Circadian Misalignment Induced by Chronic Night Shift Work Promotes Endoplasmic Reticulum Stress Activation Impacting Directly on Human Metabolism
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Biochemical Analysis
2.3. Anthropometric Indicators and Body Composition
2.4. R.N.A. Extract and Gene Expression Analysis
2.5. RT-qPCR
2.6. Statistical Analysis
3. Results
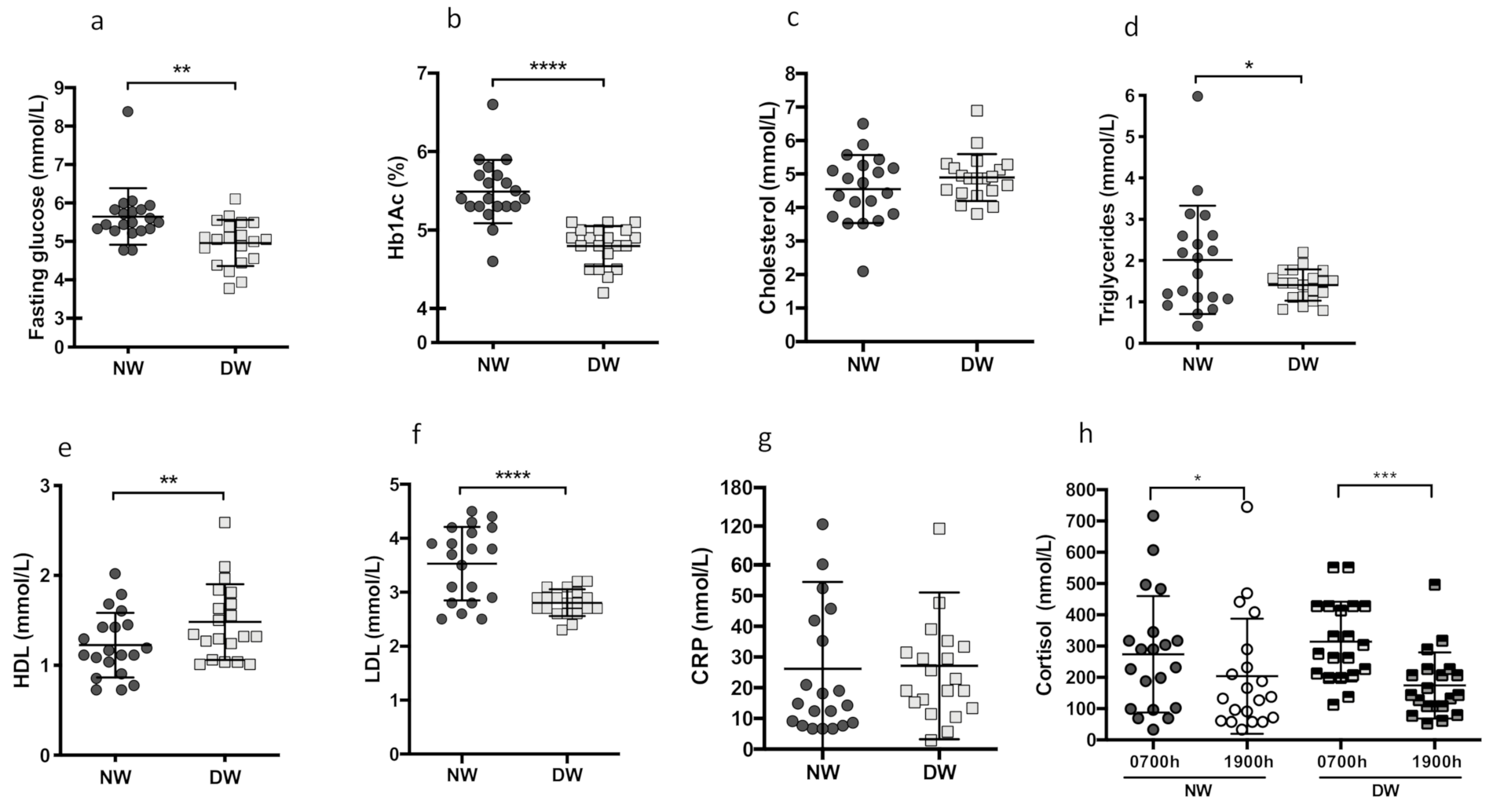
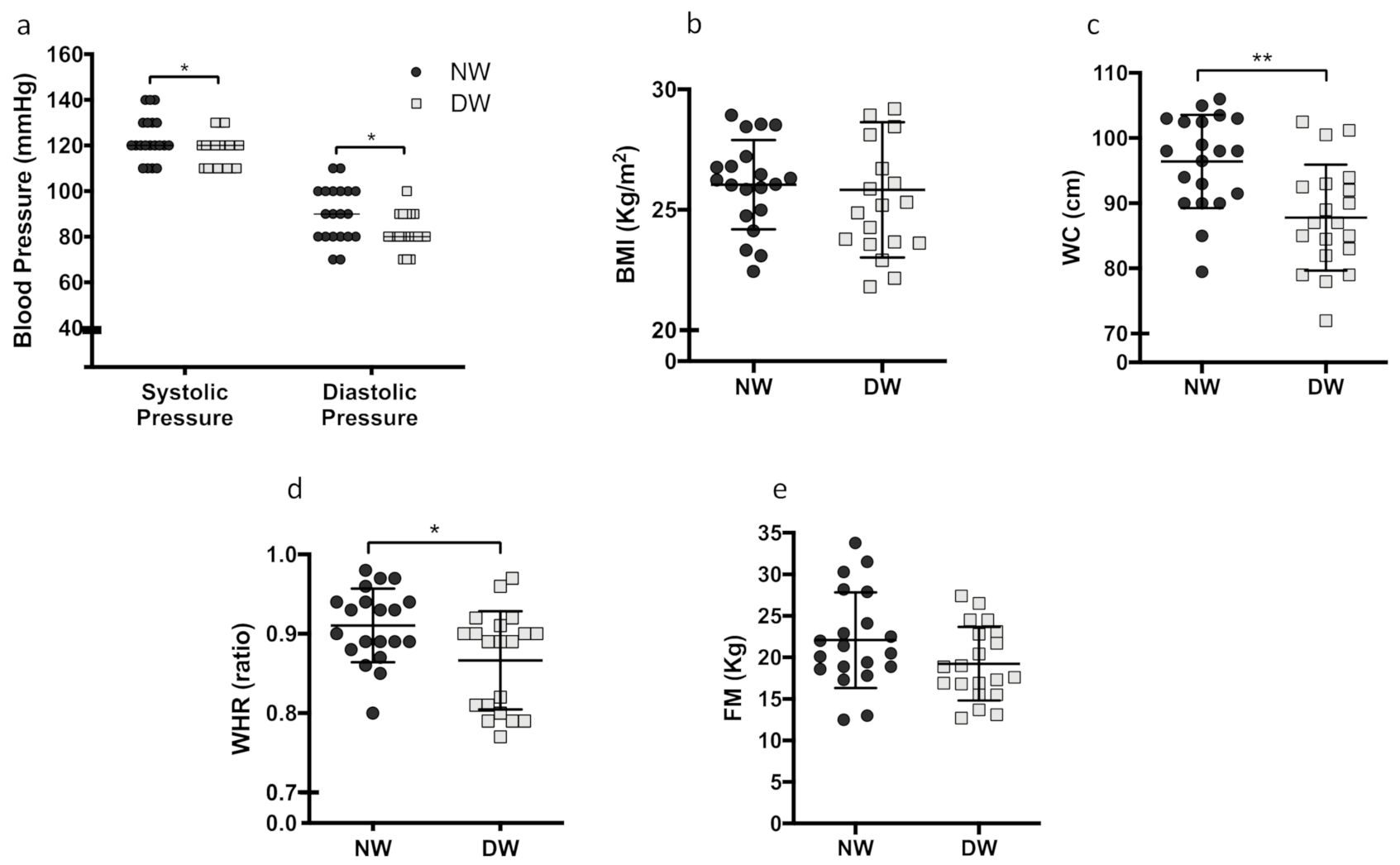
3.1. Metabolic Parameters and Anthropometric Measures
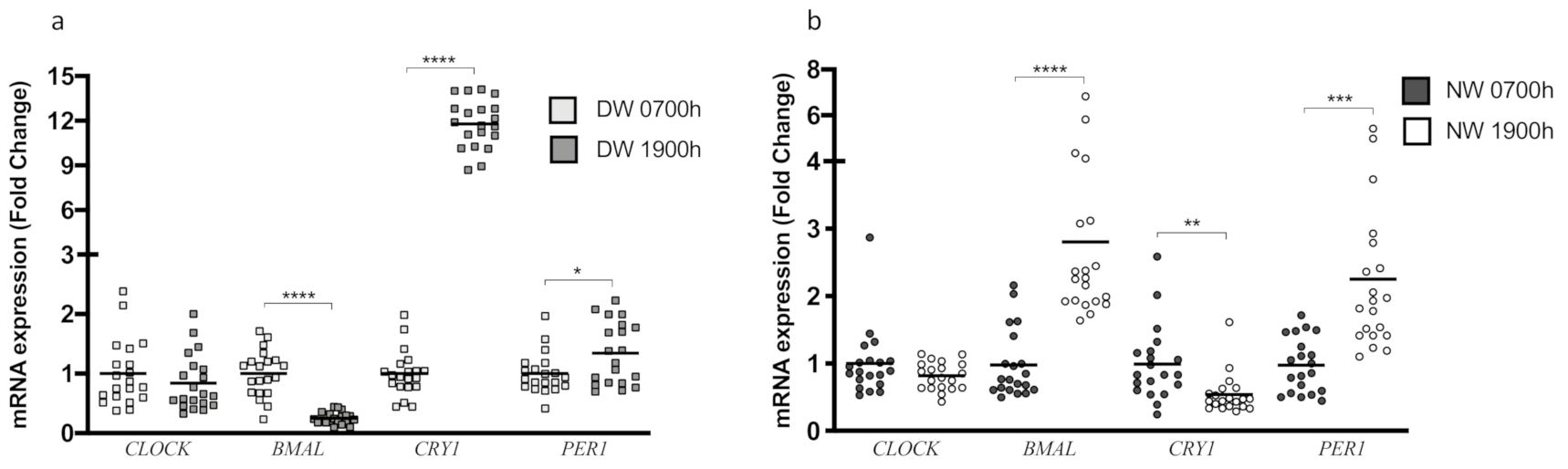
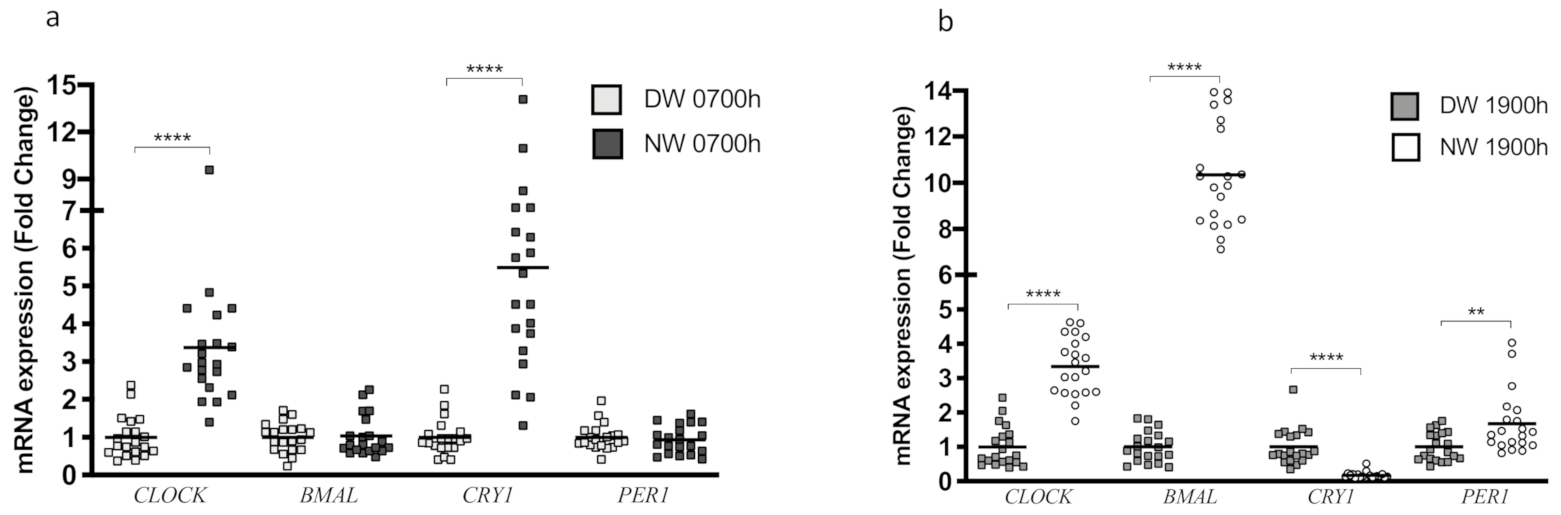
3.2. CLOCK Genes and the Circadian Rhythm
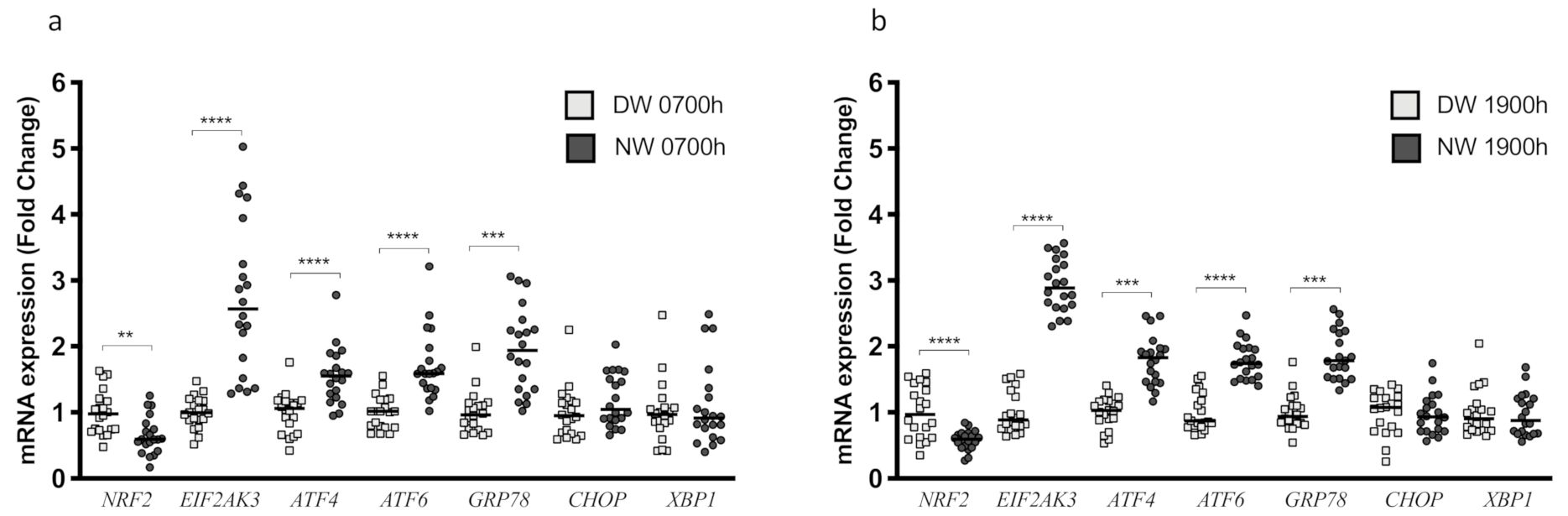
3.3. Endoplasmic Reticulum Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.B.; Currie, A.; Peile, E.; Stranges, S.; Miller, M.A. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Dorn, J.M.; Cappuccio, F.P.; Donahue, R.P.; Rafalson, L.B.; Hovey, K.M.; Freudenheim, J.L.; Kandala, N.B.; Miller, M.A.; Trevisan, M. A population-based study of reduced sleep duration and hypertension: The strongest association may be in premenopausal women. J. Hypertens 2010, 28, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Ayas, N.T.; White, D.P.; Al-Delaimy, W.K.; Manson, J.E.; Stampfer, M.J.; Speizer, F.E.; Patel, S.; Hu, F.B. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 2003, 26, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Gekakis, N.; Staknis, D.; Nguyen, H.B.; Davis, F.C.; Wilsbacher, L.D.; King, D.P.; Takahashi, J.S.; Weitz, C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998, 280, 1564–1569. [Google Scholar] [CrossRef]
- Panda, S.; Antoch, M.P.; Miller, B.H.; Su, A.I.; Schook, A.B.; Straume, M.; Schultz, P.G.; Kay, S.A.; Takahashi, J.S.; Hogenesch, J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002, 109, 307–320. [Google Scholar] [CrossRef]
- Storch, K.F.; Lipan, O.; Leykin, I.; Viswanathan, N.; Davis, F.C.; Wong, W.H.; Weitz, C.J. Extensive and divergent circadian gene expression in liver and heart. Nature 2002, 417, 78–83. [Google Scholar] [CrossRef]
- Costa, G. Multidimensional aspects related to shiftworkers’ health and well-being. Rev. Saude Publica 2004, 38, 86–91. [Google Scholar] [CrossRef]
- Dashti, H.S.; Follis, J.L.; Smith, C.E.; Tanaka, T.; Cade, B.E.; Gottlieb, D.J.; Hruby, A.; Jacques, P.F.; Lamon-Fava, S.; Richardson, K.; et al. Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am. J. Clin. Nutr 2015, 101, 135–143. [Google Scholar] [CrossRef]
- Ajabnoor, G.M.; Bahijri, S.; Shaik, N.A.; Borai, A.; Alamoudi, A.A.; Al-Aama, J.Y.; Chrousos, G.P. Ramadan fasting in Saudi Arabia is associated with altered expression of CLOCK, DUSP and IL-1alpha genes, as well as changes in cardiometabolic risk factors. PLoS ONE 2017, 12, e0174342. [Google Scholar] [CrossRef] [PubMed]
- Rutter, J.; Reick, M.; McKnight, S.L. Metabolism and the control of circadian rhythms. Annu. Rev. Biochem. 2002, 71, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Kawamoto, T.; Takagi, Y.; Fujimoto, K.; Sato, F.; Noshiro, M.; Kato, Y.; Honma, K. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature 2002, 419, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Lowrey, P.L.; Takahashi, J.S. Genetics of the mammalian circadian system: Photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu. Rev. Genet. 2000, 34, 533–562. [Google Scholar] [CrossRef] [PubMed]
- Roa, S.L.R.; Martinez, E.Z.; Martins, C.S.; Antonini, S.R.; De Castro, M.; Moreira, A.C. Postnatal Ontogeny of the Circadian Expression of the Adrenal Clock Genes and Corticosterone Rhythm in Male Rats. Endocrinology 2017, 158, 1339–1346. [Google Scholar] [CrossRef]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Gorgun, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef]
- Foss-Freitas, M.C.; Ferraz, R.C.; Monteiro, L.Z.; Gomes, P.M.; Iwakura, R.; De Freitas, L.C.C.; Foss, M.C. Endoplasmic reticulum stress activation in adipose tissue induces metabolic syndrome in individuals with familial partial lipodystrophy of the Dunnigan type. Diabetol. Metab. Syndr. 2018, 10, 6. [Google Scholar] [CrossRef]
- Shi, Y.; Taylor, S.I.; Tan, S.L.; Sonenberg, N. When translation meets metabolism: Multiple links to diabetes. Endocr. Rev. 2003, 24, 91–101. [Google Scholar] [CrossRef]
- Shi, Y.; Taylor, S.I.; Tan, S.L.; Sonenberg, N. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell Biol. 1998, 18, 7499–7509. [Google Scholar] [CrossRef]
- Harding, H.P.; Calfon, M.; Urano, F.; Novoa, I.; Ron, D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol. 2002, 18, 575–599. [Google Scholar] [CrossRef]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Spiegelman, D.; Manson, J.; Schernhammer, E.S.; Colditz, G.A.; Kawachi, I. Work characteristics and incidence of type 2 diabetes in women. Am. J. Epidemiol. 2007, 165, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Nakagawa, H.; Miura, K.; Soyama, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; Suwazono, Y.; Nogawa, K. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand. J. Work Environ. Health 2005, 31, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; La Fleur, S.; Fliers, E. Circadian control of glucose metabolism. Mol. Metab. 2014, 3, 372–383. [Google Scholar] [CrossRef]
- Perez-Tilve, D.; Stern, J.E.; Tschop, M. The brain and the metabolic syndrome: Not a wireless connection. Endocrinology 2006, 147, 1136–1139. [Google Scholar] [CrossRef]
- Brum, M.C.; Filho, F.F.; Schnorr, C.C.; Bottega, G.B.; Rodrigues, T.C. Shift work and its association with metabolic disorders. Diabetol. Metab. Syndr. 2015, 7, 45. [Google Scholar] [CrossRef]
- Al-Naimi, S.; Hampton, S.M.; Richard, P.; Tzung, C.; Morgan, L.M. Postprandial metabolic profiles following meals and snacks eaten during simulated night and day shift work. Chronobiol. Int. 2004, 21, 937–947. [Google Scholar] [CrossRef]
- Ghiasvand, M.; Heshmat, R.; Golpira, R.; Haghpanah, V.; Soleimani, A.; Shoushtarizadeh, P.; Tavangar, S.M.; Larijani, B. Shift working and risk of lipid disorders: A cross-sectional study. Lipids Health Dis. 2006, 5, 9. [Google Scholar] [CrossRef]
- Patel, P.; Abate, N. Body fat distribution and insulin resistance. Nutrients 2013, 5, 2019–2027. [Google Scholar] [CrossRef]
- Bahijri, S.; Borai, A.; Ajabnoor, G.; Abdul Khaliq, A.; AlQassas, I.; Al-Shehri, D.; Chrousos, G. Relative metabolic stability, but disrupted circadian cortisol secretion during the fasting month of Ramadan. PLoS ONE 2013, 8, e60917. [Google Scholar] [CrossRef]
- Van Amelsvoort, L.G.; Schouten, E.G.; Kok, F.J. Impact of one year of shift work on cardiovascular disease risk factors. J. Occup. Environ. Med. 2004, 46, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hannerz, H.; Albertsen, K.; Nielsen, M.L.; Tuchsen, F.; Burr, H. Occupational factors and 5-year weight change among men in a danish national cohort. Health Psychol. 2004, 23, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Ordovas, J.M.; Madrid, J.A. The chronobiology, etiology and pathophysiology of obesity. Int. J. Obes. 2010, 34, 1667–1683. [Google Scholar] [CrossRef]
- Van Someren, E.J.; Riemersma-Van Der Lek, R.F. Live to the rhythm, slave to the rhythm. Sleep Med. Rev. 2007, 11, 465–484. [Google Scholar] [CrossRef]
- Knutsson, A. Health disorders of shift workers. Occup Med. 2003, 53, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Gangwisch, J.E.; Malaspina, D.; Boden-Albala, B.; Heymsfield, S.B. Inadequate sleep as a risk factor for obesity: Analyses of the NHANES I. Sleep 2005, 28, 1289–1296. [Google Scholar] [CrossRef]
- Dijk, D.J.; Duffy, J.F.; Silva, E.J.; Shanahan, T.L.; Boivin, D.B.; Czeisler, C.A. Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS ONE 2012, 7, e30037. [Google Scholar] [CrossRef]
- Korompeli, A.; Sourtzi, P.; Tzavara, C.; Velonakis, E. Rotating shift-related changes in hormone levels in intensive care unit nurses. J. Adv. Nurs. 2009, 65, 1274–1282. [Google Scholar] [CrossRef]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef]
- Moreira, A.C.; Antonini, S.R.; De Castro, M. Mechanisms in Endocrinology: A sense of time of the glucocorticoid circadian clock: From the ontogeny to the diagnosis of Cushing’s syndrome. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2018, 179, R1–R18. [Google Scholar] [CrossRef]
- McHill, A.W.; Melanson, E.L.; Higgins, J.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Wright, K.P., Jr. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. USA 2014, 111, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.S.; Park, H.K.; Linton, J.A.; Lee, J.W.; Lee, H. Visceral adiposity and expression of clock genes in peripheral blood mononuclear cells: A pilot study. Chronobiol. Int. 2017, 34, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Reszka, E.; Peplonska, B.; Wieczorek, E.; Sobala, W.; Bukowska, A.; Gromadzinska, J.; Lie, J.A.; Kjuus, H.; Wasowicz, W. Rotating night shift work and polymorphism of genes important for the regulation of circadian rhythm. Scand. J. Work Environ. Health 2013, 39, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Skornyakov, E.; Chowdhury, N.R.; Gajula, R.P.; Middleton, B.; Satterfield, B.C.; Porter, K.I.; Van Dongen, H.P.A.; Gaddameedhi, S. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 7825–7830. [Google Scholar] [CrossRef] [PubMed]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Darlington, T.K.; Wager-Smith, K.; Ceriani, M.F.; Staknis, D.; Gekakis, N.; Steeves, T.D.; Weitz, C.J.; Takahashi, J.S.; Kay, S.A. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 1998, 280, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Shearman, L.P.; Sriram, S.; Weaver, D.R.; Maywood, E.S.; Chaves, I.; Zheng, B.; Kume, K.; Lee, C.C.; Van der Horst, G.T.; Hastings, M.H.; et al. Interacting molecular loops in the mammalian circadian clock. Science 2000, 288, 1013–1019. [Google Scholar] [CrossRef]
- Griffin, E.A., Jr.; Staknis, D.; Weitz, C.J. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 1999, 286, 768–771. [Google Scholar] [CrossRef]
- Schafer, M.; Werner, S. Nrf2—A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015, 88, 243–252. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Battaglia-Hsu, S.F.; Arnold, C. Endoplasmic Reticulum Stress in Metabolic Disorders. Cells 2018, 7, 63. [Google Scholar] [CrossRef]
- Koyanagi, S.; Hamdan, A.M.; Horiguchi, M.; Kusunose, N.; Okamoto, A.; Matsunaga, N.; Ohdo, S. cAMP-response element (CRE)-mediated transcription by activating transcription factor-4 (ATF4) is essential for circadian expression of the Period2 gene. J. Biol. Chem. 2011, 286, 32416–32423. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, H.; Li, C.; Xiao, Y.; Yang, D.; Zhang, M.; Zhou, D.; Liu, W.; Wang, A.; Jin, Y. ER stress activation impairs the expression of circadian clock and clock-controlled genes in NIH3T3 cells via an ATF4-dependent mechanism. Cell Signal. 2019, 57, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.; Wang, Y.; Carreras, A.; Hirotsu, C.; Zhang, J.; Peris, E.; Gozal, D. Chronic sleep fragmentation during the sleep period induces hypothalamic endoplasmic reticulum stress and PTP1b-mediated leptin resistance in male mice. Sleep 2015, 38, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef] [PubMed]
- Wible, R.S.; Ramanathan, C.; Sutter, C.H.; Olesen, K.M.; Kensler, T.W.; Liu, A.C.; Sutter, T.R. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife 2018, 7, e31656. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Day Workers (n = 20) | Night Workers (n = 20) |
|---|---|---|
| Age, y | 38 (6.8) | 40 (4.9) |
| Sex, % male | 10 (50%) | 10 (50%) |
| Weight, kg | 70.1 (12.4) | 77.3 (10.0) |
| Height, m | 1.64 (0.07) | 1.72 (0.08) |
| Sleep time work day, h | 8.1 (0.5) | 9.1 (0.6) |
| Sleep time free day, h | 8.5 (0.5) | 8.2 (0.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz-Bannitz, R.; Beraldo, R.A.; Coelho, P.O.; Moreira, A.C.; Castro, M.; Foss-Freitas, M.C. Circadian Misalignment Induced by Chronic Night Shift Work Promotes Endoplasmic Reticulum Stress Activation Impacting Directly on Human Metabolism. Biology 2021, 10, 197. https://doi.org/10.3390/biology10030197
Ferraz-Bannitz R, Beraldo RA, Coelho PO, Moreira AC, Castro M, Foss-Freitas MC. Circadian Misalignment Induced by Chronic Night Shift Work Promotes Endoplasmic Reticulum Stress Activation Impacting Directly on Human Metabolism. Biology. 2021; 10(3):197. https://doi.org/10.3390/biology10030197
Chicago/Turabian StyleFerraz-Bannitz, Rafael, Rebeca A. Beraldo, Priscila Oliveira Coelho, Ayrton C. Moreira, Margaret Castro, and Maria Cristina Foss-Freitas. 2021. "Circadian Misalignment Induced by Chronic Night Shift Work Promotes Endoplasmic Reticulum Stress Activation Impacting Directly on Human Metabolism" Biology 10, no. 3: 197. https://doi.org/10.3390/biology10030197
APA StyleFerraz-Bannitz, R., Beraldo, R. A., Coelho, P. O., Moreira, A. C., Castro, M., & Foss-Freitas, M. C. (2021). Circadian Misalignment Induced by Chronic Night Shift Work Promotes Endoplasmic Reticulum Stress Activation Impacting Directly on Human Metabolism. Biology, 10(3), 197. https://doi.org/10.3390/biology10030197






