Smoking-, Alcohol-, and Age-Related Alterations of Blood Monocyte Subsets and Circulating CD4/CD8 T Cells in Head and Neck Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Blood Collection and Patient Data
2.3. Staining of Monocyte Subsets in Whole Blood
2.4. Staining of T-Cell Subsets in Isolated PBMC
2.5. FACS Analysis
2.6. Statistical Analysis
3. Results
3.1. Smoking-, Alcohol-, and Age-Related Monocyte Subset Distribution
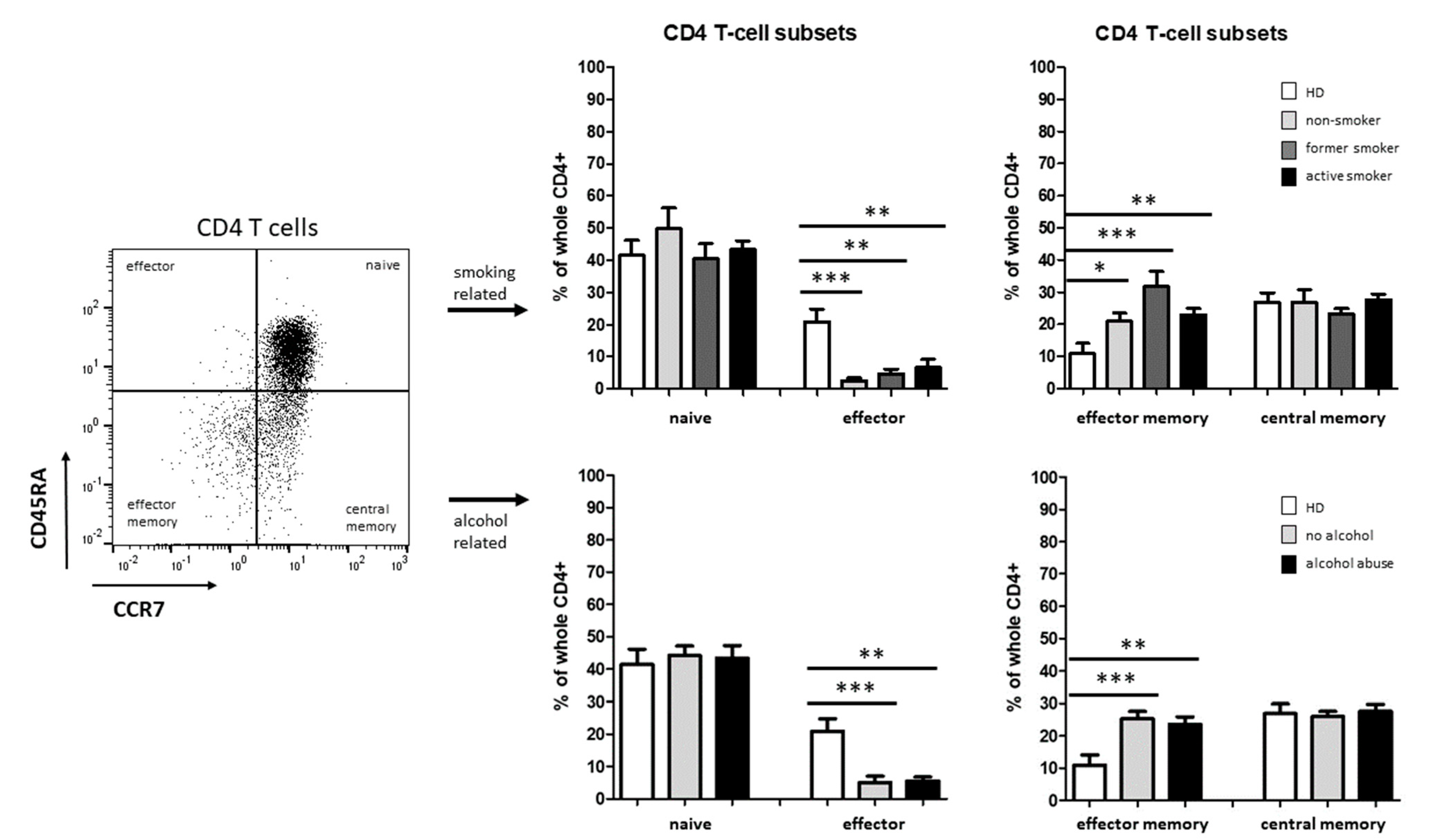
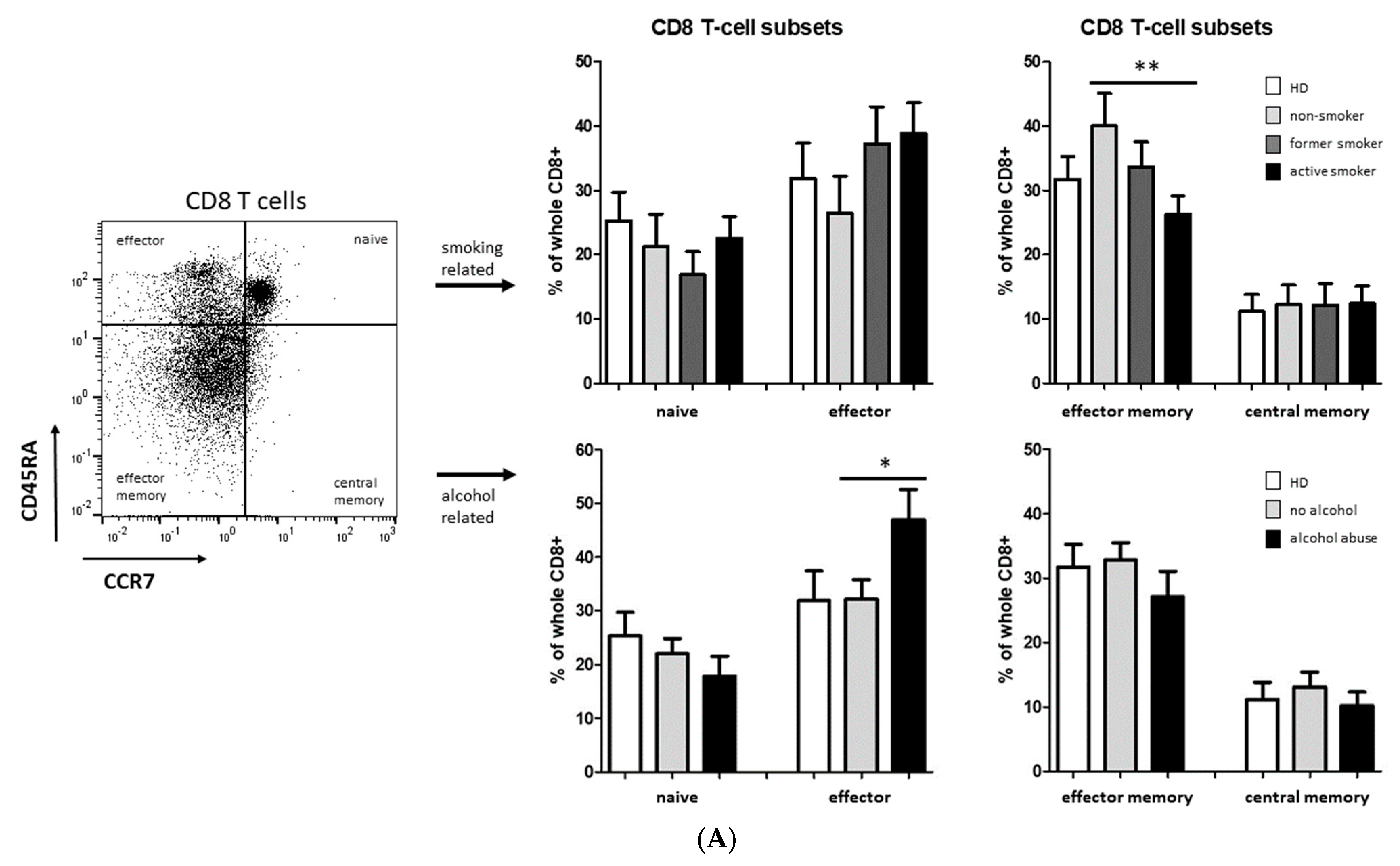
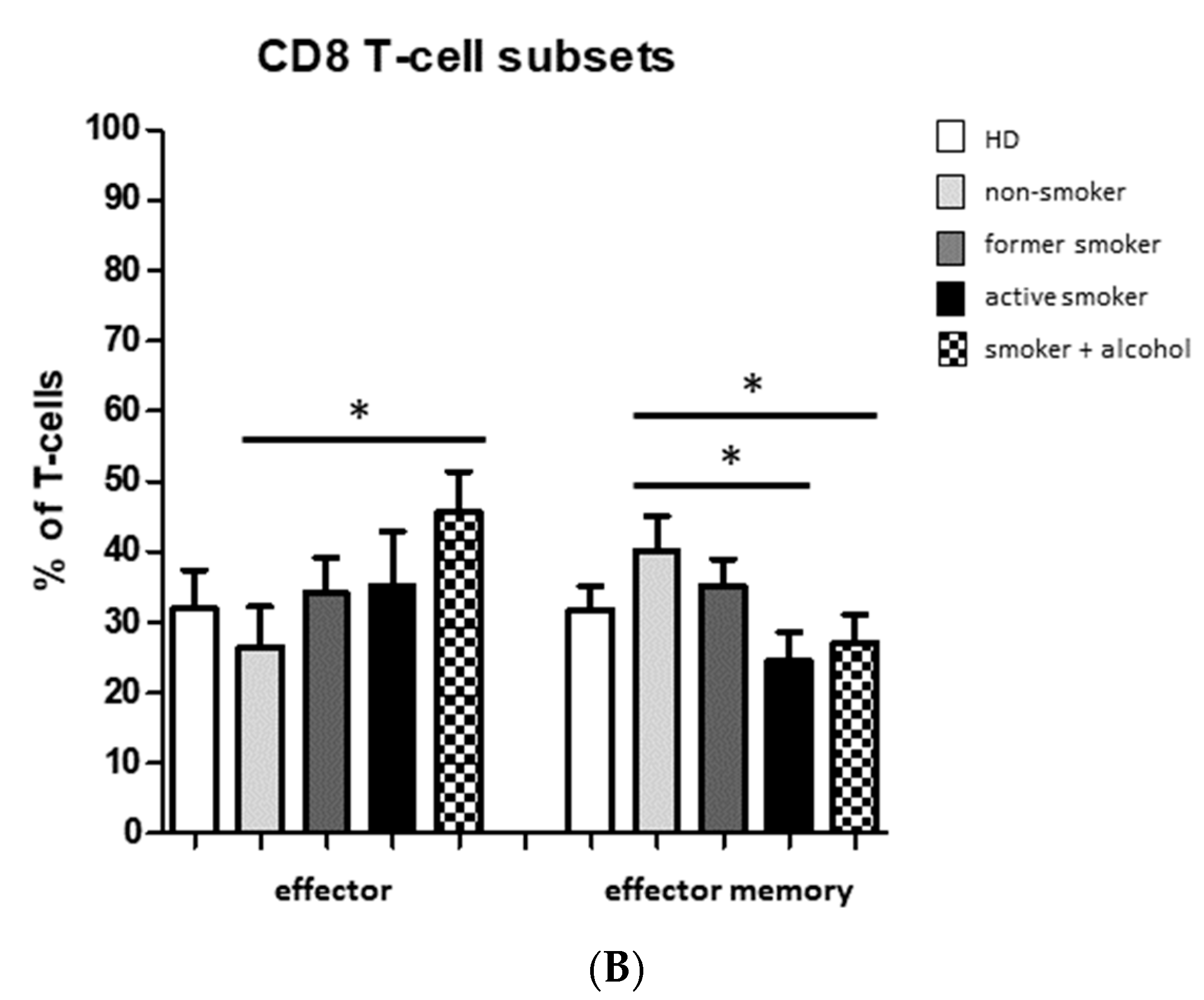
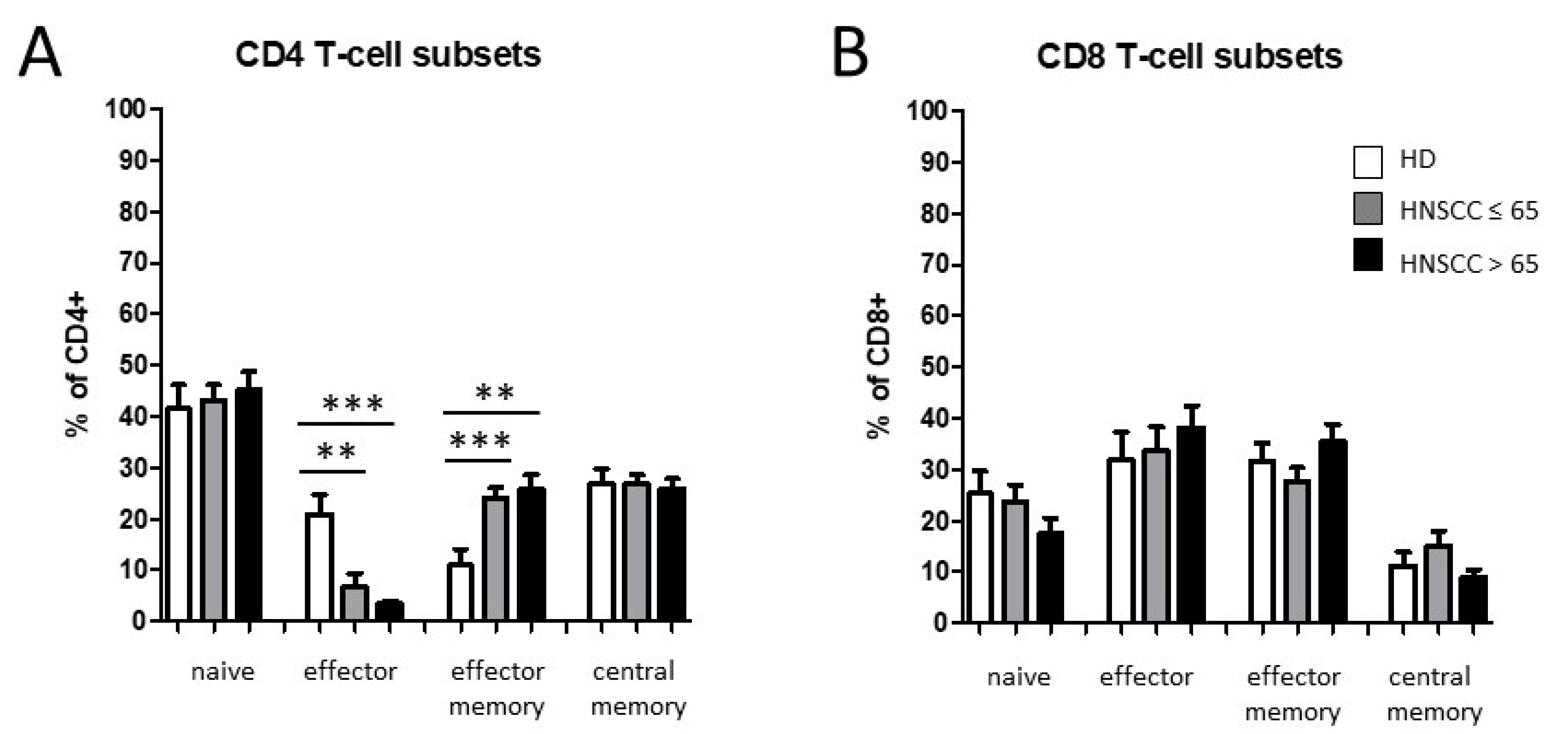
3.2. Smoking-, Alcohol-, and Age-Related Distribution of T-Cell Subsets
4. Discussion
4.1. Smoking-, Alcohol-, and Age-Related Alteration of Monocyte Subsets
4.2. Alteration of Circulating T Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Li, S.; Zhang, T.; Cui, F.; Shi, J.H.; Zhao, F.; Sheng, X. Characterization of Molecular Subtypes in Head and Neck Squamous Cell Carcinoma With Distinct Prognosis and Treatment Responsiveness. Front. Cell Dev. Biol. 2021, 9, 711348. [Google Scholar] [CrossRef] [PubMed]
- Cogliano, V.J.; Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; et al. Preventable exposures associated with human cancers. J. Natl. Cancer Inst. 2011, 103, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Dobrossy, L. Epidemiology of head and neck cancer: Magnitude of the problem. Cancer Metastasis Rev. 2005, 24, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.; Joachim, C.; Deloumeaux, J.; Gaete, S.; Michineau, L.; Herrmann-Storck, C.; Duflo, S.; Luce, D. Head and neck cancer risk factors in the French West Indies. BMC Cancer 2021, 21, 1071. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Gregoire, V.; Lefebvre, J.L.; Licitra, L.; Felip, E.; EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21 (Suppl. S5), v184–v186. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef]
- Schrank, T.; Weir, W.; Lal, A.; Landess, L.; Lenze, N.; Hackman, T. Quantifying smoking exposure, genomic correlates, and related risk of treatment failure in p16+ squamous cell carcinoma of the oropharynx. Laryngoscope Investig. Otolaryngol. 2021, 6, 1376–1382. [Google Scholar] [CrossRef]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F., Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar]
- Wyss, A.; Hashibe, M.; Chuang, S.C.; Lee, Y.C.; Zhang, Z.F.; Yu, G.P.; Winn, D.M.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am. J. Epidemiol. 2013, 178, 679–690. [Google Scholar] [CrossRef]
- Piaggeschi, G.; Rolla, S.; Rossi, N.; Brusa, D.; Naccarati, A.; Couvreur, S.; Spector, T.D.; Roederer, M.; Mangino, M.; Cordero, F.; et al. Immune Trait Shifts in Association With Tobacco Smoking: A Study in Healthy Women. Front. Immunol. 2021, 12, 637974. [Google Scholar] [CrossRef] [PubMed]
- de la Iglesia, J.V.; Slebos, R.J.C.; Martin-Gomez, L.; Wang, X.; Teer, J.K.; Tan, A.C.; Gerke, T.A.; Aden-Buie, G.; van Veen, T.; Masannat, J.; et al. Effects of Tobacco Smoking on the Tumor Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Praud, D.; Rota, M.; Rehm, J.; Shield, K.; Zatonski, W.; Hashibe, M.; La Vecchia, C.; Boffetta, P. Cancer incidence and mortality attributable to alcohol consumption. Int. J. Cancer 2016, 138, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Stickel, F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 2006, 387, 349–360. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef]
- Garro, A.J.; Espina, N.; Farinati, F.; Salvagnini, M. The effects of chronic ethanol consumption on carcinogen metabolism and on O6-methylguanine transferase-mediated repair of alkylated DNA. Alcohol. Clin. Exp. Res. 1986, 10, 73S–77S. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, L.; Lopez-Hoyos, M.; Munoz-Cacho, P.; Martinez-Taboada, V.M. Aging is associated with circulating cytokine dysregulation. Cell. Immunol. 2012, 273, 124–132. [Google Scholar] [CrossRef]
- De Maeyer, R.P.H.; Chambers, E.S. The impact of ageing on monocytes and macrophages. Immunol. Lett. 2021, 230, 1–10. [Google Scholar] [CrossRef]
- Seidler, S.; Zimmermann, H.W.; Bartneck, M.; Trautwein, C.; Tacke, F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010, 11, 30. [Google Scholar] [CrossRef]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Zhang, Y.; Fullerton, J.N.; Boelen, L.; Rongvaux, A.; Maini, A.A.; Bigley, V.; Flavell, R.A.; Gilroy, D.W.; Asquith, B.; et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017, 214, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L. Blood Monocytes and Their Subsets: Established Features and Open Questions. Front. Immunol. 2015, 6, 423. [Google Scholar] [CrossRef] [PubMed]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef] [PubMed]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Reviews. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Moniuszko, M.; Bodzenta-Lukaszyk, A.; Kowal, K.; Lenczewska, D.; Dabrowska, M. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin. Immunol. 2009, 130, 338–346. [Google Scholar] [CrossRef]
- Polasky, C.; Steffen, A.; Loyal, K.; Lange, C.; Bruchhage, K.L.; Pries, R. Redistribution of Monocyte Subsets in Obstructive Sleep Apnea Syndrome Patients Leads to an Imbalanced PD-1/PD-L1 Cross-Talk with CD4/CD8 T Cells. J. Immunol. 2021, 206, 51–58. [Google Scholar] [CrossRef]
- Nyugen, J.; Agrawal, S.; Gollapudi, S.; Gupta, S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 2010, 30, 806–813. [Google Scholar] [CrossRef]
- Merino, A.; Buendia, P.; Martin-Malo, A.; Aljama, P.; Ramirez, R.; Carracedo, J. Senescent CD14+CD16+ monocytes exhibit proinflammatory and proatherosclerotic activity. J. Immunol. 2011, 186, 1809–1815. [Google Scholar] [CrossRef]
- Ong, S.M.; Hadadi, E.; Dang, T.M.; Yeap, W.H.; Tan, C.T.; Ng, T.P.; Larbi, A.; Wong, S.C. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Landsman, L.; Bar-On, L.; Zernecke, A.; Kim, K.W.; Krauthgamer, R.; Shagdarsuren, E.; Lira, S.A.; Weissman, I.L.; Weber, C.; Jung, S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 2009, 113, 963–972. [Google Scholar] [CrossRef]
- Panek, C.A.; Ramos, M.V.; Mejias, M.P.; Abrey-Recalde, M.J.; Fernandez-Brando, R.J.; Gori, M.S.; Salamone, G.V.; Palermo, M.S. Differential expression of the fractalkine chemokine receptor (CX3CR1) in human monocytes during differentiation. Cell. Mol. Immunol. 2015, 12, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, L.; Hilkens, C.M.; Morrison, V.L. beta(2) Integrins As Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front. Immunol. 2017, 8, 1966. [Google Scholar] [CrossRef] [PubMed]
- Han, C.F.; Jin, J.; Xu, S.; Liu, H.B.; Li, N.; Cao, X.T. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 2010, 11, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gordon, R.A.; Huynh, L.; Su, X.D.; Min, K.H.P.; Han, J.H.; Arthur, J.S.; Kalliolias, G.D.; Ivashkiv, L.B. Indirect Inhibition of Toll-like Receptor and Type I Interferon Responses by ITAM-Coupled Receptors and Integrins. Immunity 2010, 32, 518–530. [Google Scholar] [CrossRef]
- Caruntu, A.; Scheau, C.; Tampa, M.; Georgescu, S.R.; Caruntu, C.; Tanase, C. Complex Interaction Among Immune, Inflammatory, and Carcinogenic Mechanisms in the Head and Neck Squamous Cell Carcinoma. Adv. Exp. Med. Biol. 2021, 1335, 11–35. [Google Scholar] [CrossRef]
- Sakakura, K.; Takahashi, H.; Motegi, S.I.; Yokobori-Kuwabara, Y.; Oyama, T.; Chikamatsu, K. Immunological features of circulating monocyte subsets in patients with squamous cell carcinoma of the head and neck. Clin. Immunol. 2021, 225, 108677. [Google Scholar] [CrossRef]
- Tavenier, J.; Rasmussen, L.J.H.; Houlind, M.B.; Andersen, A.L.; Panum, I.; Andersen, O.; Petersen, J.; Langkilde, A.; Nehlin, J.O. Alterations of monocyte NF-kappa B p65/RelA signaling in a cohort of older medical patients, age-matched controls, and healthy young adults. Immun. Ageing 2020, 17, 25. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Kuang, D.M.; Peng, C.; Zhao, Q.; Wu, Y.; Chen, M.S.; Zheng, L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology 2010, 51, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Heeren, A.M.; Punt, S.; Bleeker, M.C.; Gaarenstroom, K.N.; van der Velden, J.; Kenter, G.G.; de Gruijl, T.D.; Jordanova, E.S. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2016, 29, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Ha, S.J.; Hong, M.H.; Heo, S.J.; Koh, Y.W.; Choi, E.C.; Kim, E.K.; Pyo, K.H.; Jung, I.; Seo, D.; et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci. Rep. 2016, 6, 36956. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ge, S.; Sang, S.; Hu, C.; Deng, S. Evaluation of PD-L1 Expression Level in Patients With Non-Small Cell Lung Cancer by (18)F-FDG PET/CT Radiomics and Clinicopathological Characteristics. Front. Oncol. 2021, 11, 789014. [Google Scholar] [CrossRef]
- Cass, S.P.; Mekhael, O.; Thayaparan, D.; McGrath, J.J.C.; Revill, S.D.; Fantauzzi, M.F.; Wang, P.; Reihani, A.; Hayat, A.I.; Stevenson, C.S.; et al. Increased Monocyte-Derived CD11b(+) Macrophage Subpopulations Following Cigarette Smoke Exposure Are Associated With Impaired Bleomycin-Induced Tissue Remodelling. Front. Immunol. 2021, 12, 740330. [Google Scholar] [CrossRef]
- Stockfelt, M.; Christenson, K.; Andersson, A.; Bjorkman, L.; Padra, M.; Brundin, B.; Ganguly, K.; Asgeirsdottir, H.; Linden, S.; Qvarfordt, I.; et al. Increased CD11b and Decreased CD62L in Blood and Airway Neutrophils from Long-Term Smokers with and without COPD. J. Innate Immun. 2020, 12, 480–489. [Google Scholar] [CrossRef]
- McDermott, D.H.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Merrell, M.N.; Epstein, N.; Quyyumi, A.A.; Murphy, P.M. Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ. Res. 2001, 89, 401–407. [Google Scholar] [CrossRef]
- Tacke, F.; Alvarez, D.; Kaplan, T.J.; Jakubzick, C.; Spanbroek, R.; Llodra, J.; Garin, A.; Liu, J.H.; Mack, M.; van Rooijen, N.; et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007, 117, 185–194. [Google Scholar] [CrossRef]
- Guo, J.; Chen, T.; Wang, B.; Zhang, M.; An, H.; Guo, Z.; Yu, Y.; Qin, Z.; Cao, X. Chemoattraction, adhesion and activation of natural killer cells are involved in the antitumor immune response induced by fractalkine/CX3CL1. Immunol. Lett. 2003, 89, 1–7. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, M.; Wang, B.; Yuan, Z.; Guo, Z.; Chen, T.; Yu, Y.; Qin, Z.; Cao, X. Fractalkine transgene induces T-cell-dependent antitumor immunity through chemoattraction and activation of dendritic cells. Int. J. Cancer 2003, 103, 212–220. [Google Scholar] [CrossRef]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-associated thrombosis: The when, how and why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef] [PubMed]
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pages, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.A.; Amir, E. HYPE or HOPE: The prognostic value of infiltrating immune cells in cancer. Br. J. Cancer 2017, 117, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 541–550. [Google Scholar] [CrossRef]
- Wight, A.J.; Ogden, G.R. Possible mechanisms by which alcohol may influence the development of oral cancer—A review. Oral. Oncol. 1998, 34, 441–447. [Google Scholar] [CrossRef]
- Browman, G.P.; Wong, G.; Hodson, I.; Sathya, J.; Russell, R.; McAlpine, L.; Skingley, P.; Levine, M.N. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N. Engl. J. Med. 1993, 328, 159–163. [Google Scholar] [CrossRef]
- Whincup, P.; Papacosta, O.; Lennon, L.; Haines, A. Carboxyhaemoglobin levels and their determinants in older British men. BMC Public Health 2006, 6, 189. [Google Scholar] [CrossRef]
- Rades, D.; Setter, C.; Schild, S.E.; Dunst, J. Effect of smoking during radiotherapy, respiratory insufficiency, and hemoglobin levels on outcome in patients irradiated for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1134–1142. [Google Scholar] [CrossRef]
- Fleshner, N.; Garland, J.; Moadel, A.; Herr, H.; Ostroff, J.; Trambert, R.; O’Sullivan, M.; Russo, P. Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer 1999, 86, 2337–2345. [Google Scholar] [CrossRef]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Turksma, A.W.; Bontkes, H.J.; van den Heuvel, H.; de Gruijl, T.D.; von Blomberg, B.M.; Braakhuis, B.J.; Leemans, C.R.; Bloemena, E.; Meijer, C.J.; Hooijberg, E. Effector memory T-cell frequencies in relation to tumour stage, location and HPV status in HNSCC patients. Oral Dis. 2013, 19, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Le Page, A.; Dupuis, G.; Larbi, A.; Witkowski, J.M.; Fulop, T. Signal transduction changes in CD4(+) and CD8(+) T cell subpopulations with aging. Exp. Gerontol. 2018, 105, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.S.; Schuler, P.J.; Doescher, J.; Theodoraki, M.N.; Laban, S.; Brunner, C.; Hoffmann, T.K.; Wigand, M.C. Age-related changes in T lymphocytes of patients with head and neck squamous cell carcinoma. Immun. Ageing 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Patients (n = 64) | |
|---|---|---|
| n | % | |
| Gender | ||
| m | 47 | 73 |
| f | 17 | 27 |
| Age (years) | ||
| ≤65 | 38 | 59 |
| >65 | 26 | 41 |
| Tumor Site | ||
| pharynx | 37 | 58 |
| larynx | 13 | 20 |
| oral cavity | 14 | 22 |
| Tumor Stage | ||
| T1-T2 | 40 | 63 |
| T3-T4 | 24 | 37 |
| HPV Status | ||
| positive | 20 | 31 |
| negative | 44 | 69 |
| Alcohol Abuse | ||
| yes | 14 | 22 |
| no | 50 | 78 |
| Tobacco Consumption | ||
| yes | 45 | 70 |
| no | 19 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idel, C.; Loyal, K.; Rades, D.; Hakim, S.G.; Schumacher, U.; Bruchhage, K.-L.; Pries, R. Smoking-, Alcohol-, and Age-Related Alterations of Blood Monocyte Subsets and Circulating CD4/CD8 T Cells in Head and Neck Cancer. Biology 2022, 11, 658. https://doi.org/10.3390/biology11050658
Idel C, Loyal K, Rades D, Hakim SG, Schumacher U, Bruchhage K-L, Pries R. Smoking-, Alcohol-, and Age-Related Alterations of Blood Monocyte Subsets and Circulating CD4/CD8 T Cells in Head and Neck Cancer. Biology. 2022; 11(5):658. https://doi.org/10.3390/biology11050658
Chicago/Turabian StyleIdel, Christian, Kristin Loyal, Dirk Rades, Samer G. Hakim, Udo Schumacher, Karl-Ludwig Bruchhage, and Ralph Pries. 2022. "Smoking-, Alcohol-, and Age-Related Alterations of Blood Monocyte Subsets and Circulating CD4/CD8 T Cells in Head and Neck Cancer" Biology 11, no. 5: 658. https://doi.org/10.3390/biology11050658
APA StyleIdel, C., Loyal, K., Rades, D., Hakim, S. G., Schumacher, U., Bruchhage, K.-L., & Pries, R. (2022). Smoking-, Alcohol-, and Age-Related Alterations of Blood Monocyte Subsets and Circulating CD4/CD8 T Cells in Head and Neck Cancer. Biology, 11(5), 658. https://doi.org/10.3390/biology11050658






