Effect of Tea Tree Essential Oil@Chitosan Microcapsules on Surface Coating Properties of Pine Wood
Abstract
1. Introduction
2. Methods and Test Materials
2.1. Test Materials
2.2. Microcapsule Screening Method
2.3. Pine Wood Finishing Methods
2.4. Testing and Characterisation
3. Results and Discussion
3.1. Microcapsule Microstructure and Chemical Composition Analysis
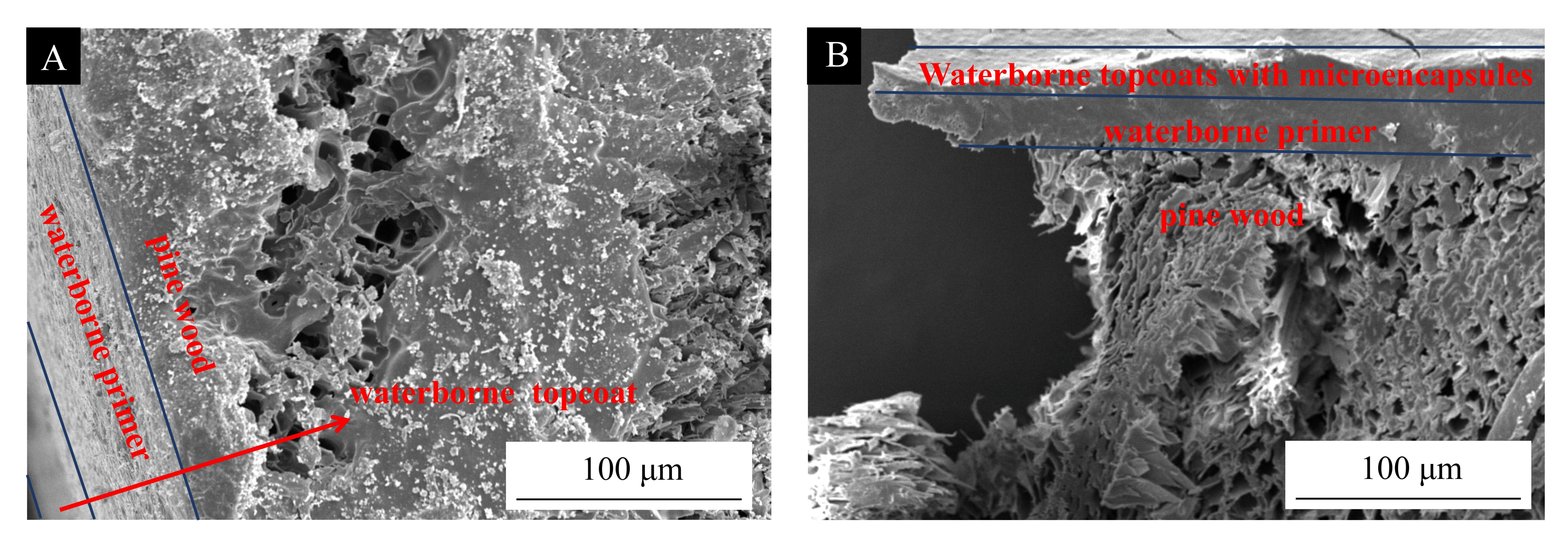
3.2. Analysis of Micro-Morphology and Chemical Composition of Pine Wood Surface Coatings
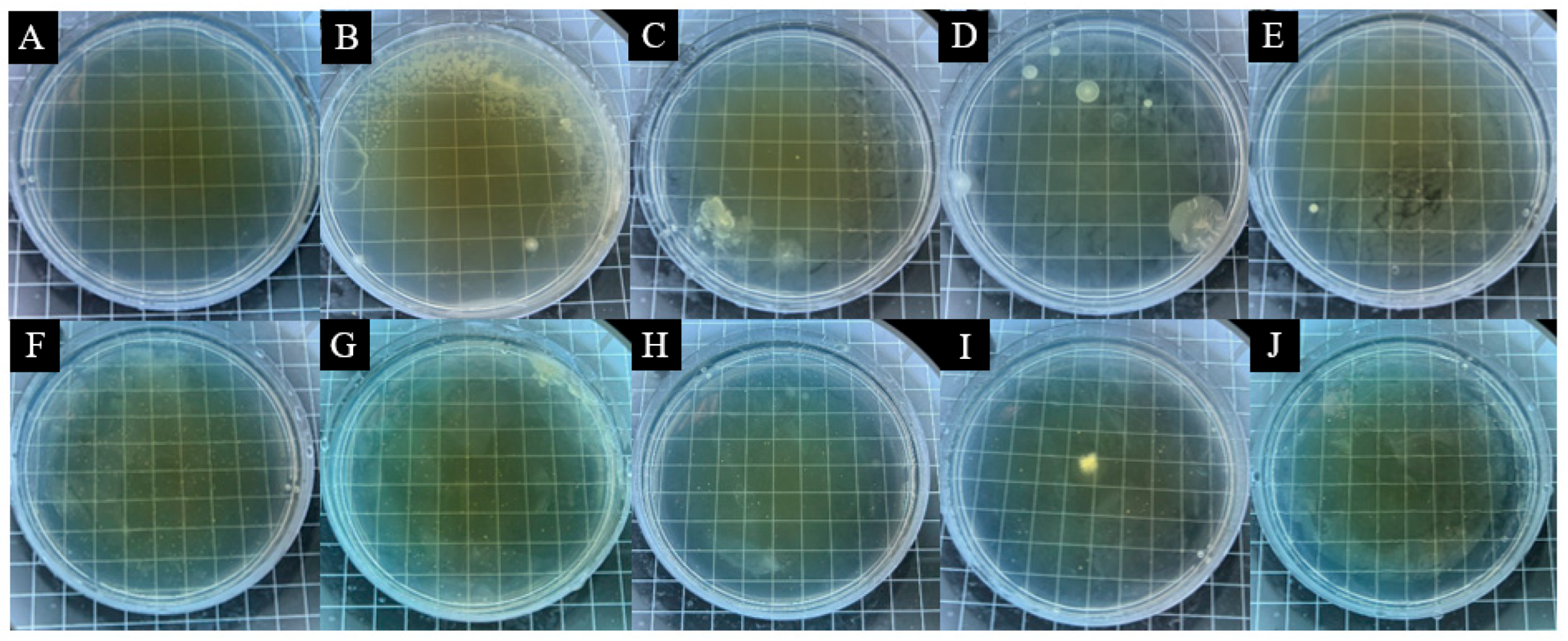
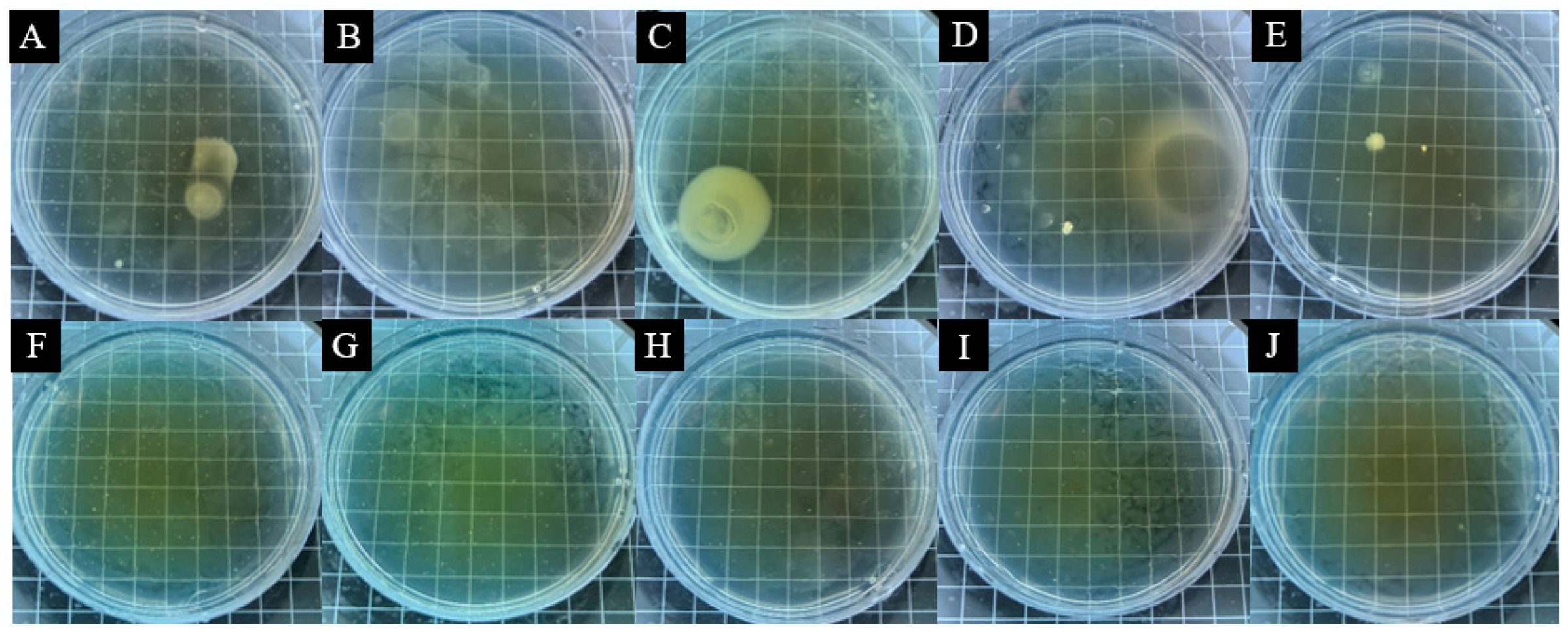
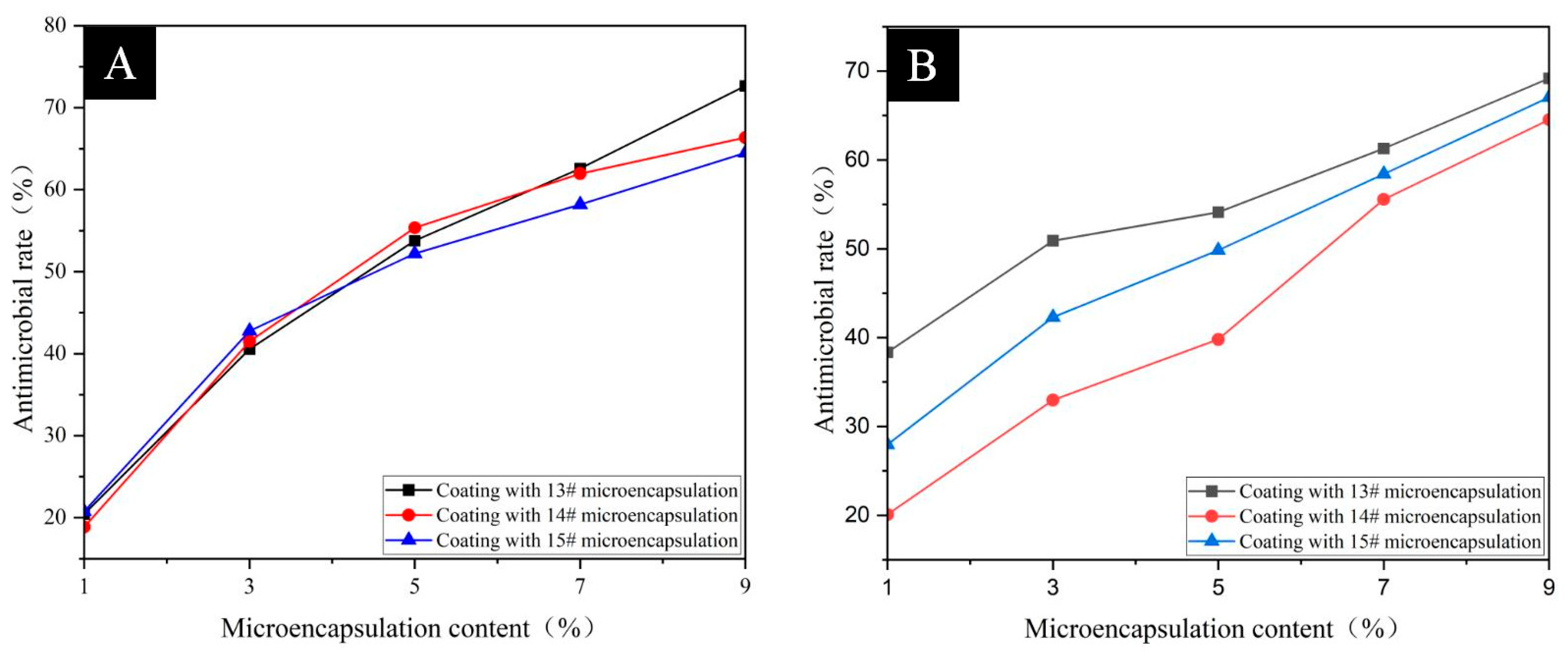
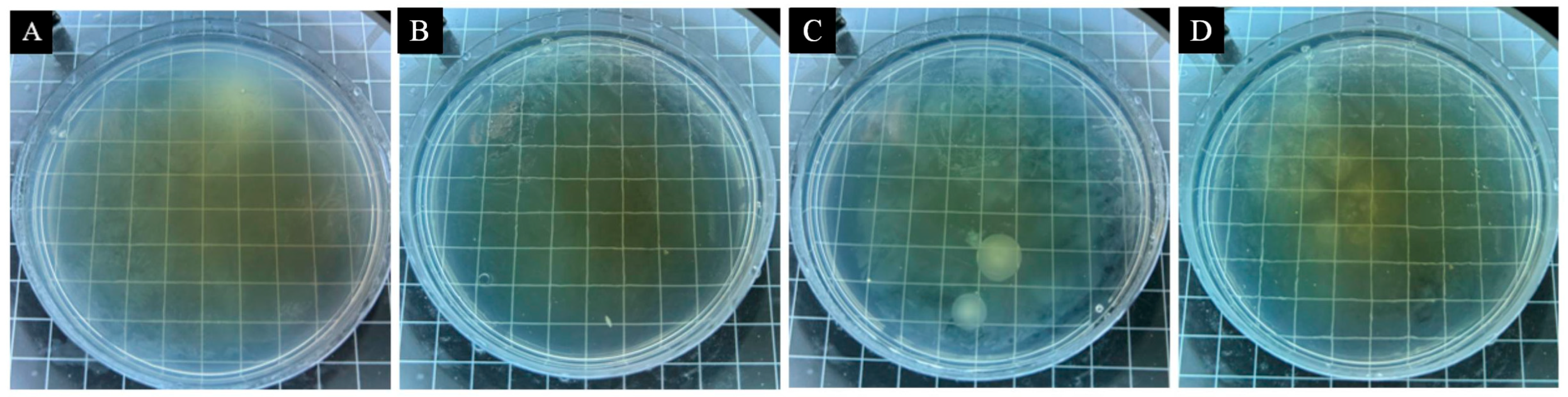
3.3. Analysis of Antimicrobial Properties of Surface Coatings on Pine Wood
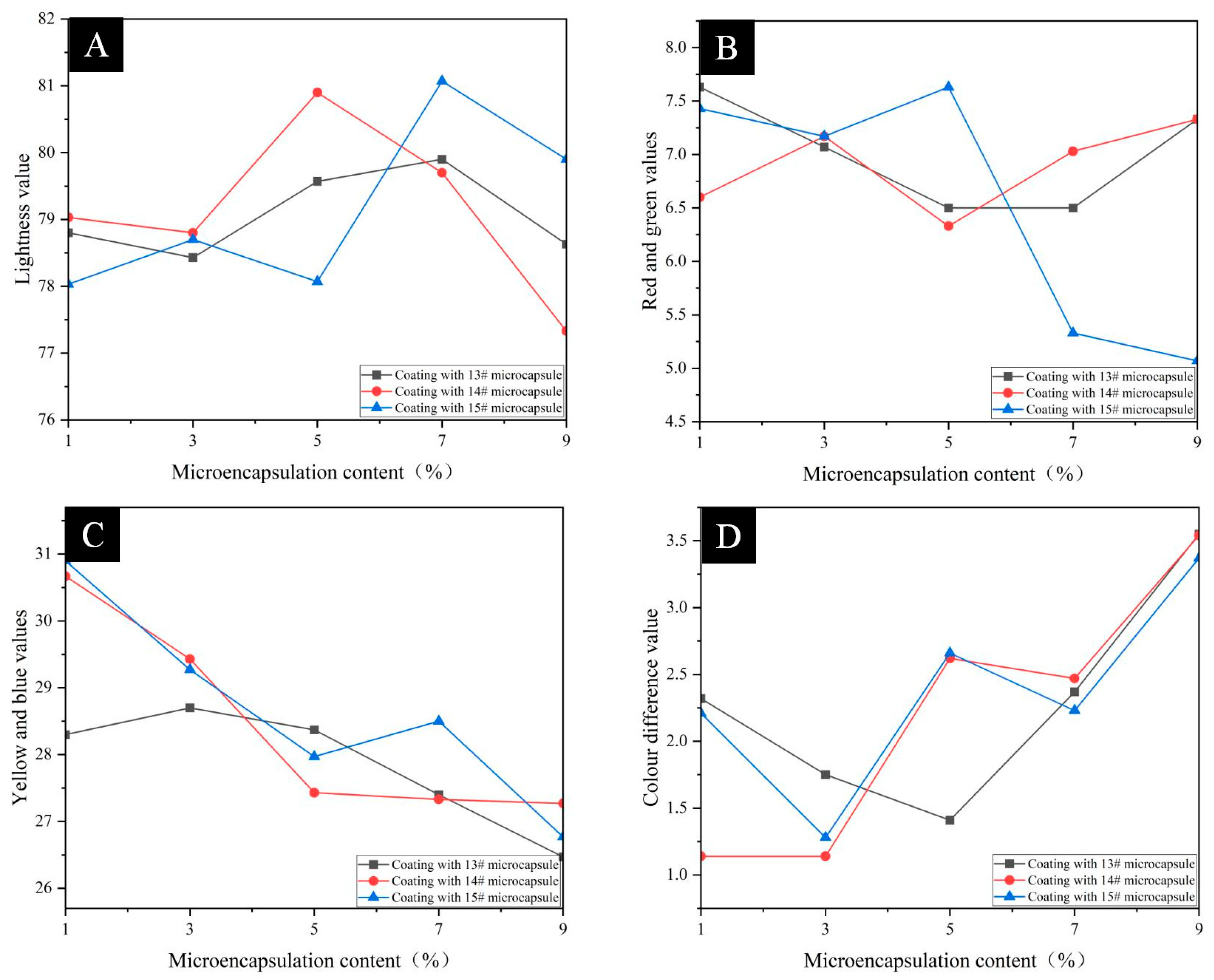
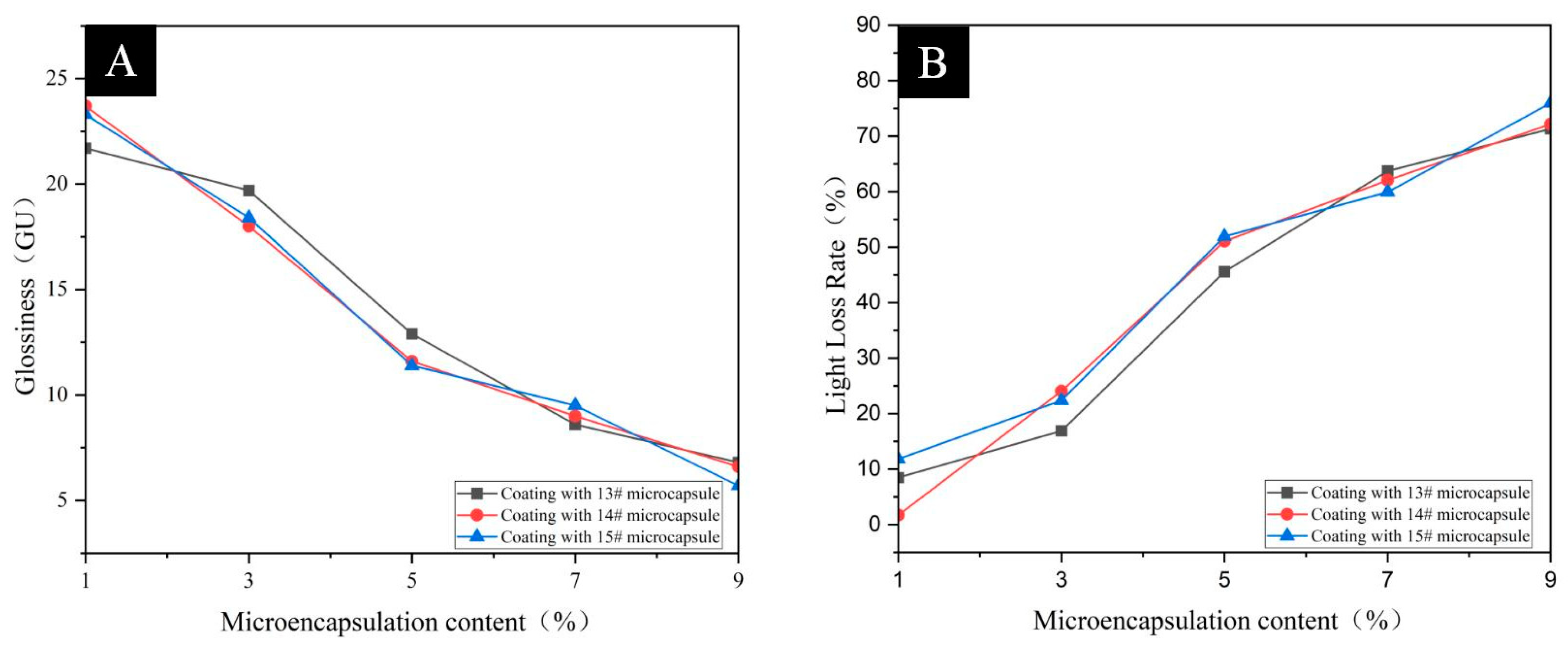
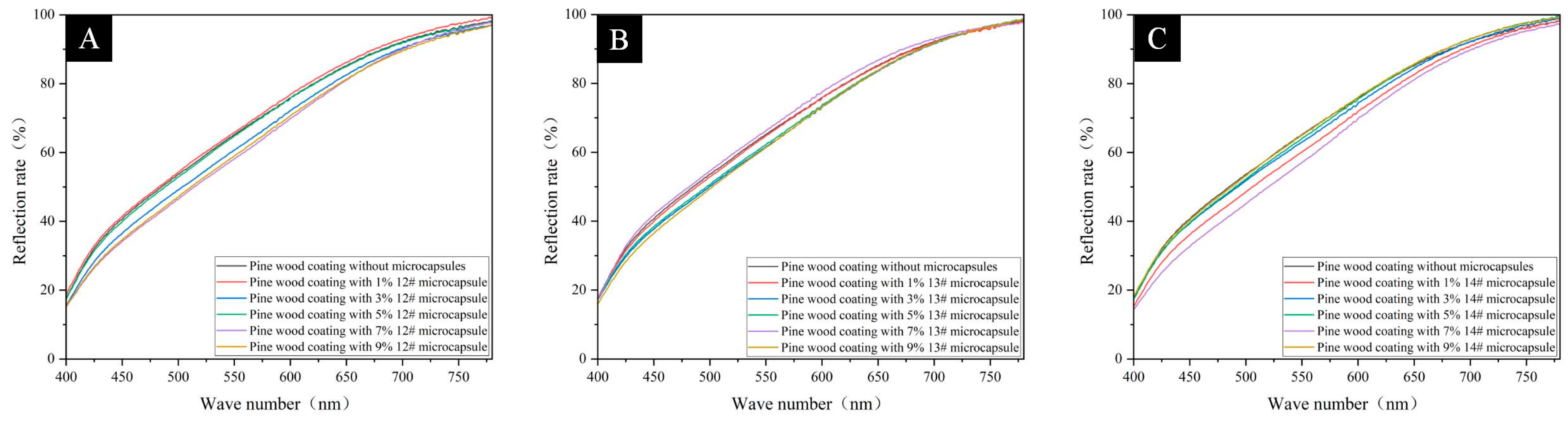
3.4. Analysis of Optical Properties of Pine Wood Surface Coatings
3.5. Analysis of Mechanical Properties of Pine Wood Surface Coatings
3.6. Analysis of Weather Resistance of Pine Wood Surface Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, W.; Yang, Z.; Shi, N.; Yu, X. Experimental study on effects of the selected load parameters on fatigue life of the mortise-and-tenon furniture joint. Wood Mater. Sci. Eng. 2025, 19, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wang, T.; Wang, X. Additive manufacturing of furniture corner guards based on thermoplastic polyurethane filament. BioResources 2025, 20, 5398–5406. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, W.; Kasal, A.; Erdil, Y.Z. The state of the art of biomechanics applied in ergonomic furniture design. Appl. Sci. 2023, 13, 12120. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Yue, X.; Xiong, X. Design of furniture mortise-and-tenon joints: A review of mechanical properties and design recommendations. Wood Mater. Sci. Eng. 2025, 1–15. [Google Scholar] [CrossRef]
- Yu, R.; Liu, Y.; Konukcu, C.A.; Hu, W. A method of simulating seat load for numerical analysis of wood chair structure. Wood Res-Slovak. 2024, 69, 432–444. [Google Scholar] [CrossRef]
- Hu, W.; Yu, R.; Yang, P. Characterizing roughness of wooden mortise and tenon considering effects of measured position and assembly condition. Forests 2024, 15, 1584. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, W. Optimizing the Interface Compatibility of Transparent Wood for Green Phase-Change Thermal Storage. Wood Sci. Technol. 2025, 59, 45. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, W.; Liu, X.; Wang, X. (Alice). Study on Permeability of Cunninghamia Lanceolata Based on Steam Treatment and Freeze Treatment. Wood Res. 2021, 66, 721–731. [Google Scholar] [CrossRef]
- Wu, S.; Tao, X.; Xu, W. Thermal Conductivity of Poplar Wood Veneer Impregnated with Graphene/Polyvinyl Alcohol. Forests 2021, 12, 777. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, D.; Xu, W. Effect of Paint Process on the Performance of Modified Poplar Wood Antique. Coatings 2021, 11, 1174. [Google Scholar] [CrossRef]
- Li, S.; Hu, W. Study on mechanical strength of cantilever handrail joints for chair. BioResources 2023, 18, 209–219. [Google Scholar] [CrossRef]
- Fu, S.; Xiong, X.; Wan, R.; Zhang, M.; Xu, X. The Development and Future Challenges of China’s Furniture Industry. Drewno 2025, 68, 199709. [Google Scholar] [CrossRef]
- Hu, W.; Fu, W.; Zhao, Y. Optimal design of the traditional Chinese wood furniture joint based on experimental and numerical method. Wood Res-Slovak. 2024, 69, 50–59. [Google Scholar] [CrossRef]
- Xu, W.; Fang, X.Y.; Han, J.T.; Wu, Z.H. Effect of Coating Thickness on Sound Absorption Property of Four Wood Species Commonly Used for Piano Soundboards. Wood Fiber Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of Sanding Processes on the Surface Properties of Modified Poplar Coated by Primer Compared with Mahogany. Coatings 2020, 10, 856. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of Polyurethane Non-Transparent Coating Process on Paint Film Performance Applied on Modified Poplar. Coatings 2022, 12, 39. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gu, Y.T.; Xu, W.; Lu, T.; Li, W.J.; Fan, H.B. Compressive Properties of Green Velvet Material Used in Mattress Bedding. Appl. Sci. 2021, 11, 11159. [Google Scholar] [CrossRef]
- Wan, R.Y.; Xiong, X.Q.; Fu, S.J.; Xiong, D.J.; Xu, X.T. Life cycle assessment and optimization scenarios of sofas: A case study in China. Int. J. Life Cycle Assess. 2025, 1–17. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.Y.; Wang, T.Y.; Chu, Q.; Wang, X.W. Fused deposition 3D printing of bonsai tree guiding mold based on acrylonitrile-butadiene-styrene copolymer. BioResources 2024, 19, 5839–5846. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.Y.; Wang, T.Y.; Chu, Q.; Wen, S.Q. Design and Rapid Prototyping of Packaging Liner for Rosewood Craft based on Gyroid Infill Structure. BioResources 2025, 20, 842–851. [Google Scholar] [CrossRef]
- Abolhasani, H.; Farzi, G.; Davoodi, A.; Vakili-Azghandi, M.; Das, O.; Neisiany, R.E. Development of self-healable acrylic water-based environmental-friendly coating as an alternative to chromates coatings. Prog. Org. Coat. 2023, 176, 107402. [Google Scholar] [CrossRef]
- Han, K.; Liu, J.; Hao, F.; Wang, J.; Yuan, J.; Pan, Z.; Pan, M. An adaptive waterborne fluorocarbon coatings with Anti-Flashing Rust, Antibiofouling, and Self-Repairing properties. Chem. Eng. J. 2024, 495, 153644. [Google Scholar] [CrossRef]
- Wu, A.; Sun, Y.; Wang, Z.; Cao, G.; Zhang, H. Polyaniline/cellulose nanocrystal nanorods integrated into waterborne polyurethane coatings for enhanced corrosion resistance. Colloids Surf. A-Physicochem. Eng. Asp. 2025, 713, 136531. [Google Scholar] [CrossRef]
- Mastouri, A.; Efhamisisi, D.; Tarmian, A.; Boukherroub, R.; Lexa, M.; Karami, E.; Frigione, M. Sustainable superhydrophobic and self-cleaning wood via wax within Epoxy/PDMS nano-composite coatings: Durability related to surface morphology. Prog. Org. Coat. 2024, 186, 107951. [Google Scholar] [CrossRef]
- Barthwal, S.; Uniyal, S.; Barthwal, S. Nature-inspired superhydrophobic coating materials: Drawing inspiration from nature for enhanced functionality. Micromachines 2024, 15, 391. [Google Scholar] [CrossRef]
- Lu, Q.X.; Cheng, R.F.; Jiang, H.Q.; Xia, S.W.; Zhan, K.; Yi, T.F.; Morrell, J.J.; Yang, L.; Wan, H.; Du, G.B. Superhydrophobic wood fabricated by epoxy/Cu2(OH)3Cl NPs/stearic acid with performance of desirable self-cleaning, anti-mold, dimensional stability, mechanical and chemical durability. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 647, 1029162. [Google Scholar] [CrossRef]
- Jian, H.; Liang, Y.; Deng, C.; Xu, J.; Liu, Y.; Shi, J.; Wen, M.Y.; Park, H.J. Research progress on the improvement of flame retardancy, hydrophobicity, and antibacterial properties of wood surfaces. Polymers 2023, 15, 951. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally friendly anticorrosive polymeric coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Lin, D.; Wang, X.; Zhang, M.; Yuan, S.; Xu, F.; Bao, D.; Wang, H. Development of a robust and eco-friendly waterborne anti-corrosion composite coating with multiple synergistic corrosion protections. Compos. Part B Eng. 2022, 232, 109624. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, X.; Wu, Z. Application and prospect of self-healing microcapsules in surface coating of wood. Colloid Interface Sci. Commun. 2023, 56, 100736. [Google Scholar] [CrossRef]
- Wang, X.J.; Sun, W.P.; Li, W.G.; Zuo, C.L.; Jiang, Y.; Wang, S.X. Development of Waterborne Heavy-Duty Anticorrosive Coatings with Modified Nanoscale Titania. Coatings 2022, 12, 1651. [Google Scholar] [CrossRef]
- Tang, J.L.; Wang, Y.C.; He, M.J.; Huang, L.Q.; Wang, X.L.; Yu, J.Y. Electrothermochromic Fabrics with a Single-Layer Functional Coating Based on Silver Nanowires/Thermochromic Microcapsules/Waterborne Polyurethane Paints. ACS Appl. Mater. Interfaces 2024, 16, 70963–70972. [Google Scholar] [CrossRef]
- Xue, D.; Zhao, T. The electrothermal color-changing fabric based on high-sensitivity thermochromic microcapsules. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 678, 132458. [Google Scholar] [CrossRef]
- Boh Podgornik, B.; Šandrić, S.; Kert, M. Microencapsulation for functional textile coatings with emphasis on biodegradability—A systematic review. Coatings 2021, 11, 1371. [Google Scholar] [CrossRef]
- Ahuja, A.; Rastogi, V.K. PFAS free, food-grade, water and grease-resistant coating based on crosslinked shellac for molded pulp products. Prog. Org. Coat. 2024, 196, 108734. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Encapsulation systems for antimicrobial food packaging components: An update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef]
- Rezić, I.; Somogyi Škoc, M.; Majdak, M.; Jurić, S.; Stracenski, K.S.; Vinceković, M. Functionalization of Polymer Surface with Antimicrobial Microcapsules. Polymers 2022, 14, 1961. [Google Scholar] [CrossRef]
- Bagheri Darvish, H.; Bahrami, A.; Jafari, S.M.; Williams, L. Micro/nanoencapsulation strategy to improve the efficiency of natural antimicrobials against Listeria monocytogenes in food products. Crit. Rev. Food Sci. Nutr. 2021, 61, 1241–1259. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.F.; Li, Y.; Liu, H.; Yang, Z.; Wu, H.; Hu, Y. Formation of cinnamon essential oil/xanthan gum/chitosan composite microcapsules basing on Pickering emulsions. Colloid Polym. Sci. 2022, 300, 1187–1195. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, X. Preparation of Tea Tree Essential Oil–Chitosan Microcapsules and Its Effect on the Properties of Water-Based Coating. Polymers 2025, 17, 849. [Google Scholar] [CrossRef]
- GB/T 6739-2022; Determination of Coating Hardness by Pencil Method for Coloured Paints and Varnishes. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- GB/T 4893.9-2013; Physical and Chemical Performance Tests of the Paint Film on the Surface of Furniture—Part 9: Determination of Impact Resistance. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 4893.4-2013; Physical and Chemical Performance Tests of the Paint Film on the Surface of Furniture—Part 4: Determination of Adhesion by Cross-Cutting Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 21866-2008; Determination Method and Antibacterial Effect of Antibacterial Coatings (Paint Films). Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- GB/T 4789.2-2022; National Food Safety Standard—Microbiological Examination of Food—Aerobic Plate Count. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
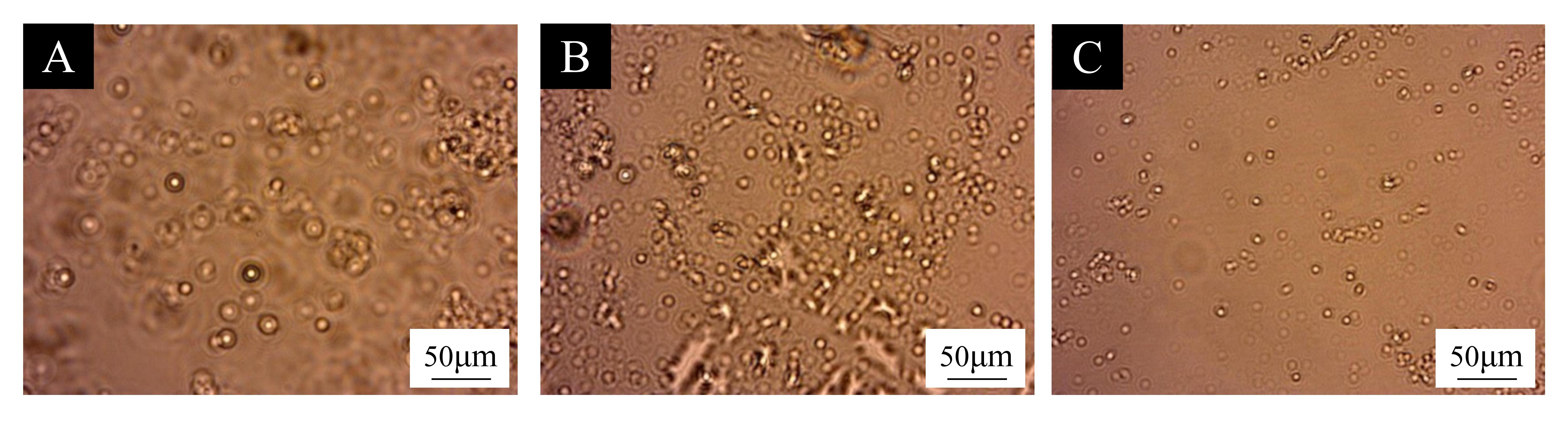
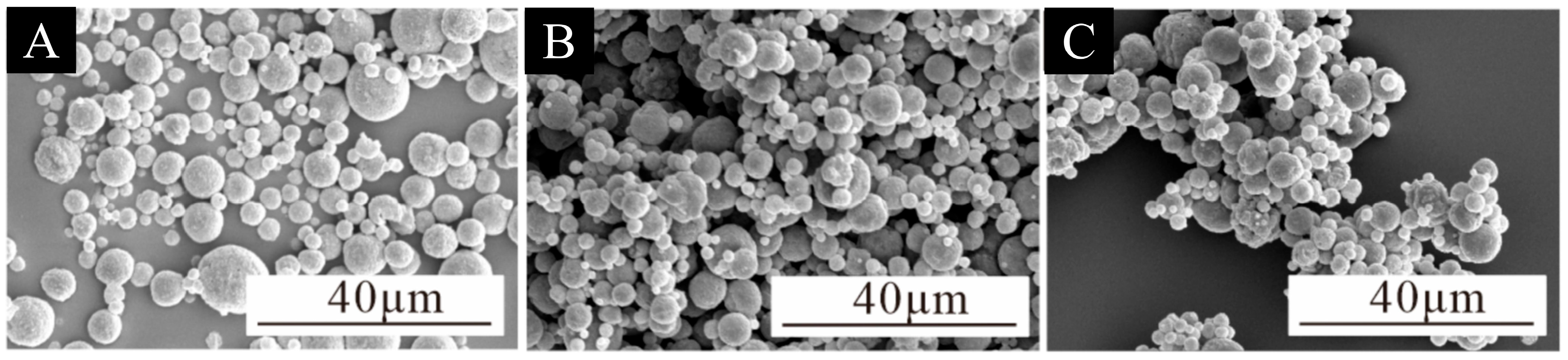
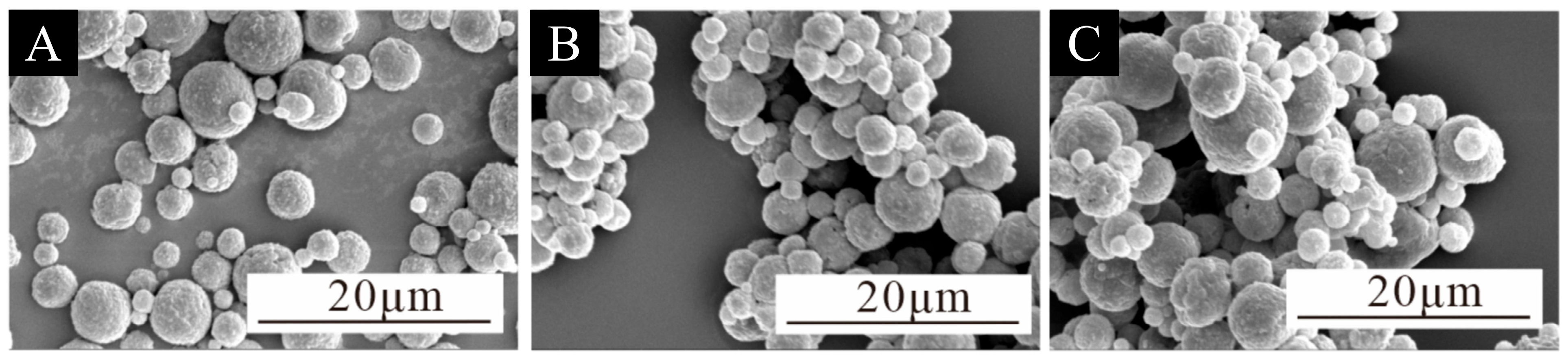


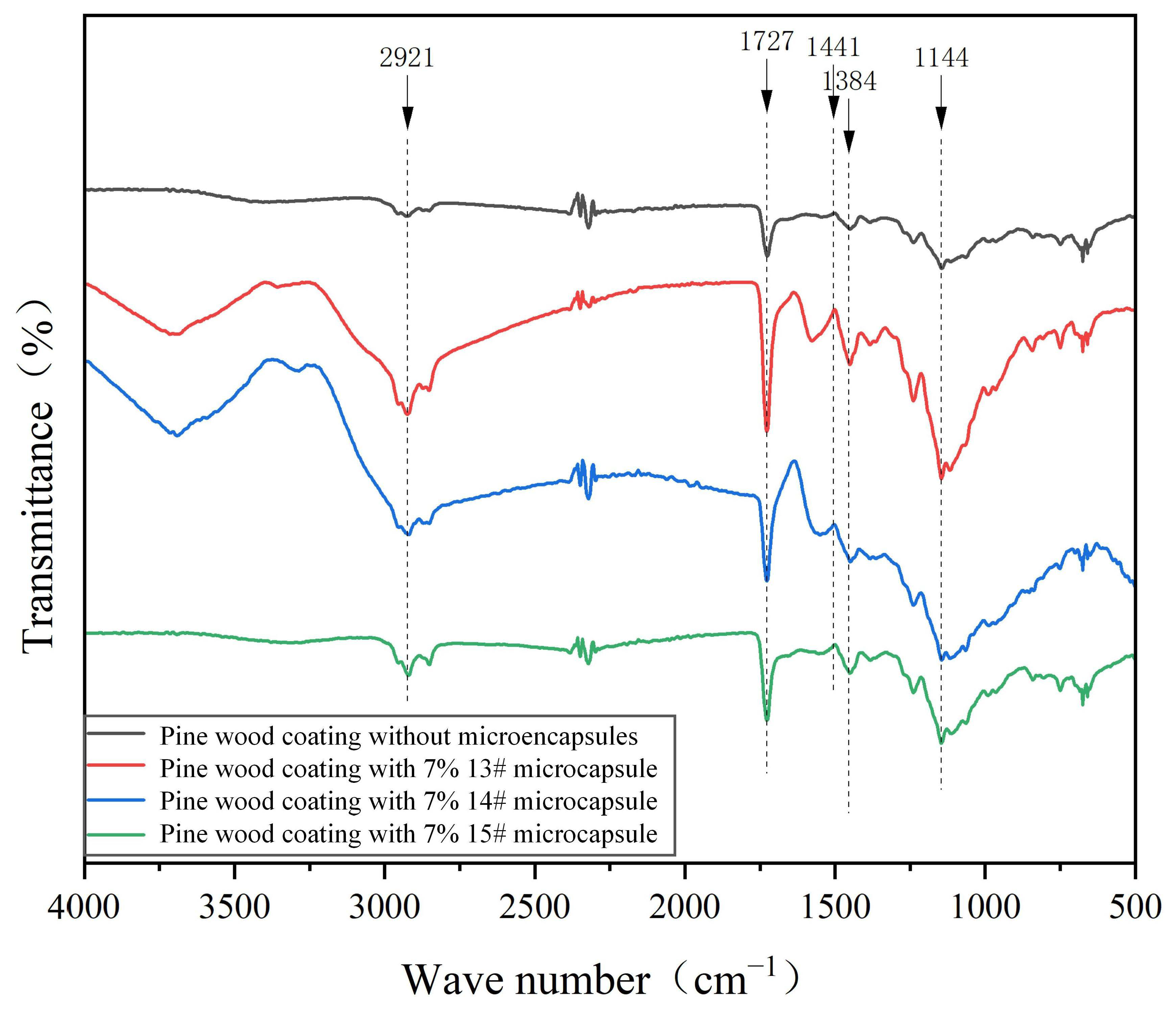

| Test Materials | Purity | Manufacturer |
|---|---|---|
| Tea tree essential oil | - | Wuhan Huaxiang Biotechnology Co., Ltd., Wuhan, China |
| Chitosan | AR | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Tween-80 | AR | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| SDBS | AR | Shandong Xinjucheng Chemical Technology Co., Ltd., Jinan, China |
| Acetic acid | AR | Shandong Chengkai New Material Co., Ltd., Linyi, China |
| Sodium tripolyphosphate | AR | Tianjin Huasheng Chemical Reagent Co., Ltd., Tianjin, China |
| Dulux primer | - | Nanjing Jinyou Biotechnology Co., Ltd., Nanjing, China |
| Nutrient agar medium | - | Zhongshan Baimicrobial Technology Co., Ltd., Guangdong, China |
| Nutrient broth medium | - | Zhongshan Baimicrobial Technology Co., Ltd., Guangdong, China |
| Sodium chloride | AR | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Escherichia coli | - | Beijing Baocang Biotechnology Co., Ltd., Beijing, China |
| Staphylococcus aureus | - | Beijing Baocang Biotechnology Co., Ltd., Beijing, China |
| Sample (#) | CS (g) | Acetic Acid (mL) | Deionized Water for Acetic Acid (mL) | Tween80 (g) | SDBS (g) | Deionized Water for Emulsifier (mL) | TTO (g) | STPP (g) |
|---|---|---|---|---|---|---|---|---|
| 13 | 2.000 | 0.100 | 99.000 | 1.600 | 6.400 | 192.000 | 2.400 | 0.400 |
| 14 | 2.000 | 0.100 | 99.000 | 2.000 | 8.000 | 190.000 | 2.400 | 0.400 |
| 15 | 2.000 | 0.100 | 99.000 | 2.400 | 9.600 | 188.000 | 2.400 | 0.400 |
| Equipment | Model | Manufacturer |
|---|---|---|
| Pencil hardness tester | HT-6510P | Aipu Measuring Instrument Co., Ltd., Quzhou, China |
| Coating Impactor | QCJ-50 | Jiaxin Measuring Instrument Co., Ltd., Dongguan, China |
| Adhesion Tester | QFH-A | Aipu Measuring Instrument Co., Ltd., Quzhou, China |
| Microencapsulation Content (%) | Primer Quality (g) | Microencapsulation Content (%) | Topcoat Quality (g) |
|---|---|---|---|
| 0 | 0.720 | 0 | 0.720 |
| 1 | 0.720 | 0.007 | 0.713 |
| 3 | 0.720 | 0.022 | 0.698 |
| 5 | 0.720 | 0.036 | 0.684 |
| 7 | 0.720 | 0.050 | 0.670 |
| 9 | 0.720 | 0.065 | 0.655 |
| Sample | Microencapsulation Content (%) | Average Number of Bacteria Recovered (CFU·Piece−1) | Antimicrobial Rate (%) | ||
|---|---|---|---|---|---|
| E. coli | S. aureus | E. coli | S. aureus | ||
| Pine wood Surface Add 13# Coating of microcapsules | 0 | 318 | 279 | - | - |
| 1 | 253 | 172 | 20.44 | 38.35 | |
| 3 | 189 | 137 | 40.57 | 50.90 | |
| 5 | 147 | 128 | 53.77 | 54.12 | |
| 7 | 119 | 108 | 62.58 | 61.29 | |
| 9 | 87 | 86 | 72.64 | 69.18 | |
| Pine wood Surface Add 14# Coating of microcapsules | 0 | 318 | 279 | - | - |
| 1 | 258 | 223 | 18.87 | 20.07 | |
| 3 | 186 | 187 | 41.51 | 32.97 | |
| 5 | 142 | 168 | 55.35 | 39.78 | |
| 7 | 121 | 124 | 61.95 | 55.56 | |
| 9 | 107 | 99 | 66.35 | 64.52 | |
| Pine wood Surface Add 15# Coating of microcapsules | 0 | 318 | 279 | - | - |
| 1 | 252 | 201 | 20.75 | 27.96 | |
| 3 | 182 | 161 | 42.77 | 42.29 | |
| 5 | 152 | 140 | 52.20 | 49.82 | |
| 7 | 133 | 116 | 58.18 | 58.42 | |
| 9 | 113 | 92 | 64.47 | 67.03 | |
| Sample | Microencapsulation Content (%) | Chromaticity Value | ΔE | ||
|---|---|---|---|---|---|
| L | a | b | |||
| Coating without microencapsulation | 0 | 79.73 | 6.60 | 29.77 | - |
| Pine wood Surface Add 13# Coating of microcapsules | 1 | 78.80 | 7.63 | 28.30 | 2.32 |
| 3 | 78.43 | 7.07 | 28.70 | 1.75 | |
| 5 | 79.57 | 6.50 | 28.37 | 1.41 | |
| 7 | 79.90 | 6.50 | 27.40 | 2.37 | |
| 9 | 78.63 | 7.33 | 26.47 | 3.55 | |
| Pine wood Surface Add 14# Coating of microcapsules | 1 | 79.03 | 6.60 | 30.67 | 1.14 |
| 3 | 78.80 | 7.17 | 29.43 | 1.14 | |
| 5 | 80.90 | 6.33 | 27.43 | 2.62 | |
| 7 | 79.70 | 7.03 | 27.33 | 2.47 | |
| 9 | 77.33 | 7.33 | 27.27 | 3.54 | |
| Pine wood Surface Add 15# Coating of microcapsules | 1 | 78.03 | 7.43 | 30.90 | 2.21 |
| 3 | 78.70 | 7.17 | 29.27 | 1.28 | |
| 5 | 78.07 | 7.63 | 27.97 | 2.66 | |
| 7 | 81.07 | 5.33 | 28.50 | 2.23 | |
| 9 | 79.90 | 5.07 | 26.77 | 3.37 | |
| Sample | Microencapsulation Content (%) | Glossiness (GU) | Light Loss Rate (%) | ||||
|---|---|---|---|---|---|---|---|
| 20° | 60° | 85° | 20° | 60° | 85° | ||
| Coating without microencapsulation | 0 | 5.80 | 23.70 | 43.90 | - | - | - |
| Pine wood Surface Add 13# Coating of microcapsules | 1 | 5.60 | 21.70 | 37.60 | 3.45 | 8.44 | 14.35 |
| 3 | 4.70 | 19.70 | 33.30 | 18.97 | 16.88 | 24.15 | |
| 5 | 3.50 | 12.90 | 23.40 | 39.66 | 45.57 | 46.70 | |
| 7 | 2.60 | 8.60 | 8.80 | 55.17 | 63.71 | 79.95 | |
| 9 | 2.50 | 6.80 | 6.40 | 56.90 | 71.31 | 85.42 | |
| Pine wood Surface Add 14# Coating of microcapsules | 1 | 5.80 | 23.70 | 37.00 | 1.72 | 1.69 | 15.72 |
| 3 | 4.30 | 18.00 | 29.30 | 25.86 | 24.05 | 33.28 | |
| 5 | 3.20 | 11.60 | 18.40 | 44.83 | 51.05 | 58.09 | |
| 7 | 3.00 | 9.00 | 2.10 | 48.28 | 62.03 | 95.22 | |
| 9 | 2.40 | 6.60 | 4.20 | 58.62 | 72.15 | 90.43 | |
| Pine wood Surface Add 15# Coating of microcapsules | 1 | 5.50 | 23.30 | 43.90 | 5.17 | 11.81 | 4.56 |
| 3 | 4.50 | 18.40 | 30.30 | 22.41 | 22.36 | 30.98 | |
| 5 | 3.00 | 11.40 | 14.30 | 48.28 | 51.90 | 67.43 | |
| 7 | 2.80 | 9.50 | 11.40 | 51.72 | 59.92 | 74.03 | |
| 9 | 2.40 | 5.70 | 2.20 | 58.62 | 75.95 | 94.99 | |
| Microencapsulation Content (%) | R | ||
|---|---|---|---|
| 13# | 14# | 15# | |
| 0 | 0.6447 | 0.6447 | 0.6447 |
| 1 | 0.6388 | 0.6566 | 0.6042 |
| 3 | 0.6407 | 0.6406 | 0.6613 |
| 5 | 0.6465 | 0.6780 | 0.6530 |
| 7 | 0.6860 | 0.6493 | 0.6566 |
| 9 | 0.6803 | 0.6571 | 0.6727 |
| Microencapsulation Content (%) | Hardness | ||
|---|---|---|---|
| 13# | 14# | 15# | |
| 0 | HB | HB | HB |
| 1 | HB | HB | HB |
| 3 | H | H | H |
| 5 | 2H | H | H |
| 7 | 2H | 2H | 2H |
| 9 | 2H | 2H | 2H |
| Microencapsulation Content (%) | Adhesion (Level) | ||
|---|---|---|---|
| 13# | 14# | 15# | |
| 0 | 0 | 0 | 0 |
| 1 | 1 | 1 | 1 |
| 3 | 1 | 1 | 1 |
| 5 | 1 | 1 | 1 |
| 7 | 1 | 1 | 1 |
| 9 | 2 | 2 | 2 |
| Microencapsulation Content (%) | Impact Resistance (Level) | ||
|---|---|---|---|
| 13# | 14# | 15# | |
| 0 | 4 | 4 | 4 |
| 1 | 4 | 4 | 4 |
| 3 | 4 | 4 | 4 |
| 5 | 4 | 4 | 4 |
| 7 | 3 | 4 | 3 |
| 9 | 3 | 3 | 3 |
| Microencapsulation Content (%) | Roughness (μm) | ||
|---|---|---|---|
| 13# | 14# | 15# | |
| 0 | 0.356 | 0.356 | 0.356 |
| 1 | 0.389 | 0.410 | 0.487 |
| 3 | 0.487 | 0.459 | 0.613 |
| 5 | 0.758 | 0.629 | 1.209 |
| 7 | 1.320 | 1.270 | 1.359 |
| 9 | 2.279 | 4.989 | 2.815 |
| Sample (#) | Average Number of Bacteria Recovered (CFU·Piece−1) | Antimicrobial Rate (%) | ||
|---|---|---|---|---|
| E. coli | S. aureus | E. coli | S. aureus | |
| 0 | 142 | 162 | - | - |
| 13 | 85 | 99 | 40.14 | 38.89 |
| Sample (#) | Microencapsulation Content (%) | Antibacterial Rate After 48 h (%) | Antibacterial Rate After 4 Months (%) | Optical Properties | Mechanical Properties | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | E. coli | S. aureus | ΔE | 60° Light Loss Rate (%) | R | Hardness | Adhesion (Level) | Impact Resistance (Level) | Roughness (μm) | ||
| 13 | 7 | 62.58 | 61.29 | 40.14 | 38.89 | 2.37 | 63.71 | 0.6860 | 2H | 1 | 3 | 1.320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Zhu, Y.; Yan, X. Effect of Tea Tree Essential Oil@Chitosan Microcapsules on Surface Coating Properties of Pine Wood. Coatings 2025, 15, 938. https://doi.org/10.3390/coatings15080938
Zhang N, Zhu Y, Yan X. Effect of Tea Tree Essential Oil@Chitosan Microcapsules on Surface Coating Properties of Pine Wood. Coatings. 2025; 15(8):938. https://doi.org/10.3390/coatings15080938
Chicago/Turabian StyleZhang, Nana, Ye Zhu, and Xiaoxing Yan. 2025. "Effect of Tea Tree Essential Oil@Chitosan Microcapsules on Surface Coating Properties of Pine Wood" Coatings 15, no. 8: 938. https://doi.org/10.3390/coatings15080938
APA StyleZhang, N., Zhu, Y., & Yan, X. (2025). Effect of Tea Tree Essential Oil@Chitosan Microcapsules on Surface Coating Properties of Pine Wood. Coatings, 15(8), 938. https://doi.org/10.3390/coatings15080938




