Inter-Correlation among the Hydrophilic–Lipophilic Balance, Surfactant System, Viscosity, Particle Size, and Stability of Candelilla Wax-Based Dispersions
Abstract
1. Introduction
- Particle size: small particle sizes with a narrow distribution are required; hence, particles are evenly molten after curing. Non-molten particles induce cracks and compromise the water vapor barrier;
- Suspension stability: a slow flotation process is required, which ideally exceeds the storage time of the dispersion.
2. Materials and Methods
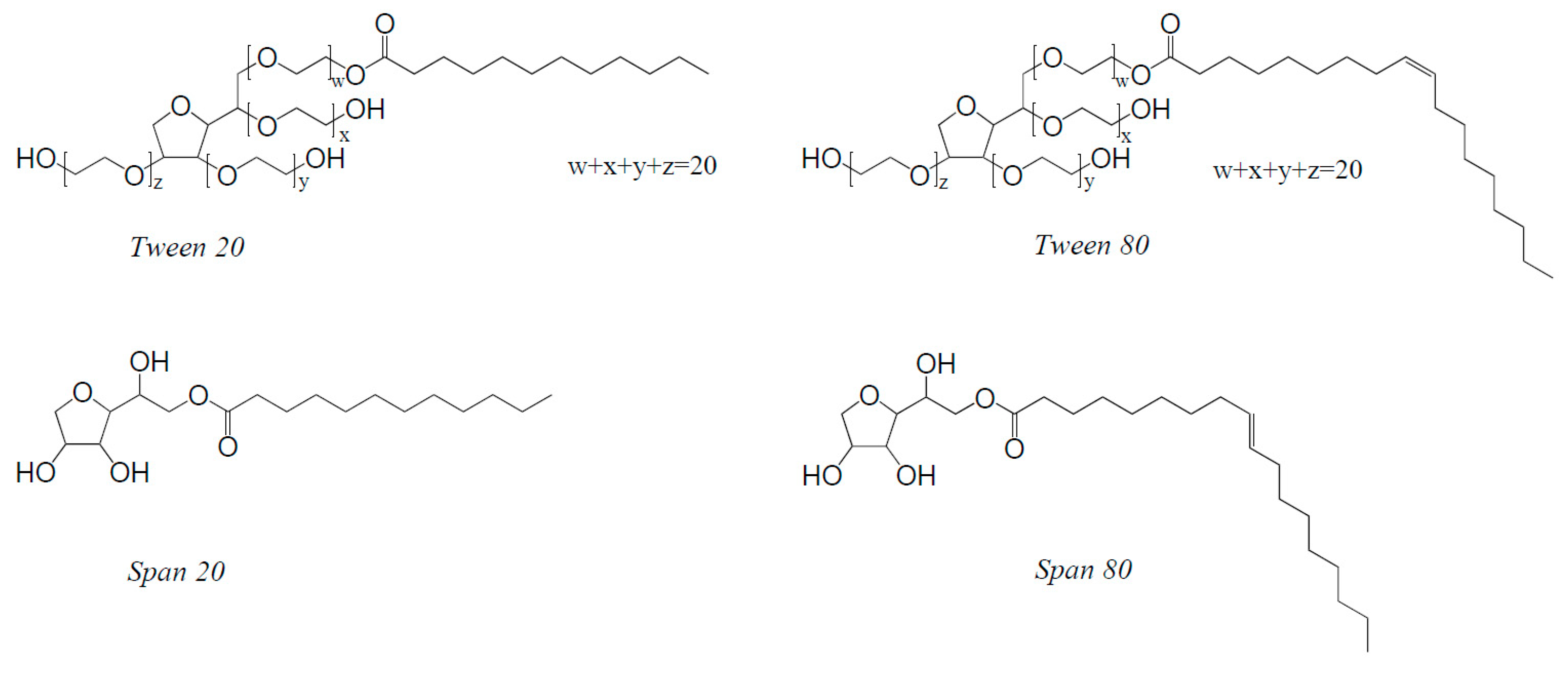
2.1. Materials
2.2. Melt Emulsification
2.3. Suspension Stability
2.4. Particle Size Distribution
2.5. Dynamic Viscosity
2.6. Scanning Electron Microscope (SEM)
2.7. Analysis of Wax Composition
2.8. Statistical Analysis
3. Results and Discussion
3.1. Short Time Stability: Interaction between Particle Size, Phase Separation, and Viscosity of the Continuous Phase
3.1.1. Effect of Continuous Phase Viscosity on Particle Size
3.1.2. Effect of Particle Size on Phase Separation
- Without any interactions between particles, monodisperse spheres form a layer with a dense structure. For hexagonal close packing, the maximum theoretical volume fraction to be attained is 74% [62]. Cases with negligible interparticle interactions between small particles typically lead to the formation of a thin flotation layer, because the unit cell of the structure is small (Figure 2a,b);
- With decreasing particle size, the surface/weight ratio increases; thus, the impact of the interaction forces increases. Typically, gravitational force dominates the interparticle forces for larger particles (>50 µm) [61]. Hence, larger particles do not agglomerate, whereas smaller particles flocculate if the attractive forces (i.e., Van der Waals force) dominate over the repulsive forces (i.e., electrostatic force) [63]. During particle agglomeration, the gap between the agglomerates and other particles increases. Thus, the particles form a thick flotation layer, and the volume density of the layer decreases [61,63] (Figure 2c).
3.1.3. Effect of Continuous Phase Viscosity on Phase Separation
3.2. Short Time Stability: Effect of Surfactants
3.2.1. Effect of the HLB Value
Viscosity
Particle Size
- High HLB values (>10) led to a low viscosity. The low viscosity of the continuous phase led to an increased droplet collision frequency. Therefore, droplet re-coalescence increases with high HLB values [35];
- High HLB (>12) values were achieved via the incorporation of an additional tween agent, which has a high molecular weight, possibly leading to a low critical packing parameter (CPP) [11,38]. Thus, the amount of the absorbed surfactant molecules on the surface of the wax particles should be low for high HLB values. Hence, a high HLB value possibly increases re-coalescence;
- The high water solubility of the surfactant at high HLB values (>10) possibly increased the adsorption rate of surfactant on the wax surface, due to the increased molecular mobility. Hence, a high HLB value possibly decreases re-coalescence;
- The better suitability of HLB values in terms of chemical similarities between the emulsifier and the disperse phase led to better stabilization and prevention of re-coalescence.
Stability
3.2.2. Effect of the Surfactant System and the Molecular Structure
Viscosity
Particle Size
Stability
3.3. Long-Term Stability: Changes in Particle Size Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Köhler, K.; Hensel, A.; Kraut, M.; Schuchmann, H.P. Melt emulsification–Is there a chance to produce particles without additives? Particuology 2011, 9, 506–509. [Google Scholar] [CrossRef]
- Shah, M.R.; Imran, M.; Ullah, S. Lipid-Based Nanocarriers for Drug Delivery and Diagnosis, 1st ed.; Elsevier Science: Oxford, UK, 2017. [Google Scholar]
- Chen, C.-H.; Kuo, W.-S.; Lai, L.-S. Effect of surfactants on water barrier and physical properties of tapioca starch/decolorized hsian-tsao leaf gum films. Food Hydrocoll. 2009, 23, 714–721. [Google Scholar] [CrossRef]
- Zhong, Q.-P.; Xia, W.-S. Physicochemical properties of edible and preservative films from chitosan/cassava starch/gelatin blend plasticized with glycerol. Food Technol. Biotechnol. 2008, 46, 262–269. [Google Scholar]
- Sun, Q.; Sun, C.; Xiong, L. Mechanical, barrier and morphological properties of pea starch and peanut protein isolate blend films. Carbohyd. Polym. 2013, 98, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Petersson, M.; Stading, M. Water vapour permeability and mechanical properties of mixed starch-monoglyceride films and effect of film forming conditions. Food Hydrocoll. 2005, 19, 123–132. [Google Scholar] [CrossRef]
- Kim, K.W.; Ko, C.J.; Park, H.J. Mechanical properties, water vapor permeabilities and solubilities of highly carboxymethylated starch-based edible films. J. Food Sci. 2002, 67, 218–222. [Google Scholar] [CrossRef]
- Botrel, D.A.; Ferreira Soares, N.F.; Camilloto, G.P.; de Barros Fernandes, R.V. Starch-based edible coating on extending shelf life of fresh-cut pear. Cienc. Rural 2010, 40, 1814–1820. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Starch–gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Ustunol, Z.; Mert, B. Water solubility, mechanical, barrier, and thermal properties of cross-linked whey protein isolate-based films. J. Food Sci. 2004, 69, 129–133. [Google Scholar] [CrossRef]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of whey-protein-coated films and laminates as novel recyclable food packaging materials with excellent barrier properties. Int. J. Polym. Sci. 2012, 5, 1–7. [Google Scholar] [CrossRef]
- Schmid, M.; Cinelli, P.; Wildner, J.; Bazzichi, A.; Lazzeri, A. Whey protein based barrier layers to enhance the barrier properties in polylactic acid films while maintaining biodegradability. In Proceedings of the International Conference on Bio-Based Polymers and Composites (BiPoCo), Siofok, Hungary, 27–31 May 2012. [Google Scholar]
- Sabato, S.F.; Ouattara, B.; Yu, H.; D’Aprano, G.; Le Tien, C.; Mateescu, M.A.; Lacroix, M. Mechanical and barrier properties of cross-linked soy and whey protein based films. J. Agric. Food Chem. 2001, 49, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Zhao, Y.Y. Barrier and mechanical properties of milk protein-based edible films containing nutraceuticals. J. Agric. Food Chem. 2003, 51, 1914–1918. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Nandane, A.S.; Jain, R. Value addition of fruits and vegetables by edible packaging: Scope and constraints. In Proceedings of the National Symposium on Emerging Innovative Technologies for Assurance of Quality and Safety in Processed Food, Kharagpur, India, 24–25 February 2011. [Google Scholar]
- Morillon, V.; Debeaufort, F.; Blond, G.; Capelle, M.; Voilley, A. Factors affecting the moisture permeability of lipid-based edible films: A review. Crit. Rev. Food Sci. Nutr. 2002, 42, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Khwaldia, K.; Arab-Tehrany, E.; Desobry, S. Biopolymer coatings on paper packaging materials. Compr. Rev. Food Sci. Food Saf. 2010, 9, 82–91. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Baraniak, B. Effect of candelilla wax on functional properties of biopolymer emulsion films—A comparative study. Food Hydrocoll. 2014, 41, 195–209. [Google Scholar] [CrossRef]
- Brock, T.; Groteklaes, M.; Mischke, P.; Strehmel, B. Lehrbuch der Lacktechnologie, 5th ed.; Vincentz Network: Hannover, Germany, 2017. [Google Scholar]
- Müller, B.; Poth, U. Lackformulierung und Lackrezeptur, 4th ed.; Vincentz Network: Hannover, Germany, 2005. [Google Scholar]
- Fanselow, S.; Emamjomeh, S.E.; Wirth, K.-E.; Schmidt, J.; Peukert, W. Production of spherical wax and polyolefin microparticles by melt emulsification for additive manufacturing. Chem. Eng. Sci. 2016, 141, 282–292. [Google Scholar] [CrossRef]
- Luckham, P.F. The physical stability of suspension concentrates with particular reference to pharmaceutical and pesticide formulations. Pestic. Sci. 1989, 25, 25–34. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Zaki, N.N.; Badawi, A.-F.M. Effect of binary surfactant mixtures on the stability of asphalt emulsions. J. Chem. Technol. Biotechnol. 1997, 69, 350–356. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.-F.; Lu, L.-J.; Li, M.-X.; Xu, J.-C.; Deng, H.-P. Turbiscan lab® expert analysis of the biological demulsification of a water-in-oil emulsion by two biodemulsifiers. J. Hazard. Mater. 2011, 190, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Califano, F.; James, N. Effects of viscosity on phase separation of liquid mixtures with a critical point of miscibility. J. Eng. Technol. Res. 2013, 5, 79–86. [Google Scholar] [CrossRef]
- Holmberg, K. Surfactants and Polymers in Aqueous Solution, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2003. [Google Scholar]
- Griffin, W.C. Classification of surface-active agents by ”HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Rowe, E.L. Effect of emulsifier concentration and type on the particle size distribution of emulsions. J. Pharm. Sci. 1965, 54, 260–264. [Google Scholar] [CrossRef] [PubMed]
- The HLB System: A Time-Saving Guide to Emulsifier Selection, 2nd ed.; ICI Americas, Inc.: Wilmington, NC, USA, 1984.
- Wan, L.S.C.; Heng, P.W.S.; Chan, L.W. Influence of hydrophile-lipophile balance on alginate microspheres. Int. J. Pharm. 1993, 95, 77–83. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M. The relevance HLB of surfactants on the stability of asphalt emulsion. Colloids Surf. A 2002, 204, 73–83. [Google Scholar] [CrossRef]
- Schubert, M.A.; Müller-Goymann, C.C. Characterisation of surface-modified solid lipid nanoparticles (SLN): Influence of lecithin and nonionic emulsifier. Eur. J. Pharm. Biopharm. 2005, 61, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Kim, K.; Voorhees, P.W. Ostwald ripening of spheroidal particles in multicomponent alloys. Acta Mater. 2018, 152, 327–337. [Google Scholar] [CrossRef]
- Campos, A.; Gómez, C.M.; García, R.; Figueruelo, J.E.; Soria, V. Extension of the flory-huggins theory to study incompatible polymer blends in solution from phase separation data. Polymer 1996, 37, 3361–3372. [Google Scholar] [CrossRef]
- Hou, B.; Chen, W.; Cao, Z. A process for the preparation of emulsified wax. Petrol Sci. Technol. 2007, 25, 1549–1555. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. An experimental study of stability of oil–water emulsion. Fuel Process. Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Euston, S.R.; Hirst, R.L. Comparison of the concentration-dependent emulsifying properties of protein products containing aggregated and non-aggregated milk protein. Int. Dairy J. 1999, 9, 693–701. [Google Scholar] [CrossRef]
- Li, C.; Liu, Q.; Mei, Z.; Wang, J.; Xu, J.; Sun, D. Pickering emulsions stabilized by paraffin wax and laponite clay particles. J. Colloid Interface Sci. 2009, 336, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, J.; Levic, S.; Manojlovic, V.; Nedovic, V.; Bugarski, B. Carnauba wax microparticles produced by melt dispersion technique. Chem. Pap. 2011, 65, 213–220. [Google Scholar] [CrossRef]
- Kheradmandnia, S.; Vasheghani-Farahani, E.; Nosrati, M.; Atyabi, F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomedicine 2010, 6, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Asumadu-Mensah, A.; Smith, K.W.; Ribeiro, H.S. Solid lipid dispersions: Potential delivery system for functional ingredients in foods. J. Food Sci. 2013, 78, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Bosquez-Molina, E.; Guerrero-Legarreta, I.; Vernon-Carter, E. Moisture barrier properties and morphology of mesquite gum–candelilla wax based edible emulsion coatings. Food Res. Int. 2003, 36, 885–893. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D.J. Dependence of creaming and rheology of monodisperse oil-in-water emulsions on droplet size and concentration. Colloids Surf. A 2000, 172, 79–86. [Google Scholar] [CrossRef]
- Stokes, G.G. Mathematical and Physical Papers. Volumes I–V; Cambridge University Press: Cambridge, UK, 1901. [Google Scholar]
- Lemarchand, C.; Couvreur, P.; Vauthier, C.; Costantini, D.; Gref, R. Study of emulsion stabilization by graft copolymers using the optical analyzer Turbiscan. Int. J. Pharm. 2003, 254, 77–82. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayré, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayre, I.; Puech, K.; Snabre, P. Characterisation of instability of concentrated dispersions by a new optical analyser: The turbiscan MA 1000. Colloids Surf. A 1999, 152, 111–123. [Google Scholar] [CrossRef]
- Buron, H.; Mengual, O.; Meunier, G.; Cayré, I.; Snabre, P. Optical characterization of concentrated dispersions: Applications to laboratory analyses and on-line process monitoring and control. Polym. Int. 2004, 53, 1205–1209. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, W.-I.; Kang, M.; Jin, H.-J. Multiple light scattering measurement and stability analysis of aqueous carbon nanotube dispersions. J. Phys. Chem. Solids 2008, 69, 1209–1212. [Google Scholar] [CrossRef]
- Azema, N. Sedimentation behaviour study by three optical methods—Granulometric and electrophoresis measurements, dispersion optical analyser. Powder Technol. 2006, 165, 133–139. [Google Scholar] [CrossRef]
- Vie, R.; Azema, N.; Quantin, J.C.; Touraud, E.; Fouletier, M. Study of suspension settling: A approach to determine suspension classification and particle interactions. Colloid Surf. A 2007, 298, 192–200. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Aghlara, A.; Hamid, N.S.A.; Yusof, S.; Chern, B.H. Influence of pectin and CMC on physical stability, turbidity loss rate, cloudiness and flavor release of orange beverage emulsion during storage. Carbohyd. Polym. 2008, 73, 83–91. [Google Scholar] [CrossRef]
- Xu, R.L. Particle Characterization: Light Scattering Methods; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Alderliesten, M. Mean particle diameters. Part I: Evaluation of definition systems. Part. Part. Syst. Charact. 1990, 7, 233–241. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef] [PubMed]
- Köhler, K.; Schuchmann, H.P. Emulgiertechnik: Grundlagen, Verfahren und Anwendungen; Behr’s Verlag: Hamburg, Germany, 2012. [Google Scholar]
- Malvern Mastersizer “Getting Started” Manual; Malvern Instruments: Worcestershire, UK, 1997; Available online: http://pmbrc.org/index.php/download_file/view/190/ (accessed on 21 November 2018).
- Blanshard, J.M.V.; Mitchell, J.R. Food Structure: Its Creation and Evaluation; Butterworth-Heinemann: Oxford, UK, 1988. [Google Scholar]
- Müller, W. Mechanische Grundoperationen und ihre Gesetzmäßigkeiten, 2nd ed.; Oldenburg Wissenschaftsverlag: München, Germany, 2014; p. 316. [Google Scholar]
- Tiller, F.M.; Yeh, C.S.; Leu, W.F. Compressibility of paniculate structures in relation to thickening, filtration, and expression—A review. Sep. Sci. Technol. 1987, 22, 1037–1063. [Google Scholar] [CrossRef]
- Batchelor, G.K. Sedimentation in a dilute dispersion of spheres. J. Fluid Mech. 1972, 52, 245–268. [Google Scholar] [CrossRef]
- Barnea, E.; Mizrahi, J. A generalized approach to the fluid dynamics of particulate systems. Chem. Eng. J. 1973, 5, 171–189. [Google Scholar] [CrossRef]
- Richardson, J.F.; Zaki, W.N. Sedimentation and fluidisation: Part I. Chem. Eng. Res. Des. 1997, 75, 82–99. [Google Scholar] [CrossRef]
- Xu, W.; Nikolov, A.; Wasan, D.T. The effect of many-body interactions on the sedimentation of monodisperse particle dispersions. J. Colloid Interface Sci. 1998, 197, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Salas, G.E.S. Sedimentationsverhalten von Submikrometerpartikeln in wässrigen Suspensionen. Ph.D. Thesis, Technical University of Dresden, Dresden, Germany, 2007. [Google Scholar]
- Swarbrick, J.; Rubino, J.T.; Rubino, O.P. Coarse Dispersions. In Remington: The Science and Practice of Pharmacy; Troy, D., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; p. 332. [Google Scholar]
- Mitsui, T. New Cosmetic Science; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Kim, E.-H.; Cho, W.-G. Candelilla wax nanoemulsions prepared by phase inversion composition (PIC) method. J. Oil Appl. Sci. 2014, 31, 203–209. [Google Scholar] [CrossRef]
- Pasquali, R.C.; Taurozzi, M.P.; Bregni, C. Some considerations about the hydrophilic–lipophilic balance system. Int. J. Pharm. 2008, 356, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Kretzschmar, H.-J. Wasser. VDI-Wärmeatlas, 11th ed.; Springer: Berlin/Heidelberg, Germany, 2013; p. 189. [Google Scholar]
- Ohba, N. Hydrophile-lipophile balance values for O/W emulsions stabilized by nonionic surfactants. II. “Required hydrophile-lipophile balance values” of the oil mixture. Bull. Chem. Soc. Jpn. 1962, 35, 1021–1025. [Google Scholar] [CrossRef]
- Tulloch, A.P. Comparison of some commercial waxes by gas liquid chromatography. J. Am. Oil Chem. Soc. 1973, 50, 367–371. [Google Scholar] [CrossRef]
- Scora, G.A.; Ahmed, M.; Scora, R.W. Epicuticular hydrocarbons of candelilla (euphorbia antisiphylitica) from three different geographical areas. Ind. Crop. Prod. 1995, 4, 179–184. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Alonzo-Macias, M.; González-Chávez, M.M. Thermal and textural properties of organogels developed by candelilla wax in safflower oil. J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Regert, M.; Langlois, J.; Colinart, S. Characterisation of wax works of art by gas chromatographic procedures. J. Chrom. A 2005, 1091, 124–136. [Google Scholar] [CrossRef]
- Holloway, P.J. Chemistry of leaf waxes in relation to wetting. J. Sci. Food Agr. 1969, 20, 124–128. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Sjoblom, J. Encyclopedic Handbook of Emulsion Technology; Marcel Dekker Inc.: New York, NY, USA, 2001. [Google Scholar]
- Balzer, D.; Lüders, H. Nonionic Surfactants: Alkyl Polyglucosides; Marcel Dekker Inc.: New York, NY, USA, 2000. [Google Scholar]
- Gao, X.; Gao, F.; Chen, L.; Yao, Y.; Chen, T.; Lin, S. Tuning the morphology of amphiphilic copolymer aggregates by compound emulsifier via emulsion–solvent evaporation. J. Saudi Chem. Soc. 2016, 22, 297–306. [Google Scholar] [CrossRef]
- Parker, M.E.; Bronlund, J.E.; Mawson, A.J. Moisture sorption isotherms for paper and paperboard in food chain conditions. Packag. Technol. Sci. 2006, 19, 193–209. [Google Scholar] [CrossRef]
- Chick, J.; Hernandez, R. Physical, thermal, and barrier characterization of casein-wax-based edible films. J. Food Sci. 2002, 67, 1073–1079. [Google Scholar] [CrossRef]
- Device Manual Turbiscan® LAB; Formulaction: L’Union, France, 2014.






| Effect of Parameters | On | Literature |
|---|---|---|
| particle size | phase separation 1 | [24] |
| viscosity of continuous phase | particle size distribution 1 | [25,26] |
| viscosity of continuous phase | phase separation 1 | [27] |
| HLB value | viscosity of continuous phase 1 | – |
| HLB value | particle size distribution 1 | [28,29,30,31,32] |
| HLB value | phase separation 1 | [33] |
| emulsifier system | viscosity of continuous phase 1 | – |
| emulsifier system | particle size distribution 1 | [34,35] |
| emulsifier system | phase separation 1 | [36] |
| HLB value | change in particle size distribution over storage time 1 | [24,36,37] |
| stirring intensity, time, and speed | particle size | [35,38,39] |
| mixing temperature | particle size | [35,38,39] |
| phase concentration | particle size | [20,30,35,39,40,41,42] |
| surfactant concentration | particle size | [30,33,35,36,38,43] |
| aggregation state of the disperse phase | particle size | [44,45] |
| phase concentration | phase separation | [46] |
| Parameters | d1,0 (0.16–2.72 µm) | d3,2 (2.11–9.27 µm) | d4,3 (38.98–213.63 µm) | Viscosity (1.95–2.72 mPa·s) |
|---|---|---|---|---|
| Viscosity (1.95–2.72 mPa·s) | − | − | 0 | |
| ESI (3.53–34.04%) | 0 | 0 | 0 | 0 |
| TSI (2.40–12.30%) | 0 | 0 | 0 | 0 |
| v (0.20–31.86 mm·h−1) | + | 0 | + | − |
| Substances | HLB | Molecular Weight (g·mol−1) | η (mPa·s) |
|---|---|---|---|
| Tween 20 | 16.7 | 1227 | 396 ± 7 |
| Span 20 | 8.6 | 346 | 3476 ± 63 |
| Tween 80 | 15 | 1310 | 455 ± 72 |
| Span 80 | 4.3 | 428 | 1000 ± 14 |
| Water | – | – | 0.890 1 |
| Substance | Amount |
|---|---|
| alkane C23 | 0.01% |
| alkane C24 | 0.02% |
| alkane C25 | 0.03% |
| alkane C26 | 0.05% |
| alkane C27 | 0.09% |
| alkane C28 | 0.20% |
| alkane C29 | 2.11% |
| alkane C30 | 0.56% |
| alkane C31 | 30.06% |
| alkane C32 | 0.67% |
| alkane C33 | 3.83% |
| alkane C34 | 0.07% |
| alkane C35 | 0.38% |
| lupeol | 0.83% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindner, M.; Bäumler, M.; Stäbler, A. Inter-Correlation among the Hydrophilic–Lipophilic Balance, Surfactant System, Viscosity, Particle Size, and Stability of Candelilla Wax-Based Dispersions. Coatings 2018, 8, 469. https://doi.org/10.3390/coatings8120469
Lindner M, Bäumler M, Stäbler A. Inter-Correlation among the Hydrophilic–Lipophilic Balance, Surfactant System, Viscosity, Particle Size, and Stability of Candelilla Wax-Based Dispersions. Coatings. 2018; 8(12):469. https://doi.org/10.3390/coatings8120469
Chicago/Turabian StyleLindner, Martina, Magdalena Bäumler, and Andreas Stäbler. 2018. "Inter-Correlation among the Hydrophilic–Lipophilic Balance, Surfactant System, Viscosity, Particle Size, and Stability of Candelilla Wax-Based Dispersions" Coatings 8, no. 12: 469. https://doi.org/10.3390/coatings8120469
APA StyleLindner, M., Bäumler, M., & Stäbler, A. (2018). Inter-Correlation among the Hydrophilic–Lipophilic Balance, Surfactant System, Viscosity, Particle Size, and Stability of Candelilla Wax-Based Dispersions. Coatings, 8(12), 469. https://doi.org/10.3390/coatings8120469






