Abstract
This study employed sodium laurate solution as the raw material to fabricate superhydrophobic coatings on cement-based substrates via a facile one-step spraying method. To optimize the processing parameters, the influence of solution concentration on substrate wettability was investigated, leading to the identification of the optimal concentration. Subsequently, superhydrophobic coatings were fabricated under these optimized conditions, and their wettability, mechanical durability, chemical corrosion resistance, and surface repairability were systematically characterized. The results revealed that the coating fabricated with a 0.3% sodium laurate solution exhibited an obvious regular, flaky, rough microstructure, achieving a water contact angle (WCA) of 154° ± 2° and a sliding angle (SA) of 2.9°. The coating demonstrated superhydrophobicity (WCA > 150° and SA < 10°), self-cleaning capability, mechanical durability, chemical corrosion resistance, and environmental stability; furthermore, the abraded surface can be restored to be superhydrophobic by simple and rapid repair.
1. Introduction
Cement-based materials rank among the most extensively utilized construction materials worldwide [1]. The cement hydration process encompasses a series of chemical reactions and physical transformations, generating numerous micropores, cracks, defects, and hydroxyl groups. These features collectively impart an intrinsic hydrophilic property to cement-based materials [2,3]. Consequently, water droplets rapidly infiltrate the material through capillary action induced by surface micropores, substantially compromising concrete durability [4]. Additionally, increasing environmental pollution has resulted in elevated atmospheric concentrations of pollutants and particulate matter. These contaminants persistently accumulate on building surfaces, exacerbating maintenance challenges, diminishing aesthetic appeal, and accelerating material degradation [4,5]. To address these issues, developing superhydrophobic coatings with enhanced self-cleaning and anti-fouling functionalities represents a critical strategy for mitigating the inherent hydrophilicity of cement-based materials. Surface superhydrophobicity can be achieved through either reducing surface free energy, constructing micro- and nanoscale surface roughness, or a synergistic combination of both strategies [6].
Reducing the surface free energy of cementitious materials is typically accomplished through chemical modifications using silanes [7] and siloxanes [8]. These hydrophobic agents are applied via surface impregnation, spraying, or brushing to render the material surface hydrophobic. However, silane/siloxane modifiers are costly. Achieving superhydrophobicity further requires pre-engineered surface roughness structures integrated with micro- and nano-particles [9]. These limitations restrict their large-scale application on concrete surfaces and existing structural components.
The fabrication of micro-nano rough structures on superhydrophobic surfaces typically utilizes micro- and nano-particles such as silica [10,11], metakaolin [12], and diatomaceous earth [13]. Additionally, copper mesh coatings applied to cementitious material surfaces are widely adopted to generate micro-nanostructures [11]. The dual strategy of combining low-surface-energy material modification with micro-nano roughness construction synergistically enhances the hydrophobicity of cementitious materials. However, superhydrophobic material preparation generally requires the incorporation of multiple components, involving a cumbersome material preparation process and complex, cost-prohibitive coating techniques. Furthermore, the micro-nano structures exhibit limited mechanical robustness, significantly hindering the practical application of superhydrophobic surfaces.
Although conventional superhydrophobic coatings typically rely on binary synergistic systems, recent studies have demonstrated that single-component materials can simultaneously achieve low surface energy and micro-nanoscale roughness, thereby enabling superhydrophobicity. Wang et al. [14] screened sodium laurate from six sodium carboxylates based on solubility and hydrophobic modification efficacy as the raw material for superhydrophobic surface fabrication. By immersing hardened cement paste in a 1% sodium laurate solution for 5 s, they obtained a superhydrophobic surface with a WCA of 154.3° and a SA of 8.7°. Post-immersion analysis revealed calcium laurate coverage on the cement surface. This low-surface-energy reaction product formed an irregular lamellar microstructure. The resulting microstructure directly contributed to the superhydrophobic behavior. This method significantly enhanced hydrophobicity. Sodium laurate is non-toxic, harmless, and safe under normal conditions. However, the immersion process has limitations: low preparation efficiency and poor engineering applicability. Furthermore, this study did not systematically evaluate the mechanical durability, chemical corrosion resistance, environmental stability, or mechanical properties of the fabricated coatings. To address these gaps, this work aims to develop a simpler, faster, and economically viable superhydrophobic coating technique while comprehensively investigating its performance characteristics.
Among various modification techniques, the coating method represents a widely adopted approach for fabricating superhydrophobic surfaces on cementitious materials. This method involves applying hydrophobic modifier-containing coatings onto the substrates via dipping, brushing, or spraying, followed by curing and drying processes to form a superhydrophobic coating with a rough structure [15]. Notably, the spraying technique offers distinct advantages as a simple and cost-effective preparation strategy. Its applicability remains unaffected by substrate geometry or dimensions, making it particularly suitable for industrial-scale production of large-area superhydrophobic coatings. Consequently, developing low-cost, readily available superhydrophobic materials compatible with a rapid one-step spraying protocol is critical. Such materials must simultaneously confer low surface energy and micro-nano roughness to minimize production costs while ensuring enhanced hydrophobicity and durability of cementitious substrates.
This study fabricated superhydrophobic coatings on cementitious substrates via a one-step spraying technique utilizing sodium laurate solution, effectively addressing the limitations of low preparation efficiency and poor applicability. To optimize cost-effectiveness, the influence of sodium laurate concentration on substrate wettability was systematically investigated, enabling the development of coatings with reduced material consumption. The surface morphology and chemical composition of the superhydrophobic coatings were characterized by SEM and EDS. Their wettability, self-cleaning capability, mechanical durability, chemical corrosion resistance, environmental stability, and surface reparability were systematically tested to comprehensively evaluate the coatings’ performance.
2. Experimental Section
2.1. Materials
Sodium laurate (98%), purchased from Hubei Yamade Biomedical Co., Wuhan, China. The cement was P·O 42.5 ordinary silicate cement, purchased from Tianjin Jidong Cement Co., Tianjin, China. The experimental water was deionized water.
2.2. Preparation of Superhydrophobic Coating
Substrate preparation: Cement mortar specimens (40 mm × 40 mm × 10 mm) were fabricated using P·O 42.5 ordinary Portland cement with a water-cement ratio of 0.36. After 24-h curing at ambient temperature and demolding, the specimens were uniformly polished with 400-grit sandpaper, ultrasonically cleaned twice, and air-dried at room temperature before experimental use.
Preparation of superhydrophobic coating: As illustrated in Figure 1, sodium laurate solution was prepared by heating and stirring until clarification. Subsequently, the substrate was placed horizontally, and 5 mL (3.13 L/m2) of the solution was uniformly sprayed onto the substrate using a spray gun operated at 600 kPa pressure, maintaining a nozzle-to-substrate distance of 2~3 cm. The coated specimen was then air-dried at ambient temperature (5~35 °C, relative humidity ≤ 60%) for about 0.5 h to form the superhydrophobic coating.
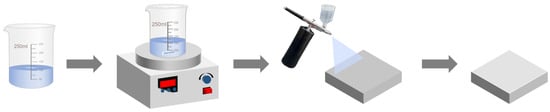
Figure 1.
Schematic of the superhydrophobic coating fabrication process.
2.3. Characterization
The water contact angle (WCA) of the coating surface was measured with a contact angle goniometer (JC2000D1, Zhongchen Digital Technology Apparatus Co., Ltd., Shanghai, China) using 4 μL deionized water droplets. The Bond number of the contact angle measuring instrument was less than or equal to 1, and the Weber number was less than 1. The final WCA value was determined by averaging measurements taken at six randomly selected locations [16,17,18].
The sliding angle (SA) was measured by incrementally adjusting the inclination of the specimen [19]. Initially, the specimen was placed against multiple steel plates of equal thickness (1 mm). A 10 μL water droplet was gently deposited onto the surface from a height of 1 cm using a syringe. The tilt angle was progressively reduced through systematic removal of steel plates. This process continued until the droplet failed to roll off smoothly. The critical tilt angle at this transition point was recorded as the SA. Calculate according to the following formula:
In the formula:
α—sliding angle of the coating surface (°);
H—tilt height of the specimen (mm);
L—vertical projection length of the specimen (mm).
A Superhydrophobic surface is characterized by a WCA exceeding 150° and a SA below 10° [20].
The wettability of the coating was further validated through water jetting analysis [21].
Surface morphology of the coating was characterized using a field emission scanning electron microscope (JSM-7800F, JEOL, Tokyo, Japan). Three-dimensional morphology and surface roughness were analyzed using a super-depth-of-field 3D imaging system (VHX-600e, KEYENCE, Osaka, Japan) at 200× magnification. Imaging was performed at the center position of the test block with a shooting height of 185.2 μm and a width of 653.0 μm. Elemental composition and distribution of the coating surface were determined via an energy dispersive spectrometer (B110G500, EDAX, Pleasanton, CA, USA).
2.4. Superhydrophobic Coating Performance Tests
Self-cleaning performance test: Chalk ash was used to simulate particulate pollutants [22]. The specimen was tilted at 20°, and 2 mL of deionized water was slowly dispensed from a disposable dropper positioned 2 cm above the surface. Residual chalk ash was observed to evaluate self-cleaning efficacy.
Droplet adhesion analysis: A 4 μL water droplet was formed on the needle tip of the contact angle goniometer. The droplet’s interaction with the coating surface (approaching, contacting, extruding, and detaching) was manually controlled to assess adhesion behavior [16].
Mechanical peeling resistance test: international-standard 3M tape was adhered to the coating surface under a 200 g weight. The tape was rolled twice across the surface and subsequently peeled off at 1 s/cm. The WCA was measured after each peeling cycle, with testing concluded after 10 rev [16,17,18,23].
Abrasion resistance test: The coated specimen was placed upside down on 400-grit sandpaper under a 200 g load. The specimen was reciprocated linearly over a 0.1 m stroke length. The WCA was measured every five cycles (0.5 m cumulative abrasion), with testing concluding after 10 cycles (5m total abrasion) [16,17,18,23].
Chemical corrosion resistance evaluation: Solution droplets (pH = 2 HCl, pH = 14 NaOH, 2 mol/L NaCl) were deposited on the coating surface. Test block was tilted to approximately 15° and the solution was dropped onto the coating surface from a height of 2–4 cm. Their contact angles (CA) were measured and compared against the baseline WCA to determine chemical stability [17,18,22,23,24].
Environmental stability assessment: The coated specimens were aged under ambient indoor conditions (5~35 °C, relative humidity ≤ 60%, atmospheric pressure 101.3 kPa). The WCA was measured at 0, 15, 30, 45, 75, 90, and 120 days to evaluate durability [17,22,24,25].
Surface repairability test: Post-abrasion (10 cycles), 1 mL of 0.3% sodium laurate solution was sprayed onto the damaged surface of the specimen and air-dried. Repaired surface WCA was compared with pre-repair values to assess restoration capability [23].
3. Results and Discussion
3.1. Surface Wettability Characteristics of Superhydrophobic Coatings
The effect of sodium laurate solution concentration on the wettability of the coating surface is shown in Figure 2. When the concentration is 0.1%, the WCA measures 148.6°, indicating hydrophobic behavior. At concentrations ranging from 0.3% to 1.5%, the WCA exceeds 150°, with no significant increase observed as concentration rises. This suggests that once superhydrophobicity is achieved, further increases in solution concentration minimally impact surface wettability. Notably, at 0.3% concentration, the average WCA reaches 154.2°, confirming superhydrophobicity. Considering coating fabrication costs, 0.3% sodium laurate solution was selected as the optimal concentration for preparing superhydrophobic coatings in subsequent experiments.
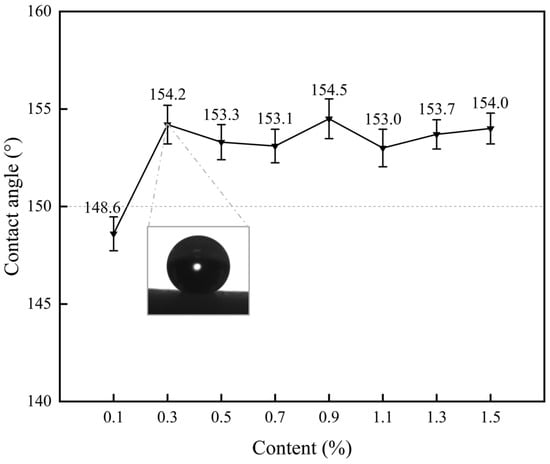
Figure 2.
Effect of sodium laurate solution concentration on coating surface wettability.
The SA serves as a critical parameter for characterizing surface wettability. It is defined as the critical tilt angle at which a water droplet begins to roll off the surface [3]. To validate compliance with this criterion, the sliding angle of the coating was measured (Figure 3). The obtained value of 2.9° confirms adherence to the superhydrophobic standard. Consequently, the coating fabricated with 0.3% sodium laurate solution fulfills the requirements for superhydrophobic surfaces.
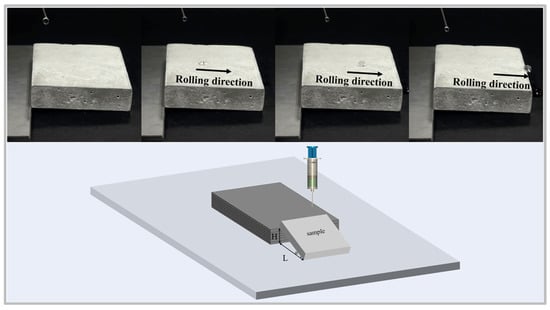
Figure 3.
Sliding angle measurement methodology and schematic representation of the superhydrophobic coating surface.
As demonstrated in Figure 4a, the hydrophilic nature of the untreated specimen causes rapid water penetration and surface wetting upon contact. In contrast, Figure 4b illustrates the water jet behavior on the superhydrophobic coating surface. When subjected to hydrodynamic impact, water droplets exhibit coherent directional rebound rather than surface wetting. This phenomenon arises from two key factors: (1) the low surface energy of the superhydrophobic coating minimizes droplet adhesion, and (2) air pockets trapped within the micro-nano structure which create a metastable Cassie-Baxter state. At high kinetic energy, this composite solid-liquid-air interface prevents wetting while enabling directional water ejection. These observations confirm the coating’s exceptional superhydrophobicity capacity to withstand hydrodynamic stresses.
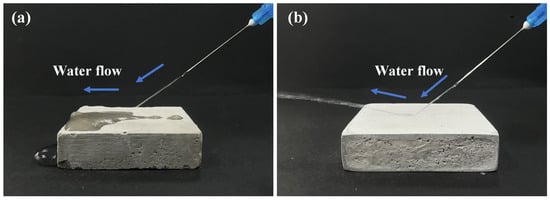
Figure 4.
Comparison of water jetting effects: (a) Uncoated specimen; (b) Superhydrophobic-coated specimen.
3.2. Surface Morphology Analysis of Superhydrophobic Coatings
To explore the surface morphology of the superhydrophobic coating, the surfaces of a uncoated specimen and a superhydrophobic-coated specimen were examined using a scanning electron microscope (SEM) at magnifications of 500× and 5000×, respectively. Micro-nano rough structures were observed on the superhydrophobic coating surface. As shown in Figure 5a, the uncoated specimen exhibits a rough surface with irregular pores and cracks. In contrast, Figure 5b reveals a uniform layer of reaction products formed on the superhydrophobic coating surface after spraying with 0.3% sodium laurate solution. At 5000× magnification, these products display a flake-like hierarchical structure, attributed to the reaction between sodium laurate and calcium hydroxide in cement, which forms calcium laurate deposits on the cement-based substrate. The reaction mechanism is expressed as follows [26]:
Ca(OH)2 + 2CH3(CH2)10COONa = (CH3(CH2)10COO)2Ca + 2NaOH
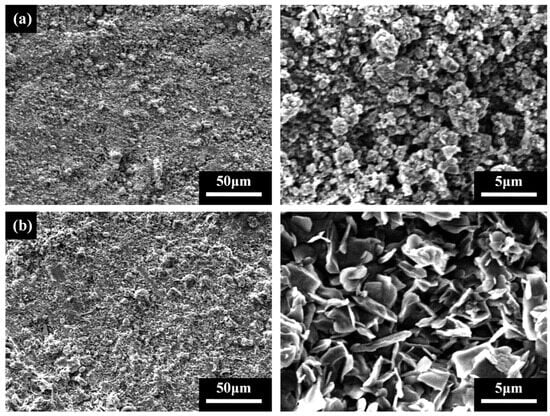
Figure 5.
SEM images of specimen surfaces: (a) Uncoated specimen; (b) Superhydrophobic-coated specimen.
Figure 6 illustrates the formation mechanism of the superhydrophobic coating. Calcium laurate, the reaction product, is a calcium salt composed of dodecanoic acid (C12 alkyl chain) and calcium ions. The twelve-carbon alkyl chains in calcium laurate confer low surface energy, while the flake-like morphology creates a hierarchical rough structure. This synergistic combination of low surface energy and multi-scale rough structure is critical for achieving superhydrophobicity.
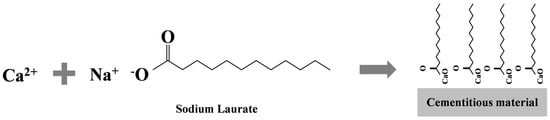
Figure 6.
Formation mechanism of the superhydrophobic coating.
3.3. Surface Composition Analysis of Superhydrophobic Coatings
To investigate the elemental composition and distribution of the superhydrophobic coating, EDS analysis was conducted on both uncoated and superhydrophobic-coated specimens. As shown in Figure 7a, the surface of the uncoated specimen primarily contains O, Si, and Ca elements, with Ca exhibiting the highest concentration (74.65%). In contrast, Figure 7b reveals that the superhydrophobic-coated specimen surface contains Ca, O, C, Si, and Mg, where Ca remains predominant (73.26%) and C content reaches 8.19%. The marked increase in C content confirms the formation of calcium laurate (C12 alkyl chains), which imparts low surface energy and superhydrophobicity.
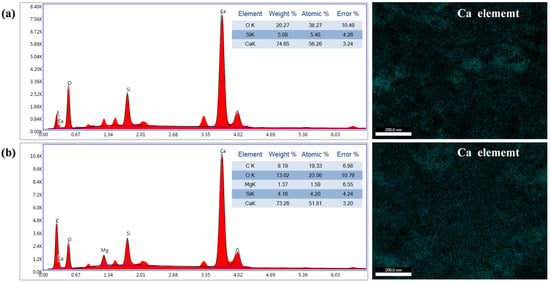
Figure 7.
EDS spectra and Ca elemental distribution: (a) Uncoated specimen; (b) Superhydrophobic-coated specimen.
EDS elemental mapping (Figure 7) demonstrates heterogeneous Ca distribution in uncoated specimens, which is attributed to localized enrichment of calcium hydroxide crystals and C-S-H gel formation during cement hydration. Pores and cracks in hardened cement paste further promote calcium ion migration to defect edges. After calcium laurate formation fills surface defects in hardened cement paste, the Ca element distribution on the superhydrophobic-coated specimen surface becomes uniform. Calcium laurate exhibits inherent density and hydrophobicity, effectively blocking calcium ion migration into pores and cracks. This homogeneous calcium laurate distribution ensures consistent superhydrophobic performance across the coating surface. The combined EDS and morphological analyses conclusively demonstrate the formation and uniform distribution of low-surface-energy calcium laurate, which governs the exceptional hydrophobicity of the coating.
3.4. Self-Cleaning Properties
The self-cleaning effect of the superhydrophobic coating primarily originates from its unique surface structure, which enables efficient removal of inorganic pollutants (e.g., dust and soil) through water droplet roll-off under inclined conditions [27]. Figure 8 compares the self-cleaning performance between uncoated and superhydrophobic-coated specimens. As shown in Figure 8a, contaminated droplets on the uncoated specimen surface exhibit strong wetting behavior, leading to pollutant spreading and persistent surface contamination. In contrast, Figure 8b demonstrates that pollutants on the superhydrophobic-coated specimen adhere to rolling droplets and are completely removed through continuous droplet impact. These results confirm the exceptional self-cleaning capability of the coating, effectively eliminating surface dust on cementitious materials while maintaining long-term cleanliness.
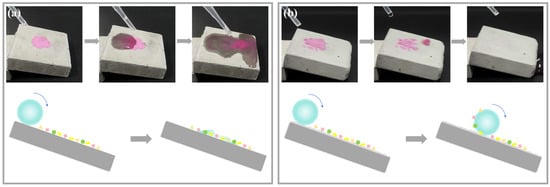
Figure 8.
Cleaning effect comparison: (a) Uncoated specimen, (b) Superhydrophobic-coated specimen.
3.5. Adhesion Characteristics of Water Droplets to Coatings
The adhesion behavior of water droplets on the superhydrophobic surface was analyzed by recording the dynamic interaction process (approach, contact, compression, and detachment) using a contact angle goniometer, as illustrated in Figure 9. The water droplets exhibit negligible adhesion to the superhydrophobic surface during contact and compression phases. Even after significant deformation, the droplets fully detach from the surface without residual wetting. Strong adhesion exists between the water droplet and the needle. Negligible adhesion occurs between the droplet and the superhydrophobic coating. This adhesion contrast prevents droplet retention on the coating surface. The coating exhibits non-wetting behavior and intrinsic superhydrophobicity.

Figure 9.
Dynamic interaction process of a water droplet: (a) approaching, (b) contacting, (c) compressing, (d) detaching, and (e) completely detaching from the superhydrophobic surface.
3.6. Mechanical Peeling Resistance
The adhesion durability of the superhydrophobic coating to cementitious substrates was evaluated via cyclic tape peeling tests with post-peel contact angle measurement. Figure 10 illustrates the dynamic relationship between the WCA and peeling cycles. Initial superhydrophobicity (WCA > 150°) was maintained through two cycles, followed by a decline to 143.9° at three cycles, marking a transition to hydrophobic behavior. After 10 cycles, hydrophobicity persisted with WCA values ≥ 140°. SEM imaging revealed compressive deformation-induced flattening of calcium laurate flakes (Figure 10a,b) and progressive material loss via tape adhesion, both contributing to roughness reduction and superhydrophobicity loss. This explains why the WCA of the coating decreased with increasing peeling cycles. The coating retained hydrophobicity after 10 cycles, attributed to the low-intensity mechanical damage induced by tape adhesion. This demonstrates that the sodium laurate-derived superhydrophobic coating exhibits both substrate adhesion and moderate resistance to mechanical peeling stresses.
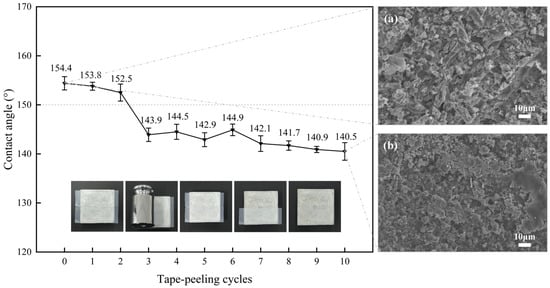
Figure 10.
Variation of surface contact angle of the superhydrophobic coating with peeling cycles and SEM images of the surface before and after peeling: (a) Before peeling, (b) After peeling.
3.7. Abrasion Characteristics of Sandpaper
Sandpaper abrasion serves as a critical method for evaluating the mechanical stability of superhydrophobic surfaces [28]. The abrasion resistance of superhydrophobic coatings significantly impacts their practical applicability, as physical/chemical degradation compromises both surface roughness and chemical composition, thereby diminishing superhydrophobic performance.
As shown in Figure 11, the specimen exhibited superhydrophobicity with an initial WCA of 154.5° prior to abrasion testing. The coating retained superhydrophobic (WCA > 150°) after two friction cycles (1 m total abrasion distance). However, the WCA gradually decreased with increasing friction distance, reaching 122.1° after 10 cycles (5 m total distance). SEM analysis at 1000× magnification of the abraded surface revealed severe structural degradation, including pronounced scratches and reduced coverage of calcium laurate flakes. Residual calcium laurate structures exhibited significant deformation. Sandpaper abrasion caused more severe damage than tape peeling due to direct interfacial friction between the coating and abrasive sandpaper under weighted compression, progressive material transfer of calcium laurate to the sandpaper surface, and compressive deformation of the low-surface-energy calcium laurate structures. The WCA reduction directly correlated with the cumulative mechanical degradation of the roughness. Despite progressive hydrophobicity loss, the coating demonstrated measurable resistance to abrasive wear.
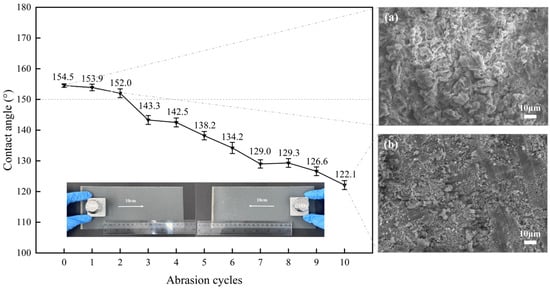
Figure 11.
Variation of surface contact angle of superhydrophobic coating with the number of friction cycles and the SEM images of the surface before and after friction: (a) Before friction, (b) After friction.
As shown in Figure 12a, the undamaged sodium laurate coating exhibited an undulating rough morphology with a surface roughness of 246.3 μm. In contrast, Figure 12b,c demonstrate significant surface flattening after 10 peeling cycles (roughness: 177.4 μm) and 10 abrasion cycles (roughness: 57.1 μm). Mechanical degradation compromised the roughness structure, directly correlating with hydrophobicity reduction.
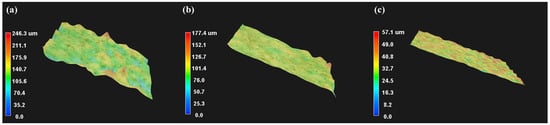
Figure 12.
3D morphology and surface roughness of the coating: (a) Undamaged 0.3% sodium laurate superhydrophobic coating; (b) Coating after 10 peeling cycles; (c) Coating after 10 sandpaper abrasion cycles.
3.8. Chemical Corrosion Resistance Properties
Figure 13 illustrates the dynamic interactions of hydrochloric acid (pH = 2), sodium hydroxide solution (pH = 14), and sodium chloride (2 mol/L) solution with the sodium laurate superhydrophobic coating. As shown in Figure 13a, droplets of hydrochloric acid (pH = 2) slide slowly over the superhydrophobic coating surface, leaving visible trails and generating CO2 bubbles. This phenomenon arises because atmospheric exposure causes carbonation of the cement substrate, forming calcium carbonate (CaCO3). The hydrochloric acid corrodes the coating and reacts with CaCO3, producing CO2 gas. Consequently, the coating loses superhydrophobicity, and bubbles form during the reaction. As shown in Figure 13b, sodium hydroxide solution (pH = 14) initially rolls off the superhydrophobic coating surface in a spherical form without adhesion. However, continuous addition of the solution at the same location degrades the coating, resulting in droplets sliding slowly across the surface. This behavior arises from the decomposition of calcium laurate by sodium hydroxide. Progressive sodium hydroxide addition reduces calcium laurate content, diminishing the low-surface-energy components critical to hydrophobicity. As shown in Figure 13c, a 2 mol/L sodium chloride solution droplet rapidly rebounds from or rolls off the superhydrophobic coating surface upon impact. Even under continuous droplet deposition, the solution maintains smooth mobility without adhesion. This behavior stems from the absence of direct chemical reactivity between sodium chloride and calcium laurate, rendering chlorides non-corrosive to the coating.
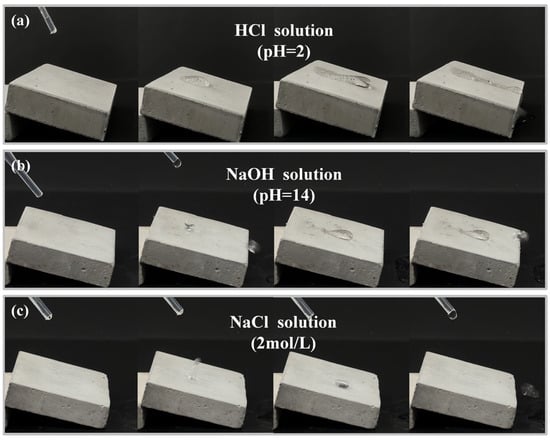
Figure 13.
Interaction dynamics of droplets with superhydrophobic coatings (a): HCl (pH = 2); (b) NaOH (pH = 14); and (c) NaCl (2 mol/L).
To evaluate the chemical corrosion resistance of the superhydrophobic coating, the CA of HCl, NaOH, and NaCl solutions dropped on the surface were measured and compared with that of the WCA, as illustrated in Figure 14. When HCl and NaOH solutions were dropped onto the superhydrophobic coating surface, the measured CA were 142.0° and 138.1°, respectively. This significant reduction in CA indicates that the coating loses superhydrophobicity due to chemical corrosion-induced damage to its hierarchical rough structure, demonstrating limited resistance to strong acids and alkalis. In contrast, the NaCl solution exhibited a CA of 152.6°, showing negligible deviation from the original WCA (153.8°). This minimal change confirms that the salt solution causes almost no erosion, preserving the coating’s microstructure and hydrophobicity. These results are consistent with the dynamic interaction behaviors observed in Figure 13, where HCl and NaOH degraded the coating, while NaCl maintained non-wetting performance.
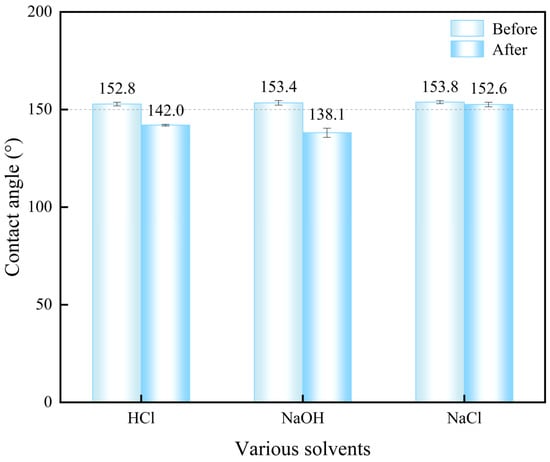
Figure 14.
Contact angle comparison of HCl, NaOH, and NaCl solutions on the superhydrophobic coating surface.
3.9. Environmental Stability
For practical applications requiring long-term durability, the environmental stability of the sodium laurate superhydrophobic coating was evaluated by monitoring the WCA evolution under indoor ambient conditions. Specimens were exposed to a controlled indoor environment, and the surface WCA was measured periodically over 120 days. As shown in Figure 15, the WCA remained consistently above 150° throughout the exposure period. This negligible decline confirms that indoor environmental factors (e.g., temperature, humidity, airborne contaminants) do not compromise the superhydrophobicity or structural integrity of the coatings. These results demonstrate the ability of the coating to maintain stable non-wetting performance under prolonged indoor exposure, validating its suitability for long-term applications. Subsequent experiments will investigate the durability of the superhydrophobic coating in practical environments to ensure its better application in practice.
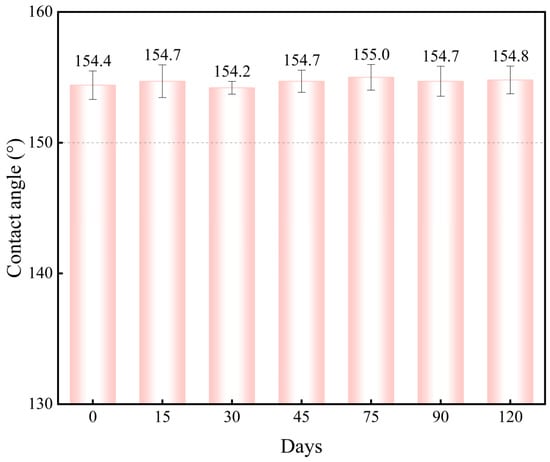
Figure 15.
Surface contact angle variation of the superhydrophobic coating over exposure days.
3.10. Surface Repairability
Superhydrophobic coatings typically exhibit limited mechanical durability, with hydrophobicity degradation or complete loss under mechanical stress. To address this, a rapid repair strategy was developed for mechanically damaged coatings. The mechanical durability of the superhydrophobic coating was significantly compromised after 10 m of abrasion (equivalent to 10 friction cycles), resulting in a WCA reduction to 123.9° and degrading the surface from superhydrophobic to hydrophobic (Figure 16a). To restore functionality, 1 mL of 0.3% sodium laurate solution was sprayed onto the damaged surface and air-dried at ambient conditions, successfully recovering superhydrophobicity with a WCA of 155.2°. SEM analysis at 5000× magnification revealed distinct structural evolution before and after repair. Prior to repair, the original flake-like hierarchical roughness was irreversibly compacted into a flattened morphology (Figure 16b), directly responsible for the observed superhydrophobicity loss. Following repair, the calcium laurate flake structure regenerated, re-establishing multi-scale surface roughness (Figure 16b), which is essential for restoring superhydrophobicity. These results confirm that the coating can be rapidly and repeatedly repaired through simple solution reapplication, demonstrating practical applicability in scenarios requiring sustained hydrophobic performance under mechanical wear.
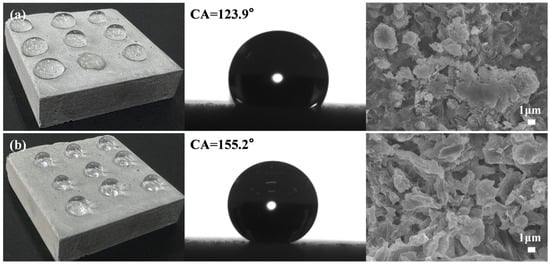
Figure 16.
SEM analysis of superhydrophobic coating surface structural evolution: (a) Damaged surface; (b) Regenerated surface.
4. Conclusions
The sodium laurate superhydrophobic coating, fabricated via a facile one-step spraying method using 0.3% sodium laurate solution, exhibits exceptional superhydrophobicity with a WCA of 154° ± 2° and a SA of 2.9°. This coating demonstrates multifunctional durability: (1) It maintains self-cleaning capability through contaminant removal via droplet roll-off; (2) Preserves the WCA > 150° after 120-day indoor exposure, confirming environmental stability; (3) Withstands mechanical stresses including 10 cycles of 400-grit sandpaper abrasion (10 m total distance, WCA > 120°) and 10 tape peeling tests (WCA > 140°); (4) Resists chemical corrosion from pH = 2 HCl and pH = 14 NaOH (CA > 140°), while remaining inert to 2 mol/L NaCl (CA > 150°). Mechanically damaged surfaces (WCA = 123.9°) can be rapidly regenerated by respraying 1 mL of 0.3% sodium laurate solution, restoring superhydrophobicity (WCA = 155.2°) through microstructural reconstruction of calcium laurate flakes. The one-step spraying technique offers practical scalability due to its applicability to substrates of arbitrary geometry and dimensions, ambient-temperature operation, and low material consumption, making it economically viable for large-area applications. To fully realize its industrial potential, future studies must address the coating’s long-term stability in chemically aggressive environments.
Author Contributions
Conceptualization, R.X. and L.Z.; methodology, R.X. and Y.Y.; validation, X.X., G.L., B.P. and C.Y.; investigation, G.L., B.P. and Y.Y.; writing—original draft preparation, R.X. and Y.Y.; writing—review and editing, R.X., Y.Y., C.Y. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (GrantNos. 52178265 and 52178264).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
Authors Ben Peng, Guanghua Lu and Changsheng Yue were employed by the company Central Research Institute of Building and Construction Co., Ltd. Author Xiujun Xing was employed by the company Metallurgical Corporation of China Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhao, J.; Gao, X.; Chen, S.; Lin, H.; Li, Z.; Lin, X. Hydrophobic or superhydrophobic modification of cement-based materials: A systematic review. Compos. Part B 2022, 243, 110104. [Google Scholar] [CrossRef]
- Shen, C.; Zhu, Y.; Shi, W.; He, K.; Xiao, X.; Xu, X.; Shi, J.; Xu, G. Mechanically stable superhydrophobic surface on cement-based materials. Chem. Phys. 2020, 538, 110912. [Google Scholar] [CrossRef]
- Yao, H.; Xie, Z.; Huang, C.; Yuan, Q.; Yu, Z. Recent progress of hydrophobic cement-based materials: Preparation, characterization and properties. Constr. Build. Mater. 2021, 299, 124255. [Google Scholar] [CrossRef]
- Zhang, F.; Pei, W.; Li, D.; Zhang, M.; Wang, C.; Lai, Y. A self-adaption robust superhydrophobic cement mortar for resistance of cold environment. Cold Reg. Sci. Technol. 2024, 228, 104323. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Klapsa, W.; Anna, D. Self-Cleaning Coatings and Surfaces of Modern Building Materials for the Removal of Some Air Pollutants. Materials 2021, 14, 2161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hou, P.; He, S.; Han, M.; Xiang, P.; Xiao, T.; Tan, X. The robust superhydrophobic SiO2/Diatomite/PDMS/KH-570/Me-MQ composite coating for self-cleaning application of building surface. Colloids Surf. A 2022, 634, 127936. [Google Scholar] [CrossRef]
- Cui, L.; Xiang, T.; Hu, B.; Lv, Y.; Rong, H.; Liu, D.; Zhang, S.; Gou, M.; Lv, Z.; Chen, D. Design of monolithic superhydrophobic concrete with excellent anti-corrosion and self-cleaning properties. Colloids Surf. A 2024, 685, 133345. [Google Scholar] [CrossRef]
- Gu, Z.; Zhao, M.; Liu, Q.; Mao, C.; Zhang, L.; Sun, X.; Lv, S. Photothermal superhydrophobic coating for concrete: Highly effective anti/deicing performance and corrosion resistance. Mater. Today Commun. 2024, 41, 110623. [Google Scholar] [CrossRef]
- Horgnies, M.; Chen, J. Superhydrophobic concrete surfaces with integrated microtexture. Cem. Concr. Compos. 2014, 52, 81–90. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, W.; Li, F.; Yuan, L.; Diao, Y.; Liu, Y.; Pu, Y.; Zhang, Y.; Zhao, Y.; Jiang, O.; et al. Sprayable coating based on fluorinated silica nanocomposites with superhydrophobic and antibacterial properties for advanced concrete. Prog. Nat. Sci. Mater. Int. 2022, 32, 472–481. [Google Scholar] [CrossRef]
- Du, H.; Shen, Y.; Zhang, W.; Kong, X.; Fu, Y. Fabrication of superhydrophobic concrete with stable mechanical properties and self-cleaning properties. J. Build. Eng. 2023, 67, 105950. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Q.; Qu, L.; Zhang, H.; Shi, Z.; Xu, S.; Li, X. Study of mortar layer property of superhydrophobic metakaolin based cement mortar. J. Build. Eng. 2022, 45, 103578. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Wang, H.; Wang, H. Fabrication of super-robust and nonfluorinated superhydrophobic coating based on diatomaceous earth. Surf. Coat. Technol. 2019, 362, 90–96. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Ou, J.; Li, W. Fast fabrication of superhydrophobic surfaces on hardened cement paste using sodium laurate aqueous solution. Constr. Build. Mater. 2021, 278, 122385. [Google Scholar] [CrossRef]
- Wang, F.; Xie, T.; Ou, J.; Xue, M.; Li, W. Cement based superhydrophobic coating with excellent robustness and solar reflective ability. J. Alloys Compd. 2020, 823, 153702. [Google Scholar] [CrossRef]
- Wu, Y.; She, W.; Shi, D.; Jiang, T.; Hao, T.; Liu, J.; Zhang, Q.; You, J.; Li, R. An extremely chemical and mechanically durable siloxane bearing copolymer coating with self-crosslinkable and anti-icing properties. J. Compos. Part B Eng. 2020, 195, 108031. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, B.; Li, S.; Luo, G.; Zheng, B.; Zhang, J. Durable superamphiphobic coatings with high static and dynamic repellency towards liquids with low surface tension and high viscosity. J. Colloid Interface Sci. 2020, 578, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Wen, L.; Yang, K.; Li, X.; Xu, S.; Pi, P.; Wen, X. Robust and durable fluorinated 8-MAPOSS-based superamphiphobic fabrics with buoyancy boost and drag reduction. J. Chem. Eng. 2020, 383, 123125. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Zhang, L.; Liao, X.; Wang, J.; Chen, Z.; He, J.; Zeng, X. Dual-Functional Superhydrophobic Textiles with Asymmetric Roll-Down/Pinned States for Water Droplet Transportation and Oil–Water Separation. ACS Appl. Mater. Interfaces 2018, 10, 4213. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Q.; Xu, S. A review on applications of superhydrophobic materials in civil engineering. Adv. Eng. Mater. 2021, 24, 2101238. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Q.; Wang, N.; Qu, L.; Song, Q. Study of corrosion property and mechanical strength of eco-friendly fabricated superhydrophobic concrete. J. Clean. Prod. 2021, 323, 129267. [Google Scholar] [CrossRef]
- Zhi, D.; Lu, Y.; Sathasivam, S.; Parkin, I.; Zhang, X. Large-scale fabrication of translucent and repairable superhydrophobic spray coatings with remarkable mechanical, chemical durability and UV resistance. J. Mater. Chem. A 2017, 5, 10622–10631. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Zhao, X.; Zhao, X.; Tian, N.; Zhang, J. Totally waterborne, nonfluorinated, mechanically robust, and self-healing superhydrophobic coatings for actual anti-icing. ACS Appl. Mater. Interfaces 2018, 10, 39391–39399. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ma, J.; Yang, C.; Zhang, J.; Cheng, J. Robust, multiresponsive, superhydrophobic, and oleophobic nanocomposites via a highly efficient multifluorination strategy. ACS Appl. Mater. Interfaces 2021, 13, 28949–28961. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Li, J.; Liu, W.; Zhang, Y. Preparation and performance evaluation of new durable superhydrophobic wall coatings. Surf. Technol. 2021, 50, 239–246. [Google Scholar]
- Wang, F.; Zhang, M.; Lei, S.; Ou, J.; Li, W. Rapid preparation of superhydrophobic surface on cement stone. Appl. Phys. A Mater. Sci. Process. 2019, 125, 386. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, X.; Zhang, X. Facile preparation of mechanically robust superhydrophobic concrete with self-cleaning property. Mater. Res. Express 2019, 6, 015001. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Q.; Xu, S.; Wang, N.; Zheng, X. Fabrication of superhydrophobic concrete used in marine environment with anti-corrosion and stable mechanical properties. Constr. Build. Mater. 2020, 251, 118946. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).