Abstract
A facile way to prepare Cu(OH)2 inorganic nanofiltration membranes with neatly arranged multilayers has been developed based on the reaction of a sodium hydroxide solution and a copper ammonia solution at the liquid–liquid interfaces. The effects of the concentration, temperature, and time of the liquid–liquid reaction on membrane structure and pore sizes were studied by SEM, TEM, and X-ray diffraction. The growth mechanism of the membrane was discussed and the formation process model was proposed. It was found that the reaction temperature was a key factor in obtaining a Cu(OH)2 monolayer, and this could be used to adjust the thickness and pore size of the monolayer. The as-prepared Cu(OH)2 membranes exhibited excellent filtration performance with the pure water fluxes of 156.2 L·m−2 h−1 bar−1 and retention rates of 100% for methylene blue (50 ppm) at a pressure of 0.1 MPa. This successfully opens up a new method of synthesizing multilayer nanoarrays’ Cu(OH)2 structure for nanofiltration.
1. Introduction
A nanofiltration (NF) membrane with a microporous structure of about 1 nm is a new type of separation membrane with a molecular weight cut-off value that lies between those of a reverse osmosis membrane and an ultrafiltration membrane []. NF membranes can effectively filter and separate heavy metals, microorganisms, small organic molecules and even monovalent bivalent salts in water at a low pressure based on the coupling effect of steric hindrance effect and Donnan effect []. Up to date, because of the high rejection and good processability, organic based membranes are still the mainstream of commercial NF membranes. However, there are problems such as poor oxidation resistance and biological pollution. Therefore, the preparation of inorganic nanofiltration membranes with biofouling remains challenging. The wide application of loose inorganic nanofiltration membranes of graphene oxide in textile wastewater treatment has demonstrated the advantages of efficient separation of dyes and salts [].
Self-assembly is an effective method of preparing membranes, whereby the components of a system organize into ordered or functional structures [,,]. Liquid–liquid interfaces offer highly controlled, flexible, and adaptable platforms for nanoparticle, nanowire or nanobelts in order to create to obtain the sophisticated construction and specific functional materials []. Copper is known for its excellent resistance to biological pollution, and its compounds are ideal materials for inorganic nanofiltration membranes such as copper oxide and copper hydroxide [].
The orthorhombic structure of Cu(OH)2 makes it an ideal material for nanoassembly-based preparation of nanofunctional devices. The magnetic properties of Cu(OH)2 are remarkably sensitive to the intercalation of molecular anions, making the material suitable for the sensor applications []. The Cu(OH)2 nanoparticles can be used as supercapacitors for growth on a substrate [,]. Even more intriguingly, Cu(OH)2 nanowires can also be used to prepare membrane for efficient separation of oil-water [,].
Two-dimensional (2D) inorganic nanomaterials have attracted considerable interest in the production of inorganic nanofiltration membranes for the separations of organic dyes and inorganic salts [,,,]. Conversely, most commercial nanofiltration membranes are generally organic polymer-based, and are fabricated using the interfacial polymerization process. However, there are currently few research reports on the in situ preparation of multilayer copper hydroxide nanoinorganic films. Here, the Cu(OH)2 nanomembranes with multilayer structure were synthesized for the first time using a liquid–liquid interface reaction. NF membranes have a layered structure similar to graphite, which can be peeled off layer by layer using adhesive tape. Compared to organic NF membranes, Cu(OH)2 NF membranes have excellent antibacterial and bactericidal abilities, as well as resistance to biological pollution. Furthermore, layer-by-layer nanoassembly provides an easy way to form a multilayer nanomembrane that is inexpensive and can be reproduced with high efficiency.
2. Materials and Methods
2.1. Reagents
Copper (II) sulfate pentahydrate (CuSO4·5H2O), ammonia (NH3·H2O), sodium hydroxide (NaOH), methylene blue and methyl blue were used, which are supplied from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, and all reagents were used without further purification.
2.2. Preparation of Cu(OH)2 Membranes
The fabrication method is as follows: Firstly, 0.25 M CuSO4, 1.2 M NaOH and 0.25 M NH3·H2O solutions were prepared using distilled water. Secondly, a copper ammonia solution was prepared. The NH3·H2O solution (60 mL) was quickly added into the CuSO4 solution (100 mL), and the resulting suspension was stirred by a magnetic stirrer for 30 min. Thirdly, Cu(OH)2 hollow nanocage spheres were prepared. A NaOH solution (24 mL) was added dropwise to the synthesized copper ammonia solution at a rate of 2 mL/min via a metering pump while the reaction solution was vigorously stirred. Once the dropwise addition was complete, the reaction solution was stirred for a further 30 min. This synthesis was carried out in a water bath and the reaction temperatures were controlled at 25 °C, 30 °C and 35 °C. Finally, the copper hydroxide slurries were filtered by a vacuum filtration device with a membrane diameter of 0.22 μm to produce three types of Cu(OH)2 membrane, the T-25 membrane, the T-30 membrane, and the T-35 membrane.
2.3. Characterization
The samples were examined using scanning electron microscopy (SEM, Nova Nano SEM 450, FEI, Hillsboro, OR, USA) and high-resolution transmission electron microscopy (HRTEM, Thermo Fisher Scientific Talos F200X G2, Waltham, MA, USA). X-ray diffraction (XRD, D/Max 2500PC, Rigaku, Japan) was performed using Cu-Kα radiation (λ = 0.15406 nm).
2.4. Water Flux and Filtration Performance
At present, household water purification equipment generally requires the installation of a booster pump to meet the water purification pressure requirements of nanofiltration water purifiers and reverse osmosis water purifiers. Therefore, we chose low pressure values to study the feasibility of using Cu(OH)2 NF membranes for interception and filtration under tap water pressure []. The water flux and filtration performance of the membrane were characterized using a laboratory-made suction filtration device at 0.1 MPa [] shown as in Figure 1 and ultraviolet–visible spectrophotometry (UV-2500, SHIMADZU, Kyoto, Japan).
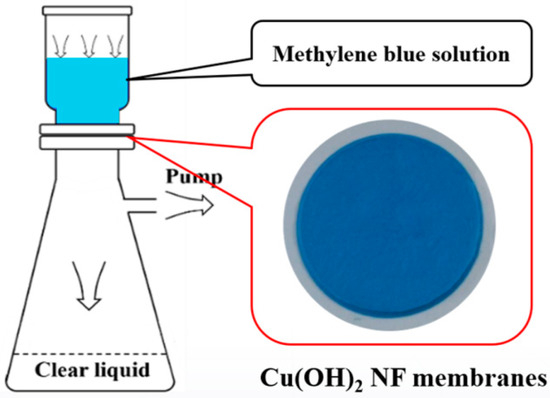
Figure 1.
A laboratory-made suction filtration device of Cu(OH)2 NF membranes at 0.1 MPa.
3. Results and Discussion
3.1. Characterization of Cu(OH)2 Nanowire Bundles
Figure 2 shows TEM and HRTEM images of Cu(OH)2 nanowire bundles. The widths labeled as A, B, and C represent the widths of nanowires or bundles, as indicated in the Figure 2b,e. The Cu(OH)2 bundles are all composed of nanowires, which are assembled from smaller diameter nanowires as shown in Figure 2f. As the temperature increases, the diameter of the nanowire grows rapidly. The diffraction pattern of the nanowire in Figure 2a indicates that the Cu(OH)2 nanowire is a polycrystalline structure, with diffraction rings that correspond to the (110), (111), (023), (220) and (222) crystal planes of Cu(OH)2. According to the HRTEM image in Figure 2c, the nanowires are assembled from nanoparticles, and the interplanar spacing of their three grains is 0.284 nm, 0.2078 nm and 0.2354 nm, coinciding with the (110), (131), and (022) crystal planes of Cu(OH)2, respectively. The above analysis indicates that the membrane is self-assembled step by step from nanoparticles, nanowires and nanobeams [].
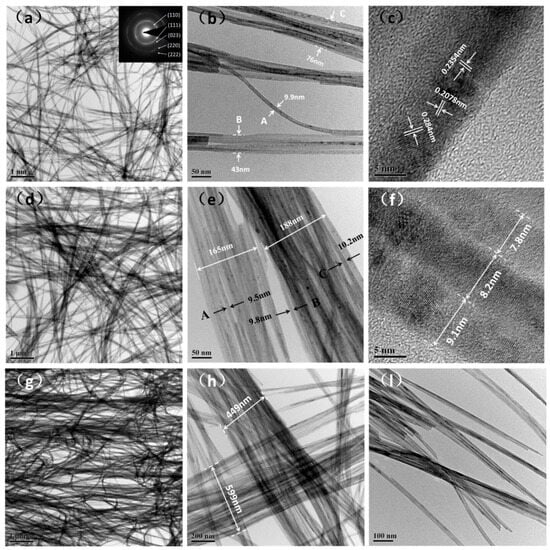
Figure 2.
TEM images and HRTEM lattice fringes of Cu(OH)2 nanowire bundles: (a–c) T-25; (d–f) T-30; (g–l) T-35.
3.2. Characterization of Cu(OH)2 Membranes
When filtered [], multiple single-layer membranes are formed by stacking numerous hollow nanocage spheres in the solution. Figure 3 shows the surface morphology and cross-sectional structure of Cu(OH)2 membranes. The surface of the membranes is flat and free of cracks. The length of the Cu(OH)2 nanowires on the membrane surface exceeds 30 µm. The nanowires on the outer surface are thicker (100–300 nm), while those on the subsurface are thinner (20–25 nm). Based on the principle of liquid–liquid interface reaction, the nanoparticles self-assemble along the droplet surface to form nanowires or nanobundles. The outermost interface of the droplet reacts violently, forming nanowires with larger diameters, while the diameters of the nanowires gradually decrease in the subsurface and inner layers. This can be further explained by a graphical abstract (GA). Nanowire bundles are stacked on top of each other, forming a porous structure. The diameter of the nanobeam and pore size gradually increase with rising reaction temperature. According to the SEM images of the cross-section of the Cu(OH)2 membranes shown in Figure 3c,f,l, it can be seen that a multi-layer membrane structure is formed by assembling a single-layer film with a thickness of about 300–500 nm. Cu(OH)2 membranes assembled from multi-layer membranes can achieve layer-by-layer filtration of the filtrate, improving the filtration accuracy.
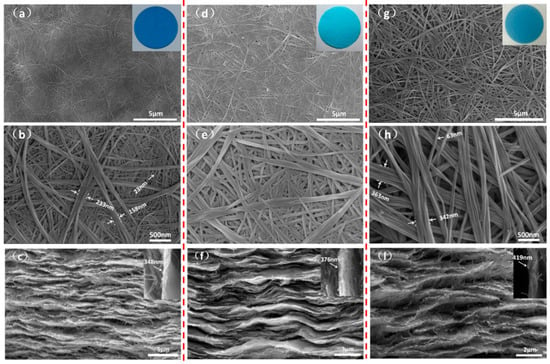
Figure 3.
The surface morphology and cross-section structure of Cu(OH)2 membranes: (a–c) T-25; (d–f) T-30; (g–l) T-35.
3.3. Formation Mechanism of Multilayered Cu(OH)2 Membranes
Figure 4 shows the growth mechanism of the Cu(OH)2 nanofiltration membrane. When a NaOH solution is added dropwise to a [Cu(NH3)4]SO4 solution, a liquid–liquid reaction interface with a high concentration and activity forms on the outer surface of the NaOH droplet []. The nanoparticles then self-assemble along the droplet surface to form nanowires or nanobundles, which gradually become thicker and surround the NaOH droplet. As the reaction progresses, the liquid–liquid interface moves towards the inner layer of the NaOH droplet. The [Cu(NH3)4]SO4 solution continues to undergo a liquid–liquid reaction as the nanobeam passes through the surface of the droplet. However, due to the spatial confinement effect formed by the outer nanobundles of the droplet and the resulting decrease in [Cu(NH3)4]SO4 concentration, smaller-diameter nanowires or nanobundles are formed inside the NaOH droplet. Once the reaction has finished, numerous Cu(OH)2 nanowire spheres are formed in the solution. During stirring, the Cu(OH)2 nanowire spheres were not damaged, indicating their strength. Freeze-drying the Cu(OH)2 solution while maintaining the droplet’s shape allows for clear observation of the Cu(OH)2 nanowire spheres’ morphology, as shown in Figure 4b. This further confirms the proposed model of the three-dimensional, droplet-shaped structure of Cu(OH)2 nanowire bundles. After filtering the Cu(OH)2 suspension, a flat, single-layer membrane was formed, as shown in Figure 4c. Finally, multiple single-layer membranes were assembled into a nanofiltration membrane.
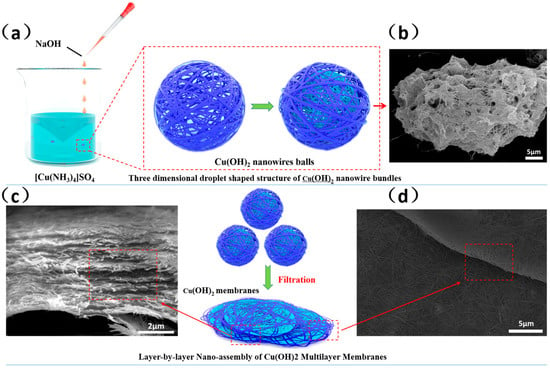
Figure 4.
Schematic diagram of formation mechanism of multilayer self-assembled Cu(OH)2 membranes. (a) The liquid–liquid reaction interface; (b) Cu(OH)2 nanowire spheres; (c) Multiple single-layer membranes (d) The edge of the membrane formed by Cu(OH)2 nanowire spheres.
3.4. Filtration Performance of Cu(OH)2 Membranes
As is well known, methylene blue is a commonly used dye and one of the commonly used retention materials in membrane material research. Its molecular diameter in pure water is about 1.09–1.40 nm. Therefore, by testing the retention rate of methylene blue, it can be indirectly determined whether the pore size of the membrane meets the pore size requirements of NF membranes. To characterize the retention performance of the Cu(OH)2 membranes, methyl blue and methylene blue were used in tests with a concentration of 50 ppm. As shown in Figure 5a, the T-25 and T-30 membranes completely cut off methyl blue from the solution. However, the rejection rate of the T-35 membrane for methyl blue was approximately 92%. The retention rates of the T-25 and T-30 membranes for methylene blue were 99% and 52%, respectively. The T-35 membrane did not filter methylene blue at all (see Figure 5b). The pure water fluxes of the three Cu(OH)2 membranes were 58.9, 133.7 and 159.2 L·m−2·h−1·bar−1 under 0.1 MPa pressure, respectively.
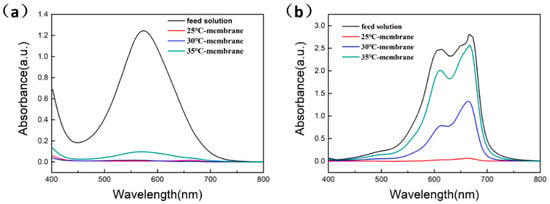
Figure 5.
UV–vis spectra of the feed solution and filtration performance of different Cu(OH)2 membranes: (a) Filtration of methyl blue; (b) Filtration of methylene blue.
Compared with other nanowire membranes [,], the Cu(OH)2 nanomembranes have smaller pores and can effectively filter dyes with different molecular weights. Furthermore, the method of preparing the Cu(OH)2 membranes is simple and inexpensive. Most importantly, the Cu(OH)2 membranes are fully free-standing and mechanically robust enough for filtration, even at higher applied pressures. The Young’s modulus of the three types of Cu(OH)2 nanobeams were measured using an atomic force microscope (AFM, shown in Figure S3). The respective values were found to be 579 MPa, 1.31 GPa, and 8.14 GPa.
4. Conclusions
In summary, a new kind of Cu(OH)2 NF membrane with a multilayered nanostructure was prepared based on the principle of liquid–liquid interface reaction. The formation mechanism of the multilayer nanomembranes via the self-assembly of nanowires and nanobundles to form larger nanocage spheres in the solution has also been systematically studied. When filtered, multiple single-layer membranes are formed by stacking hollow nanocage spheres. The reaction temperature plays a crucial role in controlling the pore size. The research results of the paper further explore the feasibility of preparing CuO NF membranes, and even Cu NF membranes, using Cu(OH)2 NF membranes as templates. Based on current research findings, the filtration retention pressure of Cu(OH)2 NF membranes is lower, which have potential application prospects in the purification of drinking water and sewage treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings15080895/s1, Figure S1: Macroscopic photos (a,b) and SEM image (c) of Cu(OH)2 membrane of T-30 membrane; Figure S2: X-ray diffraction patterns of the T-25 (a), T-30 (b), T-35 (c) samples; Figure S3: AFM morphology images and sampling force curves of Cu(OH)2 nanobeam: (a) T-25; (b) T-30, and (c) T-35.
Author Contributions
Conceptualization, Y.X. and G.L.; methodology, W.S.; validation, W.S. and Y.X.; formal analysis, W.S.; investigation; data curation, G.L.; writing—original draft preparation, W.S.; writing—review and editing, W.S. and G.L.; supervision, Y.X.; project administration, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Hundred Projects” of Nankai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
Author G.L. was employed by the company Weichai Lovol Intelligent Agricultural Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Di, J.; Wang, H.; Zhang, L.; Chen, Z.; Zhang, Y.; Mamba, B.B.; Qiu, M.; Guo, J.; Shao, L. Recent progress in advanced polyamide nanofiltration membranes via interfacial polymerization for desalination and beyond. Desalination 2024, 592, 118167. [Google Scholar] [CrossRef]
- Kong, P.; Sun, Z.; Gui, H.; Chen, Z.; Song, Y.; Wang, Y.; Wang, Y.; Kipper, M.J.; Tang, J.; Huang, L. Advances in the application of graphene oxide composite loose nanofiltration membranes for dye and salt separation. J. Environ. Chem. Eng. 2024, 12, 114278. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, Y.; Dai, L.; Dong, W.; He, H.; Li, H.; Nie, Z.; Sang, Y. Photo-Induced Self-assembly of Copolymer-Capped Nanoparticles into Colloidal Molecules. Angew. Chem. Int. Ed. Engl. 2023, 63, e202313406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Bai, Z.X.; Lin, G.; Xia, Y.Q.; Tong, L.F.; Li, T.R.; Liu, C.C.; Liu, S.N.; Jia, K.; Liu, X.B. Tannic acid-mediated interfacial layer-by-layer self-assembly of nanofiltration membranes for high-efficient dye separation. Appl. Surf. Sci. 2022, 602, 154264. [Google Scholar] [CrossRef]
- Qinpiao, Y.; Liang, Y.; Xie, G. Recent Advances of Stimuli-Responsive Liquid–Liquid Interfaces Stabilized by Nanoparticles. ACS Nano 2024, 18, 32364–32385. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Gu, J.L.; Du, J.; Yao, K.F. Novel Cu–Ag bimetallic porous nanomembrane prepared from a multi-component metallic glass. RSC Adv. 2015, 5, 50565–50571. [Google Scholar] [CrossRef]
- Arvand, M.; Gholizadeh, T.M.; Zanjanchi, M.A. MWCNTs/Cu(OH)2 nanoparticles/IL nanocomposite modified glassy carbon electrode as a voltammetric sensor for determination of the non-steroidal anti-inflammatory drug diclofenac. Mater. Sci. Eng. C. 2012, 32, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Gurav, K.; Patil, U.; Shin, S.; Agawane, G.; Suryawanshi, M.; Pawar, S.; Patil, P.; Lokhande, C.; Kim, J. Room temperature chemical synthesis of Cu(OH)2 thin films for supercapacitor application. J. Alloys Compd. 2013, 573, 27–31. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Zhou, S.; Zhao, N.; Wong, C.-P. Facile and scalable fabrication of three-dimensional Cu(OH)2nanoporous nanorods for solid-state supercapacitors. J. Mater. Chem. A 2015, 3, 17385–17391. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, T.-T.; Han, X.-L.; Wang, Y.-Q.; Li, J.-D. Fabrication of Cu(OH)2 Nanowires Blended Poly(vinylidene fluoride) Ultrafiltration Membranes for Oil-Water Separation. Chin. J. Polym. Sci. 2018, 36, 612–619. [Google Scholar] [CrossRef]
- Li, Q.; Deng, W.; Li, C.H.; Sun, Q.Y.; Huang, F.Z.; Zhao, Y.; Li, S.K. High-Flux Oil/Water Separation with Interfacial Capillary Effect in Switchable Superwetting Cu(OH)2@ZIF-8 Nanowire Membranes. ACS Appl. Mater. Interfaces 2018, 10, 40265–40273. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Liu, T.; Yao, F.Y.; Hu, D.W.; Miao, L.; Uemura, S.; Kusunose, T.; Feng, Q. Ultrahydrophilic Inorganic Nanosheet-Based Nanofiltration Membranes for High Efficiency Separations of Inorganic Salts and Organic Dyes. Langmuir 2014, 40, 21280–21290. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Li, G.; Liu, Y.; Zhou, S.; Liu, F. Loose Polyester Nanofiltration Membrane Designed with Hydroxyl-Ammonium for Efficient Dye/Salt Separation. Membranes 2025, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, W.; Zheng, K.; Zhou, S. Novel polyurethane loose nanofiltration membrane with excellent water permeance for efficient dye desalination. Sep. Purif. Technol. 2025, 364, 132467. [Google Scholar] [CrossRef]
- Li, L.J.; Xue, R.H.; Liu, T.; Zhao, B.; Hu, D.W.; Uemura, S.; Kusunose, T.; Feng, Q. High-Performance Inorganic Nanofiltration Membranes Based on Large-Size Layered Titanate Nanosheets. Langmuir 2015, 41, 11854–11865. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Sun, J.; Xue, Y.; Zhao, G.; Liu, T.; Liu, X.; He, Q.; Xie, K. High-performance CrN tight ultrafiltration membranes prepared by in situ gas–solid catalytic reaction. Mater. Des. 2021, 204, 109669. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, Y.J.; Wu, J.; Shao, Y.T.; Cai, A.Y.; Dong, L.Y. Ultralong Hydroxyapatite Nanowire-Based Filter Paper for High-Performance Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 4288–4301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, H.; Fan, X.; Lv, F.; Chen, S.; Quan, X. Fabrication of TiO2 nanofiber membranes by a simple dip-coating technique for water treatment. Surf. Coat. Tech. 2016, 298, 45–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).