Abstract
Bone regeneration demands biomaterials capable of supporting tissue integration and mimicking the native piezodynamic properties of bone. In this study, hydroxyapatite–barium titanate (HA-BT) composite coatings with varying BT content (10, 30, and 50 wt%) were developed to enhance the piezoelectric response and corrosion resistance of Ti6Al4V implants. The coatings were synthesized via high-energy ball milling and atmospheric plasma spraying (APS). XRD analysis with Rietveld refinement confirmed the presence of HA along with secondary phases (TTCP, β-TCP, CaO). Electrochemical tests revealed lower corrosion current densities for the coatings containing ≤30% BT, indicating improved stability in physiological environments. Cytotoxicity assays (MTT) demonstrated biocompatibility across all formulations. Piezoresponse force microscopy (DART-SS-PFM) confirmed enhanced d33-eff values for the 50% BT coating (>15 pm/V); however, biological assays under low-intensity pulsed ultrasound (LIPUS) stimulation showed increased osteocalcin expression for ≤30% BT, while 50% BT induced cellular stress. Overall, HA-BT coatings with up to 30% BT exhibited optimal electrochemical stability, favorable piezoelectric performance, and enhanced biological response, underscoring their potential for orthopedic implant applications and regenerative tissue engineering.
1. Introduction
An increased demand for materials that can replace damaged organs of the human body is evident. Biomaterials have been successfully developed and used to improve the quality of human life, not just for an ageing population but also for younger people suffering from trauma or bone disease or with specific functional and aesthetic dental needs. However, it is a challenge for synthetic biomaterials to mimic all the functions performed by the complex musculoskeletal system, which are based on the mechanical, physicochemical, and biological properties of bone tissue [1,2,3]. Therefore, only carefully designed materials, anticipating a match of diverse properties, can satisfy such clinical needs. To tackle this challenge, several research groups have dedicated their efforts to tailoring the properties of various metallic, ceramic, and polymer biomaterials to obtain truly efficient biomedical implants that mimic all the regenerative properties of the native tissue [4,5]. Titanium (Ti) and its alloys, such as Ti6Al4V, are the most used metallic implants, characterized by their high corrosion resistance, good mechanical properties, and acceptable biocompatibility. However, in addition to its non-bioactive response to the surrounding tissue and after long periods of implantation, there is a chance that some toxic ions may be released due to implant wear, which limits its biomedical application [6]. Biocompatible calcium phosphate (CaP) coatings on Ti6Al4V implants are used as a strategy to take advantage of both the bioactive properties of this ceramic and the mechanical properties of the metallic substrate to favor bone regeneration and integrity between the bone tissue and the implant surface [7]. Hydroxyapatite (HA, Ca10(PO4)6(OH)2) has a chemical composition and structure very similar to the mineral component of natural bone [8]. HA is the most studied bioceramic owing to its excellent biocompatibility and bioactivity; when applied on biocompatible metals, it can promote osteoconductivity that enhances cell attachment and proliferation for bone regeneration [9,10,11,12].
Several techniques to produce hydroxyapatite-based coatings on metallic bone implants are reported in the literature, including plasma spraying, magnetron sputtering, pulsed laser deposition, electrospray deposition, electrophoretic deposition, biomimetic deposition, sol–gel combined with dip or spin coating, electrodeposition, and hydrothermal synthesis [13,14,15]. Among them, air plasma spraying (APS) is a thermal-spray process in which powder particles are melted in a plasma jet and propelled toward the substrate material. This is a well-established industrial method for the deposition of HA coatings due to its reliability, efficiency, and reasonable cost. Moreover, plasma spraying is the only thermal-spray process that has been approved by the FDA for use in the deposition of HA coatings for medical implants [16,17,18]. During the plasma spray process, the HA powder subjected to a high temperature flame can be decomposed into CaP secondary phases, such as TTCP, TCP, and CaO. Recently, the positive effects of both TTCP and TCP, particularly β-TCP, were demonstrated due to their ability to promote cell adhesion and proliferation, contributing to bone tissue regeneration processes [19,20]. It is well known that pure HA coatings possess inherent brittleness, including low tensile and fracture strength, which restricts its clinical application to non-bearing or low-load components [21]. The gap in the functionality of HA-coated bone implants can be filled by incorporating multifunctional composites that may enhance mechanical properties, corrosion resistance, and osteoconductivity for improved orthopedic care [22,23]. Among the available multifunctional materials, piezoelectric materials exhibit an electromechanical response to an external stimulus in either a direct or converse piezoelectric configuration [24]. The direct piezoelectric effect involves the generation of an electric voltage from an applied mechanical stress, whereas the converse piezoelectric effect is a reverse scenario, where an applied electric voltage results in a mechanical response [25]. Piezodynamics uses piezoelectric materials, which generate electrical charges in response to mechanical stress, to create controlled vibrations or forces. In nature, some tissues show piezodynamic behavior as they generate electric charges that promote cell activity. Wolff’s Law describes how bone tissue responds to mechanical stress [26]. The direct piezoelectric constant (d33) of bone typically falls between 0.5 and 2.3 pC/N [24,27,28,29,30,31], whereas the indirect piezoelectric constant is between 0.1 and 10 pm/V [32,33].
Different attempts have been reported to obtain composite coatings of piezoelectric materials that combine the electromechanical nature of lead-free ferroelectric ceramics, such as barium titanate (BaTiO3, BT), with biologically active ceramics like HA [34,35,36]. Also, there are studies reporting that HA/BT composites for scaffold and coatings development show good cell interaction and promote osseointegration [35,37,38,39,40]. More recently, Fan et al. and Chen et al. reported a novel approach involving the combination of BaTiO3-coated Ti6Al4V substrates with low-intensity pulsed ultrasound (LIPUS) to overcome the limited sustainability of electroactive stimulation in clinical applications of piezodynamic therapy [39,41]. LIPUS is a specific type of ultrasound operating in a frequency and intensity range of 0.045–3 MHz and 0.02–1 W/cm2, respectively; it has been demonstrated that it promotes cellular viability, proliferation, differentiation, and migration to accelerate tissue healing [42,43,44,45,46,47,48,49]. Remarkably, the mechanical action of ultrasound combined with the piezoelectric effect of BT induces beneficial biological responses that promote accelerated bone tissue regeneration, offering the advantages of reduced treatment cost and shorter recovery time [39,41].
Lab-on-a-chip devices are used for research in the biomedical field. In the field of tissue engineering, piezoelectric materials have been utilized for the development of biosensors, drug delivery systems, and clinical diagnostic tools, among other applications [50,51,52,53]. To our knowledge, this is the first study that integrates the piezodynamic response of HA-BT coatings using a lab-on-a-chip in vitro model.
The aim of this study is to evaluate the piezodynamic effect of HA-BT coatings deposited on Ti6Al4V substrates using APS. As we reported in our previous research [54], additions of BT (10, 30, and 50% w/w) to HA powders generate positive impacts on the mechanical properties (principally the adherence) of APS coatings. In this research, the phase distribution, corrosion resistance, piezoelectric, and biocompatibility properties of HA-BT coatings were carefully investigated. Subsequently, the coatings were subjected to mechanical stimulation through LIPUS in a lab-on-a-chip model, exploiting their piezodynamic properties to increase cellular activity. Finally, the feasibility of producing HA-BT coatings with corrosion protective and biomimetic bone piezodynamic properties on Ti6Al4V substrates was presented.
2. Materials and Methods
Ball milling homogenization of HA-BT powders has been shown to have a significant positive impact on various properties, including mechanical performance and phase distribution, as reported in previous studies [54]. This method was employed in the present work to produce a homogeneous precursor material for coating fabrication. The bioactive HA-BT coatings were obtained by plasma spray deposition using hydroxyapatite particles (CaP Biomaterials, LLC, East Troy, WI, USA) and barium titanate nanoparticles (US Research Nanomaterials, Inc., Houston, TX, USA). The average particle sizes of the starting powders prior to ball milling homogenization were approximately 30–60 µm for HA and 180–205 nm for BT.
To evaluate the influence of the piezoelectric response induced by low-intensity pulsed ultrasound (LIPUS) treatment on osteoblast activity, coatings with varying weight percent ratios (w/w%) of HA and BT were fabricated, as summarized in Table 1.

Table 1.
Nomenclature and weight percent ratio of different coatings.
For the homogenization process, the powders were milled using yttria-stabilized zirconia (YSZ) milling balls in a high-energy ball mill (SPEX 8000M, SPEX CERTIPREP, Metuchen, NJ, USA). The milling duration was set to 30 min, and the ball-to-powder weight ratio was maintained at 1:2. High-purity ethanol (99.99%, JT. Baker) was used as a process control agent at a volume of 0.2 mL.
A grade 25 titanium alloy (Ti6Al4V ELI) was used as the substrate material. The substrates were cylindrical in shape, with a diameter of 18 ± 0.03 mm and a thickness of 3 ± 0.10 mm. Disk-shaped specimens were obtained by transverse sectioning using a low-speed precision cutting machine (IsoMet, Buehler, Lake Bluff, IL, USA) equipped with a 4-inch diamond wafering blade (MetLab Co., Niagara Falls, NY, USA). The cross-sectioned samples were subsequently ground with silicon carbide (SiC) emery paper of varying grit sizes (180, 220, 400, and 600).
Prior to coating deposition, the samples were surface-treated via grit blasting using corundum particles with an average size of 700 µm (grid size ANSI G-24). Following this treatment, the samples were ultrasonically cleaned in pure ethanol (JT Baker, Phillipsburg, NJ, USA) to remove any residual grease or surface contaminants and then dried using compressed air.
The HA-BT coatings were deposited via plasma spray using an SG-100 Plasma Spray Torch (TAFA, Concord, NH, USA). The deposition parameters employed are summarized in Table 2.

Table 2.
Parameters used for plasma spray deposition of HA coating and for HA-BT coatings.
XRD measurements were performed to identify the crystal structure of the primary HAp and BT powders and the phases present in both powders and coatings. XRD patterns were obtained employing a BRUKER D8 ADVANCE diffractometer (Karslruhe, Germany) with Cu Kα radiation (λ = 1.5418 Å) as the X-ray source. The diffraction data were collected through a Bragg–Brentano geometry in the 2θ range of 20° to 60° using the step-scanning mode with a step of 0.02° and step counting time of 100 s. The identification of phases in the HA and BT primary powders, HA-BT composite powders, and HA-BT coatings was performed employing Match! Crystal impact software version 3.11 [55], whereas the crystalline unit cells of the HAp and BT were modeled by employing VESTA software version 3 (ver. 3.90.5a) [56]. For the HA-BT coatings, lattice parameters and the weight fraction of each phase were determined from the XRD patterns by the Rietveld refinement method [57] using the FullProf Suite software package (version april2025) [58] considering a five-phase model: (i) HA (hexagonal, P63/m, no. 176) [59], (ii) BT (tetragonal, P4mm, no. 99) [60], (iii) β-tricalcium phosphate, β-TCP (Rombohedral, R3c, no. 161) [61], (iv) calcium oxide, CaO (cubic, Fm-3m, no. 225) [62], and (v) tricalcium phosphate, TTCP (monoclinic, P21, no. 4) [63]. For the refinement, the following parameters were considered: (i) scale factor; (ii) zero displacement correction; (iii) unit cell and background parameters; and (iv) peak profile parameters (full width at half maximum, FWHM, and shape parameters) using a pseudo-Voigt function.
Cross-sectional backscattered electron (BSE) micrographs were obtained using a scanning electron microscope (SEM, HITACHI SU3500, Naka, Japan) to assess the thickness of the HA-BT coatings. BSE imaging and energy-dispersive X-ray spectroscopy (EDS, Oxford Aztec, Abingdon, UK), both integrated into the SEM system, were employed to analyze surface and cross-sectional contrast, enabling the evaluation of phase distribution and elemental composition within the HA/BT coatings. For cross-sectional SEM analysis, samples were sectioned following the same cutting procedure described in the substrate preparation methodology. Subsequently, the samples were embedded in resin, subjected to standard metallographic preparation, and polished using alumina suspensions.
To calculate the corrosion current density (Icorr), the linear polarization resistance (LPR) technique was used. Samples were exposed to simulated body fluid (SBF). The SBF solution was prepared according to the Kokubo methodology [64]. The LRP analysis was performed employing a potentiostat/galvanostat (Autolab, METROHM, Ultrech, The Netherlands) with a three-electrode array: a saturated Ag/AgCl reference electrode, a Pt bar as the auxiliary electrode, and the coated titanium sample as the working electrode. The analysis conditions implemented were as follows: (i) a working temperature of 36.5 °C to simulate human body temperature; (ii) stabilization of the working electrode by immersion in SBF for 1200 s and measurement of open circuit potential; and (iii) a potential polarization of ±30 mV vs. Ecorr with a scan rate of 10 mV/min. Cview 3.5h software (SCRIBNER ASSOCIATES, INC., Hampshire, UK) [65] was used for the data analysis and calculation of the corrosion rate.
The Stern–Geary model was employed for the calculation of Icorr (see Equation (1)); the model considers that a polarization window close to open circuit potential (EOCP) resembles the electrochemical behavior of a linear behavior.
where Rp denotes the polarization resistances and B is the Stern–Geary constant, calculated as a function of the anodic (Ba) and cathodic (Bc) slopes of Tafel as (Equation (2)):
Piezoelectric hysteresis loops were obtained using switching spectroscopy piezoresponse force microscopy combined with dual AC resonance tracking (SS-PFM DART) [66]. The measurements were carried out using an atomic force microscope (AFM) system (MFP-3D, Asylum Research, Oxford Instruments, Oxfordshire, UK) operated in vertical mode, with an applied AC driving voltage amplitude of 5 V.
Conductive AFM silicon tips (model ASYELEC-01, Asylum Research, Oxfordshire, UK), coated with a 5/20 nm layer of Ti/Ir, were employed. The cantilever had a spring constant of 0.2 N/m and a free resonance frequency of approximately 70 kHz. The drive frequency during contact resonance was set to 295 kHz (well above the free resonance frequency of the cantilever) and was applied between the bottom electrode (Ti6Al4V substrate) and the conductive AFM tip.
Local polarization hysteresis loops were recorded by applying a DC bias voltage ranging from −60 V to +60 V to the sample using the DART method at room temperature. In this work, DC “OFF” hysteresis loops were analyzed in order to minimize electrostatic artifacts typically associated with PFM hysteresis measurements performed in DC “ON” mode [67].
Equation (3) was used to determine the effective d33 [68]:
where D and V correspond to the displacement and voltage obtained by the measuring during the PFM analysis, respectively, and D1 and V1 correspond to the displacement and voltage values of the intersection of the displacement–voltage curve, respectively.
To assess both cell viability and cytotoxic effects by indirect contact, test samples were immersed in RPMI-1640 cell culture medium and incubated at 36.5 ± 5 °C for 1, 3, 7, and 21 days to promote the release of possible cytotoxic components.
In a 96-well culture plate, the ion release period was determined, and the resulting supernatants were collected. Serial dilutions (1/2, 1/4, 1/8, 1/16, 1/32, 1/64, and 1/128) were subsequently prepared for use in the MTT-formazan assay.
Each well was initially filled with 50 μL of supplemented RPMI-1640 medium and seeded with 2.5 × 104 cells. Following a 24 h incubation period at 36.5 °C, 50 μL of the corresponding diluted extract was added to each well. The cells were then incubated for an additional 24 h under the same conditions.
Afterward, the supernatant was carefully removed, and 20 μL of MTT solution was added to each well. The plate was incubated for 3 h at 36.5 °C to allow for the formation of formazan crystals. Upon completion of the incubation, 100 μL of DMSO was added to each well to dissolve the formazan crystals. The plate was further incubated for 1 h before the absorbance measure.
Cell viability was assessed by measuring absorbance at λ = 595 nm using a microplate reader (iMark™ Microplate Reader, Bio-Rad, Hercules, CA, USA). The percentage of cell viability and cytotoxicity was calculated using the following equations:
% Cell Viability (CV)
% Cytotoxicity (CT)
where ODC represents the optic density of the control and ODS denotes the optic density of the samples.
To evaluate the piezodynamic effect and its influence on cellular activity, a custom-designed lab-on-a-chip device was developed for this study. The device features a multilayer architecture designed to accommodate up to five individual samples simultaneously, each of which can be independently subjected to LIPUS. Additionally, the design allows for essential cell culture maintenance procedures, such as medium exchange or removal, through integrated inlet and outlet ports.
The lab-on-a-chip was fabricated using polytetrafluoroethylene (PTFE) for the bottom layer, polydimethylsiloxane (PDMS) mixed at a base-to-curing agent ratio of 10:1 for the intermediate layers, and polymethyl methacrylate (PMMA) for the top layer. The PTFE and PMMA components were precisely machined using CNC laser technology, while the PDMS layers were produced by casting from a negative mold created via 3D printing using polylactic acid (PLA).
Figure 1 presents an illustration of the multilayer configuration along with a photograph of the assembled device containing cultured cells and growth medium.
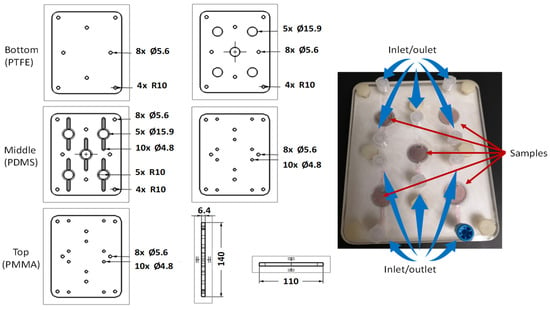
Figure 1.
Schematic representation of the lab-on-a-chip cell layers and a photograph of the assembled device containing the samples.
For immunofluorescence assessment, a lab-on-a-chip device was employed. A cell density of 1.5 × 104 cells per sample was seeded and cultured for 7 days. To evaluate the influence of piezoresponse on cellular activity, samples were exposed to LIPUS or maintained without stimulation (control group). In the LIPUS-treated group, the samples were subjected to ultrasound at a frequency of 1 MHz, an intensity of 0.1 W/cm2, and a pulsed repetition frequency of 100 Hz for 3 min. Following the treatment period, the samples were processed for immunofluorescence staining.
For immunofluorescence staining, cells were fixed for 30 min at 36.5 ± 0.5 °C using a solution of 2.5% (v/v) glutaraldehyde in 0.1 M sodium cacodylate buffer. After fixation, the samples were incubated for 20 min in 0.1% (w/v) sodium borohydride solution to quench residual glutaraldehyde autofluorescence. Subsequently, the cell membranes were permeabilized by incubation with 0.2% (v/v) Triton X-100 in phosphate-buffered saline (PBS) for 20 min. Non-specific binding sites were blocked by incubating the samples in a solution containing 10% (v/v) non-fat dry milk in PBST (PBS supplemented with 0.2% (v/v) Tween 20) for 1 h.
Thereafter, the samples were incubated with a 1:1000 dilution of a polyclonal primary antibody against osteocalcin (OCN, Human-Rabbit/IgG, Invitrogen, Carlsbad, CA, USA) in PBST for 1 h. This was followed by incubation with a 1:1000 dilution of a fluorescent secondary antibody (FITC-conjugated polyclonal goat anti-rabbit IgG, Invitrogen) under the same conditions. For cytoskeletal staining, the samples were treated with a 1:1000 dilution of rhodamine-labeled phalloidin in PBST for 30 min. Finally, nuclei were stained and mounted using a DAPI-containing mounting medium.
The samples were analyzed using a laser scanning confocal microscope (model LSM T-PMT, Carl Zeiss, Oberkochen, Germany). Micrographs were processed and analyzed using ZEN 3.0 software [69].
3. Results
Figure 2a presents the X-ray diffractograms of the HA and BT primary powders as well as the different HA-BT composite powders. The diffraction peaks observed in the XRD pattern of the BT powder confirm a tetragonal crystal structure, which is consistent with the standard XRD pattern of BaTiO3 (JCPDS card no. 79-2264, ■). The tetragonal structure of the synthesized BT powder is further evidenced by the (200)/(002) peak doublet located at approximately 45°, characteristic of the tetragonal (P4mm, space group 99) phase [70]. The intensity ratio of I002/I200 (~0.5) aligns with previously reported values by Zhu et al. [71]. The red circle indicates the displacement of the Ti4+ ion in the TiO6 octahedron, which causes the ferroelectric distortion in the tetragonal perovskite structure.
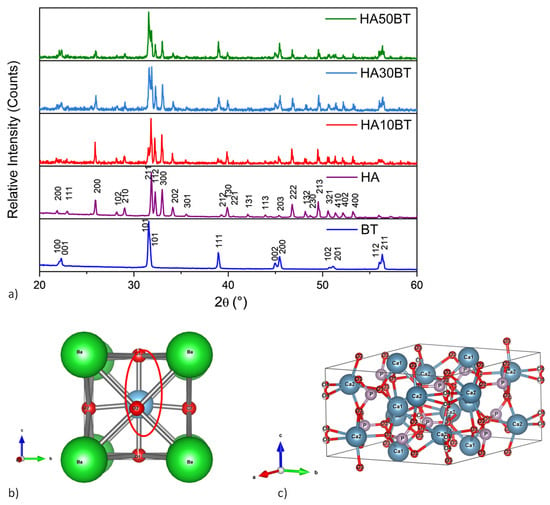
Figure 2.
(a) XRD patterns of the raw materials (HA and BT) and the HA-BT powder composites with varying weight percentages (w/w%) after the high-energy ball milling process. (b) Crystal structure model of BT and (c) HA, visualized using VESTA software.
Figure 2b shows the crystal structure of tetragonal BT visualized using VESTA software. This visualization reveals a distortion of the cubic perovskite structure, where the c-axis is slightly elongated relative to the a-axes. In this configuration, the Ti4+ ion is displaced from the center of the oxygen octahedron, generating a net dipole moment within a non-centrosymmetric arrangement. Such structural asymmetry is a prerequisite for piezoelectric behavior [72].
The XRD peaks corresponding to the HA primary powder (Figure 2a) are consistent with a hexagonal crystalline structure, as indicated by JCPDS card no. 9-0432. Figure 2c illustrates the centrosymmetric hexagonal unit cell of HA modeled in VESTA software, showing a repeating arrangement of Ca3(PO4)2 layers and hydroxyl groups aligned along the c-axis.
In all HA-BT composite powders containing 10, 30, and 50 wt% of BT (Figure 2), no additional diffraction peaks were detected beyond those corresponding to the HA and BT phases. This indicates that neither the variation in BT content nor the high-energy ball milling process induced the formation of secondary phases, such as other calcium phosphates, oxides, or metallic species.
Figure 3 presents the XRD patterns of the HA-BT composite coatings deposited onto Ti6Al4V substrates using the APS technique. The coatings were fabricated from HA-BT composite powders containing 10, 30, and 50 wt% BT. In addition to the characteristic peaks corresponding to HA and BT, diffraction peaks attributed to β-tricalcium phosphate (β-TCP, Ca3(PO4)2, ●), tetracalcium phosphate (TTCP, Ca4(PO4)2O, ¤), and calcium oxide (CaO, ○) were also detected. These phases are consistent with data from the JCPDS database (No. 00-009-0169 for β-TCP, No. 25–1137 for TTCP, and No. 01-077-2376 for CaO). Notably, no additional peaks indicative of oxide phase formation resulting from decomposition of the BaTiO3 phase during the APS process were observed.
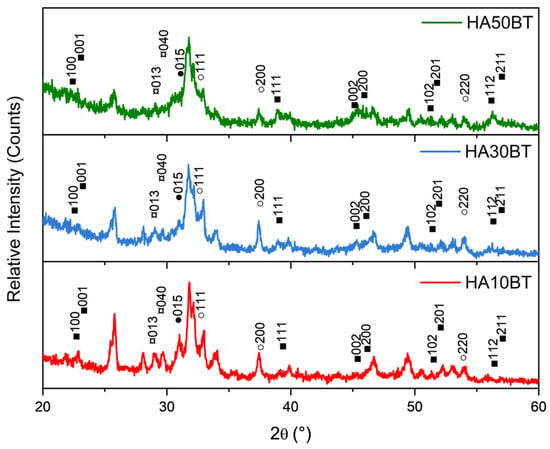
Figure 3.
XRD patterns of HA-BT composite coatings showing characteristic crystallographic planes corresponding to typical phases formed during the thermal degradation of HA. The detected phases include barium titanate (BaTiO3, ■), β-tricalcium phosphate (β-TCP, Ca3(PO4)2, ●), tetracalcium phosphate (TTCP, Ca4(PO4)2O, ¤), and calcium oxide (CaO, ○).
A further analysis of the X-ray diffractograms using the Rietveld refinement method confirms the presence of calcium-deficient phosphate phases (Ca/P atomic ratio < 1.67), namely β-tricalcium phosphate (β-TCP), tetracalcium phosphate (TTCP), and calcium oxide (CaO), in addition to the HA and BT phases.
Figure 4 illustrates the goodness of fit (χ2 = 1.35) obtained from the Rietveld refinement performed on the HA10BT coating. Table 3 summarizes the weight fractions, lattice parameters, reliability factors (Rwp, Rexp), and the goodness-of-fit (χ2) derived from the Rietveld analysis for the HA10BT, HA30BT, and HA50BT coatings.
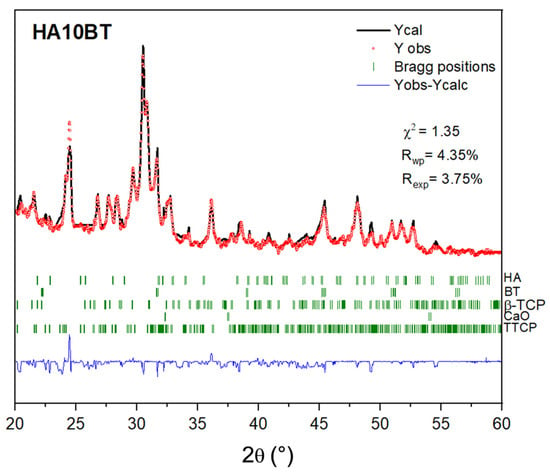
Figure 4.
Rietveld refinement of XRD pattern with the respective Bragg positions of crystal phases and the residual in blue (Yobs-Ycalc).

Table 3.
Weight fraction, lattice parameters (a, b and c), reliability factors (Rwp, Rexp), and goodness-of-fit (χ2).
The data presented in Table 3 indicate that the HA-BT coatings contain lower amounts of HA and BT phases than the theoretical weight percentages estimated during the preparation of the HA-BT composite powders. This discrepancy is attributed to the formation of secondary calcium phosphate phases during processing. The Rietveld analysis also confirms that increasing the BT content (w/w%) in the coatings leads to a decrease in the weight fraction of the HA phase, while the TTCP phase exhibits a corresponding increase. Moreover, the lattice parameters of the TTCP phase expand as both the HA phase weight fraction and its lattice parameters decrease.
XRD patterns of the HA-BT coatings reveal that the atmospheric plasma spraying process, characterized by rapid heating and cooling cycles, induces structural modifications in the material. These changes lead to the formation of secondary calcium phosphate phases, including β-tricalcium phosphate (β-TCP), tetracalcium phosphate (TTCP), and calcium oxide (CaO), all of which exhibit a Ca/P atomic ratio lower than that of stoichiometric HA. The absence of detectable diffraction peaks corresponding to metallic oxide phases indicates that barium titanate (BaTiO3) remains structurally stable and does not decompose during the process.
Therefore, the observed secondary phases are attributed to the thermal degradation of the HA component, with measured decomposition levels of 2.68%, 4.44%, and 8.64% in the HA10BT, HA30BT, and HA50BT coatings, respectively.
According to the findings reported by Gross et al., Cihlár et al., Wang et al., Heimann, Singh et al., and Kotian et al., the high-temperature decomposition of HA follows a predictable sequence: lower plasma enthalpy results in reduced decomposition, thereby minimizing the formation of β-TCP and TTCP and limiting the subsequent breakdown of TTCP into CaO [73,74,75,76,77,78,79]. Upon cooling, residual HA crystallizes alongside β-TCP, TTCP, and CaO. In this study, the relatively low degree of HA decomposition resulted in the formation of these specific secondary phases.
Notably, international standards such as ISO 13779-3 and ASTM F1609 specify that HA coatings for surgical implants must contain a minimum of 50 wt% HA phase to ensure sufficient bioactivity and performance [80,81]. Despite the presence of secondary phases, the HA10BT and HA30BT coatings in this study retained HA content above this threshold, indicating their potential suitability for biomedical applications.
Cross-sectional and surface BSE micrographs of the HA/BT coatings were obtained to evaluate coating thickness and phase distribution, respectively. Figure 5 presents the cross-sectional images of (a) HA10BT, (b) HA30BT, and (c) HA50BT.
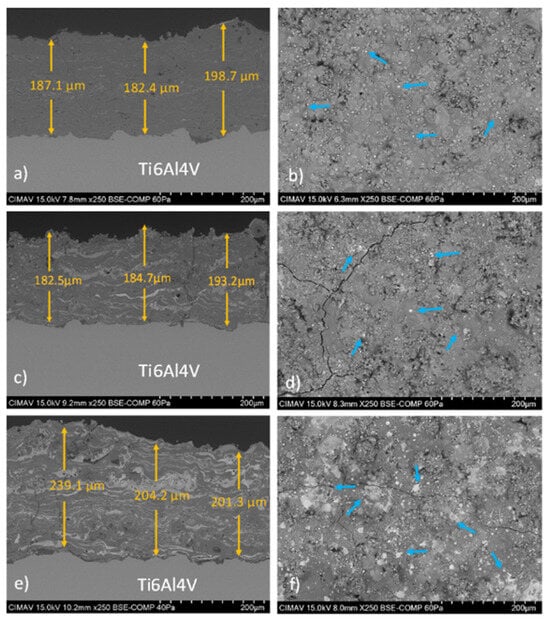
Figure 5.
BSE cross-sectional and surface micrographs of the HA/BT coatings are shown for (a,b) HA10BT, (c,d) HA30BT, and (e,f) HA50BT. Based on the BSE signal contrast, the BT phase is identified as bright regions (highlighted by blue arrows), whereas the homogeneous gray areas are attributed to the HA phase.
All coatings exhibited uniform thicknesses ranging from 180 to 240 µm, exceeding the minimum requirement of 50 µm specified by ISO 13779-3 and ASTM F1609-08 for ensuring coating stability in biological environments, and remaining below the threshold at which delamination is likely to occur [80,81]. The coatings also displayed dense microstructures and strong interfacial adhesion, indicating robust mechanical integration (an essential factor for long-term implant performance) [22].
As the BT content increased, more pronounced agglomeration of the BT phase was observed, particularly in the form of lamellar bright regions in the cross-sectional BSE images. Surface BSE analysis of the HA10BT and HA30BT coatings (Figure 5b,e) revealed good phase dispersion with minimal BT agglomeration. In contrast, the HA50BT coating (Figure 5f) exhibited larger and more numerous bright regions (highlighted by blue arrows), indicative of significant clustering associated with the higher BT content.
In addition, BSE imaging of all HA/BT coatings revealed the presence of surface cracks, a common feature linked to the atmospheric plasma spraying (APS) deposition process due to the high temperatures involved and rapid heat dissipation during cooling. However, no fractures at the coating–substrate interface were observed.
The strong interfacial integrity aligns with the mechanical performance previously reported in our adhesion and nanoindentation tests [54]. Briefly, adhesive strength measured according to ASTM C633 increased by 68%, 106%, and 87% for HA10BT, HA30BT, and HA50BT, respectively, compared to pure HA; the highest value, 44 ± 2 MPa, was achieved with 30 wt% BT. Nanoindentation confirmed an increase in elastic modulus (E) and nanohardness (H) with increasing BT content:
- (i)
- HA10BT: E = 105 ± 1 GPa, H = 7.1 ± 0.2 GPa;
- (ii)
- HA30BT: E = 123 ± 2 GPa, H = 9.1 ± 0.3 GPa;
- (iii)
- HA50BT: E = 136 ± 3 GPa, H = 13.0 ± 0.5 GPa.
Collectively, the microstructural observations and mechanical data demonstrate that controlled incorporation of BT enhances both the cohesion and functional stiffness of HA-based coatings without compromising interfacial reliability.
As shown in Figure 6 and Table 4, the presence of HA in the composite coating matrix promotes a protective effect on the underlying substrate. This is evidenced by a shift in the corrosion potential (Ecorr) toward more positive values and a reduction in the corrosion current density (Icorr), which together indicate a lower corrosion rate. Similar findings have been reported by Kaur et al., Khlifi et al., and Canpolat et al., who observed that the incorporation of HA in ceramic and composite coatings enhances substrate protection by improving the coating’s resistance to electrochemical degradation [7,82,83]. These results suggest a potential improvement in the service life of metallic orthopedic implants.
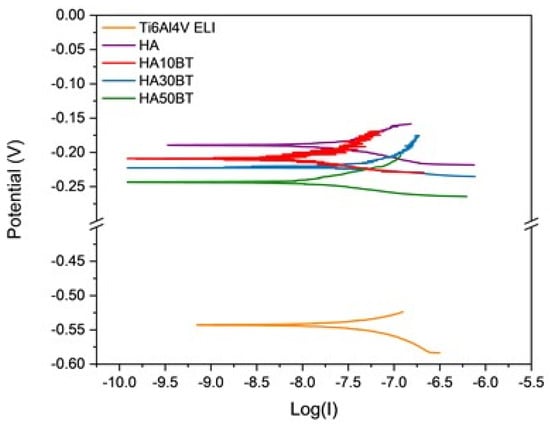
Figure 6.
Linear polarization resistance (LPR) curves for different sample coatings.

Table 4.
Corrosion potential and corrosion current density values for substrate and different coatings.
Moreover, as shown in Table 4, the incorporation of BT at concentrations of 10 wt% and 30 wt% resulted in a reduction in the corrosion current density (Icorr), indicating an enhanced protective effect compared to the pure HA coating. However, when the BT content was increased to 50 wt%, a decrease in the corrosion potential (Ecorr) and an increase in Icorr were observed, suggesting a decline in the coating’s protective performance.
This behavior is primarily attributed to the ferroelectric nature of BT. The inherent ferroelectric properties of the coating may induce localized electric potentials at the substrate surface, which can promote electrochemical reactions and ultimately lead to an increase in Icorr, thereby compromising the overall protective capability of the coating.
As previously stated, the primary objective of the coatings developed in this study is to mimic certain functional properties of native bone tissue, particularly its piezoelectric response.
Figure 7 provides a comprehensive overview of the PFM characterization process. In panel (a), regions of interest were identified using scanning electron microscopy (SEM) in backscattered electron (BSE) mode prior to PFM analysis, which was performed using dual AC resonance tracking piezoresponse force microscopy (DART-PFM). Panel (b) splays the acquired signals from the pure HA coating, including the amplitude–voltage plot, which does not exhibit the characteristic “butterfly” curve, and the corresponding phase (°) versus voltage (V) response, which lacks a hysteresis loop. These results indicate the absence of piezoelectric behavior in the HA coating. Panel (c) displays a representative coating amplitude–voltage plot exhibiting a characteristic “butterfly” loop, indicative of local piezoelectric displacement (in picometers). The corresponding phase (°) versus voltage (V) plot also reveals polarization switching behavior, which is a hallmark of ferroelectric materials. Panel (d) illustrates the effective piezoelectric coefficient (d33) as a function of applied voltage, calculated using Equation (2). Finally, panel (e) compares the average d33 values obtained for coatings with varying BT weight percentages (w/w%) against the d33 value measured for natural bone tissue.
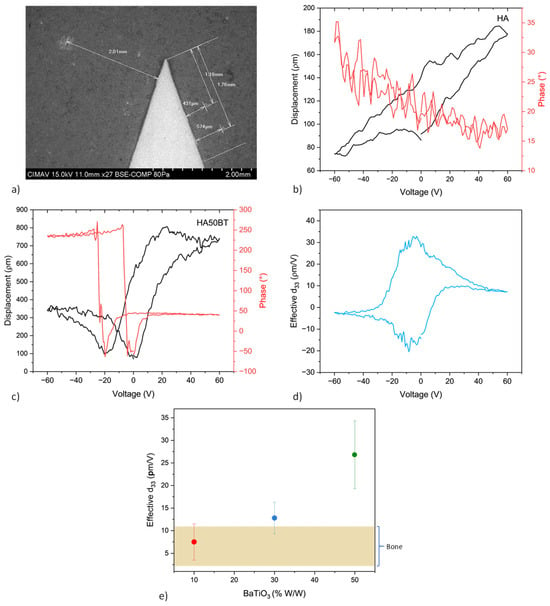
Figure 7.
Piezoresponse analysis of HA-BT coatings. (a) SEM micrograph of the HA50BT coating showing regions identified by backscattered electron imaging prior to PFM-DART analysis; (b) displacement–voltage curve and no signal of typical ferroelectric hysteresis loop of pure HA coating; (c) typical displacement–voltage (D–V) “butterfly” curve accompanied by the hysteresis loop of phase–voltage response of HA50BT coating; (d) effective piezoelectric coefficient (d33) calculated as a function of applied voltage of HA50BT coating; (e) comparative plot of the effective d33 values for HA-BT coatings and natural bone tissue.
In comparison with previous studies [32,33], which report an effective piezoelectric coefficient for natural bone within the range of approximately 0.1–10 pm/V, the HA-BT composite coatings developed in this work exhibited significantly enhanced piezo response. The highest d33 value, approximately 35 pm/V, was recorded for the HA50BT coating. This value substantially exceeds the typical d33 range reported for native bone, indicating that, at higher BT concentrations, the piezoelectric behavior diverges from that of biological bone tissue. While such enhancement may offer advantages in stimulating cellular responses relevant to bone regeneration, it also highlights the need for precise control over BT content to preserve biomimetic characteristics.
Figure 8 and Figure 9 present the cell viability and cytotoxicity percentages calculated from UV–Vis spectroscopy data using Equations (4) and (5). Throughout all exposure times, the HA-BT coatings demonstrated good biocompatibility, with cell viability values consistently exceeding 100%.
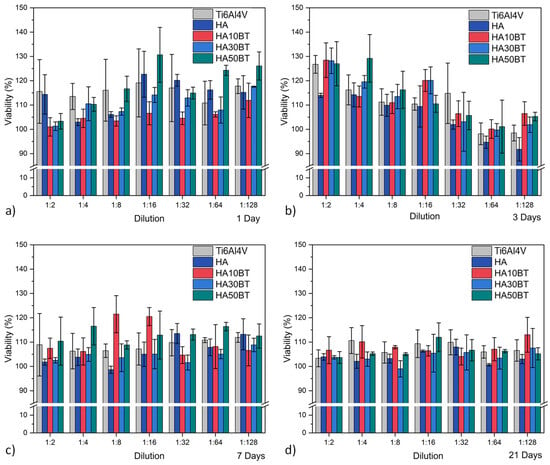
Figure 8.
HOS cell viability behavior assessed by MTT assay for different experimental groups, categorized by exposure time: (a) after 1 day; (b) 3 days; (c) 7 days; and (d) 21 days.
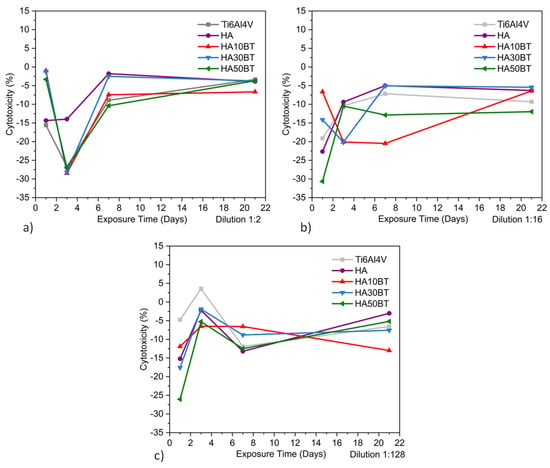
Figure 9.
Indirect HOS cell cytotoxicity behavior by MTT assay of different groups: (a) supernatant dilution 1:2, (b) dilution 1:16, and (c) dilution 1:128.
At early time points (1 and 3 days), the coatings containing BT exhibited lower cellular activity compared to the pure HA coating, particularly at both low and high dilution levels. This difference is likely attributed to the higher concentration of the osteoconductive HA phase in the pure HA coating, which may enhance early-stage cell attachment and proliferation.
However, at longer exposure times (7 and 21 days), the variations in cellular activity between the coated and uncoated samples decreased, indicating a progressive convergence in biological response. This trend suggests that the interaction between cells and the material stabilizes over time, leading to comparable levels of biocompatibility across all sample groups.
According to the results presented in Figure 9 and Table 5, the cytotoxicity values obtained from the MTT assay comply with the thresholds established by ISO 10993 [84] and ASTM F895 [85], which define acceptable cytotoxicity as total cytotoxicity (TC) below 10%. All tested samples exhibited cytotoxicity levels within this non-toxic range.

Table 5.
Cytotoxic values obtained by MTT assay of the specific dilution (D) and exposure time (ET) for different samples.
Furthermore, in correlation with the XRD analysis, it was observed that the phases present in the HA-BT coatings, including those resulting from the thermal decomposition of HA, such as β-tricalcium phosphate (β-TCP), tetracalcium phosphate (TTCP), and calcium oxide (CaO), did not induce significant cytotoxic effects. These findings are consistent with previous studies on HA and HA-BT coatings and composites, which similarly reported no adverse effects on cell proliferation [18,36,86,87,88,89].
Based on these results, both HA and BT can be considered promising biomaterials, showing potential for use in composite formulations for medical applications, particularly as bioactive coatings to enhance the performance and osseointegration of metallic orthopedic implants.
It is well established that the activation of ionic channels and integrins involved in cell adhesion plays a critical role in the interaction between cells and biomaterials, as it triggers the activation of multiple intracellular signaling pathways [90,91]. These pathways regulate key biological processes, such as cell proliferation, differentiation, and the expression of specific osteogenic genes, including osteocalcin (OCN), osterix, alkaline phosphatase (ALP), and RunX2. In this context, a surface that promotes effective cell adhesion can significantly influence the biological response of surrounding tissues, thereby enhancing the integration and performance of the biomaterial.
OCN expression in osteoblasts serves as a key biochemical marker of bone mineralization and plays a critical role in skeletal development. As reported in multiple studies [92,93,94,95], OCN facilitates calcium binding within the extracellular bone matrix and supports the differentiation of osteoblasts into osteocytes, an essential transition for proper bone formation and remodeling.
Figure 10 and Figure 11 show the immunofluorescent expression of OCN in human osteosarcoma (HOS) cells (ATCC CRL-1543), comparing samples with and without exposure to piezodynamic stimulation via LIPUS. In the absence of LIPUS, cellular activity remained stable across all conditions. No significant differences were observed between the HA-BT coatings and the control group, with OCN expression showing uniform distribution. These findings indicate that the HA-BT coatings do not impair osteogenic marker expression, further supporting their suitability for bioactive applications in bone tissue engineering.
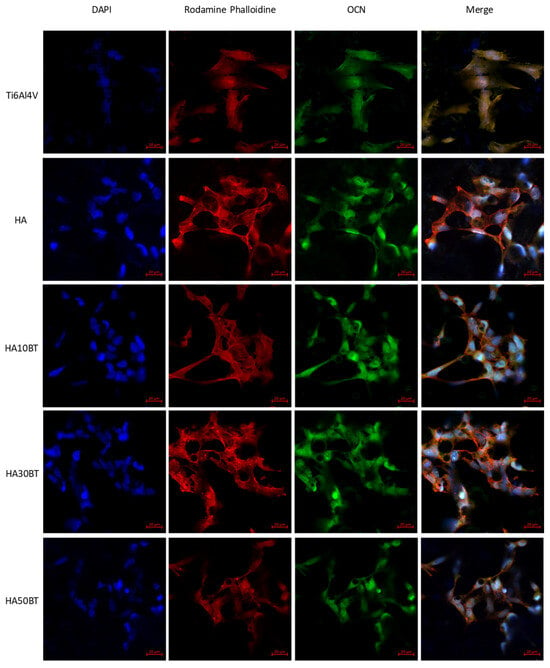
Figure 10.
Immunofluorescent staining of HOS without LIPUS stimulation in different groups. Blue, DAPI labeling for nucleus; red, Rhodamine-phalloidin for F-actin; and green, FITC conjugate polyclonal Goat-Anti-rabbit/IgG for OCN.
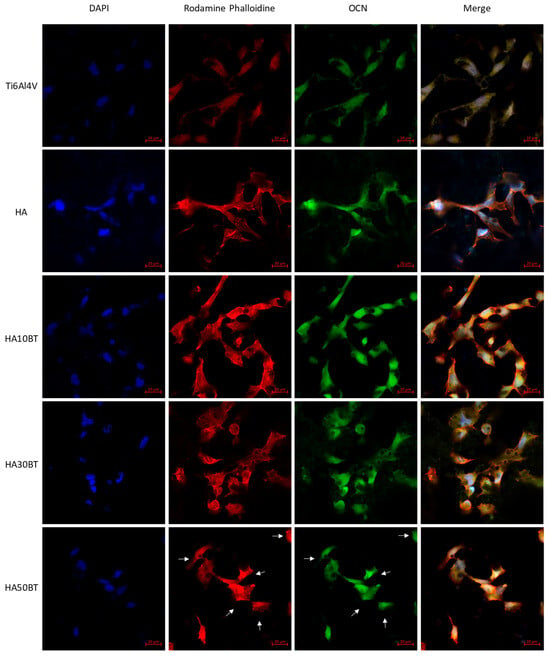
Figure 11.
Immunofluorescent staining of HOS stimulated by LIPUS in different groups. Blue, DAPI labeling for nucleus; red, Rhodamine-phalloidin for F-actin; and green, FITC conjugate polyclonal Goat-Anti-rabbit/IgG for OCN. The white arrows indicate cytoplasmic shrinkage induced by the mechanical stress of the piezodynamic stimulus.
Cell morphology was evaluated using F-actin staining with rhodamine-conjugated phalloidin and nuclear counterstaining with DAPI. These analyses confirmed the biocompatibility of the HA-BT coatings, which effectively supported cell adhesion. The incorporation of BT within the HA matrix did not disrupt cytoskeletal organization or adhesion behavior, as cells maintained a characteristic fibroblast-like morphology and intact actin filament structures.
In contrast to the Ti6Al4V substrate and pure HA coatings, which lack intrinsic piezoelectric activity, the samples subjected to piezodynamic stimulation (Figure 11) showed significant differences in osteocalcin (OCN) expression. This indicates that the piezoelectric properties of the HA-BT coatings contribute to enhanced osteogenic activity under dynamic mechanical stimulation. These results, combined with effective d33 piezoresponse measurements obtained through dual AC resonance tracking piezoresponse force microscopy (DART-PFM), highlight the influence of BT content on cellular behavior under mechanical stimulation.
When the BT concentration in the HA matrix was maintained at or below 30 wt%, the synergistic interaction between the composite matrix and the application of low-intensity pulsed ultrasound (LIPUS) became particularly evident. Under LIPUS stimulation, the intrinsic piezoelectric effect of BT contributed to enhanced cell adhesion behavior. As previously mentioned, this improved adhesion facilitates the activation of multiple intracellular signaling pathways, including the upregulation of osteocalcin (OCN), a key biomarker of osteogenic differentiation, as well as the activation of mitogenic pathways that promote cell proliferation.
In contrast, at higher BT concentrations (50 wt%), cells exhibited signs of mechanical stress, including reduced proliferation and altered morphology, indicating impaired cell adhesion. This response is attributed to the elevated effective d33 value (~35 pm/V), which exceeds the physiological piezoelectric range of natural bone and may lead to overstimulation of cellular mechanotransduction pathways. This effect primarily impacts cell adhesion and associated biological processes, such as osteogenic protein expression and cell proliferation.
Although previous studies have explored HA-BT composites or the incorporation of BT for piezodynamic responsiveness [39,47,96], many did not directly evaluate the piezoelectric coefficient. Some reported the direct d33 value [97]; however, comprehensive characterization of piezoelectric properties remains limited. Accurate measurement and understanding of these electromechanical properties are essential for the development of biomimetic materials capable of replicating the natural electromechanical environment of bone tissue.
4. Conclusions
Based on the results obtained in the present study, which included the development and characterization of HA-BT biocoatings deposited by atmospheric plasma spraying (APS) as well as the evaluation of their crystalline structure, biocompatibility, cytotoxicity, piezoresponse analysis, and corrosion resistance, the following conclusions were drawn:
- The atmospheric plasma spraying (APS) technique has proven to be an effective method for fabricating HA-BT composite coatings. Although the high temperatures inherent to this process can induce partial thermal degradation of hydroxyapatite (HA), leading to the formation of secondary phases such as β-tricalcium phosphate (β-TCP), tricalcium phosphate (TCP), and tetracalcium phosphate (TTCP), the processing parameters employed in this study successfully minimized their formation. Additionally, a strong coating–substrate interface was achieved. As a result, the produced coatings meet the compositional and thickness requirements specified by ISO 13779 and ASTM F1609-08 standards.
- The incorporation of BT into the HA matrix significantly enhances the piezoelectric response of the coatings while also improving their corrosion resistance, particularly at BT concentrations up to 30 wt%. Importantly, this level of BT incorporation does not compromise biocompatibility or impair cell adhesion to the coating surface.
- However, when evaluating the piezodynamic response under low-intensity pulsed ultrasound (LIPUS) stimulation, it was observed that excessive BT content (50 wt%), associated with higher effective piezoelectric coefficients, can induce cellular stress, negatively affecting cell adhesion and the different pathways related to this behavior, like OCN expression and cell proliferation. Therefore, an optimal BT concentration (30 wt%) was identified that closely mimics the piezoelectric behavior of natural bone, promoting favorable cellular responses through piezodynamic stimulation without inducing adverse effects.
- Overall, the results demonstrate the multifunctional potential of HA-BT composite coatings for bone regeneration applications, combining enhanced corrosion resistance with bioactive piezoelectric properties that support tissue integration and cellular activity.
Given the favorable in vitro performance of the HA-BT biocoating, the development of in vivo trials using animal models is feasible. The aim would be to assess whether piezodynamic stimulation enhances the bone bioregeneration process. It is recommended to conduct pathological, clinical, and imaging evaluations as well as to investigate potential adverse effects associated with the coating and its response to mechanical stimulation.
Author Contributions
Conceptualization, R.G.B. and V.M.O.C.; methodology, I.L.-B., O.O.M.M., R.G.B. and V.M.O.C.; formal analysis, K.C.G., I.L.-B., R.G.B. and V.M.O.C.; investigation, O.O.M.M., R.G.B. and V.M.O.C.; resources, V.M.O.C.; data curation, E.B.N., A.C.B., R.G.B. and V.M.O.C.; writing—original draft preparation, K.C.G., R.G.B. and V.M.O.C.; writing—review and editing, K.C.G., C.A.P.-S., R.G.B. and V.M.O.C.; funding acquisition, V.M.O.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors thank the Centro de Investigación en Materiales Avanzados (CIMAV), which supported this work. The authors acknowledge Claudia Ramírez Valdespino, Oscar Omar Solís Canto, Janette Guadalupe Moreno González, Verónica Moreno Brito, and Johanna Patricia Garay Rivas for their technical assistance during this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Scholz, M.S.; Blanchfield, J.P.; Bloom, L.D.; Coburn, B.H.; Elkington, M.; Fuller, J.D.; Bond, I.P. The use of composite materials in modern orthopaedic medicine and prosthetic devices: A review. Compos. Sci. Technol. 2011, 71, 1791–1803. [Google Scholar] [CrossRef]
- Choudhury, P.; Agrawal, D.C. Hydroxyapatite (HA) coatings for biomaterials. In Nanomedicine: Technologies and Applications; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A review on hydroxyapatite coatings for the biomedical applications: Experimental and theoretical perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Gkomoza, P.; Vardavoulias, M.; Pantelis, D.I.; Sarafoglou, C. Comparative study of structure and properties of thermal spray coatings using conventional and nanostructured hydroxyapatite powder, for applications in medical implants. Surf. Coat. Technol. 2019, 357, 748–758. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, W.; Xiong, Z.; Hu, Y.; Xiao, J. Effects of biomimetic hydroxyapatite coatings on osteoimmunomodulation. Biomater. Adv. 2022, 134, 112640. [Google Scholar] [CrossRef]
- Blum, M.; Sayed, M.; Mahmoud, E.M.; Killinger, A.; Gadow, R.; Naga, S.M. In vitro evaluation of biologically derived hydroxyapatite coatings manufactured by high velocity suspension spraying. J. Therm. Spray Technol. 2021, 30, 1891–1904. [Google Scholar] [CrossRef]
- Khlifi, K.; Dhiflaoui, H.; Rhouma, A.B.; Faure, J.; Benhayoune, H.; Laarbi, A.B.C. Nanomechanical behavior, adhesion and corrosion resistance of hydroxyapatite coatings for orthopedic implant applications. Coatings 2021, 11, 477. [Google Scholar] [CrossRef]
- Carrera, K.; Huerta, V.; Orozco, V.; Matutes, J.; Urbieta, A.; Fernández, P.; Herrera, M. Formation of oxygen vacancies in Cr3+-doped hydroxyapatite nanofibers and their role in generating paramagnetism. Biomed. Mater. Devices 2025, 3, 529–544. [Google Scholar] [CrossRef]
- Pereira, H.F.; Cengiz, I.F.; Silva, F.S.; Reis, R.L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 26. [Google Scholar] [CrossRef]
- Popescu-Pelin, G.; Ristoscu, C.; Duta, L.; Pasuk, I.; Stan, G.E.; Stan, M.S.; Mihailescu, I.N. Fish bone derived bi-phasic calcium phosphate coatings fabricated by pulsed laser deposition for biomedical applications. Mar. Drugs 2020, 18, 623. [Google Scholar] [CrossRef]
- Duta, L.; Stan, G.E.; Popescu-Pelin, G.; Zgura, I.; Anastasescu, M.; Oktar, F.N. Influence of post-deposition thermal treatments on the morpho-structural, and bonding strength characteristics of lithium-doped biological-derived hydroxyapatite coatings. Coatings 2022, 12, 1883. [Google Scholar] [CrossRef]
- Wang, Q.; Du, J.; Sun, Q.; Xiao, S.; Huang, W. Evaluation of the osteoconductivity and the degradation of novel hydroxyapatite/polyurethane combined with mesoporous silica microspheres in a rabbit osteomyelitis model. J. Orthop. Surg. 2023, 31, 10225536231206921. [Google Scholar] [CrossRef] [PubMed]
- Kien, P.T.; Quan, T.N.; Tuyet Anh, L.H. Coating characteristic of hydroxyapatite on titanium substrates via hydrothermal treatment. Coatings 2021, 11, 1226. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Advanced biomaterials and coatings. Coatings 2022, 12, 965. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Benhayoune, H. Bioactive calcium phosphate coatings for bone implant applications: A review. Coatings 2023, 13, 1091. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Ardhaoui, M.; Benyounis, K.; Looney, L.; Stokes, J.T. Plasma sprayed hydroxyapatite coatings: Understanding process relationships using design of experiment analysis. Surf. Coat. Technol. 2015, 283, 29–36. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Cinca, N.; Garcia-Giralt, N.; Dosta, S.; Cano, I.G.; Nogués, X.; Guilemany, J.M. In-vitro comparison of hydroxyapatite coatings obtained by cold spray and conventional thermal spray technologies. Mater. Sci. Eng. C 2020, 107, 110306. [Google Scholar] [CrossRef]
- Kowalski, S.; Gonciarz, W.; Belka, R.; Góral, A.; Chmiela, M.; Lechowicz, Ł.; Kaca, W.; Żórawski, W. Plasma-sprayed hydroxyapatite coatings and their biological properties. Coatings 2022, 12, 1317. [Google Scholar] [CrossRef]
- Qi, J.; Yu, T.; Hu, B.; Wu, H.; Ouyang, H. Current biomaterial-based bone tissue engineering and translational medicine. Int. J. Mol. Sci. 2021, 22, 10233. [Google Scholar] [CrossRef]
- Feng, J.; Liu, J.; Wang, Y.; Diao, J.; Kuang, Y.; Zhao, N. Beta-TCP scaffolds with rationally designed macro-micro hierarchical structure improved angio/osteo-genesis capability for bone regeneration. J. Mater. Sci. Mater. Med. 2023, 34, 36. [Google Scholar] [CrossRef]
- Li, T.T.; Ling, L.; Lin, M.C.; Peng, H.-K.; Ren, H.-T.; Lou, C.-W.; Lin, J.-H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Xu, H.H.K. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Khare, D.; Basu, B.; Dubey, A.K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 2020, 258, 120280. [Google Scholar]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018, 38, 2. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.; Popowski, K.; Cheng, K.; Greenbaum, A.; Ligler, F.S.; Moatti, A. Enhancement of bone regeneration through the converse piezoelectric effect, a novel approach for applying mechanical stimulation. Bioelectricity 2021, 3, 255–271. [Google Scholar] [CrossRef]
- Stock, J.T. Wolff’s law (bone functional adaptation). Int. Encycl. Biol. Anthropol. 2018, 4, 1–2. [Google Scholar]
- Bur, A. Measurements of the dynamic piezoelectric properties of bone as a function of temperature and humidity. J. Biomech. 1976, 9, 495–507. [Google Scholar] [CrossRef]
- Halperin, C.; Mutchnik, S.; Agronin, A.; Molotskii, M.; Urenski, P.; Salai, M.; Rosenman, G. Piezoelectric effect in human bones studied in nanometer scale. J. Appl. Phys. 2004, 96, 4–7. [Google Scholar] [CrossRef]
- Galkhowski, B.; Petrisor, B.; Dick, D. Bone stimulation for fracture healing. Indian J. Orthop. 2009, 43, 132–138. [Google Scholar]
- Reis, J.; Frias, C.; Canto, C.; Lu, M.; Marques, T.; Sim, O.; Potes, J. A new piezoelectric actuator induces bone formation in vivo: A preliminary study. J. Biomed. Biotechnol. 2012, 2012, 613403. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Li, J.; Yu, T.; Ruan, J.; Yang, F. Advanced Piezoelectric Materials, Devices, and Systems for Orthopedic Medicine. Adv. Sci. 2025, 12, 2410400. [Google Scholar] [CrossRef]
- Kwon, J.; Cho, H. Piezoelectric Heterogeneity in Collagen Type I Fibrils Quantitatively Characterized by Piezoresponse Force Microscopy. ACS Biomater. Sci. Eng. 2020, 6, 6680–6689. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, K.; Liu, X.; Liu, X.; Wang, J.; Suo, M.; Li, Z. Piezoelectric Biomaterials for Providing Electrical Stimulation in Bone Tissue Engineering: Barium Titanate. J. Orthop. Transl. 2025, 51, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Zarkoob, H.; Ziaei-Rad, S.; Fathi, M.; Dadkhah, H. Synthesis, Characterization and Bioactivity Evaluation of Porous Barium Titanate with Nanostructured Hydroxyapatite Coating for Biomedical Application. Adv. Eng. Mater. 2012, 14, B322–B329. [Google Scholar] [CrossRef]
- Senthilkumar, G.; Kaliaraj, G.S.; Vignesh, P.; Vishwak, R.S.; Joy, T.N.; Hemanandh, J. Hydroxyapatite–Barium/Strontium Titanate Composite Coatings for Better Mechanical, Corrosion and Biological Performance. Mater. Today Proc. 2021, 44, 3618–3621. [Google Scholar] [CrossRef]
- Nair, A.S.; Viannie, L.R. Piezoelectric Barium Titanate/Hydroxyapatite Composite Coatings on Ti-6Al-4V Alloy via Electrophoretic Deposition. Heliyon 2024, 10, e11598. [Google Scholar] [CrossRef]
- Prakasam, M.; Albino, M.; Lebraud, E.; Maglione, M.; Elissalde, C.; Largeteau, A. Hydroxyapatite–Barium Titanate Piezocomposites with Enhanced Electrical Properties. J. Am. Ceram. Soc. 2017, 100, 2621–2631. [Google Scholar] [CrossRef]
- Tavangar, M.; Heidari, F.; Hayati, R.; Tabatabaei, F.; Vashaee, D.; Tayebi, L. Manufacturing and Characterization of Mechanical, Biological and Dielectric Properties of Hydroxyapatite–Barium Titanate Nanocomposite Scaffolds. Ceram. Int. 2020, 46, 9086–9095. [Google Scholar] [CrossRef]
- Fan, B.; Guo, Z.; Li, X.; Li, S.; Gao, P.; Xiao, X.; Hou, W. Electroactive Barium Titanate Coated Titanium Scaffold Improves Osteogenesis and Osseointegration with Low-Intensity Pulsed Ultrasound for Large Segmental Bone Defects. Bioact. Mater. 2020, 5, 1087–1101. [Google Scholar] [CrossRef]
- Dias, I.J.G.; Pádua, A.S.; Pires, E.A.; Borges, J.P.M.R.; Silva, J.C.; Lança, M.C. Hydroxyapatite–Barium Titanate Biocoatings Using Room Temperature Coblasting. Crystals 2023, 13, 579. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Jiao, Y.; Li, J.; Li, Y.; Hao, Y.L.; Zuo, Y. In Vitro Study on the Piezodynamic Therapy with a BaTiO3-Coating Titanium Scaffold under Low-Intensity Pulsed Ultrasound Stimulation. ACS Appl. Mater. Interfaces 2021, 13, 49542–49555. [Google Scholar] [CrossRef]
- Doan, N.; Reher, P.; Meghji, S.; Harris, M. In Vitro Effects of Therapeutic Ultrasound on Cell Proliferation, Protein Synthesis, and Cytokine Production by Human Fibroblasts, Osteoblasts, and Monocytes. J. Oral Maxillofac. Surg. 1999, 57, 409–419. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Riverón, F.; Zarco, R.; Medina, E.; Medina, A.; Alvarado, L.; Mercado, P. Ultrasonido de Baja Intensidad en el Tratamiento de la Consolidación Ósea de Radio y Cúbito. Rev. Mex. Fís. 2001, 47, 13. [Google Scholar]
- Della Rocca, G.J. The Science of Ultrasound Therapy for Fracture Healing. Indian J. Orthop. 2009, 43, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.-H.; Cheung, W.-H.; Pounder, N.M.; De Ana, F.J.; Harrison, A.; Leung, K.-S. Effects of different therapeutic ultrasound intensities on fracture healing in rats. Ultrasound Med. Biol. 2012, 38, 745–752. [Google Scholar] [CrossRef]
- Padilla, F.; Puts, R.; Vico, L.; Raum, K. Stimulation of bone repair with ultrasound: A review of the possible mechanical effects. Ultrasonics 2014, 54, 1125–1145. [Google Scholar] [CrossRef]
- Cao, H.; Feng, L.; Wu, Z.; Hou, W.; Li, S.; Hao, Y.; Wu, L. Effect of low-intensity pulsed ultrasound on the biological behavior of osteoblasts on porous titanium alloy scaffolds: An in vitro and in vivo study. Mater. Sci. Eng. C 2017, 80, 7–17. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Liu, X.; Liu, X.; Zhu, B.; Guo, S.; Wang, C.; Wang, D.; Li, S.; Zhang, Z. Effects of low-intensity pulsed ultrasound in tendon injuries. J. Ultrasound Med. 2023, 42, 1923–1939. [Google Scholar] [CrossRef]
- Tang, L.; Wu, T.; Li, J.; Yu, Y.; Ma, Z.; Sun, L.; Ta, D.; Fan, X. Study on synergistic effects of nanohydroxyapatite/high-viscosity carboxymethyl cellulose scaffolds stimulated by LIPUS for bone defect repair in rats. ACS Biomater. Sci. Eng. 2024, 10, 1018–1030. [Google Scholar] [CrossRef]
- Temiz, Y.; Lovchik, R.D.; Kaigala, G.V.; Delamarche, E. Lab-on-a-chip devices: How to close and plug the lab? Microelectron. Eng. 2015, 132, 156–175. [Google Scholar] [CrossRef]
- Lafleur, J.P.; Jönsson, A.; Senkbeil, S.; Kutter, J.P. Recent advances in lab-on-a-chip for biosensing applications. Biosens. Bioelectron. 2016, 76, 213–233. [Google Scholar] [CrossRef]
- Lins, F.M.; Castor, K.; Monteiro, R.; Mota, F.B.; Rocha, L.F.M. Mapping the lab-on-a-chip patent landscape through bibliometric techniques. World Pat. Inf. 2019, 58, 101904. [Google Scholar]
- Sanjay, G.; Shreedhara, L.; Mallya, V.; Sarpangala, P.; Guttal, K.; Nandimath, K. Lab-on-a-chip—The advent of instantaneous diagnosis for a plethora of diseases. J. Acad. Dent. Educ. 2023, 9, 64–72. [Google Scholar] [CrossRef]
- Gómez, R.; Guzmán, Z.S.; Carrera, K.I.; Leal-Berumen, I.; Hurtado, A.; Herrera, G.; Orozco, V.M. Impact evaluation of high energy ball milling homogenization process in the phase distribution of hydroxyapatite-barium titanate plasma spray biocoating. Coatings 2021, 11, 728. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Match! 3.11—Phase Analysis Using Powder Diffraction; Crystal Impact: Bonn, Germany, 2003. [Google Scholar]
- Momma, K.; Izumi, F. Visualization for Electronic and Structural Analysis (VESTA), Version 3.5.7; JP-Minerals: Tsukuba-shi, Japan, 2021. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Yacoubi, A.E.; Massit, A.; Moutaoikel, S.E.; Rezzouk, A.; Idrissi, B.C.E. Rietveld refinement of the crystal structure of hydroxyapatite using X-ray powder diffraction. Am. J. Mater. Sci. Eng. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Al-Shakarchi, E.K.; Mahmood, N.B. Three techniques used to produce BaTiO3 fine powder. J. Mod. Phys. 2011, 2, 9. [Google Scholar] [CrossRef]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, S.; Pal, B. Superior adsorptive removal of brilliant green and phenol red dyes mixture by CaO nanoparticles extracted from egg shells. J. Nanostruct. Chem. 2022, 12, 207–221. [Google Scholar] [CrossRef]
- Komuro, N.; Mikami, M.; Saines, P.J.; Cheetham, A.K. Structure-property correlations in Eu-doped tetra calcium phosphate phosphor: A key to solid-state lighting application. J. Lumin. 2015, 162, 25–30. [Google Scholar] [CrossRef]
- Kokubo, T. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Jhonson, D. CView, Version 3.5h; Scribner Associates, Inc.: Southern Pines, NC, USA, 2020. [Google Scholar]
- Denning, D.; Guyonnet, J.; Rodriguez, B.J. Applications of piezoresponse force microscopy in materials research: From inorganic ferroelectrics to biopiezoelectrics and beyond. Int. Mater. Rev. 2016, 61, 46–70. [Google Scholar] [CrossRef]
- Qiao, H.; Kwon, O.; Kim, Y. Electrostatic effect on off-field ferroelectric hysteresis loop in piezoresponse force microscopy. Appl. Phys. Lett. 2020, 116, 172903. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, J.; Yang, Y.; Wang, Y.; Wu, Y.; He, H.; Jiang, Z. Piezoelectric properties of rhombic LiNbO3 nanowires. RSC Adv. 2012, 2, 7380–7383. [Google Scholar] [CrossRef]
- Carl Zeiss Microscopy GmbH; ZEN 3.0, Blue Edition; Carl Zeiss Microscopy Gmbh: Jena, Germany, 2019.
- Aimi, A.; Horiuchi, K.; Yamaguchi, Y.; Ito, S.; Fujimoto, K. Disordered off-center direction of Ti4+ in pseudo-cubic type BaTiO3 prepared by mixed hydroxide process. J. Ceram. Soc. Jpn. 2021, 129, 73–78. [Google Scholar] [CrossRef]
- Zhu, W.; Akbar, S.A.; Asiaie, R.; Dutta, P.K. Synthesis, microstructure and electrical properties of hydrothermally prepared ferroelectric BaTiO3 thin films. J. Electroceram. 1998, 2, 21–31. [Google Scholar] [CrossRef]
- Khomchenko, V.A.; Kiselev, D.A.; Vieira, J.M.; Jian, L.; Kholkin, A.L.; Lopes, A.M.L.; Maglione, M. Effect of diamagnetic Ca, Sr, Pb, and Ba substitution on the crystal structure and multiferroic properties of the BiFeO3 perovskite. J. Appl. Phys. 2008, 103, 024107. [Google Scholar] [CrossRef]
- Gross, K.A.; Gross, V.; Berndt, C.C. Thermal analysis of amorphous phases in hydroxyapatite coatings. J. Am. Ceram. Soc. 1998, 81, 106–112. [Google Scholar] [CrossRef]
- Cihlár, J.A.; Buchal, A.; Trunec, M. Kinetics of thermal decomposition of hydroxyapatite bioceramics. J. Mater. Sci. 1999, 34, 6121–6131. [Google Scholar] [CrossRef]
- Wang, H.; Lee, J.K.; Moursi, A.; Lannutti, J.J. Ca/P ratio effects on the degradation of hydroxyapatite in vitro. J. Biomed. Mater. Res. Part A 2003, 67, 599–608. [Google Scholar] [CrossRef]
- Heimann, R. B Structure, properties, and biomedical performance of osteocondctive bioceramic coatings. Surf. Coat. Technol. 2013, 233, 27–38. [Google Scholar] [CrossRef]
- Singh, T.P.; Singh, H.; Singh, H. Characterization of thermal sprayed hydroxyapatite coatings on some biomedical implant materials. J. Appl. Biomater. Funct. Mater. 2014, 12, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Heimann, R.B. Plasma-sprayed hydroxylapatite coatings as biocompatible intermediaries between inorganic implant surfaces and living tissue. J. Therm. Spray Technol. 2018, 27, 1212–1237. [Google Scholar] [CrossRef]
- Kotian, R.; Rao, P.P.; Madhyastha, P. X-ray diffraction analysis of hydroxyapatite-coated in different plasma gas atmosphere on Ti and Ti-6Al-4V. Eur. J. Dent. 2019, 11, 438–446. [Google Scholar] [CrossRef]
- ISO 13779-3:2018; Implants for Surgery—Hydroxyapatite—Part 3: Chemical Analysis and Characterization of Crystallinity and Phase Purity. International Organization for Standardization: Geneva, Switzerland, 2010.
- ASTM F1609-08; Standard Specification for Calcium Phosphate Coatings for Implantable Materials. ASTM International: West Conshohocken, PA, USA, 2008.
- Kaur, S.; Sharma, S.; Bala, N. A comparative study of corrosion resistance of biocompatible coating on titanium alloy and stainless steel. Mater. Chem. Phys. 2019, 238, 121923. [Google Scholar] [CrossRef]
- Canpolat, Ö.; Çanakçı, A.; Gültekin, G. Effect of HA ratio on the morphology and characteristics of Ti-HA composite coatings on Ti6Al4V-ELI synthesized via mechanical milling. Tribol. Mater. Surf. Interfaces 2024, in press. [Google Scholar] [CrossRef]
- ISO 10993-3; Biological Evaluation of Medical Devices—Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity. International Organization for Standardization: Geneva, Switzerland, 2018.
- ASTM F895-08; Standard Test Method for Agar Diffusion Cell Culture Screening for Cytotoxicity. ASTM International: West Conshohocken, PA, USA, 2008.
- Ossa, C.P.O.; Rogero, S.O.; Tschiptschin, A.P. Cytotoxicity study of plasma-sprayed hydroxyapatite coating on high nitrogen austenitic stainless steels. J. Mater. Sci. Mater. Med. 2006, 17, 1095–1100. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Fan, T.; Tan, Z.; Zhou, Z.; He, D. In vitro evaluation of hydroxyapatite coatings with (002) crystallographic texture deposited by micro-plasma spraying. Mater. Sci. Eng. C 2017, 75, 596–601. [Google Scholar] [CrossRef]
- Vouilloz, F.J.; Castro, M.S.; Vargas, G.E.; Gorustovich, A.; Fanovich, M.A. Reactivity of BaTiO3-Ca10(PO4)6(OH)2 phases in composite materials for biomedical applications. Ceram. Int. 2017, 43, 4212–4221. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Zhou, Z.; Guo, X.; Liu, Y.; Hou, W.; Li, H. In vitro bioactivity and antibacterial performances of atmospheric plasma sprayed c-axis preferential oriented hydroxyapatite coatings. Surf. Coat. Technol. 2021, 417, 127209. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, L.; Jing, L.; Wang, X.; Zhou, Q.; Chang, J. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 73, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Hou, C.; Bao, B.; Wang, S.; Maté Sánchez de Val, J.E.; Wenhui, W. The Molecular Interaction of Collagen with Cell Receptors for Biological Function. Polymers 2022, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Neumann, M.; Hanisch, U.; Reinstorf, A.; Pompe, W.; Zwipp, H.; Biewener, A. Osteocalcin enhances bone remodeling around hydroxyapatite/collagen composites. J. Biomed. Mater. Res. Part A 2005, 73, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Y.; Huang, Y.; Wu, H.; Liu, Y.; Liu, Y.; Lee, O. Osteocalcin mediates biomineralization during osteogenic maturation in human mesenchymal stromal cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef]
- Hosseini, S.; Naderi-Manesh, H.; Vali, H.; Eslaminejad, B.; Sayahpour, F.; Sheibani, S.; Faghihi, S. Contribution of osteocalcin-mimetic peptide enhances osteogenic activity and extracellular matrix mineralization of human osteoblast-like cells. Colloids Surf. B Biointerfaces 2019, 173, 662–671. [Google Scholar] [CrossRef]
- Manolagas, S. Osteocalcin promotes bone mineralization but is not a hormone. PLoS Genet. 2020, 16, e1008584. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Wu, C.; Du, Y.; Tang, H.; Zheng, S.; Wu, G. In vitro assessment of immunomodulatory and osteogenic properties in 3D-printed hydroxyapatite/barium titanate piezoelectric ceramic scaffolds. Ceram. Int. 2024, 50, 8751–8759. [Google Scholar] [CrossRef]
- Ehterami, A.; Kazemi, M.; Nazari, B.; Saraeian, P.; Azami, M. Fabrication and characterization of highly porous barium titanate based scaffold coated by Gel/HA nanocomposite with high piezoelectric coefficient for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2018, 79, 195–202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).