Microbial Biosurfactant as Sustainable Inhibitor to Mitigate Biocorrosion in Metallic Structures Used in the Offshore Energy Sector
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Biosurfactant Production
2.3. Biosurfactant Extraction
2.4. Evaluation of the Biosurfactant as an Inhibitor of Microorganisms Responsible for Metal Biocorrosion
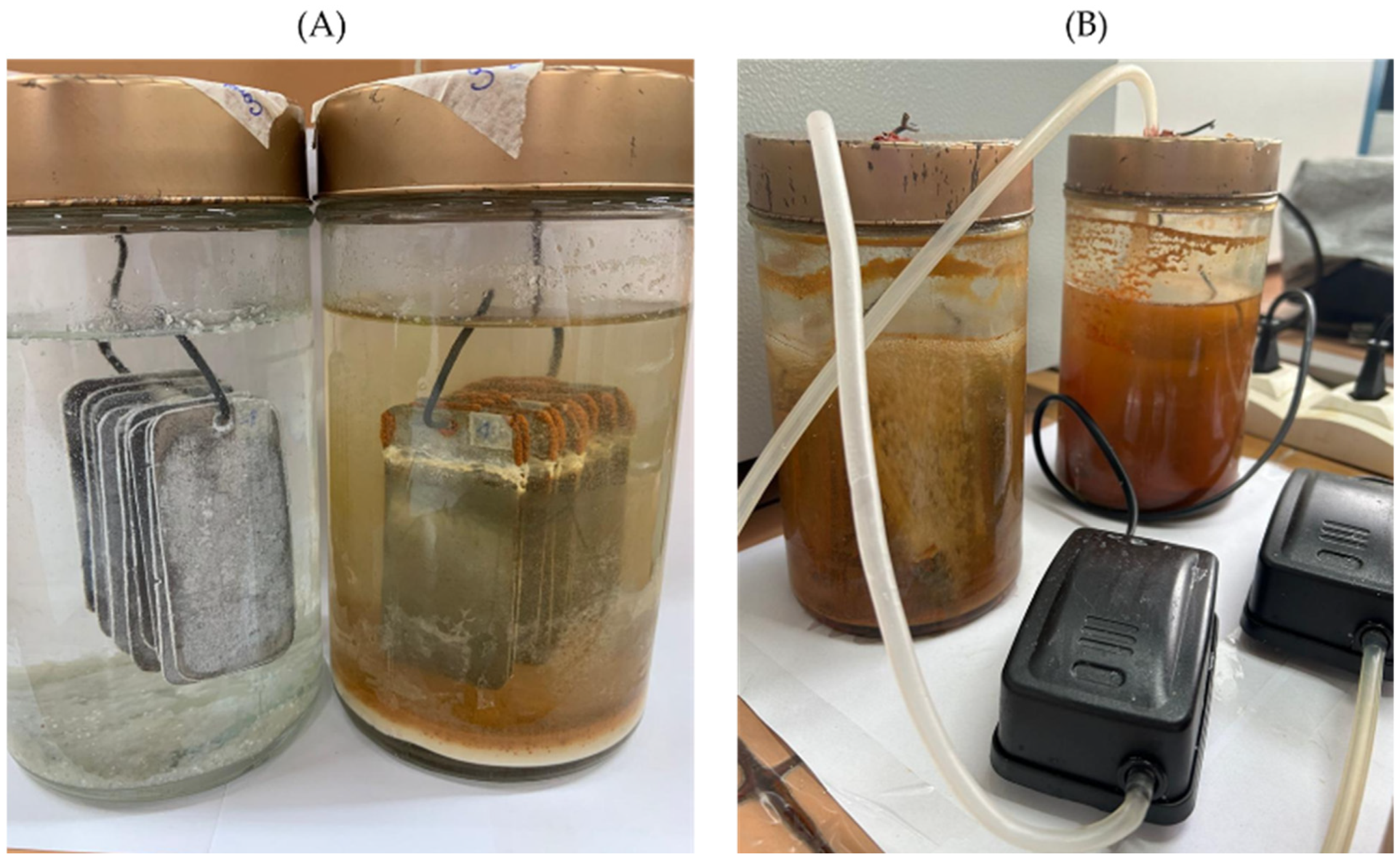
2.4.1. Mass Loss Tests
2.4.2. Microbiological Analyses
Total Aerobic Heterotrophic Bacteria
Total Anaerobic Heterotrophic Bacteria
Sulfate Reducing Bacteria
2.4.3. Scanning Electron Microscopy Paired with Energy Dispersive Spectroscopy
2.5. Statistical Analysis
3. Results and Discussion
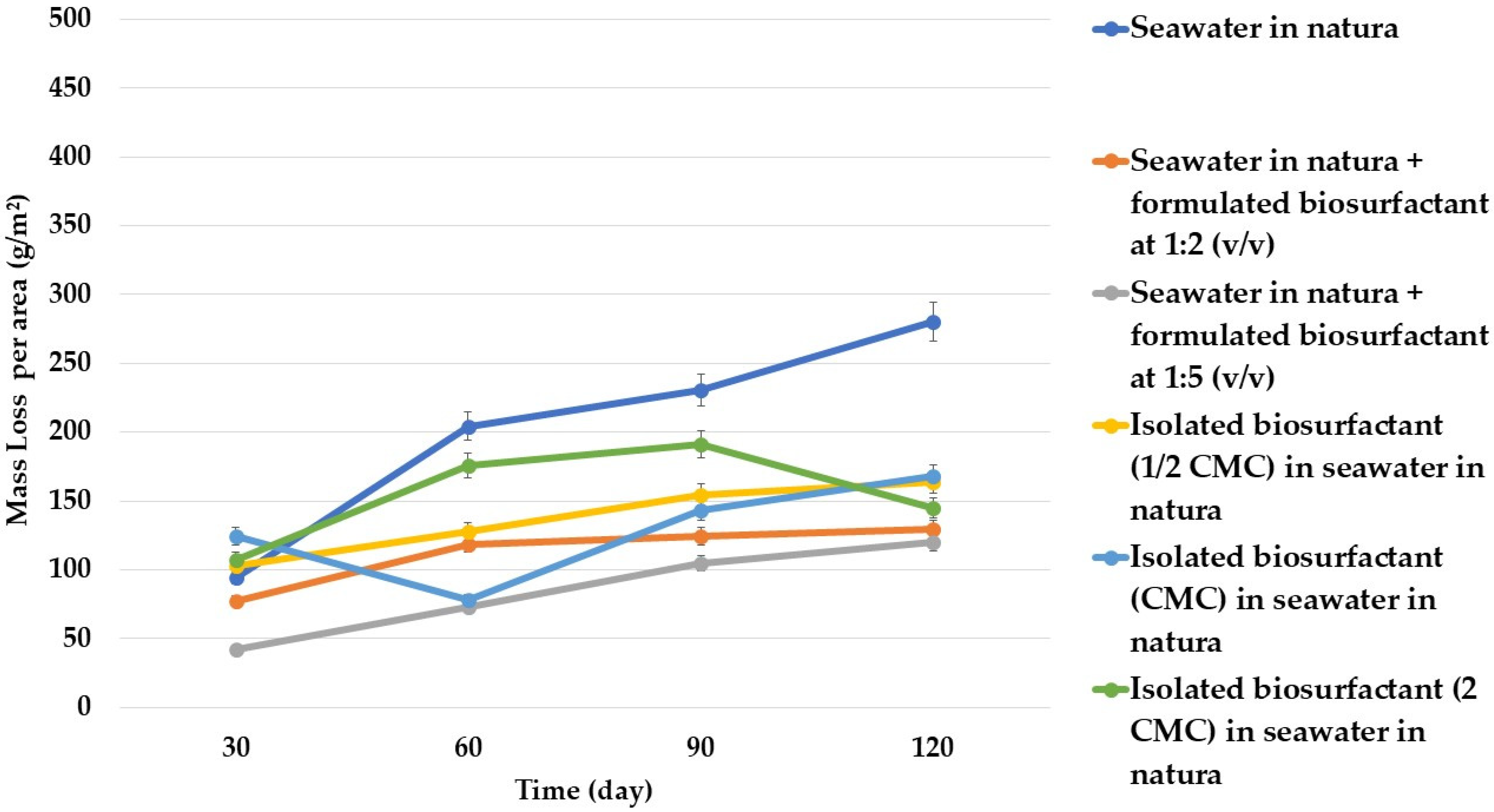
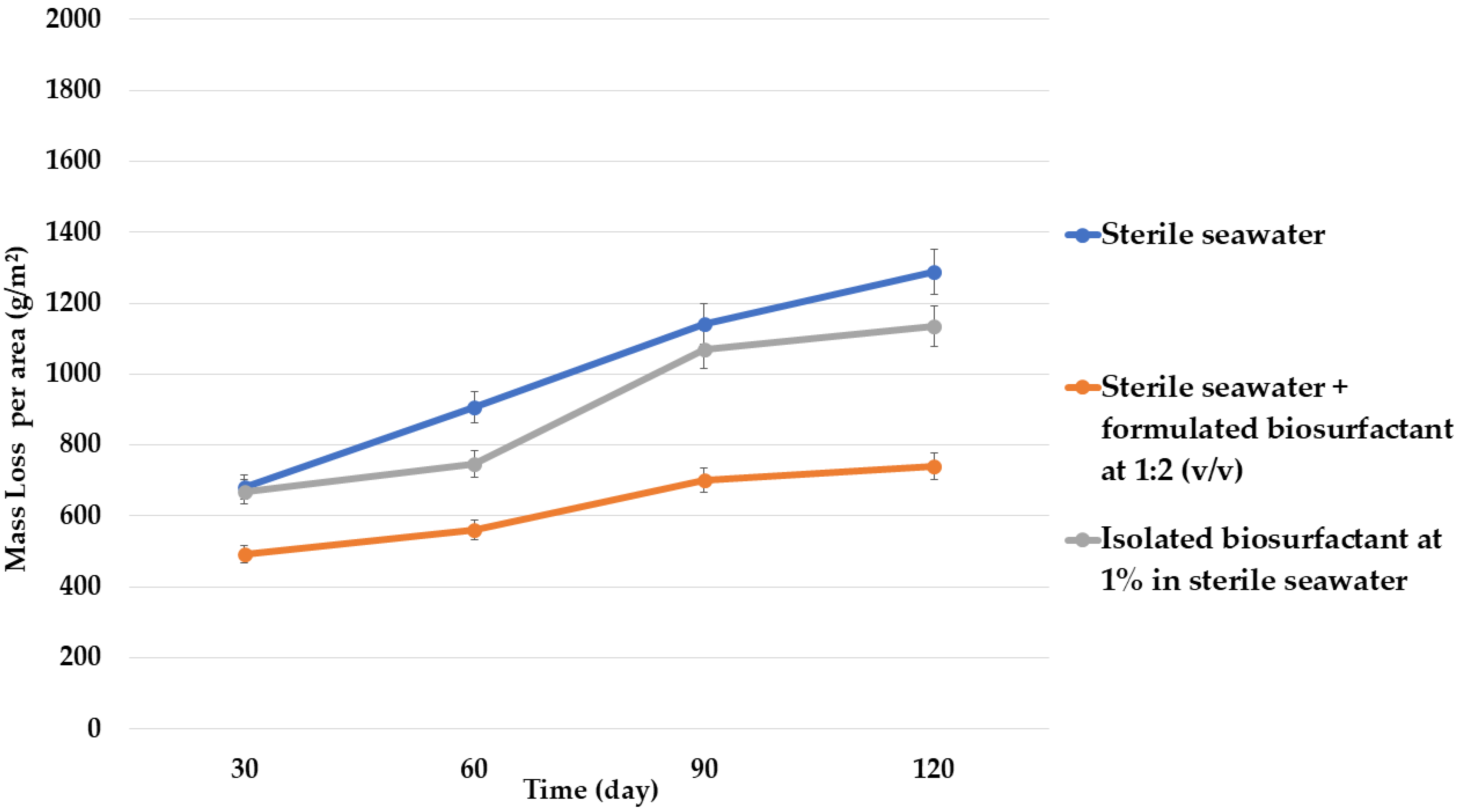
3.1. Evaluation of Biosurfactant as a Biocorrosion Inhibiting Agent
3.2. Autochthonous Microorganisms in Static and Dynamic Systems
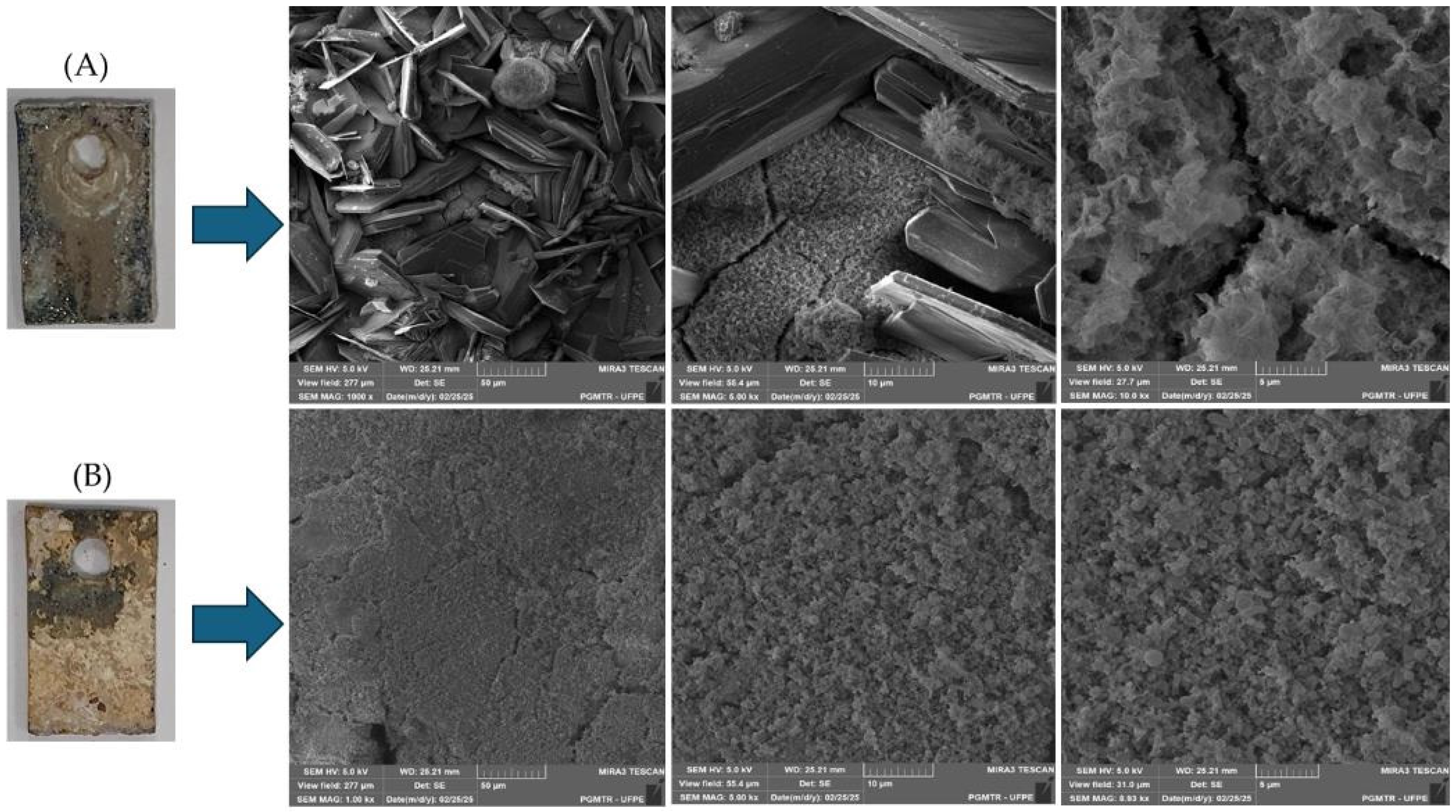
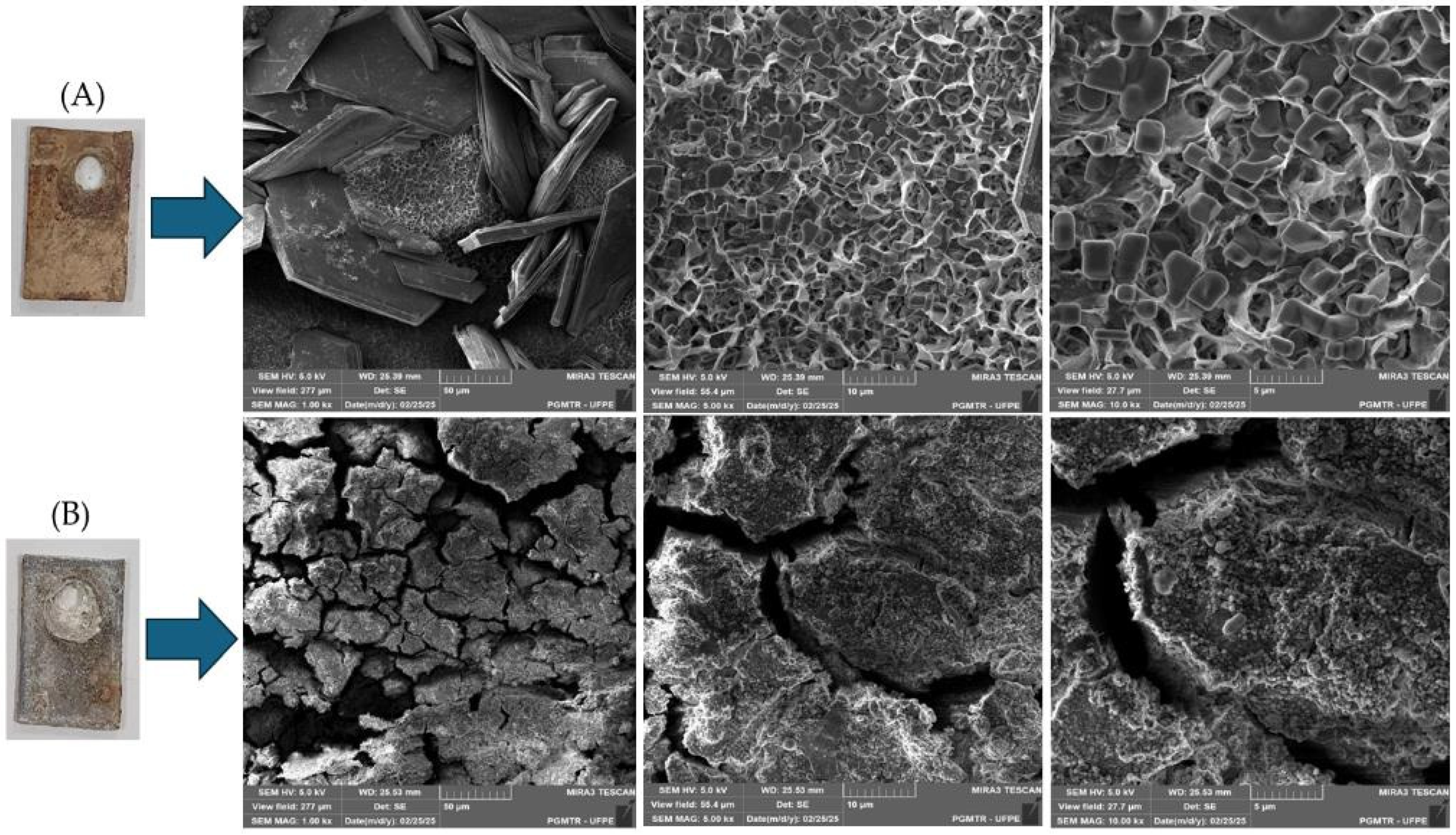
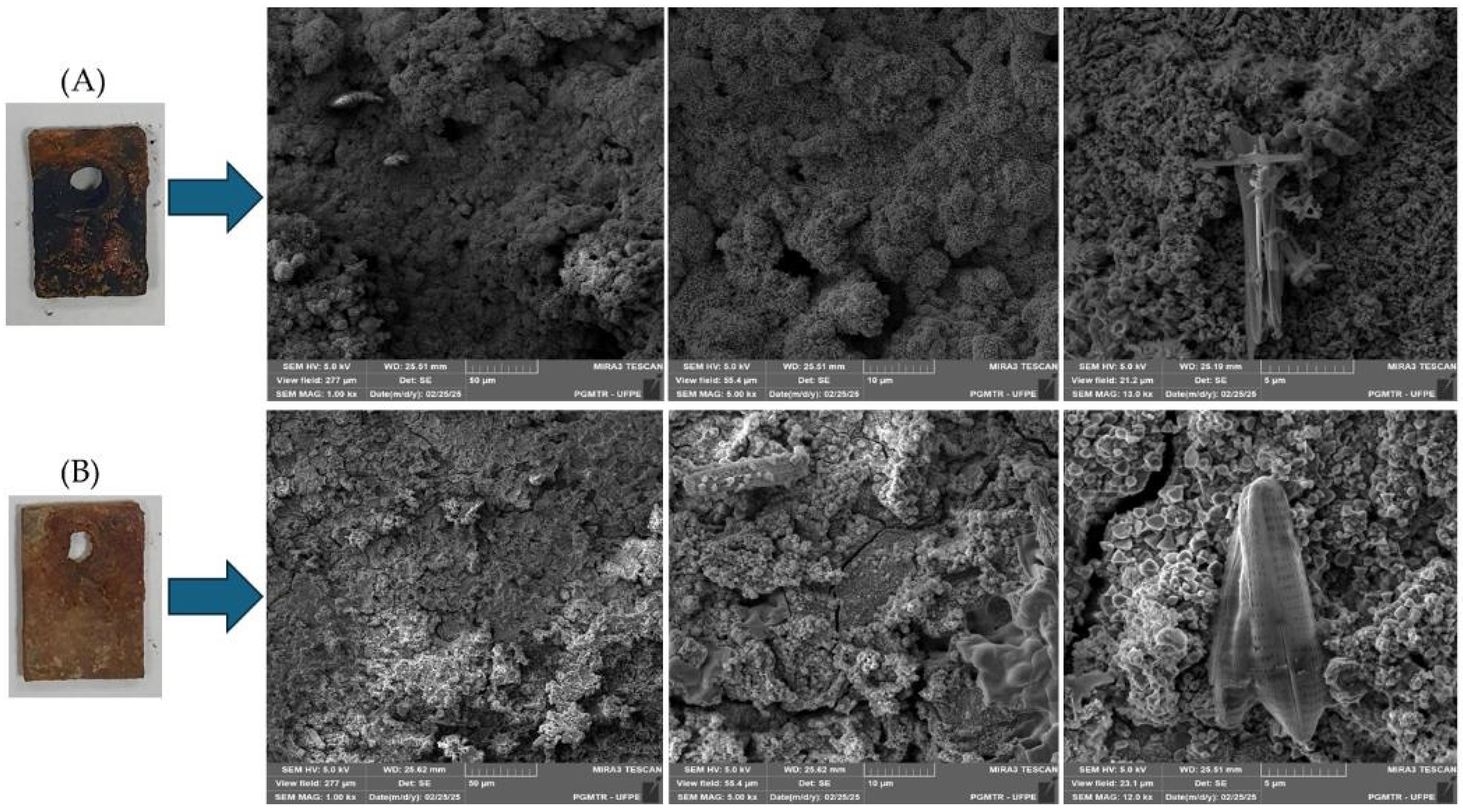
3.3. Scanning Electron Microscopy
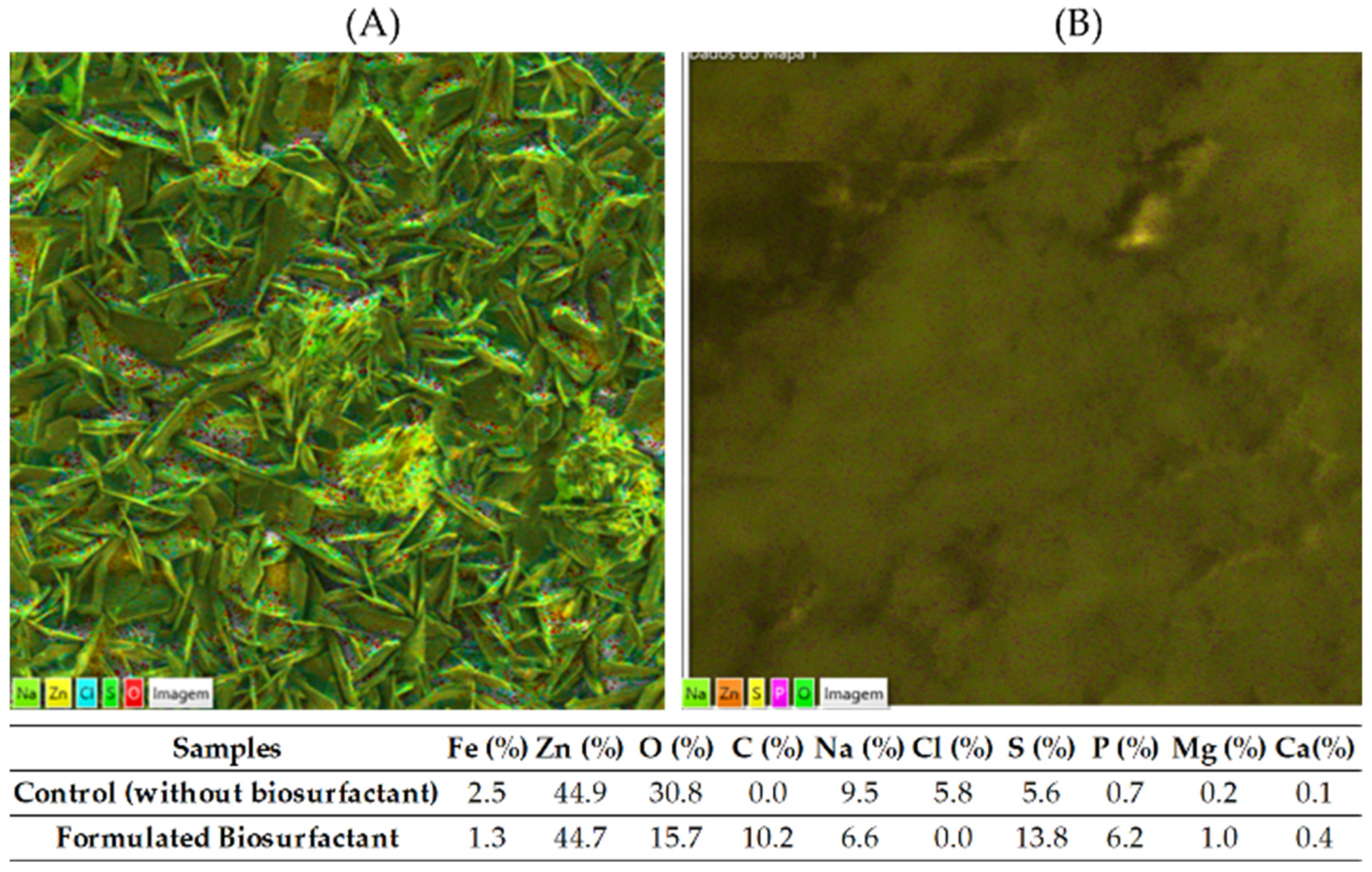
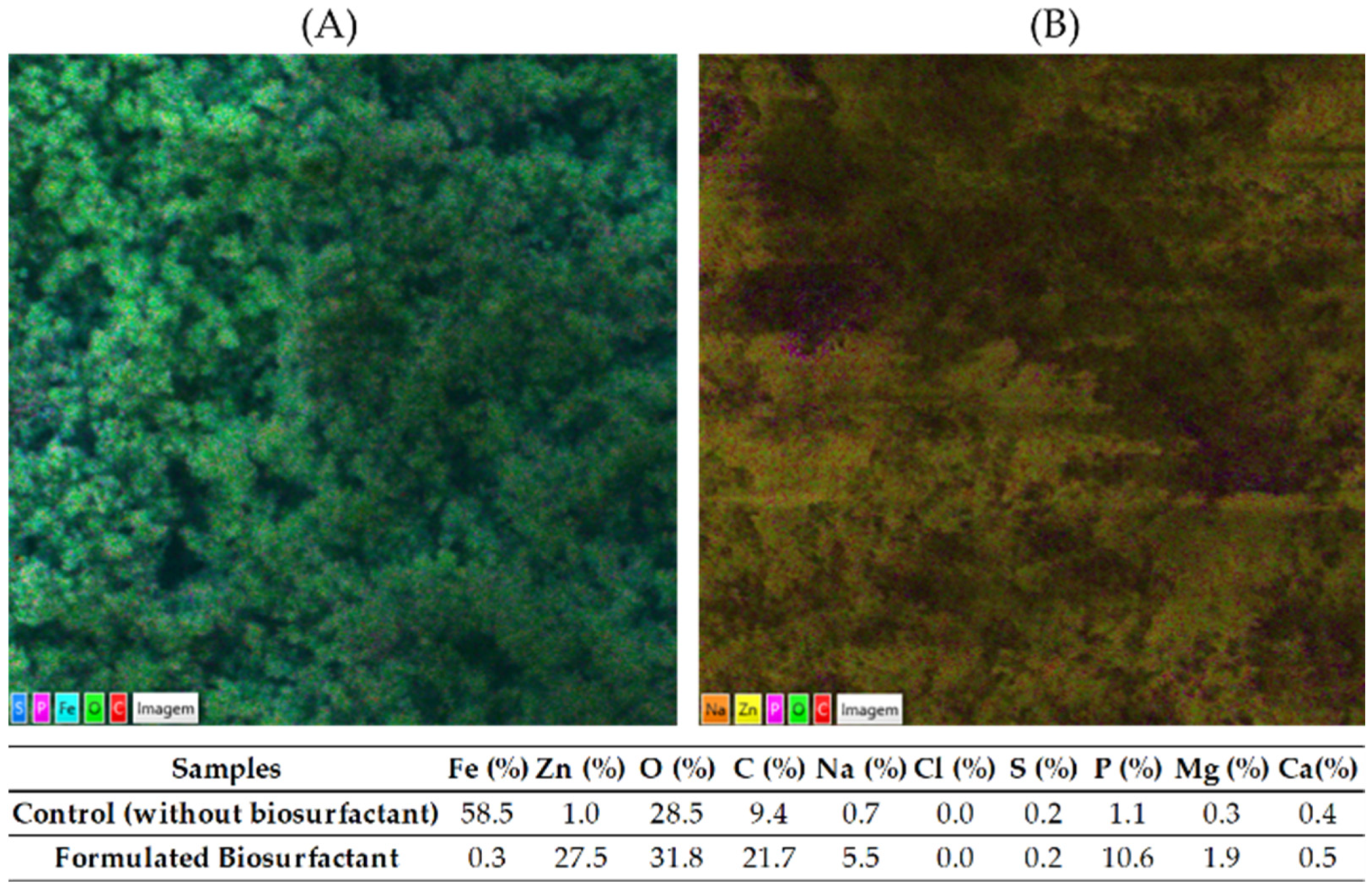
3.4. Characterization by Energy Dispersive Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, X.; Wang, C.; Liu, J.; Sand, W.; Nabuk Etim, I.-I.; Zhang, Y.; Xu, A.; Duan, J.; Zhang, R. The Microbiologically Influenced Corrosion and Protection of Pipelines: A Detailed Review. Materials 2024, 17, 4996. [Google Scholar] [CrossRef]
- Sani, S.; Aliyu, A.; Bagudo, A.I.; Aliero, A.A.; Umar, A.A.; Haruna, M.; Usman, M. Review on Bacteria Associated with Metal Rusting. UMYU Sci. J. 2024, 3, 213–223. [Google Scholar] [CrossRef]
- Shurayfan, Z.M.M.; Shubayli, S.I.A.; Maha, S.M.A.; Allaghabi, S.A.; Manaa, A.M.E.; Hzazi, F.A.A.; Hamdi, G.A.Y. Biofilms: Mechanisms of Formation and Strategies for Control in Clinical Settings. Egypt. J. Chem. 2024, 67, 793–816. [Google Scholar] [CrossRef]
- Erdei-Tombor, P.; Kiskó, G.; Taczman-Brückner, A. Biofilm Formation in Water Distribution Systems. Processes 2024, 12, 280. [Google Scholar] [CrossRef]
- Liu, W.; Bi, W.; Hu, Y.; Lu, W.; Feng, W.; Wang, Y.; Li, Y.; Liu, J. Influence of Initial pH and Sulfate-Reducing Bacteria Concentration on the Microbiologically Influenced Corrosion of Buried Pipeline Steel. Mater. Corros. 2024, 75, 1193–1203. [Google Scholar] [CrossRef]
- Dominici, L.E.; Del Panno, M.T.; Viera, M.R. Effect of Tetrakis(hydroxymethyl)phosphonium Sulfate (THPS) on the Microbial Community and Corrosion of Carbon Steel in a Simulated Crude-Oil Storage Tank Environment. Geoenergy Sci. Eng. 2024, 236, 212775. [Google Scholar] [CrossRef]
- Li, Q.; Gong, L.; Chen, X.; Gadd, G.M.; Liu, D. Dual role of microorganisms in metal corrosion: A review of mechanisms of corrosion promotion and inhibition. Front. Microbiol. 2025, 16, 1552103. [Google Scholar] [CrossRef]
- Sivakumar, D.; Ramasamy, R.; Thiagarajan, R.; Rangaiya, Y.; Thirumalairaj, B.; Krishnamoorthy, U.; Siddiqui, M.I.H.; Natrayan, L.; Kumar, A.; Shah, M.A. Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview. Open Chem. 2024, 22, 20240036. [Google Scholar] [CrossRef]
- Dini, S.; Bekhit, A.E.-D.A.; Roohinejad, S.; Vale, J.M.; Agyei, D. The Physicochemical and Functional Properties of Biosurfactants: A Review. Molecules 2024, 29, 2544. [Google Scholar] [CrossRef]
- Faccioli, Y.E.d.S.; de Oliveira, K.W.; Campos-Guerra, J.M.; Converti, A.; Soares da Silva, R.d.C.F.; Sarubbo, L.A. Biosurfactants: Chemical Properties, Ecofriendly Environmental Applications, and Uses in the Industrial Energy Sector. Energies 2024, 17, 5042. [Google Scholar] [CrossRef]
- Oliveira, K.W.; Selva Filho, A.A.P.; Faccioli, Y.E.S.; Araújo, G.P.; Converti, A.; Soares da Silva, R.d.C.F.; Sarubbo, L.A. Eco-Friendly Biosurfactant: Tackling Oil Pollution in Terrestrial and Aquatic Ecosystems. Fermentation 2025, 11, 199. [Google Scholar] [CrossRef]
- Simões, C.R.; da Silva, M.W.P.; de Souza, R.F.M.; Hacha, R.R.; Merma, A.G.; Torem, M.L.; Silvas, F.P.C. Biosurfactants: An Overview of Their Properties, Production, and Application in Mineral Flotation. Resources 2024, 13, 81. [Google Scholar] [CrossRef]
- Faccioli, Y.E.S.; Silva, G.O.; Soares da Silva, R.C.F.; Sarubbo, L.A. Application of a Biosurfactant from Pseudomonas cepacia CCT 6659 in Bioremediation and Metallic Corrosion Inhibition Processes. J. Biotechnol. 2022, 351, 109–121. [Google Scholar] [CrossRef]
- Lavanya, M.; Machado, A.A. Surfactants as Biodegradable Sustainable Inhibitors for Corrosion Control in Diverse Media and Conditions: A Comprehensive Review. Sci. Total Environ. 2024, 908, 168407. [Google Scholar] [CrossRef]
- Welikala, S.; Al-Saadi, S.; Gates, W.P.; Panter, C.; Singh Raman, R.K. Sulphate Reducing Bacteria (SRB) biofilm development and its role in microbial corrosion of carbon steel. Front. Mater. 2024, 11, 1360869. [Google Scholar] [CrossRef]
- Soares da Silva, R.C.F.; Almeida, D.G.; Meira, H.M.; Silva, E.J.; Farias, C.B.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Production and Characterization of a New Biosurfactant from Pseudomonas cepacia Grown in Low-Cost Fermentative Medium and Its Application in the Oil Industry. Biocatal. Agric. Biotechnol. 2017, 12, 206–215. [Google Scholar] [CrossRef]
- Deng, M.-C.; Li, J.; Hong, Y.-H.; Xu, X.-M.; Chen, W.-X.; Yuan, J.-P.; Peng, J.; Yi, M.; Wang, J.-H. Characterization of a novel biosurfactant produced by marine hydrocarbon-degrading bacterium Achromobacter sp. HZ01. J. Appl. Microbiol. 2016, 120, 889–899. [Google Scholar] [CrossRef]
- Pal, Y.P.; Mali, S.N.; Pratap, A.P. Optimization of the primary purification process of extracting sphorolipid from the fermentation broth to achieve a higher yield and purity. Tenside Surfactants Deterg. 2022, 59, 441–449. [Google Scholar] [CrossRef]
- Porto de Suape. Programa de Monitoramento da Qualidade e Controle Ambiental—Monitoramento Oceanográfico. Governo do Estado de Pernambuco—Suape, 2024. Available online: https://www.suape.pe.gov.br/pt/131-meio-ambiente/gestao-ambiental/ambiente-portuario/programa-de-monitoramento-da-qualidade-e-controle-ambiental/monitoramento-oceanografico (accessed on 20 June 2025).
- Fawzy, A.; Al Bahir, A.; Alqarni, N.; Toghan, A.; Khider, M.; Ibrahim, I.M.; Abulreesh, H.H.; Elbanna, K. Evaluation of Synthesized Biosurfactants as Promising Corrosion Inhibitors and Alternative Antibacterial and Antidermatophytes Agents. Sci. Rep. 2023, 13, 2585. [Google Scholar] [CrossRef]
- ASTM G1-03(2017)e1; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017. Available online: https://www.astm.org/g0001-03r17e01.html (accessed on 25 June 2025).
- Oliveira, S.H. Estudo da Utilização da Xantana e Hipoclorito de Sódio Como Estratégia Para Controle da Biocorrosão. Ph.D. Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2010. [Google Scholar]
- Chaouiki, A.; Chafiq, M.; Al-Moubaraki, A.H.; Bakhouch, M.; El Yazidi, M.; Gun Ko, Y. Electrochemical Behavior and Interfacial Bonding Mechanism of New Synthesized Carbocyclic Inhibitor for Exceptional Corrosion Resistance of Steel Alloy: DFTB, MD and Experimental Approaches. Arab. J. Chem. 2022, 15, 104323. [Google Scholar] [CrossRef]
- Silva, N.; Junqueira, V.C.A.; Silveira, N.F.A.; Taniwaki, M.H.; Gomes, R.A.R.; Okazaki, M.M. Manual de Métodos de Análise Microbiológica de Alimentos e Água, 5th ed.; Blucher: São Paulo, Brazil, 2017. [Google Scholar]
- McKenzie, J.; Hamilton, W.A. The assay of in-situ activities of sulphate-reducing bacteria in a laboratory marine corrosion model. Int. Biodeterior. Biodegrad. 1992, 29, 285–297. [Google Scholar] [CrossRef]
- Nevius, B.A.; Bagwell, C.E.; Brigmon, R.L. Characterization of Microbial Communities in TCE-Contaminated Seep Zone Sediments. J. South Carol. Acad. Sci. 2004, 2, 25–29. [Google Scholar]
- Postgate, J.R. The Sulphate-Reducing Bacteria, 2nd ed.; Cambridge University Press: Cambridge, UK, 1984; ISBN 978-0521257916. [Google Scholar]
- Beech, I.B. Corrosion of Technical Materials by Microorganisms. Int. Biodeterior. Biodegrad. 2004, 53, 177–183. [Google Scholar] [CrossRef]
- Tamilselvi, B.; Bhuvaneshwari, D.S.; Karuppasamy, P.; Padmavathy, S.; Nikhil, S.; Siddegowda, S.B.; Murthy, H.C.A. Investigation of Corrosion Inhibition of Mild Steel in 0.5 M H2SO4 with Lachancea fermentati Inhibitor Extracted from Rotten Grapefruits (Vitis vinifera): Adsorption, Thermodynamic, Electrochemical, and Quantum Chemical Studies. ACS Phys. Chem. Au 2024, 4, 67–84. [Google Scholar] [CrossRef]
- Olivia, R.; Ang, C.H.; Clotilda, P.; Caroline, M.; Rudy, T.; Joe, N. Corrosion Inhibition of Mild Steel Bars by Biosurfactant Produced by Penicillium citrinum. IOP Conf. Ser. Earth Environ. Sci. 2023, 1135, 012057. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, X.; Sun, M.; Li, Z.; Zhang, D.; Lei, Y.; Zhang, M.; Fan, Y.; Xu, D.; Wang, F. Rhamnolipid as an Eco-Friendly Corrosion Inhibitor for Microbiologically Influenced Corrosion. Corros. Sci. 2022, 204, 110390. [Google Scholar] [CrossRef]
- Parthipan, P.; Sabarinathan, D.; Angaiah, S.; Rajasekar, A. Glycolipid Biosurfactant as an Eco-Friendly Microbial Inhibitor for the Corrosion of Carbon Steel in Vulnerable Corrosive Bacterial Strains. J. Mol. Liq. 2018, 261, 473–479. [Google Scholar] [CrossRef]
- Mulligan, C.N. Environmental Applications for Biosurfactants. Environ. Pollut. 2005, 133, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, M.; Duarte, N.; Ribeiro, I. Exploring Biosurfactants as Antimicrobial Approaches. Pharmaceuticals 2024, 17, 1239. [Google Scholar] [CrossRef] [PubMed]
- Stratford, M.; Wilson, P.D.G. Agitation effects on microbial cell-cell interactions. Lett. Appl. Microbiol. 1990, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.L.D.; Leal, C.; Brito-Gitirana, L. Exploring the Synergistic Effects of Agitation on the Interaction Between Nanoparticles of Polyethylene and Benzophenone in Microalgae Tetraselmis sp. Am. J. Environ. Sci. 2023, 19, 127–133. [Google Scholar] [CrossRef]
- Purwasena, I.A.; Astuti, D.I.; Fauziyyah, N.A.; Putri, D.A.S.; Sugai, Y. Inhibition of Microbial Influenced Corrosion on Carbon Steel ST37 Using Biosurfactant Produced by Bacillus sp. Mater. Res. Express 2019, 6, 115405. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, Z. Potential of biosurfactants in corrosion inhibition. In Industrial Applications of Biosurfactants and Microorganisms: Green Technology Avenues from Lab to Commercialization; Aslam, R., Aslam, J., Hussain, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 277–305. [Google Scholar] [CrossRef]
- Mao, T.; Huang, H.; Liu, D.; Shang, X.; Wang, W.; Wang, L. Novel Cationic Gemini Ester Surfactant as an Efficient and Eco-Friendly Corrosion Inhibitor for Carbon Steel in HCl Solution. J. Mol. Liq. 2021, 339, 117174. [Google Scholar] [CrossRef]
- Rahman, K.S.; Thahira-Rahman, J.; Lakshmanaperumalsamy, P.; Banat, I. Towards efficient crude oil degradation by a mixed bacterial consortium. Bioresour. Technol. 2002, 85, 257–261. [Google Scholar] [CrossRef]
- Das, K.; Mukherjee, A.K. Characterization of Biochemical Properties and Biological Activities of Biosurfactants Produced by Pseudomonas aeruginosa Mucoid and Non-Mucoid Strains Isolated from Hydrocarbon-Contaminated Soil Samples. Appl. Microbiol. Biotechnol. 2005, 69, 192–199. [Google Scholar] [CrossRef]
- Soares da Silva, R.d.C.F.; Selva Filho, A.A.P.; Faccioli, Y.E.S.; Silva, Y.K.; Oliveira, K.W.; Araujo, G.P.; Rocha e Silva, N.M.P.; Converti, A.; Sarubbo, L.A. Application of Pseudomonas cepacia CCT 6659 Biosurfactant as a Metal Corrosion Inhibitor in a Constructed Accelerated Corrosion Chamber (ACC). Fermentation 2024, 10, 602. [Google Scholar] [CrossRef]
- Salgar-Chaparro, S.J.; Lepkova, K.; Pojtanabuntoeng, T.; Darwin, A.; Machuca, L.L. Nutrient Level Determines Biofilm Characteristics and Subsequent Impact on Microbial Corrosion and Biocide Effectiveness. Appl. Environ. Microbiol. 2020, 86, e02885-19. [Google Scholar] [CrossRef] [PubMed]
- Videla, H.A. Biocorrosão, Biofouling e Biodeterioração de Materiais, 1st ed.; Edgard Blucher: São Paulo, Brazil, 2003; p. 160. [Google Scholar]
- Stancu, M.M. Role of Indigenous Bacteria in Corrosion of Two Types of Carbon Steel. Microorganisms 2022, 10, 2451. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhang, J.; Mathivanan, K.; Wang, F.; Hou, B. Advances in Understanding Biofilm-Based Marine Microbial Corrosion. Corros. Rev. 2024, 43, 301–314. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Du, M.; Shan, X.; Tian, Z. Study on the Screening of Marine Beneficial Bacteria and the Inhibition of Sulfate-Reducing Bacteria Corrosion in Marine Oil Field Produced Water. Anti-Corros. Methods Mater. 2024, 71, 439–449. [Google Scholar] [CrossRef]
- Kaundal, T.; Sharma, A.; Batra, N. The Antioxidant, Antimicrobial and Antibiofilm Potential of Biosurfactants Derived from Enterococcus faecalis BHT-2. J. Surfactants Deterg. 2024, 27, 793–800. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhang, D.; Ma, H. Biofilm Characterization and Corrosion Behavior of Carbon Steel in Marine Environments. Bioelectrochemistry 2020, 132, 107414. [Google Scholar] [CrossRef]
- Didouh, H.; Khurshid, H.; Hadj Meliani, M.; Suleiman, R.K.; Umoren, S.A.; Bouhaik, I.S. Exploring NRB Biofilm Adhesion and Biocorrosion in Oil/Water Recovery Operations Within Pipelines. Bioengineering 2024, 11, 1046. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, D.; Zhang, D.; Gao, Y.; Xu, D.; Wang, F. Harnessing Active Biofilm for Microbial Corrosion Protection of Carbon Steel against Geobacter sulfurreducens. Bioelectrochemistry 2024, 157, 108654. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, J.; Liu, H.; Ren, P.; Yan, F. Effect of Dissolved Oxygen Content on Tribo-Corrosion Behavior of Monel 400 Alloy in Seawater. Metals 2023, 14, 6. [Google Scholar] [CrossRef]
- Khan, M.S.; Liang, T.; Liu, Y.; Zhang, H.; Li, H.; Guo, S.; Pan, H.; Yang, K.; Zhao, Y.-C. Microbiologically Influenced Corrosion Mechanism of Ferrous Alloys in Marine Environment. Superalloys 2022, 12, 1458. [Google Scholar] [CrossRef]
- Kazimierczak, J.; Kania, H.; Staszuk, M. The Effect of Surfactants on the Structure and Properties of Electrogalvanized Coatings. Coatings 2019, 9, 93. [Google Scholar] [CrossRef]
- Yin, S.; Yang, H.; Dong, Y.; Qu, C.; Liu, J.; Guo, T.; Duan, K. Environmentally Favorable Magnesium Phosphate Anti-Corrosive Coating on Carbon Steel and Protective Mechanisms. Sci. Rep. 2021, 11, 197. [Google Scholar] [CrossRef]
- Kalyani, D.S.; Rao, S.S.; Babu, M.S.; Rao, B.V.A.; Sreedhar, B. Electrochemical and Surface Analytical Studies of Carbon Steel Protected from Corrosion in a Low-Chloride Environment Containing a Phosphonate-Based Inhibitor. Res. Chem. Intermed. 2015, 41, 5007–5032. [Google Scholar] [CrossRef]
- Zhao, Y.; Ni, M.; Pan, Y.; Li, L.; Ding, Y. Cultivation of Phosphate-Accumulating Biofilm: Study of the Effects of Acyl-Homoserine Lactones (AHLs) and Cyclic Dimeric Guanosine Monophosphate (c-Di-GMP) on the Formation of Biofilm and the Enhancement of Phosphate Metabolism Capacity. Sci. Total Environ. 2024, 928, 172408. [Google Scholar] [CrossRef] [PubMed]
- Boxin, W.; Zheng, C.; Mengchao, N.; Jin, X.; Bokai, L.; Tangqing, W.; Changkun, Y.; Cheng, S. Synergistic Microbial Interactions and Electrochemical Mechanisms Driving Microbiologically Influenced Corrosion in Offshore Platform Produced Seawater at 60 °C. Corros. Sci. 2025, 248, 112797. [Google Scholar] [CrossRef]




| Parameter | Value Range | Unit | Notes |
|---|---|---|---|
| Salinity | 0.06–38.05 | ‰ | Estuarine-to-marine variability |
| Temperature | 27.16–32.09 | °C | Tropical marine zone |
| pH | up to 8.54 | – | - |
| Dissolved Oxygen | ≥5.0 | mg/L | Supports aerobic biofilm development |
| Total Phosphorus | up to 0.13 | mg/L | Nutrient availability |
| Iron (Fe, dissolved) | up to 0.739 | mg/L | Detected in natural concentration |
| Zinc (Zn) | 0.12 | mg/L | Trace metal |
| Oils and Greases | Not detected | – | Visual inspection |
| Test | Solution |
|---|---|
| Control | Seawater in natura |
| Condition 1 | Seawater in natura + formulated biosurfactant at 1:2 (v/v) |
| Condition 2 | Seawater in natura + formulated biosurfactant at 1:5 (v/v) |
| Condition 3 | Isolated biosurfactant at 1/2 CMC in seawater in natura |
| Condition 4 | Isolated biosurfactant at CMC in seawater in natura |
| Condition 5 | Isolated biosurfactant (2 CMC) in seawater in natura |
| Control | Sterile seawater |
| Condition 1 | Sterile seawater + formulated biosurfactant at 1:2 (v/v) |
| Condition 2 | Isolated biosurfactant at 1% in sterile seawater |
| 30 Days | 60 Days | 90 Days | 120 Days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluid Conditions | TAHB | TAnHB | SRB | TAHB | TAnHB | SRB | TAHB | TAnHB | SRB | TAHB | TAnHB | SRB |
| Seawater in natura | + | + | + | − | + | + | + | + | + | + | + | + |
| Seawater in natura + formulated biosurfactant at 1:2 (v/v) | + | + | − | + | + | + | + | + | + | + | + | − |
| Seawater in natura + formulated biosurfactant at 1:5 (v/v) | + | + | − | + | + | − | + | + | − | + | + | − |
| Isolated biosurfactant (1/2 CMC) in seawater in natura | + | − | + | − | − | + | − | − | − | − | − | − |
| Isolated biosurfactant (CMC) in seawater in natura | + | + | + | + | − | + | − | − | − | − | − | − |
| Isolated biosurfactant (2 CMC) in seawater in natura | + | − | + | + | − | + | − | − | − | − | − | + |
| Sterile seawater | + | − | + | − | − | + | − | − | + | + | − | + |
| Sterile seawater + formulated biosurfactant at 1:2 (v/v) | + | + | − | + | + | − | + | + | − | + | + | − |
| Isolated biosurfactant at 1% in sterile seawater | + | − | − | + | + | − | + | + | − | + | − | − |
| 30 Days | 60 Days | 90 Days | 120 Days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluid Conditions | TAHB | TAnHB | SRB | TAHB | TAnHB | SRB | TAHB | TAnHB | SRB | TAHB | TAnHB | SRB |
| Seawater in natura | + | + | + | + | + | + | + | + | + | − | − | − |
| Seawater in natura + formulated biosurfactant at 1:2 (v/v) | + | + | + | − | − | − | − | − | − | + | − | − |
| Seawater in natura + formulated biosurfactant at 1:5 (v/v) | + | + | + | + | − | + | + | + | − | + | + | − |
| Isolated biosurfactant (1/2 CMC) in seawater in natura | + | − | + | + | − | + | + | + | − | + | + | − |
| Isolated biosurfactant (CMC) in seawater in natura | + | + | + | − | + | − | − | + | − | − | + | − |
| Isolated biosurfactant (2 CMC) in seawater in natura | + | − | + | + | + | + | + | + | − | − | + | − |
| Sterile seawater | − | + | − | − | + | + | + | + | − | − | − | − |
| Sterile seawater + formulated biosurfactant at 1:2 (v/v) | + | + | − | + | − | + | − | − | − | − | + | − |
| Isolated biosurfactant at 1% in sterile seawater | + | − | + | − | + | − | − | + | − | − | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccioli, Y.E.S.; França, I.B.; Oliveira, K.W.; Roque, B.A.C.; Selva Filho, A.A.P.; Converti, A.; Soares da Silva, R.d.C.F.; Sarubbo, L.A. Microbial Biosurfactant as Sustainable Inhibitor to Mitigate Biocorrosion in Metallic Structures Used in the Offshore Energy Sector. Coatings 2025, 15, 937. https://doi.org/10.3390/coatings15080937
Faccioli YES, França IB, Oliveira KW, Roque BAC, Selva Filho AAP, Converti A, Soares da Silva RdCF, Sarubbo LA. Microbial Biosurfactant as Sustainable Inhibitor to Mitigate Biocorrosion in Metallic Structures Used in the Offshore Energy Sector. Coatings. 2025; 15(8):937. https://doi.org/10.3390/coatings15080937
Chicago/Turabian StyleFaccioli, Yslla Emanuelly S., Irinan B. França, Kaio Wêdann Oliveira, Bruno Augusto C. Roque, Alexandre Augusto P. Selva Filho, Attilio Converti, Rita de Cássia F. Soares da Silva, and Leonie A. Sarubbo. 2025. "Microbial Biosurfactant as Sustainable Inhibitor to Mitigate Biocorrosion in Metallic Structures Used in the Offshore Energy Sector" Coatings 15, no. 8: 937. https://doi.org/10.3390/coatings15080937
APA StyleFaccioli, Y. E. S., França, I. B., Oliveira, K. W., Roque, B. A. C., Selva Filho, A. A. P., Converti, A., Soares da Silva, R. d. C. F., & Sarubbo, L. A. (2025). Microbial Biosurfactant as Sustainable Inhibitor to Mitigate Biocorrosion in Metallic Structures Used in the Offshore Energy Sector. Coatings, 15(8), 937. https://doi.org/10.3390/coatings15080937










