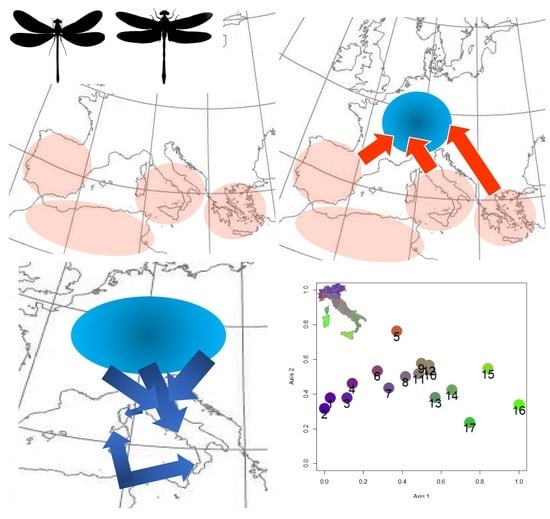
Odonate Diversity Patterns in Italy Disclose Intricate Colonization Pathways
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Odonata List. Available online: https://www2.pugetsound.edu/academics/academic-resources/slater-museum/biodiversity-resources/dragonflies/world-odonata-list2/ (accessed on 24 May 2022).
- Sahlén, G.; Bernard, R.; Cordero Rivera, A.; Ketelaar, R.; Suhling, F. Critical species of Odonata in Europe. Int. J. Odonatol. 2004, 7, 385–398. [Google Scholar] [CrossRef]
- Kalkman, V.J.; Boudot, J.-P.; Bernard, R.; Conze, K.-J.; De Knijf, G.; Dyatlova, E.; Ferreira, S.; Jović, M.; Ott, J.; Riservato, E.; et al. European Red List of Dragonflies; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Daguet, C.; French, G.; Taylor, P. The Odonata Red Data List for Great Britain; Joint Nature Conservation Committee: Peterborough, UK, 2008. [Google Scholar]
- Ott, J.; Conze, K.J.; Günther, A.; Lohr, M.; Mauersberger, R.; Roland, H.-J.; Suhling, F. Rote Liste und Gesamtartenliste der Libellen Deutschlands (Odonata). Libellula Suppl. 2015, 14, 395–422. [Google Scholar]
- Riservato, E.; Fabbri, R.; Festi, A.; Grieco, C.; Hardersen, S.; Landi, F.; Utzeri, C.; Rondinini, C.; Battistoni, A.; Teofili, C. Lista Rossa IUCN delle Libellule Italiane; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2014. [Google Scholar]
- Dijkstra, K. Field Guide to the Dragonflies of Britain and Europe; British Wildlife Publishing: London, UK, 2006. [Google Scholar]
- Galliani, C.; Scherini, R.; Piglia, A. Odonati d’Italia. Guida al Riconoscimento e allo Studio di Libellule e Damigelle; Libreria della Natura: Milano, Italy, 2015. [Google Scholar]
- Galliani, C.; Scherini, R.; Piglia, A. Dragonflies and Damselflies of Europe. A Photographic Field Guide to all the Species; WBA Handbooks: Verona, Italy, 2017. [Google Scholar]
- Dijkstra, K.D.; Schröter, A. Field Guide to the Dragonflies of Britain and Europe, 2nd ed.; Bloomsbury Publishing: London, UK, 2020. [Google Scholar]
- Smallshire, D.; Swash, A. Europe’s Dragonflies: A Field Guide to the Damselflies and Dragonflies; Princeton University Press: Princeton, NJ, USA, 2020. [Google Scholar]
- Boudot, J.P.; Doucet, G.; Grand, D. Dragonflies and Damselflies of Britain and Western Europe. A Photographic Guide; Bloomsbury Publishing: London, UK, 2021. [Google Scholar]
- Boudot, J.-P.; Kalkman, V.J.; Amorín, M.A.; Bogdanović, T.; Rivera, A.C.; Degabriele, G.; Dommanget, J.-L.; Ferreira, S.; Garrigós, B.; Jović, M.; et al. Atlas of the Odonata of the Mediterranean and North Africa. Libellula Suppl. 2009, 9, 1–256. [Google Scholar]
- Boudot, J.-P.; Kalkman, V.J. (Eds.) Atlas of the Dragonflies and Damselflies of Europe; KNNV: Utrecht, The Netherlands, 2015. [Google Scholar]
- Kalkman, V.J.; Boudot, J.P.; Bernard, R.; De Knijf, G.; Suhling, F.; Termaat, T. Diversity and conservation of European dragonflies and damselflies (Odonata). Hydrobiologia 2018, 811, 269–282. [Google Scholar] [CrossRef]
- Galimberti, A.; Assandri, G.; Maggioni, D.; Ramazzotti, F.; Baroni, D.; Bazzi, G.; Chiandetti, I.; Corso, A.; Ferri, V.; Galuppi, M.; et al. Italian odonates in the Pandora’s box: A comprehensive DNA barcoding inventory shows taxonomic warnings at the Holarctic scale. Mol. Ecol. Resour. 2021, 21, 183–200. [Google Scholar] [CrossRef]
- Keil, P.; Simova, I.; Hawkins, B.A. Water-energy and the geographical species richness pattern of European and North African dragonflies (Odonata). Insect Conserv. Divers. 2008, 1, 142–150. [Google Scholar] [CrossRef]
- Heiser, M.; Schmitt, T. Do different dispersal capacities influence the biogeography of the western Palearctic dragonflies (Odonata)? Biol. J. Linn. Soc. 2010, 99, 177–195. [Google Scholar] [CrossRef]
- Heiser, M.; Dapporto, L.; Schmitt, T. Coupling impoverishment analysis and partitioning of beta diversity allows a comprehensive description of Odonata biogeography in the Western Mediterranean. Org. Divers. Evol. 2014, 14, 203–214. [Google Scholar] [CrossRef]
- Pinkert, S.; Dijkstra, K.D.B.; Zeuss, D.; Reudenbach, C.; Brandl, R.; Hof, C. Evolutionary processes, dispersal limitation and climatic history shape current diversity patterns of European dragonflies. Ecography 2018, 41, 795–804. [Google Scholar] [CrossRef]
- Dapporto, L.; Habel, J.C.; Dennis, R.L.H.; Schmitt, T. The biogeography of the western Mediterranean: Elucidating contradictory distribution patterns in butterflies. Biol. J. Linn. Soc. 2011, 103, 571–577. [Google Scholar] [CrossRef]
- Fattorini, S.; Ulrich, W. Drivers of species richness in European Tenebrionidae (Coleoptera). Acta Oecol. 2012, 36, 255–258. [Google Scholar] [CrossRef]
- Fattorini, S.; Ulrich, W. Spatial distributions of European Tenebrionidae point to multiple postglacial colonization trajectories. Biol. J. Linn. Soc. 2012, 105, 318–329. [Google Scholar] [CrossRef]
- Ulrich, W.; Fattorini, S. Longitudinal gradients in the phylogenetic community structure of European Tenebrionidae (Coleoptera) do not coincide with the major routes of postglacial colonization. Ecography 2013, 36, 1106–1116. [Google Scholar] [CrossRef]
- Dapporto, L.; Fattorini, S.; Vodă, R.; Dincă, V.; Vila, R. Biogeography of western Mediterranean butterflies: Combining turnover and nestedness components of faunal dissimilarity. J. Biogeogr. 2014, 41, 1639–1650. [Google Scholar] [CrossRef]
- Fattorini, S. Tenebrionid beetle distributional patterns in Italy: Multiple colonization trajectories in a biogeographical crossroad. Insect Conserv. Divers. 2014, 7, 144–160. [Google Scholar] [CrossRef]
- Schmitt, T.; Fritz, U.; Delfino, M.; Ulrich, W.; Habel, J.C. Biogeography of Italy revisited: Genetic lineages confirm major phylogeographic patterns and a pre-Pleistocene origin of its biota. Front. Zool. 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.A.; Diniz-Filho, J.A.F. ‘Latitude’ and geographic patterns in species richness. Ecography 2004, 27, 268–272. [Google Scholar] [CrossRef]
- Pianka, E.R. Latitudinal gradients in species diversity: A review of concepts. Am. Nat. 1966, 100, 33–46. [Google Scholar] [CrossRef]
- Rohde, K. Latitudinal gradients in species diversity: The search for the primary cause. Oikos 1992, 65, 514–527. [Google Scholar] [CrossRef]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Ashton, K.G. Are ecological and evolutionary rules being dismissed prematurely? Divers. Distrib. 2001, 7, 289–295. [Google Scholar] [CrossRef]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S. Testing the latitudinal gradient: A narrow-scale analysis of tenebrionid richness (Coleoptera, Tenebrionidae) in the Aegean archipelago (Greece). Ital. J. Zool. 2006, 73, 203–211. [Google Scholar] [CrossRef]
- Cancello, E.M.; Silva, R.R.; Vasconcellos, A.; Reis, Y.T.; Oliveira, L.M. Latitudinal variation in termite species richness and abundance along the Brazilian Atlantic forest hotspot. Biotropica 2014, 46, 441–450. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Schemske, D.W.; Mittelbach, G.G. “Latitudinal gradients in species diversity”: Reflections on Pianka’s 1966 article and a look forward. Am. Nat. 2017, 189, 599–603. [Google Scholar] [CrossRef]
- Kinlock, N.L.; Prowant, L.; Herstoff, E.M.; Foley, C.M.; Akin-Fajiye, M.; Bender, N.; Umarani, M.; Ryu, H.Y.; Sen, H.Y.; Gurevitch, J.; et al. Explaining global variation in the latitudinal diversity gradient: Meta-analysis confirms known patterns and uncovers new ones. Glob. Ecol. Biogeogr. 2018, 27, 125–141. [Google Scholar] [CrossRef]
- Beaugrand, G.; Kirby, R.; Goberville, E. The mathematical influence on global patterns of biodiversity. Ecol. Evol. 2020, 10, 6494–6511. [Google Scholar] [CrossRef]
- Cushman, J.; Lawton, J.; Manly, B. Latitudinal patterns in European ant assemblages: Variation in species richness and body size. Oecologia 1993, 95, 30–37. [Google Scholar] [CrossRef]
- Baselga, A. Determinants of species richness, endemism and turnover in European longhorn beetles. Ecography 2008, 31, 263–271. [Google Scholar] [CrossRef]
- Schuldt, A.; Assmann, T. Environmental and historical effects on richness and endemism patterns of carabid beetles in the western Palaearctic. Ecography 2009, 32, 705–714. [Google Scholar] [CrossRef]
- Ulrich, W.; Fiera, C. Environmental correlates of species richness of European springtails (Hexapoda: Collembola). Acta Oecol. 2009, 35, 45–52. [Google Scholar] [CrossRef]
- Bąkowski, M.; Ulrich, W.; Laštůvka, Z. Environmental correlates of species richness of Sesiidae (Lepidoptera) in Europe. Eur. J. Entomol. 2010, 107, 563–570. [Google Scholar] [CrossRef]
- Fattorini, S.; Baselga, A. Species richness and turnover patterns in European tenebrionid beetles. Insect Conserv. Divers. 2012, 5, 331–345. [Google Scholar] [CrossRef]
- Heino, J.; Alahuhta, J.; Fattorini, S. Macroecology of ground beetles: Species richness, range size and body size show different geographical patterns across a climatically heterogeneous area. J. Biogeogr. 2019, 46, 2548–2557. [Google Scholar] [CrossRef]
- Battisti, C. ‘Peninsula effect’ and Italian peninsula: Matherials for a review and implications in applied biogeography. Biogeographia 2006, 27, 153–188. [Google Scholar] [CrossRef][Green Version]
- Battisti, C. Peninsular patterns in biological diversity: Historical arrangement, methodological approaches and causal processes. J. Nat. Hist. 2014, 48, 43–44. [Google Scholar] [CrossRef]
- Dennis, R.L.H. Butterflies and Climate Change; Manchester University Press: Manchester, UK, 1993. [Google Scholar]
- Whittaker, R.J.; Nogues-Bravo, D.; Araujo, M.B. Geographical gradients of species richness: A test of the water-energy conjecture of Hawkins et al. (2003) using European data for five taxa. Glob. Ecol. Biogeogr. 2007, 16, 76–89. [Google Scholar] [CrossRef]
- Svenning, J.C.; Skov, F. Ice age legacies in the geographical distribution of tree species richness in Europe. Glob. Ecol. Biogeogr. 2007, 16, 234–245. [Google Scholar] [CrossRef]
- Corbet, P.S. A Biology of Dragonflies; Witherby: London, UK, 1962. [Google Scholar]
- Corbet, P.S. Dragonflies: Behaviour and Ecology of Odonata, 2nd ed.; Brill Academic Publishers: Leiden, The Netherlands, 2004. [Google Scholar]
- Keddy, P.; Laughlin, D. A Framework for Community Ecology: Species Pools, Filters and Traits; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Hubbell, S.P. The Unified Theory of Biogeography and Biodiversity; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Suhling, F.; Martens, A.; Suhling, I. Long-distance dispersal in Odonata: Examples from arid Namibia. Austral Ecol. 2017, 42, 544–552. [Google Scholar] [CrossRef]
- Baroni Urbani, C.; Ruffo, S.; Vigna Taglianti, A. Materiali per una biogeogeografia italiana fondata su alcuni generi di Coleotteri Cicindelidi, Carabidi e Crisomelidi. Mem. Soc. Entomol. Ital. 1978, 56, 35–92. [Google Scholar]
- Konvicka, M.; Fric, Z.; Benes, J. Butterfly extinctions in European states: Do socioeconomic conditions matter more than physical geography? Glob. Ecol. Biogeogr. 2006, 15, 82–92. [Google Scholar] [CrossRef]
- Dapporto, L.; Dennis, R.L.H. Conservation biogeography of large Mediterranean islands. Butterfly impoverishment, conservation priorities and inferences for an ecological island paradigm. Ecography 2009, 32, 169–179. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Williams, W.R.; Shreeve, T.G. A multivariate approach to the determination of faunal units among European butterfly species (Lepidoptera: Papilionoidea, Hesperioidea). Zool. J. Linn. Soc. 1991, 101, 1–49. [Google Scholar] [CrossRef]
- Pinkert, S.; Barve, V.; Guralnick, R.; Jetz, W. Global geographical and latitudinal variation in butterfly species richness captured through a comprehensive country-level occurrence database. Glob. Ecol. Biogeogr. 2022, 31, 830–839. [Google Scholar] [CrossRef]
- Hortal, J. Uncertainty and the measurement of terrestrial biodiversity gradients. J. Biogeogr. 2008, 35, 1202–1214. [Google Scholar] [CrossRef]
- Heino, J.; Alahuhta, J.; Fattorini, S.; Schmera, D. Predicting beta diversity of terrestrial and aquatic beetles using ecogeographical variables: Insights from the replacement and richness difference components. J. Biogeogr. 2019, 46, 304–315. [Google Scholar] [CrossRef]
- Keil, P.; Hawkins, B.A. Grids versus regional species lists: Are broad-scale patterns of species richness robust to the violation of constant grain size? Biodiv. Conserv. 2009, 18, 3127–3137. [Google Scholar] [CrossRef]
- Società Italiana per lo Studio e la Conservazione Delle Libellule—ODONATA.IT. Available online: https://www.odonata.it/libe-italiane/ (accessed on 1 February 2022).
- Conci, C.; Nielsen, C. Odonata. Fauna d’Italia; Calderini: Bologna, Italy, 1956. [Google Scholar]
- D’Antonio, C.; Utzeri, C. Insecta Odonata. In Checklist and Distribution of the Italian Fauna. 10,000 Terrestrial and Freshwater Species; Ruffo, S., Stoch, F., Eds.; Memorie del Museo Civico di Storia Naturale di Verona, 2° serie, Sez. Scienze della Vita; Comune di Verona: Verona, Italy, 2007; pp. 131–132. [Google Scholar]
- Baselga, A.; Jiménez-Valverde, A.; Niccolini, G. A multiple-site similarity measure independent of richness. Biol. Lett. 2007, 3, 642–645. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 2012, 21, 1223–1232. [Google Scholar] [CrossRef]
- Smith, S.A.; Bermingham, E. The biogeography of lower Mesoamerican freshwater fishes. J. Biogeogr. 2005, 32, 1835–1854. [Google Scholar] [CrossRef]
- Fattorini, S. Influence of recent geography and paleogeography on the structure of reptile communities in a land-bridge archipelago. J. Herpetol. 2010, 44, 242–252. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing. 2022. Available online: http://www.r-project.org/ (accessed on 15 March 2022).
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Schliep, K. ape: Analyses of Phylogenetics and Evolution. Available online: https://CRAN.R-project.org/package=ape (accessed on 15 March 2022).
- Hijmans, R.J.; Williams, E.; Vennes, C. Geosphere: Spherical Trigonometry. Available online: https://CRAN.R-project.org/package=geosphere (accessed on 15 March 2022).
- Vallejos, R.; Osorio, F.; Bevilacqua, M. Spatial Relationships between Two Georeferenced Variables: With Applications in R; Springer: New York, NY, USA, 2020. [Google Scholar]
- Osorio, F.; Vallejos, R.; Cuevas, F.; Mancilla, D. SpatialPack: Tools for Assessment the Association between Two Spatial Processes. Available online: https://CRAN.R-project.org/package=SpatialPack (accessed on 15 March 2022).
- Bartoń, K. MuMIn: Multi-Model Inference. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 15 March 2022).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E. Vegan: Community Ecology Package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 April 2022).
- Dapporto, L.; Ramazzotti, M.; Fattorini, S.; Talavera, G.; Vila, R.; Dennis, R.L.H. Recluster: An unbiased clustering procedure for beta-diversity turnover. Ecography 2013, 36, 1070–1075. [Google Scholar] [CrossRef]
- Dapporto, L.; Ramazzotti, M.; Fattorini, S.; Vila, R.; Talavera, G.; Dennis, R.L.H. Recluster: Ordination Methods for the Analysis of Beta-Diversity Indices. Available online: https://CRAN.R-project.org/package=recluster (accessed on 15 March 2022).
- Fattorini, S. Beetle species-area relationships and extinction rates in protected areas. Insects 2020, 11, 646. [Google Scholar] [CrossRef]
- Massa, B. Il gradiente faunistico nella penisola Italiana e nelle isole. Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 1982, 1923, 353–374. [Google Scholar]
- Fratianni, S.; Acquaotta, F. The climate of Italy. In Landscapes and Landforms of Italy; Soldati, M., Marchetti, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 29–38. [Google Scholar]
- Pignatti, S. Phytogeography and chorology. Definitions and problems. Ann. Bot. 1988, 46, 7–23. [Google Scholar]
- Pignatti, S. Ecologia del Paesaggio; UTET: Torino, Italy, 1994. [Google Scholar]
- Fusco, F. Vegetation response to early Pleistocene climatic cycles in the Lamone valley (Northern Apennines, Italy). Rev. Palaeobot. Palynol. 2007, 145, 1–23. [Google Scholar] [CrossRef]
- Bertini, A. Pliocene to Pleistocene palynoflora and vegetation in Italy: State of the art. Quat. Int. 2010, 225, 5–24. [Google Scholar] [CrossRef]
- Giachino, P.M.; Vailati, D. I Coleotteri Colevidi dell’Appennino settentrionale e centrale: Inventario, analisi faunistica e origine del popolamento (Coleoptera Cholevidae). Biogeographia 2007, 28, 365–420. [Google Scholar] [CrossRef]
- Rattu, A.; Leo, P.; Moratin, R.; Hardersen, S. Diplacodes lefebvrii in Sardinia, a new species for the Italian fauna (Odonata: Libellulidae). Fragm. Entomol. 2014, 46, 121–124. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Guillén, R.A.; Córdoba-Aguilar, A.; Cordero-Rivera, A.; Wellenreuther, M. Genetic divergence predicts reproductive isolation in damselflies. J. Evol. Biol. 2014, 27, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Swaegers, J.; Mergeay, J.; Van Geystelen, A.; Therry, L.; Larmuseau, M.H.D.; Stoks, R. Neutral and adaptive genomic signatures of rapid poleward range expansion. Mol. Ecol. 2015, 24, 6163–6176. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Lorenzo-Carballa, M.O.; Torres-Cambas, Y.; Cordero-Rivera, A.; Thompson, D.J.; Watts, P.C. New EPIC nuclear DNA sequence markers to improve the resolution of phylogeographic studies of Coenagrionids and other odonates. Int. J. Odonatol. 2014, 17, 135–147. [Google Scholar] [CrossRef]
- Wellenreuther, M.; Sánchez-Guillén, R.A. Nonadaptive radiation in damselflies. Evol. Appl. 2016, 9, 103–118. [Google Scholar] [CrossRef]




| Total Odonata | Zygoptera | Anisoptera | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p-Value | sw | Estimate | SE | p-Value | Estimate | SE | p-Value | sw | |
| Intercept | −9.791 | 34.717 | 0.782 | 7.758 | 3.188 | 0.029 | −7.515 | 30.226 | 0.806 | ||
| Area | 0.001 | <0.0001 | <0.00001 | 1 | 1.921 × 10−4 | 5.991 × 10−5 | 0.006 | 4.578 × 10−4 | 1.132 × 10−4 | <0.001 | 1 |
| Pmean | 0.049 | 0.011 | <0.00001 | 1 | 0.015 | 0.004 | 0.001 | 0.034 | 0.009 | <0.001 | 1 |
| Latitude | 0.547 | 0.723 | 0.459 | 0.42 | 0.347 | 0.598 | 0.568 | 0.28 | |||
| Tmean | −0.208 | 0.422 | 0.629 | 0.23 | −0.312 | 0.474 | 0.516 | 0.34 | |||
| Tmin | −0.148 | 0.362 | 0.689 | 0.17 | −0.179 | 0.382 | 0.645 | 0.20 | |||
| Tmax | −0.134 | 0.329 | 0.692 | 0.17 | −0.144 | 0.331 | 0.668 | 0.18 | |||
| Matrix Correlation | Biogeographical Distances | ||||||
|---|---|---|---|---|---|---|---|
| Matrix A × Matrix B | Matrix C (Controlling) | Sørensen (βsor) | Simpson (βsim) | Nestedness (βnest) | |||
| r | p | r | p | r | p | ||
| Odonata | |||||||
| Climatic distances | Centroids | 0.620 | <0.001 | 0.580 | <0.001 | 0.290 | 0.038 |
| Centroids | Climatic distances | 0.404 | <0.001 | 0.454 | <0.001 | 0.075 | 0.246 |
| Zygoptera | |||||||
| Climatic distances | Centroids | 0.574 | 0.002 | 0.569 | <0.001 | 0.129 | 0.207 |
| Centroids | Climatic distances | 0.182 | 0.105 | 0.207 | 0.050 | −0.002 | 0.456 |
| Anisoptera | |||||||
| Climatic distances | Centroids | 0.583 | <0.001 | 0.440 | 0.003 | 0.346 | 0.003 |
| Centroids | Climatic distances | 0.478 | 0.002 | 0.541 | <0.001 | 0.069 | 0.296 |
| Sørensen Index | Simpson Index | |||
|---|---|---|---|---|
| r | p | r | p | |
| Total Odonata | ||||
| Western Europe | 0.86 | 0.025 | 0.921 | 0.24 |
| Central Europe | 0.881 | 0.019 | 0.950 | 0.009 |
| Eastern Europe | 0.791 | 0.025 | 0.495 | 0.066 |
| Northern Africa | −0.896 | 0.015 | −0.804 | 0.041 |
| Zygoptera | ||||
| Western Europe | 0.469 | 0.088 | 0.404 | 0.108 |
| Central Europe | 0.698 | 0.029 | 0.803 | 0.019 |
| Eastern Europe | 0.635 | 0.039 | 0.768 | 0.023 |
| Northern Africa | −0.789 | 0.023 | −0.611 | 0.09 |
| Anisoptera | ||||
| Western Europe | 0.860 | 0.025 | 0.921 | 0.024 |
| Central Europe | 0.881 | 0.019 | 0.951 | 0.009 |
| Eastern Europe | 0.791 | 0.025 | 0.495 | 0.066 |
| Northern Africa | −0.896 | 0.015 | −0.804 | 0.041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattorini, S. Odonate Diversity Patterns in Italy Disclose Intricate Colonization Pathways. Biology 2022, 11, 886. https://doi.org/10.3390/biology11060886
Fattorini S. Odonate Diversity Patterns in Italy Disclose Intricate Colonization Pathways. Biology. 2022; 11(6):886. https://doi.org/10.3390/biology11060886
Chicago/Turabian StyleFattorini, Simone. 2022. "Odonate Diversity Patterns in Italy Disclose Intricate Colonization Pathways" Biology 11, no. 6: 886. https://doi.org/10.3390/biology11060886
APA StyleFattorini, S. (2022). Odonate Diversity Patterns in Italy Disclose Intricate Colonization Pathways. Biology, 11(6), 886. https://doi.org/10.3390/biology11060886