Abstract
Bio-based and/or biodegradable food contact materials are being developed as alternatives to conventional petroleum-based materials. Like other food contact materials, these are subject to regulatory requirements. The characterization of these biomaterials enables the identification of chemical substances that could potentially migrate from these materials into food and may pose a risk to consumer health. In this work, commercial samples of food contact materials labeled as bio-based and/or biodegradable were analyzed. To tentatively identify compounds, two analytical methods were optimized: purge and trap (P&T) for volatile compounds and methanolic extract injection for the determination of semi-volatile compounds, both using gas chromatography coupled with mass spectrometry (GC-MS). Compound toxicity was estimated using an in silico methodology, namely Cramer’s rules. More than 200 compounds of different natures were tentatively identified, but only 29 are included in Regulation (EU) 10/2011 on plastic materials intended to come into contact with food, and 38 of them were classified as high-toxicity compounds.
1. Introduction
Petroleum-based materials have a significant negative impact on the environment. Although their use as food contact materials (FCMs) is reducing, food packaging and products produced from non-renewable resources are still mainstream in the industry. This environmental concern has increased consumer interest and demand for more sustainable alternatives, such as bio-based and/or biodegradable materials [1,2].
There are two main groups of bioplastics according to the European Association of Bioplastics: plastics based on renewable resources (bio-based) and biodegradable plastics, which do not necessarily have to be biologically based [3].
Bio-based polymers can be defined as man-made or man-processed organic macromolecules synthesized or derived from renewable resources (plants and/or animals) or by microorganisms using a carbon source through fermentative processes. Occasionally, they can return to nature as carbon dioxide, water, inorganic compounds, and biomass through the composting process, leaving no distinguishable and/or toxic residues [4,5]. Their “bio” origin does not guarantee biodegradation; it depends on the chemical structure of the polymers.
Biodegradable plastics are those that easily biodegrade in nature through the action of microorganisms and other living organisms, reducing the molar masses of macromolecules. They can be composed of both natural and fossil sources [4,6,7].
Biodegradable and/or bio-based polymers can be classified into three main categories. The first group includes biodegradable and bio-based polymers, like cellulose, starch, polylactic acid (PLA), and polyhydroxyalkanoates (PHA) [4,8,9]. The second category comprises biodegradable but not bio-based polymers, which originate from petrochemicals, including polybutylene succinate (PBS) and poly(butylene adipate-co-terephthalate) (PBAT). Lastly are bio-based but not biodegradable polymers, which are synthesized similarly to petroleum-based plastics but originate from bio-based raw materials, such as bio-based polyethylene terephthalate (bio-PET) and bio-based polypropylene (bio-PP) [4].
Food contact bioplastics do not yet have specific regulations but must comply with Regulation (EC) No. 1935/2004 [10] on materials and articles intended to come into contact with food and must not transfer their constituents to food in quantities which could endanger human health. Regulation (EU) 10/2011 [11] on plastic food contact materials, which includes a list of substances that can migrate with their specific migration limits, should also be taken as a reference.
For these bioplastic materials to achieve properties similar to those of petroleum-based polymers (mechanical properties, permeability, etc.), it is common to incorporate additives such as plasticizers, antioxidants, and slip agents, among others. However, regarding food safety, the number of studies on the characterization of these bioplastic food contact materials is limited [12,13,14,15,16,17]. For example, Asensio et al. [17] analyzed natural biomaterials, identifying a total of 67 compounds used in manufacturing paper, adhesives, and food packaging. Migration tests in three liquid simulants revealed numerous compounds related to the food contact industry, such as decanal, nonanal, and phthalic anhydride, among others. In other study, Tsochatzis et al. [13] investigated the chemical safety of polysaccharide films made from pea starch, organocatalytic acetylated pea starch, and pectin using two official food simulants representing hydrophilic and lipophilic foods. Semi-volatile and non-volatile migrating compounds were identified and semi-quantified. Measurable migration levels of substances such as glycerol, monoacetylated maltose, and dibutyl phthalate were determined. These materials, like conventional plastics, can release substances and reach food. Potential migrants from food packaging include intentionally added substances (IAS), such as monomers and additives, and non-intentionally added substances (NIAS), such as reaction and/or degradation products, etc. Therefore, the characterization of these bioplastic materials is an imperative.
According to some authors [2,18], analytical methods currently used for the detection and identification of chemical compounds from petroleum-derived plastic FCMs, as well as migration assays, are expected to be suitable for bioplastic FCMs, for example, GC-MS methods for the determination of volatile and semi-volatile substances, liquid chromatography coupled to mass spectrometry (LC-MS) for the analysis of non-volatile compounds, etc. [4]. GC-MS is the most commonly used technique for identifying unknown volatile compounds due to its high reproducibility, robustness, and the availability of standardized commercial libraries [19].
In this work, the characterization of a set of commercial samples of food contact materials labeled as bio-based and/or biodegradable was carried out. First, Fourier-transform infrared spectroscopy with attenuated total reflection (FTIR-ATR) was used to identify the type of polymer in the samples. Once determined, two non-targeted methods for the tentative identification of both volatile and semi-volatile compounds were optimized: the P&T and GC-MS techniques allow the volatile compounds to become concentrated in a sorbent material, and the solvent extraction technique followed by GC-MS enables the detection of semi-volatile substances. Finally, for chemical compounds with no previous toxicity tests, their toxicity was estimated using an in silico methodology, i.e., Cramer’s rules.
This study aligns with the objectives of the proposed revision of the Packaging and Packaging Waste Regulation [20], which emphasizes not only the sustainability of packaging materials but also their chemical safety. The potential presence of potentially hazardous substances in bio-based and biodegradable food contact materials highlights the urgent need for standardized assessment frameworks.
2. Materials and Methods
2.1. Reagents and Analytical Standards
Methanol (MeOH) (CAS-No: 67-56-1) and n-hexane for gas chromatography (CAS-No: 110-54-3) were provided by Merck (Darmstadt, Germany).
Toluene-d8 (≥99%) (CAS-No: 2037-26-5), provided by Sigma-Aldrich (Schnelldorf, Germany), was used as the internal standard for the P&T method, while diethyl phthalate-3,4,5,6-d4 (DEP-d, 99,3%) (CAS-No: 93951-87-2), purchased from Fluka (Steinheim, Germany), was used as the internal standard for the determination of semi-volatile compounds.
Analytical standards with high purity (>99%) were used in this study for the confirmation of some of the compounds tentatively identified. Diethyl phthalate (CAS-No: 84-66-2), methenamine (CAS-No: 100-97-0), and diisobutyl phthalate (CAS-No: 84-69-5) were purchased from Sigma-Aldrich (Schnelldorf, Germany). Tributyl acetylcitrate (CAS-No: 77-90-7) was obtained from Fluka (Steinheim, Germany), and tetradecane (CAS-No: 629-59-4) was provided by Panreac (Barcelona, Spain). All standards were prepared using MeOH as a solvent, except for tetradecane which was dissolved in hexane.
Various preventive measures were performed to reduce possible contamination. To handle the samples, the use of plastic materials was avoided by using glass instead, previously washed with an organic solvent, muffled (to avoid contamination with ubiquitous compounds such as phthalates), and covered with aluminum foil until use.
2.2. Samples
A total of seven samples of commercial food contact materials labeled as bio-based and/or biodegradable were analyzed in this study. None of the samples had previously been in contact with foodstuffs. All of them were purchased from stores in Santiago de Compostela (Spain) and consisted of plastic materials, except for the pasta packaging (BSC) and the green paper straws (CBV), which were made of paper and cardboard. Sample information is detailed in Table 1.

Table 1.
Information on food contact materials analyzed in this study.
2.3. Fourier-Transform Infrared Spectroscopy with Attenuated Total Reflectance (ATR-FTIR)
To identify the type of material, an ATR (attenuated total reflectance)-FTIR (Fourier-transform infrared) spectrometer (ATR-PRO ONE, FTIR 4700, Jasco, Tokyo, Japan) fitted with a diamond optical crystal and controlled by the Spectra Manager™ v.2 software (Jasco, Japan) was used.
Before the analysis, samples were cut, cleaned with an organic solvent, and dried. FTIR spectra were acquired in the region from 4000 to 650 cm−1, on the inside and outside surfaces of the material. For spectrum identification, KnowItAll 17.4.135.B software was used, comparing the recorded samples’ spectra with the infrared spectra available in the libraries of polymers and related compounds from Bio-Rad Laboratories, Inc. (Philadelphia, PA, USA). Only entries from the library with an impact quality index (HQI) greater than 80 were taken into account in the identification.
2.4. Sample Preparation
Food contact materials were cut into small pieces of approximately 5 mm and stored in Petri glass dishes until analysis.
For the P&T GC-MS method, 1 g of the sample was weighed and introduced directly into EPA glass vials with a PTFE/silicone septum for this equipment, along with 50 µL of a 10 mg/L solution of toluene-d8 as an internal standard.
For the determination of semi-volatile compounds, solid–liquid extraction had previously been carried out. In each glass vial, the amount of optimized sample was weighed, and 5 mL of MeOH was added. Vials were left in the oven for 24 h at 70 °C. After this time, extracts were concentrated 15 times by evaporating to dryness with a stream of nitrogen at 40 °C (RapidVap Vertex Evaporator, Labconco, Kansas City, MO, USA) and subsequent reconstitution with MeOH, and 10 µL of a 10 mg/L solution of DEP-d was added as an internal standard. Then, the extract was filtered using a PTFE membrane filter of 0.45 µm (Membrane Solutions, Auburn, WA, USA) into a glass vial for its encapsulation and subsequent injection into the gas chromatograph. All tests were performed in triplicate.
2.5. P&T GC-MS Method for the Determination of Volatile Compounds
The P&T technique was used for the extraction of volatile compounds. The analysis was conducted in an ATOMX XYZ multi-matrix P&T system (Teledyne, CA, USA) operated with Atomx XYZ TekLink™ software. The purge temperature was 80 °C and the purge time was 20 min. The purge flow was 40 mL/min with helium as the purge gas, the desorption time was 2 min, and the temperature and desorption flow were 250 °C and 300 mL/min, respectively.
The GC-MS equipment used was a Trace 1300 Series GC gas chromatograph coupled to a Trace ISQ LT single-quadrupole mass spectrometer detector, both from Thermo Scientific (San José, CA, USA). For the separation of volatile compounds from the samples, a Rxi-624SilMS column from Restek® (Bellefonte, PA, USA) of 30 m length × 0.25 mm internal diameter and 1.40 µm film thickness was used.
The chromatographic conditions applied were the following: Helium (3× quality, from Nippon Gases, Madrid, Spain) was used as the carrier gas, at a constant flow of 1 mL/min. The initial oven temperature was set at 35 °C for 2 min, then increased at a rate of 9 °C/min until reaching 300 °C, holding this temperature for 10 min. The transfer line temperature and electron source temperature were set at 300 °C. Mass spectra were obtained with a mass-selective detector in which the ionization mode was electron impact (EI) at a voltage of 70 eV. To acquire the data, a full scan was performed with an m/z range of between 20 and 500. Xcalibur 2.0.7 software (Thermo Scientific Inc., San José, CA, USA) was used for acquisition and processing. For the tentative identification of the compounds, the commercial mass spectral libraries Wiley Registry™ 12th edition, with nearly one million mass spectra per EI, and NIST/EPA/NIH 11 version 2.0, with 30,898 mass spectra, were used.
2.6. GC-MS for the Determination of Semi-Volatile Compounds
The gas chromatograph and mass spectrometer equipment used was the same as indicated in Section 2.5, but with an AI 1310 automatic injector (Thermo Fischer Scientific, San José, CA, USA). For the separation of the semi-volatile compounds in the samples, an Rxi-5Sil MS column of 30 m length × 0.25 mm internal diameter and 0.25 µm film thickness from Restek® (Bellefonte, PA, USA) was used as the stationary phase.
For the screening analysis, 1.0 µL aliquots from the extracts of the samples were injected in splitless mode, with an injector temperature of 250 °C. Helium (3× quality, from Nippon Gases) was used as the carrier gas at a constant flow of 1 mL/min. An initial oven temperature of 40 °C was maintained for 2 min, subsequently increased following a temperature ramp of 9 °C/min until reaching 300 °C, and maintained at that temperature for 10 min. EI ionization was set at a voltage of 70 eV. Both the temperatures of the transfer line and the ion source were set at 300 °C. The acquisition of the chromatograms was carried out in full scan mode, over an m/z range of between 20 and 500. The same software and commercial mass spectral libraries were used as in Section 2.5.
2.7. Toxicity Estimation
Cramer’s decision tree (1978) is a tool used to carry out a first classification and ordering of substances according to the expected level of toxicity, based on their molecular structure, in order to estimate the toxicological risk of exposure to these compounds. For this purpose, the software Toxtree v3.1.0.1851 (Ideaconsult Ltd., Sofia, Bulgaria) [21] was used. This model classifies substances into three classes, according to their level of toxicity: class I (low toxicity), class II (intermediate toxicity), and class III (high toxicity). The Threshold of Toxicological Concern (TTC) values established were 1800 μg/person/day for Cramer class I, 540 μg/person/day for Cramer class II, and 90 μg/person/day for Cramer class III [22].
3. Results and Discussion
3.1. Characterization of the Materials by FTIR-ATR
The FTIR analysis allowed for the identification of the type of material used in the seven samples. The results are shown in Table 1. The two samples that corresponded to paper and cardboard are composed of cellulose (CBV and BSC samples); another two samples are based on PLA (VLT and CBX samples); one sample is composed of poly(lactic-co-glycolic) acid (PLGA), a copolymer formed by the polymerization of lactic acid (LA) and glycolic acid (GA) [23] (sample CFR); and two samples are composed of biodegradable polystyrene, a polymer mentioned by Pinaeva & Noskov (2024) (VCT and VBT samples) [24]. In all cases, the material of the internal surface matches the material of the external surface, except for the BSC sample, whose external surface could not be identified because it is probably a multilayered material or has coatings on the external surface, which makes its identification difficult by FTIR-ATR spectroscopy. All identifications presented an HQI greater than 87.
Figure 1 shows the IR spectrum of the VLT sample (blue) and the IR spectrum of the first entry in the IR spectral libraries (PLA, in red) overlapped. Characteristic bands of PLA [25] present in the spectrum of the VLT sample are indicated. The IR spectrum of the other sample can be seen in Figure S1 (Supplementary Materials).
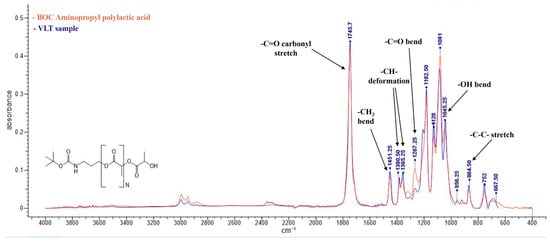
Figure 1.
IR spectrum of the VLT sample (blue line) and the first entry of the IR spectral libraries that corresponds to PLA (red line). Characteristic bands are indicated by arrows. In the lower left part of the image, the molecular structure of PLA is shown.
3.2. Screening of Volatile Compounds by P&T GC-MS
3.2.1. P&T GC-MS Method Optimization
Several tests were carried out for the optimization of the method in the VCT sample, including the amount of sample to be analyzed (0.5, 1, or 2.5 g), the purge temperature (30, 60, or 80 °C), and the purge time (10, 20, or 30 min).
It was determined that 80 °C allowed a greater number of compounds to be identified than 60 °C or 30 °C. Regarding the time parameter, 20 min was chosen instead of 10 min or 30 min, since no significant differences were found between the number of compounds identified between 20 and 30 min, but better results were obtained when comparing 20 with 10 min. Finally, regarding the amount of sample used, 1 g of sample prevails over 0.5 g and 2.5 g, since the number of compounds that could be identified using 1 g was practically the same as using 2.5 g and greater than if 0.5 g was used.
3.2.2. Tentative Identification of Volatile Compounds
In this study, the optimized P&T GC-MS method was used to tentatively identify volatile compounds, which may be potential migrants.
Only compounds with a high spectral coincidence with the available libraries were considered, that is, those with an SI (search index) and RSI (reverse search index) greater than 700.
A total of 68 compounds of different natures were tentatively identified in the FCM samples analyzed (Table 2). The BSC sample was the one with the most volatile compounds, with a total of 29 compounds. Figure 2 shows the P&T GC-MS chromatogram of the CBV sample with some of the tentatively identified compounds.

Table 2.
Volatile compounds tentatively identified using P&T GC-MS in the studied FCM.
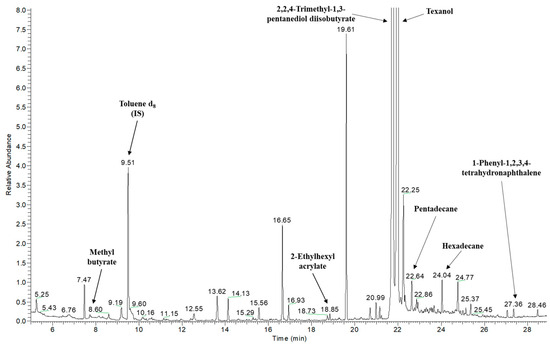
Figure 2.
P&T GC-MS chromatogram of the CBV sample as an example with some of the tentatively identified compounds indicated by arrows.
Numerous compounds of different chemical nature (alkanes, aldehydes, ketones, alcohols, esters, etc.) were tentatively identified, related to the manufacture of FCM. Some of these compounds are IAS, such as monomers, additives, or other starting substances. Among the additives, phthalates, and other plasticizers, photoinitiators and slip agents were identified, among others. In addition, numerous NIAS were also detected in all seven samples, such as reaction and/or degradation products.
Within the IAS, numerous plasticizers were detected, like 2,2,4-trimethyl-1,3-pentanediol diisobutyrate, in the CBV sample, which is a safer and environmentally friendly alternate non-phthalate plasticizer and ink solvent, included in Regulation (EU) 10/2011 with an SML of 5 mg/kg [11,62,70]. Regarding phthalate esters (PAEs), only one compound was tentatively identified, diisobutyl phthalate (DIBP) in the CBX sample, belonging to Cramer’s class I. PAEs are a group of compounds widely used as plasticizers to increase the durability and flexibility of products. Numerous studies classify them as endocrine disruptors [71].
Several compounds related to adhesives were found. For example, compound 1-hexanol-2-ethyl acetate (BSC sample), which is a Cramer’s class I compound, was previously found in adhesives [53,54]. The compound 1-hexanol-2-ethyl, identified in the BSC sample, is included in Regulation 10/2011 with an SML of 30 mg/kg, whose use is permitted as a monomer or another starting substance, and it has also been found in adhesives and paper manufacturing [11,17,47].
In the CBX and CFR samples (both based on PLA or derivatives), a PLA oligomer was identified, namely DL-lactide [28], which was classified as Cramer class I. Methyl lactate, a compound found in the VLT and CBX samples, is a degradation product of PLA belonging to Cramer’s class I [57].
Some characteristic compounds of cellulose and paper were found in the CBV and BSC samples. Many of the analyzed compounds originated from the degradation of a biopolymer chain such as alkanes and alkenes, which are considered NIAS of chain degradation [28]. Pentadecane and 3-methylpentadecane, which are both volatile organic compounds (VOCs) from recycled cellulose, were identified in both samples and belong to Cramer’s class I [53]. Propanoic acid, 2-methyl, 3-hydroxy-2,2,4-trimethylpentyl ester is a coalescent agent (a chemical additive that helps paint form a solid film) identified in the CBV samples, which belongs to Cramer’s class II [63]. 2-Ethylhexyl acrylate is an acrylic monomer used for solvent-free photopolymerizable paper coating, which was also found in the CBV sample, and it is included in Regulation (EU) 10/2011 with an SML of 0.05 mg/kg [11,58]. Propylene glycol is a softener additive used for cellulose regeneration found in the CBV sample, and it is included in Regulation (EU) 10/2011 with an SML of 60 mg/kg [11,34].
Compounds related to polystyrene were found in the VBT and VCT samples, like benzene, 1-methylpropyl [38] and benzene, 1-propenyl [40], both found in the VCT sample and belonging to Cramer’s class I. Benzene, 1,1′-(1,3-propanediyl)bis is an isomer of styrene dimers identified in the VCT sample, belonging to Cramer’s class III, which means high toxicity [67]. Benzene, 1,3-diethyl is an aromatic VOC of polystyrene found in the VBT sample (Cramer’s class I) [40].
Numerous compounds identified in the analyzed samples correspond to NIAS: 1-hexanol-2-ethyl, a product of the thermal decomposition or hydrolysis of plasticizers such as bis(2-ethylhexyl) phthalate (DEHP) or bis-(2-ethylhexyl) adipate (DEHA), was detected in the BSC sample [48]. Other highly abundant NIAS in the samples were polymerization by-products and degradation products of polystyrene, belonging to Cramer’s class I (decanal and propylbenzene, both in VCT and VBT [38]; 2-phenylpropenal in VCT [38]), as well as Cramer’s class III (2,4-diphenyl-1-butene in the VBT and VCT samples [38,68]), among others.
Some compounds related to printing ink were found in the analyzed samples, because the materials were analyzed on both sides, including the external side, which in some of the samples was printed or colored (BSC, CBV). Although this side is not in direct contact with food, it is interesting to take these substances into account due to possible contamination because of the set-off phenomenon, for example. Set-off occurs when, after printing, products are stacked without assembly, and part of the fresh ink may be stamped on the internal side of the adjacent product, which will be in contact with food [44]. In this study, some ink-related compounds were tentatively identified, such as 1,2,4-methenoazulene, decahydro-1,5,5,8A-tetramethyl (in the BSC sample, Cramer’s class I) [60,61] and benzaldehyde (VBT, CFR, and VLT samples, included in Regulation (EU) 10/2011 with an SML of 60 mg/kg) [11,35]. A compound that works as a photoinitiator for curing UV inks, methanone, (1-hydroxycyclohexyl)phenyl, which is also a component of some paints, coatings, and printing inks, was found in the VLT sample [44,69]. Other ink components were tentatively identified, such as α-pinene in the BSC sample, which is also used in adhesives, coatings, and adhesion agents [41]. This compound is listed in Regulation (EU) 10/2011 with a specific migration limit (SML) of 60 mg/kg [11]. Additionally, 1,2,3-trichlorobenzene was detected in the CBV, VBT, and VCT samples; this substance is used in lacquers, resins, and pigments and is classified as a Cramer class III compound [52].
This work shows the presence of numerous compounds that are not included in the list of substances allowed by Regulation (EU) 10/2011 [11]. Only 10 of the 68 compounds tentatively identified and grouped in Table 2 are included in it. In this context, Cramer’s method results in an adequate first step for estimating the toxicity of these potential migrants.
3.3. Screening of Semi-Volatile Compounds by GC-MS
3.3.1. GC-MS Method of Optimization
To carry out the extraction of compounds in the analyzed samples, the extraction solvent (MeOH), time, and temperature conditions were selected based on previous laboratory studies [32], with modifications. The amount of sample to be analyzed by this technique was optimized (0.5, 1 or 2.5 g). Finally, 1 g of sample was used since the results were significantly better than using 0.5 g, identifying a great number of chromatographic peaks (approximately 10), without major differences with respect to using 2.5 g. Subsequently, 5 mL of MeOH, the solvent chosen for both extraction and reconstitution, was added to each of the samples.
3.3.2. Tentative Identification of Semi-Volatile Compounds
In this work, the optimized GC-MS method was used for the tentative identification of semi-volatile compounds in the analyzed samples.
More than 100 compounds were tentatively identified in the samples, and the results are shown in Table 3. Five of the compounds were confirmed through the injection of the corresponding standard under the same conditions used for the sample analysis and their subsequent comparison regarding the mass spectrum and retention time. The remaining compounds were tentatively identified by comparing the obtained mass spectra with those available in the libraries. As with the P&T method, only compounds with an SI and RSI greater than 700 were considered.

Table 3.
Semi-volatile compounds tentatively identified using GC/MS in the studied FCM.
Figure 3 shows the GC-MS chromatogram of the VCT sample, showing the tentative identification of several peaks. Only 19 of the total compounds tentatively identified are included in the list of substances allowed by Regulation (EU) 10/2011 [11].
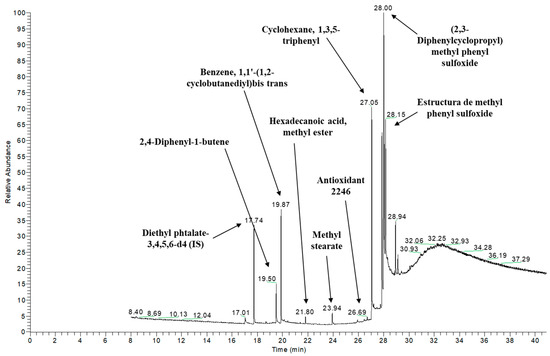
Figure 3.
GC-MS chromatogram of the VCT sample with some of the tentatively identified compounds indicated by arrows.
Using the described technique, numerous compounds of different chemical natures (alkanes, aldehydes, alcohols, ketones, carboxylic acids, esters, etc.) related to the manufacturing of FCMs were tentatively identified as monomers and additives. Within the second group, numerous plasticizers (phthalates and others), antioxidants, solvents, stabilizers, slip agents, ultraviolet filters, photoinitiators, etc., were found.
Since the extraction was carried out by immersion, the material was extracted from both sides, and some ink-related components were identified in the samples, such as surfactants, which allow water-insoluble pigments to be compatible in aqueous-based inks. An example of an ink surfactant found in the CBV sample was 2,4,7,9-tetramethyl-5-decyn-4,7-diol, which was classified as a highly toxic chemical compound (Cramer class III) [82].
Numerous phthalates were detected in the samples, such as diethyl phthalate (DEP) (CBV, VBT, VLT, BSC, VCT), diisobutyl phthalate (DIBP) (BSC), bis(2-ethylhexyl)hexahydro phthalate (CFR), and bis(2-ethylhexyl) phthalate (DEHP) (CBV, VBT, VCT), which, in addition to their function as plasticizers, are often used in printing ink formulations and as solvents to maintain color [17,45,121]. DEHP is included in Regulation (EU) 10/2011 and authorized for use in plastic FCMs with an SML of 1.5 mg/kg, with restrictions of an SML of 60 mg/kg in terms of the sum of group substances [11], while DEP, bis(2-ethylhexyl)hexahydro phthalate, and DIBP are not included. DIBP and DEHP are classified as carcinogenic, endocrine-disrupting, and reproductively toxic substances by the European Chemical Agency (ECHA) [123]. For this reason, attempts have been made to replace them with other plasticizers. Some of the alternative plasticizers to phthalates found in the samples are bis(2-ethylhexyl) fumarate (DEHF) (BSC) [47]; 2,2,4-trimethyl-1,3-pentanediol (CBV) [76], which was classified as Cramer class II; and heptadecanol (CBV, CBX, CFR, VLT) [26,95]. 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate (CBV) is a plasticizer and ink solvent [62] included in Regulation (EU) 10/2011, whose SML is 5 mg/kg [11]. This compound was also identified in the P&T analysis. Another class of alternative plasticizers to phthalates are citric acid esters, such as tributyl citrate (CFR) and tributyl acetylcitrate (ATBC) (VBT, CBX, CFR) [64]. ATBC is included in Regulation (EU) 10/2011 with restrictions of an SML of 60 mg/kg in terms of the sum of group substances [11]. Bis(2-ethylhexyl) hexanedioate (DEHA) is another plasticizer [47] included in Regulation (EU) 10/2011, with an SML of 18 mg/kg, also with restrictions of an SML of 60 mg/kg in terms of the sum of group substances [11]. Isopropyl myristate, found in the BSC sample, is a plasticizer used for cellulose, a pigment dispersant, and a binding agent [64]. Polyethylene glycol (PEG) is highly recommended as a plasticizer for PLA, which is why several compounds from the ethylene glycol family were identified in the PLA samples [84]. Recently, fatty acid methyl esters (FAMEs) have emerged as sustainable alternatives that can be used as greener plasticizers, such as 11-octadecenoic acid, methyl ester (BSC), which belongs to Cramer’s class I [98].
Some adhesive-related compounds, such as ethanol, 2-(2-ethoxyethoxy) (CBV, BSC), also used in paints, dyes, inks and surface coatings [72,73]; or 4-methylbenzenesulfonamide, in sample CFR (Cramer’s class III), have been identified [72]. Pentadecanoic acid and heptadecanoic acid, both found in the CBV sample, are two compounds related to adhesives used for the manufacturing of paper [17]. Polypropylene glycol and dodecanoic acid (BSC) [17,72] are two compounds used in adhesives, both authorized by Regulation (EU) 10/2011 for polymerization, with an SML of 60 mg/kg [11]. In addition to its use in adhesives, palmitic acid, a compound found in most samples (CBV, CBX, CFR, VLT, BSC), is used as a lubricant and slip agent in paper manufacturing [17,46,47,64].
Some compounds used as lubricants were detected in this study, such as 1-eicosanol (BSC) [74,102]. Oleic acid (CBV, BSC) and stearic acid (CBV, VLT) are two compounds included in Regulation (EU) 10/2011, with an SML of 60 mg/kg [11], and are used in the manufacturing of paper and adhesives, as well as lubricants [17,32,47].
Two compounds that function as UV filters, that is, protect the product by absorbing UV light, were identified in the BSC and CBV samples: 2-ethylhexyl salicylate and 2-propenoic acid, 3-(4-methoxyphenyl), 2-ethylhexyl ester, respectively [64,93]. Both compounds are classified as Cramer’s class I.
Numerous degradation products were found in the studied FCMs (NIAS) as antioxidant degradation products: 2,4-di-tert-butylphenol (CFR) [44,45,64] and 3-pentenoic acid, 4-phenyl (CBV) [44], both of low toxicity according to Cramer’s rules. The compounds benzene 1,1′-(1,2-cyclobutanediyl)bis, cis (VCT) [38,44] and 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione (CBV, CFR) [47], which are also degradation products of antioxidants, are classified as highly toxic according to Cramer’s decision tree. It should be noted that the slipping and anti-blocking agent N,N-diethyldodecanamide (CBV) has a chemical structure similar to the antistatic agent N,N-bis(2-hydroxyethyl)dodecanamide, which is subject to restriction [104].
It is interesting to note that, in the BSC sample, not only was the adhesive methyl dehydroabietate identified, but as was its degradation product, dehydroabietal [64,69,86]. While the adhesive belongs to Cramer’s class I, indicating low toxicity, its degradation product falls into a high-toxicity category.
Numerous compounds could be identified with both GC-MS techniques, such as 1-hexanol, 2-ethyl (BSC), included in Regulation (UE) 10/2011 with an SML of 30 mg/kg [11]; DL-lactide (CBX, CFR), Cramer’s class I; 2,2,4-trimethyl-1,3-pentanediol diisobutyr-ate (TXIB) (CBV), included in Regulation (EU) 10/2011 with an SML of 5 mg/kg [11]; 1,6-dioxacyclododecane-7,12-dione in the CFR sample; and benzene, 1,1′-(1,3-propanediyl)bis (VCT) and 2,4-diphenyl-1-butene (VBT, VCT), both belonging to Cramer’s class III. These results highlight the complementarity of the two techniques, which provide a complete screening analysis of the compounds present in the samples.
4. Conclusions
In this work, two GC-MS methods were used for the tentative identification of a wide range of potential migrant compounds, both volatile and semi-volatile, present in commercial FCM samples labeled as bio-based and/or biodegradable. This screening approach allowed for the tentative identification of over 200 compounds of different natures, including intentionally added substances (IAS), such as plasticizers, lubricants, UV filters, antioxidants, or photoinitiators, as well as non-intentionally added substances (NIAS), such as antioxidant or plasticizer degradation products, among others. Currently, there is no specific legislation for these bio-based and/or biodegradable FCMs, and only 29 of the identified compounds are included in Regulation (EU) 10/2011.
The toxicity of these tentatively identified migrant compounds was assessed using an in silico method, specifically Cramer’s decision tree. The results classified most of the compounds in class I. Overall, approximately 12% of the tentatively identified compounds were categorized as high toxicity (class III), highlighting the importance of further toxicological evaluation.
Consequently, it is important to highlight that the results demonstrate that even alternative materials marketed as biodegradable and/or bio-based are not exempt from chemical complexity. The presence of potentially hazardous substances among the tentatively identified migrants reinforces the idea that these materials should not be assumed to be inherently safer than conventional fossil plastics. This underscores the need for a more comprehensive assessment framework that includes both chemical characterization and toxicological analysis, in accordance with current and future regulatory requirements.
Therefore, it is necessary to highlight the need to carry out further studies for the characterization and quantification of these new food contact materials, since their use is expected to increase in the coming years, progressively replacing petrochemical-derived FCMs. A deeper understanding of their potential risks to consumer health is essential to ensure their safety and to guide future regulatory developments.
Supplementary Materials
The following supporting information can be downloaded a: https://www.mdpi.com/article/10.3390/coatings15070751/s1, Figure S1. ATR-FTIR spectra of the inner side of the samples, CBV (a), VBT (b), CBX (c), CFR (d), BSC (e), and VCT (f) (blue), and the IR spectrum of the first entry on the IR spectra libraries (red) overlapped.
Author Contributions
Conceptualization, A.L.-C. and A.R.B.d.Q.; methodology, A.L.-C. and E.L.S.; software, E.L.S.; validation, E.L.S.; formal analysis, E.L.S.; investigation, E.L.S.; data curation, A.L.-C.; writing—original draft preparation, E.L.S.; writing—review and editing, A.L.-C., L.B.-P., A.R.B.d.Q., R.S.; supervision, A.L.-C., L.B.-P., A.R.B.d.Q.; project administration, A.R.B.d.Q.; funding acquisition, A.R.B.d.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerio de Ciencia e Innovación, Agencia Estatal de Investigación, and by Fondo Europeo de Desarrollo Regional (FEDER). Ref. No. PID2021-124729NB- I00 “MIGRABIOQUANT” (MCIN/AEI/10.13039/501100011033/FEDER, UE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in [Identification of potential migrants in food contact materials labeled as bio-based and/or biodegradable by GC-MS].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Donkor, L.; Kontoh, G.; Yaya, A.; Bediako, J.K.; Apalangya, V. Bio-based and sustainable food packaging systems: Relevance, challenges, and prospects. Appl. Food Res. 2023, 3, 100356. [Google Scholar] [CrossRef]
- Bonwick, G.; Bradley, E.; Lock, I.; Romero, R. Bio-Based Materials for Use in Food Contact Applications; Fera Science 2019 (FR/001658); Report to the Food Standards Agency; Fera Science Ltd.: York, UK, 2019. Available online: https://www.food.gov.uk/sites/default/files/media/document/bio-based-materials-for-use-in-food-contact-applications_0.pdf (accessed on 20 January 2025).
- European Bioplastics. Bioplastics Facts and Figures 2020. Available online: https://docs.european-bioplastics.org/publications/EUBP_Facts_and_figures.pdf (accessed on 17 May 2024).
- Lestido-Cardama, A.; Barbosa-Pereira, L.; Sendón, R.; Bustos, J.; Losada, P.P.; De Quirós, A.R.B. Chemical safety and risk assessment of bio-based and/or biodegradable polymers for food contact: A review. Food Res. Int. 2025, 202, 115737. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(ethylene terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Geueke, B. Dossier—Bioplastics as Food Contact Materials; Food Packaging Forum: Zurich, Switzerland, 2014; Volume 10. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Birania, S.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Rohilla, P.; Kumar, R. Advances in development of biodegradable food packaging material from agricultural and agro-industry waste. J. Food Process Eng. 2021, 45, e13930. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Regulation (EC) No. 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. Available online: http://data.europa.eu/eli/reg/2004/1935/2021-03-27 (accessed on 13 September 2024).
- European Comission. Commission Regulation (UE) No. 10/2011, on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. Available online: http://data.europa.eu/eli/reg/2011/10/2023-08-31 (accessed on 9 September 2024).
- Ubeda, S.; Aznar, M.; Alfaro, P.; Nerín, C. Migration of oligomers from a food contact biopolymer based on polylactic acid (PLA) and polyester. Anal. Bioanal. Chem. 2019, 411, 3521–3532. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Vidal, N.P.; Bai, W.; Diamantidou, D.; Theodoridis, G.; Martinez, M.M. Untargeted screening and in silico toxicity assessment of semi-and non-volatile compounds migrating from polysaccharide-based food contact materials. Food Chem. 2023, 425, 136499. [Google Scholar] [CrossRef]
- Lin, J.; Wu, W.L.; Zhong, A.H.; Xian, Y.P.; Zhong, H.N.; Dong, B.; Liang, M.; Hu, J.P.; Wu, Y.N.; Yang, X.F.; et al. Non-targeted analysis and risk assessment of intentionally and non-intentionally added substances migrating from the emerging biodegradable food contact material poly (butylene adipate-co-terephthalate)/modified starch blend film. Food Packag. Shelf Life 2023, 40, 101190. [Google Scholar] [CrossRef]
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic products leach chemicals that induce in vitro toxicity under realistic use conditions. Environ. Sci. Technol. 2021, 55, 11814–11823. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, S.; Aznar, M.; Nerín, C.; Kabir, A. Fabric phase sorptive extraction for specific migration analysis of oligomers from biopolymers. Talanta 2021, 233, 122603. [Google Scholar] [CrossRef] [PubMed]
- Asensio, E.; Montañés, L.; Nerín, C. Migration of volatile compounds from natural biomaterials and their safety evaluation as food contact materials. Food Chem. Toxicol. 2020, 142, 111457. [Google Scholar] [CrossRef] [PubMed]
- Riboni, N.; Bianchi, F.; Cavazza, A.; Piergiovanni, M.; Mattarozzi, M.; Careri, M. Mass spectrometry-based techniques for the detection of non-intentionally added substances in bioplastics. Separations 2023, 10, 222. [Google Scholar] [CrossRef]
- Gómez-Ramos, M.M.; Ucles, S.; Ferrer, C.; Fernández-Alba, A.R.; Hernando, M.D. Exploration of environmental contaminants in honeybees using GC-TOF-MS and GC-Orbitrap-MS. Sci. Total Environ. 2019, 647, 232–244. [Google Scholar] [CrossRef]
- Regulation (EU) 2025/40 of the European Parliament and of the Council of 19 December 2024 on Packaging and Packaging Waste, Amending Regulation (EU) 2019/1020 and Directive (EU) 2019/904, and Repealing Directive 94/62/EC. Available online: http://data.europa.eu/eli/reg/2025/40/oj (accessed on 19 June 2025).
- Patlewicz, G.; Jeliazkova, N.; Safford, R.; Worth, A.; Aleksiev, B. An evaluation of the implementation of the Cramer classification scheme in the Toxtree software. SAR QSAR Environ. Res. 2008, 19, 495–524. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific opinion on exploring options for providing advice about possible human health risks based on the concept of threshold of toxicological concern (TTC). EFSA J. 2012, 10, 2750. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Pinaeva, L.G.; Noskov, A.S. Biodegradable biopolymers: Real impact to environment pollution. Sci. Total Environ. 2024, 947, 174445. [Google Scholar] [CrossRef]
- Lee, H.W.; Insyani, R.; Prasetyo, D.; Prajitno, H.; Sitompul, J. Molecular Weight and Structural Properties of Biodegradable PLA Synthesized with Different Catalysts by Direct Melt Polycondensation. J. Eng. Technol. Sci. 2015, 47, 364–373. [Google Scholar] [CrossRef]
- Ibarra, V.G.; Rodríguez Bernaldo De Quirós, A.; Paseiro Losada, P.; Sendón, R. Non-target analysis of intentionally and non intentionally added substances from plastic packaging materials and their migration into food simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- European Printing Ink Association. A sector of CEPE Aisbl. Comprising Packaging Ink Raw Materials Applied to the Non-Food Contact Surface of Food Packaging; Inventory list–Version December 2013; European Printing Ink Association: Brussels, Belgium, 2019. [Google Scholar]
- Paiva, R.; Wrona, M.; Nerín, C.; Gavril, G.L.; Cruz, S.A. Volatile Compounds and Off-odors Analysis of Recycled PLA for Packaging Applications: An Essential Factor for Ensuring Food Safety and Quality. J. Polym. Environ. 2024, 32, 6687–6697. [Google Scholar] [CrossRef]
- Salazar, R.; Domenek, S.; Plessis, C.; Ducruet, V. Quantitative determination of volatile organic compounds formed during Polylactide processing by MHS-SPME. Polym. Degrad. Stab. 2017, 136, 80–88. [Google Scholar] [CrossRef]
- Vera, P.; Uliaque, B.; Canellas, E.; Escudero, A.; Nerín, C. Identification and quantification of odorous compounds from adhesives used in food packaging materials by headspace solid phase extraction and headspace solid phase microextraction coupled to gas chromatography–olfactometry–mass spectrometry. Anal. Chim. Acta 2012, 745, 53–63. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Loureiro, P.V. Materials for Food Contact: Contribution to the Study of Chemical Safety. Ph.D. Thesis, Universidade de Santiago de Compostela, Santiago de Compostela, Spain, 2023. [Google Scholar]
- Li, D.; Zeng, Y.; Ye, Z.K.; Li, H.K.; Li, Y.Z.; Dong, B.; Su, Q.Z.; Lin, Q.B.; Xiao, J.; Zhong, H.N. Analysis of volatile organic compounds and potential odour compounds in food contact paperboard using headspace two-dimensional GC-QTOF-MS. Food Addit. Contam. Part A 2023, 40, 1482–1493. [Google Scholar] [CrossRef]
- An, H.; Lu, Z.; Wang, Z.; Abbas, M.Q.; Du, Z. Safety assessment and quality control of regenerated cellulose food packaging in different processes. Food Control. 2024, 163, 110543. [Google Scholar] [CrossRef]
- Riganakos, K.; Koller, W.; Ehlermann, D.; Bauer, B.; Kontominas, M. Effects of ionizing radiation on properties of monolayer and multilayer flexible food packaging materials. Radiat. Phys. Chem. 1999, 54, 527–540. [Google Scholar] [CrossRef]
- Panseri, S.; Chiesa, L.; Zecconi, A.; Soncini, G.; De Noni, I. Determination of Volatile Organic Compounds (VOCs) from Wrapping Films and Wrapped PDO Italian Cheeses by Using HS-SPME and GC/MS. Molecules 2014, 19, 8707–8724. [Google Scholar] [CrossRef]
- Hwang, J.B.; Lee, S.; Yeum, J.; Kim, M.; Choi, J.C.; Park, S.; Kim, J. HS-GC/MS method development and exposure assessment of volatile organic compounds from food packaging into food simulants. Food Addit. Contam. Part A 2019, 36, 1574–1583. [Google Scholar] [CrossRef]
- Song, X.; Wrona, M.; Nerin, C.; Lin, Q.; Zhong, H. Volatile non-intentionally added substances (NIAS) identified in recycled expanded polystyrene containers and their migration into food simulants. Food Packag. Shelf Life 2019, 20, 100318. [Google Scholar] [CrossRef]
- Ehret-Henry, J.; Ducruet, V.; Luciani, A.; Feigenbaum, A. Styrene and ethylbenzene migration from polystyrene into dairy products by dynamic purge-and-trap gas chromatography. J. Food Sci. 1994, 59, 990–992. [Google Scholar] [CrossRef]
- Cabanes, A.; Valdés, F.; Fullana, A. A review on VOCs from recycled plastics. Sustain. Mater. Technol. 2020, 25, e00179. [Google Scholar] [CrossRef]
- Mosquera, M.E.G.; Jiménez, G.; Tabernero, V.; Vinueza-Vaca, J.; García-Estrada, C.; Kosalková, K.; Sola-Landa, A.; Monje, B.; Acosta, C.; Alonso, R.; et al. Terpenes and Terpenoids: Building Blocks to Produce Biopolymers. Sustain. Chem. 2021, 2, 467–492. [Google Scholar] [CrossRef]
- Marin, N.; Collura, S.; Sharypov, V.I.; Beregovtsova, N.G.; Baryshnikov, S.V.; Kutnetzov, B.N.; Membrado, L.; Cebolla, V.L.; Marin, N.; Weber, A.J. Copyrolysis of wood biomass and synthetic polymers mixtures. Part II: Characterisation of the liquid phases. J. Anal. Appl. Pyrolysis 2002, 65, 41–55. [Google Scholar] [CrossRef]
- Donetzhuber, A.; Johansson, B.; Johansson, K.; Lövgren, M.; Sarin, E. Analytical characterization of the gas phases in paper and board products. Nord. Pulp Pap. Res. J. 1999, 14, 48–60. [Google Scholar] [CrossRef]
- Lago, M.A.; Ackerman, L.K. Identification of print-related contaminants in food packaging. Food Addit. Contam. Part A 2016, 33, 518–529. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; Bustos, J.; Lomo, M.L.; Losada, P.P.; De Quirós, A.R.B. Dietary Exposure Estimation to Chemicals Transferred from Milk and Dairy Products Packaging Materials in Spanish Child and Adolescent Population. Foods 2020, 9, 1554. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of volatile compounds and their sensory impact in a biopolymer based on polylactic acid (PLA) and polyester. Food Chem. 2019, 294, 171–178. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; Bustos, J.; Santillana, M.I.; Losada, P.P.; De Quirós, A.R.B. GC-MS Screening for the Identification of Potential Migrants Present in Polymeric Coatings of Food Cans. Polymers 2019, 11, 2086. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Loureiro, P.V.; Sendón, R.; Losada, P.P.; De Quirós, A.R.B. Application of chromatographic analysis for detecting components from polymeric can coatings and further determination in beverage samples. J. Chromatogr. A 2021, 1638, 461886. [Google Scholar] [CrossRef] [PubMed]
- Nerín, C.; Acosta, D.; Rubio, C. Potential migration release of volatile compounds from plastic containers destined for food use in microwave ovens. Food Addit. Contam. 2002, 19, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Bentayeb, K.; Ackerman, L.K.; Begley, T.H. Ambient Ionization–Accurate Mass Spectrometry (AMI-AMS) for the Identification of Nonvisible Set-off in Food-Contact Materials. J. Agric. Food Chem. 2012, 60, 1914–1920. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Chen, L.; Wu, X.; Wu, S.; Su, Q.; Dong, B.; Li, D.; Ma, T.; Zhong, H.; Wang, X.; et al. Characterization of volatile organic compounds in food contact paperboards and elucidation of their potential origins from the perspective of the raw materials. Food Packag. Shelf Life 2023, 37, 101062. [Google Scholar] [CrossRef]
- Domeño, C.; Aznar, M.; Nerín, C.; Isella, F.; Fedeli, M.; Bosetti, O. Safety by design of printed multilayer materials intended for food packaging. Food Addit. Contam. Part A 2017, 34, 1239–1250. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Migration of odorous compounds from adhesives used in market samples of food packaging materials by chromatography olfactometry and mass spectrometry (GC–O–MS). Food Chem. 2014, 145, 237–244. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerín, C. Risk assessment derived from migrants identified in several adhesives commonly used in food contact materials. Food Chem. Toxicol. 2015, 75, 79–87. [Google Scholar] [CrossRef]
- Salem, M.Z.; Zidan, Y.E.; El Hadidi, N.M.; Mansour, M.M.; Elgat, W.A.A. Evaluation of usage three natural extracts applied to three commercial wood species against five common molds. Int. Biodeterior. Biodegrad. 2016, 110, 206–226. [Google Scholar] [CrossRef]
- Ilayaraja, N.; Radhakrishnan, S.; Renganathan, N.G. Electrochemical fluorination of dimethyl glutarate and its characterization. Ionics 2010, 16, 137–144. [Google Scholar] [CrossRef]
- Román-Ramírez, L.A.; McKeown, P.; Shah, C.; Abraham, J.; Jones, M.D.; Wood, J. Chemical Degradation of End-of-Life Poly(lactic acid) into Methyl Lactate by a Zn(II) Complex. Ind. Eng. Chem. Res. 2020, 59, 11149–11156. [Google Scholar] [CrossRef]
- Silva, F.M.; Pinto, R.J.; Barros-Timmons, A.; Freire, C.S. Solventless Photopolymerizable Paper Coating Formulation for Packaging Applications. Polymers 2023, 15, 1069. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mann, B.; Sharma, R.; Verma, A.; Panjagari, N.R.; Gandhi, K. Identification of polymer additives from multilayer milk packaging materials by liquid-solid extraction coupled with GC-MS. Food Packag. Shelf Life 2022, 34, 100975. [Google Scholar] [CrossRef]
- Asensio, E.; Peiro, T.; Nerín, C. Determination the set-off migration of ink in cardboard-cups used in coffee vending machines. Food Chem. Toxicol. 2019, 130, 61–67. [Google Scholar] [CrossRef]
- Yang, Q.H.; Lin, Q.B.; Hua, X.Y.; Liao, J.; Lu, S.Q.; Yan, L.Y.; Ma, H.S. Identification and health risk assessment of volatile and semi-volatile migrants along with chemical elements in food contact water-borne coating paper. Food Packag. Shelf Life 2024, 45, 101337. [Google Scholar] [CrossRef]
- Aurela, B. Migration of Substances from Paper and Board Food Packaging Materials. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2001. Available online: https://core.ac.uk/download/pdf/14916631.pdf (accessed on 28 November 2024).
- Knight, W.R. Recent Advances in Waterborne Acrylic Nanocomposite Paints and Coatings. Ph.D. Thesis, Lehigh University, Bethlehem, PA, USA, 2021. Available online: https://preserve.lib.lehigh.edu/ (accessed on 4 December 2024).
- Lestido-Cardama, A.; Rodríguez Bernaldo De Quirós, A.; Bustos, J.; Lomo, M.L.; Losada, P.P.; Sendón, R. Estimation of Dietary Exposure to Contaminants Transferred from the Packaging in Fatty Dry Foods Based on Cereals. Foods 2020, 9, 1038. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, Q.; Xie, C.; Liu, Y.; Zhong, H.; Gu, W.; McClements, D.J.; Ma, D. Screening and safety assessment of migrating substances released from biodegradable packaging materials into milk. Food Control 2024, 166, 110755. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerín, C. UPLC–ESI-Q-TOF-MSE and GC–MS identification and quantification of non-intentionally added substances coming from biodegradable food packaging. Anal. Bioanal. Chem. 2015, 407, 6781–6790. [Google Scholar] [CrossRef]
- Choi, J.O.; Jitsunari, F.; Asakawa, F.; Sun Lee, D. Migration of styrene monomer, dimers and trimers from polystyrene to food simulants. Food Addit. Contam. 2005, 22, 693–699. [Google Scholar] [CrossRef]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Chromatographic pattern in recycled high-impact polystyrene (HIPS)—Occurrence of low molecular weight compounds during the life cycle. Polym. Degrad. Stab. 2010, 95, 172–186. [Google Scholar] [CrossRef]
- Skjevrak, I.; Brede, C.; Steffensen, I.; Mikalsen, A.; Alexander, J.; Fjeldal, P.; Herikstad, H. Non-targeted multi-component analytical surveillance of plastic food contact materials: Identification of substances not included in EU positive lists and their risk assessment. Food Addit. Contam. 2005, 22, 1012–1022. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Beg, M.A. Structural characterization of potential endocrine disrupting activity of alternate plasticizers di-(2-ethylhexyl) adipate (DEHA), acetyl tributyl citrate (ATBC) and 2, 2, 4-trimethyl 1, 3-pentanediol diisobutyrate (TPIB) with human sex hormone-binding globulin. Reprod. Toxicol. 2019, 83, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lestido-Cardama, A.; Vázquez-Loureiro, P.; Sendón, R.; Bustos, J.; Santillana, M.I.; Losada, P.P.; De Quirós, A.R.B. Characterization of Polyester Coatings Intended for Food Contact by Different Analytical Techniques and Migration Testing by LC-MSn. Polymers 2022, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- CFR. Indirect Food Additives: Adhesives and Components of Coatings- Code of Federal Regulations Title 21- Part 175-Subpart B. (s. f.). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=175.105 (accessed on 3 December 2024).
- Rahim, A.A.; Saad, B.; Osman, H.; Hashim, N.; Yahya, S.; Talib, K.M. Simultaneous determination of diethylene glycol, diethylene glycol monoethyl ether, coumarin and caffeine in food items by gas chromatography. Food Chem. 2011, 126, 1412–1416. [Google Scholar] [CrossRef]
- Binderup, M.L.; Pedersen, G.A.; Vinggaard, A.M.; Rasmussen, E.S.; Rosenquist, H.; Cederberg, T. Toxicity testing and chemical analyses of recycled fibre-based paper for food contact. Food Addit. Contam. 2002, 19, 13–28. [Google Scholar] [CrossRef]
- Bush, J.; Gilbert, J.; Goenaga, X. Spectra for the Identification of Monomers in Food Packaging; Springer Science & Business Media: Dordrcht, The Netherlands, 1993; Volume 14515. [Google Scholar]
- Aker, A.; Caron-Beaudoin, É.; Ayotte, P.; Ricard, S.; Gilbert, V.; Avard, E.; Lemire, M. Non-persistent exposures from plasticizers or plastic constituents in remote Arctic communities: A case for further research. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 400–407. [Google Scholar] [CrossRef]
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—a review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Guart, A.; Wagner, M.; Mezquida, A.; Lacorte, S.; Oehlmann, J.; Borrell, A. Migration of plasticisers from Tritan™ and polycarbonate bottles and toxicological evaluation. Food Chem. 2013, 141, 373–380. [Google Scholar] [CrossRef]
- Dandan Doganci, M.; Doganci, E.; Balci, H.; Cetin, M. Antibacterial and cytotoxic performance of methenamine-based poly (lactic acid)/poly (ethylene glycol)(PLA/PEG) composite films. J. Appl. Polym. Sci. 2024, 141, e55412. [Google Scholar] [CrossRef]
- Astill, B.; Terhaar, C.; Fassett, D. The toxicology and fate of 2,2,4-trimethyl-1,3-pentanediol diisobutyrate. Toxicol. Appl. Pharmacol. 1972, 22, 387–399. [Google Scholar] [CrossRef]
- Bentz, K.C. Synthesis and Characterization of Linear and Branched Polylactic Acid For Use in Food Packaging Applications. Master’s Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, 2011. Available online: https://www.proquest.com/dissertations-theses/synthesis-characterization-linear-branched/docview/2838330030/se-2 (accessed on 6 January 2025).
- Kleinschnitz, M.; Schreier, P. Identification and semi-quantitative determination of a migration contaminant from beverage carton packages into mineral water by on-line solid phase extraction gas chromatography-mass spectrometry (SPE-GC-MS). Chromatographia 1998, 48, 581–583. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Z.; Sun, X.; Ma, X.; Song, J.; Sui, H.; Debrah, A.A. Non-targeted analysis and risk assessment of non-volatile compounds in polyamide food contact materials. Food Chem. 2020, 345, 128625. [Google Scholar] [CrossRef]
- Courgneau, C.; Domenek, S.; Guinault, A.; Avérous, L.; Ducruet, V. Analysis of the structure-properties relationships of different multiphase systems based on plasticized poly (lactic acid). J. Polym. Environ. 2011, 19, 362–371. [Google Scholar] [CrossRef]
- Singh, S.; Pereira, J.; Guerreiro, P.; Selbourne, C.; Paula, C.; Cunha, A.; Sousa, C.; Poças, F. Safety profile of ZnO active packaging PBAT based biomaterial for food packaging. First tier evaluation. Food Control 2024, 161, 110389. [Google Scholar] [CrossRef]
- Sapozhnikova, Y. Non-targeted screening of chemicals migrating from paper-based food packaging by GC-Orbitrap mass spectrometry. Talanta 2021, 226, 122120. [Google Scholar] [CrossRef]
- Cui, H.; Gao, W.; Lin, Y.; Zhang, J.; Yin, R.; Xiang, Z.; Zhang, S.; Zhou, S.; Chen, W.; Cai, K. Development of microwave-assisted extraction and dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry for the determination of organic additives in biodegradable mulch films. Microchem. J. 2021, 160, 105722. [Google Scholar] [CrossRef]
- Trumbo, D.L.; Giddings, C.L.; Wilson, L.R.A. Terpene–anhydride resins as coating materials. J. Appl. Polym. Sci. 1995, 58, 69–76. [Google Scholar] [CrossRef]
- Gavril, G.L.; Wrona, M.; Bertella, A.; Świeca, M.; Râpă, M.; Salafranca, J.; Nerín, C. Influence of medicinal and aromatic plants into risk assessment of a new bioactive packaging based on polylactic acid (PLA). Food Chem. Toxicol. 2019, 132, 110662. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Compounds responsible for off-odors in several samples composed by polypropylene, polyethylene, paper and cardboard used as food packaging materials. Food Chem. 2020, 309, 125792. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Ghosh, T.; Purohit, S.D.; Prasannavenkadesan, V.; Rhim, J. Lignin as a sustainable and functional material for active food packaging applications: A review. J. Clean. Prod. 2024, 469, 143151. [Google Scholar] [CrossRef]
- Rai, S.; Dutta, P.K.; Mehrotra, G.K. Natural antioxidant and antimicrobial agents from agrowastes: An emergent need to food packaging. Waste Biomass Valorization 2020, 11, 1905–1916. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Su, Q.Z.; Mercado, D.; Nerín, C. Migration of volatile substances from recycled high density polyethylene to milk products. Food Packag. Shelf Life 2023, 35, 101020. [Google Scholar] [CrossRef]
- Gratia, A.; Merlet, D.; Ducruet, V.; Lyathaud, C. A comprehensive NMR methodology to assess the composition of biobased and biodegradable polymers in contact with food. Anal. Chim. Acta 2015, 853, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Park, S.; Volpe, S.; Torrieri, E.; Masi, P. Active packaging based on PLA and chitosan-caseinate enriched rosemary essential oil coating for fresh minced chicken breast application. Food Packag. Shelf Life 2021, 29, 100708. [Google Scholar] [CrossRef]
- Iskandar, A.F.A.; Santoso, U.; Supriyadi, S. Chemical characteristics of waru leaf (Hibiscus tiliaceus) as food packaging material. Indones. Food Nutr. Prog. 2023, 20, 72–78. [Google Scholar] [CrossRef]
- Sustaita-Rodriguez, A.; Vega-Rios, A.; Bugarin, A.; Ramos-Sanchez, V.H.; Camacho-Davila, A.A.; Rocha-Gutierrez, B.; Chavez-Flores, D. Chemoenzymatic epoxidation of highly unsaturated fatty acid methyl ester and its application as poly (lactic acid) plasticizer. ACS Sustain. Chem. Eng. 2021, 9, 17016–17024. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, H.J.; Komolprasert, V. Analytical procedure for quantifying five compounds suspected as possible contaminants in recycled paper/paperboard for food packaging. J. Agric. Food Chem. 2000, 48, 5856–5859. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Alberto Lopes, J.; Hoekstra, E.; Emons, H. Development and validation of a multi-analyte GC-MS method for the determination of 84 substances from plastic food contact materials. Anal. Bioanal. Chem. 2020, 412, 5419–5434. [Google Scholar] [CrossRef]
- Aznar, M.; Domeño, C.; Nerín, C.; Bosetti, O. Set-off of non volatile compounds from printing inks in food packaging materials and the role of lacquers to avoid migration. Dye. Pigment. 2014, 114, 85–92. [Google Scholar] [CrossRef]
- Guan, W.; He, Y.; McClements, D.J.; Chen, J.; Ma, D. Risk assessment of migrants released from multilayer packaging materials: Direct immersion-solid-phase microextraction coupled to gas chromatography-mass spectrometry. Food Packag. Shelf Life 2024, 46, 101407. [Google Scholar] [CrossRef]
- Miralles, P.; Yusà, V.; Pineda, A.; Coscollà, C. A fast and automated strategy for the identification and risk assessment of unknown substances (IAS/NIAS) in plastic food contact materials by GC-Q-Orbitrap HRMS: Recycled LDPE as a proof-of-concept. Toxics 2021, 9, 283. [Google Scholar] [CrossRef]
- Bhanot, V.; Gupta, S.; Panwar, J. Phylloplane fungus Curvularia dactyloctenicola VJP08 effectively degrades commercially available PS product. J. Environ. Manag. 2024, 351, 119920. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.J.; Hernando, M.D.; Uclés, S.; Rajski, L.; Cimmino, S.; Fernández-Alba, A.R. Identification of non-intentionally added substances in food packaging nano films by gas and liquid chromatography coupled to orbitrap mass spectrometry. Talanta 2017, 172, 68–77. [Google Scholar] [CrossRef]
- Fasano, E.; Bono-Blay, F.; Cirillo, T.; Montuori, P.; Lacorte, S. Migration of phthalates, alkylphenols, bisphenol A and di (2-ethylhexyl) adipate from food packaging. Food Control 2012, 27, 132–138. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Letcher, R.J.; Zhang, Y.; Jian, K.; Zhang, J.; Su, G. A review on organophosphate Ester (OPE) flame retardants and plasticizers in foodstuffs: Levels, distribution, human dietary exposure, and future directions. Environ. Int. 2019, 127, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhou, R.; Yin, Y.; Liu, Y.; Zhao, N.; Li, H.; Zhang, A.; Li, X.; Fu, J. Occurrence of Organophosphate Esters in Food and Food Contact Materials and Related Human Exposure Risks. J. Agric. Food Chem. 2025, 73, 4455–4465. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers derived from biomass resources: A short review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef]
- Sanchis, Y.; Yusà, V.; Coscollà, C. Analytical strategies for organic food packaging contaminants. J. Chromatogr. A 2017, 1490, 22–46. [Google Scholar] [CrossRef]
- Gelbke, H.; Banton, M.; Block, C.; Dawkins, G.; Eisert, R.; Leibold, E.; Pemberton, M.; Puijk, I.M.; Sakoda, A.; Yasukawa, A. Risk assessment for migration of styrene oligomers into food from polystyrene food containers. Food Chem. Toxicol. 2018, 124, 151–167. [Google Scholar] [CrossRef]
- Nieves Calvo, S. Estudios de Migración en Biomateriales Para su Uso a Alta Temperatura en Contacto con Alimentos. Bachelor’s Thesis, University of Zaragoza, Zaragoza, Spain, 2020. Available online: https://zaguan.unizar.es/record/96429?ln=es# (accessed on 3 January 2025).
- Yang, J.; Li, Y.; Wang, Y.; Ruan, J.; Zhang, J.; Sun, C. Recent advances in analysis of phthalate esters in foods. TrAC Trends Anal. Chem. 2015, 72, 10–26. [Google Scholar] [CrossRef]
- Ibarra, V.G.; Sendón, R.; Bustos, J.; Losada, P.P.; De Quirós, A.R.B. Estimates of dietary exposure of Spanish population to packaging contaminants from cereal based foods contained in plastic materials. Food Chem. Toxicol. 2019, 128, 180–192. [Google Scholar] [CrossRef]
- Kirchkeszner, C.; Petrovics, N.; Nyiri, Z.; Szabó, B.S.; Eke, Z. Role of gas chromatography–single quadrupole mass spectrometry in the identification of compounds migrating from polypropylene-based food contact plastics. Microchem. J. 2022, 181, 107772. [Google Scholar] [CrossRef]
- Osorio, J.; Dreolin, N.; Aznar, M.; Nerín, C.; Hancock, P. Determination of volatile non intentionally added substances coming from a starch-based biopolymer intended for food contact by different gas chromatography-mass spectrometry approaches. J. Chromatogr. A 2019, 1599, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.L.; Honkalampi-Hämäläinen, U.; Weber, A.; Andersson, M.A.; Bertaud, F.; Castle, L.; Dahlman, O.; Hakulinen, P.; Hoornstra, D.; Lhuguenot, J.-C.; et al. The BIOSAFEPAPER project for in vitro toxicity assessments: Preparation, detailed chemical characterisation and testing of extracts from paper and board samples. Food Chem. Toxicol. 2008, 46, 2498–2509. [Google Scholar] [CrossRef] [PubMed]
- Hurd, Maycee. Thermal Oxidative Degradation of Polystyrene Plastics Used for Food Packaging During Incomplete Waste Incineration. Ph.D. Thesis, University of New Mexico, Albuquerque, NM, USA, 2024. Available online: https://digitalrepository.unm.edu/ce_etds/336 (accessed on 5 January 2025).
- Rung, C.; Welle, F.; Gruner, A.; Springer, A.; Steinmetz, Z.; Munoz, K. Identification and evaluation of (non-) intentionally added substances in post-consumer recyclates and their toxicological classification. Recycling 2023, 8, 24. [Google Scholar] [CrossRef]
- Gigli, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Finelli, L.; Munari, A.; Dalla Rosa, M. Fully aliphatic copolyesters based on poly (butylene 1, 4-cyclohexanedicarboxylate) with promising mechanical and barrier properties for food packaging applications. Ind. Eng. Chem. Res. 2013, 52, 12876–12886. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Leanza, M.; Rapisarda, M. Investigations into the characterization, degradation, and applications of biodegradable polymers by mass spectrometry. Mass Spectrom. Rev. 2023, 1–42. [Google Scholar] [CrossRef]
- Yoon, W.J.; Oh, K.S.; Koo, J.M.; Kim, J.R.; Lee, K.J.; Im, S.S. Advanced Polymerization and Properties of Biobased High Tg polyester of Isosorbide and 1,4-Cyclohexanedicarboxylic Acid through in Situ Acetylation. Macromolecules 2013, 46, 2930–2940. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Störmer, Á.; Franz, R. Dialkylketones in Paperboard Food Contact Materials—Method of Analysis in Fatty Foods and Comparative Migration into Liquid Simulants Versus Foodstuffs. Molecules 2020, 25, 915. [Google Scholar] [CrossRef]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: http://data.europa.eu/eli/reg/2006/1907/oj (accessed on 8 February 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).