Abstract
We introduce a fully solution-processed interlayer strategy for n–p CdS/PbS thin film solar cells that combines a sol–gel ZnO compact coating with an electrospun ZnO nanofiber network. The synthesis and characterization of ZnO, CdS, and PbS thin films, complemented by electrospun ZnO nanofibers, are aimed at low-cost photovoltaic applications. Sol–gel ZnO films exhibited a hexagonal wurtzite structure with a bandgap (Eg) of approximately 3.28 eV, functioning effectively as electron transport and hole-blocking layers. CdS films prepared by chemical bath deposition (CBD) showed mixed cubic and hexagonal phases with an Eg of about 2.44 eV. PbS films deposited at low temperature displayed a cubic galena structure with a bandgap of approximately 0.40 eV. Scanning Electron Microscopy revealed uniform ZnO and CdS surface coatings and a conformal 1D ZnO network with nanofibers measuring about 50 nm in diameter (ranging from 49.9 to 53.4 nm), which enhances interfacial contact coverage. PbS films exhibited dense grains ranging from 50 to 150 nm, and EDS confirmed the expected stoichiometries. Electrical characterization indicated low carrier densities and high resistivities consistent with low-temperature processing, while mobilities remained within reported ranges. The incorporation of ZnO layers and nanofibers significantly improved device performance, particularly at the CdS/PbS heterojunction. The device achieved a Voc of 0.26 V, an Jsc of 3.242 mA/cm2, and an efficiency of 0.187%. These improvements are attributed to enhanced electron transport selectivity and reduced interfacial recombination provided by the percolated 1D ZnO network, along with effective hole blocking by the compact film and increased surface area. Fill-factor limitations are linked to series resistance losses, suggesting potential improvements through fiber densification, sintering, and control of the compact layer thickness. This work is a proof-of-concept of a fully solution-processed and low-temperature CdS/PbS architecture. Efficiencies remain modest due to low carrier concentrations typical of low-temperature CBD films and the deliberate omission of high-temperature annealing/ligand exchange. Overall, this non-vacuum, low-temperature coating method establishes electrospun ZnO as a tunable functional interlayer for CdS/PbS devices and offers a practical pathway to elevate power output in scalable productions. These findings highlight the potential of nanostructured intermediate layers to optimize charge separation and transport in low-cost PbS/CdS/ZnO solar cell architectures.
1. Introduction
Thin-film solar cells remain a compelling route to cost-effective photovoltaics because they enable low-temperature, solution-processed stacks and precise control of interfacial energetics []. Within II-VI/IV-VI heterojunctions, CdS/PbS architectures are attractive for their broad spectral coverage and full compatibility with chemical bath deposition (CBD) [,,,,]. However, compact electron-transport layers (ETLs) such as sol–gel ZnO often struggle to achieve conformal coverage across polycrystalline topographies, which can create local recombination sites and series resistance that cap the fill factor [,,]. This motivates coating strategies that simultaneously enhance carrier selectivity and physical contact at the junction, ideally through non-vacuum processes amenable to scale-up [,].
Zinc oxide (ZnO) is an abundant, non-toxic n-type semiconductor with a wide direct bandgap (~3.37 eV) and suitable electron mobility (~200 cm2/V·s under ideal conditions), widely used as an electron transport layer (ETL) and interfacial modifier in photovoltaic heterojunctions [,,,,]. Cast as electrospun one-dimensional (1D) nanofibers, ZnO provides percolating electron pathways, increased active surface area, and improved conformal coverage over grain-scale asperities—attributes linked to reduced interfacial recombination, and enhanced charge collection [,,,,,,,,]. From a band alignment perspective, the ZnO compact film acts as a hole-blocking layer, and the nanofiber network promotes vectorial electron transport; the expected energetics at the ZnO/CdS/PbS interfaces further support selective extraction and suppressed interface-assisted recombination [,,,].
Recent studies have demonstrated that Mg- and Ga-doped ZnO electron-transport layers (ETLs) can significantly enhance band alignment, reduce trap density, and improve carrier mobility in solution-processed photovoltaic devices. For example, Mg-doped ZnO has been employed as an ETL and demonstrated a raised conduction band minimum, reduced recombination, and increased PCE in perovskite solar cells [,]. Similarly, Ga-doped ZnO thin films have been successfully used in inverted organic and perovskite photovoltaics, achieving higher efficiencies and improved charge transport compared to undoped ZnO []. In contrast, the present work focuses on intrinsic sol–gel ZnO as a low-cost, fully solution-processed ETL for CdS/PbS thin-film solar cells, where the measured mobility and resistivity fall within ranges reported for undoped ZnO systems [].
Several studies have explored vertically aligned ZnO nanorods as efficient one-dimensional electron-transport channels in thin-film photovoltaics [,], where their anisotropic geometry facilitates directional carrier extraction. However, nanorods typically require seed layers and hydrothermal growth conditions that limit their compatibility with low-temperature, fully solution-processed stacks. In contrast, electrospun ZnO nanofibers provide a high-coverage, conformal network that adapts to the rough topography of CBD-grown CdS and PbS films, forming continuous percolation pathways without substrate-specific nucleation. This extended interfacial contact and larger accessible surface area make the nanofiber network particularly advantageous for charge separation and interface passivation in non-vacuum CdS/PbS architectures.
CdS/PbS heterojunction solar cells operate through band offsets that enable charge separation and selective electron extraction; however, CBD-grown layers often exhibit high defect densities and low carrier concentrations that increase interface-assisted recombination []. These limitations underscore the need for interlayer engineering strategies that can enhance surface coverage, electron selectivity, and charge transport in fully solution-processed architectures. While ZnO concerns include toxic ETL, PbS inherently presents toxicity concerns; thus, developing scalable, low-temperature device architectures such as the one proposed here may facilitate future transitions toward Pb-reduced or Pb-free absorber technologies.
It should be noted that this study is intentionally framed as a proof-of-concept of a fully solution-processed CdS/PbS architecture. Both CdS and PbS are deposited by low-temperature chemical bath deposition, and no high-temperature annealing or ligand-exchange treatments were employed; as a result, absolute efficiencies remain modest. This controlled processing window enables a targeted isolation of the electrospun ZnO nanofiber interlayer’s role in electron selectivity, interfacial contact, and recombination mitigation.
In the present n–p stack, CdS serves as the n-type window/buffer, whereas PbS is the primary near-infrared absorber; both layers are prepared by low-temperature CBD [,,,,,,,]. To isolate morphological and electrical contributions, we fabricate three device configurations: (i) compact ZnO only, (ii) compact ZnO plus electrospun nanofibers, and (iii) a control without ZnO. As shown below, introducing the compact plus fiber bilayer yields relative gains in open-circuit voltage and short-circuit current, as well as higher output power, consistent with improved coverage and electron selectivity. Although absolute efficiencies remain at proof-of-concept levels typical of a low-temperature CBD stack, light/dark J-V analysis indicates series resistance-limited fill factors, and we outline low-temperature lever-fiber densification/sintering and compact-layer thickness control to reduce Rs and raise FF in scalable coating workflows [,]. Overall, this work positions electrospun ZnO as a tunable functional interlayer for thin-film chalcogenide devices and contributes a coating-centric route to interfacial optimization in fully solution-processed architectures.
2. Materials and Methods
Fluorine-doped tin oxide (FTO) glass substrates (sheet resistance ~7–15 Ωsq−1 were cleaned by sequential ultrasonication (10 min each) in (i) neutral detergent in DI water, (ii) deionized water, (iii) acetone, (iv) isopropanol, and (v) ethanol, followed by N2 blow to remove residual moisture.
2.1. Deposition of ZnO Thin Film (Compact Layer)
A compact ZnO layer was deposited using the sol–gel method, employing zinc acetate dihydrate as the Zn precursor. The precursor solution was prepared by adding, for each mole of Zn, 2.5 moles of ethylene glycol, 0.15 moles of glycerol, 5.92 moles of 1-propanol, and 2.0 moles of triethylamine, following the methodology described elsewhere []. The coating process was carried out at a withdrawal speed of 2 cm/min, ensuring uniform film formation. Subsequently, the films were subjected to a thermal treatment at 500 °C for 6 h under air.
Films were deposited by dip-coating, and the coated substrates were subsequently heat-treated to induce crystallization of the ZnO compact layer on FTO. A single coating layer was applied at a withdrawal speed of 2 cm/min, ensuring uniform film formation. Subsequently, the films were subjected to a thermal treatment at 500 °C for 6 h under an air atmosphere.
2.2. Fabrication of Electrospun ZnO Nanofibers on Compact ZnO
Electrospinning was employed to form a 1D ZnO network on top of the compact film, to enhance interfacial coverage and carrier selectivity.
A polymer solution was prepared by dissolving PVA (10% w/v) in a water/ethanol mixture (9:1); 0.07 g of zinc acetate was then added, and the mixture was stirred until complete dissolution. The solution was loaded into a syringe and processed using a Giga Electrospinning system, using an applied voltage of 19 kV, a tip-to-substrate distance of 15 cm, and a feed rate of 1 mL/h. After deposition, the fibers were heat-treated in air at 500 °C for 6 h to remove the PVA and obtain ZnO fibers [].
2.3. Deposition of CdS Thin Film
The CdS thin film was subsequently deposited by the chemical bath deposition (CBD) onto three types of substrates—(a) clean FTO, (b) FTO with compact ZnO, and (c) FTO with compact ZnO and ZnO nanofibers-using a bath prepared with CdCl2·2.5H2O, (0.05 M), sodium citrate (Na3C6H5O7, 0.5 M), potassium hydroxide (KOH, 0.5 M), a borate buffer at pH 10, and 7.5 mL of thiourea (CS(NH2)2).; the deposition was carried out at 60 °C for 60 min following the procedure reported by Pérez-García C. E. et al. [].
2.4. Deposition of PbS Thin Film
Finally, PbS was deposited by CBD on the CdS layer from a solution containing lead acetate (Pb(C2H3O2)2, 0.5 M), triethanolamine (C6H15NO3, 1 M), sodium hydroxide (NaOH, 2 M), and thiourea (CS(NH2)2, 1 M), with the deposition performed at 26 °C for 4–5 h as described by C.E. Pérez-García et al. [].
2.5. Fabrication of the Devices and Application of Contacts
After forming all the semiconductor layers, silver top contacts were applied to the PbS surface using commercial silver paint (SPI Supplies®, West Chester, PA, USA), and the devices were heated at 80 °C for 1 h in air to improve adhesion and conductivity [].
Three device configurations were fabricated to evaluate the role of the ZnO layers (Figure 1):
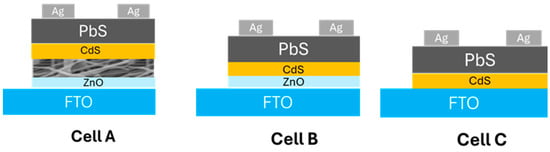
Figure 1.
Cross-sectional schematics of the three device architectures evaluated. Cell A: FTO/ZnO compact layer (sol–gel)/electrospun ZnO nanofiber network/CdS (CBD)/PbS (CBD)/Ag top contacts; the nanofiber network acts as a functional interlayer to improve interfacial coverage and electron selectivity. Cell B: FTO/ZnO compact layer/CdS/PbS/Ag, used to isolate the effect of the compact ZnO without nanofibers. Cell C: FTO/CdS/PbS/Ag (no ZnO), serving as the baseline control. Schematics are not to scale.
- •
- Cell A: FTO/compact ZnO (sol–gel)/ZnO nanofibers/CdS/PbS/Ag
- •
- Cell B: FTO/compact ZnO (sol–gel)/CdS/PbS/Ag
- •
- Cell C: FTO/CdS/PbS/Ag.
2.6. Characterization Techniques
The film thickness of the deposited layers (ZnO, CdS, and PbS) was measured using a stylus profilometer (KLA Tencor D100, Milpitas, CA, USA). A masked step edge was created during deposition for each layer, and the resulting step height was used to determine the film thickness.
X-ray diffraction (XRD) patterns were recorded with a Panalytical Empyrean diffractometer equipped with Cu Kα radiation (λ = 1.5406 Å), operating in the 2θ range of 20–70° for ZnO and 20–80° for CdS and PbS, with a step size of 0.02° and a scan rate of 2°·min−1. The crystallite size (D) for each film was estimated using the Debye–Scherrer equation, as implemented in the Jade software (MDI Jade 6.5).
Surface morphology and chemical composition were analyzed using a JEOL JSM-6010 Plus scanning electron microscope (Akishima, Tokyo, Japan) (SEM) equipped with an energy-dispersive X-ray spectroscopy (EDS) detector.
Optical properties of ZnO and CdS thin films were examined by UV–Vis absorption spectroscopy using a Thermo Scientific Genesis 10S spectrophotometer (Waltham, MA, USA) in the 300–800 nm wavelength range. The optical band gap (Eg) was estimated from Tauc plots, assuming direct allowed transitions.
For PbS, the optical band gap was evaluated through diffuse reflectance infrared spectroscopy (DRIFTS) using a Nicolet iS50 Fourier-transform infrared (FTIR) spectrometer (Waltham, MA, USA), which allowed the estimation of E9 in the near-infrared (NIR) region.
Hall-effect measurements were performed using an Ecopia HMS-3000 (Anyang-si, Gyeonggi-do, Republic of Korea) system in a van der Pauw configuration, with ohmic silver-paint contacts, to determine carrier type, concentration, mobility, and resistivity.
Current–voltage (I–V) measurements were carried out under simulated AM 1.5 G illumination (100 mW·cm−2) using an Oriel solar simulator (Newport model 91160, Irvine, CA, USA), to evaluate the photovoltaic performance of the fabricated heterostructures.
3. Results and Discussion
3.1. Film Thickness Determination
To quantify the thickness of each layer, profilometric measurements were performed. Step edges were defined on each sample and scanned to obtain the thickness of the compact ZnO underlayer, the CBD-CdS buffer (60 min), and the CBD-PbS absorber deposited for 4 h and 5 h, respectively. Table 1 summarizes the values obtained. As expected for chemical bath deposition, the PbS thickness increases with deposition time. In contrast, the CdS thickness after 60 min falls within the range typically used for n-type window/buffer layers in thin film photovoltaics.

Table 1.
Thicknesses of compact ZnO, CdS, and PbS thin films.
The ZnO value reported corresponds to the compact sol–gel layer prior to the deposition of electrospun fibers.
3.2. Structural Analysis (XRD)
X-ray diffraction (XRD) patterns were obtained for the compact ZnO film (glass/ITO), electrospun ZnO nanofibers deposited on glass/ITO, CBD-CdS on glass, and CBD-PbS on glass. All diffractograms were processed with uniform background subtraction and substrate peak annotation. Reference PDF cards were used solely for phase identification: ZnO wurtzite (hexagonal) [PDF 21-4186], CdS wurtzite (hexagonal) [PDF 00-041-104] and zinc blende (cubic) [PDF 01-089-0440], and PbS galena (cubic) [PDF 05-0592].
Crystallite sizes (D) were estimated from the diffraction peak broadening using the Debye–Scherrer Equation (1):
where K is the shape factor, λ the X-ray wavelength, β the FWHM of the selected peak (in radians) after background fitting, and θ the Bragg angle. The analysis was performed on the dominant reflections of each phase. The resulting sizes confirm the nanocrystalline nature of all films and fibers.
The sol–gel ZnO compact film exhibits diffraction features consistent with the hexagonal wurtzite structure, with the characteristic reflections [(hkl) = 100] observed at [2θ = 30.67°] (Figure 2a). The estimated crystallite size is 26.3 nm, confirming its nanocrystalline character. The electrospun ZnO nanofiber network, after heat treatment, retains the wurtzite phase, indicating complete conversion of the precursor and retention of the 1D crystal structure (Figure 2b). The crystallite size obtained by Debye–Scherrer is 14.8 nm.
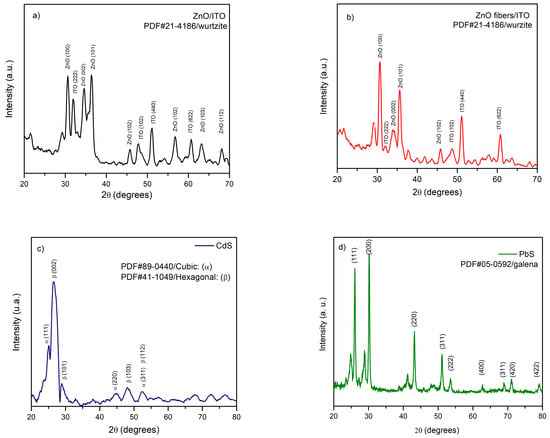
Figure 2.
(a) XRD pattern of the sol–gel compact ZnO film on [FTO/ITO], indexed to the hexagonal wurtzite phase [PDF 21-4186]. (b) XRD pattern of electrospun ZnO nanofibers after heat treatment, also indexed to wurtzite [PDF 21-1486]. (c) XRD of CBD-CdS showing mixed wurtzite (hexagonal) [PDF 41-1049] and zinc-blende (cubic) [PDF 89-0440] polymorphs characteristic of low-temperature deposition. (d) XRD of CBD-PbS, indexed to the cubic galena structure [PDF 05-0592].
The CBD-CdS film exhibits coexistence of the wurtzite (hexagonal) and zinc-blende (cubic) polymorphs, typical of low-temperature chemical growth. The diffraction peaks appear broadened due to reduced film thickness and small coherent domains (Figure 2c), corresponding to an average crystallite size of 18.5 nm.
The CBD-PbS samples are indexed to the cubic galena structure, with crystallite sizes of 17.5 nm (4 h) and 20.4 nm (5 h), and no secondary lead oxysulfide phases were detected under the present acquisition conditions (Figure 2d) [,]. The increase in crystallite size with deposition time indicates a slight improvement in crystalline order, consistent with the extended reaction kinetics of the CBD process.
The preservation of wurtzite in electrospun ZnO after calcination at (500 °C, 6 h) indicates complete conversion of the polymer/acetate precursor without inducing secondary phases. The mixed CdS polymorphs reflect the low-temperature CBD kinetics, whereas cubic galena PbS is stabilized under our alkaline bath.
3.3. Surface Morphology and Elemental Composition (SEM/EDS)
The surface morphology of the compact ZnO film, electrospun ZnO nanofibers, and the CBD-grown CdS and PbS films was analyzed by scanning electron microscopy (SEM). The micrographs were used to assess coating homogeneity, nanofiber formation, and film continuity across the stack.
Figure 3a shows the compact ZnO film with a continuous, feature-uniform surface typical of sol–gel coatings. Electrospun ZnO fibers deposited on the conductive oxide (Figure 3b) form a randomly oriented network, as expected for collection on a planar grounded plate. Higher magnification (Figure 3c) resolves individual fibers of 53.4 nm and 49.9 nm, indicating a narrow size distribution consistent with a stable electrospinning process. Such diameter control is relevant for applications where one-dimensional transport and interfacial area govern device response.
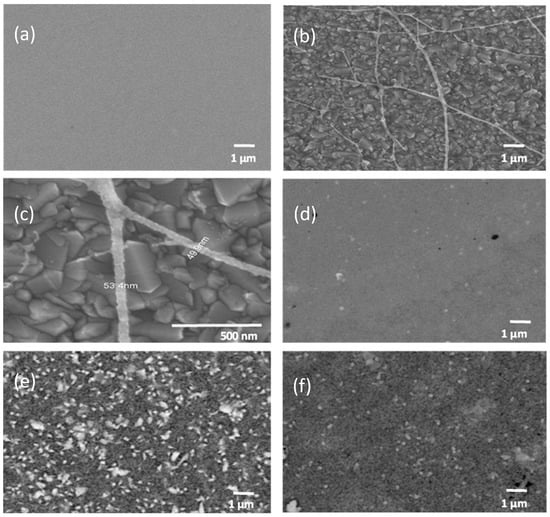
Figure 3.
(a) Compact ZnO (sol–gel), 10,000×. (b) Electrospun ZnO nanofibers, 10,000×. (c) Electrospun ZnO nanofibers, 200,000× (individual fibers highlighted). (d) CBD–CdS, 60 min, 10,000×. (e) CBD–PbS, 5 h, 10,000×. (f) CBD–PbS, 4 h, 10,000×.
The CdS film deposited for 60 min (Figure 3d) exhibits a continuous coating with the usual CBD-derived texture, including local porosity and agglomerations. Point EDS measurements at five positions (Table 2) yield average compositions of S (atomic %) = 45.09 and Cd (atomic %) = 54.91, close to CdS stoichiometry and within expected dispersion for thin CBD layers probed at moderate accelerating voltage.

Table 2.
Elemental composition of the CBD-CdS film (five positions).
For PbS, SEM images acquired after 4 h and 5 h (Figure 3e,f) reveal dense, homogeneous surfaces composed of irregular grains in the 50–150 nm range, uniformly distributed across the substrate. The commonly reported “cabbage-like” PbS surface texture [] is not observed under our low-temperature conditions, consistent with reduced atom mobility and more uniform nucleation that suppresses overgrowth. This type of morphology is favorable for increasing the effective surface area and enhancing interaction with the CdS window layer in the solar cell. EDS analysis (Table 3) confirms the presence of the main elements, with average atomic fractions of Pb (51.35%) and S (48.64%).

Table 3.
Elemental composition of the CBD-PbS film (five positions).
To further verify the chemical uniformity of the CdS and PbS films, elemental mapping (EDS mapping) was performed for all layers. The complete elemental distribution maps are provided in the Supplementary Information (Figures S1 and S2).
3.4. Optical Absorption and Bandgap Determination
UV analyzed the optical response of the thin films–Vis spectroscopy for the CdS and ZnO layers, and by Fourier-transform infrared (FTIR) diffuse reflectance for the PbS film. The spectra of ZnO and CdS were recorded in the 300–1000 nm range and used to construct Tauc plots, assuming direct allowed electronic transitions to estimate the optical band gaps (2), i.e.,
where α is the absorption coefficient, hν is the photon energy (measured in eV), A is the material-dependent constant, n equals 2 for direct transitions, and Eg is the optical bandgap. For PbS, near-infrared reflectance R(ν) in the 4000–2000 cm−1 range (energy window capturing the fundamental edge of PbS) was transformed using the Kubelka-Munk function (3);
Tauc analysis was performed with (F(R) = hν)2 vs. hν, consistent with direct-allowed transitions in PbS.
The ZnO compact film exhibits a sharp absorption edge at ~350 nm and >90% transmittance in the visible spectrum, making it suitable as a front electron-selective coating. Linear fitting of the Tauc region yields Eg = 3.28 eV (Figure 4a), consistent with phase-pure wurzite ZnO [,]. The CdS film shows an absorption edge near 500 nm, separating the strong-absorption region from the high-transmittance window: the corresponding Tauc fit gives Eg = 2.44 eV (Figure 4b), within the expected range for CBD-grown CdS, thin films [].
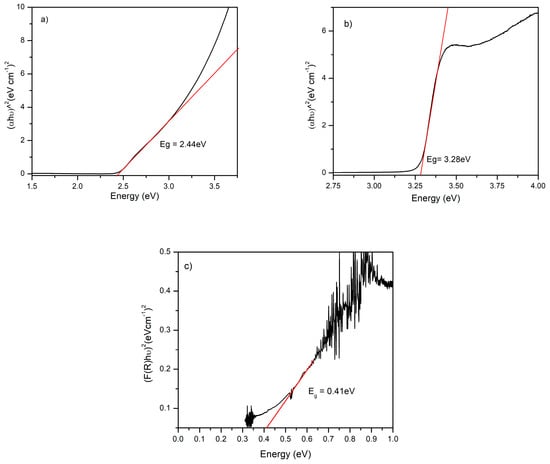
Figure 4.
Bandgap determination by Tauc analysis. (a) ZnO: (αhν)2 vs. hν (UV–Vis), (b) CdS: (αhν)2 vs. hν (UV–Vis), (c) PbS: (F(R) hν)2 vs. hν. Fit windows are highlighted in red.
For PbS, FTIR-based Tauc analysis yields Eg = 0.40 eV for the 4 h deposited film (Figure 4c). The proximity to the bulk value (≈0.41 eV at room temperature) suggests limited quantum confinement effects, in line with the SEM observed grain sizes (50–150 nm) and dense coverage (α) was estimated from transmittance using Beer-Lambert with thickness (d) from profilometry (α = −lnT/d). FTIR reflectance was collected in diffuse mode and converted to F(R) using the Kubelka-Munk equation before Tauc analysis.
3.5. Hall Transport and Resistivity of ZnO, CdS, and PbS Films
Carrier type, carrier concentration (n or p), Hall mobility (µ), and resistivity (ρ) were determined using Hall-effect measurements in a Van der Pauw configuration (Table 4).

Table 4.
Hall-effect transport parameters of the CdS, PbS, and ZnO films.
Negative Hall coefficients for ZnO and CdS indicate n-type conductivity, while PbS films exhibit p-type behavior under our CBD conditions. The CdS (60 min) sample displays a low carrier density (~4.8 × 1014 cm−3) with modest mobility (4.35 cm2V−1s−1), yielding a highly resistive film below the 1015–1017 cm−3 range commonly reported for CBD-CdS and consistent with higher ρ values [,]. Such behavior may be associated with sulfur-rich stoichiometry and the formation of intrinsic defects during low-temperature deposition conditions typical of CBD growth.
The PbS films deposited for 4 h and 5 h show high hole mobilities (146 and 143 cm2V−1s−1, respectively), but low carrier concentrations (7.2 × 1015 and 1.7 × 1016 cm−3), i.e., one to two orders below the typical CBD-PbS level. This explains the relatively high ρ values compared with reported values for PbS films grown at higher temperatures or with optimized sulfur activity [].
The ZnO films synthesized by the sol–gel method exhibit a very low electron concentration (~1.5 × 1014 cm−3) and moderate mobility (5.85 cm2V−1s−1), thus acting primarily as an electron-selective, hole-blocking coating; reported sol–gel ZnO often shows 1016–1018 cm−3, 5–40 cm2V−1s−1, 1–102 Ωcm [,]. This confirms that the ZnO synthesized in this work is highly resistive, which is likely due to a low density of donor defects, such as oxygen vacancies or interstitial Zn atoms. From a photovoltaic standpoint, this behavior is advantageous, as the ZnO layer, particularly when combined with electrospun ZnO nanofibers, acts as a hole-blocking and structural support layer, rather than a conductive transport medium.
Taken together, the high mobility but low effective doping in CBD-PbS and the highly resistive, electron-selective ZnO align with the series-resistance-limited fill factors reported later; the nanofiber scaffold reduces lateral transport bottlenecks by providing percolation pathways while still blocking holes at the front contact.
3.6. Device Efficiency
The current–voltage (I–V) characteristics of the solar cells were measured under simulated AM 1.5 G illumination (100 mW·cm−2) by scanning the applied voltage between −1.0 and +1.0 V, as shown in Figure 5a.
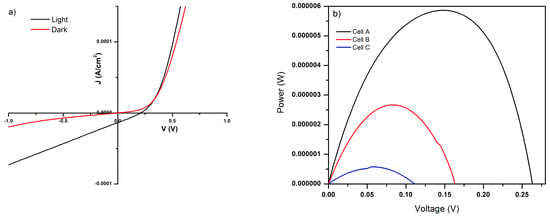
Figure 5.
(a) J–V characteristic curves for the CdS/PbS heterojunction; the red line corresponds to dark conditions and the black line to illuminated conditions. (b) Power–voltage curves for the heterojunctions.
From these curves, the photovoltaic parameters-short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF), and power conversion efficiency (η) were calculated. The cell efficiency was determined using the following equation:
The data presented in Table 5 show how the performance of the CdS/PbS solar cell is affected by the use of intermediate layers.

Table 5.
Solar cell parameters.
Cell C exhibited limited photovoltaic performance, with a Voc of 0.11 V, an Isc of 1.86 × 10−5 A, and an efficiency of 1.83 × 10−2%. These results indicate strong interfacial recombination and low crystalline quality in the PbS film obtained by chemical bath deposition at low temperature. This observation is consistent with previous reports, where PbS layers grown under similar conditions exhibit a high density of surface defects and limited carrier mobility, resulting in weak photovoltaic response [,].
The incorporation of a compact ZnO layer deposited by sol–gel method significantly improved the device performance, increasing the Voc to 0.16 V and the Isc to 6.7 × 10−5 A, with an efficiency of 8.37 × 10−2%. This improvement can be attributed to the function of ZnO as an electron transport layer, acting as a hole-blocking barrier and reducing recombination losses at the FTO/CdS interface. Similar improvements have been observed in PbS- and CdS-based heterojunctions incorporating metal oxide interlayers, where ZnO facilitates charge separation and extraction [,].
Absolute efficiencies are lower than optimized CdS/PbS devices because the CBD films exhibit intrinsically low carrier concentrations and no post-annealing or ligand-exchange treatments were applied. These constraints are deliberate to isolate the ZnO nanofiber interlayer on charge extraction and interface quality.
It is important to note that all ZnO, CdS, and PbS layers were deposited under fixed conditions using constant deposition times, resulting in comparable thicknesses across the three device configurations. Therefore, the performance differences between Cells A, B, and C cannot be attributed to variations in film thickness. The improvements observed in the ZnO-containing devices arise from the interfacial and morphological effects introduced by the compact ZnO layer and the electrospun nanofiber network, rather than dimensional changes.
The best performance was obtained with the Cell A configuration, which integrates electrospun ZnO nanofibers on top of the sol–gel ZnO layer, achieving a Voc of 0.26 V, an Isc of 1.0 × 10−4 A, and an efficiency of 0.187%. Although this efficiency remains below the typical values reported for CdS/PbS solar cells (ranging from 0.033% to 1.35%) [,], it represents a nearly tenfold improvement over Cell B and an enhancement of one order of magnitude compared to the reference Cell C (Figure 5b).
The increase in device efficiency is primarily attributed to the morphological advantages of the ZnO nanofibers. Their high aspect ratio and interconnected structure increase the interfacial area with CdS, promoting efficient charge separation and providing direct pathways for electron transport toward the FTO electrode. Consequently, the incorporation of electrospun ZnO nanofibers effectively reduces recombination losses and enhances carrier collection efficiency in the CdS/PbS heterostructure.
The fill factor (FF) remained low in all configurations (0.22–0.28), reflecting significant resistive losses and poor interface quality. The fill factor was measured using the following Equation (5):
In particular, the low carrier density in CdS and ZnO limits charge transport and contributes to the low Isc values. Likewise, the absence of post-deposition thermal treatments for PbS could have prevented the reduction in surface defects, affecting carrier lifetimes and, consequently, efficiency.
The improvement observed in Cell A is primarily attributed to the morphological effect of the electrospun ZnO nanofibers, whose high-aspect-ratio and interconnected structure provides continuous electron percolation pathways and improved conformal contact with the CBD-grown CdS and PbS layers, thereby reducing interface-assisted recombination. Although no intentional band-structure engineering was applied, the expected conduction band alignment between ZnO, CdS, and PbS supports selective electron extraction and hole blocking, which complements the morphological contribution and further promotes charge separation across the heterojunction.
Compared to the literature, where typical Voc values for PbS/CdS heterostructures range between 0.3 and 0.6 V and short-circuit currents (Jsc) are in the 10–20 mA/cm2 range [,], the values obtained in this work are one to two orders of magnitude lower. Nevertheless, the systematic increase observed upon introducing ZnO layers, particularly when combined with electrospun ZnO nanofibers, demonstrates the effectiveness of this strategy in enhancing charge separation and transport. These results confirm the potential of this approach to improve the photovoltaic performance of PbS-based solar cells synthesized through low-cost, solution-based techniques, such as chemical bath deposition.
4. Conclusions
The results show that, although the carrier mobilities fall within the characteristic ranges of each material, the films exhibit low carrier concentrations and high resistivities compared to literature reports. This behavior highlights the strong influence of low-temperature synthesis conditions in both the CBD and sol–gel methods, which can be further exploited to optimize the electronic properties of each layer within the PbS/CdS/ZnO solar cell architecture. These findings indicate that optimizing the deposition conditions for CdS and PbS and applying moderate post-deposition annealing and surface doping of ZnO are key strategies to enhance device performance. The integration of ZnO nanofibers is a promising approach to overcome the limitations of low crystallinity and high defect density in low-cost photovoltaic devices.
The results confirm that incorporating ZnO into the CdS/PbS heterostructure (Cell C) significantly enhances the device’s photoelectric response. The observed increase in Voc and Isc demonstrates that ZnO facilitates charge separation and transport, reducing losses associated with carrier recombination. Consequently, the overall efficiency of Cell B increases by approximately one order of magnitude compared with Cell C, which lacked the ZnO interlayer.
Furthermore, it was demonstrated that the morphology of the ZnO layer plays a decisive role in photovoltaic performance by influencing the series resistance and fill factor. In particular, incorporating electrospun ZnO nanofibers forms a three-dimensional conductive network that enhances electron transport and improves interlayer connectivity, resulting in a further significant increase in efficiency, about one order of magnitude higher than that of the CdS/PbS heterostructure.
These results emphasize the importance of morphological control in semiconductor heterostructures and suggest that integrating nanostructured intermediate layers, such as ZnO fibers, is a promising strategy to optimize charge collection and maximize solar cell performance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings15121371/s1, Figure S1: SEM–EDS elemental mapping of the CBD-CdS thin film, showing Cd and S distribution; Figure S2: SEM–EDS elemental mapping of the CBD-PbS thin film, showing Pb and S distribution.
Author Contributions
Conceptualization, R.H.-H. and C.E.P.-G.; methodology, R.H.-H., A.G.-P. and C.E.P.-G.; validation, R.H.-H., F.d.M.-F. and J.S.-C.; formal analysis, A.G.-P., L.L.-J. and C.E.P.-G.; investigation, R.H.-H. and C.E.P.-G.; resources, F.d.M.-F., J.S.-C. and L.L.-J.; data curation, R.H.-H. and C.E.P.-G.; writing—original draft preparation, R.H.-H., A.G.-P. and C.E.P.-G.; writing—review and editing, A.G.-P., L.L.-J. and C.E.P.-G.; visualization, A.G.-P.; supervision, C.E.P.-G.; project administration, A.G.-P. and C.E.P.-G.; funding acquisition, C.E.P.-G. and A.G.-P. All authors have read and agreed to the published version of the manuscript.
Funding
Hernández-Hernández acknowledges the financial support received from CONAHCYT through a graduate scholarship. The authors are also grateful to the Universidad Autónoma de Querétaro (UAQ) for the financial support provided through the project FONFIVE-FQU202416.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank the Centro de Investigación en Materiales Avanzados (CIMAV), Monterrey Unit, for providing access to their facilities and for the technical assistance with SEM and XRD characterizations.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ZnO | Zinc oxide |
| CdS | Cadmium sulfide |
| PbS | Lead sulfide |
| CBD | Chemical bath deposition |
| EDS | Energy dispersive spectroscopy |
| Voc | Open circuit voltage |
| Isc | Short-circuit intensity |
| ETL | Electron transport layer |
| FF | Fill-factor |
| 1D | One-dimensional |
| FTO | Fluorine-doped tin oxide |
| DI | Distilled water |
| PVA | Polyvinyl alcohol and polyvinyl acetate |
| XRD | X-ray diffraction |
| ITO | Indium tin oxide |
| FTIR | Fourier-transform infrared |
References
- Sivaraj, S.; Rathanasamy, R.; Kaliyannan, G.V.; Panchal, H.; Jawad Alrubaie, A.; Musa Jaber, M.; Said, Z.; Memon, S. A Comprehensive Review on Current Performance, Challenges and Progress in Thin-Film Solar Cells. Energies 2022, 15, 8688. [Google Scholar] [CrossRef]
- Villarreal Gómez, L.J.; Iglesias, A.L.; Miranda Soto, V.; Olivas Sarabia, A.; Valdez Castro, R.; López Maldonado, E.A.; Oropeza Guzmán, M.T.; Romero Soto, C.A.; Lizarraga Medina, E.G.; Vazquez Arce, J.L. Study of Electrospun Nanofibers Loaded with Ru(Ii) Phenanthroline Complexes as a Potential Material for Use in Dye-Sensitized Solar Cells (DSSCs). RSC Adv. 2023, 13, 36023–36034. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Tran, T.N.; Le, M.T.; Ullah, A.; Phan, D.N.; Kim, I.S. Enhanced Dye Removal and Antibacterial Efficacy of Copper-Doped ZnO Nanoparticles on Cellulose Nanofibers. Adv. Mater. Interfaces 2024, 11, 1–11. [Google Scholar] [CrossRef]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A Review on Electrospun Nanofibers Based Advanced Applications: From Health Care to Energy Devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.K.; Nair, M.T.S.; García, V.M.; Arenas, O.L.; Peña, Y.; Castillo, A.; Ayala, I.T.; Gomezdaza, O.; Sánchez, A.; Campos, J.; et al. Semiconductor Thin Films by Chemical Bath Deposition for Solar Energy Related Applications. Sol. Energy Mater. Sol. Cells 1998, 52, 313–344. [Google Scholar] [CrossRef]
- Sengupta, S.; Aggarwal, R.; Raula, M. A Review on Chemical Bath Deposition of Metal Chalcogenide Thin Films for Heterojunction Solar Cells. J. Mater. Res. 2023, 38, 142–153. [Google Scholar] [CrossRef]
- Castillo-Sánchez, Y.B.; González, L.A. Chemically Deposited PbS Thin Films by Reaction Media with Glycine for Use in Photovoltaics. Mater. Sci. Semicond. Process 2021, 121, 105405. [Google Scholar] [CrossRef]
- Aouf, D.; Henni, A.; Selloum, D.; Khane, Y.; Fenniche, F.; Zerrouki, D.; Belkhalfa, H.; Dizge, N. Facile Preparation and Characterization of Nanostructured ZnS/PbS Heterojunction Thin Films for Enhanced Microbial Inhibition and Photocatalytic Degradation. Mater. Chem. Phys. 2023, 295, 127059. [Google Scholar] [CrossRef]
- Khurshid, S.; Latif, H.; Rasheed, S.; Sharif, R.; Sattar, A.; Amjad, R.J. Enhancement in Absorption Spectrum by ITO Coated, down Converting Glass as a Photoanode Substrate for Efficient PbS/CdS Quantum Dots Sensitized ZnO Nano-Rods Array Solar Cell. Opt. Mater. 2022, 124, 111991. [Google Scholar] [CrossRef]
- Mezher, M.J.; Kudhier, M.A.; Dakhil, O.A. Using ZnO-CdS Composite Nanofibers in the Photolytic Activity Under Sunlight Irradiation. arXiv 2023. [Google Scholar] [CrossRef]
- Hernández-León, P.A.; Castillo-Alvarado, F.L.; González-Cisneros, A.; Durán-Ledezma, A.A. C-V Model of CdS/CdTe Thin-Film Solar Cells Dependent on Applied Voltage Frequency. Rev. Mex. Fis. 2023, 69, 041604. [Google Scholar] [CrossRef]
- Pérez-García, C.E.; Ramírez-Bon, R.; Vorobiev, Y.V. PbS Thin Films Growth with CBD and PCBD Techniques: A Comparative Study. Chalcogenide Lett. 2015, 12, 579–588. [Google Scholar]
- Mohtaram, F.; Borhani, S.; Ahmadpour, M.; Fojan, P.; Behjat, A.; Rubahn, H.G.; Madsen, M. Electrospun ZnO Nanofiber Interlayers for Enhanced Performance of Organic Photovoltaic Devices. Sol. Energy 2020, 197, 311–316. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.P.; Kim, D.H.; Hwang, D.K. Electrospun ZnO Nanofibers as a Photoelectrode in Dye-Sensitized Solar Cells. J. Nanosci. Nanotechnol. 2015, 15, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- López-Covarrubias, J.G.; Soto-Muñoz, L.; Iglesias, A.L.; Villarreal-Gómez, L.J. Electrospun Nanofibers Applied to Dye Solar Sensitive Cells: A Review. Materials 2019, 12, 3190. [Google Scholar] [CrossRef]
- Choi, S.H.; Ankonina, G.; Youn, D.Y.; Oh, S.G.; Hong, J.M.; Rothschild, A.; Kim, I.D. Hollow ZnO Nanofibers Fabricated Using Electrospun Polymer Templates and Their Electronic Transport Properties. ACS Nano 2009, 3, 2623–2631. [Google Scholar] [CrossRef]
- Arifin, Z.; Hadi, S.; Jati, H.N.; Prasetyo, S.D. Suyitno Effect of Electrospinning Distance to Fabricate ZnO Nanofiber as Photoanode of Dye-Sensitized Solar Cells. AIP Conf. Proc. 2020, 2217, 030095. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for Application in Photocatalysis: From Thin Films to Nanostructures. Mater. Sci. Semicond. Process 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of Nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doǧan, S.; Avrutin, V.; Cho, S.J.; Morko̧, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Lauth, J.; Failla, M.; Klein, E.; Klinke, C.; Kinge, S.; Siebbeles, L.D.A. Photoexcitation of PbS Nanosheets Leads to Highly Mobile Charge Carriers and Stable Excitons. Nanoscale 2019, 11, 21569–21576. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: From Basics towards Applications. Phys. Status Solidi Basic Res. 2007, 244, 3027–3073. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide-from Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Silva, Y.S.d.S.; Marques, M.d.F.V. Organic Solar Cells with Nanofibers in the Active Layer Obtained by Coaxial Electrospinning. Adv. Energy Convers. Mater. 2023, 4, 96–120. [Google Scholar] [CrossRef]
- Tanveer, M.; Habib, A.; Khan, M.B. Structural and Optical Properties of Electrospun ZnO Nanofibres Applied to P3HT:PCBM Organic Photovoltaic Devices. J. Exp. Nanosci. 2015, 10, 640–650. [Google Scholar] [CrossRef]
- Hakim, A.A.N.; Rashid, A.R.A.; Arsad, N.; Surani, A.H. Zinc Oxide Thin Film Synthesized by Sol-Gel Method. Solid. State Phenom. 2020, 307, 51–57. [Google Scholar] [CrossRef]
- Najm, A.S.; Chelvanathan, P.; Tiong, S.K.; Ferdaous, M.T.; Shahahmadi, S.A.; Yusoff, Y.; Sopian, K.; Amin, N. Numerical Insights into the Influence of Electrical Properties of N-CdS Buffer Layer on the Performance of SLG/Mo/p-Absorber/n-CdS/n-ZnO/Ag Configured Thin Film Photovoltaic Devices. Coatings 2021, 11, 52. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Yap, B.K.; Islam, M.A.; Yong, T.C.; Kiong, T.S.; Chan, K.Y.; Thien, G.S.H.; Basher, M.K.; Tamam, N.; Khandaker, M.U. Spin-Coated Mg-Doped ZnO Thin Films as Electron Transport Layers for Efficient and Stable Perovskite Solar Cells. Sci. Rep. 2025, 15, 36618. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wu, Y.; Song, J.; Wang, W.; Li, Z. Efficient Planar Perovskite Solar Cells with ZnO Electron Transport Layer. Coatings 2022, 12, 1981. [Google Scholar] [CrossRef]
- Sharma, R.; Lee, H.; Borse, K.; Gupta, V.; Joshi, A.G.; Yoo, S.; Gupta, D. Ga-Doped ZnO as an Electron Transport Layer for PffBT4T-2OD: PC70BM Organic Solar Cells. Org. Electron. 2017, 43, 207–213. [Google Scholar] [CrossRef]
- Yeon, D.H.; Mohanty, B.C.; Lee, C.Y.; Lee, S.M.; Cho, Y.S. High-Efficiency Double Absorber PbS/CdS Heterojunction Solar Cells by Enhanced Charge Collection Using a ZnO Nanorod Array. ACS Omega 2017, 2, 4894–4899. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Marsudi, M.A.; Amal, M.I.; Ananda, M.B.; Stephanie, R.; Ardy, H.; Diguna, L.J. ZnO Nanostructured Materials for Emerging Solar Cell Applications. RSC Adv. 2020, 10, 42838–42859. [Google Scholar] [CrossRef]
- Bakry, M.; Ismail, W.; Abdelfatah, M.; El-Shaer, A. Low-Cost Fabrication Methods of ZnO Nanorods and Their Physical and Photoelectrochemical Properties for Optoelectronic Applications. Sci. Rep. 2024, 14, 23788. [Google Scholar] [CrossRef] [PubMed]
- Obaid, A.S.; Hassan, Z.; Mahdi, M.A.; Bououdina, M. Fabrication and Characterisations of N-CdS/p-PbS Heterojunction Solar Cells Using Microwave-Assisted Chemical Bath Deposition. Sol. Energy 2013, 89, 143–151. [Google Scholar] [CrossRef]
- Anero, M.L.A.; Montallana, A.D.S.; Vasquez, M.R. Fabrication of Electrospun Poly(Vinyl Alcohol) Nanofibers Loaded with Zinc Oxide Particles. Results Phys. 2021, 25, 104223. [Google Scholar] [CrossRef]
- Hodes, G.; Gal, D.; Schock, H. Chemical Solution Deposition of Semiconductor Films; Taylor & Francis: Abingdon, UK, 2000; Volume 362, ISBN 0824708512. [Google Scholar]
- Ortega-Borges, R.; Lincot, D. Mechanism of Chemical Bath Deposition of Cadmium Sulfide Thin Films in the Ammonia-Thiourea System: In Situ Kinetic Study and Modelization. J. Electrochem. Soc. 1993, 140, 3464–3473. [Google Scholar] [CrossRef]
- Dofia, J.M.; Herrero, J. Chemical Bath Deposition of CdS Thin Films: An Approach to the Chemical Mechanism Through Study of the Film Microstructure. J.Electrochem. Soc. 1997, 144, 4081–4091. [Google Scholar]
- Seghaier, S.; Kamoun, N.; Brini, R.; Amara, A.B. Structural and Optical Properties of PbS Thin Films Deposited by Chemical Bath Deposition. Mater. Chem. Phys. 2006, 97, 71–80. [Google Scholar] [CrossRef]
- Pérez-García, C.E.; Meraz-Dávila, S.; Arreola-Jardón, G.; De Moure-Flores, F.; Ramírez-Bon, R.; Vorobiev, Y.V. Characterization of PbS Films Deposited by Successive Ionic Layer Adsorption and Reaction (SILAR) for CdS/PbS Solar Cells Application. Mater. Res. Express 2020, 7, 015530. [Google Scholar] [CrossRef]
- Glatthaar, R.; Huster, F.; Okker, T.; Cela Greven, B.; Seren, S.; Hahn, G.; Terheiden, B. Contact Formation of Silver Paste and Atmospheric Pressure Chemical Vapor Deposition (n) Poly-Silicon Passivating Contacts on Planar and Textured Surfaces. Phys. Status Solidi Appl. Mater. Sci. 2022, 219, 2200501. [Google Scholar] [CrossRef]
- Ismail, W.; Ibrahim, G.; Habib, M.A.; Alduaij, O.K.; Abdelfatah, M.; El-Shaer, A. Advancement of Physical and Photoelectrochemical Properties of Nanostructured CdS Thin Films toward Optoelectronic Applications. Nanomaterials 2023, 13, 1764. [Google Scholar] [CrossRef]
- Carrasco-Chavez, L.A.; Rubio-Valle, J.F.; Jiménez-Pérez, A.; Martín-Alfonso, J.E.; Carrillo-Castillo, A. Study of CdS/CdS Nanoparticles Thin Films Deposited by Soft Chemistry for Optoelectronic Applications. Micromachines 2023, 14, 1168. [Google Scholar] [CrossRef]
- Barote, M.A.; Yadav, A.A.; Chavan, T.V.; Masumdar, E.U. Characterization and Photoelectrochemical Properties of Chemical Bath Deposited N-PbS Thin Films. Dig. J. Nanomater. Biostruct. (DJNB) 2011, 6, 979–990. [Google Scholar]
- Nasrin, T.; Selvanathan, V.; Islam, M.A.; Haque, M.M.; Rashid, A.W.; Ludin, N.A.; Chelvanathan, P.; Kiong, T.S.; Alanazi, A.M.; AlMohamadi, H.; et al. Exploring Ionic Liquid Assisted Chemical Bath Deposition of a Highly Uniform and Transparent Cadmium Sulfide Thin Film for Photovoltaic Applications. RSC Adv. 2025, 15, 4892–4903. [Google Scholar] [CrossRef] [PubMed]
- Ampadu, E.K.; Kim, J.; Oh, E. Lateral Pbs Photovoltaic Devices for High Performance Infrared and Terahertz Photodetectors. Nanomaterials 2021, 11, 1692. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.H.; Jeon, D.; Lee, S.N. High-Performance Sol–Gel-Derived CNT-ZnO Nanocomposite-Based Photodetectors with Controlled Surface Wrinkles. Materials 2024, 17, 5325. [Google Scholar] [CrossRef]
- Ierides, I.; Ligorio, G.; McLachlan, M.A.; Guo, K.; List-Kratochvil, E.J.W.; Cacialli, F. Inverted Organic Photovoltaics with a Solution-Processed Mg-Doped ZnO Electron Transport Layer Annealed at 150 °C. Sustain. Energy Fuels 2022, 6, 2835–2845. [Google Scholar] [CrossRef]
- Liu, X.; Ji, Y.; Xia, Z.; Zhang, D.; Cheng, Y.; Liu, X.; Ren, X.; Liu, X.; Huang, H.; Zhu, Y.; et al. In-Doped ZnO Electron Transport Layer for High-Efficiency Ultrathin Flexible Organic Solar Cells. Adv. Sci. 2024, 11, e2402158. [Google Scholar] [CrossRef]
- Lv, Q.; Li, R.; Fan, L.; Huang, Z.; Huan, Z.; Yu, M.; Li, H.; Liu, G.; Qiao, G.; Liu, J. High Detectivity of PbS Films Deposited on Quartz Substrates: The Role of Enhanced Photogenerated Carrier Separation. Sensors 2023, 23, 8413. [Google Scholar] [CrossRef]
- Yang, M.; Liu, H.; Wen, S.; Du, Y.; Gao, F. Optimizing the Infrared Photoelectric Detection Performance of Pbs Quantum Dots through Solid-State Ligand Exchange. Materials 2022, 15, 9058. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.B.; Ha, Y.; Choi, S.; Jung, D. Development of a Highly Reliable PbS QDs-Based SWIR Photodetector Based on Metal Oxide Electron/Hole Extraction Layer Formation Conditions. Nanomaterials 2025, 15, 1107. [Google Scholar] [CrossRef]
- Li, Y.; Wei, L.; Chen, X.; Zhang, R.; Sui, X.; Chen, Y.; Jiao, J.; Mei, L. Efficient PbS/CdS Co-Sensitized Solar Cells Based on TiO2 Nanorod Arrays. Nanoscale Res. Lett. 2013, 8, 67. [Google Scholar] [CrossRef]
- Obaid, A.S.; Mahdi, M.A.; Hassan, Z.; Bououdina, M. Preparation of Chemically Deposited Thin Films of CdS/PbS Solar Cell. Superlattices Microstruct. 2012, 52, 816–823. [Google Scholar] [CrossRef]
- Encinas-Terán, A.; Pineda-León, H.A.; Gómez-Colín, M.R.; Márquez-Alvarez, L.R.; Ochoa-Landín, R.; Apolinar-Iribe, A.; Gastélum-Acuña, S.L.; Mendívil-Reynoso, T.; Castillo, S.J. Synthesis and Characterization of a Semiconductor Diodic Bilayer PbS/CdS Made by the Chemical Bath Deposition Technique. ACS Omega 2024, 9, 24321–24332. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).