Abstract
Anti-icing glass is particularly important for applications where ice formation can pose safety risks or impair functionality. The challenge of anti-icing modification for glass lies in maintaining water repellency while addressing the issue of transparency and durability. In this work, leveraging the robustness and wear resistance of inorganic/organic composite materials, a highly transparent coating, with strong adhesive properties to glass substrates and repellency to liquids has been developed. Briefly, 3-glycidoxypropyl polyhedral oligomeric silsesquioxane (GPOSS) is employed as a precursor to fabricate a high-strength, high-transparency coating through modification with acrylic acid and perfluorooctyl acrylate. The inorganic component imparts strength and wear resistance to the coating, while the organic component provides hydrophobic and near oleophobic features. Furthermore, a custom-built mechanical test instrument evaluated the absolute value of the de-icing shear force. The results reveal that at −20 °C, the fluorinated modified coating only exhibit a minimum de-icing pressure of 40.3 kPa, which is 75% lower than the unmodified glass substrate. As-prepared coating exhibits a transmittance of up to 99% and can endure a high-pressure water impact of 30 kPa for 1 min without cracking. Compared to existing anti-icing coating methods, the core innovation of the fluorinated GPOSS-based coating developed in this study lies in its inorganic/organic composite structure, which simultaneously achieves high transparency, mechanical durability, and enhanced anti-icing performance.
1. Introduction
In regions characterized by cold climates, the prevalence of freezing phenomenon and the subsequent adhesion of ice to substrate surfaces pose significant challenges. For instance, ice formation can induce instability and result in frost and ice accumulation on car windshield [1,2,3,4]. Existing passive anti-icing strategies, such as superhydrophobic (SHP) coatings and smooth liquid-infused porous surfaces (SLIPS), face critical limitations: SHP coatings suffer from reduced transparency and mechanical fragility due to micro/nano structures, while SLIPS lose lubricant stability after repeated de-icing cycles [1,2,3,4] and smooth liquid-infused porous surfaces (SLIPS) [5,6,7,8]. Although liquid-like surfaces (LLSs) offer low contact angle hysteresis, their reliance on reactive functional groups for glass adhesion often requires energy-intensive surface treatments (e.g., laser/chemical etching), raising environmental concerns [9,10,11,12].
Inorganic–organic composite coatings show promise, combining mechanical robustness (inorganic components) with flexibility (polymer matrices). For example, polyhedral oligomeric silsesquioxane (POSS)-based materials exhibit high hardness and transparency, but traditional perfluorocarboxylic acid (PFCA) modifiers are costly and environmentally problematic [13,14,15,16]. Existing coatings struggle to balance high transparency, mechanical durability, and anti-icing performance simultaneously [17,18]. The challenge lies in developing a scalable, low-toxicity coating that achieves strong glass adhesion, ultra-low ice shear force, and resistance to wear/impact without compromising optical clarity.
This study addresses this gap by introducing a fluorinated GPOSS-based coating, leveraging inorganic/organic hybrid architecture to enhance mechanical strength, while acrylic acid and perfluorooctyl acrylate impart hydrophobicity. The goal is to demonstrate a durable, transparent coating with superior anti-icing properties, overcoming the limitations of conventional SHP, SLIPS, and LLS systems.
2. Materials and Methods
2.1. Materials
3-methacryloxypropyl polyhedral oligomeric silsesquioxane (GPOSS) was purchased from Guangzhou Xin Technology Co., Ltd., Guangzhou, China. Acrylic acid (99%), 1H, 1H, 2H, 2H-perfluorodecyl acrylate (HDFDA) (97%), a mixture of hexafluoroantimonate and triarylsulfonium hexafluoroantimonate salts (UVI-6976, 50 wt.% in propylene carbonate), hexadecane (99%), diiodomethane (99%), n-hexane (98.5%), n-decane (99.0%), n-octane (99.0%), dodecane (99.0%), chloroform (99.8%), 2-butanone (≥99.0%), acetonitrile (99.98%), CDCl3 (99.8% D), and tetrabutylammonium bromide (TBAB) (≥98%) were purchased from Aladdin Industrial Co., Ltd, Shanghai, China. Friction cloth was acquired from Testfabrics and used as received without further treatment. Glass slides measuring 2.3 × 10 cm with a thickness of 4 mm, microscope slides (7.62 × 2.54 cm), acetone for cleaning, and drying equipment were utilized.
2.2. Preparation of the Fluorine-Modified Glass Coating
To synthesize the fluorine-containing wear-resistant coating GAF5, GPOSS (5 g, 3.74 mmol), acrylic acid (0.0673 g, 9.34 × 10−1 mmol), and TBAB (50 mg) were refluxed in 20 mL of acetonitrile at 85 °C for 6 h. Subsequently, the temperature was lowered to 75 °C and HDFDA (0.4840 g, 0.934 mmol) along with ammonium thiosulfate (50 mg) were added. The mixture was then heated and refluxing for an additional 10 h. After cooling to room temperature, the solvent was removed by rotary evaporation. The resultant residue was subsequently dissolved in 30 mL of chloroform. The solution was then washed twice with 50 mL of 0.10 M NaHCO3 solution, followed by two washes with 50 mL of deionized water each. Anhydrous magnesium sulfate was added to the washed chloroform solution, and the mixture was subsequently centrifuged and filtered. The solvent was concentrated to a viscous state by rotary evaporation, and subsequently dissolved in 10 mL of acetonitrile and filtered. The resulting pale yellow viscous product, weighing 4.0231 g, was dried under vacuum and stored in 50 mL of tert-butyl ketone. To investigate the influence of varying fluorine content on the hydrophobic modification of GPOSS, a series of GAF samples with different fluorine contents were prepared by adjusting the stoichiometric ratio of GPOSS and HDFDA. Specifically, the molar ratio of GPOSS to HDFDA was systematically increased from 1:0.05 to 1:0.25 in increments of 0.05. The corresponding samples were designated as GAF1 through GAF5. The composition of the fluorine-containing coating is detailed in Table S1. A volume of 3 μL of UVI-6976 was added to 1 mL of butanone solution, and the mixed solution was drop-coated onto a glass substrate. The coated sample was dried at 75 °C for 10 h in an oven and subsequently cured in a curing chamber for 120 s.
The molecular weight of GPOSS is 1337.88, and it is an oily transparent liquid with a viscosity of 4~6 Pa s (25 C). The thickness of the coating is controlled by the solution solubility, and the thickness is increased to control the thickness by increasing the solution solubility. The synthesis process involves combining glycidoxypropyltrimethoxysilane (GPOSS) and heptadecafluoroacrylate via acrylic acid (AA) monomer intermediate to prepare a fluorine-containing copolymer denoted as GAF. The synthesis route of GAF is depicted in Figure 1a,b (see Scheme S1 in Supporting Information). Detailed procedures are provided in Experimental Section.
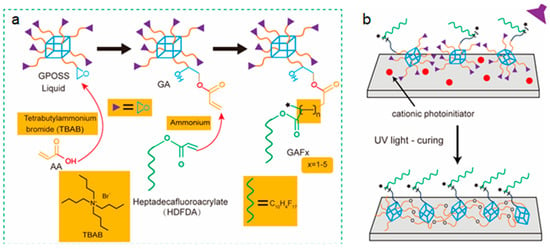
Figure 1.
(a) The process diagram for preparing GAF (fluorinated modified GPOSS); (b) schematic diagram of GAF coating curing.
Ice shearing results demonstrate that the GAF coating exhibits excellent adhesive and cohesive properties, withstanding a pressure of at least 40.3 kPa. Notably, the coating exhibits a low contact angle hysteresis (CAH) value for both water and oil, along with exceptional wear and impact resistance. Additionally, the GAF coating achieves a transmittance exceeding 98%. Furthermore, even after 500 cycles of friction testing, the coating retains its excellent hydrophobic properties, showing outstanding durability.
2.3. Characterization
The morphology and structure of the coating surface were examined using atomic force microscopy (AFM, Dimension ICON). The cross-sectional characteristics of the prepared coating were analyzed using field emission scanning electron microscopy (FE-SEM, Hitachi SU-8010, Hitachi Manufacyuring Co., Ltd., Tokyo, Japan), with samples sputter-coated with gold prior to examination. The surface chemical composition of the samples was characterized by X-ray photoelectron spectroscopy (XPS) using an ESCALAB 250Xi instrument (Thermo Fisher Scientific Inc., Waltham, MA, USA). The results calibrated with respect to the adventitious carbon peak (C1s) set at 284.80 eV. The static contact angles (CAs) and CAHs of liquids were measured using a contact angle measuring instrument (JC2000D, Shanghai Zhongchen Digital Technic Apparatus Co., Ltd., Shanghai, China) at room temperature. For all measurements, the volume of each droplet was consistently 2 μL. CAH values were determined by gradually increasing and then decreasing the droplet volume with a syringe needle while imaging the droplet’s movement. The difference between the advancing contact angle (θACA) and receding contact angles (θRCA) was measured. Three replicate measurements were conducted for both CAs and CAHs. UV–vis spectra were acquired using a spectrophotometer (Lambda950, PerkinElmer, Bucks, UK). The modulus and hardness of the prepared samples were measured using nanoindentation (T1750, Hysitron, Eden Prairie, MN, USA), which involved monitoring the force exerted on a diamond tip with a defined shape as it was pressed into the film and subsequently retracted from the sample at the maximum force. Fourier-transform infrared spectroscopy (FTIR) was performed in attenuated total reflection (ATR) mode using a Nicolet iS 5 instrument (Thermo Fisher, USA). The 1H NMR spectra of the products and reactants were acquired using a nuclear magnetic resonance spectrometer (Bruker Avance III HD 400 MHz, Bruker Corporation, Karlsruhe, Germany). The decomposition temperature of the cured GAF5 was evaluated using differential scanning calorimetry (DSC 25, TA Instruments, New Castke, DE, USA) and thermogravimetric analysis (TGA, STA449-F3, Netzsch, Selb, Germany). The thickness of the coating was measured using a Yamaid desktop digital thickness gauge (Model CD24778, supplied by Yueqing Huaxia Instrument Co., Ltd., Yueqing, China).
Additionally, we conducted wear resistance tests, as shown in Figure 2a, and water impact tests, as illustrated in Figure 2b. The wear resistance and solvent resistance of the sample surfaces were assessed using a wear resistance tester (model ZL7013, provided by Dazhong Instrument Co., Ltd., Beijing, China). The impact resistance of the coating samples was evaluated using a programmable rain shower test chamber (model HT-IP9K-1000L, Dongguan Huitai Machine Co., Ltd., Donguan, China.) equipped with a 1 mm nozzle diameter and adjustable pressure settings for high-pressure water impact. Furthermore, we developed a deicing apparatus that enables controlled pulling of a glass sheet attached to an ice block to measure shear force, as depicted in Figure 2c. By precisely quantifying the absolute value of the deicing shear force, comparative evaluations can be performed to gain a better understanding of its performance.
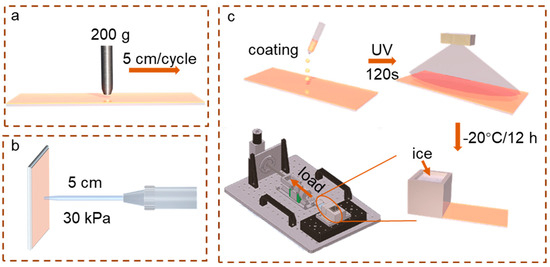
Figure 2.
(a) Wear resistance test; (b) water impact test; (c) deicing shear force test.
3. Results and Discussion
Component Characterization
Figure 3a illustrates the FTIR spectra of GPOSS, its intermediate product GA (reaction product of GPOSS with acrylic acid), and the final product GAF5 (highest concentration of fluorinated modified GPOSS). The GPOSS modified with acrylic acid is designated as GA. In comparison to GPOSS, the FTIR spectrum of GA shows new characteristic absorption peaks at 3471 cm−1 and 1740 cm−1, corresponding to the O-H and C=O functional groups, respectively. This indicates that the epoxy groups in GPOSS have undergone ring-opening reactions with the carboxyl group of acrylic acid. When comparing GAF5 with GA, no significant differences in the characteristic peaks are observed. The C-F, C-F2, and C-F3 characteristic absorption peaks around 670 cm−1, 1230 cm−1, and 1200 cm−1 are obscured by the epoxy characteristic peaks of GPOSS and other overlapping peaks, making them undetectable in the infrared spectrum. Additionally, the comparison of the coating before and after curing is shown in Figure 3a. At 3420 cm−1, 1740 cm−1, and 1058 cm−1, the characteristic absorption peaks of O-H, C=O and epoxy groups of GAF5 were observed, respectively. After curing, the characteristic absorption peak of O-H of GAF5 increased, and the characteristic absorption peak of epoxy group decreased. To further verify the fluorine modification, XPS characterization was conducted, as illustrated in Figure 3b. Furthermore, the synthesis of GAF5 was confirmed by nuclear magnetic resonance (1H NMR), as presented in Figure S1.
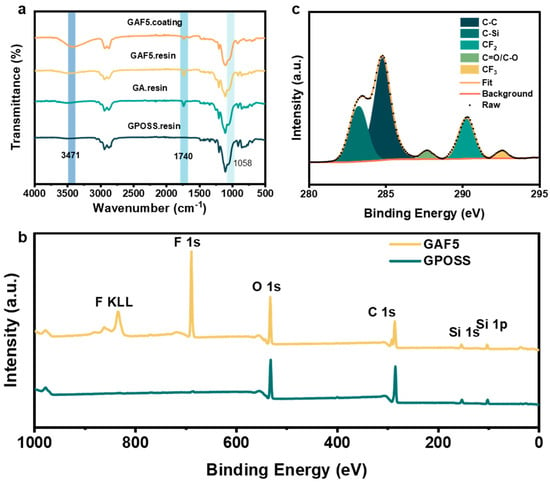
Figure 3.
(a) The FT-IR spectra of GPOSS resin, GA resin, GAF5 resin, GAF5 coating; (b) X-ray Photoelectron Spectroscopy (XPS) spectra of GPOSS and GAF5; (c) the C 1s XPS peak—fitting results of GAF5.
The cured GPOSS coating show strong characteristic elemental peaks for O 1s and C 1s at approximately 532.4 eV and 284.8 eV, respectively. Additionally, the observed peaks at 152.4 eV and 103.6 eV are attribute to Si 2s and Si 2p, respectively. Notably, the cured GAF5 coating displays newly emerged elemental peaks at 689.4 eV and 835.4 eV, corresponding to F 1s signal and F KLL signals. These XPS results confirm the successful synthesis of GAF5.
Based on the analysis of Figure 3c, the C 1s spectrum of GAF5 can be deconvoluted into five distinct peaks, with binding energies approximately at 283.21 eV, 284.76 eV, 287.70 eV, 290.24 eV, and 292.56 eV. These peaks are, respectively, attributed to C-Si, C-C, C-O/C=O, CF2, and CF3 species. The observed spectral features provide compelling evidence that, following the chemical reaction, 1H, 1H, 2H, 2H-perfluorodecyl acrylate successfully reacted with GPOSS.
The corresponding UV-vis spectrums are presented in Figure 4a. It can be observed that within the wavelength range of 400–800 nm, there were no significant differences in the transmittance of these coatings compared to that of the bare glass. Moreover, the transmittance of the coating itself, independent of the glass substrate, was characterized. The obtained results are compiled in Figure 4a. Furthermore, Figure 4a shows the variation in transmittance values at a wavelength of 500 nm with respect to the thickness of the GAF5 coating. The transmittance values do not increase with the coating thickness but remain consistently high at 98.4 ± 0.6%, even at a thickness of 100 μm. This result again suggests the uniform distribution of fluorinated GPOSS within the GAF5 coating. From the spectrum, both GPOSS and GAF1-5 coatings exhibit a transmittance of nearly 99% within the 400–800 nm range. Additionally, the fluorine content in GAF does not significantly influence their transmittance. In the subsequent characterization, measurements of the static contact angle of water on GPOSS and GAF1-5 coatings were conducted. The results indicate that the static contact angle of water on the unmodified GPOSS coating was 69°. After fluorination treatment, there was a significant increase in the static contact angle of water, from 69° to 109° for GAF1. As the fluorine content increased, the static contact angle of the coating with water also increased, ultimately reaching 120° for GAF5, as shown in Figure 4c. To further investigate the hydrophobic performance, the surface energy of as-prepared coating was also calculated. The hydrophobic performance of a coating is closely correlated with its surface energy. To further validate the hydrophobic performance of the coating, the Zisman critical surface tension method [18] was employed. Specifically, the static contact angle of a series of normal alkanes (n-hexane, n-octane, decane, dodecane, and hexadecane) on the coating surface were measured. After converting the obtained values into cosine values, a linear regression analysis was performed using the known surface tension of the corresponding test liquids. The surface tension of the coating surface values was defined as the surface tension value at the intersection of the extrapolated line with cos(θ) = 1. The fitting results are shown in Figure 4d. The results indicate that GAF5 exhibited the lowest critical surface tension, measured at 6.10 mN/m. These findings also correlate with the static contact angle measurements of the coating with water, demonstrating that coatings with lower critical surface tension exhibit larger static contact angles with water.
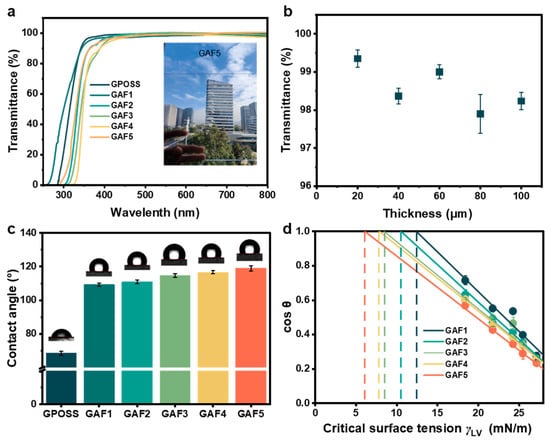
Figure 4.
(a) The UV–vis spectra of GPOSS and GAF1-5 coatings (GPOSS modified by fluorination at different concentrations), highlighting the variations in the coating curves within the 400–600 nm range (illustrated by a GAF5 coated glass measuring 24 × 18 cm); (b) the relationship between the transmittance variation of GAF5 coating at 500 nm and its thickness. (c) Static contact angle mapping of water droplets on GPOSS and GAF1-5 coated surfaces; (d) critical surface tension measurements for the GAF1-5 coating.
The results from the Atomic Force Microscopy (AFM) study further elucidate the structural morphology of the prepared coatings. The AFM images (Figure 5a–f) show minimal discernible differences between the GPOSS and GAF1-5 coatings. The similarity in the surface morphology across different samples suggests that the AFM characterization lacks sufficient sensitivity in identifying the type and concentration of fluorine side chains on the surface of the modified coatings. Therefore, the arrangement of the GPOSS units determines the appearance of the surface image. The Scanning Electron Microscopy (SEM) images in Figure 5g–i reveal the cross-sectional morphology of the GPOSS, GA, and GAF5 coatings. These images indicate that all three coatings exhibit a consistent thickness of approximately 20 μm, suggesting no significant correlation between coating thickness and fluorine content. Additionally, we examined the thermodynamic stability of the coating, as shown in Figure S2.
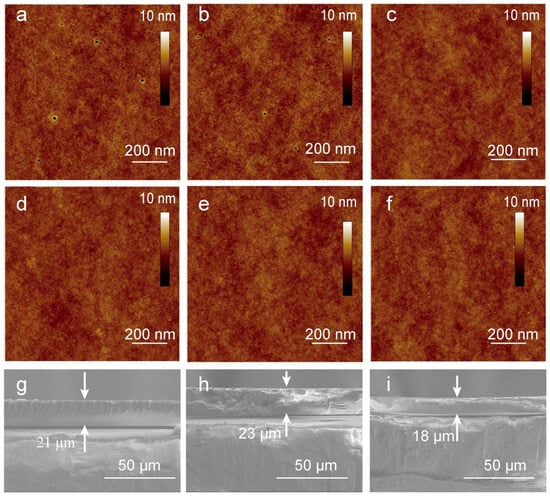
Figure 5.
(a–f) Atomic force microscopy images of GPOSS and GAF1-5 (GPOSS modified by fluorination at different concentrations); (g–i) scanning electron microscopy images of the cross-sections of GPOSS, GA (reaction product of GPOSS with acrylic acid), and GAF5 (highest concentration of fluorinated modified GPOSS).
As illustrated in Figure 6, the wear hardness of two types of coatings was characterized through nanoindentation tests. Figure 6a shows the load, hold, and release curves for both GPOSS and GFAF5 coatings. Hardness is determined through the load–displacement curve, where the slope of the curve (Pmax/hmax) was determined as the hardness. The hardness (H) and elastic modulus (E) of the GPOSS coating are 0.6567 GPa and 3.7985 GPa, respectively, while those of the GAF5 coating are 0.7179 GPa and 3.8490 GPa, as shown in Figure 6b. A quantitative analysis using the Oliver-Pharr method reveals that the H/E ratio for the GPOSS coating is 17.3%, with an elastic recovery (we) value of 82.7%, whereas for the GAF5 coating, the H/E ratio is 18.7%, with a we value of 81.3%. Corresponding calculation method is depicted in Supporting Information. The results indicate that the H/E and we values for both coatings are similar, thereby meeting the criteria for high wear resistance [6,19]. Considering that automotive windshields are frequently exposed to harsh conditions, the durability of glass coatings has become a critical concern. In this study, we conducted hydro-pressure testing on GAF5 coatings to simulate the environmental conditions encountered during car washes. The test equipment is shown in Figure S3. To simulate these challenging environments and assess the service life of the coating, an impact resistance test was conducted using a spray test with water pressure of 30 kPa for 60 s (Movie S3). Notably, during the initial 60 s impact cycle, the glass surface coating remained intact. However, when the impact duration extended to 120 s (Movie S4), the coating eventually fractured, demonstrating its excellent impact resistance, as shown in Figure 6c. To further evaluate the abrasion resistance of the coating, a soft abrasive cloth was used as the friction medium, applying a load of 200 g for water immersion wear resistance testing. The GAF5 coating endured 500 cycles of friction (Movies S5 and S6). Remarkably, even after this rigorous testing, the GAF5 coating surface showed no signs of wear, as shown in Figure 6d. The contact angle was 120° before the abrasion test, 113° for the abrasion test, and the contact angle after the abrasion test decreased, but did not change much, as shown in Figure S4.
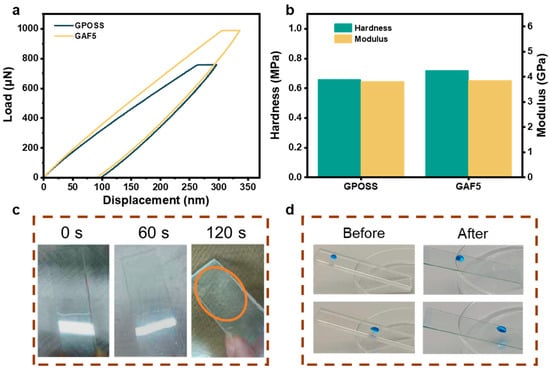
Figure 6.
The results of the nanoindentation testing for the sample preparation. (a) Nanoindentation system—displacement curve; (b) hardness and modulus of GPOSS and GAF5 (highest concentration of fluorinated modified GPOSS). (c) The impact resistance performance of GAF5 coating; (d) the wear resistance test of GAF5 coating.
To evaluate the liquid repellency of the as-prepared coating, tests on the static contact angle, advancing angle, and receding angle of the sample with respect to water and other organic reagents were conducted. Samples include GPOSS and GAF1-5 (GPOSS modified by fluorination at different concentrations). Table 1 compiles the static contact angles of hexane, octane, decane, dodecane, and hexadecane. These static contact angle measurements were subsequently utilized to determine the critical surface tension of the coating. Concurrently, Table 2 compiles the advancing and receding contact angles of water, diiodomethane, and hexadecane on the surface of the as-prepared samples, along with the calculated contact angle hysteresis (expressed as CAHs = θACA − θRCA). Generally, a smaller contact angle hysteresis indicates superior liquid-repellent properties of the coating. The results show that the CAH value for the GPOSS coating without fluorine modification is 38°. After fluorine modification, the CAH value of the coating decreases significantly to a minimum value of 19°. For organic solvents, hexadecane droplets cannot coalesce on the unmodified GPOSS coating, resulting in the very low contact angle of 3.1°, as shown in Figure S5. After fluorine modification, the coating exhibits enhanced liquid repellency to hexadecane. Based on calculations, the CAH value of hexadecane on the as-prepared coating is only 11°.

Table 1.
The static contact angle CA of different liquids on various coatings.

Table 2.
Advancing contact angle (θACA), receding contact angle (θRCA), and contact angle hysteresis (CAH) of H2O, CH2I2, and C16H34 on various coatings.
A critical parameter for evaluating the anti-icing performance of a coating is the ability to remove ice from the coating surface under low shear force. To quantify this anti-icing effect, de-icing shear force data were collected using a specially designed evaluation instrument, as illustrated in Figure 7a. The working mechanism of this instrument is to convert the analog shear force generated by moving ice into a digital signal. However, the analog signals produced during de-icing are often too weak to be reliably detected, necessitating the use of a signal amplifier. The amplified electrical signal is received and processed by the data acquisition system. The de-icing data acquisition kit includes a motherboard, a signal amplifier, and a data acquisition device, as shown in Figure 7b. Specifically, the motherboard connects to the power supply and controls the motor, which can control the speed at which the ice is removed from the glass sample by adjusting the motor speed. As shown in Figure 7c, the physical de-icing mechanical assembly includes a load motor that transfers force to the fixed coating test sample via a transmission rod. The tension sensor is mounted on a load linear guide rail to ensure consistent force application on the test sample.
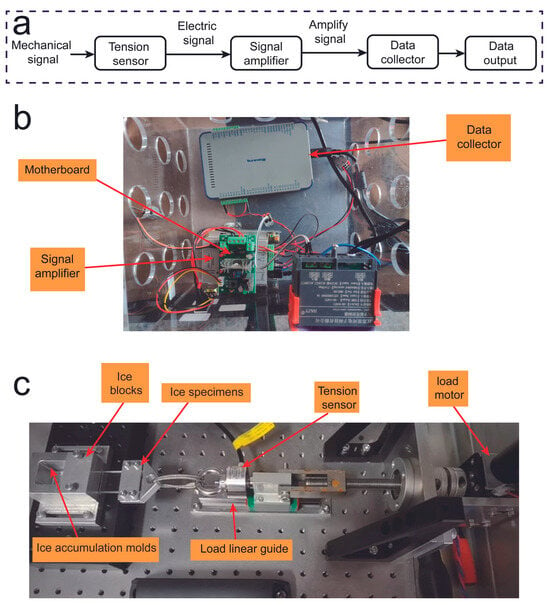
Figure 7.
(a) The signal transmission flowchart for de-icing tests; (b) the physical diagram of the de-icing data acquisition device; (c) the physical diagram of the de-icing mechanical device.
During the testing process (Movie S1), ice cubes were prepared using a cubic mold with a side length of 2.3 cm to ensure consistent contact area dimensions (Figure S6). Meanwhile, the tested glass surfaces were uniformly sized at 4 mm × 2.3 mm × 10 cm. The testing environment was maintained at −20 °C, and tests were conducted once a steady temperature was reached, as illustrated in Figure S7.
Low ice adhesion strength, freezing time delay, reduced freezing temperature and frost resistance are the criteria for evaluating anti-icing surfaces [20]. Therefore, the de-icing shear force test is carried out on the fluorinated modified glass. To obtain a more accurate de-icing shear force for the coating, three samples of the same type were tested. The de-icing shear force data from of each sample were aggregated, and an analysis of variance was performed on the collected data. Figure 8a presents a comparison of ice removal pressure and critical surface tension for GPOSS and GAF1-5 coatings, showing that the average de-icing pressure of the GAF5 coating is 45 kPa with a minimum of 40.3 kPa, significantly lower than the unmodified GPOSS coating, and revealing a close relationship between de-icing pressure and the coating’s critical surface tension where lower surface tension corresponds to reduced de-icing shear force. Furthermore, we investigated the impact of the drive rod speed on the de-icing efficiency and de-icing shear force. The de-icing shear forces were measured for slow speed (0.3 mm/s) and fast speed (1.4 mm/s). Figure 9b compares critical surface tension and shear force under slow and fast conditions, indicating that the de-icing shear forces of GAF5 coatings are significantly lower than that of GPOSS regardless of speed, with minimal differences between speeds, suggesting that the coating’s anti-icing performance is mainly determined by its chemical structure rather than drive rod speed. Figure 8c contrasts pressures of GPOSS and GAF1-5 under different de-icing frequencies, showing that the unmodified GPOSS has high de-icing pressure with little change across tests, while GAF series coatings, though showing a gradual increase in pressure with repeated de-icing, still maintain much lower values than GPOSS, demonstrating their durability in practical applications. Figure 8d illustrates the pressure variation of GAF5 under different de-icing operation frequencies, where the first five de-icing shear test data of GAF5 are below 48 kPa and then the de-icing shear force increases with the number of operations, reflecting that while repeated de-icing may slightly affect the coating’s surface, GAF5 still retains excellent anti-icing performance overall due to its chemical cross-linking structure. Taken together, these figures comprehensively validate the superior anti-icing performance and durability of the fluorinated GAF coatings, particularly GAF5, through quantitative data and comparative analyses under various conditions.
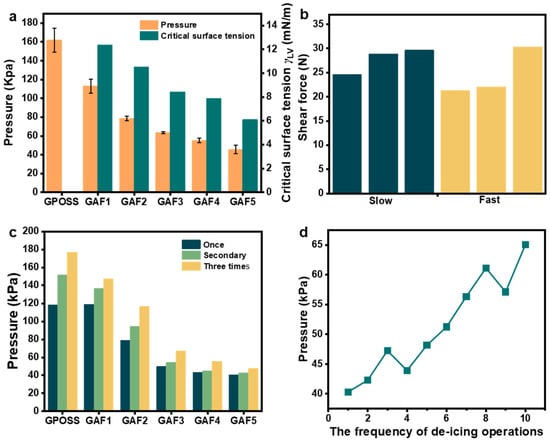
Figure 8.
(a) The ice removal pressure and critical surface tension chart for GPOSS and GAF1-5 (GPOSS modified by fluorination at different concentrations); (b) comparison of critical surface tension and shear force under slow and fast conditions; (c) the same experimental sample was tested for de-icing shear force three times in succession; (d) graph of the shear pressure of GAF5 coating and the number of de-icing.
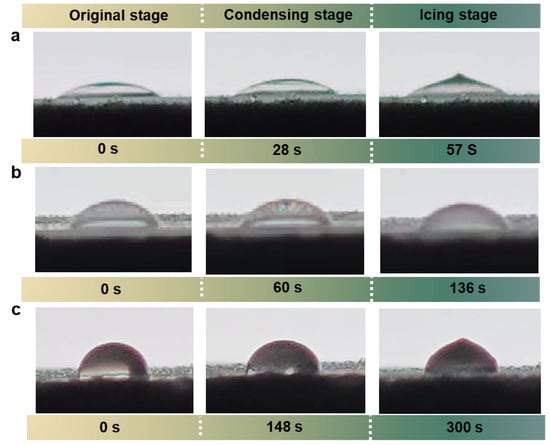
Figure 9.
Comparison of ice formation processes on (a) bare glass substrate, (b) GPOSS coating, (c) GAF5 (highest concentration of fluorinated modified GPOSS) coating under condensation conditions. The droplet size is 15 μL.
Figure 9 captures the ice formation dynamics on three distinct surfaces—bare glass (Figure 9a), GPOSS coated glass (Figure 9b), and GAF5 coated glass (Figure 9c)—under controlled condensation conditions at −20 °C, illustrating the critical role of fluorine modification in enhancing anti-icing performance. For the bare glass substrate (Figure 9a), ice nucleation initiates rapidly upon condensation, with dense ice crystals forming uniformly across the surface within 28 s, and by 57 s, the entire surface is covered by a thick, cohesive ice layer that demonstrates strong ice adhesion and rapid freezing kinetics typical of hydrophilic surfaces. On the GPOSS coated glass (Figure 9b), the ice formation process is slightly delayed compared to bare glass, with initial ice crystals appearing at 60 s, though the coating’s moderate hydrophobicity allows partial water droplet aggregation before freezing; by 136 s, while the ice layer is less dense than on bare glass, significant ice accumulation still occurs, indicating limited anti-icing improvement from GPOSS alone. In stark contrast, the GAF5 coated glass (Figure 9c) exhibits remarkable anti-icing behavior, with water droplets on the fluorinated surface remaining in a supercooled liquid state for an extended period and no visible ice nucleation until 148 s; even at 300 s, the surface only shows isolated, sparse ice patches rather than a continuous ice layer. This dramatic reduction in ice adhesion and coverage highlights the GAF5 coating’s ability to suppress ice formation through low surface energy and enhanced liquid repellency, directly verifying its superior anti-icing performance compared to unmodified or minimally modified counterparts. Taken together, Figure 9 provides visual evidence of how fluorine-modified inorganic/organic composite coatings like GAF5 can fundamentally alter ice-substrate interactions, offering a promising strategy for practical anti-icing applications in cold environments.
These coatings effectively protect object surfaces from erosion and contamination caused by liquids like water and oil. This study evaluates the performance of as-prepared modified coatings in terms of stain resistance and self-cleaning capabilities across a range of liquid types. In Figure 10a, three distinct droplets were observed: blue (water), light blue (ethanol), and pink (hexadecane). The results show that these liquids exhibited excellent repellency on the GAF5 coated surface at an inclination angle of 25°, allowing substrate rubbing. As a liquid-like coating, GAF5 demonstrated outstanding anti-soiling properties and remarkable repellency toward low-viscosity polar and non-polar liquids. Furthermore, to demonstrate the coating’s ability to repel high-viscosity liquids, glycerol was selected as the test liquid. As shown in Figure 10b, a yellow glycerol droplet exhibited slow rubbing behavior at a 30° inclination angle (Movie S2). Besides good anti-icing performance in glass coatings, self-cleaning glass is also widely used in architecture and automotive industries [21]. Therefore, in addition to evaluating the anti-icing performance of the glass, this study also assessed the self-cleaning capability of the modified glass. To simulate natural environmental conditions, a thin layer of a fine particle and oil mixture was evenly spread across the prepared sample. At a 25° incline, water droplets exhibited free-rubbing or scratching behavior on the surface of the GAF5 coating, effectively removing surface contaminants, as shown in Figure 10c.
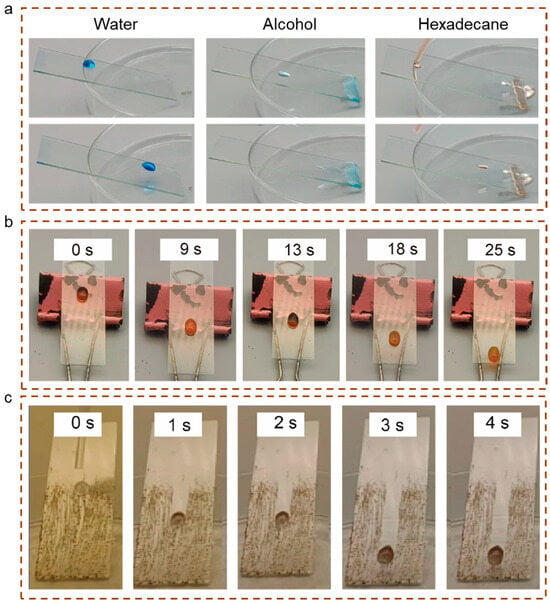
Figure 10.
(a) The anti-fouling properties of GAF5 coating (highest concentration of fluorinated modified GPOSS); (b) the flowability of glycerol on the GAF5 coating; (c) the self-cleaning experiment of the GAF5 coating.
Compared to other glass coatings of similar types, the GAF5 coating prepared in this study exhibits a higher static contact angle with water, as well as superior optical transparency and hardness, as illustrated in Figure 11. Specifically, Figure 11a presents an Ashby plot comparing static contact angles and transmittance for various coatings, where our GAF5 coating achieves a remarkable transmittance of up to 99%. Additionally, the static contact angle of GAF5 with water surpasses that of other coatings in this category. Figure 11b provides a comparison of static contact angles and hardness for different coatings, underscoring the exceptional hardness of the GAF5 coating.
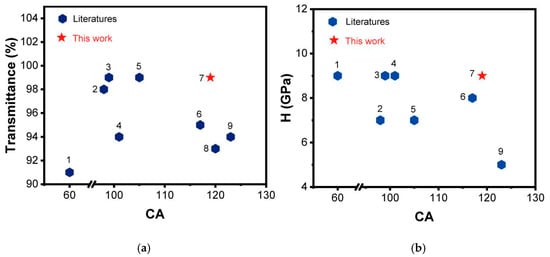
Figure 11.
(a) is a comparison of the transmittance of 1. ESMH [15], 2. GPOSS-FC2-6.1 [13], 3. GPOSS-PDMS [16], 4. NP-GLIDE [22], 5. GPOSS-FC6-6.2 [13], 6. F-GMPOSS/PVDFP [23], 7. GAF5 (highest concentration of fluorinated modified GPOSS), 8. FSV-0.5-60-no [24] and 9. TCMS [25] coatings; (b) compares the hardness of ESMH, GPOSS-FC2-6.1, GPOSS-PDMS, NP-GLIDE, GPOSS-FC6-6.2, F-GMPOSS/PVDFP, GAF5, and TCMS.
4. Conclusions
In conclusion, this work presents a facile two-step method for preparing highly transparent, wear-resistant, anti-icing glass coatings. The drop-coated high-transparency coatings can be applied to glass substrates of various dimensions, achieving a transmittance of approximately 99% at a wavelength of 550 nm, while the fluorine-modified coatings exhibit low contact angle hysteresis (CAH) with diverse liquid droplets (e.g., water, organic solvents) and demonstrate robust durability, retaining excellent water droplet sliding performance after 500 cycles of cotton cloth abrasion and withstanding high-pressure water impact (30 kPa) for 1 min without damage. Notably, no significant trade-off was observed among transparency (99% transmittance), durability (mechanical wear and impact resistance), and anti-icing performance (minimum de-icing pressure of 40.3 kPa at −20 °C), as the inorganic/organic composite structure synergistically enhances all three properties. However, the study has limitations: it does not evaluate the coatings’ long-term UV resistance or performance under dynamic icing conditions. For future research, investigating the coatings’ durability under prolonged UV exposure and their anti-icing efficiency in dynamic environmental scenarios would be critical to advancing their practical implementation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings15070745/s1, Scheme S1. Synthesis route for GAF coating. Table S1. Quantities of reactants in the preparation of GAF coating are presented. Figure S1. NMR hydrogen spectrum (CDCl3, 400MHz). Figure S2. The thermodynamic plot of cured GAF5. Figure S3. Impact testing instruments test the durability of coatings. Figure S4. Static contact angle of GAF5 coating before and after abrasion test. Figure S5. Static contact angle of hexadecane on the surface of the GPOSS coating. Figure S6. After the prepared glass coating has undergone de-icing tests, the surface structure of the ice cubes is smooth. Figure S7. The environmental chamber provides an environment for de-icing testing.
Author Contributions
Conceptualization, Z.Z.; Methodology, Z.W. (Zian Wang) and Z.W. (Zixiang Weng); Investigation, W.Z.; Resources, X.Z.; Funding acquisition, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key R&D Program of China (Grant No.: 2024YFE0107500), the Science and Technology Planning Project of Fujian Province (Grant No.: 2024N0063), the STS Project of Fujian-CAS (Grant No.: 2023T3007), the Major Science and Technology Project of Fuzhou City of Fujian Province (Grant No.: 2023-ZD-001), and the Science and Technology Planning Project of Putian City of Fujian Province (Grant No.: 2023GJGZ004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Conflicts of Interest
The authors hereby declare that the work presented in this manuscript has NOT been applied for a patent. The authors have no other financial or personal relationships that could inappropriately influence or bias the content of this work.
References
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between water wettability and ice adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Zhou, Y. Fabrication of transparent superhydrophobic porous silica coating for self-cleaning and anti-fogging. Ceram. Int. 2016, 42, 8706–8712. [Google Scholar] [CrossRef]
- Rickert, C.A.; Wittmann, B.; Fromme, R.; Lieleg, O. Highly transparent covalent mucin coatings improve the wettability and tribology of hydrophobic contact lenses. ACS Appl. Mater. Interfaces 2020, 12, 28024–28033. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Cebeci, F.C.; Cohen, R.E.; Rubner, M.F. Stable superhydrophobic coatings from polyelectrolyte multilayers. Nano Lett. 2004, 4, 1349–1353. [Google Scholar] [CrossRef]
- Antonini, C.; Innocenti, M.; Horn, T.; Marengo, M.; Amirfazli, A. Understanding the effect of superhydrophobic coatings on energy reduction in anti-icing systems. Cold Reg. Sci. Technol. 2011, 67, 58–67. [Google Scholar] [CrossRef]
- Qiu, C.; Li, M.; Chen, S. Anti-icing characteristics of PTFE super hydrophobic coating on titanium alloy surface. J. Alloys Compd. 2021, 860, 157907. [Google Scholar]
- Jiang, S.; Zhang, H.; Liu, X. Anti-wetting surfaces with self-healing property: Fabrication strategy and application. J. Ind. Eng. Chem. 2023, 117, 54–69. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Zhao, Z.; Yan, Y.; Zhang, D. Slippery liquid-infused porous electric heating coating for anti-icing and de-icing applications. Surf. Coat. Technol. 2019, 374, 889–896. [Google Scholar] [CrossRef]
- Barrio-Zhang, H.; Ruiz-Gutiérrez, É.; Armstrong, S.; McHale, G.; Wells, G.G.; Ledesma-Aguilar, R. Contact-angle hysteresis and contact-line friction on slippery liquid-like surfaces. Langmuir 2020, 36, 15094–15101. [Google Scholar] [CrossRef]
- Buddingh, J.V.; Hozumi, A.; Liu, G. Liquid and liquid-like surfaces/coatings that readily slide fluids. Prog. Polym. Sci. 2021, 123, 101468. [Google Scholar] [CrossRef]
- Chen, W.; Fadeev, A.Y.; Hsieh, M.C.; Öner, D.; Youngblood, J.; McCarthy, T.J. Ultrahydrophobic and ultralyophobic surfaces: Some comments and examples. Langmuir 1999, 15, 3395–3399. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, C.; Yang, J.; Wang, Y.; Zhou, Y.; Zhou, J.; Luo, J.; Zhang, J.; Huang, S.; Tian, X. Reducing the contact time of impacting water drops on superhydrophobic surfaces by liquid-like coatings. Chem. Eng. J. 2022, 448, 137638. [Google Scholar] [CrossRef]
- Bender, D.N.; Zhang, K.; Wang, J.; Liu, G. Hard yet flexible transparent omniphobic GPOSS coatings modified with perfluorinated agents. ACS Appl. Mater. Interfaces 2021, 13, 10467–10479. [Google Scholar] [CrossRef]
- Chodkowski, M.; Sulym, I.Y.; Terpiłowski, K.; Sternik, D. Surface properties of silica–MWCNTs/PDMS composite coatings deposited on plasma activated glass supports. Appl. Sci. 2021, 11, 9256. [Google Scholar] [CrossRef]
- Choi, G.M.; Jin, J.; Shin, D.; Kim, Y.H.; Ko, J.H.; Im, H.G.; Jang, J.; Jang, D.; Bae, B.S. Flexible hard coating: Glass-like wear resistant, yet plastic-like compliant, transparent protective coating for foldable displays. Adv. Mater. 2017, 29, 1700205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huang, S.; Wang, J.; Liu, G. Transparent omniphobic coating with glass-like wear resistance and polymer-like bendability. Angew. Chem. 2019, 131, 12132–12137. [Google Scholar] [CrossRef]
- Liu, W.; Wu, J.; He, W.; Xu, F. A review on perfluoroalkyl acids studies: Environmental behaviors, toxic effects, and ecological and health risks. Ecosyst. Health Sustain. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Shibuichi, S.; Yamamoto, T.; Onda, T.; Tsujii, K. Super water-and oil-repellent surfaces resulting from fractal structure. J. Colloid Interface Sci. 1998, 208, 287–294. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Tarquini, S.; Antonini, C.; Amirfazli, A.; Marengo, M.; Palacios, J. Investigation of ice shedding properties of superhydrophobic coatings on helicopter blades. Cold Reg. Sci. Technol. 2014, 100, 50–58. [Google Scholar] [CrossRef]
- Peng, H. Synthesis and application of fluorine-containing polymers with low surface energy. Polym. Rev. 2019, 59, 739–757. [Google Scholar] [CrossRef]
- Huang, S.; Liu, G.; Hu, H.; Wang, J.; Zhang, K.; Buddingh, J. Water-based anti-smudge NP-GLIDE polyurethane coatings. Chem. Eng. J. 2018, 351, 210–220. [Google Scholar] [CrossRef]
- Bender, D.N.; Liu, G. Endowment of omniphobicity and exceptional bendability to a wear-resistant POSS coating. Chem. Eng. J. 2023, 464, 142702. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, L.; Wu, L.; Weng, Z. Scalable preparation of efficiently self-healing and highly transparent omniphobic coating for glass. Prog. Org. Coat. 2023, 182, 107606. [Google Scholar] [CrossRef]
- Esfahani, M.B.; Eshaghi, A.; Bakhshi, S.R. Transparent hydrophobic, self-cleaning, anti-icing and anti-dust nano-structured silica based thin film on cover glass solar cell. J. Non-Cryst. Solids 2022, 583, 121479. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).