae | MZ648850 | MZ707114 | MZ695787 | This study |
| X. schimicola | FCATAS898 | China | fruits of Schima noronhae | MZ648851 | N/A | N/A | This study |
| X. schweinitzii | HAST 92092023 | China | bark | GU322463 | GQ848346 | GQ495957 | [36] |
| X. scruposa | HAST 497 | Martinique | wood | GU322458 | GQ848341 | GQ495952 | [36] |
| X. sicula | HAST 90071613 | China | fallen leaves | GU300081 | GQ844794 | GQ478216 | [36] |
| Xylaria sp. 6 | JDR 258 | USA | leaves of Tibouchina semidecandra | GU300082 | GQ844795 | GQ478217 | [36] |
| X. striata | HAST 304 | China | branch of Punica granatum | GU300089 | GQ844803 | GQ478224 | [36] |
| X. telfairii | HAST 90081901 | China | bark | GU324738 | GQ848351 | GQ502687 | [36] |
| X. theaceicola | FCATAS903 (HT) | China | fruits of Schima villosa | MZ648848 | MZ707115 | MZ695788 | This study |
| X. theaceicola | FCATAS904 | China | fruits of Schima villosa | MZ648849 | N/A | N/A | This study |
| X. tuberoides | HAST 475 | Martinique | wood | GU300074 | GQ844784 | GQ478209 | [36] |
| X. venustula | HAST 88113002 | China | bark | GU300091 | GQ844807 | GQ487699 | [36] |
| X. vivantii | HAST 519 (HT) | Martinique | fruits of Magnolia sp. | GU322438 | GQ844824 | GQ495931 | [36] |
| X. wallichii | FCATAS923 | China | fruits of Schima wallichii | MZ648861 | MZ707118 | MZ695793 | This study |
| X. wallichii | FCATAS924 | China | fruits of Schima wallichii | MZ648862 | N/A | MZ695794 | This study |
| X. wallichii | FCATAS911 (HT) | China | fruits of Schima wallichii | ON222810 | N/A | MZ695797 | This study |
| X. xanthinovelutina | HAST 553 | Martinique | fruit of Swietenia macrophylla | GU322441 | GQ844828 | GQ495934 | [36] |
3. Results
3.1. Molecular Phylogenetic Analysis
Ten ITS, six RPB2, and seven β-tubulin sequences were generated from this study. The concatenated ITS-RPB2-β-tubulin dataset contained 82 sequences from each gene obtained from 82 samples representing 80 xylariacean taxa and the outgroup (Table 1). The concatenated dataset had an aligned length of 2807 characters, of which 1718 were parsimony-informative. Phylogenetic trees generated from BI and ML analyses of the combined dataset of ITS-RPB2-β-tubulin were highly similar in topology. Only the ML tree is shown in Figure 1 with Bayesian posterior probabilities ≥0.95 and ML bootstrap values ≥ 50% labeled along the branches, while the tree generated by BI analysis is provided in supplementary materials (Figure S1). The ITS dataset contained 68 ITS sequences, representing 62 Xylaria taxa with 382 characters, of which 238 were parsimony-informative, and the ML tree is shown in Figure 2.
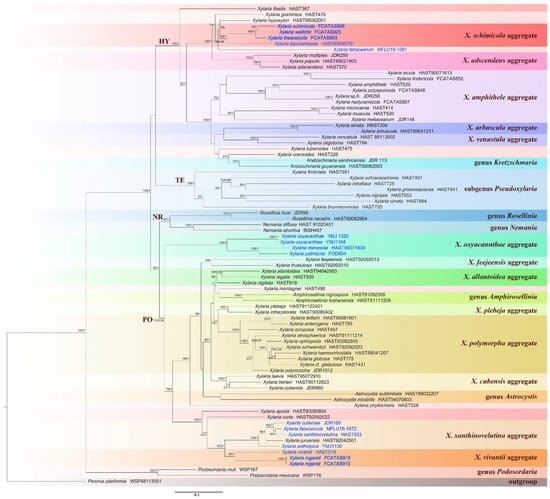
Figure 1.
Phylogenetic tree of Xylaria and related genera based on the multigene alignment of ITS-RPB2-β-tubulin derived from ML. Support values of ML and BI analyses (bootstrap support ≥50%, posterior probability value ≥0.95) are displayed above or below the respective branches (ML/BI). Species of Xylaria associated with fruits and seeds are labeled with blue font.
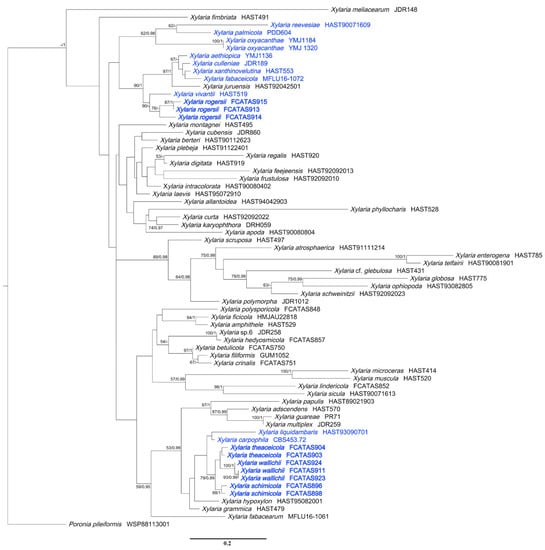
Figure 2.
Phylogenetic tree of Xylaria based on the dataset of ITS sequences derived from ML. Support values of ML and BI analyses (bootstrap support ≥50%, posterior probability value ≥0.95) are displayed above or below the respective branches (ML/BI). Species of Xylaria associated with fruits and seeds are labeled with blue font.
In the Xylariaceae ITS-RPB2-β-tubulin tree (Figure 1), Podosordaria formed a distinct branch separated from Xylaria and five other genera, Amphirosellinia, Astrocystis, Kretzschmaria, Nemania, and Rosellinia. All new Xylaria taxa studied in this paper were grouped together with already described species of Xylaria associated with fallen fruits and seeds in clades HY and PO. These new species are clearly distinct from each other and from previously known species. Xylaria spp. subgenus Pseudoxylaria were grouped in clade TE, and species of the genera Nemania and Rosellinia were clustered in clade NR, in accordance with a previous study [36]. In HY clade, X. schimicola (FCATAS896), X. theaceicola (FCATAS903), and X. wallichii (FCATAS923), the three new species growing on fruits of Schima sp., were grouped together with high bootstrap support (85/1.0) with X. liquidambaris, associated with fruits of Liquidambar formosana in a subclade. In the PO clade, X. rogersii (FCATAS913, FCATAS915) and X. vivantii (HAST 519), two species growing on fruits of Magnolia sp., were grouped together with high bootstrap support values (97/1.0). In the Xylaria ITS tree (Figure 2), X. schimicola (FCATAS896, 898), X. theaceicola (FCATAS903, 904), and X. wallichii (FCATAS911, 923, 924) were grouped together with X. liquidambaris with weak support, whereas X. rogersii (FCATAS913, 914, 915) and X. vivantii (HAST 519) were grouped together with several other fructicolous Xylaria spp. with high support (90/-), with X. rogersii (FCATAS914) clustering at some distance from the other two specimens. Similarly, Xylaria species associated with fruits and seeds were distributed differently in three separate clades of the Xylariaceae ITS-RPB2-β-tubulin tree (Figure 1 and Figure S1) and the Xylaria ITS tree (Figure 2).
3.2. Taxonomy
Xylaria rogersii Hai X. Ma & Yu Li, sp. nov., Figure 3.
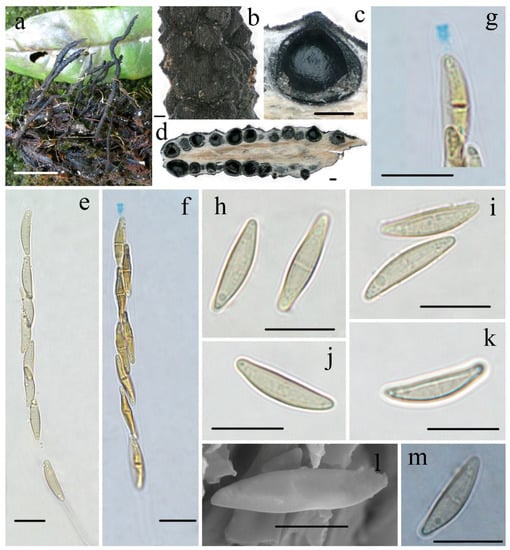
Figure 3.
Xylaria rogersii FCATAS915. (a) Stromata on fallen fruits. (b) Stromatal surface. (c,d) Section through stroma, showing perithecia. (e) Ascus in 1% SDS. (f) Ascus with ascus apical apparatus in Melzer’s reagent. (g) Ascus apical apparatus in Melzer’s reagent. (h) Ascospores with septa in water. (i) Ascospores in water. (j,k) Ascospore in 1% SDS. (l) Ascospore under SEM. (m) Ascospore in Indian ink. Scale bars: (a) = 2 cm; (b–d) = 200 µm; (e–k,m) = 10 µm; (l) = 5 µm.
MycoBank no: MB841144
Etymology—rogersii (Lat.): Referring to American mycologist Prof. Jack D. Rogers, the leading world authority on the Xylariaceae who sadly passed away on 14 June 2021.
Holotype—CHINA. Yunnan Province, Honghe Hani Autonomous Prefectures, Pingbian County, Daweishan Nature Reserve, on fruits of Magnolia sp. (Magnoliaceae), 12 November 2019, Ma Haixia, Col. M31 (FCATAS915, GenBank accession: ITS = MZ648827, RPB2 = MZ707121, β-tubulin = MZ695800).
Teleomorph—Stromata upright or prostrate, solitary or sometimes clustered, unbranched or occasionally branched, with sterile apices, on long tomentose stipes, 5–12 cm total height; fertile parts 2–6 cm high × 1.5–3.0 mm broad, cylindrical, sometimes flattened, overlain with a dark-brown fine-striped outermost layer; stipes 14–60 mm high × 1.0–3.0 mm broad, terete, sometimes contorted, tomentose, with longitudinal wrinkles, arising from swollen base; surface black, roughened with half-exposed perithecial contours and striped outermost layer; interior light-yellow, woody. Perithecia subglobose, 400–600 µm in diam. Ostioles papillate. Asci eight-spored, arranged in uniseriate or partially biseriate manner, cylindrical, long stipitate, (100–)110–130(–140) µm total length, the spore-bearing parts (63–)70–80(–85) µm long × 5.0–6.0 µm broad, the stipes 30–55 µm long, with apical apparatus staining blue in Melzer’s reagent, urn-shaped to tubular, 2.2–2.6 µm high × 1.5–1.9 µm diam. Ascospores subhyaline to light-yellow, unicellular with a septum, inequilaterally naviform-ellipsoid, with tapered to narrowly rounded ends, sometimes slightly pinched, smooth, (13.0–)13.8–15.0(–15.6) × (3.3–)3.6–4.0(–4.4) µm (M = 14.4 × 3.7 µm, Q = 3.9, n = 90/3), without a discernable germ slit, lacking a sheath or appendages visible in Indian ink or 1% SDS.
Additional specimen examined—CHINA. Yunnan Province, Honghe Hani and Yi Autonomous Prefecture, Pingbian County, Daweishan Nature Reserve, on fruits of Magnolia sp. (Magnoliaceae), 12 November 2019, Ma Haixia, Col. M1 (FCATAS913, GenBank accession: ITS = MZ648825, RPB2 = MZ707119, β-tubulin = MZ695799), Col. M5 (FCATAS914, GenBank accession: ITS = MZ648826, RPB2 = MZ707120), Col. Z190, (FCATAS916).
Notes—Xylaria rogersii was found on the fruits of Magnolia in Yunnan Province. It is characterized by long stromata with half-exposed perithecial contours and a dark-brown fine-striped outermost layer, with subhyaline to yellowish and unicellular ascospores that later form a septum. The specimens did not fit the descriptions of any known Xylaria species because of the ascospore septum. Rogers [6] described Xylaria magnoliae var. magnoliae from USA, which has a high specificity to fruits of Magnolia (Magnoliaceae). However, the Chinese collections are different from X. magnoliae var. magnoliae, which has subhyaline to yellowish ascospores lacking a discernable germ slit and long, tomentose stromatal surfaces [5,6]. Unfortunately, DNA sequences of the American material are not available in GenBank for phylogenetic analysis. However, the sequence comparison by Prof. Yu-Ming Ju (Institute of Plant and Microbial Biology, Academia Sinica, Taiwan, China) showed that there are 96.58%, 93.83%, and 95.35%, respectively, percent similarities in ITS, β-tubulin, and RPB2 between the Chinese material (FCATAS915) and X. magnoliae from the USA (J.D. Rogers RC8012, unpublished). Therefore, we described the Chinese material as a new species. The phylogenetic trees showed that X. rogersii and X. vivantii Y.M. Ju, J.D. Rogers, J. Fournier & H.M. Hsieh are sister species, forming a strongly supported branch, although X. vivantii is morphologically distinct due to its dichotomously branched stromata with a dark-brown tomentum, brown to dark-brown ascospores with an oblique germ slit surrounded by a hyaline sheath and bearing non-cellular appendages (Table 2).

Table 2.
A dichotomous key to worldwide species of Xylaria associated with fruits and seeds.
Xylaria schimicola Hai X. Ma & Yu Li, sp. nov., Figure 4.
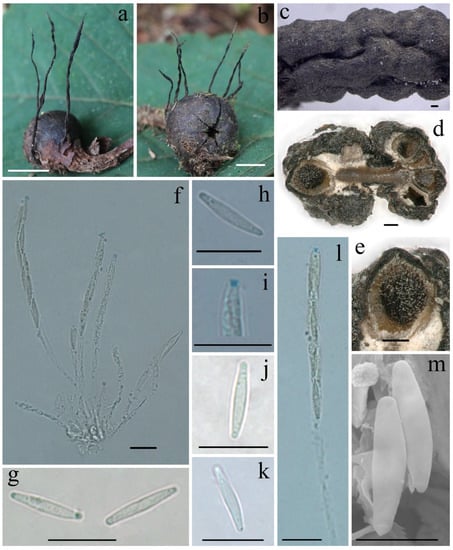
Figure 4.
Xylaria schimicola FCATAS896. (a,b) Stromata on fallen fruits. (c) Stromatal surface. (d,e) Section through stroma, showing perithecia. (f) Asci in Melzer’s reagent. (g) Ascospores in water. (h) Ascospore in Melzer’s reagent. (i) Ascus apical apparatus in Melzer’s reagent. (j) Ascospore in Indian ink. (k) Ascospore in 1% SDS. (l) Ascus with ascus apical apparatus in Melzer’s reagent. (m) Ascospores under SEM. Scale bars: (a,b) = 1 cm; (c–e) = 100 µm; (f–l) = 10 µm; (m) = 5 µm.
MycoBank no: MB840912
Etymology—Schimicola (Lat.): Referring to the host genus Schima, which the fungus inhabits.
Holotype—CHINA. Yunnan Province, Jingdong County, Ailao Mountain Nature Reserve, on fruits of Schima noronhae Reinw. ex Bl. (Theaceae), 15 October 2013, Ma Haixia, Col. 17 (FCATAS896, GenBank accession: ITS = MZ648850, RPB2 = MZ707114, β-tubulin = MZ695787).
Teleomorph—Stromata upright or prostrate, solitary or sometimes clustered, unbranched or occasionally branched from the stipes, (12–)20–50(–65) mm total height, with short to long thin stipes, tomentose when immature; fertile parts 4–26 mm high × 0.6–2.0 mm broad, narrowly fusiform to cylindrical with acute sterile apices up to 5 mm long, at times longitudinal furrowed, strongly nodulose with deep wrinkles isolating small groups of perithecia, more rarely furcate; stipes 7–50 mm high × 0.4–0.6 mm broad, smooth to downy, somewhat flattened, with longitudinal wrinkles, arising from a pannose, slightly enlarged base. Stroma surface smooth at young stage, white to cream-colored, black at mature stage, with inconspicuous to slight perithecial mounds, wrinkled, continuous, glabrous; interior whitish to buff (45) but dark-brown at center, solid, woody. Perithecia subglobose, 200–300 µm in diam. Ostioles faintly pronounced to papillate. Asci eight-spored, usually arranged in partially biseriate manner, cylindrical, long stipitate, (75–)85–95(–100) µm total length, the spore-bearing parts (41–)45–50(–55) µm long × (5–)5.5–6.5(–7.5) µm broad, the stipes 30–50 µm long, with apical apparatus staining blue in Melzer’s reagent, inverted hat-shaped to more or less rectangular, 0.7–1.3 µm high × 0.7–1.1 µm diam. Ascospores nearly hyaline to faintly light-yellow, unicellular, inequilaterally naviform-ellipsoid, with narrowly rounded ends, smooth, (9.5–)10.5–12.0(–13.0) × (1.6–)1.9–2.5(–3.0) µm (M = 11.2 × 2.2 µm, Q = 5.1, n = 60/2), lacking a discernable germ slit, no sheath or appendages visible in Indian ink or 1% SDS.
Additionalspecimen examined—CHINA. Sichuan Province, Mianning County, Lingshan Temple, on fruits of Schima noronhae, 12 July 2013, Ma Haixia Col. 259 (FCATAS898, GenBank accession: ITS = MZ648851).
Notes—Xylaria schimicola was found on the fruits of Schima noronhae in the subtropics of Southwestern China, which did not fit the descriptions of any species known of genus Xylaria [5,6,7,12]. Xylaria schimicola is characterized by nearly hyaline to faintly light-yellow ascospores lacking a germ slit. The Chinese collections somewhat resemble X. oxyacanthae Tul. & Tul., X. psidii J.D. Rogers et Hemmes, and X. palmicola with winter-season stromatal morphology, but the ascospores are distinctly different [5]. In the phylogenetic trees, Xylaria schimicola formed a sister lineage with X. wallichii and X. theaceicola, both fruiting on pericarps of Schima.
Xylaria theaceicola Hai X. Ma & Yu Li, sp. nov., Figure 5.

Figure 5.
Xylaria theaceicola FCATAS903. (a,b) Stromata on fallen fruits. (c) Stromatal surface. (d,e) Section through stroma, showing perithecia. (f) Asci in Melzer’s reagent. (g) Ascus apical apparatus in Melzer’s reagent. (h) Ascospore under SEM. (i,n) Asci and ascus apical apparatus in Melzer’s reagent. (j) Ascospores in water. (k) Ascospore in Indian ink. (l) Ascospore with germ slit in Melzer’s reagent. (m) Ascospores in 1% SDS. Scale bars: (a,b) = 1.5 cm; (c–e) = 200 µm; (f) = 20 µm; (g,i–n) = 10 µm; (h) = 5 µm.
MycoBank no: MB840914
Etymology—theaceicola (Lat.): Referring to the host family Theaceae, which the fungus inhabits.
Holotype—CHINA. Yunnan Province, Wenshan Zhuang and Miao Autonomous Prefecture, Wenshan County, Xiaoqiaogou Nature Reserve, on fruits of Schima villosa Hu (Theaceae), 16 November 2019, Ma Haixia, Col. M22 (FCATAS903, GenBank accession: ITS = MZ648848, RPB2 = MZ707115, β-tubulin = MZ695788).
Teleomorph—Stromata upright or prostrate, solitary or sometimes clustered, unbranched or occasionally branched, with acute sterile apices, on a long, thin, ill-defined stipe, 2–8 cm total height; fertile parts 0.8–25 mm long × 0.5–1.5 mm broad, thin and cylindrical, usually crowded with perithecial contours slightly exposed, and occasionally with scattered perithecia, sometimes longitudinally furrowed, slightly nodulose with wrinkles isolating small groups of perithecia, more rarely furcate; stipes 1.2–6.5 cm high × 0.4–2 mm broad, smooth, with longitudinally wrinkled, arising from a pannose, slightly enlarged base; surface smooth at young stage, mature stromata black, with inconspicuous to slightly conspicuous perithecial mounds, overlain with a brown striped outermost layer; interior white, with a dark-brown to black circle, solid, woody. Perithecia subglobose, 300–450 µm in diam. Ostioles conical, papillate. Asci eight-spored, arranged in partially biseriate manner, cylindrical, long stipitate, (85–)92–105(–110) µm total length, the spore-bearing parts (52–)55–65(–70) µm long × (5.3–)5.5–6.5(–7.1) µm broad, the stipes are 25–53 µm long, with apical apparatus staining blue in Melzer’s reagent, inverted hat-shaped to tubular, 1.0–1.5 µm high × 0.8–1.2 µm diam. Ascospores faintly light-yellowish, nearly hyaline when immature, unicellular, ellipsoid, or navicular, arc-shaped, inequilateral, with broadly rounded ends, slightly pinched at the end, smooth, (10.1–)10.7–11.6(–12) × (2.0–)2.3–2.7(–2.9) µm (M = 11.1 × 2.5 µm, Q = 4.4, n = 60/2), with a straight germ slit along the spore length, lacking a slimy sheath visible in Indian ink or 1% SDS.
Additional specimen examined—CHINA. Yunnan Province, Wenshan Zhuang and Miao Autonomous Prefecture, Wenshan County, Xiaoqiaogou Nature Reserve, on fruits of Schima villosa (Theaceae), 16 November 2019, Ma Haixia, Col. Z193 (FCATAS904 GenBank accession: ITS = MZ648849).
Notes—Xylaria theaceicola is characterized by long and usually unbranched stromata overlain with a brown, striped outermost layer, conical, papillate perithecial ostioles, faintly light-yellowish to nearly hyaline ascospores, with conspicuous straight germ slits, and growing on fruits of S. villosa (Theaceae). Xylaria schimicola, fruiting on pericarps of S. noronhae, is similar to X. theaceicola in that they share stromatal morphology, but differs on account of having ellipsoid ascospores lacking a discernable germination slit. The species also somewhat resembles X. oxyacanthae, X. psidii, and X. palmicola in stromatal morphology, but the ascospores of these species are distinctly different [5]. In the phylogenetic tree, X. theaceicola is a sister species to X. schimicola, but the relationship between the two fructicolous species of Schima is not strongly supported.
Xylaria wallichii Hai X. Ma & Yu Li, sp. nov., Figure 6.
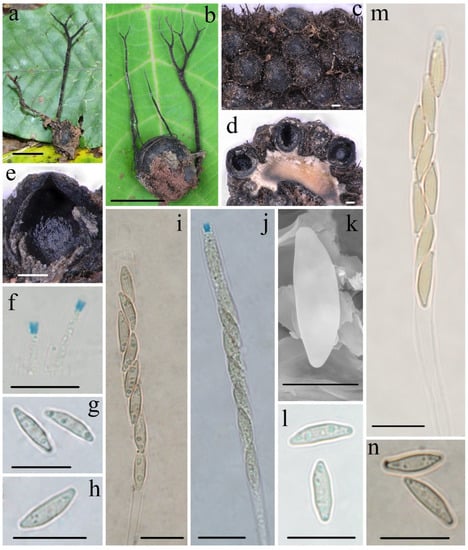
Figure 6.
Xylaria wallichii FCATAS911. (a,b) Stromata on fallen fruits. (c) Stromatal surface. (d,e) Section through stroma, showing perithecia. (f) Apical apparatus of asci in Melzer’s reagent. (g,h) Ascospores in water. (i) Ascus in Indian ink. (j,m) Asci with ascus apical apparatus in Melzer’s reagent. (k) Ascospore under SEM. (l) Ascospores in 1% SDS. (n) Ascospores in Indian ink. Scale bars: (a,b) = 1.5 cm; (c–e) = 100 µm; (f–j,l–n) = 10 µm; (k) = 5 µm.
MycoBank no: MB840915
Etymology—wallichii (Lat.): Referring to the specific epithet of its host, which the fungus inhabits.
Holotype—CHINA. Yunnan Province, Jinghong City, Dadugang Town, on fruits of Schima wallichii (DC.) Choisy (Theaceae), 21 January 2015, Ma Haixia Col. 247 (FCATAS911, GenBank accession: ITS = ON222810, β-tubulin = MZ695797).
Teleomorph—Stromata upright or prostrate, solitary to sometimes densely clustered, often dichotomously branched several times, or infrequently unbranched, 1.5–10 cm total height, long stipitate; fertile parts 2–20 mm high × 1.0–2.0 mm broad, narrowly fusiform to cylindrical, often flattened, with acute sterile apices up to 5 mm long, strongly nodulose, mostly tomentose; stipes 13–80 mm high × 0.5–2.0 mm broad, terete to rarely flattened, often ill-defined, black-brown to black, conspicuously tomentose, arising from a slightly enlarged pannose base; surface roughened with perithecial mounds and tomentose except for stromatal apices, black; interior light-yellow to light-brown, black-brown in a circle, solid, woody. Perithecia subglobose, 300–400 µm in diam. Ostioles conical, papillate. Asci eight-spored, usually arranged in uniseriate manner, sometimes in partially biseriate manner, cylindrical, long stipitate, (75–)85–105(–115) µm total length, the spore-bearing parts (50–)55–63(–68) µm long × (4.1–)4.6–5.8(–6.2) µm broad, the stipes 25–50 µm long, with apical apparatus staining blue in Melzer’s reagent, inverted hat-shaped to more or less rectangular, 1.3–2.1 µm high × 1.1–1.7 µm diam. Ascospores nearly hyaline to light-yellow, unicellular, inequilaterally naviform-ellipsoid, with tapered to narrowly rounded ends, sometimes pinched on one end, smooth, (8.2–)8.8–10.2(–11.3) × (2.4–)2.6–3.0(–3.2) µm (M = 9.3 × 2.8 µm, Q = 3.3, n = 90/3), without a discernable germ slit, lacking sheath or appendages visible in Indian ink or 1% SDS.
Additional specimen examined—CHINA. Yunnan Province, Jinghong City, Dadugang Town, on fruits of S. wallichii, 21 January 2015, Ma Haixia, Col. 229 (FCATAS909), Col. 312 (FCATAS912); Dadugang Town, Guanping Village, on fruits of S. wallichii, 21 January 2015, Ma Haixia, Col. 234 (FCATAS910); Yunnan Province, Pu’er City, Taiyanghe National Forest Park, on fruits of S. wallichii, 18 October 2013, Ma Haixia, Col. 18 (FCATAS923, GenBank accession: ITS = MZ648861, RPB2 = MZ707118, β-tubulin = MZ695793), Col. 30 (FCATAS924, GenBank accession: ITS = MZ648862, β-tubulin = MZ695794).
Notes—So far, Xylaria wallichii has only been found on fruits of S. wallichii (Theaceae) from the tropics and the transitional zone from the subtropics to tropics. This species is characterized by almost hyaline ascospores that lack a germ slit, a sheath, or appendages, and with stromata often dichotomously branched several times covered by conspicuously tomentose and perithecial mounds. The three species of the present study, X. wallichii, X. schimicola, and X. theaceicola, found on fruits of the genus Schima, have similar hyaline or nearly hyaline ascospores and form a common clade in the phylogenetic trees. However, they are clearly distinguishable based on the branching of stromata, presence or absence of germ slits, and the shape and size of ascospores. Xylaria magnoliae var. magnoliae also has pale-colored ascospores without a discernable germ slit and sheath, but differs in that is has larger ascospores (12.5–)13.5–15(–16) × (2.5–)3–3.5(–4) µm (M = 14.1 × 3.2 µm), unbranched or occasionally branched stromata, and grows on pericarps of Magnolia species (Magnoliaceae) [5]. Three other taxa, X. apeibae Mont., X. xanthinovelutina, and X. reevesiae Y.M. Ju, J.D. Rogers & H.M. Hsieh are somewhat similar to X. wallichii in stromatal morphology, but differ in their ascospores [5]. Xylaria apeibae has light-brown and larger ascospores (9.5–)10–12(–13) × (3–)3.5–4(–4.5) µm (M = 11.0 × 3.7 µm), with a straight germ slit and grows on fruits of Apeiba species (Tiliaceae) [13]. Xylaria xanthinovelutina has brown and slightly larger ascospores (9–)9.5–11(–12) × (3.5–)4–4.5(–5) µm (M = 10.3 × 4.0 µm), with a straight germ slit, a hyaline sheath, and non-cellular appendages, and grows on leguminous pods. Xylaria reevesiae has brown and slightly larger ascospores (8.5–)9–10.5(–11) × (4–)4.5–5.5(–6) µm (M = 9.7 × 5.0 µm), with a straight germ slit, and grows on fruits of Reevesia formosana (Sterculiaceae) [5]. Phylogenetically, X. wallichii is distinct from all the Xylaria species mentioned.
4. Discussion and Conclusions
Previous investigations have discovered several new species in Southwest China [43,44], and the current study confirmed the unexplored species diversity of the area. Here, four pale-spored Xylaria species from Southwest China were introduced as new taxa based on morphological characteristics, host association, and phylogenetic analyses. Combined ITS, RPB2, and β-tubulin sequence data of a representative sample of the entire genus showed that the four new species are distributed in two distinct lineages of the phylogenetic tree. Considering all known species associated with fallen fruits and seeds, fructicolous taxa formed clusters in three different clades. This suggests that the fructicolous life style of Xylaria species has evolved independently several times within the genus Xylaria. Moreover, the texture of the fruits or seeds may have promoted or influenced speciation, as reflected by the phylogenetic relationships of Xylaria species associated with fallen fruits and seeds. To further test this hypothesis, it is crucial to carry out additional studies and confirm the phylogenetic position of all Xylaria species associated with fallen fruits and seeds.
Many xylariacean endophytes are a source of bioactive secondary metabolites with antibacterial, antioxidative, anti-carcinogenic, and other properties [45,46]. Unfortunately, we could not obtain cultures from these isolates, and thus, they were not accessible for phylogenetic studies. Future research should include additional specimens of Xylaria from different hosts and substrates using an integrative approach including morphological, chemotaxonomic, and phylogenetic data.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology11060885/s1, Figure S1: Phylogenetic tree of Xylaria and related genera based on the multigene alignment of ITS-RPB2-β-tubulin in the Bayesian tree. Bayesian posterior probabilities (PP) ≥ 0.95 are labeled above or below the respective branches. Species in bold were sequenced in this study.
Author Contributions
Conceptualization and supervision, H.M.; Resources, H.M., Z.S., X.P. and Z.Y.; Investigation, methodology and data curation, Z.S. and X.P.; Formal analysis, Z.Q.; Revised the language of the text, Y.L.; Writing—review and editing, H.M. and A.Z.; Funding acquisition, H.M. and A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (no. 31770023, 31972848), Key Research and Development Program of Hainan (ZDYF2020062), and Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No. 1630012022009, 1630052022003, 1630052022042).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All newly generated sequences were deposited in GenBank (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 7 March 2022; Table 1). Data for all new taxa were deposited in MycoBank (https://www.mycobank.org/, accessed on 5 March 2022; MycoBank identifiers follow new taxa).
Acknowledgments
We express our gratitude to Yu-Ming Ju (Institute of Plant and Microbial Biology, Academia Sinica, Taiwan, China) for suggestions on some species in the study. Shoubai Liu (Hainan University, Haikou, China) helped us to identify the host.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008; p. 771. [Google Scholar]
- Index Fungorum. Available online: http://www.indexfungorum.org/names/names.asp (accessed on 1 March 2022).
- Dennis, R.W.G. Some Xylarias of tropical America. Kew Bull. 1956, 11, 401–444. [Google Scholar] [CrossRef]
- San Martín, F.; Rogers, J.D. A preliminary account of Xylaria of Mexico. Mycotaxon 1989, 34, 283–373. [Google Scholar]
- Ju, Y.M.; Rogers, J.D.; Hsieh, H.M. Xylaria species associated with fallen fruits and seeds. Mycologia 2018, 110, 726–749. [Google Scholar] [CrossRef]
- Rogers, J.D. Xylaria magnoliae sp. nov. and comments on several other fruit-inhabiting species. Can. J. Bot. 1979, 57, 941–945. [Google Scholar] [CrossRef]
- Læssøe, T.; Lodge, D.J. Three host-specific Xylaria species. Mycologia 1994, 86, 436–446. [Google Scholar] [CrossRef]
- Perera, R.H.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Jones, E.B.G.; McKenzie, E.H.C.; Stadler, M.; Lee, H.B.; Samarakoon, M.C.; Ekanayaka, A.H.; Camporesi, E.; et al. Fungi on wild seeds and fruits. Mycosphere 2020, 11, 2108–2480. [Google Scholar] [CrossRef]
- Rogers, J.D.; San Martín, F.; Ju, Y.M. A reassessment of the Xylaria on Liquidambar fruits and two new taxa on Magnolia fruits. Sydowia 2002, 54, 91–97. [Google Scholar]
- Rogers, J.D.; Yeomans, R.; Adams, M.J. The relationship of Xylaria oxyacanthae to seeds of Crataegus monogyna. N. Amer. Fungi 2008, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Fournier, J.; Ju, Y.M.; Hsieh, H.M.; Lindermann, U. Xylaria aethiopica sp. nov.—A new pod-inhabiting species of Xylaria (Xylariaceae) from Ethiopia. Ascomycete.org 2018, 10, 209–215. [Google Scholar]
- Husbands, D.R.; Urbina, H.; Lewis, S.M.; Aime, M.C. Xylaria karyophthora: A new seed-inhabiting fungus of Greenheart from Guyana. Mycologia 2018, 110, 434–447. [Google Scholar] [CrossRef]
- Teng, S.Q. Fungi of China; Science Press: Beijing, China, 1963; p. 808. [Google Scholar]
- Tai, F.L. Sylloge fungorum Sinicorum; Science Press: Beijing, China, 1979; p. 1527. [Google Scholar]
- Li, Y.L.; Li, H.J. A novel species of Xylaria. J. Nanjing Agric. Univ. 1994, 17, 145–147. [Google Scholar]
- Abe, Y.; Liu, Z. An annotated list of xylariaceous and diatrypaceous fungi collected from Mt. Fengyangshan and Mt. Baishanzu, Zhejiang Prov. in East China. Bul. Natl. Sci. Mus. Ser. B Bot. 1995, 21, 75–86. [Google Scholar]
- Xu, A.S. A new species of Xylaria. Mycosystema 1999, 18, 137–140. [Google Scholar]
- Zhu, Y.F.; Guo, L. Xylaria hainanensis sp. nov. (Xylariaceae) from China. Mycosystema 2011, 30, 526–528. [Google Scholar]
- Ma, H.X.; Vasilyeva, L.; Li, Y. A new species of Xylaria from China. Mycotaxon 2011, 116, 151–155. [Google Scholar] [CrossRef]
- Ma, H.X.; Vasilyeva, L.; Li, Y. The genus Xylaria (Xylariaceae) in the south of China—6. A new Xylaria species based on morphological and molecular characters. Phytotaxa 2013, 147, 48–54. [Google Scholar] [CrossRef]
- Ma, H.X.; Li, Y. Xylaria curta and X. partita (Xylariales) from Yunnan province. Austrian J. Mycol. 2017, 26, 99–105. [Google Scholar]
- Ma, H.X.; Li, Y. Xylaria crinalis and X. betulicola from China—Two new species with thread-like stromata. Sydowia 2018, 70, 37–49. [Google Scholar]
- Ma, H.X.; Qu, Z.; Peng, M.K.; Li, Y. Two penzigioid Xylaria species described from China based on morphological and molecular characters. Phytotaxa 2020, 436, 36–44. [Google Scholar] [CrossRef]
- Huang, G.; Guo, L.; Liu, N. Two new species of Xylaria and X. diminuta new to China. Mycotaxon 2014, 129, 149–152. [Google Scholar] [CrossRef]
- Rayner, R.W. A Mycological Color Chart; Cmi. & British Mycological Society Kew: London, UK, 1970. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Shinsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.M.; Ju, Y.M.; Rogers, J.D. Molecular phylogeny of Hypoxylon and related genera. Mycologia 2005, 97, 844–865. [Google Scholar] [CrossRef] [PubMed]
- Wangsawat, N.; Ju, Y.M.; Phosri, C.; Whalley, A.J.S.; Suwannasai, N. Twelve new taxa of Xylaria associated with termite nests and soil from Northeast Thailand. Biology 2021, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- MAFFT V.7 Online Server. Available online: https://mafft.cbrc.jp/alignment/server/ (accessed on 12 March 2022).
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A. FigTree v1.4.2, a Graphical Viewer of Phylogenetic Trees. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 2 March 2022).
- Hsieh, H.M.; Lin, C.R.; Fang, M.J.; Rogers, J.D.; Fournier, J.; Lechat, C.; Ju, Y.M. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phylogenet. Evol. 2010, 54, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.M.; Rogers, J.D.; Hsieh, H.M. Amphirosellinia gen. nov. and a new species of Entoleuca. Mycologia 2004, 96, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.M.; Hsieh, H.M. Xylaria species associated with nests of Odontotermes formosanus in Taiwan. Mycologia 2007, 99, 936–957. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; De Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdon fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; Song, Z.K.; Qu, Z.; Liu, T.D.; Ma, H.X. Three new Xylaria species (Xylariaceae, Xylariales) on fallen leaves from Hainan Tropical Rainforest National Park. Mycokeys 2022, 86, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.A.; Zare, R.; Khodaparast, S.A.; Elahinia, S.A. A new Xylaria species from Iran. Mycologia Iranica 2015, 2, 1–10. [Google Scholar]
- Peláez, F.; González, V.; Platas, G.; Sánchez-Ballesteros, J.; Rubio, V. Molecular phylogenetic studies within the Xylariaceae based on ribosomal DNA sequences. Fungal Divers. 2008, 31, 111–134. [Google Scholar]
- Dai, Y.C.; Yang, Z.L.; Cui, B.K.; Wu, G.; Yuan, H.S.; Zhou, L.W.; He, S.H.; Ge, Z.W.; Wu, F.; Wei, Y.L.; et al. Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 2021, 40, 770–805. [Google Scholar]
- Wang, K.; Chen, S.L.; Dai, Y.C.; Jia, Z.F.; Li, T.H.; Liu, T.Z.; Phurbu, D.; Mamut, R.; Sun, G.Y.; Bau, T.; et al. Overview of China’ s nomenclature novelties of fungi in the new century (2000–2020). Mycosystema 2021, 40, 822–833. [Google Scholar]
- Fan, N.W.; Chang, H.S.; Cheng, M.J.; Hsieh, S.Y.; Liu, T.W.; Yuan, G.F.; Chen, I.S. Secondary metabolites from the endophytic fungus Xylaria cubensis. Helv. Chim. Acta 2014, 97, 1689–1699. [Google Scholar] [CrossRef]
- Biasetto, C.R.; Somensi, A.; Abdalla, V.C.P.; Abreu, L.M.; Gualtieri, S.C.J.; Pfenning, L.H.; Bolzani, V.S.; Araujo, A.R. Phytotoxic constituents from endophytic fungus Xylaria cubensis associated with Eugenia brasiliensis. Quim. Nova 2019, 42, 485–488. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
