Abstract
This work was on the comparative evaluation of the property effects obtainable when acetylation is applied to parts of selected agro fibers that are obtainable within common localities. The fibers were subjected to different concentrations of acetylation treatment at ambient temperature for 3 h. The physico-chemical, morphological, and tensile properties of the fibers were examined after the treatment. It was discovered from the results that the procedures variedly influenced the constituents of the fibers, their resulting tensile properties as well as their post-acetylation treatment surface morphology. The proportion of crystalline cellulose in the starting fibers greatly influenced their post treatment composition, behaviour and properties. The results show that plantain fibers had the highest aspect ratios, followed by banana fibers with values of about 1000 and 417, respectively. These fibers exhibited the least density and are thus potential plant fibers for composite development. Banana fiber had the least density of about 1.38 g/cm3 while that of Dombeya Buettneri fiber possessed the highest value of 1.5 g/cm3. There was significant enhancement in the hemicellulose content of Combretum Racemosum, while the lignin content of the plantain fibers was highly reduced. The treatment favoured the enhancement of the tensile properties in Combretum Racemosum fibers, which had enhanced tensile strength and strain at all compositions of the treatment. Optimum tensile strength and strain values of 155 MPa and 0.046, respectively, are achieved at 4% composition. Dombeya Buettneri fibers showed the highest ultimate tensile strength among the plant fibers in the untreated condition, which was gradually decreased as the concentration of the reagents was increased. Overall, 4% acetylation treatment is optimum for tensile properties’ enhancement for most of the natural fibers evaluated.
1. Introduction
The global drive towards a circular economy through the incorporation of sustainability in manufacturing processes has encouraged the use of agro bye-derivatives such as natural fibers in applications that have hitherto been completely reliant on inorganic feedstocks. The widespread development of natural-fiber-reinforced composites has been limited by challenges in the compatibility of the matrix and the fibers which comprise the reinforcement phase. Fiber-reinforced composites are a large class within this category of materials. The reinforcement of materials such as polyester with fibers from various lignocellulosic sources has been reported by numerous researchers [1,2,3,4,5,6].
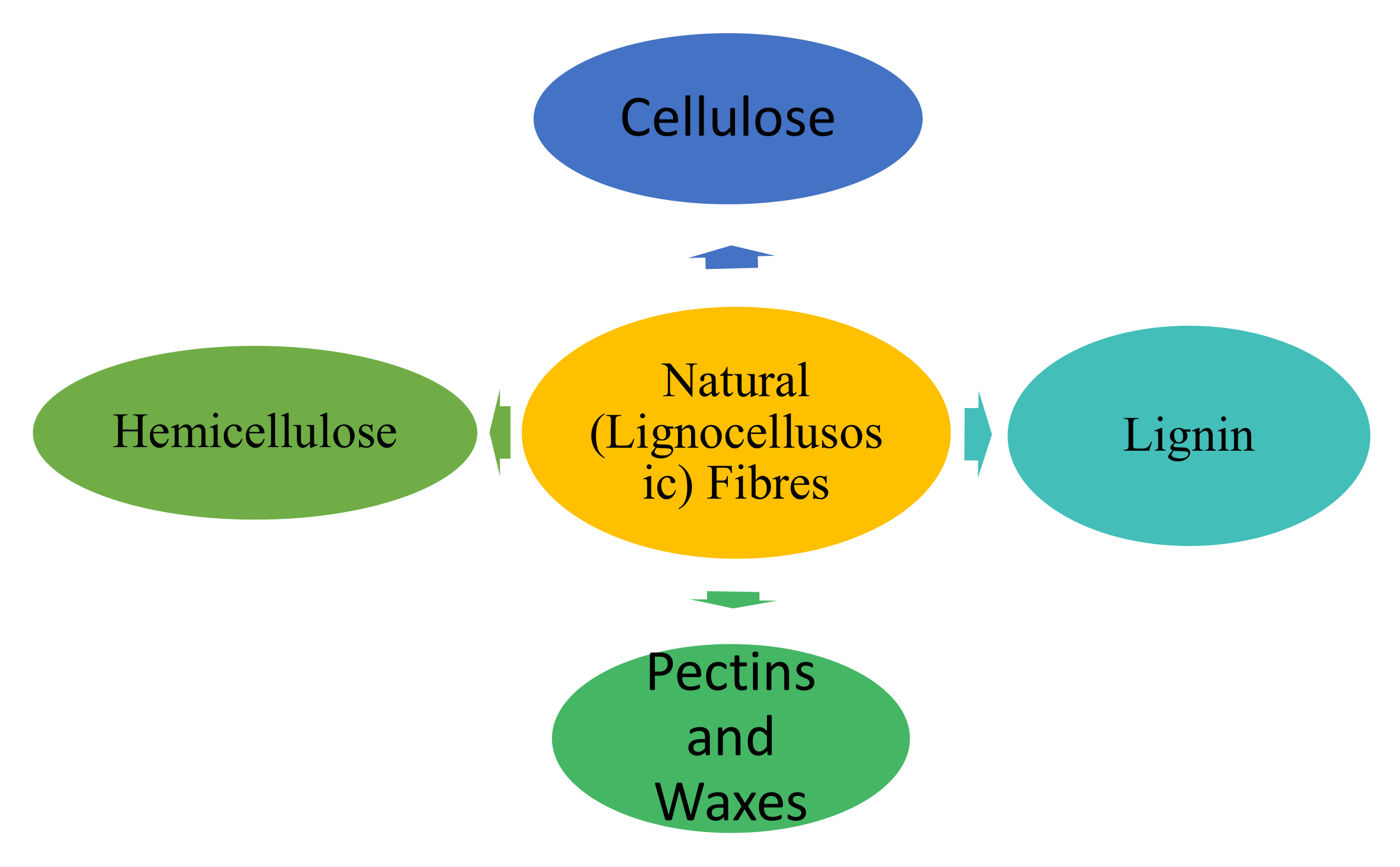
Natural fibers provide safer and more sustainable alternatives to the synthetic fibers that have mainly been used in the development of composite materials a priori. The extension of studies into renewable-resources-based natural fibers has created newer methods that are more sustainable, safer, and environmentally friendly and has also led to low-cost alternatives to artificial fibers [7]. Plants with parts that contain usable fibers are naturally grown in suitable environments throughout the world [6]. Such fibers can be extracted from stems [8], leaves, and fruits [9] of many commonly grown plants depending on which plant is most abundant in which area. However, natural fibers possess significant drawbacks in properties. The structure of natural fibers consists essentially of varied proportions of celluloses, hemicelluloses, lignin, pectins, and waxy substances [10], as shown in Figure 1.
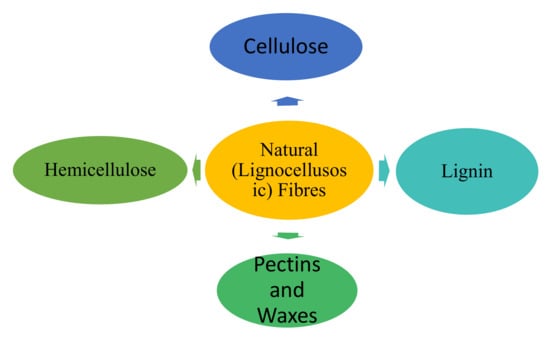
Figure 1.
Typical components of most natural fibers.
Celluloses are naturally occurring polymers that consist of D-anhydro-glucose ( repeating units interconnected by b-1,4-glyco-sidic links, with up to 10,000 degrees of monomer polymerization each of which contains three hydroxyl (OH-) groups [11]. Hemicellulose differs from cellulose in that cellulose is a linear polymer, whereas the latter comprise of several different sugar monomers and show a significant level of chain branching, which contains pendantic side groups that induce non-crystallinity and possess higher (around 50–300) degrees of polymerization than native cellulose. Lignins are amorphous [12], hydrophobic, and complicated polymers comprising both dense aliphatic and aromatic constituents which impart rigidity to plants. Pectins refer to a collection of hetero polysaccharides which aid the flexibility of the plant and the remaining parts of most plants comprise of waxes which contain various types of alcohols.
Some of these components expose the overall fiber structure to performance limiting phenomena such as moisture absorption from the surroundings, causing voids and weakening the binding between the fibers and polymer matrices. The differences in the chemical structure of the fibers and matrices also causes poor fiber–matrix adhesion. Minimal resistance to microbial action as well as inherent susceptibility to rotting also restricts successful utilization of natural fibers for composite applications. These faults result in ineffective stress (load) transfer across the fiber–matrix interface, thus limiting usage. There is therefore a need to modify the natural fibers to make them less hydrophilic [13].
Several methods have been developed to overcome this limitation to interfacial adhesion between ligno-cellulosic fibers and matrices, which affects wide-range application [2]. These methods can be classified into two main categories, namely, interphase modifying and chemical treatment. Chemical treatments [14] have continued to be the most promoted of all fiber pretreatment techniques because of its lower energy and manpower requirements. Chemical treatments help reduce OH- functional groups on the surfaces of fibers, modify the microstructure, improves surface chemistry and topography/roughness, and thus create enhanced interfacial interactions between fibers and matrix in the fibers, giving rise to better thermochemical properties in the developed composites due to improved wettability, surface morphology and general fiber–matrix compatibility [5,15,16,17,18,19,20,21,22,23,24,25].
Acetylation is a chemical treatment procedure that entails the use of a suitable catalyst (mostly acetic acid) to eliminate atoms of hydrogen in the hydrophilic hydroxyl branches of cellulose molecules by grafting and placement of acetyl groups onto the cellulose structure at these initially hydrogenated sites. Natural fibers can be acetylated with or without an acid catalyst to graft acetyl groups onto the cellulose structure. Acetylation helps to reduce the susceptibility of natural fibers to moisture absorption. Thus, improving the dimensional stability and resistance to the environmental degradation of both the reinforcement fibers as well as the resulting fiber-reinforced composite. It has been reported that acetylation yields fiber composites with better results at all fiber loadings [26]. It has been reported to cause the formation of strong covalent bonds, thereby creating an evident improvement in tensile strength and Young’s modulus of treated fibers [27].
Seena et al. [28] researched how acetylation affects the properties of banana-fiber-reinforced phenol formaldehyde composites, reporting that the tensile strength, tensile modulus and impact strength were improved in comparison to non-treated banana fiber composites. Zafeiropoulos et al. [29] studied the acetylation of flax, hemp, and wood fiber and reported its removal of noncrystalline fractions in fibers, altering the surface characteristics/topography, changing the fiber surface free energy and improving the efficiency of stress transference at the interface.
Dombeya is one dicotyledonous member of the Sterculiaceae (Cola) family of angiospermic spermatophytes (flowery seed-bearing plants), which is common worldwide. The stem is very fibrous and usually processed locally into ropes, nets, and fishing apparatus. Combretum Racemosum is a member of a large family of herbs, shrubs, and trees (combretaceae) with a tropical distribution. The plant provides a sustainable and continuous supply of natural fibers for the preparation of composites with fiber reinforcement at limited costs [8]. The global banana production has been estimated to have grown from 69 Mt in 2000–2002 to 116 Mt in 2017–2019 [30]. The trunks are often just left to rot after the fruits have been harvested in plantations. Up to 50% of the world’s plantain production is from Africa. Up to 130 different countries in the world grow the crop [30]. Its pseudostem can also be processed to obtain fibers that can be used in engineering applications.
Most of the published works on the acetylation processing of natural fibers have separately employed the process for the fibers concerned with processing variables different from case to case. The researchers employed the same acetylation treatment procedure in the processing of selected agro fibers and evaluated the resulting influence on selected properties in both treated and untreated conditions. The novelty of the work lies in the application of the treatment of newly introduced natural fibers such as plantain, Dombeya Buettneri and Combretum Racemosum which have hitherto not been widely researched. The viability of acetylation as a common chemical treatment procedure that can be applied in the batch processing of fibers from a variety of agro sources, as may be obtainable within the same or common area(s) was studied with respect to the constituents, tensile properties and surface modification.
2. Materials and Methods
2.1. Material Preparation
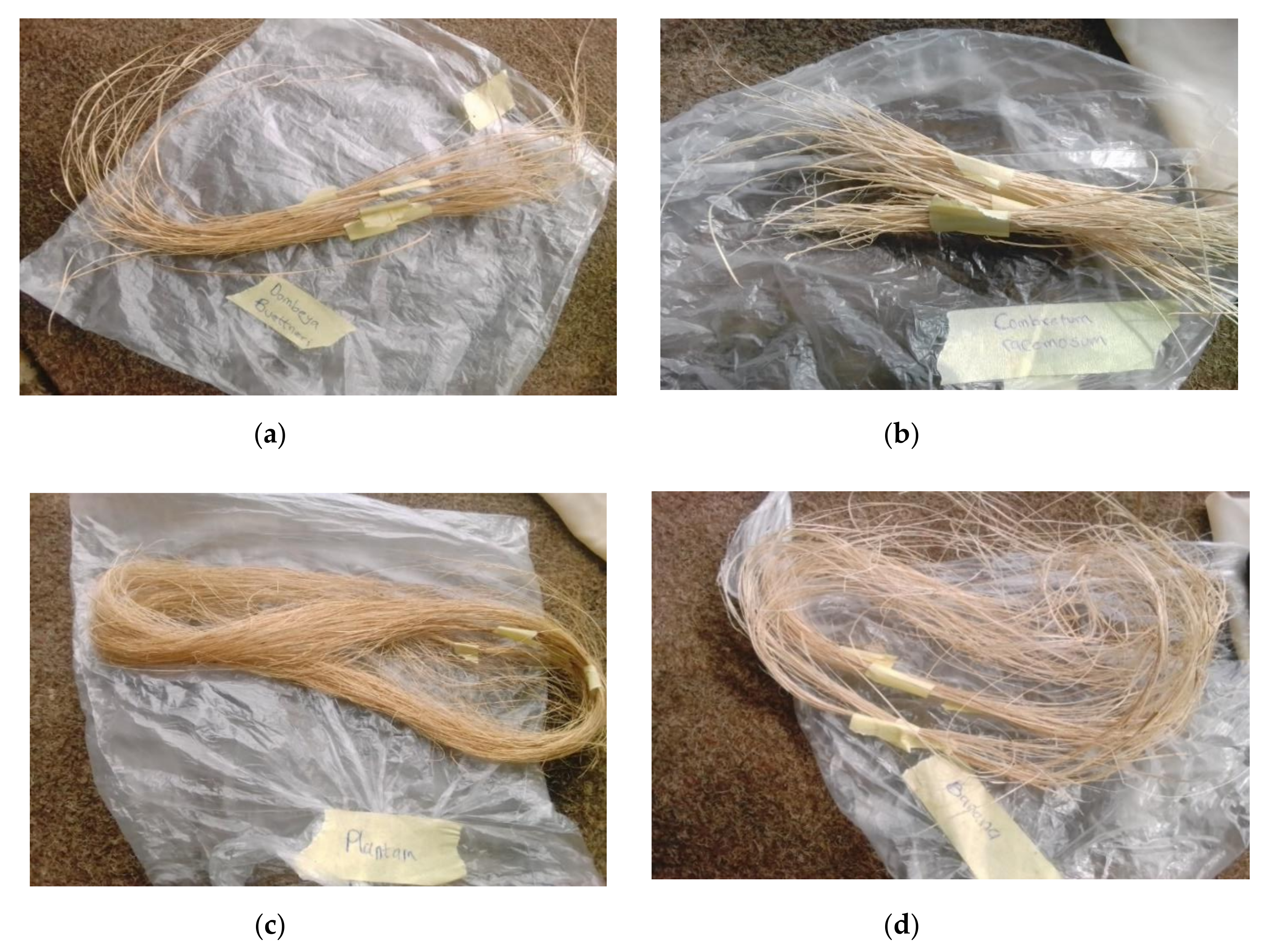
Natural fibers were obtained from Dombeya Buettneri, Combretum Racemosum, Plantain (Musa Acuminata), and banana (Musa Parasidica). They were procured from a common area within Akure metropolis. Litmus paper (blue and red), concentrated acetyl anhydride, distilled water, and soya oil were obtained from Pascal scientific store, Akure, Ondo State, Nigeria. Different extraction processes were adopted for the selected plant fibers as appropriate. The selected fibers were stem fibers. Dombeya fibers were extracted via hand stripping. The bark of the plant was peeled by hand from the stem and sun dried for 5 days within an interval of 8 h each day during sunlight to allow for the removal of moisture before further hand stripping. Combretum Racemosum fibers were extracted via water retting. The plant was soaked in tap water for 4 days, after which the fiber strands were extracted from the stem. Water retting was employed in order to better control on the environment and the extraction process. The stated retting period was selected in order to prevent excessive fiber degradation [31]. Banana and plantain have similar features and their fibers were extracted via hand stripping of the dew retted pseudostems. The extracted fibers were then sundried for 5 days within an interval of 8 h each day during sunlight. The extracted agro fibers are shown in Figure 2.
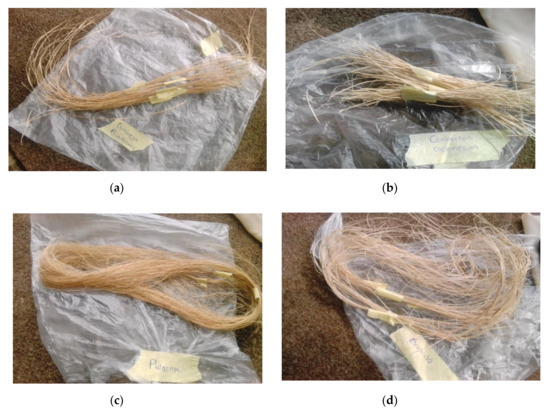
Figure 2.
Fibers obtained after extraction processes from (a) Dombeya Buettneri, (b) Combretum Racemosum, (c) plantain and (d) banana plants.
2.2. Acetylation Treatment
The extracted fibers from the four selected plants were soaked in a solution of acetyl anhydride of varying compositions, namely, 2, 4, and 6% v/v at room temperature for 3 h. They were then removed, washed with tap water, and repeatedly rinsed with distilled water until all excess acid had been removed. Neutrality was confirmed using litmus papers. Parts of the fibers from each plant type were left untreated to serve as control samples. These were also washed with tap and distilled water before both treated and untreated fibers were sun dried for 5 days. These fibers were selected for their wide spread relatively close availability in Nigeria as waste from plants.
2.3. Physico-Chemical Tests
2.3.1. Density Measurement
Apparent density was calculated for each fiber via the Archimedes principle. Known weights of fibers were immersed into an inert liquid of lower density than the fibers [32]. Water was used as the liquid medium. Apparent bulk densities for the plant fibers were subsequently determined using Equation (1). This process was carried out three times for repeatability.
The initial volume of water used was taken to be 80 cm3.
2.3.2. Aspect Ratio
About 50 mm length of each plant fiber was measured and cut out as a standard length. The nominal diameter used was obtained by finding the average of the diameters of three representative samples for each type of fiber. Moreover, since the thickness of natural fiber varies across its length, the diameter was taken across three zones at different locations at both ends and at the middle. This was done using a digital vernier caliper. The aspect ratio of each plant fiber was determined from Equation (2).
where:
- L = length of each fiber which is constant for all. D = average diameter for each fiber.
This process was carried out in triplicates for repeatability.
2.3.3. Evaluation of Fiber Fractions/Proximate Analysis
Celluloses, hemicelluloses, and lignin contents of the extracted fibers were evaluated through the same procedure adopted by Oladele et al. [14] to evaluate the presence of neutral detergent fiber (NDF) and acid detergent fiber (ADF). The weights for the neutral detergent Fibers (NDF), Acid detergent fiber (ADF), as well as acid detergent lignin (ADL) were computed. The values were then used in determining the wt.% of cellulose and hemicelluloses present. The wt.% of lignin content was obtained via gravimetric method.
Determination of Neutral Detergent Fiber (NDF)
Dried samples of the fibers were ground and then transferred inside small (5 mL) porcelain mortars. A gram of some ground fibers (pre-dried with air) was next taken and placed inside the beakers of the reflux apparatus. About 100 mL of neutral detergent solution and about 1/2 g of solution was added. This was heated to boil and was subjected to refluxing for 60 min immediately after boiling. The resulting mix was then filtered into crucibles and washed three times with boiling water and then twice with acetone, dried for 8 h at 105 °C and cooled in a desiccator. The crucible was again weighed to compute the neutral detergent fiber via Equation (3).
where:
- W1 = weight of the crucible when empty. W2 = total weight of crucible and residue.
- W3 = weight of dried sample.
Evaluation of Acid Detergent Fibers (ADF)
The methodology follows that used to determine the NDF. However, the residue obtained at the end of NDF evaluation was placed in the beaker of the reflux apparatus. About 100 mL of acid detergent solution (detergent + dil. H2SO4) was then added thereafter. This was followed by heating the mixture to boil and refluxing for 60 min upon the onset of boiling.
The mixture was filtered into empty crucibles, triply rinsed in boiling water, and next doubly rinsed with cold acetone. It was then subjected to 8 h of drying at 105 °C and finally cooled in a desiccator and weighed. ADF was computed via Equation (4).
where:
- W1 = weight of crucible (emptied). W2 = total weight of crucible and residues and;
- Ws = weight of dry samples.
Gravimetric Evaluation of Lignin Contents
The Oladele et al. [14,33]’s method was also employed for this objective. A concentrated solution containing 72% sulphuric acid was added to 1.5 g of the sample placed in a beaker soaked for 2 h. Thereafter, 8% H2SO4 was introduced, followed by 4 h of refluxing. The residue was then filtered using a purpling cloth and repeatedly rinsed with hot water. The resulting sample was scraped into an empty crucible. This was followed by 2 h of oven drying of the hydrolyzed sample at 105 °C and subsequent cooling in a desiccator and reweighing. The cooled sample was then ashed in a muffle furnace at 550 °C for 3 h after which it was cooled in a desiccator and weighed.
The percent weight of lignin was determined via Equation (5).
where:
- W1 = total weight of ash samples + crucibles, W2 = total weight of oven dried sample + crucibles,
- Ws = weight of dried extractive free sample, i.e., NDF residue.
Weight percent of lignin is also often referred to as acid detergent lignin (ADL).
Evaluation of Celluloses Content
The percent cellulose in the fiber was computed via Equation (6).
Evaluation of Hemicellulose Contents
The hemicellulose content was evaluated via Equation (7).
2.3.4. Mechanical (Tensile) Properties Evaluation
ASTM D3822M—14(2020) [34] was adopted in evaluating the tensile properties of the fibers. Tests were conducted via a universal testing machine with 2.0 mm/min crosshead speed. Strands from the treated and untreated sets of each type of fibers were tested and the average values were taken as representatives. Each of the plant fibers was fixed down manila card coupons using adhesive tape across a circular 50 mm diameter hole. Hydraulic clamps were used to grip the ends of the manila card coupons to align fibers with the machine axis. This process was carried out on three samples from each fiber sample and the average values were taken as representative values.
2.4. Scanning Electron Microscopy
Scanning electron microscopy (SEM) is a superb tool which aids in probing the morphology of material surfaces. This greatly helps to assess the surface structure of the fibers, especially after chemical treatment. Surfaces of the samples were examined by means of SEM operated at 15 kV. The sample surfaces were prepared by gold coating to impart surface conductivity before SEM observation using a Quorum sputter and SEM carbon coating machine (Q150RES, Carl Zeiss, Germany).
3. Results and Discussions
3.1. Density of Selected Plant Fibers
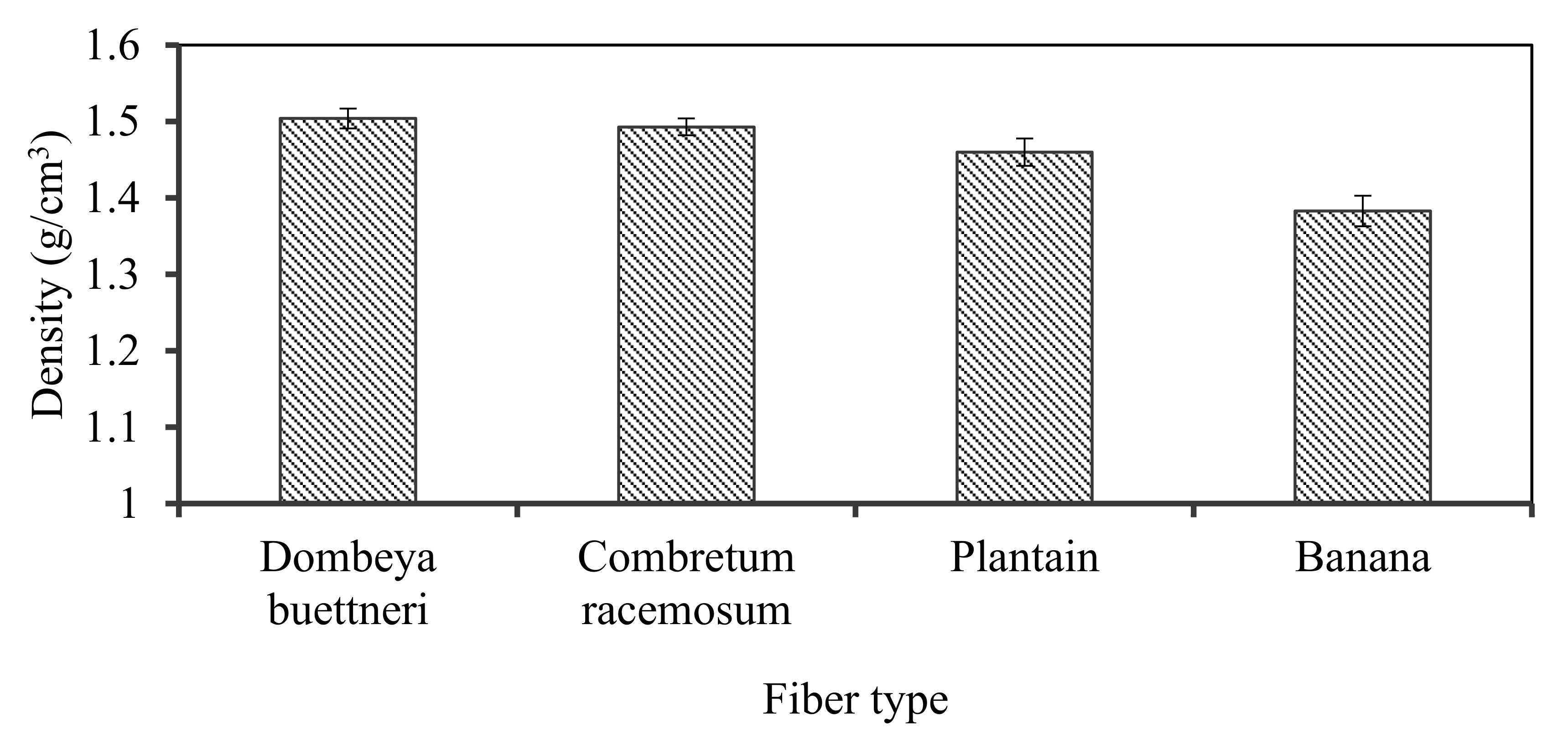
The computed fiber densities ranged between 1.38 and 1.50 g/cm3. The natural fiber density values are significantly lower compared to that of synthetic glass fibers because the reported densities for glass fibers range between 2.46 and 2.58 g/cm3 [3,35,36,37,38,39,40].
Density is a major criterion employed in the choice of plant fibers as suitable substitute(s) for synthetic ones. Being lightweight is an essential factor in material selection for various applications. The densities of the selected fibers were thus evaluated. It was revealed that banana fiber had the least density of about 1.38 g/cm3 while Dombeya fiber possessed the highest density value of 1.5 g/cm3. The physical and chemical identities of the fiber and matrix materials are retained in many composite materials development processes, making the composite formed possess a blend of properties not attainable by each constituent [41]. Plant fibers are sustainable and biodegradable materials, making their products desirable and thus preferable in many areas of applications. Figure 3 shows the variation in the densities of the fibers obtained from the selected plants that were evaluated.
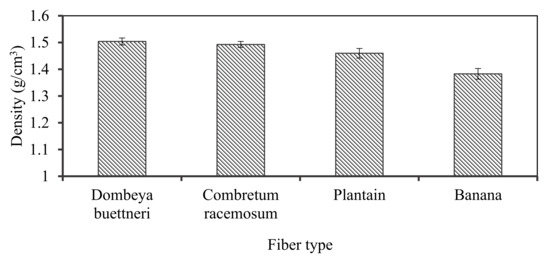
Figure 3.
Variation in the density of the selected plant-fibers.
3.2. Aspect Ratio of Plant Fibers
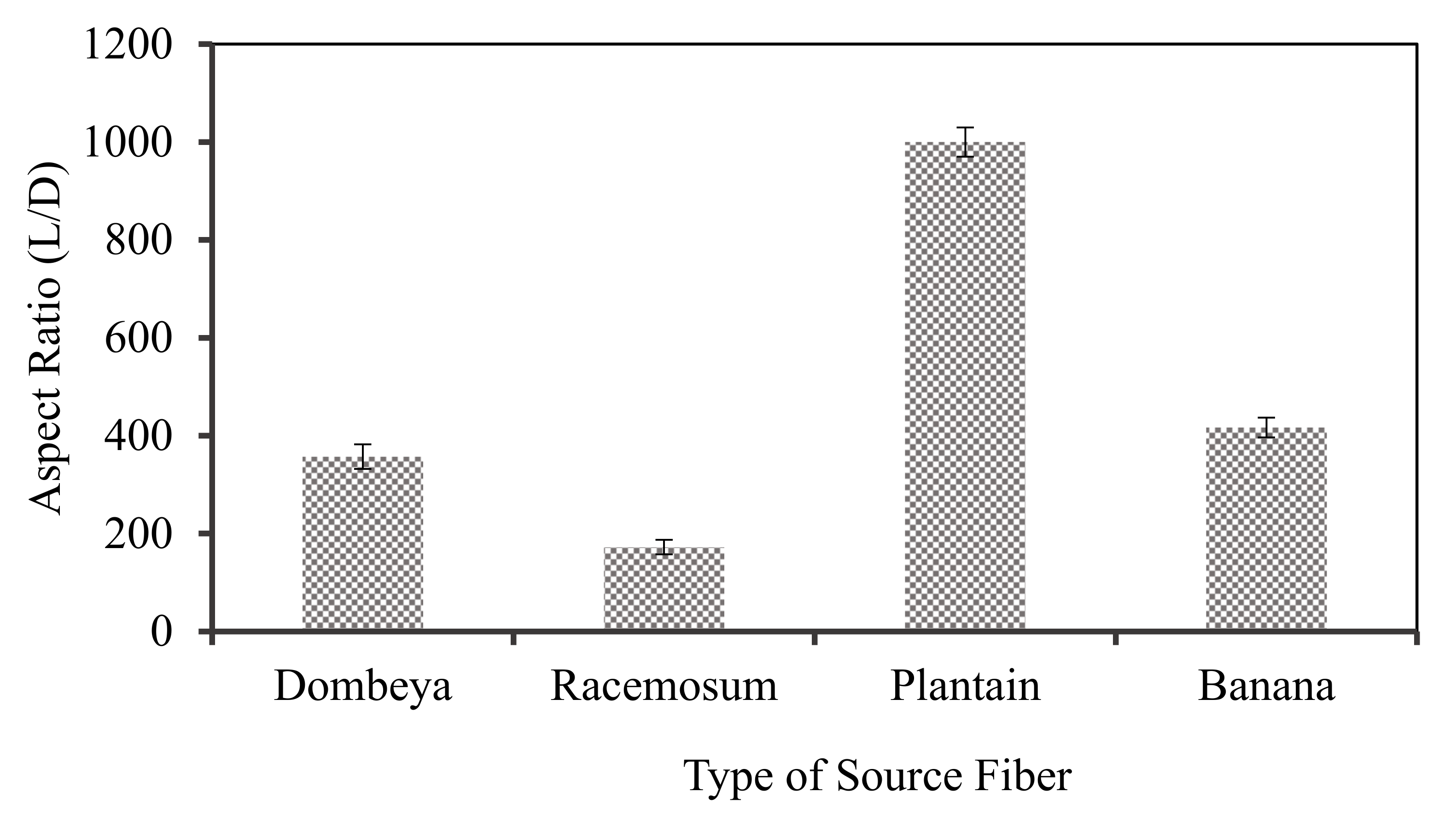
Aspect ratio is another important fiber property used to determine their suitability as reinforcement materials. Long and thin fibers (higher length to diameter) promote superior properties but are more expensive to produce. Thus, although more difficult to evenly disperse in composites, plant fibers with higher aspect ratios (longer and thinner) are more acceptable/preferable to short and thick ones. Fiber aspect ratio is computed by dividing the length by the diameter. The parameter thus gives the ratio of the fiber length to diameter. Fibers with higher aspect ratios are generally stiffer.
Aspect ratios of natural fibers differ globally due to variations in plants’ geographical location, age, and species amongst others. Figure 4 shows the variation in the aspect ratio of the selected fibers evaluated in their raw/untreated conditions. The results showed that plantain fibers followed by banana fibers had the highest aspect ratios with values of about 1000 and 417, respectively. As shown in Figure 3, these fibers have the least density and are thus more potentially applicable to lightweight composite development. Bananas and plantains are of the same family with similar physical structure but with different fruits. Their stems and leaves are readily available as waste after harvesting.
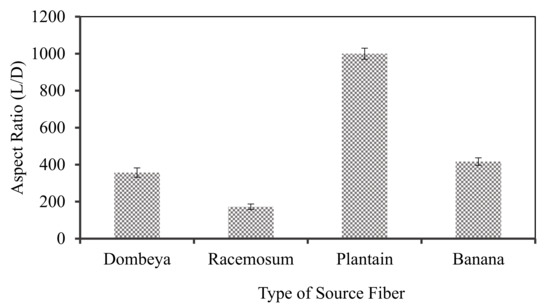
Figure 4.
Aspect ratio of the selected fibers in untreated conditions.
3.3. Effects of Acetylation on the Constituents of Selected Plant Fibers
The proximate compositions of all agro-fibers in treated and untreated situations were shown in Table 1, Table 2, Table 3 and Table 4.

Table 1.
Proximate composition of the treated and untreated Dombeya Buettneri fibers.

Table 2.
Proximate composition of the treated and untreated Combretum Racemosum fibers.

Table 3.
Proximate composition of the treated and untreated banana fibers.

Table 4.
Components (proximate) composition of raw and acetylated Plantain fibers.
3.3.1. Effects of Acetylation on the Constituent (Proximate) Composition of Dombeya Buettneri Fibers
Table 1 presents the proximate analysis results obtained after acetylation treatment of Dombeya Buettneri fibers in comparison with the untreated (control) fiber. The observed increase in cellulosic content in the natural fiber (from 26.06% for untreated fibers to 30.30, 35.72, and 40.31% for fibers in the acetylation media of 2, 4 and 6% concentrations, respectively) can be ascribed to the removal of lignin, hemicelluloses and other extractables from the fibers. Both hemicellulose and lignin contents were generally reduced in comparison to their proportions in the untreated fibers. Thus, in agreement with previous findings [33], the chemical treatment enhanced the yield of cellulose, which is the most desirable constituent essential for reinforcement. Besides, cellulose could be either crystalline or non-crystalline and this affects the response of plant fibers to chemical treatments, with highly crystalline fibers exhibiting greater resistance to chemical treatment [14,42].
3.3.2. Effects of Acetylation on the Constituent (Proximate) Composition of Combretum Racemosum Fibers
The proximate composition of the acetylated and untreated Combretum Racemosum fibers is as shown in Table 2. The results show a reduction in cellulose content as the acetylation treatment concentration increases. This reduction can be attributed to the hydrolysis of the fiber cellulose, in agreement with the submission of some researchers [14]. The results show that a higher proportion of cellulose in Combretum Racemosum fiber is likely to be non-crystalline. Non-crystalline/amorphous cellulose is more easily attacked and hydrolyzed during treatment than its crystalline counterpart. This may partly explain the reduction in cellulose content after acetylation treatment. Such effect has been observed by Castro et al. [42], amongst others.
3.3.3. Effects of Acetylation on the Constituent (Proximate) Composition of Banana (Musa Acuminata) Fibers
As shown in Table 3, a similar trend with Table 2 was observed where acetylation treatment causes a decrease in the cellulose content as the concentration increases. Banana fiber cellulose content reduced from 60.89% for the untreated fiber to 36.12, 29.62, and 21.51% for the 2, 4, and 6% v/v acetylation media, respectively. Consequently, hemicellulose content was increased for all concentrations of the acetylation media when compared with that of the untreated banana fiber. The reason for the observed trend was similar to what was noticed in Combretum Racemosum fibers as stated under the discussion of Table 2. Banana has higher amounts of amorphous cellulose, which is more hydrolysable, hence a reduction in cellulose content after treatment. This has been reported by Castro et al., and others [42,43].
3.3.4. Effects of Acetylation on the Constituent (Proximate) Composition of Plantain Fibers
Table 4 presents the relative amounts of the constituents in plantain fibers in both treated and untreated conditions. It was noticed that the raw plantain fiber has lower cellulose content (high carbohydrate source) (24.76 ± 1.24%) in comparison with banana fiber (60.89 ± 1.32%) despite being of the same genus [44,45]. Hence, acetylation also showed a distinct effect on plantain (Musa Paradisiaca) fibers compared to its effect on banana (Musa Acuminata) fibers. The cellulose content increases as the acetylation treatment concentration increases. This implies that the plantain pseudo-stems fibers contain higher amounts of crystalline type cellulose. This is in agreement with the discussion of the results for Dombeya Buettneri fiber presented in Table 1. The cellulose in banana is more degradable than the cellulose in plantain [42]. The acetylation process is accompanied by marked delignification as the acetylation media concentration is increased. The treatment reduced the lignin content by up to 62.4% (54.66 to 20.53) with increasing concentration of acetylation media. This marked delignification may also explains the apparent increment in cellulose and hemicellulose contents since their post-treatment proportion in the overall mass will now be increased compared to their initial values.
3.3.5. Effects of Acetylation on the Cellulose Content of Selected Plant Fibers
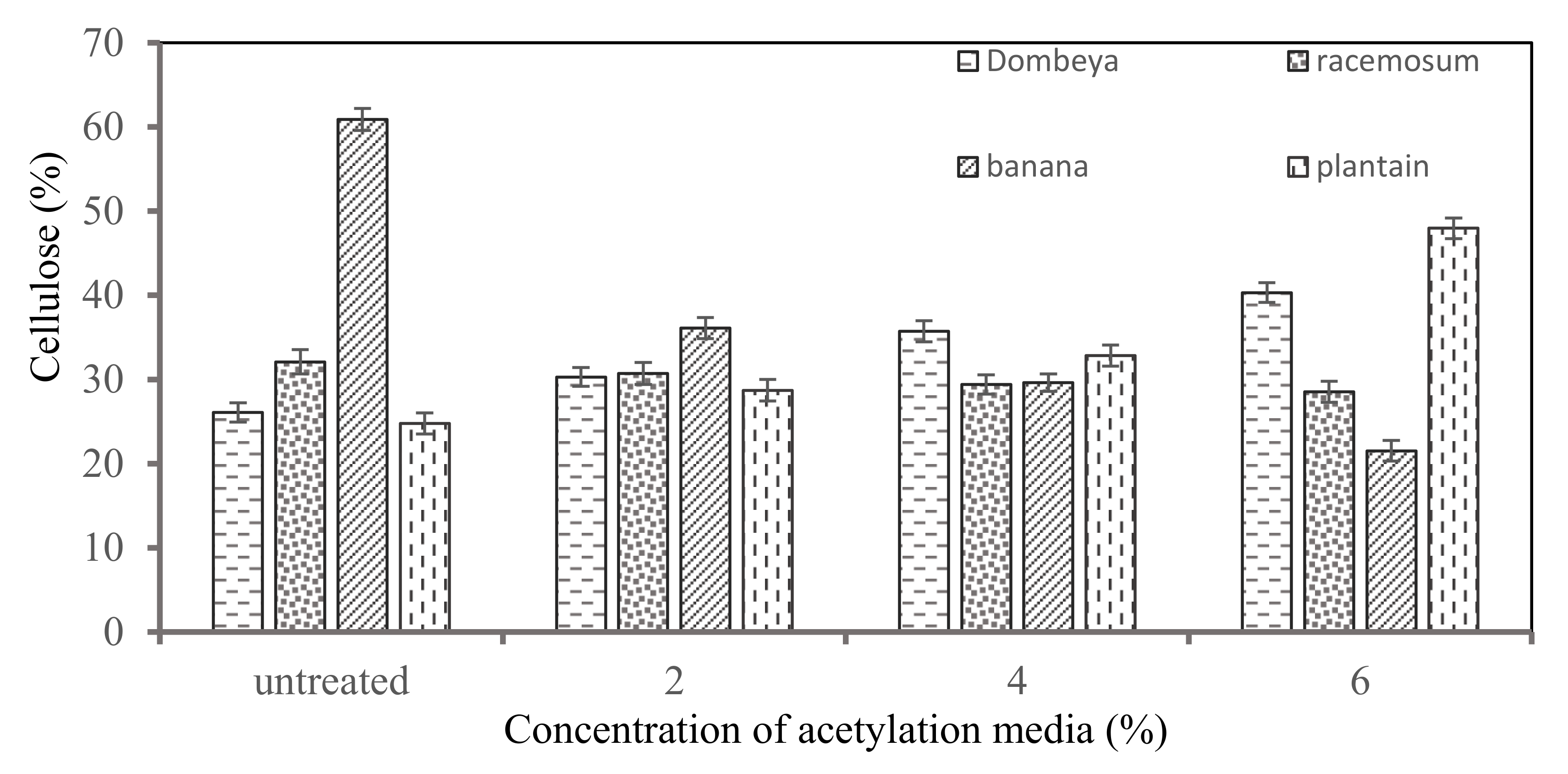
Figure 5 shows the results of the comparative cellulose contents in the selected plant fibers. From the results, it was observed that cellulose content tends to increase as the concentration of acetylation treatment media increases for Dombeya Buettneri and plantain fibers while it tends to reduce for banana and Combretum Racemosum fibers. The reason for this noticeable difference among these fibers was due to the proportion of their crystalline/amorphous cellulose content. Fibers with a higher proportion of crystalline cellulose tend to have their cellulose increase as the treatment concentration increases, while in fibers with higher initial proportion of amorphous cellulose, it tends to decrease as the treatment concentration increases. The goal of chemical treatment is not only to modify the surface morphology of the fibers, but also to increase the crystalline cellulose content by removing non-cellulosic/amorphous constituents in plant fibers. Excessive chemical treatment may reduce cellulose content due to hydrolysis of the non-amorphous/less crystalline portions of the cellulose. It was discovered that banana had the highest cellulose content in the raw/untreated condition (60.89%) while plantain had the highest value after treatment (47.97%).
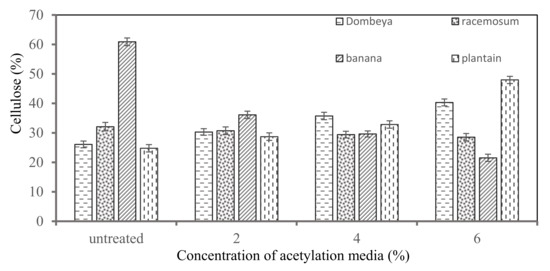
Figure 5.
Variation in cellulose content of the selected Natural fibers with increasing concentration of chemical (acetylation) media.
3.3.6. Effects of Acetylation on the Hemicellulose Content of Selected Plant Fibers
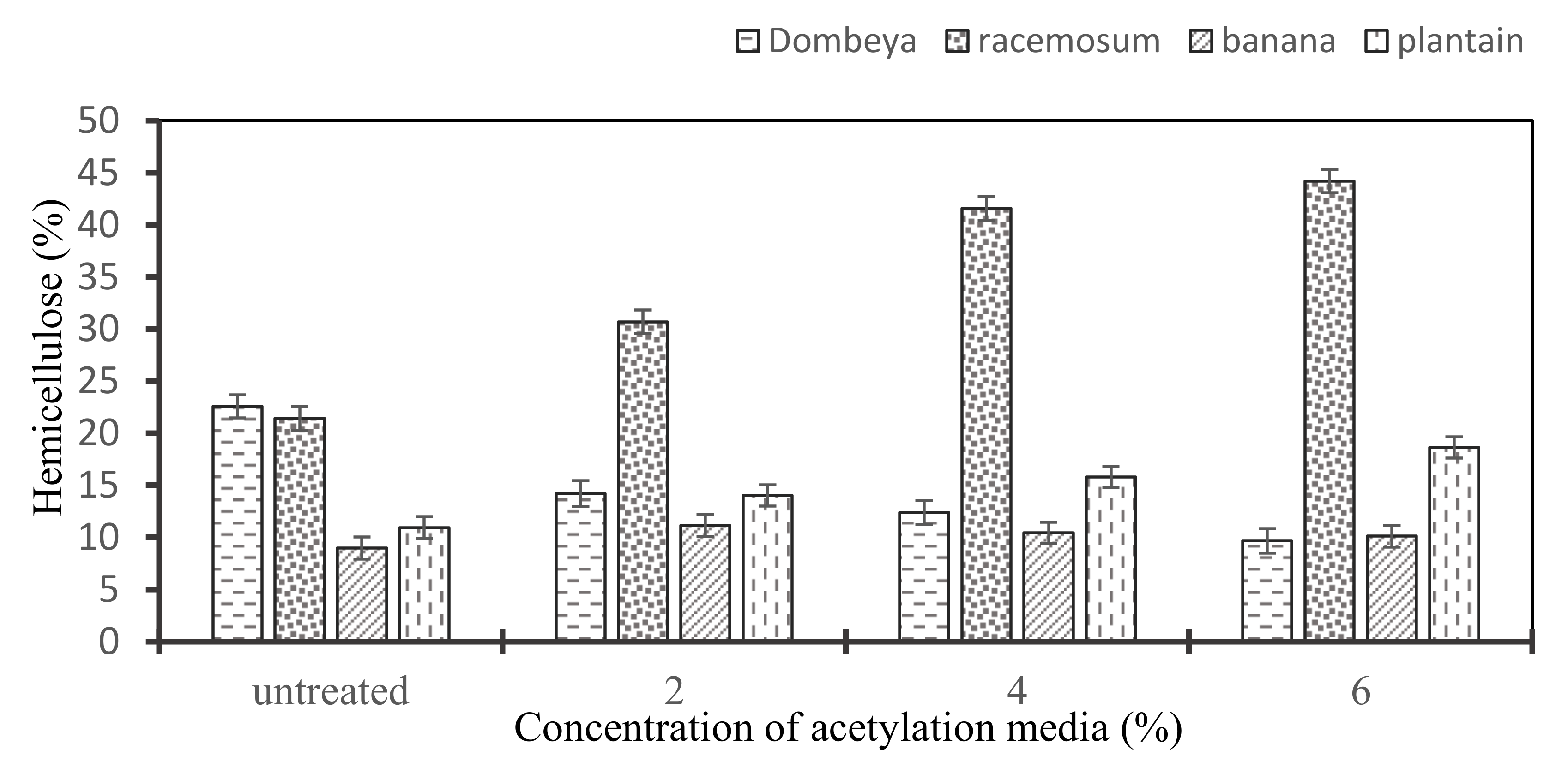
As shown in Figure 6, the treatment brought about different modifications on the hemicellulose content of the fibers. All except Dombeya Buettneri have their hemicellulose content seemingly increased, albeit in variable proportions. This may not infer any absolute inertness of hemicellulose to acetylation treatment but may rather be attributable to significant alterations in the proportions of the other constituents of the fiber structure (e.g., lignin, amorphous cellulose) after the treatment.
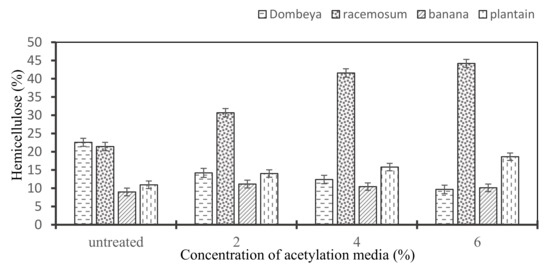
Figure 6.
Variation in the hemicellulose content of the selected natural fibers with increasing concentration of acetylation media.
3.3.7. Effects of Acetylation on the Lignin Content of Selected Plant Fibers
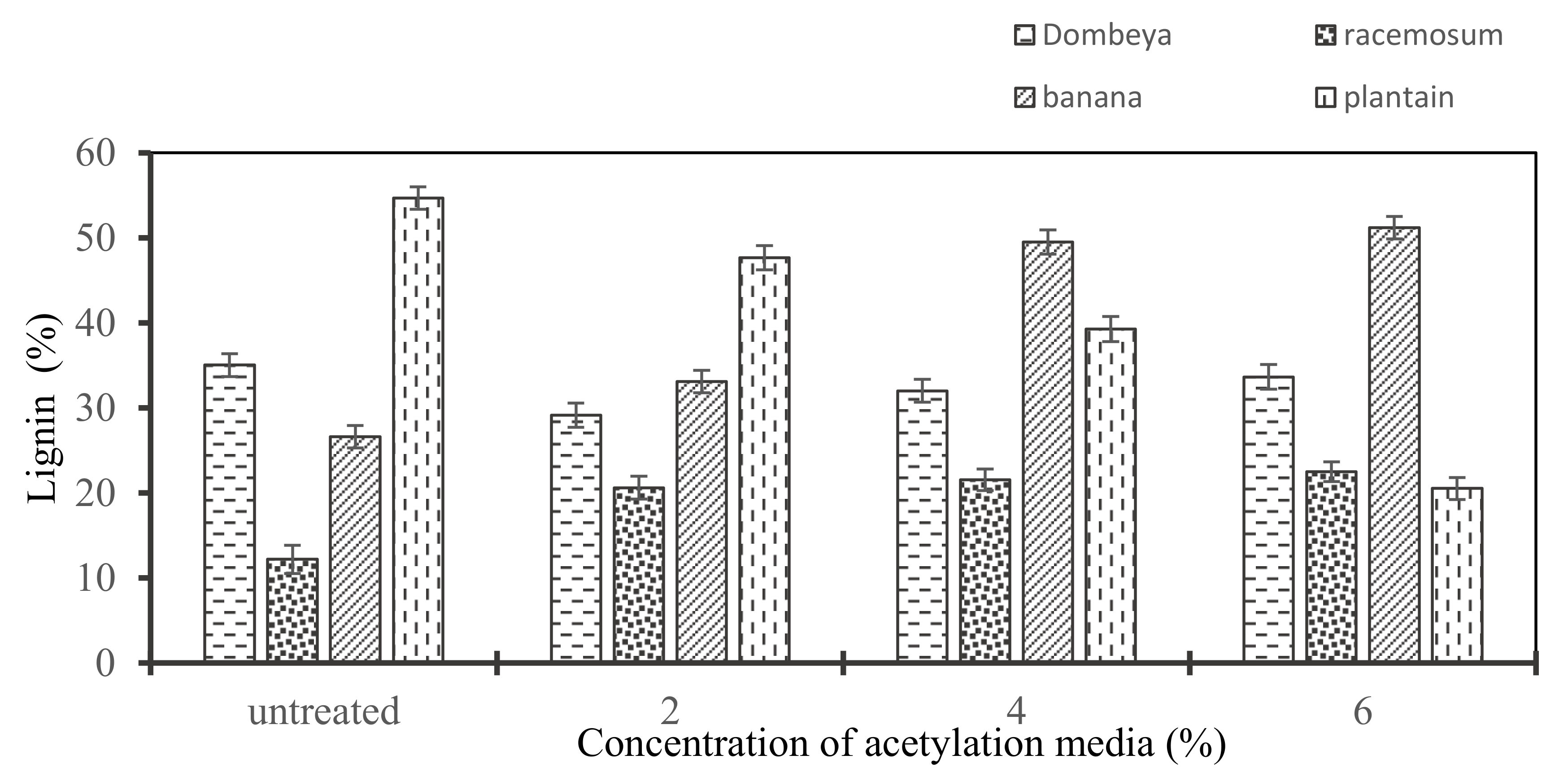
The matrix fiber bundling and cellulose stress relaxation roles of lignin in the plant fiber structure have been well researched and established. The need for delignification through chemical treatment to improve interfacial fiber–matrix adhesion and compatibility is also well established. Chemical treatment is also necessary to achieve the exposure of the cellulose fiber surface when used as reinforcement [33]. Figure 7 reveals that Dombeya Buettneri and plantain have their lignin contents reduced with increased concentration of acetylation media and this can be attributed to acetylation treatment. However, the observed increase in lignin content of banana and Combretum Racemosum may be attributed to the concurrent decrease in hemicellulose and cellulose. Hence, the residual lignin in the fiber will be reported as a higher percentage in the structure. This will be the case, since an increase in concentration of chemical treatment media has caused a further decrease in other constituents due to the hydrolysis of hemicellulose and some amount of cellulose.
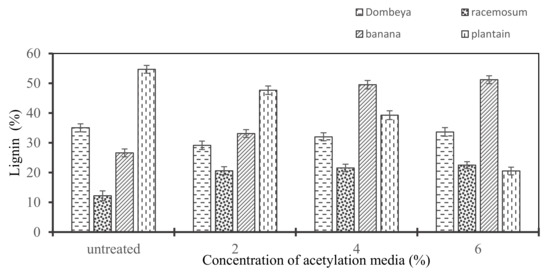
Figure 7.
Variation in lignin content of the selected natural fibers with an increasing concentration of chemical media.
3.4. Effects of Acetylation on Tensilet Properties of Selected Plant Fibers
3.4.1. Comparing the Effects of Acetylation Treatment on the Tensile Strength of Fibers from the Selected Plants
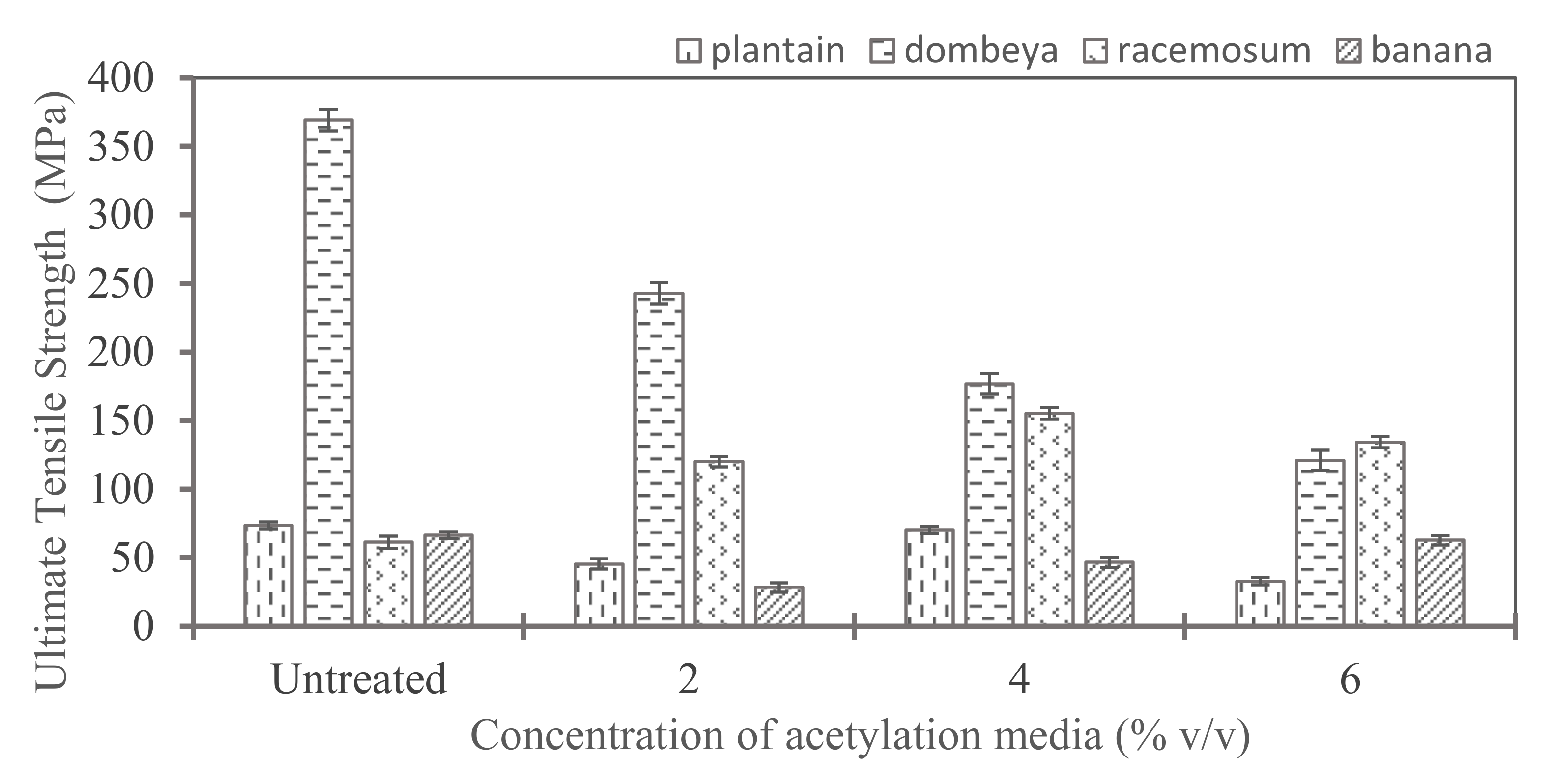
Figure 8 shows the ultimate tensile strength of acetylated fibers. Fibers from Dombeya showed the highest ultimate tensile strength in both treated and untreated conditions and retained this dominance with acetylation treatment of up to 4% concentration of the media. The values obtained are comparable with the reported values in literature. For instance, Senwitz et al. have reported up to 342 MPa tensile strength for Dombeya Burgessiae fibers [46]. For banana fibers, a maximum value of 63 Mpa was obtained for the banana fibers treated with 6% acetylation media. These values are comparable to the reported tensile strength of banana fibers in the works of Waghmare et al. [47], amongst many others.
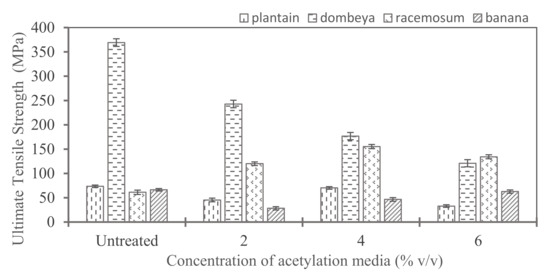
Figure 8.
Comparison of acetylation effects on ultimate tensile strength of acetylated natural fibers from different plant sources.
The results show that most of the fibers had their tensile strength reduced when the treatment concentration exceeds 4%. However, acetylation increases the tensile strength of Combretum Racemosum fibers at all evaluated concentrations. The optimal acetylation media concentration appears to be 4%. This concentration of acetylation media yielded the highest enhancement of tensile strength of Combretum Racemosum fibers.
The types of cellulose originally present in the fiber, whether crystalline or amorphous, and the relative proportions of each since they often occur together are strong and essential determining factors of the extent of tensile strength improvement or reduction that will be produced by acetylation treatment. Acetylation is suitable for batch processing of the selected natural fibers not only in tensile strength improvement when done in moderate levels but also in the removal of non-adhering constituents of the fibers. The degree of acetylation treatment is an important determinant of final properties. It is recommended that the concentration of acetylation media be moderate (about 4% maximum). Higher concentrations of acetylation media are deleterious to the tensile property improvement of natural fibers from the selected plants. Thus, 4% composition of the treatment yielded optimum tensile strength because most of the plant fibers showed the greatest strength increase when compared to the untreated and other treated plant fibers as shown in Figure 9.
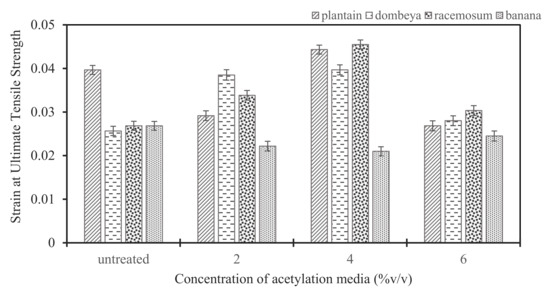
Figure 9.
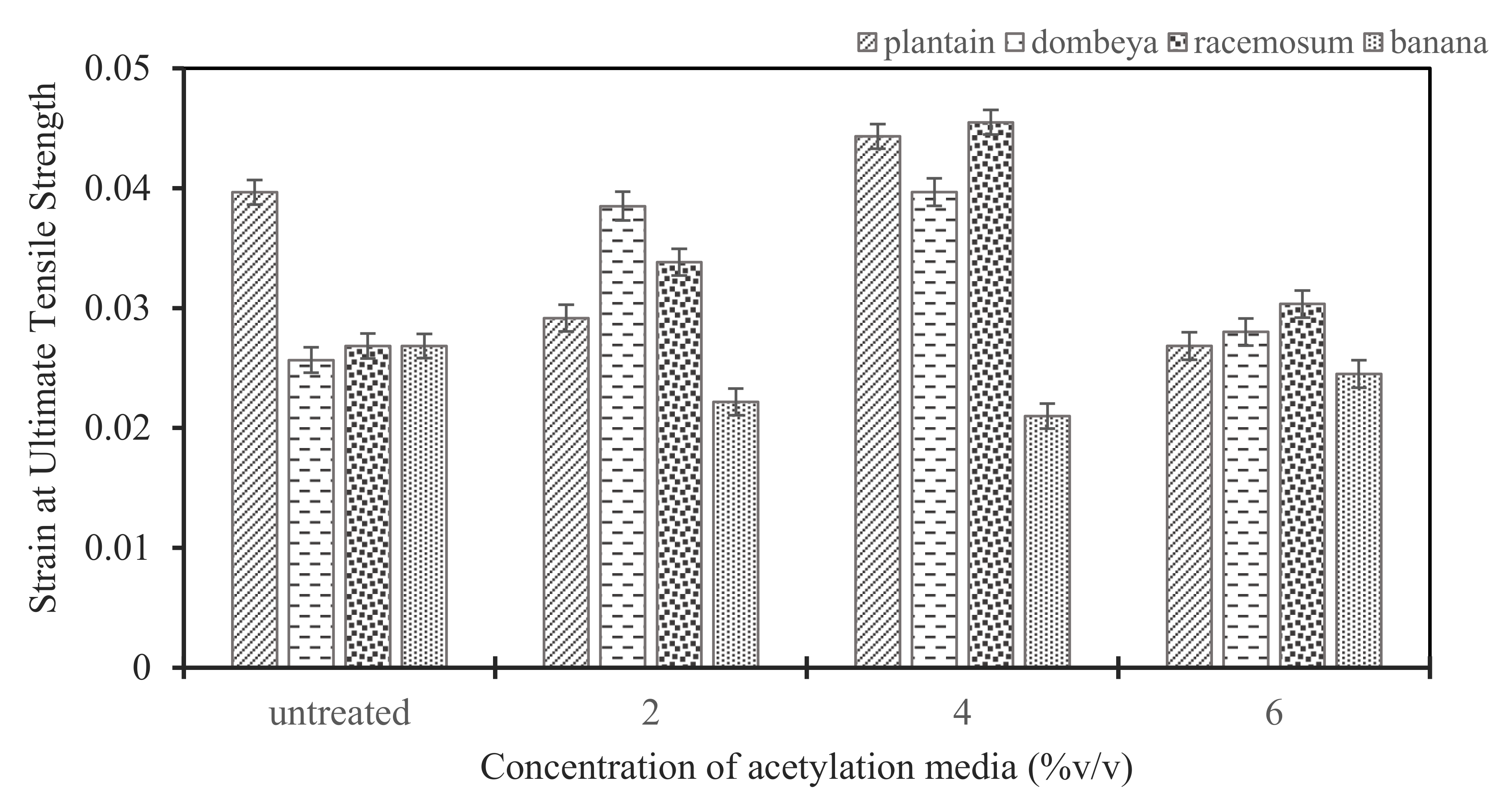
Variation in strain exhibited in treated and untreated conditions at ultimate tensile stress.
3.4.2. Effects of Acetylation Treatment on Strains of Ultimate Tensile Stress in Fibers from Selected Plants
Figure 9 shows the strain produced in each fiber with respect to the ultimate tensile stress. It was observed from the results that all fibers except banana have improved strain potential after treatment. The fibers’ strain potential increased with increment from 2 to 4% acetylation concentration where optimum values were reached, while, at 6% treatment concentration, the strains became reduced. Acetylation treatment increased the strains at ultimate tensile stress for fibers from plantain, Dombeya Buettneri and Combretum Racemosum. Hence, acetylation induced strain improvement in these fibers. A good strain property will promote ductile failure, which is preferable to brittle failure. Thus, acetylation is applicable for the enhancement of strain properties of these fibers.
3.5. Effect of Acetylation on the Surface Morphology of Selected Plant Fibers
Acetylation as a chemical treatment procedure is effective in the removal of non-cellulosic components on the surface of natural fibers. Raw and unprocessed natural fibers possess deleterious components that promote moisture uptake and prevent good fiber–matrix adhesion. Chemical treatments help to eliminate these, thus exposing the surface of the cellulose to the natural fibers and encouraging fiber–matrix interactions and adhesion. This effect can be seen in the morphology of plant fibers visible in Figure 10, Figure 11 and Figure 12, respectively.
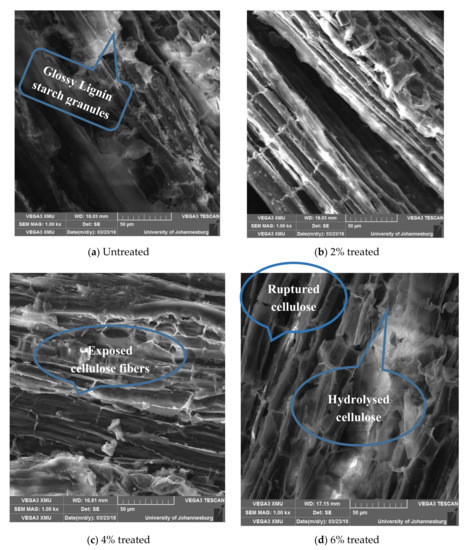
Figure 10.
Scanning electron microscopy (SEM) images showing variation in the surface morphology of Dombeya Buettnerri fibers with varying concentrations of acetylation media.
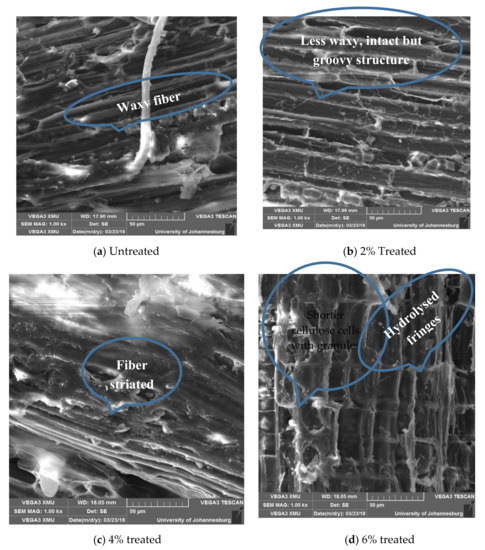
Figure 11.
SEM images showing variation surface morphology of Combretum Racemosum fibers with varying concentrations of acetylation media.
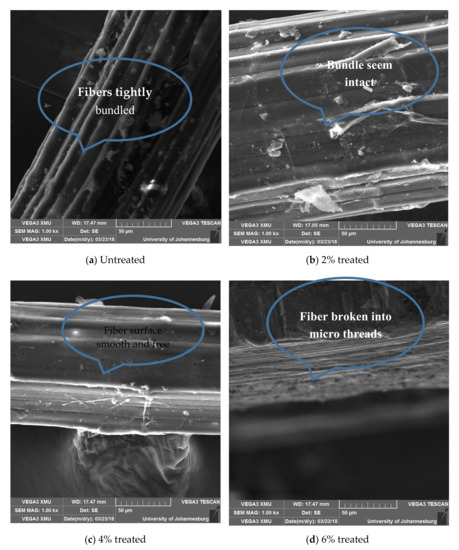
Figure 12.
SEM images showing variation in the structure and surface morphology of banana fibers with varying concentrations of acetylation media.
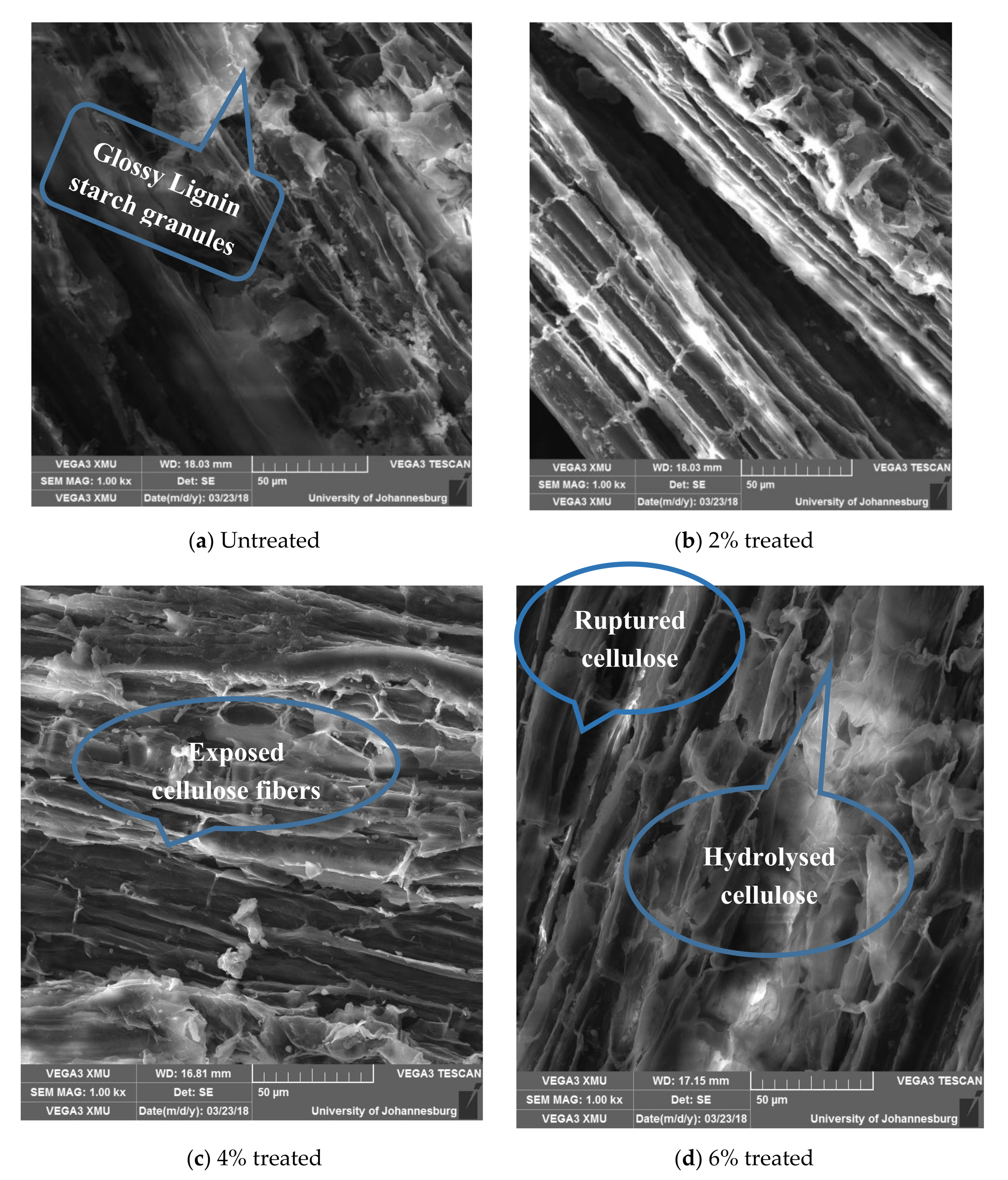
3.5.1. Effects of Acetylation on in Structure and Surface Morphology of Dombeya Buettnerri Fibers
Figure 10a shows numerous glossy, soft, viscous, and lumpy non-cellulosic materials on the fiber surfaces. After treatment with 2% acetylation media, some of the deleterious components are removed and the cellulose walls become more visible, as shown in Figure 10b. However, the fibers still appear well bonded to one another, which indicates that a mild degree of chemical treatment such as 2% acetylation mainly affected the surface of the fiber bundles but did not penetrate much. In Figure 10c, the surface of the 4% acetylated Dombeya Buettnerri fibers appears rougher, indicating a high loss of hemicellulose and delignification. Figure 10d reveals that 6% acetylation seems aggressive on the fiber and, thus, the fiber structure appears highly ruptured. Many cellulose cell walls are separated from the others in their line. Fluffy remnants of hydrolyzed fiber constituents are also observed.
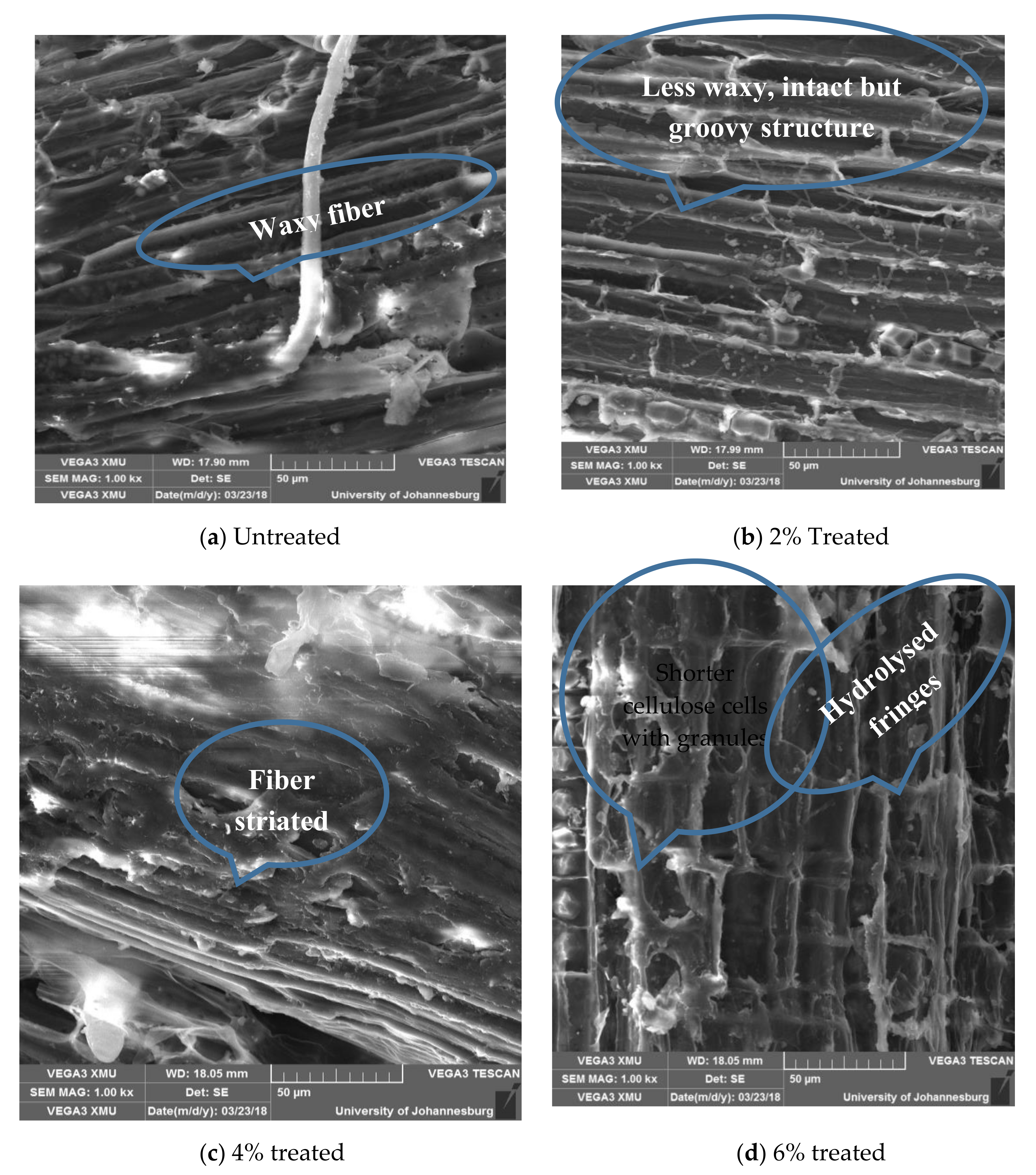
3.5.2. Effects of Acetylation on in Structure and Surface Morphology of Combretum Racemosum Fibers
The untreated surface of Conbretum Racemosum fibers appears waxy and is punctuated with granules as shown in Figure 11a. With 2% acetylation, the fiber surface became groovy as shown in Figure 11b. It was seen that 4% acetylation treatment produces a quasi-striated microfribular structure as shown in Figure 11c, but, at 6% acetylation treatment, the fiber appears to be too rough due to the aggressiveness of the treatment. Although it further exposes the surface of the cellulose fibers, which may enhance the fiber–matrix interaction, it also slightly hydrolyses this important component of the fiber structure (Figure 11d).
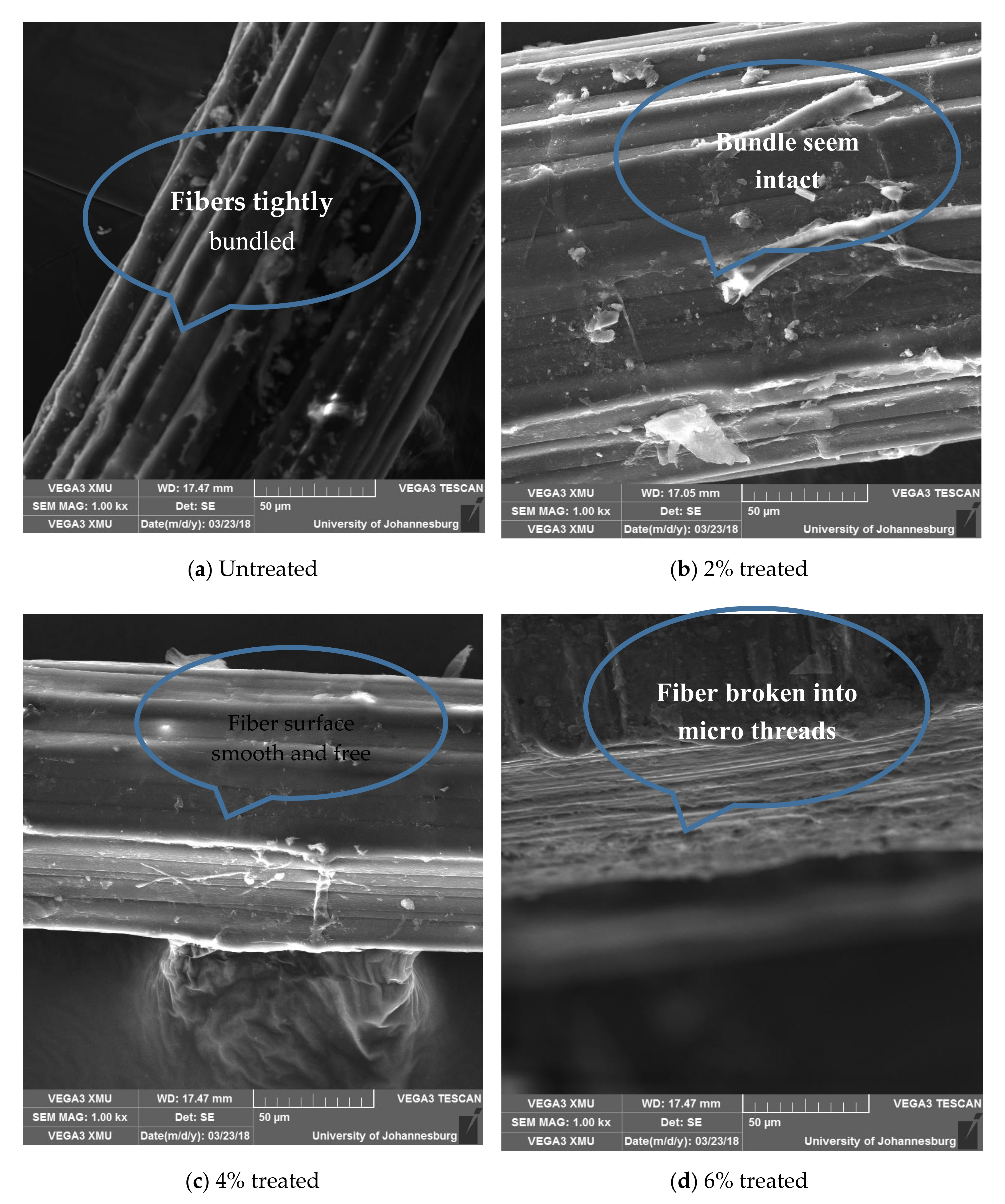
3.5.3. Effects of Acetylation on in Structure and Surface Morphology of Banana (Musa) Fiber
The structure of the untreated fibers, as shown in Figure 12a, appears bundled with sparsely dispersed granules on the surface of the bundled strands. The bundled structure seems to be maintained in Figure 12b, save for slight defibrillation and defilming, but does not infer the integrity of the structure. Treatment with 4% acetylation media served to clear off non-cellulosic components from the fiber surface as shown in Figure 12c, while in Figure 12d, the fibers appear micro-threaded.
Plantain fiber was not view due to the fact that it is of the same family with banana and, besides, banana is the most widely used fiber.
4. Conclusions
The potentialities of combined acetylation treatments for pre-processing of the selected plant fibers in both treated and untreated conditions were examined in this work. It was discovered that acetylation treatments may influence the fiber constituents, tensile and morphology in varying proportions. From the results, it was discovered that:
- Plantain fibers followed by banana fibers had the highest aspect ratios with values of about 1000 and 417, respectively. These two fibers (plantain and banana) have the least density and are thus great candidates for lightweight composite materials development. Banana fibers had the least density of about 1.38 g/cm3 while Dombeya Buettneri fibers possessed the highest value of 1.5 g/cm3.
- The treatment tends to promote improvement in cellulose content for Dombeya Buettneri, and plantain fibers, while it tends to reduce the cellulose content for banana and Combretum Racemosum fibers. This, in agreement with previous results, was due to the impact of the treatment on crystalline/amorphous cellulose content of the evaluated plant fibers as well as other non-cellulosic components. The treatment favours the enhancement of the ultimate tensile strength of combretum racemosum while the tensile strain was enhanced in most of the other fibers except banana.
- The type(s)/form(s) and the relative proportion of cellulose present in the raw and untreated fibers strongly determines the compositional and tensile property influence of acetylation on the fibers.
- The treatment protocols enhanced the surface morphology and tensile properties of natural fibers with the optimum concentration of acetylation media being 4%.
- From this work, it is expected that more investigations are to be carried out on plantain, banana, Combretum Racemosum and Dombeya Buettneri fibers as they are potential plant fibers for engineering materials applications.
Author Contributions
Conceptualization, I.O.O.; methodology, I.O.O., O.S.M.; software, I.O.O., O.S.M.; validation, I.O.O., O.S.M., A.A.A., O.P.B. and F.O.A.; formal analysis, I.O.O., O.S.M., A.A.A., O.P.B. and F.O.A.; investigation, I.O.O., O.S.M.; resources, I.O.O., O.S.M; data curation, I.O.O., O.S.M., A.A.A., O.P.B. and F.O.A.; writing—Original draft preparation, I.O.O., O.S.M., A.A.A., O.P.B. and F.O.A.; writing—review and editing, I.O.O., O.S.M., A.A.A., O.P.B. and F.O.A.; visualization, I.O.O., O.S.M., A.A.A., O.P.B. and F.O.A.; supervision, I.O.O.; project administration, O.S.M. and F.O.A.; funding acquisition, I.O.O. and A.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Authors acknowledge the Federal University of Technology Akure and SDGs-9 Research Group: Industry, Innovation and Infrastructure (3i’s), Landmark University, Omu-Aran, Kwara State, Nigeria for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yan, L. Effect of alkali treatment on vibration characteristics and mechanical properties of natural fabric reinforced composites. J. Reinf. Plast. Compos. 2012, 31, 887–896. [Google Scholar] [CrossRef]
- Salem, T.F.; Tirkes, S.; Akar, A.O.; Tayfun, U. Enhancement of mechanical, thermal and water uptake performance of TPU/jute fiber green composites via chemical treatments on fiber surface. e-Polymers 2020, 20, 133–143. [Google Scholar] [CrossRef]
- Baghban, M.H.; Mahjoub, R. Natural Kenaf Fiber and LC3 Binder for Sustainable Fiber-Reinforced Cementitious Composite: A Review. Appl. Sci. 2020, 10, 357. [Google Scholar] [CrossRef]
- Hussain, S.A.; Pandurangadu, V.; Kumar, K.P. Optimization of Mechanical Properties of Green Coconut Fiber/HDPE Composites by using flexural strength. Int. J. Adv. Sci. Technol. 2016, 92, 1–8. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef]
- Atiqah, A.; Jawaid, M.; Sapuan, S.M.; Ishak, M. Effect of Surface Treatment on the Mechanical Properties of Sugar Palm/Glass Fiber-reinforced Thermoplastic Polyurethane Hybrid Composites. Bioresources 2017, 13, 1174–1188. [Google Scholar] [CrossRef]
- Girijappa, Y.G.T.; Rangappa, S.M.; Parameswaranpillai, J.; Siengchin, S. Natural Fibers as Sustainable and Renewable Resource for Development of Eco-Friendly Composites: A Comprehensive Review. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Oladele, I.O.; Ayanleye, O.T.; Adediran, A.; Makinde-Isola, B.A.; Taiwo, A.S.; Akinlabi, E.T. Characterization of Wear and Physical Properties of Pawpaw–Glass Fiber Hybrid Reinforced Epoxy Composites for Structural Application. Fibers 2020, 8, 44. [Google Scholar] [CrossRef]
- Oladele, I.O.; Ibrahim, I.; Adediran, A.A.; Akinwekomi, A.; Adetula, Y.; Olayanju, T. Modified palm kernel shell fiber/particulate cassava peel hybrid reinforced epoxy composites. Results Mater. 2020, 5, 100053. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A Review on Natural Fiber Reinforced Polymer Composite and Its Applications. Int. J. Polym. Sci. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Ayyachamy, M.; Cliffe, F.E.; Coyne, J.M.; Collier, J.; Tuohy, M.G. Lignin: Untapped biopolymers in biomass conversion technologies. Biomass-Convers. Biorefinery 2013, 3, 255–269. [Google Scholar] [CrossRef]
- Sepe, R.; Bollino, F.; Boccarusso, L.; Caputo, F. Influence of chemical treatments on mechanical properties of hemp fiber reinforced composites. Compos. Part B Eng. 2018, 133, 210–217. [Google Scholar] [CrossRef]
- Oladele, I.; Michael, O.; Olusegun, S. Influence of Chemical Treatment on the Constituents and Tensile Properties of Selected Agro-Fibres. West Indian J. Eng. 2015, 38, 4–12. [Google Scholar]
- Alotaibi, M.D.; Alshammari, B.A.; Saba, N.; Alothman, O.Y.; Sanjay, M.; Almutairi, Z.; Jawaid, M. Characterization of natural fiber obtained from different parts of date palm tree (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2019, 135, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.M.; Mittal, V. Surface Modification of Natural Fibers. In Biodegradable and Biocompatible Polymer Composites; Elsevier: Cambridge, UK, 2017; p. 115. [Google Scholar] [CrossRef]
- Várdai, R.; Lummerstorfer, T.; Pretschuh, C.; Jerabek, M.; Gahleitner, M.; Faludi, G.; Móczó, J.; Pukánszky, B. Comparative study of fiber reinforced PP composites: Effect of fiber type, coupling and failure mechanisms. Compos. Part A Appl. Sci. Manuf. 2020, 133, 105895. [Google Scholar] [CrossRef]
- Wang, X.; Petru, M.; Yu, H. The effect of surface treatment on the creep behavior of flax fiber reinforced composites under hygrothermal aging conditions. Constr. Build. Mater. 2019, 208, 220–227. [Google Scholar] [CrossRef]
- Athith, D.; Sanjay, M.; Gowda, T.Y.; Madhu, P.; Arpitha, G.; Yogesha, B.; Omri, M.A. Effect of tungsten carbide on mechanical and tribological properties of jute/sisal/E-glass fabrics reinforced natural rubber/epoxy composites. J. Ind. Text. 2017, 48, 713–737. [Google Scholar] [CrossRef]
- Debeli, D.K.; Qin, Z.; Guo, J. Study on the Pre-Treatment, Physical and Chemical Properties of Ramie Fibers Reinforced Poly (Lactic Acid) (PLA) Biocomposite. J. Nat. Fibers 2017, 15, 596–610. [Google Scholar] [CrossRef]
- Dolez, P.I.; Arfaoui, M.A.; Dubé, M.; David, É. Hydrophobic treatments for natural fibers based on metal oxide nanoparticles and fatty acids. Procedia Eng. 2017, 200, 81–88. [Google Scholar] [CrossRef]
- Halip, J.A.; Hua, L.S.; Ashaari, Z.; Tahir, P.M.; Chen, L.W.; Uyup, M.K.A. Effect of Treatment on Water Absorption Behavior of Natural Fiber–Reinforced Polymer Composites. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Elsevier: Cambridge, UK, 2019; pp. 141–156. [Google Scholar] [CrossRef]
- Mahjoub, R.; Yatim, J.M.; Sam, A.R.M.; Hashemi, S.H. Tensile properties of kenaf fiber due to various conditions of chemical fiber surface modifications. Constr. Build. Mater. 2014, 55, 103–113. [Google Scholar] [CrossRef]
- Manimaran, P.; Senthamaraikannan, P.; Sanjay, M.; Marichelvam, M.; Jawaid, M. Study on characterization of Furcraea foetida new natural fiber as composite reinforcement for lightweight applications. Carbohydr. Polym. 2018, 181, 650–658. [Google Scholar] [CrossRef]
- Singh, J.I.P.; Dhawan, V.; Singh, S.; Jangid, K. Study of Effect of Surface Treatment on Mechanical Properties of Natural Fiber Reinforced Composites. Mater. Today Proc. 2017, 4, 2793–2799. [Google Scholar] [CrossRef]
- Chukwudi, A.D.; Uzoma, O.T.; Azuka, U.-A.A.; Sunday, E.C.; Cyprian, S. Comparison of Acetylation and Alkali Treatments on the Physical and Morphological Properties of Raffia Palm Fibre Reinforced Composite. Sci. J. Chem. 2015, 3, 72. [Google Scholar] [CrossRef][Green Version]
- Sreekala, M.; Thomas, S. Effect of fibre surface modification on water-sorption characteristics of oil palm fibres. Compos. Sci. Technol. 2003, 63, 861–869. [Google Scholar] [CrossRef]
- Joseph, S.; Koshy, P.; Thomas, S. The role of interfacial interactions on the mechanical properties of banana fibre reinforced phenol formaldehyde composites. Compos. Interfaces 2005, 12, 581–600. [Google Scholar] [CrossRef]
- Tserki, V.; Panayiotou, C.; Zafeiropoulos, N.E. A Study of the Effect of Acetylation and Propionylation on the Interface of Natural Fibre Biodegradable Composites. Adv. Compos. Lett. 2005, 14, 096369350501400202. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Preisner, M.; Kulma, A.; Zebrowski, J.; Dymińska, L.; Hanuza, J.; Arendt, M.; Starzycki, M.; Szopa, J. Manipulating cinnamyl alcohol dehydrogenase (CAD) expression in flax affects fibre composition and properties. BMC Plant Biol. 2014, 14, 50. [Google Scholar] [CrossRef]
- Ovat, F.A. Environmental Degradability of Nigerian Long Bamboo Fibre-Reinforced Polymer Composite (NLBFRPC). Eur. J. Eng. Res. Sci. 2017, 2, 48–53. [Google Scholar] [CrossRef]
- Oladele, I.; Omotoyinbo, J.; Adewara, J. Investigating the Effect of Chemical Treatment on the Constituents and Tensile Properties of Sisal Fibre. J. Miner. Mater. Charact. Eng. 2010, 9, 569–582. [Google Scholar] [CrossRef]
- D13 Committee Test Method for Tensile Properties of Single Textile Fibers. ASTM Int. 2008, ASTMD3822M. [CrossRef]
- Yang, W.; Li, Y. Sound absorption performance of natural fibers and their composites. Sci. China Ser. E Technol. Sci. 2012, 55, 2278–2283. [Google Scholar] [CrossRef]
- Pandita, S.D.; Yuan, X.; Manan, M.A.; Lau, C.H.; Subramanian, A.S.; Wei, J. Evaluation of jute/glass hybrid composite sandwich: Water resistance, impact properties and life cycle assessment. J. Reinf. Plast. Compos. 2013, 33, 14–25. [Google Scholar] [CrossRef]
- Lu, N.; Swan, R.H.; Ferguson, I.T. Composition, structure, and mechanical properties of hemp fiber reinforced composite with recycled high-density polyethylene matrix. J. Compos. Mater. 2011, 46, 1915–1924. [Google Scholar] [CrossRef]
- Tewari, M.; Singh, V.; Gope, P.; Chaudhary, A. Evaluation of Mechanical Properties of Bagasse-Glass Fiber Reinforced Composite. J. Mater. Environ. Sci. 2011, 3, 171–184. [Google Scholar]
- Aslan, M.; Tufan, M.; Küçükömeroğlu, T. Tribological and mechanical performance of sisal-filled waste carbon and glass fibre hybrid composites. Compos. Part B Eng. 2018, 140, 241–249. [Google Scholar] [CrossRef]
- Amir, M.; Irmawaty, R.; Hustim, M.; Rahim, I.R. Tensile strength of glass fiber-reinforced waste PET and Kenauf hybrid composites. IOP Conf. Ser. Earth Environ. Sci. 2020, 419, 012061. [Google Scholar] [CrossRef]
- Oladele, I.O.; Oladejo, M.O.; Adediran, A.; Makinde-Isola, B.A.; Owa, A.F.; Akinlabi, E.T. Influence of designated properties on the characteristics of Dombeya Combretum Racemosum fiber/graphite hybrid reinforced polypropylene composites. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Vélez, L.; Retegi, A.; Mondragon, I.; Gañán, P. Biodegradability of Banana and Plantain Cellulose Microfibrils Films in Anaerobic Conditions. J. Polym. Environ. 2012, 20, 774–782. [Google Scholar] [CrossRef]
- Gopinathan, P.; Subramanian, K.S.; Paliyath, G.; Subramanian, J. Genotypic variations in characteristics of nano-fibrillated cellulose derived from banana pseudostem. BioResources 2017, 12, 6984–7001. [Google Scholar] [CrossRef]
- Akpabio, U.; Udiong, D.; Akpakpan, A. The Physicochemical Characteristics of Plantain (Musa Paradisiaca) and Banana (Musa Sapientum) Pseudostem Wastes. Adv. Nat. Appl. Sci. 2012, 6, 167–172. [Google Scholar]
- Ogunsile, B.; Omotoso, M. Fibre and Chemical Properties of Some Nigerian Grown Musa Species for Pulp Production. Asian J. Mater. Sci. 2009, 1, 14–21. [Google Scholar] [CrossRef][Green Version]
- Senwitz, C.; Kempe, A.; Neinhuis, C.; Mandombe, J.L.; Branquima, M.F.; Lautenschläger, T. Almost Forgotten Resources—Biomechanical Properties of Traditionally Used Bast Fibers from Northern Angola. BioResources 2016, 11, 7595–7607. [Google Scholar] [CrossRef]
- Waghmare, P.M.; Bedmutha, P.; Sollapur, S. Review on Mechanical Properties of Banana Fiber Biocomposite. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 847–850. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).