Biotransformation of Androstenedione by Filamentous Fungi Isolated from Cultural Heritage Sites in the State Tretyakov Gallery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
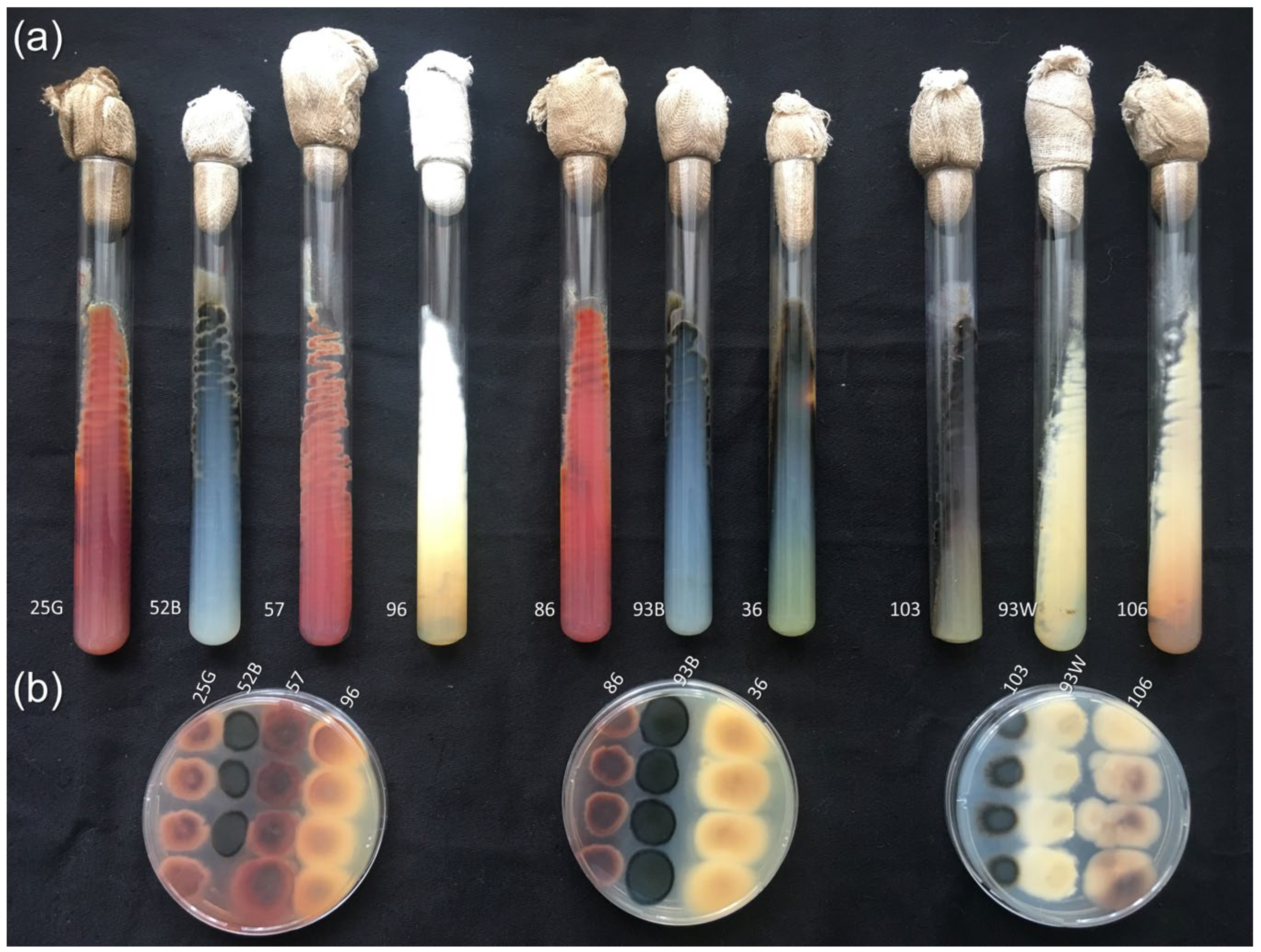
2.2. Microorganism Strains Used in the Work
2.3. Cultivation of Fungal Strains
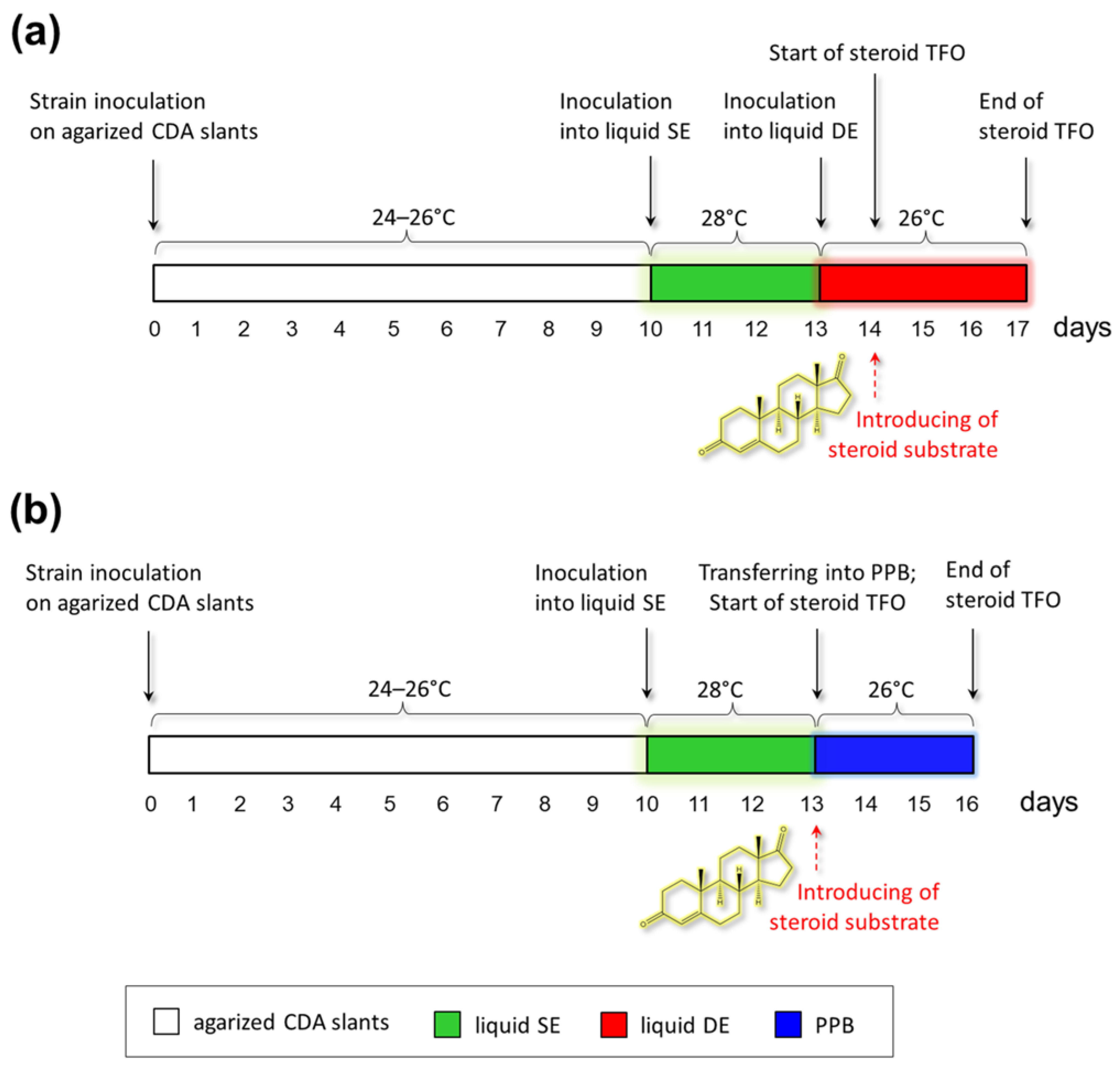
2.4. Preparation of Fungal Strains for Steroid Transformation
2.5. Steroid Biotransformation
2.6. Sample Preparation
2.7. Thin Layer Chromatography (TLC)
2.8. Gas Chromatography/Mass Spectrometry (GC/MS)
3. Results
3.1. Transformation of AD by Fungal Strains
3.1.1. Transformation of AD by Fungal Strains during Cultivation on DE Medium
3.1.2. Transformation of AD by Fungal Strains in PPB
3.2. Transformation of AD by Various Systematic Groups of Mold Fungi
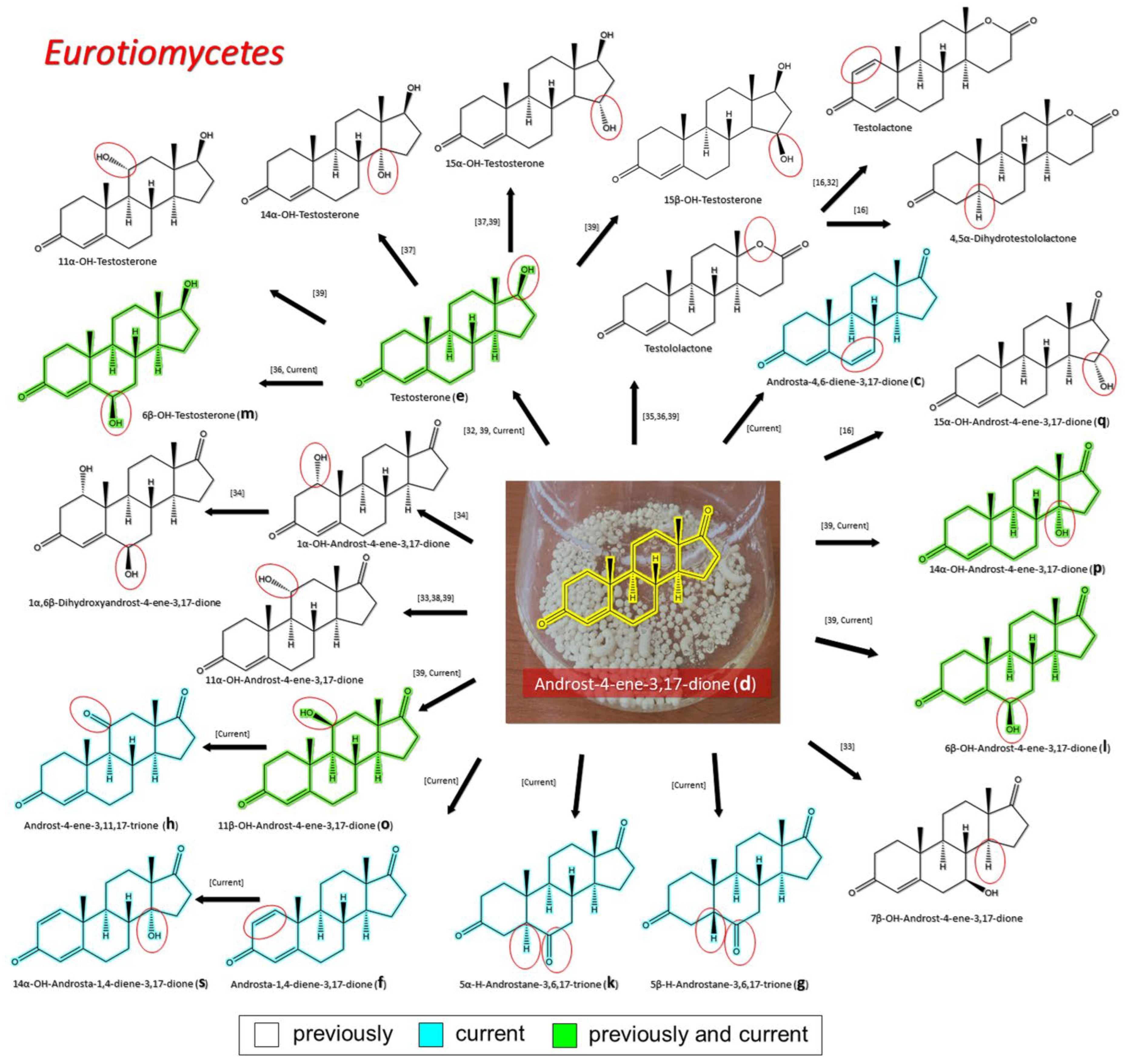
3.2.1. Transformation of AD by Representatives of Eurotiomycetes
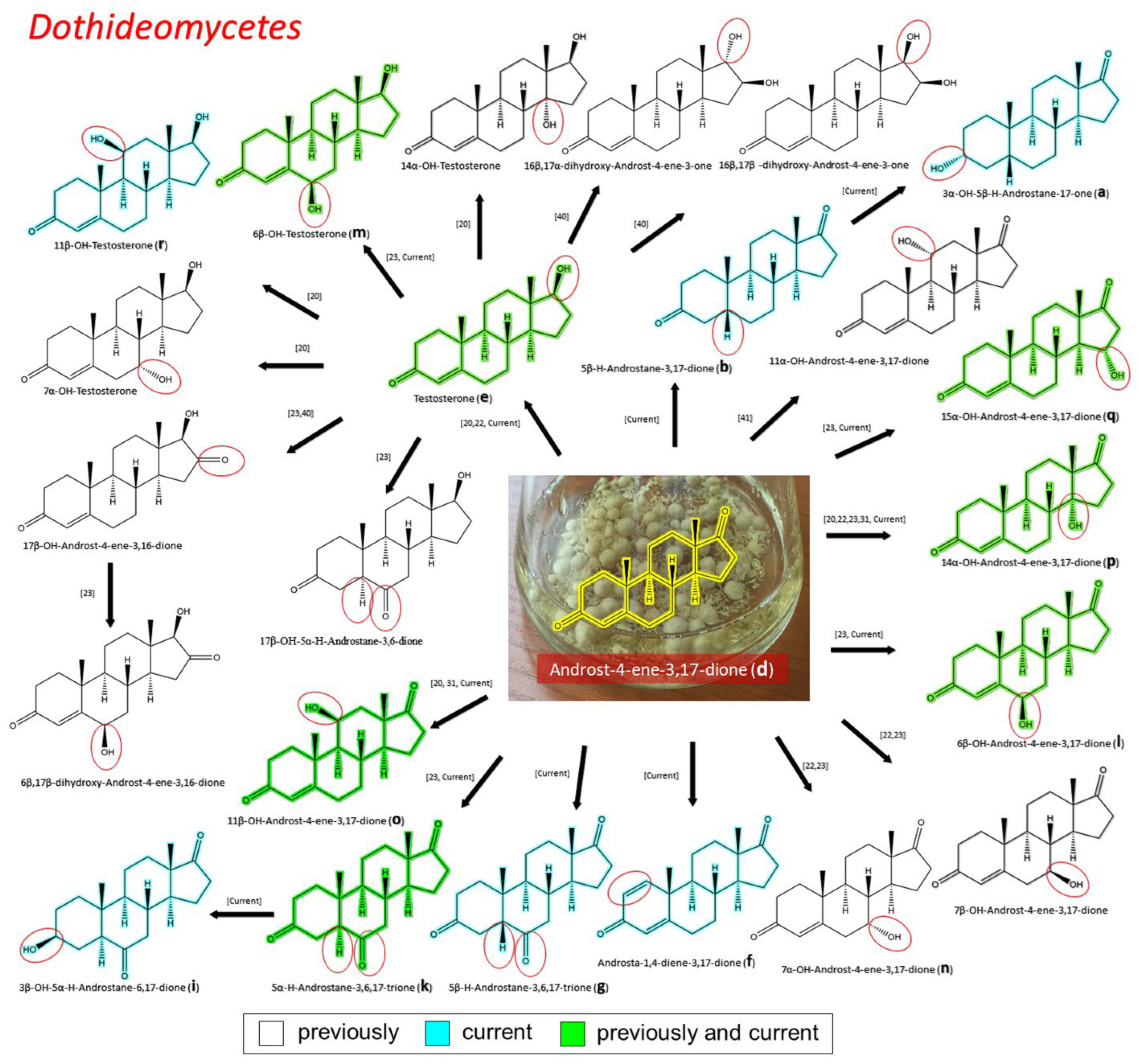
3.2.2. Transformation of AD by Representatives of Dothideomycetes
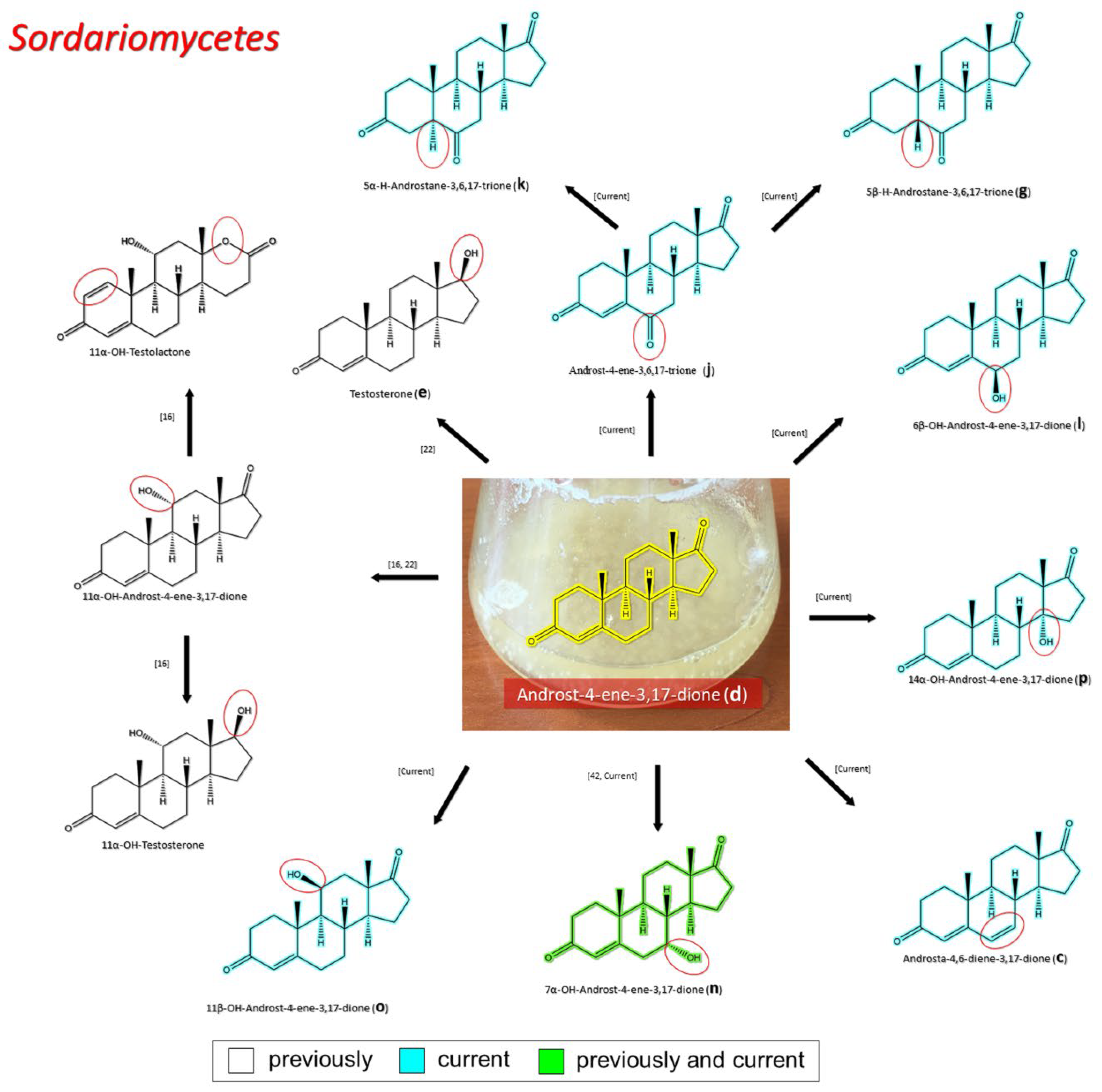
3.2.3. Transformation of AD by Representatives of Sordariomycetes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Androstenedione (Androst-4-ene-3,17-dione) |
| ADD | Androstadienedione (Androsta-1,4-diene-3,17-dione) |
| CDA | Czapek-Dox agar |
| CYP450 | Cytochromes P450 |
| GC/MS | Gas chromatography/mass spectrometry |
| DE | Defined (medium) |
| PPB | Potassium phosphate buffer |
| SE | Seed (medium) |
| STG | State Tretyakov Gallery (museum, Moscow, Russia) |
| TLC | Thin layer chromatography |
| TM1 | Transformation method 1 |
| TM2 | Transformation method 2 |
| TS | Testosterone (17β-Hydroxyandrost-4-en-3-one) |
References
- Fernández-Cabezón, L.; Galán, B.; García, J.L. New insights on steroid biotechnology. Front. Microbiol. 2018, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Cano-Flores, A.; Gómez, J.; Ramos, R. Biotransformation of Steroids Using Different Microorganisms. In Chemistry and Biological Activity of Steroids; IntechOpen: London, UK, 2020. [Google Scholar]
- Torgov, I.V. Progress in the total synthesis of steroids. Pure Appl. Chem. 1963, 6, 525–544. [Google Scholar] [CrossRef]
- Carballeira, J.D.; Quezada, M.A.; Hoyos, P.; Simeó, Y.; Hernaiz, M.J.; Alcantara, A.R.; Sinisterra, J.V. Microbial cells as catalysts for stereoselective red-ox reactions. Biotechnol. Adv. 2009, 27, 686–714. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Cruz, A.; Angelova, B.; Pinheiro, H.M.; Cabral, J.M.S. Microbial conversion of steroid compounds: Recent developments. Enzym. Microb. Technol. 2003, 32, 688–705. [Google Scholar] [CrossRef]
- Lu, W.; Feng, J.; Chen, X.; Bao, Y.J.; Wang, Y.; Wu, Q.; Ma, Y.; Zhu, D. Distinct regioselectivity of fungal P450 enzymes for steroidal hydroxylation. Appl. Environ. Microbiol. 2019, 85, e01182-19. [Google Scholar] [CrossRef] [PubMed]
- Bensasson, C.S.; Hanson, J.R.; Le Huerou, Y. The microbiological hydroxylation of 3α,5-cycloandrostanes by Cephalosporium aphidicola. Phytochemistry 1999, 52, 1279–1282. [Google Scholar] [CrossRef]
- Donova, M.V. Transformation of steroids by actinobacteria: A review. Appl. Biochem. Microbiol. 2007, 43, 1–14. [Google Scholar] [CrossRef]
- Jaderets, V.V.; Andrjushina, V.A.; Vojshvillo, N.E.; Dvojnikov, P.S.; Druzhinina, A.V.; Stytsenko, T.S.; Zejnalov, O.A.; Skrjabin, K.G. Method for Preparation 14α-Hydroxyderivatives of Δ4-3,17-Diketo-Androstene. Patent RU2407800C1, C12P33/00, C12P33/06, C12N1/14, 27 December 2010. [Google Scholar]
- Zhgun, A.A. Random Mutagenesis of Filamentous Fungi Stains for High-Yield Production of Secondary Metabolites: The Role of Polyamines. In Genotoxicity and Mutagenicity—Mechanisms and Test Methods, Chapter 2; Soloneski, S., Larramendy, M.L., Eds.; IntechOpen: London, UK, 2021; pp. 25–41. ISBN 978-1-83880-041-3. [Google Scholar]
- Avalos, J.; Limón, M.C. Fungal Secondary Metabolism. Encyclopedia 2022, 2, 1–13. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Ortega, H.E.; Torres-Mendoza, D.; Caballero, E.Z.; Cubilla-Rios, L. Structurally Uncommon Secondary Metabolites Derived from Endophytic Fungi. J. Fungi 2021, 7, 570. [Google Scholar] [CrossRef]
- Zheng, R.; Li, S.; Zhang, X.; Zhao, C. Biological Activities of Some New Secondary Metabolites Isolated from Endophytic Fungi: A Review Study. Int. J. Mol. Sci. 2021, 22, 959. [Google Scholar] [CrossRef] [PubMed]
- Kristan, K.; Rižner, T.L. Steroid-transforming enzymes in fungi. J. Steroid Biochem. Mol. Biol. 2012, 129, 79–91. [Google Scholar] [PubMed]
- Nassiri-Koopaei, N.; Faramarzi, M.A. Recent developments in the fungal transformation of steroids. Biocatal. Biotransform. 2015, 33, 1–28. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Choi, J.; Ahn, K.; Park, B.; Park, J.; Kang, S.; Lee, Y.H. Fungal cytochrome P450 database. BMC Genom. 2008, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, P.; Hur, J.S.; Yun, H. Versatile biocatalysis of fungal cytochrome P450 monooxygenases. Microb. Cell Fact. 2016, 15, 125. [Google Scholar] [CrossRef]
- Hüttel, W.; Hoffmeister, D. Fungal Biotransformations in Pharmaceutical Sciences. In Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2011; pp. 293–317. [Google Scholar]
- Karpova, N.V.; Andryushina, V.A.; Stytsenko, T.S.; Druzhinina, A.V.; Feofanova, T.D.; Kurakov, A.V. A search for microscopic fungi with directed hydroxylase activity for the synthesis of steroid drugs. Appl. Biochem. Microbiol. 2016, 52, 316–323. [Google Scholar] [CrossRef]
- Kollerov, V.V.; Shutov, A.A.; Kazantsev, A.V.; Donova, M.V. Biocatalytic modifications of pregnenolone by selected filamentous fungi. Biocatal. Biotransform. 2019, 37, 319–330. [Google Scholar] [CrossRef]
- Kollerov, V.; Shutov, A.; Kazantsev, A.; Donova, M. Biotransformation of androstenedione and androstadienedione by selected Ascomycota and Zygomycota fungal strains. Phytochemistry 2020, 169, 112160. [Google Scholar] [CrossRef]
- Yildirim, K.; Kuru, A.; Küçükbaşol, E. Microbial transformation of androstenedione by Cladosporium sphaerospermum and Ulocladium chartarum. Biocatal. Biotransform. 2020, 38, 7–14. [Google Scholar] [CrossRef]
- Zhgun, A.; Avdanina, D.; Shumikhin, K.; Simonenko, N.; Lyubavskaya, E.; Volkov, I.; Ivanov, V. Detection of potential biodeterioration risks for tempera painting in 16th century exhibits from State Tretyakov Gallery. PLoS ONE 2020, 15, e0230591. [Google Scholar] [CrossRef]
- Alexandrova, L.A.; Shevchenko, O.V.; Jasko, M.V.; Solyev, P.N.; Karpenko, I.L.; Negrya, S.D.; Efremenkova, O.V.; Vasilieva, B.F.; Efimenko, T.A.; Avdanina, D.A.; et al. 3′-Amino modifications enhance the antifungal properties of N4-alkyl-5-methylcytidines for potential biocides. New J. Chem. 2022, 46, 5614–5626. [Google Scholar] [CrossRef]
- Alexandrova, L.A.; Jasko, M.V.; Negrya, S.D.; Solyev, P.N.; Shevchenko, O.V.; Solodinin, A.P.; Kolonitskaya, D.P.; Karpenko, I.L.; Efremenkova, O.V.; Glukhova, A.A.; et al. Discovery of novel N4-alkylcytidines as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 215, 113212. [Google Scholar] [CrossRef] [PubMed]
- Zhgun, A.A.; Avdanina, D.A.; Shagdarova, B.T.; Troyan, E.V.; Nuraeva, G.K.; Potapov, M.P.; Il’ina, A.V.; Shitov, M.V.; Varlamov, V.P. Search for Efficient Chitosan-Based Fungicides to Protect the 15th–16th Centuries Tempera Painting in Exhibits from the State Tretyakov Gallery. Microbiology 2020, 89, 750–755. [Google Scholar] [CrossRef]
- Masschelein-Kleiner, L. Ancient Binding Media, Varnishes and Adhesives, 2nd ed.; Lawrence, T., Ed.; ICCROM: Rome, Italy, 1995; ISBN 92-9077-119-4. [Google Scholar]
- Stadelman, W.J.; Cotterill, O.J. (Eds.) Egg Science and Technology, 4th ed.; Routledge: New York, NY, USA, 1995; ISBN 978-1560228554. [Google Scholar]
- van den Brink, O.F.; Ferreira, E.S.B.; van der Horst, J.; Boon, J.J. A direct temperature-resolved tandem mass spectrometry study of cholesterol oxidation products in light-aged egg tempera paints with examples from works of art. Int. J. Mass Spectrom. 2009, 284, 12–21. [Google Scholar] [CrossRef]
- Wallace, W.E.; Ji, W.; Tchekhovskoi, D.V.; Phinney, K.W.; Stein, S.E. Mass Spectral Library Quality Assurance by Inter-Library Comparison. J. Am. Soc. Mass Spectrom. 2017, 28, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Andryushina, V.A.; Voishvillo, N.E.; Druzhinina, A.V.; Stytsenko, T.S.; Yaderets, V.V.; Petrosyan, M.A.; Zeinalov, O.A. 14α-Hydroxylation of steroids by mycelium of the mold fungus Curvularia lunata (VKPM F-981) to produce precursors for synthesizing new steroidal drugs. Pharm. Chem. J. 2013, 47, 103–108. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Yazdi, M.T.; Amini, M.; Mohseni, F.A.; Zarrini, G.; Amani, A.; Shafiee, A. Microbial production of testosterone and testololactone in the culture of Aspergillus terreus. World J. Microbiol. Biotechnol. 2004, 20, 657–660. [Google Scholar] [CrossRef]
- Heidary, M.; Ghasemi, S.; Habibi, Z.; Ansari, F. Biotransformation of androst-4-ene-3,17-dione and nandrolone decanoate by genera of Aspergillus and Fusarium. Biotechnol. Lett. 2020, 42, 1767–1775. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, L.; Ge, Z.; Wang, X.; Li, Y.; Liu, X.; Liu, F.; Lu, F. Microbial hydroxylation of steroids by Penicillium decumbens. J. Mol. Catal. B Enzym. 2016, 133, S346–S351. [Google Scholar] [CrossRef]
- Panek, A.; Łyczko, P.; Świzdor, A. Microbial Modifications of Androstane and Androstene Steroids by Penicillium vinaceum. Molecules 2020, 25, 4226. [Google Scholar] [CrossRef]
- Kołek, T.; Szpineter, A.; Świzdor, A. Baeyer-Villiger oxidation of DHEA, pregnenolone, and androstenedione by Penicillium lilacinum AM111. Steroids 2008, 73, 1441–1445. [Google Scholar] [CrossRef]
- Yildirim, K.; Kuru, A. The Biotransformation of Some Steroids by Aspergillus Sydowii MRC 200653. J. Chem. Res. 2016, 40, 78–81. [Google Scholar] [CrossRef]
- Ríos, L.O.D.L.; Luengo, J.M.; Fernández-Cañón, J.M. Steroid 11-alpha-hydroxylation by the fungi Aspergillus nidulans and Aspergillus ochraceus. Methods Mol. Biol. 2017, 1645, 271–287. [Google Scholar] [PubMed]
- Yildirim, K.; Kuru, A.; Keskin, E.; Salihoglu, A.; Bukum, N. Biotransformation of Androst-4-Ene-3,17-Dione by Some Fungi. J. Chem. Res. 2017, 41, 594–597. [Google Scholar] [CrossRef]
- Yildirim, K.; Kuru, A.; Yılmaz, R.F. Microbial Transformation of Some Steroids by Cladosporium Cladosporioides Mrc 70282. J. Chem. Res. 2018, 42, 408–411. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Sultan, S.; Khan, M.T.H.; Yasin, A.; Shaheen, F.; Atta-Ur-Rahman, A. Biotransformation of (+)-androst-4-ene-3,17-dione. Nat. Prod. Res. 2007, 18, 529–535. [Google Scholar] [CrossRef]
- Kozłowska, E.; Dymarska, M.; Kostrzewa-Susłow, E.; Janeczko, T. Isaria fumosorosea KCh J2 Entomopathogenic Strain as an Effective Biocatalyst for Steroid Compound Transformations. Molecules 2017, 22, 1511. [Google Scholar] [CrossRef]
- Črešnar, B.; Petrič, Š. Cytochrome P450 enzymes in the fungal kingdom. Biochim. Biophys. Acta 2011, 1814, 29–35. [Google Scholar] [CrossRef]
- Karunarathna, S.C.; Damodara Shenoy, B.; Pripdeevech, P.; Madawala, S.; Tang, A.M.C.; Karbowy-Thongbai, B.; Dissanayake, A.J.; Dutta, A.K.; Palnam Dauda, W.; Abraham, P.; et al. Robust Profiling of Cytochrome P450s (P450ome) in Notable Aspergillus spp. Life 2022, 12, 451. [Google Scholar] [CrossRef]
- Fierro, F.; Vaca, I.; Castillo, N.I.; García-Rico, R.O.; Chávez, R. Penicillium chrysogenum, a Vintage Model with a Cutting-Edge Profile in Biotechnology. Microorganisms 2022, 10, 573. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Eldarov, M.A. Polyamines Upregulate Cephalosporin C Production and Expression of β-Lactam Biosynthetic Genes in High-Yielding Acremonium chrysogenum Strain. Molecules 2021, 26, 6636. [Google Scholar] [CrossRef] [PubMed]
- Zhgun, A.A.; Dumina, M.V.; Voinova, T.M.; Dzhavakhiya, V.V.; Eldarov, M.A. Role of acetyl-CoA Synthetase and LovE Regulator Protein of Polyketide Biosynthesis in Lovastatin Production by Wild-Type and Overproducing Aspergillus terreus Strains. Appl. Biochem. Microbiol. 2018, 54, 188–197. [Google Scholar] [CrossRef]
- Domratcheva, A.G.; Zhgun, A.A.; Novak, N.V.; Dzhavakhiya, V.V. The Influence of Chemical Mutagenesis on the Properties of the Cyclosporine a High-Producer Strain Tolypocladium inflatum VKM F-3630D. Appl. Biochem. Microbiol. 2018, 54, 53–57. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Sequeira, S.O.; Macedo, M.F. Fungi in archives, libraries, and museums: A review on paper conservation and human health. Crit. Rev. Microbiol. 2019, 45, 686–700. [Google Scholar] [CrossRef]
- Felpeto-Santero, C.; Galán, B.; Luengo, J.M.; Fernández-Cañon, J.M.; Del Cerro, C.; Medrano, F.J.; García, J.L. Identification and expression of the 11β-steroid hydroxylase from Cochliobolus lunatus in Corynebacterium glutamicum. Microb. Biotechnol. 2019, 12, 856–868. [Google Scholar] [CrossRef]
- Petrič, Š.; Hakki, T.; Bernhardt, R.; Žigon, D.; Črešnar, B. Discovery of a steroid 11α-hydroxylase from Rhizopus oryzae and its biotechnological application. J. Biotechnol. 2010, 150, 428–437. [Google Scholar] [CrossRef]
- Chen, J.; Tang, J.; Xi, Y.; Dai, Z.; Bi, C.; Chen, X.; Fan, F.; Zhang, X. Production of 14α-hydroxysteroids by a recombinant Saccharomyces cerevisiae biocatalyst expressing of a fungal steroid 14α-hydroxylation system. Appl. Microbiol. Biotechnol. 2019, 103, 8363–8374. [Google Scholar] [CrossRef]
- Felpeto-Santero, C.; Galán, B.; García, J.L. Engineering the steroid hydroxylating system from Cochliobolus lunatus in Mycolicibacterium smegmatis. Microorganisms 2021, 9, 1499. [Google Scholar] [CrossRef]
- Hull, C.M.; Warrilow, A.G.S.; Rolley, N.J.; Price, C.L.; Donnison, I.S.; Kelly, D.E.; Kelly, S.L. Co-production of 11α-hydroxyprogesterone and ethanol using recombinant yeast expressing fungal steroid hydroxylases. Biotechnol. Biofuels 2017, 10, 226. [Google Scholar] [CrossRef]
- Jgoun, A.A.; Eldarov, M.A.; Solodar, L.I.; Sokolov, N.N.; Archakov, A.I.; Skryabin, K.G. Heterologous expression of eukaryotic CYP450. 1. Heterologous expression of cytochrome P450 2B4 using groups with various affinity in E. coli. Vopr. Med. Khim. 2001, 47, 391–392. [Google Scholar]
- Breskvar, K.; Hudnik-Plevnik, T. Inducibility of cytochrome P-450 and of NADPH-cytochrome C reductase in progesterone treated filamenteous fungi Rhizopus nigricans and Rhizopus arrhizus. J. Steroid Biochem. 1981, 14, 395–399. [Google Scholar] [CrossRef]
- Irrgang, S.; Schlosser, D.; Schmauder, H.P. The steroid 15α-hydroxylase of Penicillium raistrickii I 477 is inducible. Biotechnol. Lett. 1992, 14, 33–38. [Google Scholar] [CrossRef]
- Črešnar, B.; Žakelj-Mavrič, M. Aspects of the steroid response in fungi. Chem. Biol. Interact. 2009, 178, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Hyvönen, M.T.; Keinänen, T.A.; Nuraeva, G.K.; Yanvarev, D.V.; Khomutov, M.; Khurs, E.N.; Kochetkov, S.N.; Vepsäläinen, J.; Zhgun, A.A.; Khomutov, A.R. Hydroxylamine analogue of agmatine: Magic bullet for arginine decarboxylase. Biomolecules 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Regulation of secondary metabolism in fungi. Pure Appl. Chem. 1986, 58, 219–226. [Google Scholar] [CrossRef]
- Sultan, A. Steroids: A Diverse Class of Secondary Metabolites. Med. Chem. 2015, 5, 310–317. [Google Scholar] [CrossRef]

| Compound | RT (min) | MW | Structure | Encoding |
|---|---|---|---|---|
| 3α-OH-5β-H-Androstane-17-one | 11.23 | 290 |  | a |
| 5β-H-Androstane-3,17-dione | 11.50 | 288 | 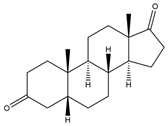 | b |
| Androsta-4,6-diene-3,17-dione (6-Dehydroandrostenedione) | 12.45 | 284 | 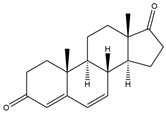 | c |
| Androst-4-ene-3,17-dione (AD, Androstenedione) | 12.51 | 286 | 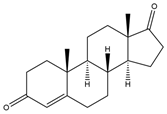 | d |
| 17β-Hydroxyandrost-4-en-3-one (TS, Testosterone) | 12.64 | 288 | 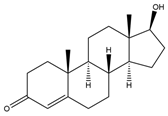 | e |
| Androsta-1,4-diene-3,17-dione (ADD, Androstadienedione, Boldione) | 12.73 | 284 |  | f |
| 5β-H-Androstane-3,6,17-trione | 12.88 | 302 | 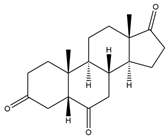 | g |
| Androst-4-ene-3,11,17-trione (Androstane-3,11,17-trione) | 13.11 | 300 | 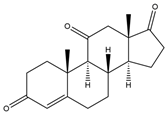 | h |
| 3β-OH-5α-H-Androstane-6,17-dione | 13.42 | 304 | 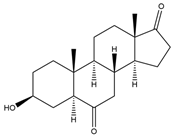 | i |
| Androst-4-ene-3,6,17-trione | 13.69 | 300 | 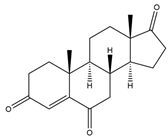 | j |
| 5α-H-Androstane-3,6,17-trione | 13.79 | 302 | 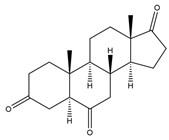 | k |
| 6β-OH-Androst-4-ene-3,17-dione (6β-OH-AD) | 13.92 | 302 |  | l |
| 6β-OH-Testosterone (6β-OH-TS) | 14.28 | 304 |  | m |
| 7α-OH-Androst-4-ene-3,17-dione (7α-OH-AD) | 14.60 | 302 |  | n |
| 11β-OH-Androst-4-ene-3,17-dione (11β-OH-AD) | 14.74 | 302 | 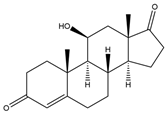 | o |
| 14α-OH-Androst-4-ene-3,17-dione (14α-OH-AD) | 14.83 | 302 | 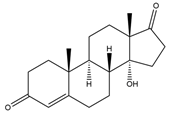 | p |
| 15α-OH-Androst-4-ene-3,17-dione (15α-OH-AD) | 14.90 | 302 | 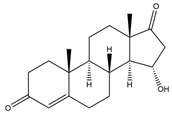 | q |
| 11β-OH-Testosterone (11β-OH-TC) | 15.33 | 304 | 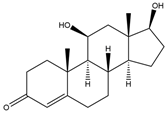 | r |
| 14α-OH-Androsta-1,4-diene-3,17-dione (14α-OH-ADD) | 15.42 | 300 | 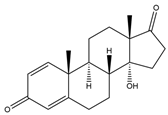 | s |
| Filamentous Fungi | The Product of Transformation | Source | |
|---|---|---|---|
| Class | Family | ||
| Eurotiomycetes | Aspergillaceae | Androst-4,6-diene-3,17-dione (c) TS (e) 1 ADD (f) 5β–H–Androstane-3,6,17-trione (g) Androstane-3,11,17-trione (h) 5α–H–Androstane-3,6,17-trione (k) 6β–OH–AD (l) 6β–OH–TS (m) 11β–OH–AD (o) 14α–OH–AD (p) 14α–OH–ADD (s) | Current |
| TS (e) Testolactone | [33] | ||
| 15α–OH–AD (q) 17α-Oxa-D-homo-5α-androstan-3,17-dione Testolactone | [16] | ||
| 7β–OH–AD 11α–OH–AD | [34] | ||
| 1α–OH–AD 1α,6β–dihydroxy–AD | [35] | ||
| Testololactone | [36,37] | ||
| 6β–OH–TS (m) 14α–OH–TS 15α–OH–TS | [38] | ||
| 11α-OH-AD | [39] | ||
| TS (e) 6β–OH–AD (l) 11α–OH–AD 11α–OH–TS 11β –OH–AD (o) 14α–OH–AD (p) 15α–OH–TS 15β –OH–TS Testololactone | [40] | ||
| Dothideomycetes | Cladosporiaceae | TS (e) ADD (f) 5β–H–Androstane-3,6,17-trione (g) 3β–OH–5α–H–Androstane-6,17-dione (i) 5α–H–Androstane-3,6,17-trione (k) 6β–OH–AD (l) 6β–OH–TS (m) 15α–OH–AD (q) | Current |
| TS (e) 5α–H–Androstane-3,6,17-trione (k) 6β–OH–AD (l) 17β–OH–androst-4-en-3,16-dione 15α–OH-AD (q) 6β,17β dihydroxyandrost-4-en-3,16-dione | [23] | ||
| TS (e) 17β-Hydroxyandrost-4-ene-3,16-dione 16β,17β-Dihydroxyandrost-4-ene-3-one 16β,17α-Dihydroxyandrost-4-ene-3-one | [41] | ||
| Pleosporaceae (except genus Curvularia) | 3α–OH–5β–H–Androstane-17-one (a) 5β–H–Androstane-6,17-dione (b) TS (e) 14α–OH–AD (p) | Current | |
| TS (e) 7α–OH–AD (n) 7β–OH–AD 7α–OH–TS 14α–OH–AD (p) | [22] | ||
| 5α–H–Androstane-3,6,17-trione (k) 7α–OH–AD (n) 7β–OH–AD 14α–OH–AD (p) 17β-Hydroxy-5α-Androstane-3,6-dione | [23] | ||
| Pleosporaceae (genus Curvularia) | 6β–OH–AD (l) 6β–OH–TS (m) 11β-OH AD (o) 14α–OH–AD (p) 11β–OH–TS (r) | Current | |
| TS (e) 7α-OH-TS 11β-OH AD (o) 14α-OH-AD (p) 11β-OH-TS (r) 14α-OH-TS | [20] | ||
| 11β-OH AD (o) 14α-OH-AD (p) | [32] | ||
| 11α–OH–AD | [42] | ||
| Sordariomycetes | Cordycipitaceae | Androst-4,6-diene-3,17-dione (c) 5β–H–Androstane-3,6,17-trione (g) Androst-4-ene-3,6,17-trione (j) 5α–H–Androstane-3,6,17-trione (k) 6β–OH–AD (l) 7α–OH–AD (n) 11β-OH-AD (o) 14α–OH–AD (p) | Current |
| 11α–OH–AD 11α–OH–TS 11α-OH-testolactone | [16] | ||
| 7α–OH–AD (n) | [43] | ||
| 11α–OH–AD | [22] | ||
| Microascaceae | 5β–H–Androstane-3,6,17-trione (g) 5α–H–Androstane-3,6,17-trione (k) 6β–OH–AD (l) 14α–OH–AD (p) | Current | |
| TS (e) | [22] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhgun, A.A.; Potapov, M.P.; Avdanina, D.A.; Karpova, N.V.; Yaderets, V.V.; Dzhavakhiya, V.V.; Kardonsky, D.A. Biotransformation of Androstenedione by Filamentous Fungi Isolated from Cultural Heritage Sites in the State Tretyakov Gallery. Biology 2022, 11, 883. https://doi.org/10.3390/biology11060883
Zhgun AA, Potapov MP, Avdanina DA, Karpova NV, Yaderets VV, Dzhavakhiya VV, Kardonsky DA. Biotransformation of Androstenedione by Filamentous Fungi Isolated from Cultural Heritage Sites in the State Tretyakov Gallery. Biology. 2022; 11(6):883. https://doi.org/10.3390/biology11060883
Chicago/Turabian StyleZhgun, Alexander A., Mark P. Potapov, Darya A. Avdanina, Natalya V. Karpova, Vera V. Yaderets, Vakhtang V. Dzhavakhiya, and Dmitry A. Kardonsky. 2022. "Biotransformation of Androstenedione by Filamentous Fungi Isolated from Cultural Heritage Sites in the State Tretyakov Gallery" Biology 11, no. 6: 883. https://doi.org/10.3390/biology11060883
APA StyleZhgun, A. A., Potapov, M. P., Avdanina, D. A., Karpova, N. V., Yaderets, V. V., Dzhavakhiya, V. V., & Kardonsky, D. A. (2022). Biotransformation of Androstenedione by Filamentous Fungi Isolated from Cultural Heritage Sites in the State Tretyakov Gallery. Biology, 11(6), 883. https://doi.org/10.3390/biology11060883









