Abstract
The utilization of waste glass as an aggregate in cement-based materials provides both environmental and economic benefits, but the alkali-silica reaction (ASR) caused by the reactive silica in glass aggregates is a significant challenge for its application. This study investigates the impact of different crushing methods on the ASR of glass aggregate mortar, with a focus on the effect of immersion crushing using calcium chloride (CaCl2) solution. Glass aggregates were prepared using conventional crushing, water immersion crushing, and CaCl2 immersion crushing methods. The ASR expansion and compressive strength of the mortar were evaluated through accelerated ASR tests, compressive strength testing, and microstructural analysis using SEM/EDS and mercury intrusion porosimetry (MIP). Results show that immersion crushing significantly mitigated ASR expansion and the associated loss in compressive strength. The CaCl2 immersion method yielded the most pronounced effect. Compared with conventional crushing, it reduced the ASR expansion by approximately 45% and improved the compressive strengths by approximately 20%. Microstructural analysis revealed that the CaCl2 treatment led to a higher Ca/Si ratio in the ASR gel, which reduced the gel’s water-absorbing swelling ability and consequently suppressed ASR-induced expansion. Additionally, the CaCl2 immersion crushing method resulted in the smallest changes in porosity and pore size distribution. These findings provide a theoretical basis for the safe use of waste glass in cement-based materials and contribute to the promotion of resource recycling in the construction industry.
1. Introduction
Concrete is the most widely used construction material in the world today. Its sustainable development faces dual challenges of resource depletion and solid waste disposal []. Replacing natural aggregates in concrete with waste materials is one of the key approaches to achieving a green transformation in the construction industry [,]. Waste glass, a typical urban solid waste, is produced in large quantities each year. It is chemically stable, difficult to degrade naturally, and landfilling consumes valuable land resources []. Therefore, crushing waste glass for use as fine aggregate or powder in concrete can not only reduce environmental burdens but also alleviate the excessive exploitation of natural sand resources, offering significant environmental and economic benefits [,].
However, the amorphous silica in glass aggregates is highly chemically reactive and tends to undergo an alkali-silica reaction (ASR) with alkali metal ions (e.g., K+, Na+) in the pore solution [,]. The ASR produces alkali-silica gel, which absorbs water and swells in humid environments, generating substantial internal stress that eventually leads to cracking, spalling, and deterioration of the mechanical properties of concrete []. This potential durability issue has severely limited the large-scale application of waste glass in concrete structures.
In recent years, various methods have been proposed to mitigate the ASR of glass aggregates. These include: (1) using low-alkali cement or incorporating supplementary cementitious materials (such as fly ash, silica fume) to lower the pore solution alkalinity and optimize pore structure [,]; (2) applying surface treatments to glass aggregates, such as annealing the glass particles at high temperatures, which could remove the residual cracks []; and (3) limiting the replacement ratio of glass aggregates []. Nevertheless, these approaches often suffer from high costs, complex processes, or limited effectiveness. Therefore, it is crucial to explore innovative strategies that address ASR from the perspective of the aggregate’s intrinsic properties and provide a fundamental solution to this problem.
Notably, the reactivity of ASR is closely related to the interfacial characteristics of glass aggregates. Studies have shown that microcracks generated during glass crushing serve as primary sites for ASR initiation [,]. In conventional crushing, the newly formed crack surfaces are directly exposed to air, and their surface chemistry depends on the bulk composition of the glass. This indicates that the aggregate preparation method plays a decisive role in the ASR process. If the chemical environment of microcrack surfaces can be modified during this critical stage, it may be possible to alter the subsequent ASR pathway.
From the perspective of ASR mechanisms, two typical types of products and reaction zones are generally observed []. On the aggregate surface, where calcium hydroxide (Ca(OH)2) from the cement paste is enriched, dissolved silicate species preferentially combine with calcium ions (Ca2+) to form calcium–silicate–hydrate (C–S–H) layers with high Ca/Si ratios []. This dense layer is not expansive and can act as a semipermeable membrane that selectively restricts ion migration [], thereby slowing down the reaction. Long-term observations have shown that the thickness of this reaction layer tends to stabilize at later stages []. In contrast, within the internal microcracks of the aggregate, where Ca(OH)2 is scarce, the dissolved silica primarily reacts with sodium and potassium ions to form ASR gels with low Ca/Si ratios. These gels exhibit strong hygroscopic swelling behavior []. The limited calcium ions diffusing into cracks are insufficient to promote effective gel carbonation. SEM/EDS analyses have confirmed that the Ca/Si ratio of gels inside cracks is significantly lower than that on the aggregate surface [,], and low Ca/Si ratios have been shown to markedly increase the swelling capacity of ASR gels []. This results in a self-accelerating cycle of “microcrack propagation → intensified ASR → expansion-induced new cracking” [,].
Based on the above analysis, calcium ions (Ca2+) play a central role in regulating the properties of ASR gels. Inspired by this understanding, our research group has innovatively proposed a pretreatment method involving immersed crushing of glass aggregates in a calcium-ion-rich solution. Preliminary studies have demonstrated that this method can significantly reduce ASR-induced expansion []. However, the underlying micro-mechanisms—particularly the regulation of gel chemistry within internal cracks—remain unclear.
This study aims to systematically elucidate the mechanism by which immersed crushing, especially in a CaCl2 solution medium, mitigates ASR, from the macroscopic to the microscopic scale. Glass aggregates were prepared via the immersed crushing process, and a multi-scale analytical approach was employed. At the macroscopic level, the evolution of mortar expansion and compressive strength was monitored to evaluate ASR inhibition. At the mesoscopic level, changes in pore structure (porosity and pore size distribution) during ASR were analyzed. At the microscopic level, SEM/EDS was used to characterize the chemical composition (particularly the Ca/Si ratio) and morphology of ASR gels to reveal the underlying mechanism. The findings of this study will provide new theoretical insights and practical pathways for the safe utilization of waste glass in cement-based materials, promoting higher-value recycling of glass solid waste.
The novelty of this study lies in the innovative application of immersion crushing in a calcium-rich solution (CaCl2) as a pretreatment method for waste glass aggregates. Unlike conventional approaches that focus on modifying the cementitious matrix or limiting aggregate content, this method directly targets the reactive microcracks within the glass aggregates at the moment of their formation. By pre-enriching the crack surfaces with Ca2+ ions, we fundamentally alter the chemical pathway of ASR gel formation in the most vulnerable regions—internal cracks—leading to the formation of less expansive, high-Ca/Si gels. This work provides the first multi-scale evidence linking the crushing medium chemistry to the microchemical properties of ASR gels and the resulting macroscopic expansion behavior, offering a novel and practical strategy for mitigating ASR in glass-containing cementitious materials.
2. Materials and Methods
2.1. Materials
Ordinary Portland cement (P·O 42.5) was used, and its physical and chemical properties are shown in Table 1 and Table 2. Natural river sand was used as the inactive fine aggregate, with a particle size range of 0–1.18 mm. Waste glass particles, with a particle size range of 1.18–4.75 mm, were used as the active fine aggregate, and their physical and chemical properties are also shown in Table 1 and Table 2. CaCl2 was used as an analytical-grade reagent, and pure water was used.

Table 1.
Physical properties of glass particles and cement.

Table 2.
Chemical properties of glass particles and cement.
2.2. Experimental Procedures
2.2.1. Glass Aggregate Preparation
Glass aggregates were prepared using three different crushing methods: (1) Conventional crushing control group (air crushing); (2) Immersion crushing control group (crushed in pure water); (3) Immersion crushing experimental group (crushed in a 1.0 mol/L CaCl2 solution). The corresponding glass aggregates were labeled as Control, IC-W, and IC-C, respectively. The preparation process is illustrated in Figure 1a. After being washed and dried, the prepared glass bottles were crushed with a hammer while immersed in water or calcium solution. After crushing, all aggregates were sieved to obtain particles within the 1.18–4.75 mm size range to ensure consistent grading and eliminate the influence of particle size distribution on the test results. Previous studies have indicated that whether the particles are cleaned after immersion crushing—i.e., whether residual solution remains on the particle surfaces after soaking—has no significant effect on the macroscopic ASR expansion []. Therefore, this study focused on one scenario, in which the obtained glass particles were rinsed with running water, dried, and then used for subsequent experiments.
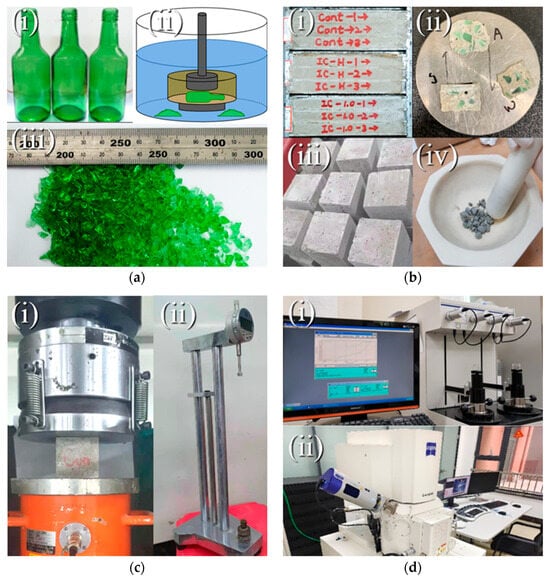
Figure 1.
Schematic diagram of the experimental procedure: (a(i)–a(iii)) aggregate preparation; (b(i)–b(iv)) specimen preparation; (c(i),c(ii)) test setup for compressive and AST test; (d(i),d(ii)) microscopic characterization.
2.2.2. Alkali-Silica Reaction (ASR) Test
To quantitatively evaluate the ASR activity of different glass aggregates, mortar bar expansion tests were conducted following the accelerated test method specified in ASTM C1260. The mortar bar specimens (25 mm × 25 mm × 285 mm) were prepared with mix proportions shown in Table 3. The sieve analysis of the sand and glass aggregate is shown in Figure 2. Glass aggregates replaced 35% of natural sand by mass, a substitution level sufficient to induce noticeable ASR expansion while maintaining adequate workability []. The bulk density of sand and glass aggregate was 1560 kg/m3. Three groups of specimens were prepared, each containing three mortar bars corresponding to the aggregate types described in Section 2.2.1, as illustrated in Figure 1b(i).

Table 3.
Mix proportions of mortar specimens.
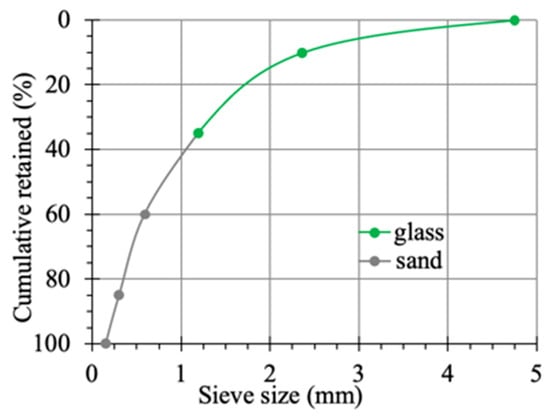
Figure 2.
Sieve analysis of sand and glass aggregate.
After 24 h of casting, the specimens were demolded and cured in water at 80 °C for 24 h. Subsequently, they were immersed in a 1.0 mol/L NaOH solution at 80 °C to accelerate ASR development. The change in specimen length was periodically measured using a comparator, as shown in Figure 1c(ii). The average length change of three specimens from each group was recorded as the ASR expansion ratio, which served as the key macroscopic indicator of ASR severity.
2.2.3. Compressive Strength Test
To investigate the degradation of mechanical performance due to ASR and the mitigating effects of different aggregates, compressive strength tests were conducted under both standard and accelerated ASR curing conditions. Mortar cubes (70.7 mm × 70.7 mm × 70.7 mm) were prepared using the same mix proportions as the mortar bars (Table 3). Two batches of specimens were produced, each consisting of three groups (corresponding to the three aggregate types used in Section 2.2.1), and each group contained three specimens, as shown in Figure 1b(iii).
The first batch was cured under standard conditions (temperature 20 ± 2 °C, relative humidity ≥ 95%) for 28 days. The second batch was immersed in a 1.0 mol/L NaOH solution at 80 °C for 28 days to simulate ASR-induced degradation. After curing, compressive strength tests were carried out using a universal testing machine (Figure 1c(i)). The average strength of the three specimens in each group was recorded as the compressive strength of that group. Comparing the strength loss between the two curing conditions provided insight into the extent of ASR-induced structural damage.
2.2.4. Microstructural Analysis
To elucidate the characteristics of ASR products and the underlying mechanism of immersed crushing from a microscopic perspective, samples were collected from the mortar bars after completion of the ASR test. Thin slices (approximately 2.0 mm thick) were prepared from the midsection of the bars using a low-speed diamond saw (Isomet 1000, Buehler Ltd., Lake Bluff, IL, USA), as shown in Figure 1b(ii), and immediately immersed in anhydrous ethanol for 24 h to terminate hydration. Then, the slices were dried at 60 °C for another 24 h, and their surfaces were polished using sandpaper before performing the SEM and EDX analysis. To enhance the electrical conductivity at the surface, the specimen was coated with platinum. Afterward, the microstructure of the glass–paste interfacial zones was observed using a scanning electron microscope (SEM) equipped with energy-dispersive X-ray spectroscopy (EDS) (Hitachi S-4800, Tokyo, Japan). The SEM images were captured at an accelerating voltage of 20 kV. Point analyses were conducted on selected ASR gel products (Figure 1d(ii)) to determine their local chemical composition. Particular attention was paid to comparing the Ca/Si ratios of ASR gels within glass aggregate cracks across different groups, as this parameter serves as key evidence for validating the study’s central hypothesis.
2.2.5. Pore Structure Analysis
The expansive stress of ASR gels leads to the initiation and propagation of microcracks in mortar, thereby altering its pore structure. After compressive strength testing, representative samples (approximately 5 mm × 5 mm × 5 mm) were extracted from the specimens, as shown in Figure 1b(iv), and immersed in anhydrous ethanol to stop hydration. Pore structure analysis was conducted using mercury intrusion porosimetry (MIP, AutoPore IV 9500, NC, USA), as shown in Figure 1d(i), to obtain the total porosity and pore size distribution data. By comparing the pore structure evolution under different curing conditions, the ASR-induced damage could be quantitatively characterized at the mesoscopic level and correlated with macroscopic mechanical performance.
3. Results and Discussion
3.1. Macro ASR Expansion
Figure 3 illustrates the effect of glass aggregate crushing methods on ASR in mortar bars. As shown in Figure 3a, the ASR expansion rate of mortar bars with different aggregates increased over time, but the rate of expansion gradually slowed down. This was primarily due to the formation of reaction products on the aggregate surface, which slowed the ingress of corrosive ions. The crushing method significantly affected the ASR expansion rate. The conventional crushing group exhibited the highest expansion rate, followed by the underwater crushing group and the CaCl2 immersion crushing group. The underwater crushing group showed a 24% reduction in expansion compared to the conventional crushing group (Figure 3b), mainly because underwater crushing significantly reduced the surface defects [] and lowered the glass’s tensile strength []. Fewer surface defects mean a reduction in ASR reaction sites, while a lower tensile strength may allow residual cracks to develop more readily into through-going fractures during the crushing process, thereby decreasing the formation of residual cracks and subsequently mitigating ASR expansion. The CaCl2 immersion crushing group showed the lowest expansion rate, reducing ASR expansion by approximately 45% compared to the conventional crushing group (Figure 3b), indicating the presence of additional influencing mechanisms beyond those in the underwater crushing process.
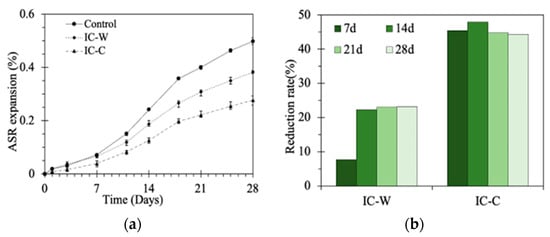
Figure 3.
Effect of crushing process on ASR: (a) ASR expansion; (b) ASR expansion reduction rate.
Figure 4 shows the surface morphology of the mortar bars after 28 days of accelerated ASR testing, as observed with a handheld digital microscope, with the observation areas located in the middle of each bar. As shown in Figure 4, large macrocracks were visible on the surface of the mortar bars containing conventionally crushed glass, with the widest cracks; fewer cracks were observed in the underwater crushed glass mortar bars; and the CaCl2 immersion crushed glass mortar bars had the best surface morphology, with only a few fine cracks. These surface morphological changes align with the macro ASR expansion trends.
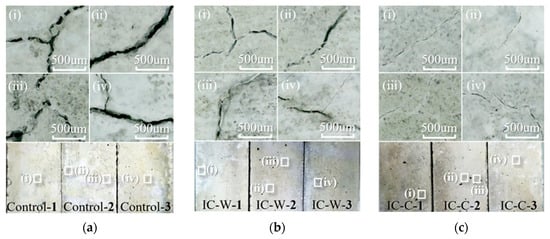
Figure 4.
Appearance of mortar bars after ASR for 28 days: (a(i)–a(iv)) Control; (b(i)–b(iv)) IC-W; (c(i)–c(iv)) IC-C.
3.2. Compressive Strength
Figure 5 presents the compressive strength of mortar specimens with glass aggregates under different curing conditions at 28 days. Under standard curing conditions, the compressive strength of all groups was approximately 45 MPa, indicating that the preparation method of glass aggregates had no significant effect on the mortar’s compressive strength. In contrast, under the accelerated ASR curing conditions, the compressive strength of all specimens significantly decreased, with the conventional crushing group experiencing the greatest reduction, down to approximately 35 MPa. The underwater crushing group and the CaCl2 immersion crushing group had compressive strengths of approximately 38 MPa and 42 MPa, respectively, which represented improvements of 8.5% and 20% compared to the conventional crushing group. This was mainly due to the absence of ASR reactions under standard curing conditions. In contrast, the faster ASR curing conditions triggered ASR reactions, leading to expansion and damage, which caused a decrease in strength. As shown in Figure 3 and Figure 4, the CaCl2 immersion crushing group exhibited the best performance in suppressing macro-expansion and crack formation, leading to the smallest reduction in compressive strength. Conversely, the conventional crushing exhibited the greatest expansion and cracking, resulting in the most significant decrease in strength. The compressive strength results of each group are consistent with the ASR expansion trends in Section 3.1.
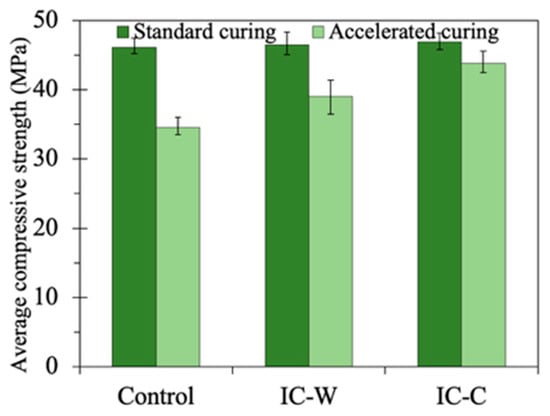
Figure 5.
Compressive strength at day 28 with different curing conditions.
3.3. Microstructural Morphology
Figure 6 presents SEM images of products formed inside and outside the cracks of glass aggregates after 28 days, along with EDS elemental distribution maps for precise localization and the chemical analysis of reaction products. The corresponding chemical compositions are summarized in Table 4. In all groups (Control, IC-W, and IC-C), a dense reaction layer was observed on the glass surface, while products within internal cracks were more porous and exhibited drying shrinkage cracks—particularly in the Control and IC-W groups. These features correspond to the typical morphology of high-Ca/Si C–S–H layers on aggregate surfaces and low-Ca/Si ASR gels inside cracks [].
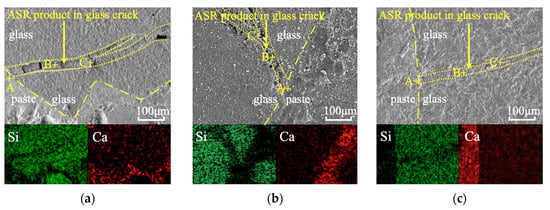
Figure 6.
SEM-EDS analysis of specimens incorporating glass aggregates: (a) Control; (b) IC-W; (c) IC-C.

Table 4.
Chemical composition of reaction products.
EDS point analyses (Table 4) revealed that products near crack openings possessed high Ca/Si ratios, confirming their identification as C–S–H gels. This occurs because the swelling ASR gel within cracks migrates outward and becomes enriched in Ca2+ at the surface. In contrast, products deeper within the cracks exhibited low Ca/Si ratios, characteristic of expansive ASR gels.
A key finding of this study is that the ASR gels within cracks in the IC-C group had significantly higher Ca/Si ratios than those in the Control and IC-W groups. This indicates that during crushing in the CaCl2 solution, the freshly formed crack surfaces instantly adsorbed large amounts of Ca2+. These pre-adsorbed ions later served as local calcium sources during ASR, promoting the formation of high-Ca/Si reaction products. According to Gholizadeh-Vayghan et al. [], the swelling capacity of ASR gels decreases markedly with increasing Ca/Si ratio. Therefore, the generation of higher-Ca/Si gels in the IC-C group fundamentally reduces the swelling potential of ASR gels, providing the microscopic mechanism for its superior ASR inhibition.
3.4. Pore Structure
Figure 7 shows the total porosity and pore size distribution of specimens with different crushing methods and curing conditions after 28 days. As seen in Figure 7, under standard curing conditions, all groups exhibited a total porosity of approximately 17%. Under accelerated ASR curing conditions, the total porosity of all groups increased to varying degrees, with the highest porosity observed in the conventional crushing group, followed by the underwater crushing group and the CaCl2 liquid immersion crushing group. This trend corresponds with the decrease in compressive strength observed in Section 3.2, as higher porosity results in lower compressive strength [].
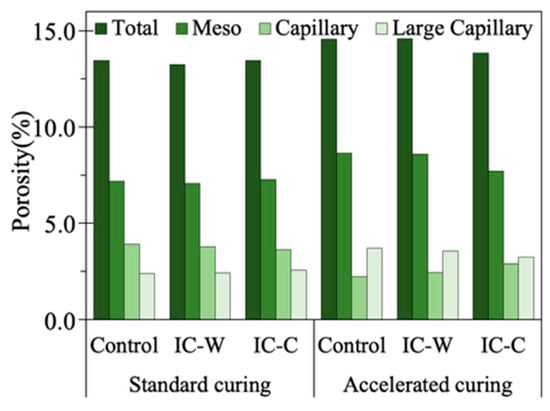
Figure 7.
Porosity and pore size distribution at day 28 with different curing conditions.
Based on pore size, the pores in the mortar were classified into three categories: mesopores (less than 50 nm), capillary pores (50–1000 nm), and macropores (greater than 1000 nm) []. Under standard curing conditions, the pore size distribution in all groups was essentially unaffected by the crushing method. However, under accelerated ASR curing conditions, the proportion of capillary pores decreased significantly, while the proportion of mesopores and macropores increased to varying extents. Among these, the specimens from the CaCl2 liquid immersion crushing group showed the lowest proportion of mesopores and macropores and the highest proportion of capillary pores. The shift in pore size distribution, characterized by an increase in mesopores and macropores, is primarily attributed to the formation of microcracks induced by ASR expansion. While the deposition of ASR gel products might have a minor, localized filling effect on some smaller pores, the MIP data conclusively shows that this potential effect is overwhelmingly offset by the net increase in harmful larger pores due to cracking.
4. Conclusions
- The immersion crushing effectively suppressed ASR and the associated deterioration in the performance of glass-containing mortar. Compared with conventional crushing, immersion crushing in water and CaCl2 solution reduced the 14-day ASR expansion of mortar by approximately 24% and 45%, respectively, and significantly mitigated the loss of compressive strength under ASR conditions. Macroscopic observations confirmed that mortars incorporating glass aggregates crushed in CaCl2 solution exhibited almost no visible surface cracks after ASR exposure.
- ASR degrades the pore structure of mortar, whereas immersion crushing effectively alleviates this degradation. ASR increased total porosity and shifted the pore size distribution toward more harmful mesopores and macropores. Specimens subjected to immersion crushing in CaCl2 solution (IC-C group) showed the smallest increases in porosity and in the proportion of harmful pores, indicating the least internal structural damage.
- The microchemical mechanism underlying ASR suppression by immersion crushing—particularly in CaCl2 solution—was revealed. In glass aggregates crushed in a CaCl2 medium, the ASR gel formed along internal microcracks exhibited a higher Ca/Si ratio. This resulted from the pre-enrichment of Ca2+ on the surfaces of newly generated cracks during crushing, which altered the gel formation pathway and composition at the reaction source. Consequently, low-expansivity, high-Ca/Si-ratio gels were produced, fundamentally inhibiting ASR-induced expansion and damage.
Author Contributions
Conceptualization, Y.S. and L.S.; methodology, Y.S. and L.S.; investigation, L.S.; writing—original draft preparation, Y.S.; writing—review and editing, Q.Z. and T.Y.; supervision, K.Y.; funding acquisition, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 52378520, 52038006), the Natural Science Foundation of Shandong Province (No. ZR2025QC515), and the research fund of Shandong Jianzhu University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Kheetan, M.J. Properties of lightweight pedestrian paving blocks incorporating wheat straw: Micro-to macro-scale investigation. Results Eng. 2022, 16, 100758. [Google Scholar] [CrossRef]
- Rakshvir, M.; Barai, S.V. Studies on recycled aggregates-based concrete. Waste Manag. Res. 2006, 24, 225–233. [Google Scholar] [CrossRef]
- Patel, D.; Shrivastava, R.; Tiwari, R.P.; Yadav, R.K. Properties of cement mortar in substitution with waste fine glass powder and environmental impact study. J. Build. Eng. 2020, 27, 100940. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Topçu, I.B.; Boğa, A.R.; Bilir, T. Alkali–silica reactions of mortars produced by using waste glass as fine aggregate and admixtures such as fly ash and Li2CO3. Waste Manag. 2008, 28, 878–884. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Use of waste glass as sand in mortar: Part II–Alkali–silica reaction and mitigation methods. Cem. Concr. Compos. 2013, 35, 118–126. [Google Scholar] [CrossRef]
- Rajabipour, F.; Giannini, E.; Dunant, C.; Ideker, J.H.; Thomas, M.D. Alkali–silica reaction: Current understanding of the reaction mechanisms and the knowledge gaps. Cem. Concr. Res. 2015, 76, 130–146. [Google Scholar] [CrossRef]
- Cota, F.P.; Melo, C.C.D.; Panzera, T.H.; Araújo, A.G.; Borges, P.H.R.; Scarpa, F. Mechanical properties and ASR evaluation of concrete tiles with waste glass aggregate. Sustain. Cities Soc. 2015, 16, 49–56. [Google Scholar] [CrossRef]
- Berry, M.; Stephens, J.; Cross, D. Performance of 100% Fly Ash Concrete with Recycled Glass Aggregate. ACI Mater. J. 2011, 108, 378–384. [Google Scholar] [CrossRef]
- Maraghechi, H.; Fischer, G.; Rajabipour, F. The role of residual cracks on alkali silica reactivity of recycled glass aggregates. Cem. Concr. Compos. 2012, 34, 41–47. [Google Scholar] [CrossRef]
- Ali, E.E.; Al-Tersawy, S.H. Recycled glass as a partial replacement for fine aggregate in self compacting concrete. Constr. Build. Mater. 2012, 35, 785–791. [Google Scholar] [CrossRef]
- Rajabipour, F.; Maraghechi, H.; Fischer, G. Investigating the alkali-silica reaction of recycled glass aggregates in concrete materials. J. Mater. Civ. Eng. 2010, 22, 1201–1208. [Google Scholar] [CrossRef]
- Idir, R.; Cyr, M.; Tagnit-Hamou, A. Pozzolanic properties of fine and coarse color-mixed glass cullet. Cem. Concr. Compos. 2011, 33, 19–29. [Google Scholar] [CrossRef]
- Ichikawa, T.; Miura, M. Modified model of alkali-silica reaction. Cem. Concr. Res. 2007, 37, 1291–1297. [Google Scholar] [CrossRef]
- Zheng, K. Pozzolanic reaction of glass powder and its role in controlling alkali–silica reaction. Cem. Concr. Compos. 2016, 67, 30–38. [Google Scholar] [CrossRef]
- Liaudat, J.; Carol, I.; López, C.M.; Leemann, A. ASR expansions at the level of a single glass-cement paste interface: Experimental results and proposal of a reaction-expansion mechanism. Constr. Build. Mater. 2019, 218, 108–118. [Google Scholar] [CrossRef]
- Gholizadeh-Vayghan, A.; Rajabipour, F. Quantifying the swelling properties of alkali-silica reaction (ASR) gels as a function of their composition. J. Am. Ceram. Soc. 2017, 100, 3801–3818. [Google Scholar] [CrossRef]
- Sun, L.; Kim, M.; Doh, J.H.; Zi, G. A novel method of crushing glass aggregates to reduce the alkali-silica reaction. KSCE J. Civ. Eng. 2021, 25, 4763–4770. [Google Scholar] [CrossRef]
- Kokura, K.; Tomozawa, M.; MacCrone, R.K. Defect formation in SiO2 glass during fracture. J. Non-Cryst. Solids 1989, 111, 269–276. [Google Scholar] [CrossRef]
- Stockdale, G.F.; Tooley, F.V.; Ying, C.W. Changes in the tensile strength of glass caused by water immersion treatment. J. Am. Ceram. Soc. 1951, 34, 116–121. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Zhou, J. Influence of porosity on compressive and tensile strength of cement mortar. Constr. Build. Mater. 2013, 40, 869–874. [Google Scholar] [CrossRef]
- Care, S. Effect of temperature on porosity and on chloride diffusion in cement pastes. Constr. Build. Mater. 2008, 22, 1560–1573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).