Recent Progress in Dielectric/Ag/Dielectric Transparent Electrodes on Flexible Substrates
Abstract
1. Introduction
2. Research Progress of the Ag Layer
2.1. Analysis of the Limitation Factors for the Optical–Electric Performance of DAD Transparent Electrodes
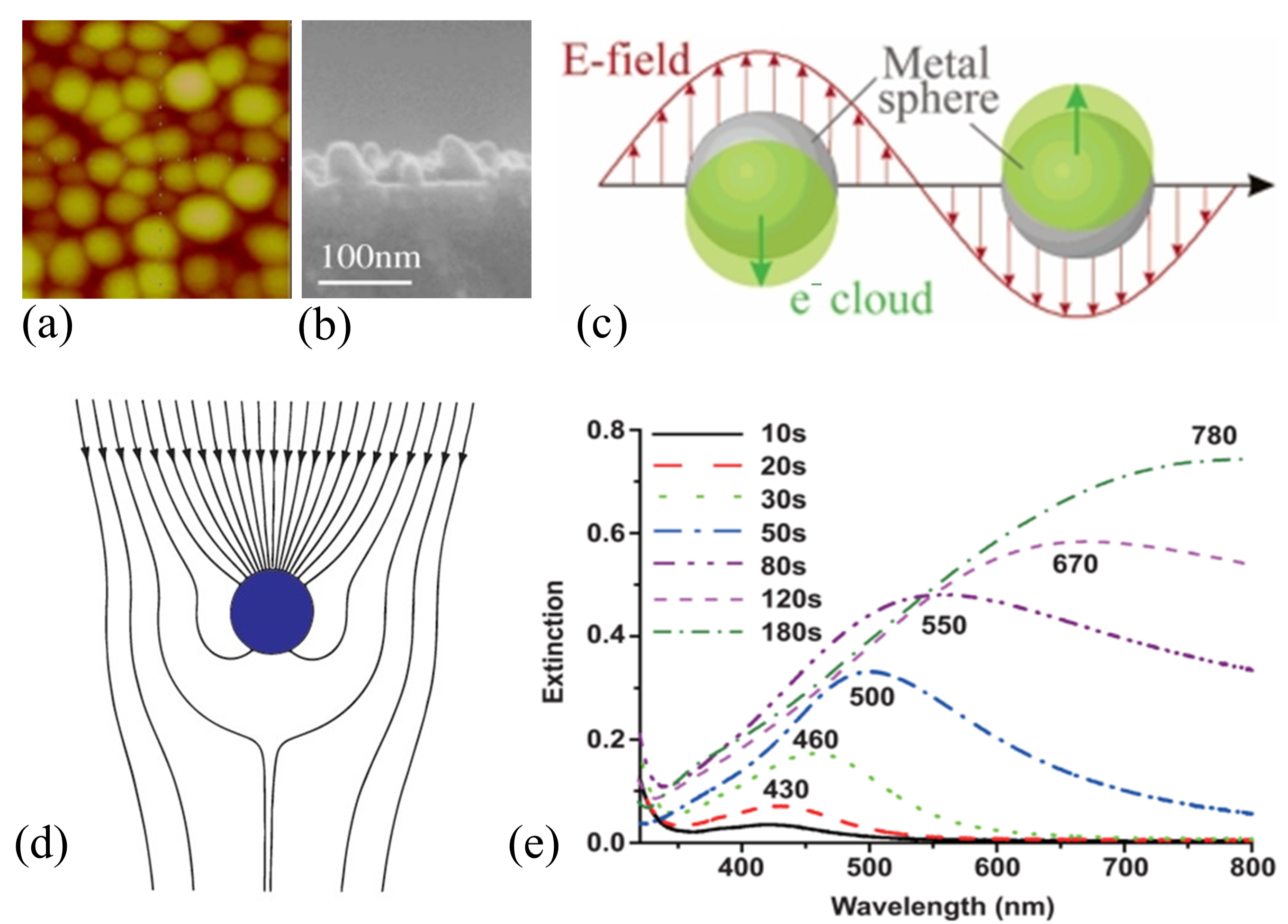
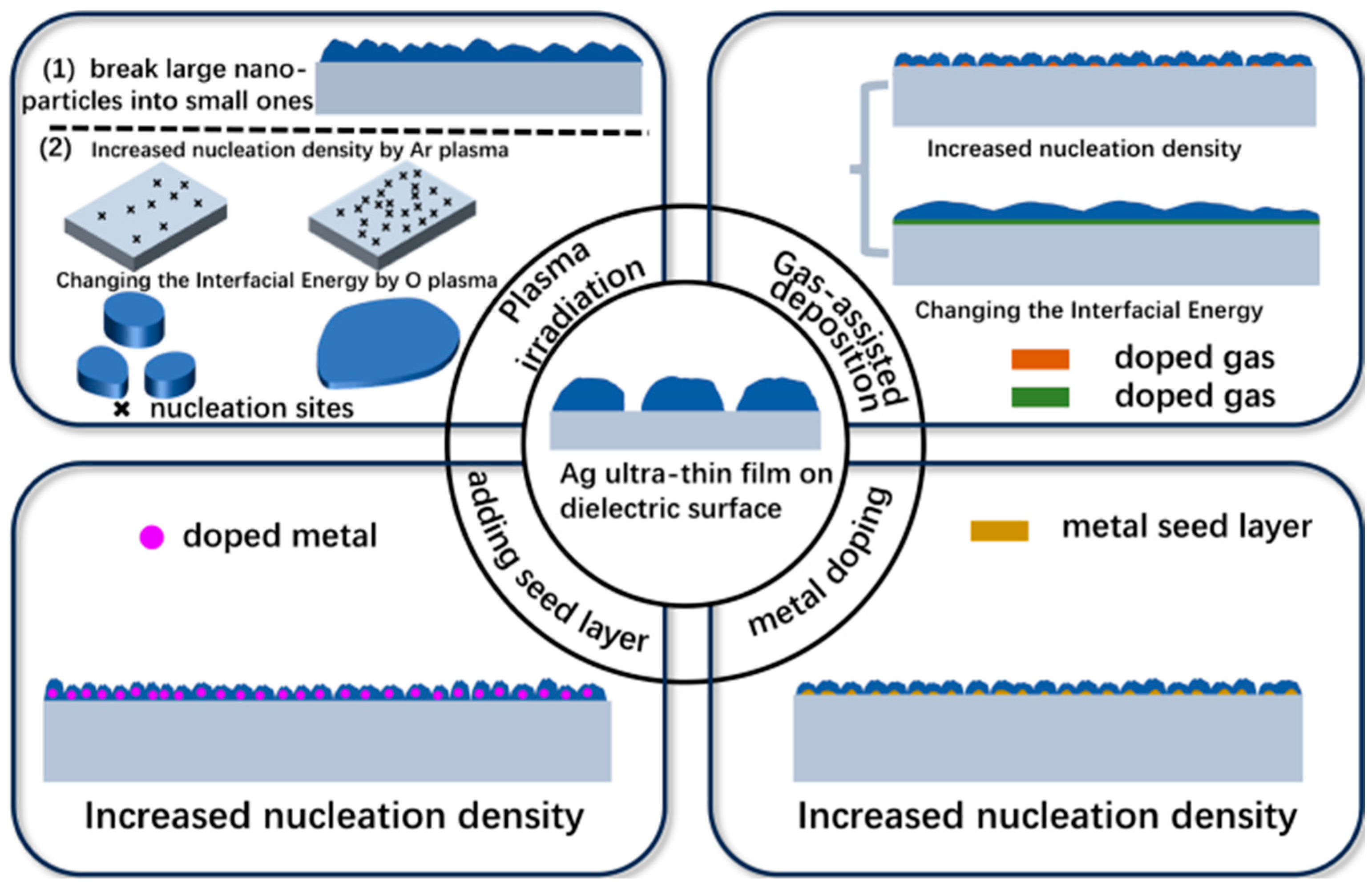
2.2. Plasma Irradiation
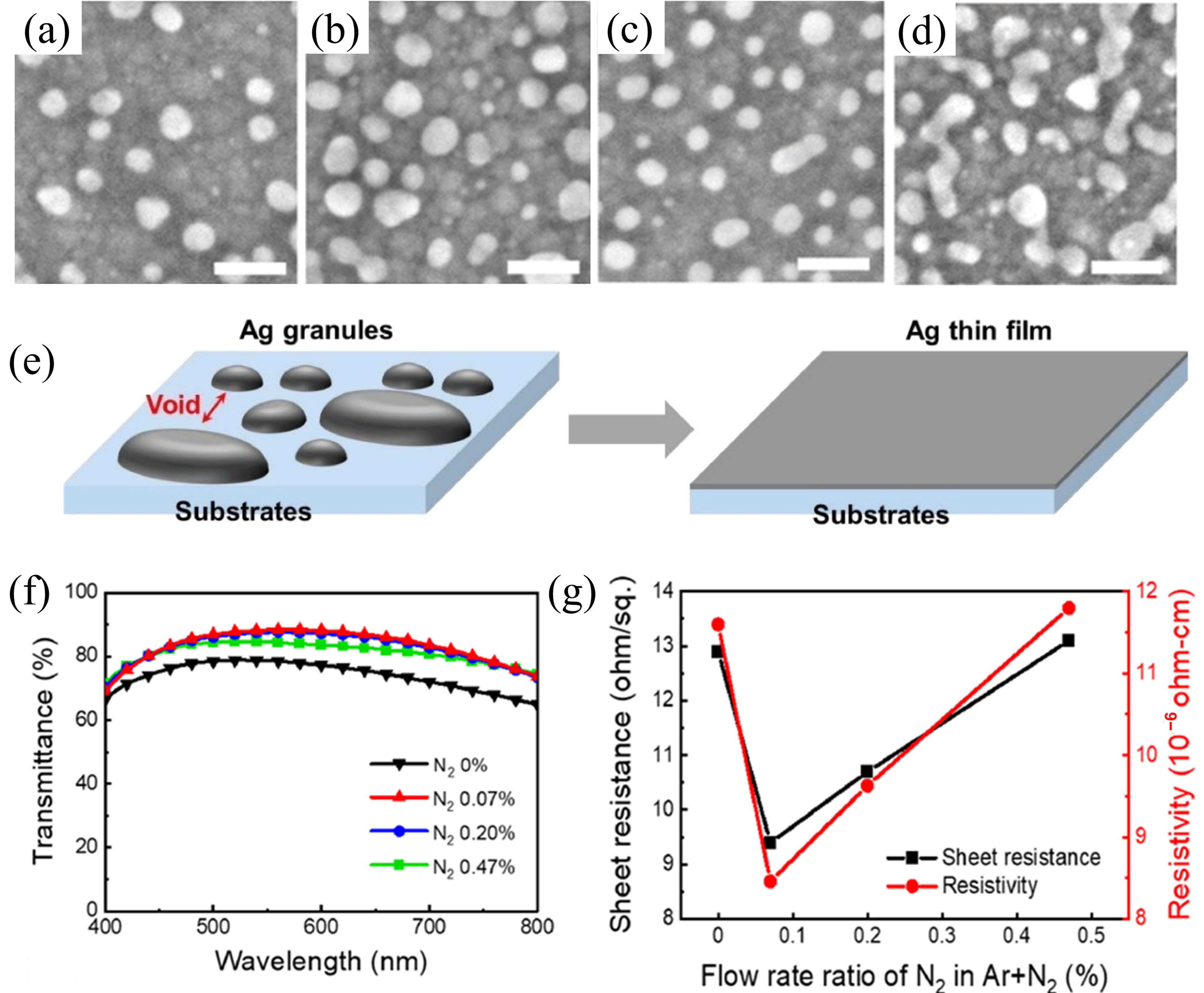
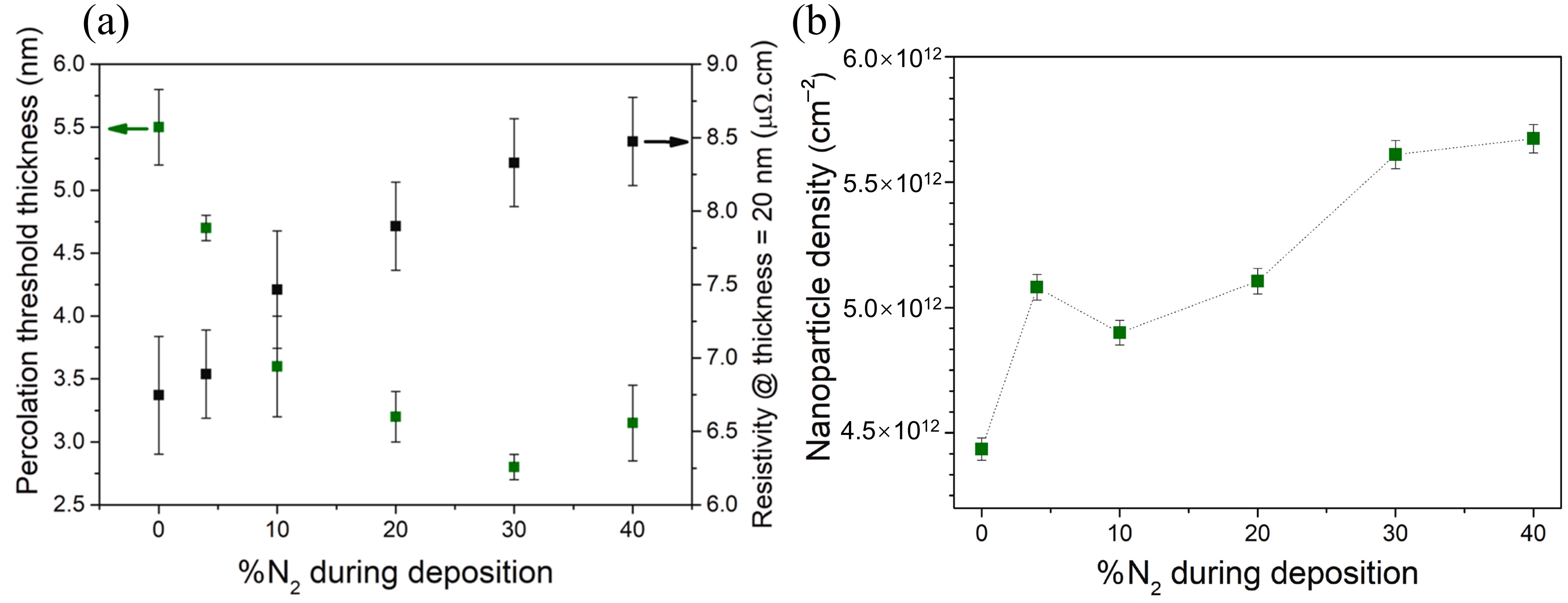
2.3. Gas-Assisted Deposition
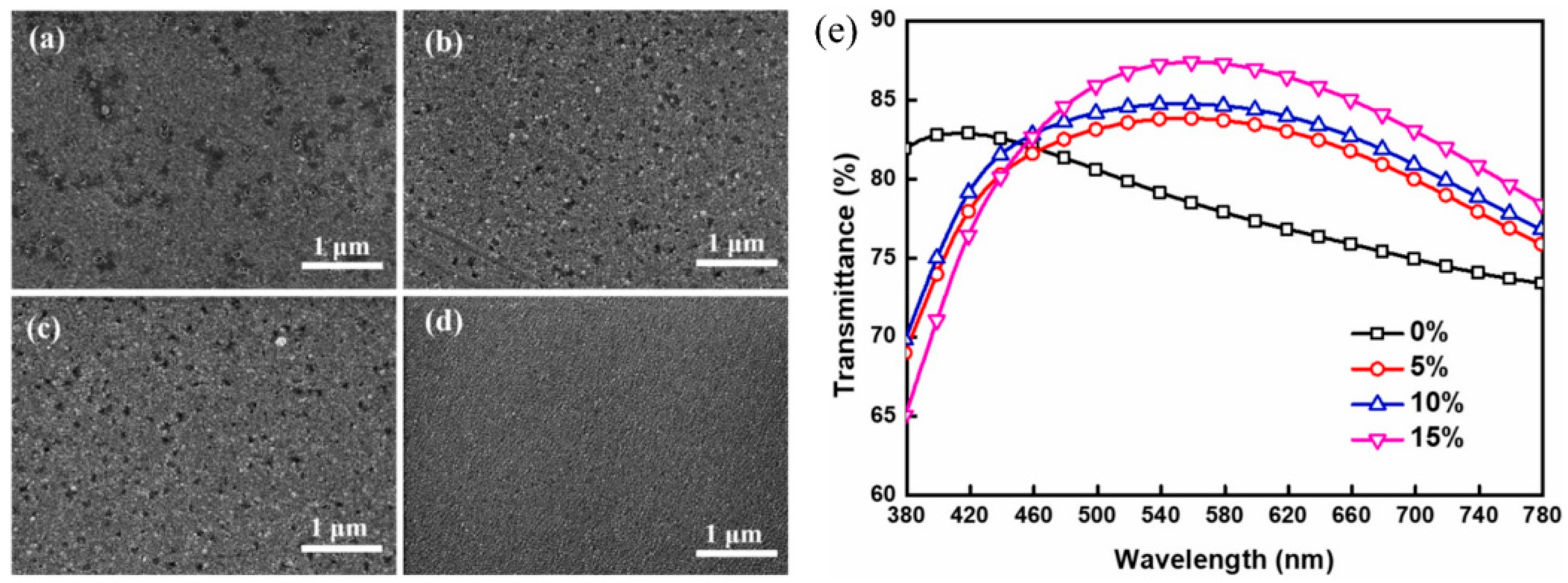
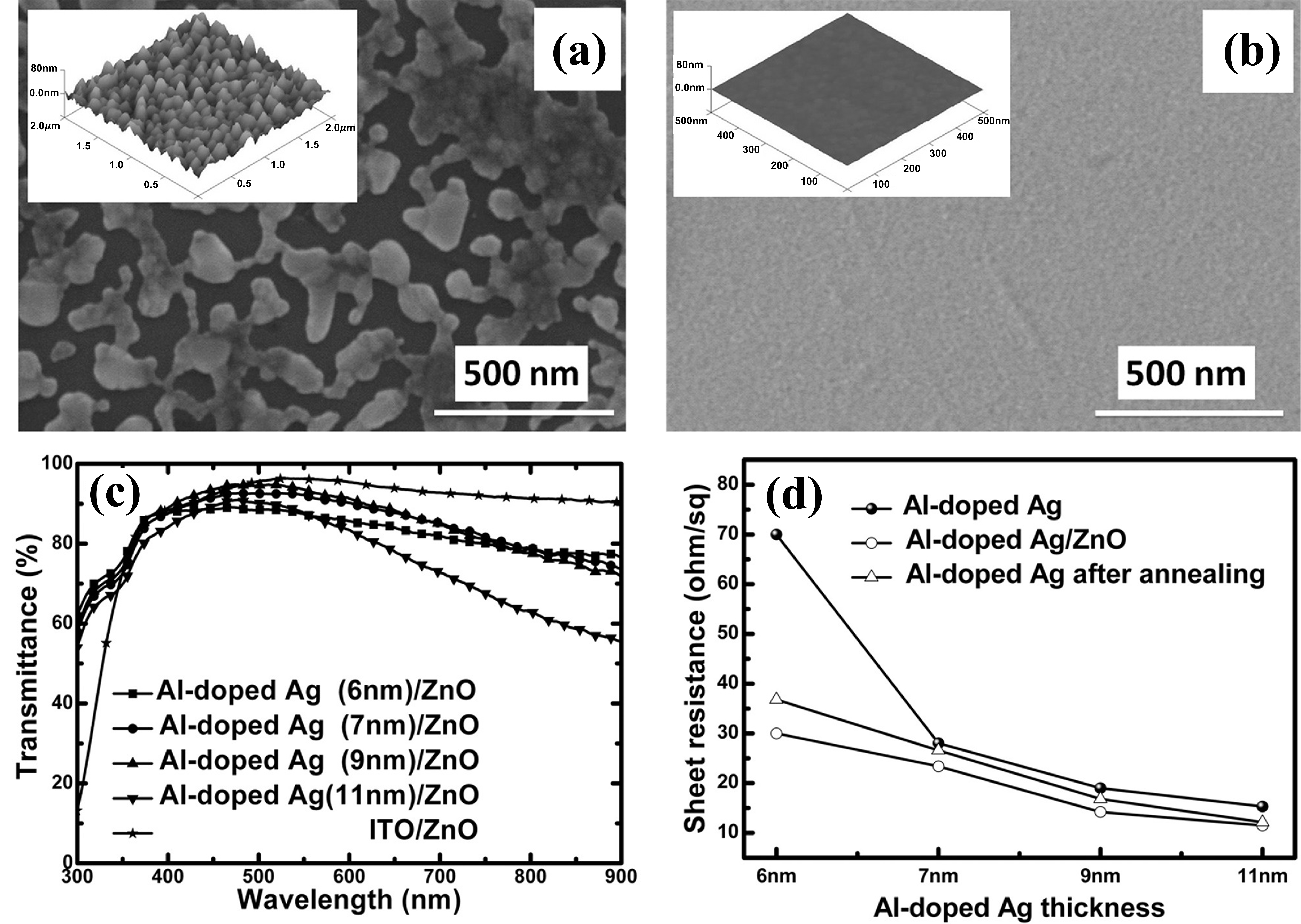
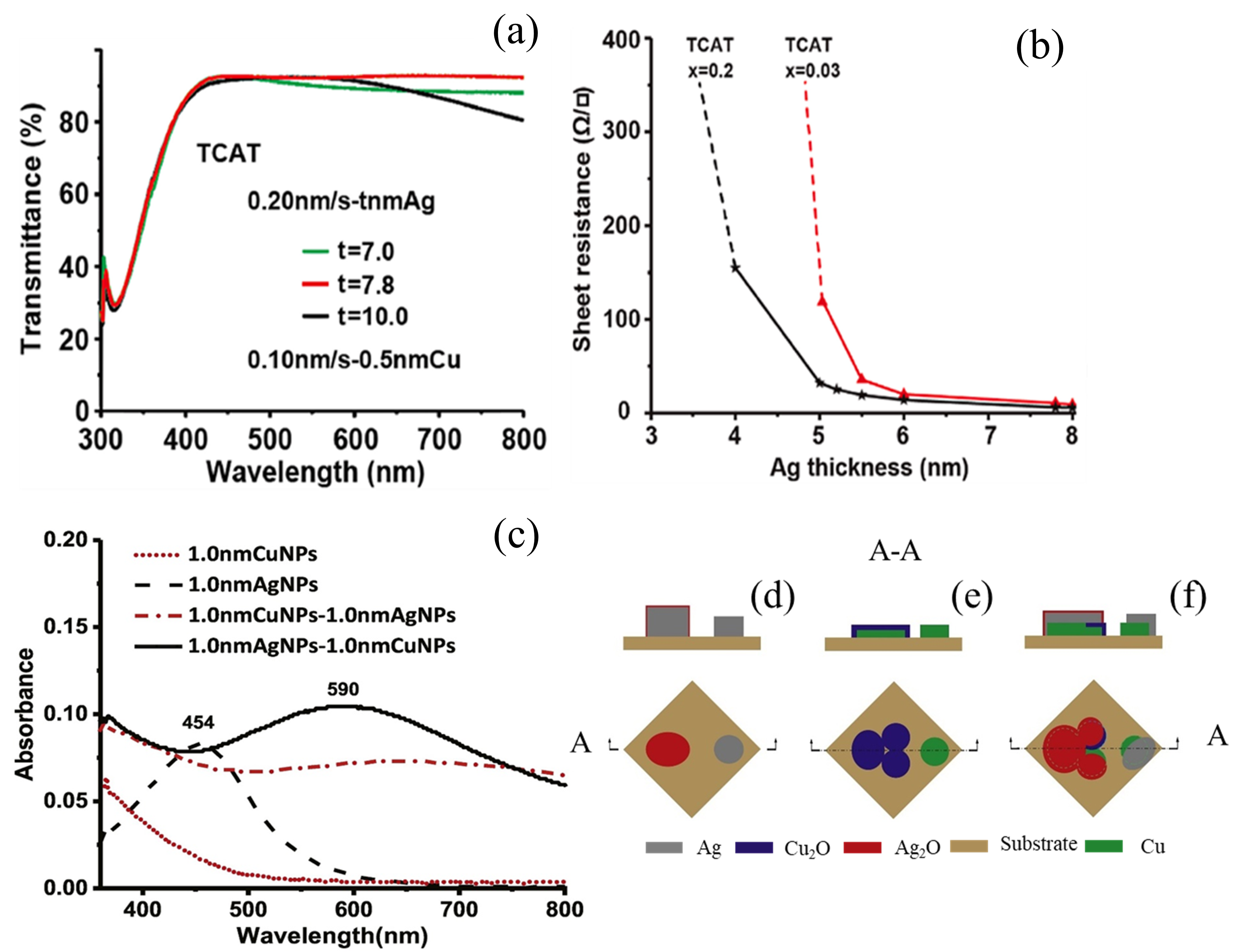
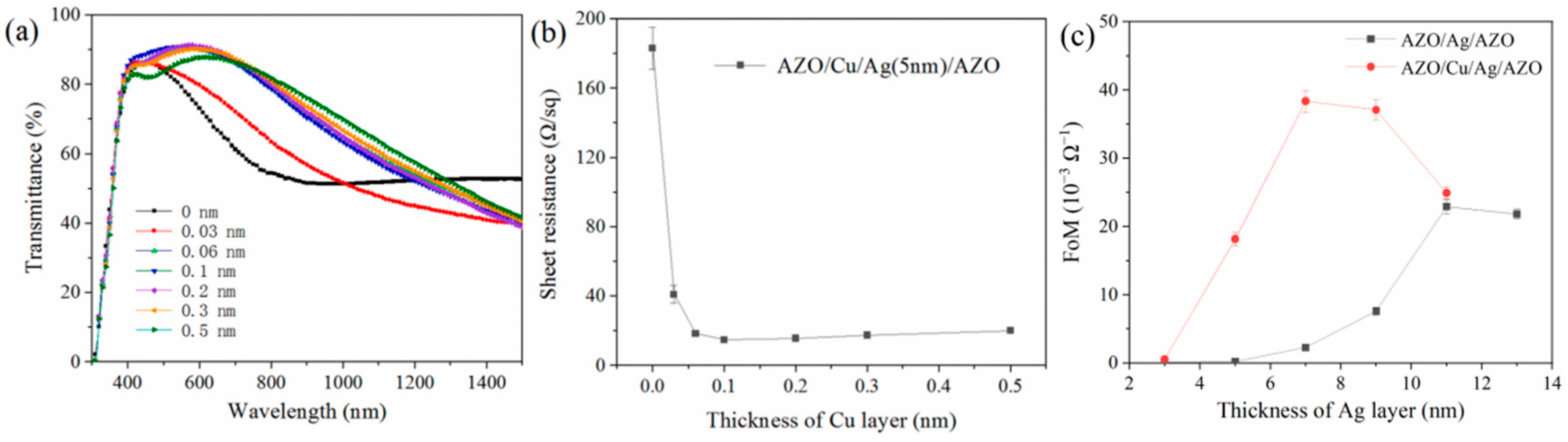
2.4. Metal Doping
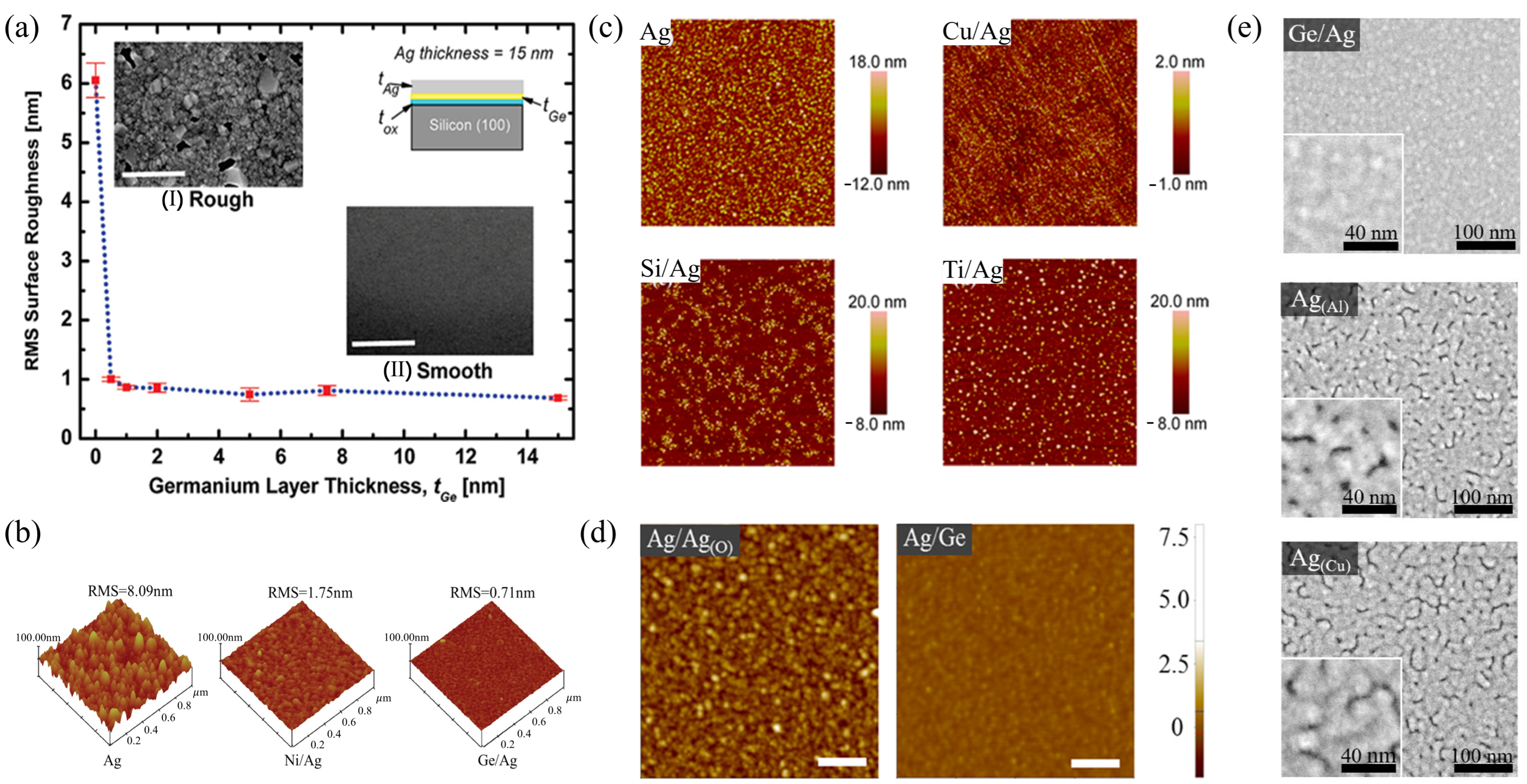
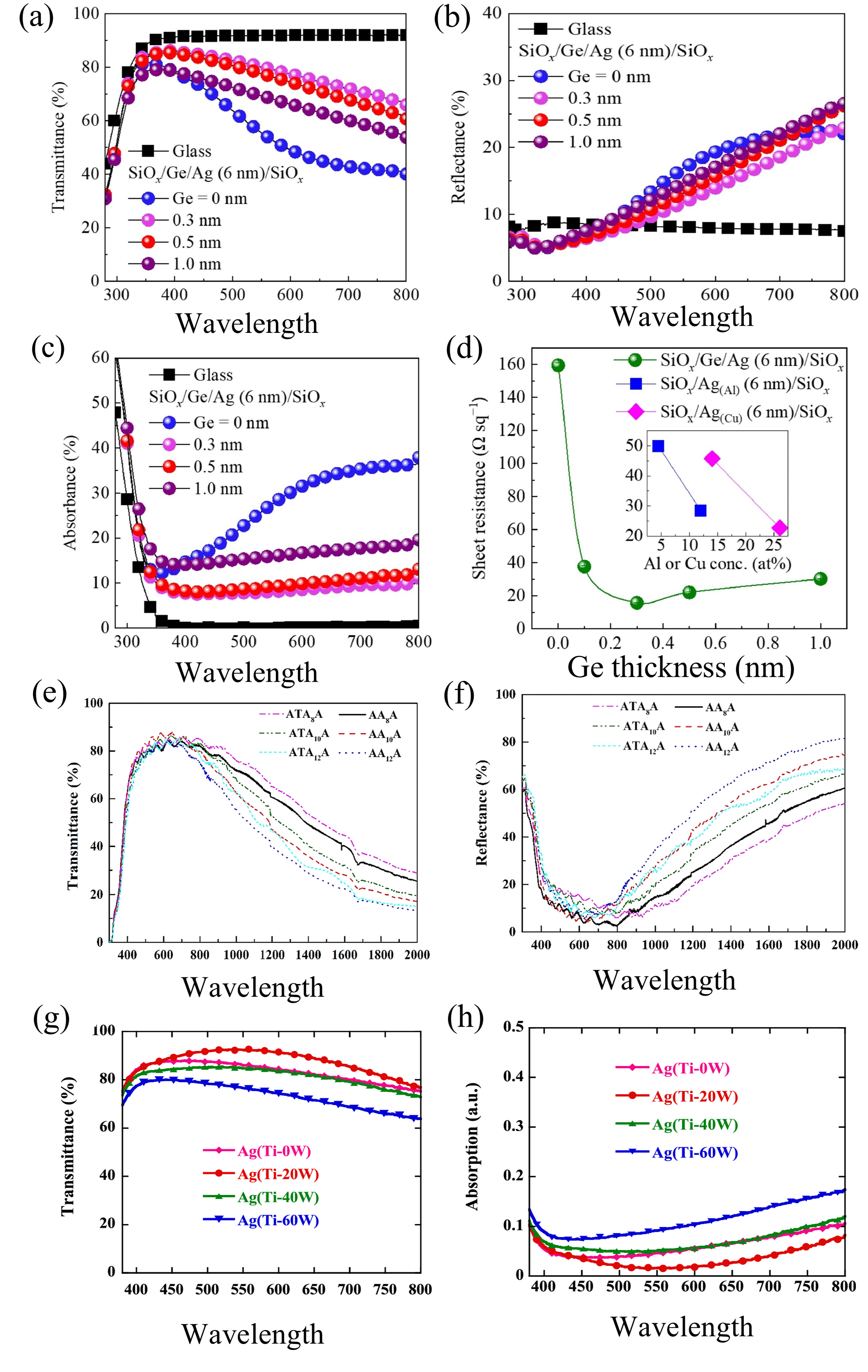
2.5. Pre-Deposited Seed Layer
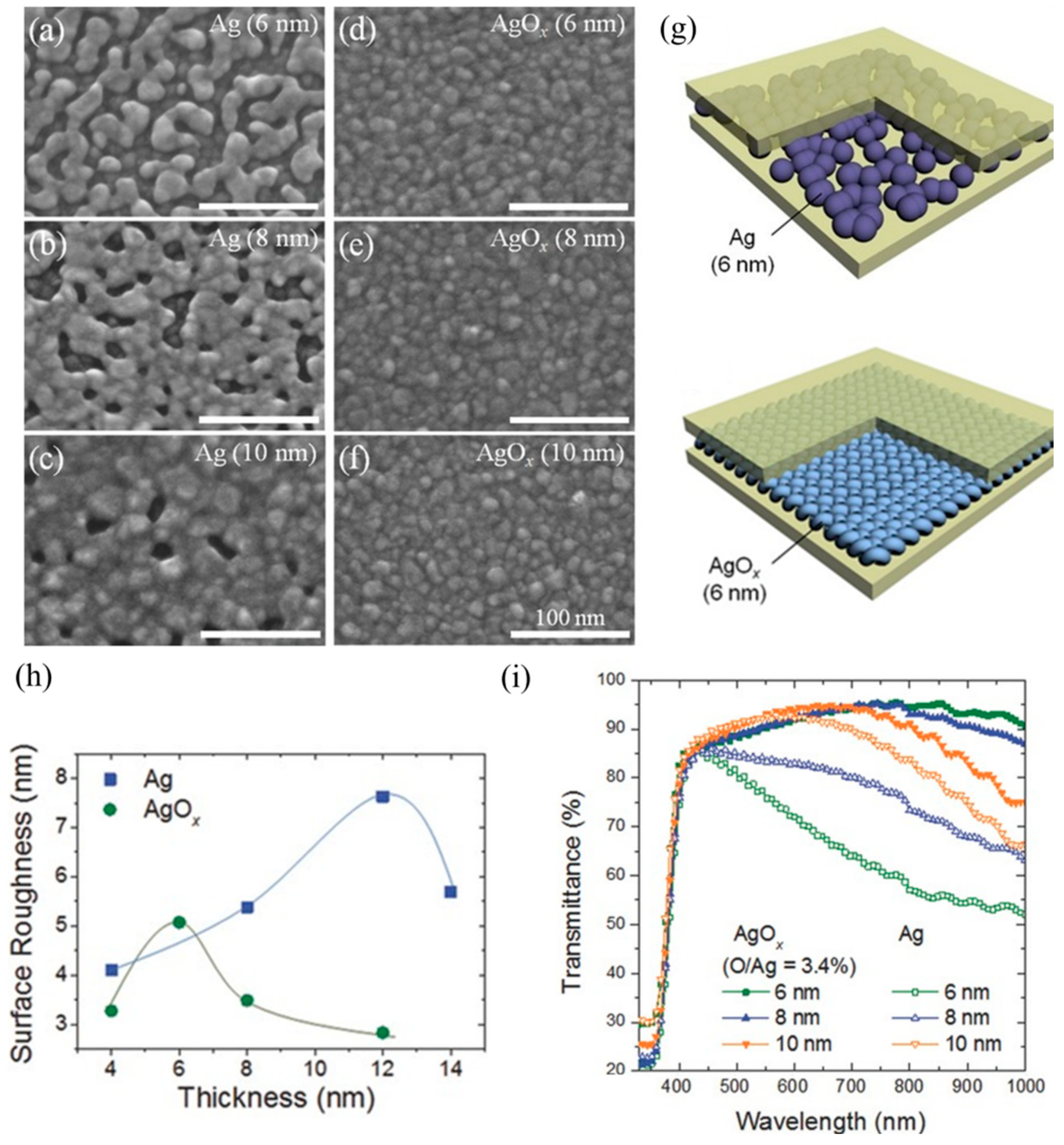
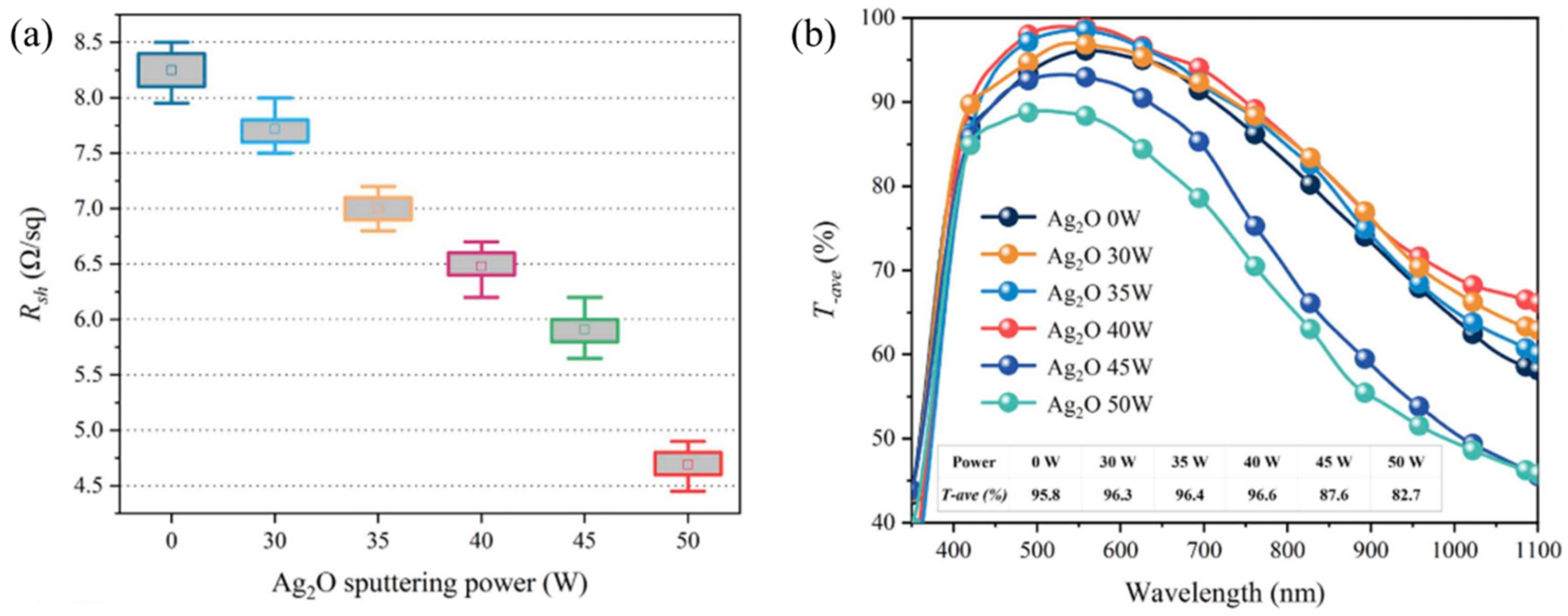
3. Progress on the Dielectric Layers of DAD Transparent Electrodes
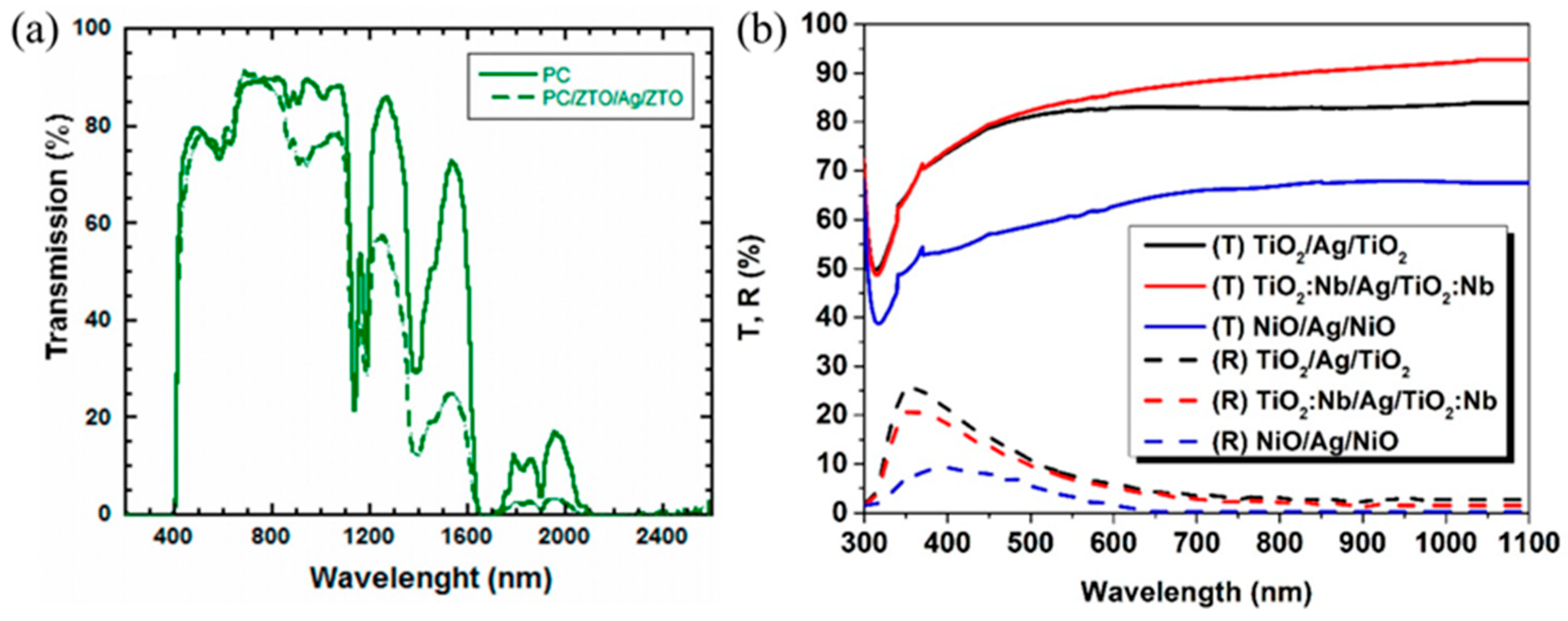
3.1. Progress in DAD with Same Dielectric Layer Materials
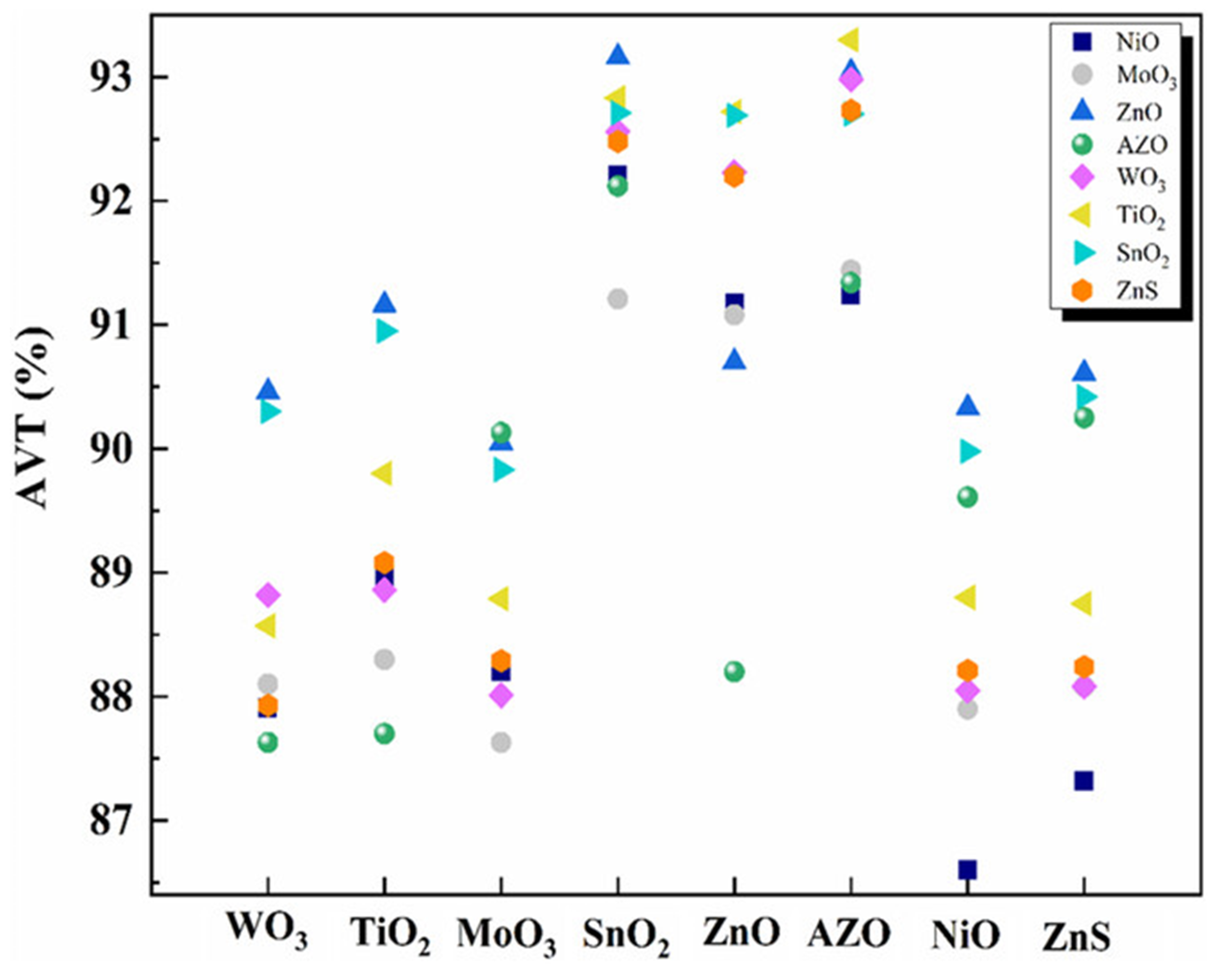
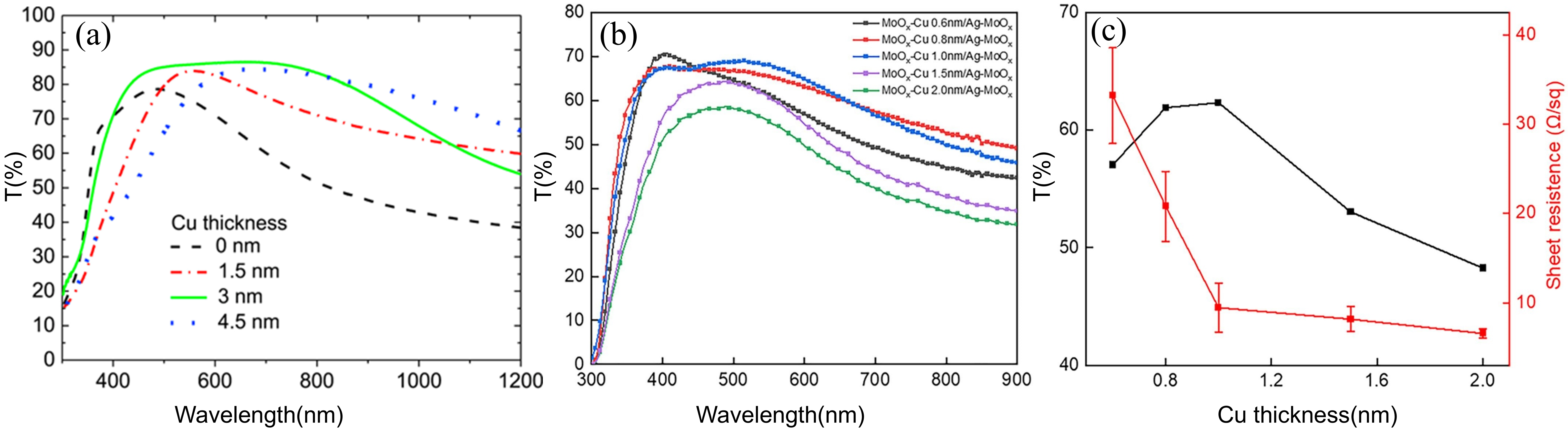
3.2. Research Progress on DAD Flexible Transparent Electrodes with Different Dielectric Layer Materials

4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, Y.; Duan, B.; Ru, M.; Gu, Q.; Li, S.; Zhao, W. Toward Flexible and Stretchable Organic Solar Cells: A Comprehensive Review of Transparent Conductive Electrodes, Photoactive Materials, and Device Performance. Adv. Energy Mater. 2025, 15, 2404233. [Google Scholar] [CrossRef]
- Triolo, C.; Lorusso, A.; Masi, S.; Mariano, F.; Torre, A.D.; Accorsi, G.; Arima, V.; Leo, S.D.; Rinaldi, R.; Patané, S.; et al. Electromagnetic Mode Management in Transparent DMD Electrodes for High Angular Color Stability in White OLEDs. ACS Photonics 2025, 12, 2413. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Papanastasiou, D.T.; Resende, J.; Bardet, L.; Sannicolo, T.; Jiménez, C.; Muñoz-Rojas, D.; Nguyen, N.D.; Bellet, D. Advances in Flexible Metallic Transparent Electrodes. Small 2022, 18, 2106006. [Google Scholar] [CrossRef]
- You, P.; Liu, Z.; Tai, Q.; Liu, S.; Yan, F. Efficient semitransparent perovskite solar cells with graphene electrodes. Adv. Mater. 2015, 27, 3632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, X.G.; Ji, K.; Zhao, S.; Liu, D.; Li, B.; Hou, P.X.; Liu, C.; Liu, L.; Stranks, S.D.; et al. High-performance bifacial perovskite solar cells enabled by single-walled carbon nanotubes. Nat. Commun. 2024, 15, 2245. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wu, X.; Chen, S.; Nedumaran, A.M.; Stephen, M.; Hou, K.; Czarny, B.; Leong, W.L. A highly conducting polymer for self-healable, printable, and stretchable organic electrochemical transistor arrays and near hysteresis-free soft tactile sensors. Adv. Mater. 2022, 3, 2200682. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, S.; Huang, J.; Gu, Y.; Huang, W.; Liu, S.; Lin, Z.; Zeng, Z.; Hu, Y.; Chen, Z.; et al. Flexible, Transparent and Conductive Metal Mesh Films with Ultra-High FoM for Stretchable Heating and Electromagnetic Interference Shielding. Nano-Micro Lett. 2024, 16, 92. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, Q.; Li, H.; Liu, M.; Li, Z.; Yang, K.; Zhao, J.; Qian, L.; Peng, Z.; Zhang, G.; et al. Transparent Heaters: Fabrication of high-performance silver mesh for transparent glass heaters via electric-field-driven microscale 3D printing and UV-assisted microtransfer. Adv. Mater. 2019, 31, 1970229. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S.; Tobah, M.; Ma, T.; Guo, L.J. Optimized optical/electrical/mechanical properties of ultrathin metal films for flexible transparent conductor applications: Review [Invited]. Opt. Mater. Express 2023, 13, 304. [Google Scholar] [CrossRef]
- Jeong, J.-A.; Kim, H.-K.; Yi, M.-S. Effect of Ag interlayer on the optical and passivation properties of flexible and transparent Al2O3/Ag/Al2O3 multilayer. Appl. Phys. Lett. 2008, 93, 033301. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. TCO/metal/TCO structures for energy and flexible electronics. Thin Solid Film. 2011, 520, 1. [Google Scholar]
- Girtan, M.; Negulescu, B. A review on oxide/metal/oxide thin films on flexible substrates as electrodes for organic and perovskite solar cells. Opt. Mater. X 2022, 13, 100122. [Google Scholar] [CrossRef]
- Malik, A.; Rani, S.; Guruprasad, S.; Basumatary, P.; Ghosh, D.S. Design and Optimization of Inverted Perovskite Solar Cells incorporating Metal Oxide-based Transparent Conductor. Adv. Theory Simul. 2025, 8, e00179. [Google Scholar]
- Masuda, Y.; Kiba, T.; Kawamura, M.; Abe, Y. Emission Wavelength Control Based on Coupling of Surface Plasmon and Microcavity Mode in Organic Light-Emitting Diodes with Metal/Dielectric/Metal Anodes. ACS Photonics 2024, 11, 2946. [Google Scholar] [CrossRef]
- Bae, S.; Jung, Y.; Pal, V.; Lee, J.K. Enhancement of Optical Transparency and Electrical Conductivity of IZO/Ag/IZO Multilayer Film by Intense Pulsed Light and its Effect on the Photovoltaic Performances of Perovskite Solar Cells. Adv. Sci. 2025, 12, 2501058. [Google Scholar]
- Nguyen, T.T.; Patel, M.; Kim, J. Multifunctional AZO/Ag (O)-Based Transparent Conductor for Flexible and Transparent Optoelectronics. Sol. RRL 2024, 8, 2400082. [Google Scholar]
- Sun, K.; Bao, Z.; Guo, X.; Zou, D.; Lv, Y.; Liang, J.; Liu, X. Crystallization Regulation and Defect Passivation of High-Performance Flexible Perovskite Light-Emitting Diodes Based on Novel Dielectric/Metal/Dielectric Transparent Electrodes. Adv. Optical Mater. 2024, 12, 2301752. [Google Scholar]
- Mohamedi, M.; Challali, F.; Touam, T.; Konstantakopoulou, M.; Bockelee, V.; Mendill, D.; Ouhenia, S.; Djouadi, D.; Chelouche, A. Ag thickness and substrate effects on microstructural and optoelectronic properties of AZO/Ag/AZO multilayer structures deposited by confocal RF magnetron sputtering. J. Appl. Phys. A 2023, 129, 545. [Google Scholar] [CrossRef]
- Hong, M.; Lee, Y.; Lee, J.; Choi, C.; Kim, J. Wavelength-dependent Light Control Transparent Film of Oxide/Metal/Oxide/Metal/Oxide Structures for Infrared Shielding Utilization. J. Korean Sol. Energy 2025, 45, 91. [Google Scholar] [CrossRef]
- Tyson, W.R.; Miller, W.A. Surface free energies of solid metals: Estimation from liquid surface tension measurements. Surf. Sci. 1977, 62, 267. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 2001, 63, 155409. [Google Scholar] [CrossRef]
- Kaiser, N. Review of the fundamentals of thin-film growth. Appl. Opt. 2002, 41, 3053. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, S.; Cheng, Z.; Wang, J.; Li, L.; Nian, Y.; Fang, Y.C. Suppressing the localized surface plasmon resonance of AgNPs to obtain ultra-high and ultra-uniform optical transmittance of dielectric-Ag-dielectric electrodes. Opt. Mater. 2021, 121, 111569. [Google Scholar]
- Dhar, A.; Alford, T.L. High quality transparent TiO2/Ag/TiO2 composite electrode films deposited on flexible substrate at room temperature by sputtering. APL Mater. 2013, 1, 012102. [Google Scholar]
- Cattin, L.; Morsli, M.; Dahou, F.; Abe, S.Y.; Kheli, A.; Bernède, J.C. Investigation of low resistance transparent MoO3/Ag/MoO3 multilayer and application as anode in organic solar cells. Thin Solid Film. 2010, 518, 4560. [Google Scholar]
- Fang, Y.C.; He, J.; Zhang, K.; Xiao, C.; Zhang, B.; Shen, J.; Niu, H.; Yan, R.; Chen, J. Ar plasma irradiation improved optical and electrical properties of TiO2/Ag/TiO2 multilayer thin film. Opt. Lett. 2015, 40, 5455. [Google Scholar]
- Fang, Y.C.; Blinn, K.; Li, X.; Weng, G.; Liu, M. Resonance of immobile Ag nanoclusters fabricated by direct current sputtering. Appl. Phys. Lett. 2013, 102, 143112. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668. [Google Scholar]
- Bohren, C.F. How can a particle absorb more than the light incident on it? Am. J. Phys. 1983, 51, 323. [Google Scholar] [CrossRef]
- Fang, Y.C.; Hong, L.; Wan, L.; Zhang, K.; Lu, X.; Wang, C.; Yang, J.; Xu, X. Localized surface plasmon of AgNPs affected by annealing and its coupling with the excitons of Rhodamine 6G. J. Vac. Sci. Technol. A 2013, 31, 041401. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238. [Google Scholar] [CrossRef]
- Haidari, G. Nano-viewpoint in modeling and investigation of the D/M/D transparent-conductive layer. Plasmonics 2021, 17, 249. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267. [Google Scholar] [CrossRef]
- Zhao, G.; Jeong, E.; Choi, E.-A.; Seung, M.Y.; Bae, J.-S.; Lee, S.-J.; Han, S.Z.; Lee, G.-H.; Yun, J. Strategy for improving Ag wetting on oxides: Coalescence dynamics versus nucleation density. Appl. Surf. Sci. 2020, 510, 145515. [Google Scholar] [CrossRef]
- Zhao, G.; Jeong, E.; Ji, F.; Lee, S.-G.; Yu, S.M.; Li, J.; Wang, T.; Chu, W.; Yun, J. Underlying principles of Ge-induced early cluster-to-layer transition for ultrathin Ag layer formation on oxides. Surf. Interf. 2024, 54, 105208. [Google Scholar] [CrossRef]
- Zhao, G.; Tan, Y.; Wang, B.; Jeong, E.; Zhang, L.; Wang, T.; Yu, H.; Min, G.; Han, S.Z.; Sun, Y.; et al. Understanding the role of engineered cluster evolution in enhancing Ag layer growth on oxides. Appl. Surf. Sci. 2024, 644, 158745. [Google Scholar]
- Zhang, C.; Kinsey, N.; Chen, L.; Ji, C.; Xu, M.; Ferrera, M.; Pan, X.; Shalaev, V.M.; Boltasseva, A.; Guo, L.J. High-performance doped silver films: Overcoming fundamental material limits for nanophotonic applications. Adv. Mater. 2017, 29, 1605177. [Google Scholar]
- Lee, T.; Kim, D.; Suk, M.E.; Bang, G.; Choi, J.; Bae, J.; Yoon, J.; Moon, W.J.; Choi, D. Regulating Ag wettability via modulating surface stoichiometry of ZnO substrates for flexible electronics. Adv. Funct. Mater. 2021, 31, 2104372. [Google Scholar] [CrossRef]
- Choi, J.; Bang, G.; Lee, T.; Tran, V.T.B.; Bae, J.S.; Choi, D. Simultaneous enhancement in visible transparency and electrical conductivity via the physicochemical alterations of ultrathin-silver-film-based transparent electrodes. Nano Lett. 2022, 22, 3133. [Google Scholar] [CrossRef]
- Logeeswaran, V.J.; Kobayashi, N.P.; Islam, M.S.; Wu, W.; Chaturvedi, P.; Fang, N.X.; Wang, S.Y.; Williams, R.S. Ultrasmooth silver thin films deposited with a germanium nucleation layer. Nano Lett. 2009, 9, 178. [Google Scholar] [CrossRef]
- Cui, X.; Li, X.; Wang, Z.; Li, Z.; Chen, X.; Tang, J.; Feng, X.; La, S.; Chen, J.; Zhang, Z.; et al. MoO3/Au/Ag/MoO3 multilayer transparent electrode enables high light utilization of semitransparent perovskite solar cells. Device 2025, 3, 100558. [Google Scholar] [CrossRef]
- Jeong, E.; Lee, S.-G.; Bae, J.-S.; Yu, S.M.; Han, S.Z.; Lee, G.-H.; Choi, E.-A.; Yun, J. Effects of substantial atomic-oxygen migration across silver-oxide interfaces during silver growth. Appl. Surf. Sci. 2021, 568, 150927. [Google Scholar] [CrossRef]
- Kim, G.; Lim, J.W.; Lee, J.; Heo, S.J. Flexible multilayered transparent electrodes with less than 50 nm thickness using nitrogen-doped silver layers for flexible heaters. Mater. Res. Bull. 2022, 149, 111703. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086. [Google Scholar] [CrossRef]
- Vo, T.T.B.; Lim, J.; Joo, S.H.; Kim, H.; Lee, T.; Bae, J.S.; Jeong, E.; Kwon, M.S.; Yun, J.; Choi, D. Smooth, chemically altered nucleating platform for abrupt performance enhancement of ultrathin Cu-layer-based transparent electrodes. Nano Lett. 2023, 23, 6528. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Joo, S.H.; Kim, H.; Choi, D. Overcoming the Trade off of Visible Transparency and Electrical Conductance via Dual Smoothing of Dielectric/Metal Interfaces in Cu-Thin-Layer-Based Transparent Electrodes. ACS Appl. Mater. Interfaces 2024, 16, 61314. [Google Scholar] [CrossRef]
- Wang, W.; Song, M.; Bae, T.-S.; Park, Y.H.; Kang, Y.-C.; Lee, S.-G.; Kim, S.-Y.; Kim, D.H.; Lee, S.; Min, G.; et al. Transparent ultrathin oxygen-doped silver electrodes for flexible organic solar cells. Adv. Funct. Mater. 2014, 24, 1551. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Jia, Z.; Qin, Z.; Chu, L.; Yang, J.; Zhang, J.; Huang, W.; Li, X. High stable, transparent and conductive ZnO/Ag/ZnO nanofilm electrodes on rigid/flexible substrates. Energies 2016, 9, 443. [Google Scholar] [CrossRef]
- Jo, H.; Yang, J.-H.; Choi, S.-W.; Park, J.; Song, J.E.; Shin, M.; Ahn, J.-H.; Kwon, J.-D. Highly transparent and conductive oxide-metal-oxide electrodes optimized at the percolation thickness of AgOx for transparent silicon thin-film solar cells. Sol. Energ. Mat. Sol. C 2019, 202, 110131. [Google Scholar] [CrossRef]
- Kong, H.; Lee, H.-Y. High performance flexible transparent conductive electrode based on ZnO/AgOx/ZnO multilayer. Thin Solid Film. 2020, 696, 137759. [Google Scholar] [CrossRef]
- Yun, J.; Jeong, E.; Zhao, G.; Lee, S.-G.; Yu, S.M.; Bae, J.-S.; Han, S.Z.; Lee, G.-H.; Ikoma, Y.; Choi, E.-A. Unconventional thickness dependence of electrical resistivity of silver film electrodes in substoichiometric oxidation states. Acta Mater. 2024, 265, 119637. [Google Scholar] [CrossRef]
- Zapata, R.; Balestrieri, M.; Gozhyk, I.; Montigaud, H.; Lazzari, R. On the O2 “Surfactant” Effect during Ag/SiO2 Magnetron Sputtering Deposition: The Point of View of In Situ and Real-Time Measurements. ACS Appl. Mater. Interfaces 2023, 15, 36951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Geng, Z.; Zhang, D.; Wang, D.; Liu, J.; Wang, Q. AZO/Ag/AZO composite film with high transmittance based on an ultrathin continuous Ag layer obtained via micro oxidation. Mat. Sci. Semicon. Proc. 2023, 165, 107643. [Google Scholar] [CrossRef]
- Li, H.; Shen, H.; Zhang, J.; Li, Y.; Bai, H.; Chen, J.; Yue, Z.; Wang, L.; Zeng, J. Enhanced mechanical endurance and power conversion efficiency in flexible Cu2ZnSnS4 solar cells using ZnO/Ag-Ag2O/ZnO film. J. Alloys Compd. 2024, 995, 174812. [Google Scholar] [CrossRef]
- Zhao, G.; Shen, W.; Jeong, E.; Lee, S.-G.; Chung, H.-S.; Bae, T.-S.; Bae, J.-S.; Lee, G.-H.; Tang, J.; Yun, J. Nitrogen-mediated growth of silver nanocrystals to form ultrathin, high-purity silver-film electrodes with broad band transparency for solar cells. ACS Appl. Mater. Interfaces 2018, 10, 40901. [Google Scholar] [CrossRef]
- Zapata, R.; Balestrieri, M.; Gozhyk, I.; Montigaud, H.; Lazzari, R. Does N2 gas behave as a surfactant during Ag thin-film sputtering deposition? Insights from in vacuo and real-time measurements. Appl. Surf. Sci. 2024, 654, 159546. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, D.; Gu, D.; Kim, H.; Ling, T.; Wu, Y.-K.; Guo, J. An ultrathin, smooth, and low-loss Al-doped Ag film and its application as a transparent electrode in organic photovoltaics. Adv. Mater. 2014, 26, 5696. [Google Scholar] [CrossRef]
- Gu, D.; Zhang, C.; Wu, Y.-K.; Guo, J. Ultrasmooth and thermally stable silver-based thin films with subnanometer roughness by aluminum doping. ACS Nano 2014, 8, 10343. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, C.; Kim, H.; Guo, J. High-performance Ta2O5/Al-doped Ag electrode for resonant light harvesting in efficient organic solar cells. Adv. Energy. Mater. 2015, 5, 1500768. [Google Scholar] [CrossRef]
- Loka, C.; Lee, K.-S. Preparation of TiO2/Ag/TiO2 (TAT) multilayer films with optical and electrical properties enhanced by using Cr-added Ag film. Appl. Surf. Sci. 2016, 415, 35. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.; Lu, Y.; Zhou, Y.; Xu, J.; Li, J.; Wang, H.; Fang, J.; Yang, Y.; Wang, W.; et al. Seed-layer-free growth of ultra-thin Ag transparent conductive films imparts flexibility to polymer solar cells. Sol. Energy Mater. Sol. Cells 2018, 184, 73. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Q.; Cui, Q.; Ji, C.; Zhang, Z.; Chen, X.; George, T.; Zhao, S.; Guo, J. High-performance large-scale flexible optoelectronics using ultrathin silver films with tunable properties. ACS Appl. Mater. Interfaces 2019, 11, 27216. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Choi, J.-W. Silver alloy-based metal matrix composites: A potential material for reliable transparent thin film heaters. J. Mater. Chem. C 2021, 9, 4670. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Xu, J.; Huang, J.; Yang, Y.; Tan, R.; Chen, G.; Fang, X.; Zhao, Y.; Song, W. Robust ultrathin and transparent AZO/Ag-SnOx/AZO on polyimide substrate for flexible thin film heater with temperature over 400 °C. J. Mater. Sci. Technol. 2020, 48, 156. [Google Scholar] [CrossRef]
- Li, H.; Shen, H.; Zhang, J.; Li, Y.; Du, Z.; Bai, H.; Chen, J.; Zheng, J.; Yue, Z.; Zeng, J. Co-sputtered Cu-Ag-based ZnO-Cu (Ag)-ZnO film enabling efficient flexible CuZnSnS solar cells with desirable mechanical endurance. Ceram. Int. 2023, 49, 36225. [Google Scholar] [CrossRef]
- Zhao, G.; Jeong, E.; Lee, S.-G.; Yu, S.M.; Ji, F.; Han, S.Z.; Lee, G.-H.; Choi, E.-A.; Yun, J. Comparison of the effect of gaseous and metallic additives on ultrathin Ag layer formation: An experimental and numerical investigation. Appl. Surf. Sci. 2025, 682, 161774. [Google Scholar] [CrossRef]
- Liu, H.; Wang, B.; Leong, E.; Yang, P.; Zong, Y.; Si, G.; Teng, J.; Maier, S. Enhanced surface plasmon resonance on a smooth silver film with a seed growth layer. ACS Nano 2010, 4, 3139. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Zhao, G.; Yu, S.M.; Lee, S.; Bae, J.; Park, J.; Rha, J.; Lee, G.-H.; Yun, J. Minimizing optical loss in ultrathin Ag films based on Ge wetting layer: Insights on Ge-mediated Ag growth. Appl. Surf. Sci. 2020, 528, 146989. [Google Scholar] [CrossRef]
- Ji, F.; Zhao, G.; Li, J.; Geng, M.; Zhang, R.; Liu, X.; Wang, T.; Zhang, L.; Min, G.; Qin, J.; et al. Establishing highly stable, transparent flexible thin film heaters: Effects of ultrathin Ti wetting layer for Ag growth. Appl. Surf. Sci. 2025, 690, 162582. [Google Scholar] [CrossRef]
- Zhu, G.; He, Z.; Zhu, K. Improved performance of AZO/Ag/AZO transparent conductive films by inserting an ultrathin Ti layer. Mater. Lett. 2024, 356, 135615. [Google Scholar] [CrossRef]
- Liao, J.; Qian, R.; Wang, G. High performances multilayer transparent conductive films with manipulated Ag growth and layer thickness. J. Mater. Sci. Mater. Electron. 2023, 34, 795. [Google Scholar] [CrossRef]
- Formica, N.; Ghosh, D.S.; Carrilero, A.; Chen, T.L.; Simpson, R.E.; Pruneri, V. Ultrastable and atomically smooth ultrathin silver films grown on a copper seed layer. ACS Appl. Mater. Interfaces 2013, 5, 3048. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Chen, L.; Huang, J.; Wang, W.; Wang, H.; Li, J.; Fan, B.; Xu, Q.; Song, W. Semitransparent perovskite solar cells with ultrathin silver electrodes for tandem solar cells. Sol. Energy 2020, 206, 294. [Google Scholar] [CrossRef]
- Bernède, J.C.; Cattin, L.; Abachi, T.; Yendoubé, L.; Mustapha, M.; Mohammed, M. Use of Cu-Ag bi-layer films in oxide/metal/oxide transparent electrodes to widen their spectra of transmittance. Mater. Lett. 2013, 112, 187. [Google Scholar] [CrossRef]
- Mouchaal, Y.; Louarn, G.; Khelil, A.; Morsli, M.; Stephant, N.; Bou, A.; Abachi, T.; Cattin, L.; Makha, M.; Torchio, P.; et al. Broadening of the transmittance range of dielectric/metal multilayer structures by using different metals. Vacuum 2015, 111, 32. [Google Scholar] [CrossRef]
- Liu, H.; Lang, R.; Jiang, S.; Lu, W.; Zhang, W.; Feng, L.; Liu, H.; Wu, L.; Liu, X.; Wang, X.; et al. Bifacial semitransparent perovskite solar cells with MoOx/Cu/Ag/MoOx multilayer transparent electrode. Sol. Energy 2021, 228, 290. [Google Scholar] [CrossRef]
- Hao, H.; Li, H.; Wang, S.; Cheng, Z.; Fang, Y.C. Epitaxial growth of Ag-Cu bimetallic nanoparticles via thermal evaporation deposition. Appl. Surf. Sci. 2020, 505, 143871. [Google Scholar] [CrossRef]
- Jiang, S.; Feng, L.; Zhang, W.; Liu, H.; Liu, H.; Liu, Y.; Li, B.; Wu, L.; Liu, X.; Wang, X.; et al. Indium-free flexible perovskite solar cells with AZO/Cu/Ag/AZO multilayer transparent electrodes. Sol. Energy Mater. Sol. Cells 2022, 246, 111895. [Google Scholar] [CrossRef]
- Sonmez, N.A.; Donmez, M.; Comert, B.; Ozcelik, S. Ag/M-seed/AZO/glass structures for low-E glass: Effects of metal seeds. Int. J. Appl. Glass Sci. 2017, 9, 383. [Google Scholar] [CrossRef]
- Zhao, G.; Shen, W.; Jeong, E.; Lee, S.; Yu, S.M.; Bae, T.; Lee, G.; Han, S.Z.; Tang, J.; Choi, E.; et al. Ultrathin silver film electrodes with ultralow optical and electrical losses for flexible organic photovoltaics. ACS Appl. Mater. Interfaces 2018, 10, 27510. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.-H.; Seong, T.-Y. Realization of highly transparent and low resistance TiO2/Ag/TiO2 conducting electrode for optoelectronic devices. Ceram. Int. 2015, 41, 3064. [Google Scholar] [CrossRef]
- Zhao, Z.; Alford, T.L. The optimal TiO2/Ag/TiO2 electrode for organic solar cell application with high device-specific Haacke figure of merit. Sol. Energy Mater. Sol. Cells 2016, 157, 599. [Google Scholar] [CrossRef]
- Abachi, T.; Cattin, L.; Louarn, G.; Lare, Y.; Bou, A.; Makha, M.; Torchio, P.; Fleury, M.; Morsli, M.; Addou, M.; et al. Highly flexible, conductive and transparent MoO3/Ag/MoO3 multilayer electrode for organic photovoltaic cells. Thin Solid Film. 2013, 545, 438. [Google Scholar] [CrossRef]
- Goetz, S.; Wibowo, R.A.; Bauch, M.; Bansal, N.; Ligorio, G.; List-Kratochvil, E.; Linke, C.; Franzke, E.; Winkler, J.; Valtiner, M.; et al. Fast sputter deposition of MoOx/metal/MoOx transparent electrodes on glass and PET substrates. J. Mater. Sci. 2021, 56, 9047. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-zadeh, K. Molybdenum oxides-from fundamentals to functionality. Adv. Mater. 2017, 29, 1701619. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.; Wibowo, R.A.; Bauch, M.; Bansal, N.; Ligorio, G.; List, K.E.; Linke, C.; Franzke, E.; Winkler, J.; Valtiner, M.; et al. Transparent electrodes based on molybdenum-titanium-oxide with increased water stability for use as hole-transport/hole-injection components. J. Mater. Sci. 2022, 57, 8752. [Google Scholar] [CrossRef]
- Noguera, C. Physics and Chemistry at Oxide Surfaces; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Yan, G.; Zhang, L.; Hong, R. High quality transparent conductive hydrogenated AZO with embedded Ag films deposited on PEN flexible substrate. J. Mater. Sci. Mater. Electron. 2018, 29, 1. [Google Scholar] [CrossRef]
- Ekmekcioglu, M.; Erdogan, N.; Astarlioglu, A.T.; Serap, Y.; Gulnur, A.; Lutfi, O.; Mehtap, O. High transparent, low surface resistance ZTO/Ag/ZTO multilayer thin film electrodes on glass and polymer substrates. Vacuum 2021, 187, 110100. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Chen, X.; Li, X.; Li, Y.; Yang, G.; Qin, H.; Xie, A.; Sun, D. The Role of Thickness Improvement and Optimization in BMZO/Ag/BMZO Sandwich Transparent Electrodes. Micro Nanostruct. 2025, 205, 208182. [Google Scholar] [CrossRef]
- Hrostea, L.; Lisnic, P.; Mallet, R.; Leontie, L.; Girtan, M. Studies on the physical properties of TiO2: Nb/Ag/TiO2: Nb and NiO/Ag/NiO three-layer structures on glass and plastic substrates as transparent conductive electrodes for solar cells. Nanomaterials 2021, 11, 1416. [Google Scholar]
- Goetz, S.; Mehanni, D.; Bansal, N.; Kubicek, B.; Wibowo, R.A.; Bauch, M.; Linke, C.; Franzke, E.; Winkler, J.; Meyer, T.; et al. Low-Temperature-Processed Transparent Electrodes Based on Compact and Mesoporous Titanium Oxide Layers for Flexible Perovskite Solar Cells. ACS Appl. Energy Mater. 2022, 5, 5318. [Google Scholar] [CrossRef]
- Chiu, P.-K.; Lee, C.-T.; Chiang, D.; Cho, W.-H.; Hsiao, C.-N.; Chen, Y.-Y.; Huang, B.-M.; Yang, J.-R. Conductive and transparent multilayer films for low-temperature TiO2/Ag/SiO2 electrodes by E-beam evaporation with IAD. Nanoscale Res. Lett. 2014, 9, 35. [Google Scholar] [CrossRef]
- Formica, N.; Paola, M.-P.; Ghosh, D.S.; Janner, D.; Chen, T.L.; Huang, M.; Garner, S.; Martorell, J.; Pruneri, V. An indium tin oxide-free polymer solar cell on flexible glass. ACS Appl. Mater. Interfaces 2015, 7, 4541. [Google Scholar] [CrossRef] [PubMed]
- Kinner, L.; Bauch, M.; Wibowo, R.A.; Ligorio, G.; Kratochvil, E.J.W.L.; Dimopoulos, T. Polymer interlayers on flexible PET substrates enabling ultra-high performance, ITO-free dielectric/metal/dielectric transparent electrode. Mater. Design 2019, 168, 107663. [Google Scholar] [CrossRef]
- Ji, C.; Liu, D.; Zhang, C.; Jay, G.L. Ultrathin-metal-film-based transparent electrodes with relative transmittance surpassing 100%. Nat. Commun. 2020, 11, 3367. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Kumar, A.; Ghosh, D.S. Oxide-metal-oxide based transparent electrodes and their potential application in semitransparent perovskite solar cells-optical modeling studies. Sol. RRL 2023, 7, 2200863. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N.; Costas, A.; Stanculescu, A.; Rasoga, O.; Stavarache, I.; Petre, G.; Popescu-Pelin, G.; Toderascu, I.; Breazu, C.; et al. Reduced graphene oxide-based multilayer transparent conductive electrodes. Vacuum 2025, 233, 113943. [Google Scholar] [CrossRef]
- Chandrakar, N.; Kumar, A.; Rani, S.; Ghosh, D.S. Oxidized copper seed layer for ultrathin and semi-transparent silver films. Thin Solid Film. 2025, 809, 140586. [Google Scholar] [CrossRef]
- Li, Q.; Tao, K.; Zhang, J.; Ren, Y.; Liu, Z. Structural, Optical and electrical properties of the flexible, asymmetric TiO2/Cu/Ag/ZnS and ZnS/Cu/Ag/TiO2 films deposited via magnetron sputtering. Coatings 2025, 15, 650. [Google Scholar] [CrossRef]

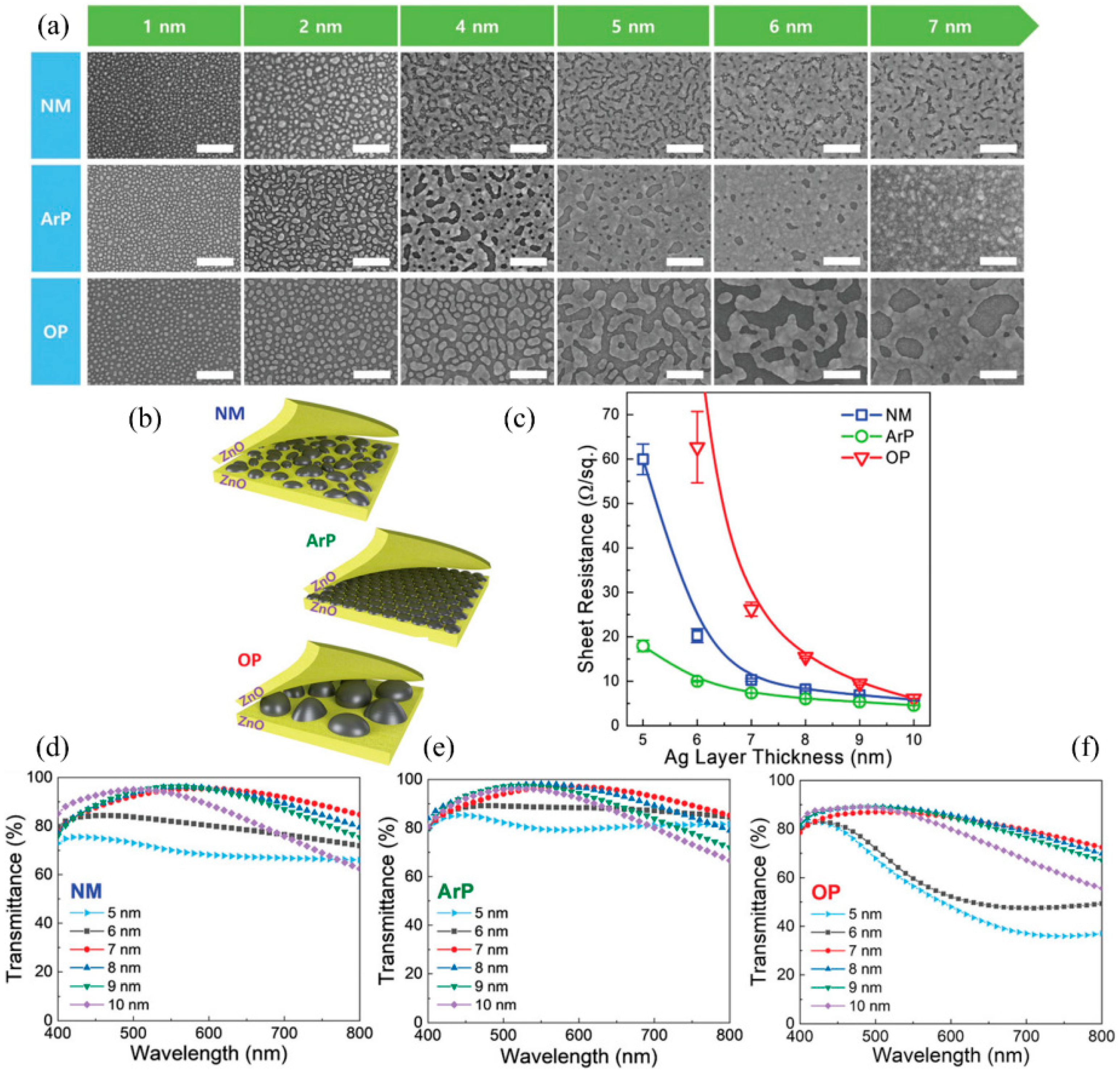
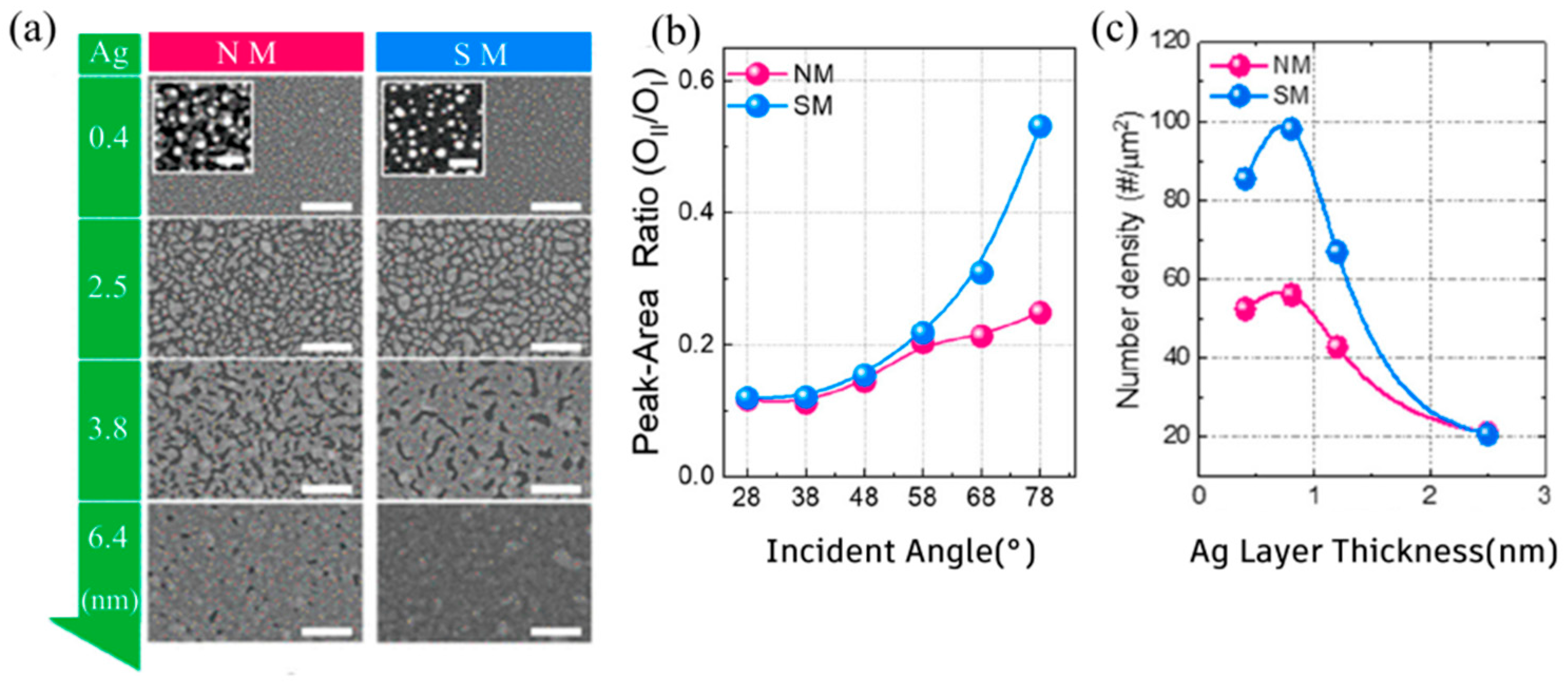
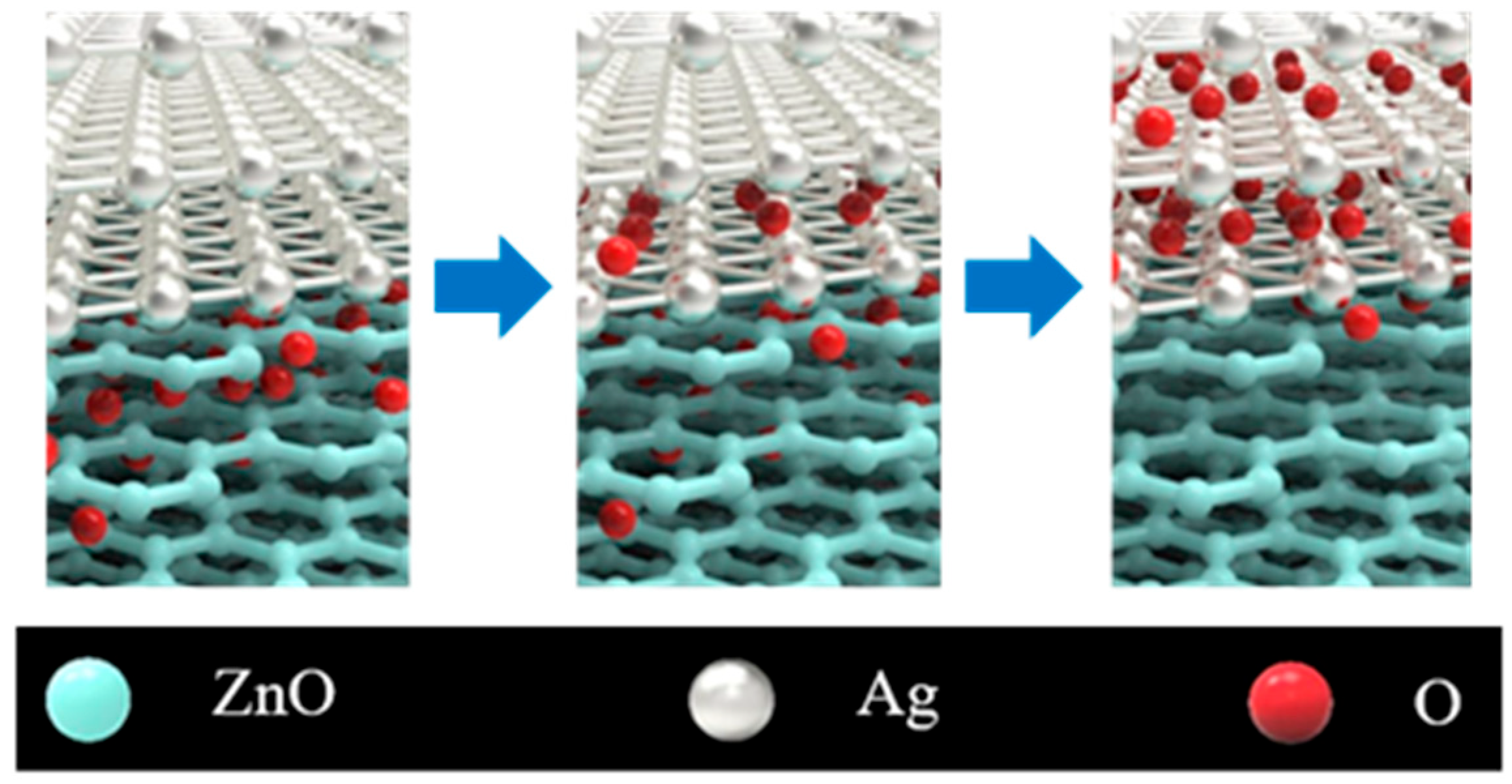
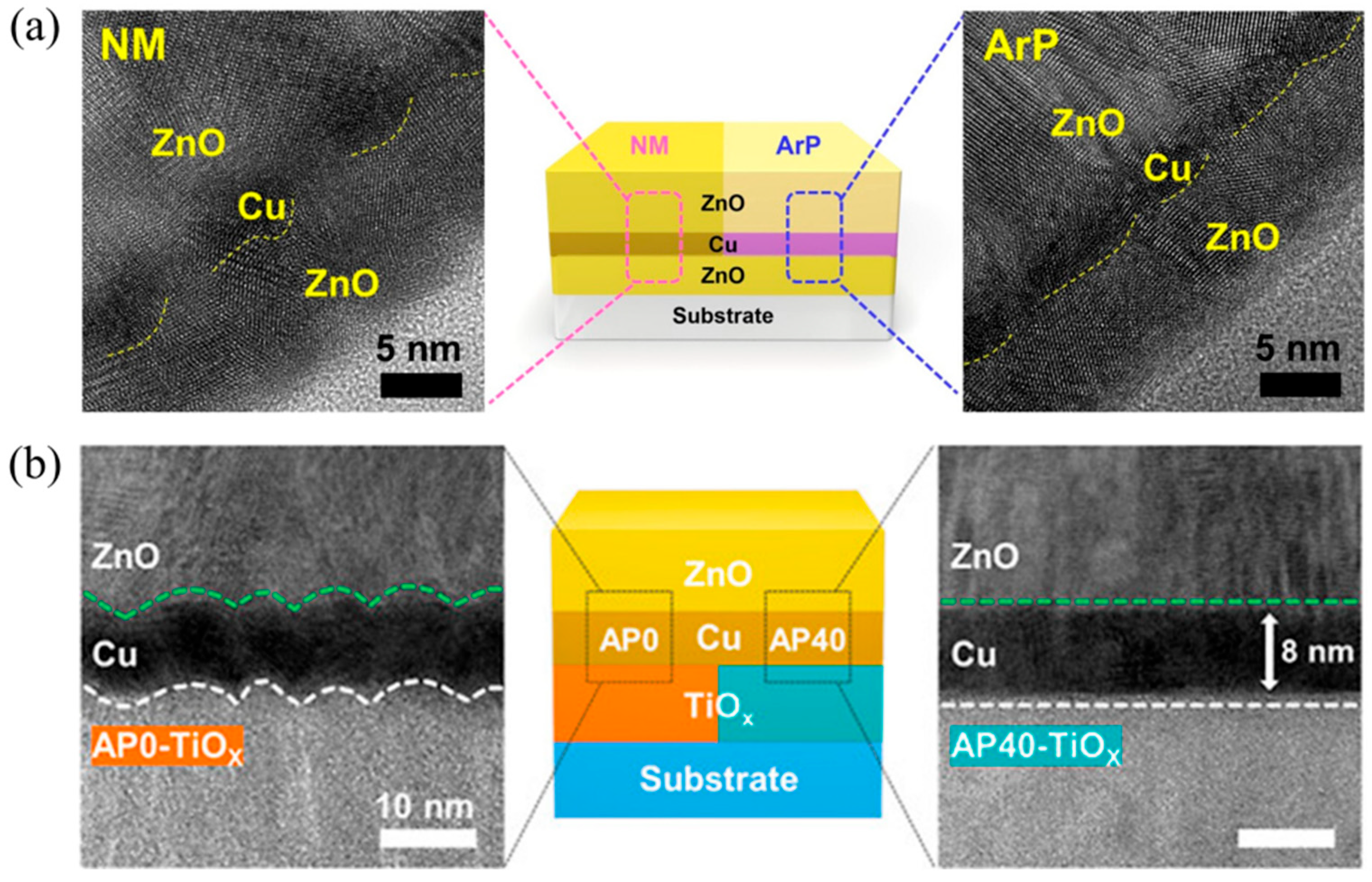

| T/Tav (%) | Rs (Ω/sq) | FOM Value (×10−3 Ω−1) | tCT (nm) | Surface Roughness | Ref. | |
|---|---|---|---|---|---|---|
| Ag:Al | 80 | 14.2 | 0.82 | [59] | ||
| Ta2O5/7 nm Ag:Al/ZnO | 96.0 at 550 nm | 23.1 | 28.78 | [60] | ||
| TiO2/Ag:Al/TiO2/MgF2 | 92.4 | 20 | 22.68 | [38] | ||
| TiO2/10 nm Ag:Cr/TiO2 | 94.2 at 550 nm | 57.15 | [61] | |||
| 6 nm Ag:Cu | 76 | 14.1 | 6 | 0.19 | [62] | |
| Ag: Ni | 80 | 18.92 | 7 | 0.57 | [63] | |
| AZO/6 nm Ag:SnOx/AZO | 88 | 10.8 | 25.79 | 6 | [65] | |
| Zn:SnOx/10 nm ATC/Zn:SnOx | 89 | 7.8 | 41.58 | 6 | [64] | |
| ZnO/Cu:Ag/ZnO | 89 | [66] |
| Method | Main Advantages | Main Drawbacks |
|---|---|---|
| Plasma irradiation | Reducing the size of AgNPs by Ar plasma or increasing the nucleation density by producing defects on the dielectric surface, or reducing the interface energy between the Ag layer and the dielectric layer by oxygen plasma; high efficiency damping LSPR, and high FOM. Improved interfacial contact. | Equipment/parameters are more complex (power, atmosphere, duration, etc.) |
| Gas-assisted deposition | Reducing the interface energy, or increasing nucleation density to damp LSPR; reduced tCT | Challenging in precise control of gas flow rate/partial pressure. |
| Metal doping | Increasing the nucleation density to damp LSPR significantly reduced tCT and roughness. | Absorbance of doped metals leading to degraded transmittance. |
| Seed layer | Increasing the nucleation density for damping LSPR effectively reduced tCT and roughness. | Absorbance or reflectance of the metal seed layer itself leading to reduced transmittance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Nian, Y.; Wang, S.; Lu, C.; Yin, L.; Wang, C.; Ma, P.; Fang, Y. Recent Progress in Dielectric/Ag/Dielectric Transparent Electrodes on Flexible Substrates. Coatings 2025, 15, 1370. https://doi.org/10.3390/coatings15121370
Wang Y, Nian Y, Wang S, Lu C, Yin L, Wang C, Ma P, Fang Y. Recent Progress in Dielectric/Ag/Dielectric Transparent Electrodes on Flexible Substrates. Coatings. 2025; 15(12):1370. https://doi.org/10.3390/coatings15121370
Chicago/Turabian StyleWang, Yawei, Yujie Nian, Shuai Wang, Cailin Lu, Lingfeng Yin, Chunmei Wang, Peiyong Ma, and Yingcui Fang. 2025. "Recent Progress in Dielectric/Ag/Dielectric Transparent Electrodes on Flexible Substrates" Coatings 15, no. 12: 1370. https://doi.org/10.3390/coatings15121370
APA StyleWang, Y., Nian, Y., Wang, S., Lu, C., Yin, L., Wang, C., Ma, P., & Fang, Y. (2025). Recent Progress in Dielectric/Ag/Dielectric Transparent Electrodes on Flexible Substrates. Coatings, 15(12), 1370. https://doi.org/10.3390/coatings15121370






