Abstract
Boriding is a widely used thermochemical treatment to improve surface hardness and wear resistance in steels used in demanding mechanical applications. However, boronizing processes using new boron paste increase costs and generate waste, creating a need for more sustainable alternatives. In this context, the reuse of dehydrated boron paste has proven effective in the formation of Fe2B layers on AISI 9254 steel. In this study, AISI 9254 steel was boronized using reused dehydrated boron paste at 1173 K, 1223 K, and 1273 K for 3600, 7200, 10,800, and 14,400 s. Optical microscopy revealed layer thicknesses ranging from 16.07 to 69.35 X-ray diffraction confirmed the formation of single-phase Fe2B, while EDS indicated elemental redistribution within the layer. The Vickers microhardness profile characterized the mechanical behavior, and the adhesion force showed HF1-HF2 ratings. The activation energy for boron diffusion in Fe2B was calculated at 106.567 kJ . Auto-affine analysis verified the fractal nature of interface growth, with a scale according to . These results confirm that reused paste allows the formation of Fe2B layers, supporting sustainable boronization strategies with controlled interfacial evolution.
1. Introduction
The formation of FeB/Fe2B bilayers and Fe2B monolayers in metallic materials enhances certain mechanical properties, thereby improving surface wear resistance under constant loading conditions. Consequently, the surface plays a crucial role in determining the service life of these components [1,2,3,4]. Considering the industrial relevance of steels AISI 9254 and the need to optimize the properties of this steel under a boronizing treatment using reused dehydrated paste, it is of great scientific and technological interest, allowing valuable information to be generated for its implementation in sectors in manufacturing, there are production areas that face the need to improve material performance, as well as to optimize materials in order to extend their service life through the formation of monolayers on metallic substrates [5,6,7,8]. Today, industries must address the challenge of preserving the planet’s natural resources while simultaneously overcoming diverse issues in the production of finished components. In this context, surface treatments that employ reused and/or recycled materials represent a viable alternative to enhance and prolong the service life of engineering components [9,10]. Likewise, various hard coatings have been applied to metallic materials, such as carburizing, decarburization, nitriding, galvanizing, aluminizing, nickel plating, anodizing, and chrome plating, which have been employed in the manufacturing industry for several decades. However, with the aim of further improving the performance of these materials, the recent incorporation of recycled dehydrated boron paste has enabled the formation of Fe2B monolayers, while also providing relevant insights into the process [11]. Thus, boriding with reused paste constitutes a thermochemical treatment capable of producing Fe2B monolayers with enhanced wear resistance, corrosion resistance, adhesion, and other properties [5,12].
Similarly, the growth of Fe2B and FeB/Fe2B layers on steels has been assessed using fractal theory to characterize kinetic roughness and crack morphology, primarily through the Hurst exponent [13]. Pioneering studies, such as that of Morales et al. [14], demonstrated that borided interfaces exhibit multifractal rather than self-affine behavior, following universal spectra associated with stochastic boron diffusion processes. The limited body of work employing the Hurst exponent for the analysis of boride layers highlights their growth behavior based on interface morphology through statistical approaches; that is, the application of growth models to define the universal value of the Hurst exponent [14,15]. Nevertheless, given the scarce literature, it is pertinent to assess this behavior in systems treated with recycled dehydrated boron paste, with the aim of contributing to the field of self-affine fractal geometry of interface morphologies associated with the growth fronts of Fe2B iron boride. Likewise, studies on the growth kinetics of borided metallic materials have increased, either using traditional diffusion models or developing novel approaches [16,17,18,19,20,21]. However, it is important to evaluate the diffusivity of this process in order to provide valuable information for future research and potential industrial applications. In addition, evaluation of diffusivity permits the determination and analysis of the diffusion rate of boron atoms during the phenomenon, with the aim of comprehending, controlling, and optimizing the process, as reported in the literature [12]. In this context, research into the microstructural and mechanical characteristics and diffusion of boron paste treatment is essential because this process depends directly on the ability of boron to penetrate the surface of the material to form Fe2B monolayers, offering technological alternatives while contributing to the preservation of natural resources. On the other hand, the high heterogeneity of steels, both in their complex chemical composition and in the random distribution of pores available when subjected to thermochemical boriding processes, enables them to play a significant role across nearly all length scales. Therefore, it is inappropriate to characterize these systems using methods that treat the medium as homogeneous. The literature reports that the morphological characterization of borided layers exhibits, for the most part, statistical persistence when analyzed using different self-affine methods [13], which indicates that the growth of the borided layer persists. In other words, the goal is to establish power-law relationships between information variation (fluctuation function) and the observation scale, represented by the window width.
This work is organized describes the materials and methods applied to AISI 9254 steel, including the boriding treatment, characterization, and layer formation. It also presents the mechanical properties of AISI 9254 steel, estimates of diffusivities and activation energy Q and morphological characterization of the borided layers.
2. Materials and Methods
2.1. Mechanical Characterization
2.1.1. Materials and Surface Treatment
Commercial AISI 9254 steel was used, with a chemical composition of 0.51–0.59% C, 1.20–1.60% Si, 0.60–0.80% Mn, max. 0.035% P, max. 0.040% S, and 0.60–0.80% Cr. The specimens had dimensions of 15 mm in diameter and 20 mm in length, and were subjected to boriding treatment at 1173 K, 1223 K, and 1273 K for 3600, 7200, 10,800, and 14,400 s, using commercial boron paste [22,23] by powder-pack process with reused dehydrated boron paste, as illustrated in Figure 1. The container was cooled in a conventional muffle furnace without inert gas, and after the treatment time, the container was removed for air cooling.
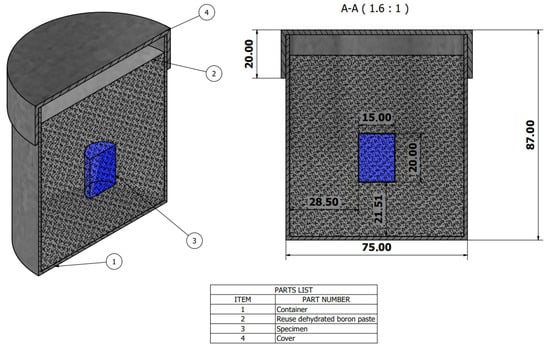
Figure 1.
Schematic view of the AISI 9254 steel container used for the boriding treatment; 1: lid; 2: reused dehydrated boron powder paste; 3: specimen; 4: container (scale in millimeters).
2.1.2. Characterization of Iron Boride
The boriding specimens were sectioned transversely for conventional metallographic processing and chemical etching with 2% nital.
The thickness and morphology of the formed borided were determined by optical microscopy using a ZEISS Axio Vert.A1 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany). To determine the phases, the borided specimens in each condition were subjected to X-ray diffraction (XRD) analysis using a Bruker D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany). CuK radiation with a wavelength of 1.54 was used in a 2 range of 20° to 90°, operating at 35 kV and 25 mA. Vickers microhardness tests were performed with a load of 50 gf and an indentation time of 10 s. Five measurements were taken for each condition using Wilson® Hardness TUKONMT 1102 equipment (Wilson Hardness, Norwood, MA, USA).
The adhesion of the boron layers was evaluated using the Daimler-Benz Rockwell C adhesion test. The well-known Rockwell C indentation test is prescribed by the VDI 3198 standard [24]. To evaluate the adhesion of the coating, a CV-700TM durometer (CV Instruments, Madrid, Spain) was used. Figure 2 shows the adhesion classification HF types according to the Daimler-Benz Rockwell-C test, VDI 3198 standard [15].
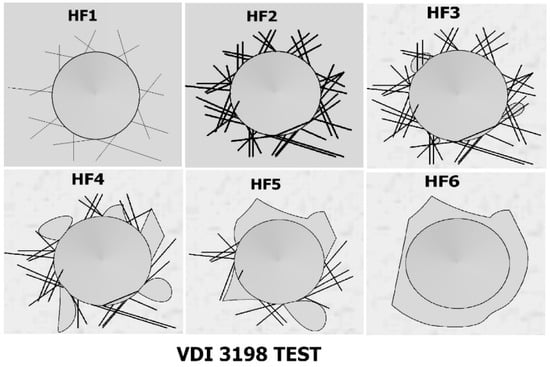
Figure 2.
Representative images of coating adhesion classification based on [15].
The indentation imprints used for classification were examined with a JEOL JSM-6010LA scanning electron microscope (JEOL Ltd., Tokyo, Japan) (SEM).
The thickness of the borided layers was obtained from the micrographs corresponding to the different temperatures and treatment times. Digital analysis was performed by binarizing the images and measuring the layer height, as illustrated in Figure 3. In total, 1224 sampling records were considered for the analysis of borided layer morphology. This digitization and interface analysis procedure has been previously documented in the literature [25].
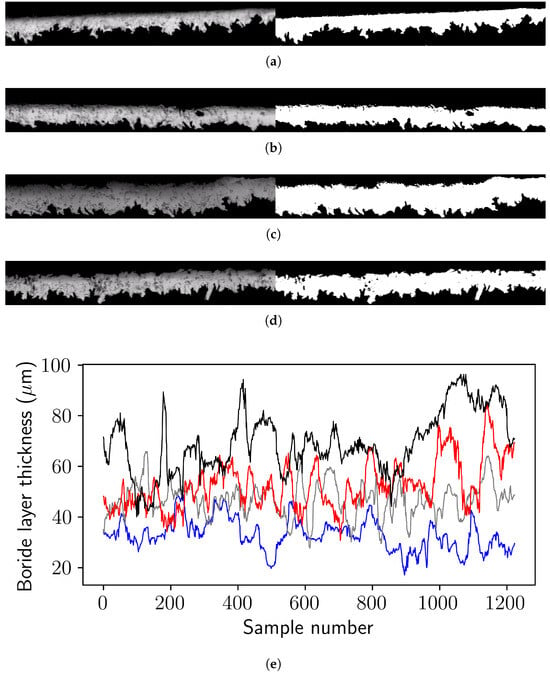
Figure 3.
(a–d) Micrographs (scale 60 ) of borided interfaces in AISI 9254 steel at a temperature of 1273 K, with treatment times of 3600, 7200, 10,800, and 14,400 s, and their corresponding binarized images. Thickness variation was obtained from 1224 sampling records of the digital processing of micrographs (a–d), (e) variation of the boride layer thickness as a function of sample number for each treatment time; blue, gray, red and black lines correspond to 3600, 7200, 10,800, and 14,400 s, respectively.
Average borided layer thicknesses were estimated, as shown in Table 1.

Table 1.
Average borided layer thicknesses obtained from measurements at different times and temperatures.
2.2. Fractal Statistical Characterization
Characterization of Borided Layer Morphology
Within the framework of fractal theory, the q-order structure function is used to establish power-law relationships of the fluctuations associated with borided layer thicknesses. These power-law relationships depend on the window and/or observation width, denoted as .
The fluctuation function () used in the analysis is constructed by accumulating the thickness profile, that is, , and fitting it with a first-degree polynomial, i.e., . Thus, is expressed as:
The structure function metric is described in Equation (2):
where represents the statistical moments of the layer thickness dataset, and is the window width. This metric was applied to four borided layer thickness series with 3600, 7200, 10,800, and 14,400 s, for temperatures 1173, 1223, and 1273 K. Each series included 1224 thickness data points, as shown in Figure 3.
If correlation exists, it is expected that , where is the generalized Hurst exponent. When , determines the second statistical moment, which coincides with the standard deviation of the series. The exponent characterizes scale invariance: antipersistent (), persistent (), or random () [26,27].
3. Results and Discussion
3.1. Thickness of the Iron Boride Layer
Figure 4 shows optical images of the microstructures of cross-sections of the boride layer obtained using the reused dehydrated boron paste process under different parameters. Figure 4a clearly shows the formation of an Fe2B layer. Similarly, in Figure 4b, an Fe2B monolayer was also obtained. In Figure 4c, the Fe2B layer is again observed, with an increase in thickness. The layers obtained under the different temperature and time conditions exhibited a saw-tooth type morphology. This morphology is attributed to the concentration of alloying elements, as reported in previous studies [2,10,12]. Likewise, with increasing time and temperature, the thickness increases, as shown in Table 1.
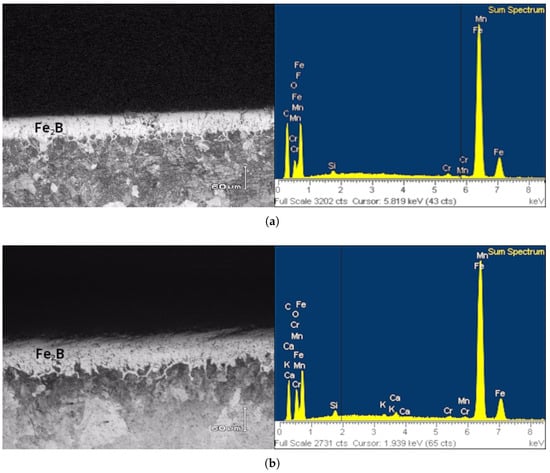
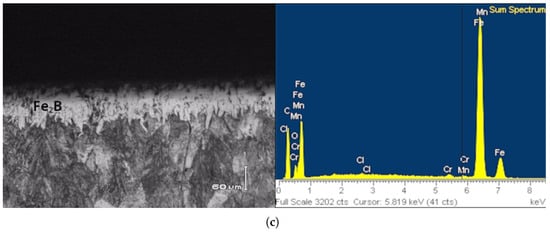
Figure 4.
Micrographs of iron boride layers under different boriding conditions; (a) 1173 K, (b) 1223 K, and (c) 1273 K for 14,400 s.
On the other hand, the limited number of studies available report that reusing the boriding medium and/or recycling the boriding process makes it possible to obtain an Fe2B monolayer [5,11,28]. However, no reports have been found on the reuse of dehydrated boron paste with the specific objective of obtaining Fe2B iron boride in the material under study.
Furthermore, the Energy Dispersive Spectroscopy (EDS) results are shown in Figure 4, confirming the presence of alloying elements in the boride layer obtained under each treatment and temperature condition. The profiles obtained by EDS show how boron and some alloying elements diffuse in the iron boride coating Fe2B In Figure 4a, Mn, Si, and Cr were identified within the Fe2B monolayer. Likewise, Figure 4b demonstrates that the alloying elements are distributed across the iron boride layer. In Figure 4c, a more pronounced distribution of Cr and Mn alloying elements is revealed within the boride layer, consistent with the observations reported in other studies [12].
3.2. X-Ray Diffraction
The X-ray diffraction (XRD) patterns of the boriding samples at 1173, 1223, and 1273 K, for different treatment times, are shown in Figure 5. In all cases, the main peaks correspond to the Fe2B phase, confirming the formation of a monolayer of iron boride on AISI 9254 steel. No signals associated with FeB were detected, suggesting that the treatment conditions and the use of reused dehydrated boron paste favored controlled boron diffusion, limiting supersaturation on the surface. The elimination of the FeB phase is important, as this phase usually appears under conditions of high boron concentration or prolonged exposure times, and its presence can induce brittleness in the coating due to its thermal expansion coefficient differing from that of the substrate. In addition, secondary peaks of MnFe2B and CrB are observed, which is consistent with the chemical composition of AISI 9254 steel, rich in manganese and chromium. These secondary borided originate from the competitive diffusion of alloying elements and may contribute to increased surface hardness, as reported in Cr- and Mn-alloyed steels [5,12,29]. Likewise, the increasing intensity of the Fe2B peaks with boronization temperature indicates an increase in the degree of crystallization of the coating, reflecting greater structural homogeneity and preferential growth in the (002) and (211) planes, characteristic of Fe2B with columnar orientation. This behavior has been reported in other works by Campos-Silva et al. [13] and Ortiz-Domínguez et al. [20], where it is associated with an improvement in the mechanical integrity of the coating. X-ray diffraction analysis confirms that the reuse of dehydrated boron paste allows for the production of single-phase Fe2B layers, free of FeB, with the secondary presence of Mn2B and CrB.
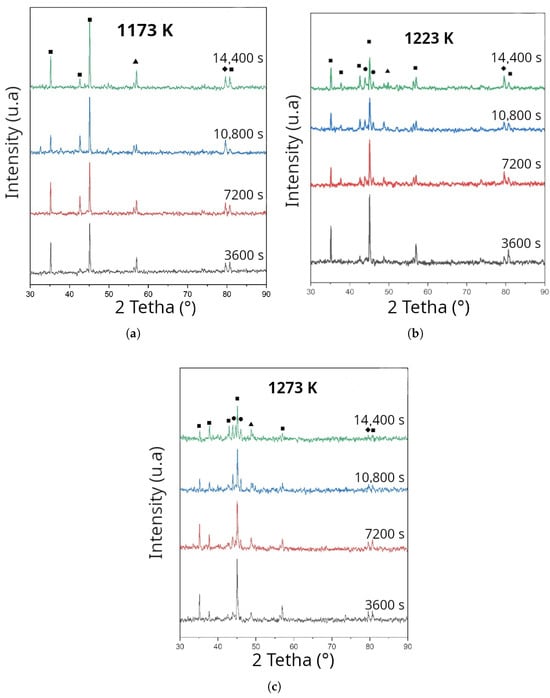
Figure 5.
X-ray diffraction images under boriding conditions; (a) 1173 K, (b) 1223 K, and (c) 1273 K. ■: , ▲: CrB, ⧫: , •: MnB.
3.3. Vickers Microhardness
The maximum microhardness values of the Fe2B monolayer were obtained as 1106.8 HV, 1452.4 HV, 1566.2 HV, and 1620.2 HV for boriding temperatures of 1173 K, 1223 K, and 1273 K, during 14,400 s. However, it was observed that the microhardness values decreased with increasing temperature. The decrease in hardness is evident when moving from the borided layer toward the diffusion layer, thereby highlighting the distinct characteristics of the borided layer compared with the non-borided matrix. The change in microhardness is attributed to the distribution of alloying elements in the Fe2B monolayer, as evidenced by several studies [2,3,5,10]. It is also clearly visible that microhardness depends on the exposure time of the material, which produced significant modifications in the Fe2B monolayer, as well as in the process with reused dehydrated boron paste.
Microhardness values of the substrate were also obtained: the hardness of the matrix remained in the range between 147.8 HV (minimum) and 165.3 HV (maximum). The boriding process with dehydrated boron paste resulted in a softening of the matrix in the borided samples. Exposure of the material to elevated temperatures induced microstructural distortions and recrystallization, which led to softening, as previously reported [30]. Figure 6 shows the microhardness profiles for each temperature and treatment time obtained by boriding with reused dehydrated boron paste.
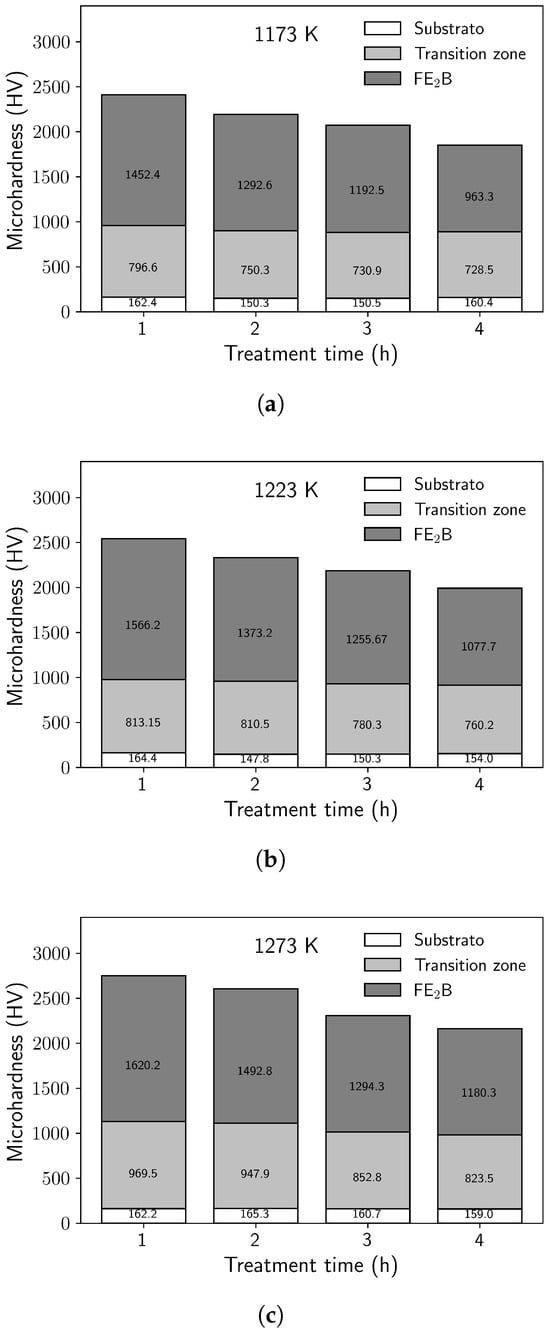
Figure 6.
Microhardness variations of borided samples; (a) 1173 K, (b) 1223 K, and (c) 1273 K.
3.4. Adhesion
The VDI 3198 standard is commonly used to evaluate the adhesion of iron boride coatings on different substrate materials [3,5,6]. The VDI 3198 standard defines the quality of adhesive strength from HF1 to HF6 to distinguish between acceptable and unacceptable adhesion.
In Figure 7, the indentation crater view from Rockwell C tests is shown. The damages, cracks, fissures, and delamination in the borided samples were examined. In Figure 7a, multiple concentric cracks within the indentation zone and cracking of the coating around the indentation were observed, corresponding to HF2 adhesion. Figure 7a evidences adhesion HF2, where cracks and fissures are clearly visible; in addition, the growth of longer lateral and radial cracks around the indentation is observed, leading to coating delamination. In Figure 7b, fine cracks were detected in the indentation area, without delamination of the coating, corresponding to HF1 adhesion. Figure 7c shows negligible delamination with few cracks, also classified as HF1 adhesion.
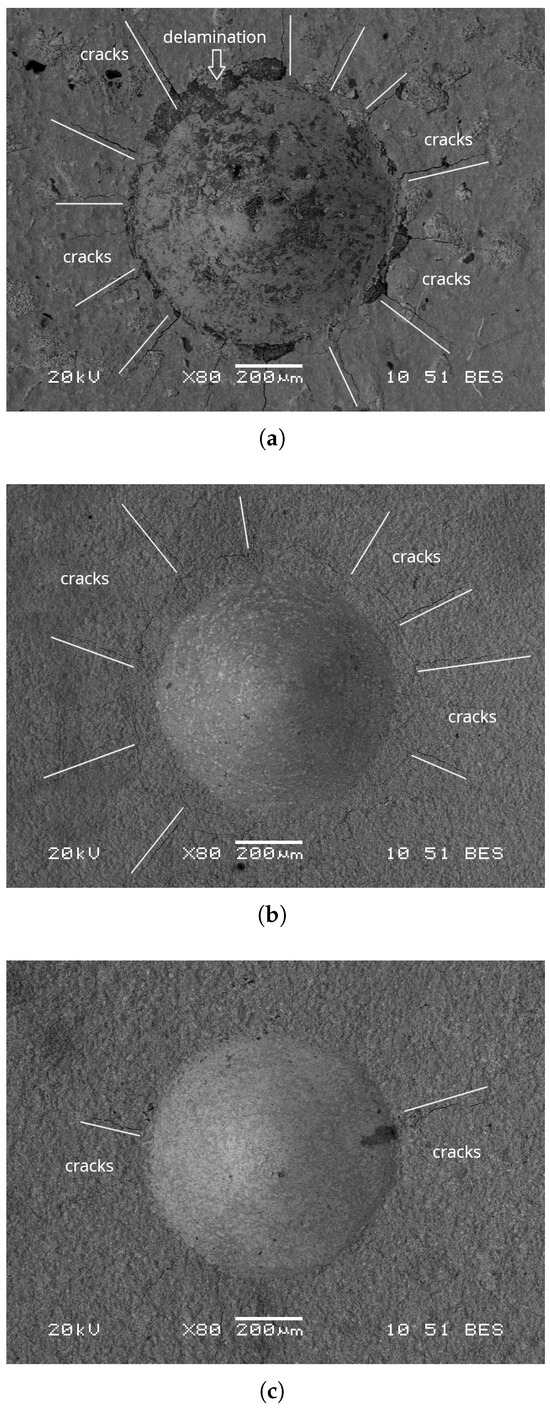
Figure 7.
SEM micrographs of the VDI 3198 adhesion test; (a) 1173 K, (b) 1223 K, and (c) 1273 K.
The adhesion results under all temperature and time conditions for the iron boride coating are summarized in Table 2. The HF code corresponds to the adhesion classification defined by the VDI 3198 standard using the Rockwell-C indentation test. An HF1 rating indicates excellent adhesion, with cracks or fissures around the indentation, while HF2 shows slightly lower adhesion characterized by radial cracks or localized delamination. Therefore, HF1 coatings have a stronger interfacial bond compared to HF2 coatings, not only. These results indicate that the thickness of the iron boride coating influenced adhesion due to anchoring in the substrate, as also evidenced in previous studies [12].

Table 2.
Adhesion type according to VDI 3198 standard for each treatment time and temperature.
On the other hand, the adhesion of the Fe2B/substrate bond depends largely on the controlled diffusion of boron, the composition of the steel, the morphology of the layer, and the residual stresses generated during treatment, as mentioned in various studies [6,12]. The different combinations of these factors give rise to varying degrees of bonding, ranging from highly adhered and resistant interfaces to weak bonds susceptible to delamination or fractures, as reported in the literature [5].
3.5. Activation Energy
Based on the determination of the layer thicknesses described above and starting from the parabolic law of normal diffusion, , where represents the mean square thickness of the layers, t is the treatment time, and k is the diffusivity, the diffusivities were estimated considering the temperatures and treatment times shown in Table 1. The diffusivities are represented by the slope in Figure 8 and are found to be = 1.33 × , = 2.11 × , and = 3.15 × .
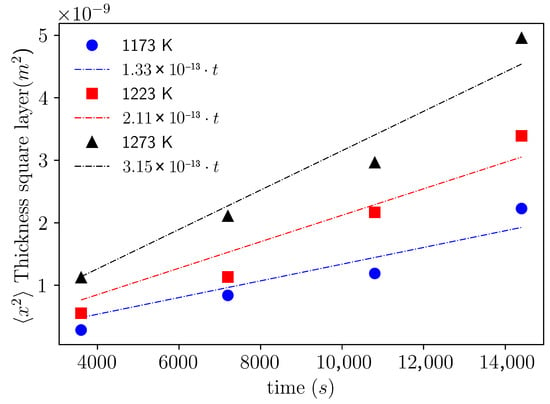
Figure 8.
Evolution of the mean square thickness () of the boride layer as a function of boriding time.
The relationship between diffusivities , the activation energy Q (kJ ), and the processes at different temperatures T (K) is given by the Arrhenius equation [31]:
where is a constant and R is the universal gas constant. Thus,
which can also be written as
In this way, the activation energy for the diffusion of boron layers in AISI 9254 steel is determined from the slope obtained by plotting versus (see Figure 9). Therefore, the calculated activation energy has a value of kJ .
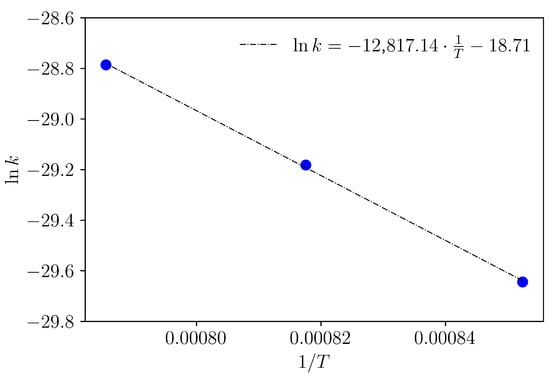
Figure 9.
Arrhenius-type relationship between diffusion coefficients and the inverse of the boriding temperature. Activation energy determination.
The use of reused dehydrated boron paste on AISI 9254 steel allows obtaining this activation energy, which indicates a greater ease for the onset of the boron diffusion process in the steel. However, as observed in Figure 8, the thicknesses and diffusivities are lower compared with those of other steels when non-reused paste is employed. In addition, various studies report that thicknesses and diffusivities of boron vary significantly depending on the type of steel and the reuse status of the boronizing process [32,33,34,35,36,37].
The diffusivities exhibit a linear growth behavior with respect to the treatment temperatures, which implies an activation energy of the order mentioned above. In contrast, other boriding studies on different steels with dehydrated paste report higher diffusivities and show a power-law growth trend with temperature, which implies higher activation energies, as reported in [23,38,39,40].
3.6. Fractal Characterization of the Morphology of Borided Layers
The results of the fractal characterization obtained by applying the structure function Equation (2) to the fluctuation function Equation (1) are shown in Figure 10. It can be observed that the power-law relationships with respect to the window width reveal long-term correlations. This behavior was found at least for , establishing self-affine fractal behavior, since the slopes or Hurst exponents for these statistical moments are of nearly the same order (see Figure 10).
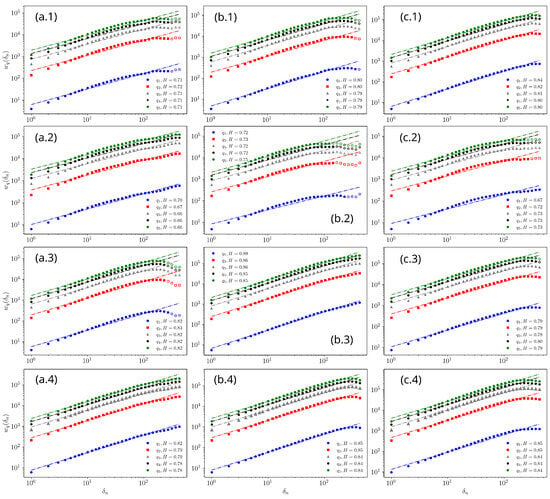
Figure 10.
Hurst exponents of the fluctuation function : (a.1–a.4) 1173 K, (b.1–b.4) 1223 K, (c.1–c.4) 1273 K.
The autocorrelation of the borided layer thicknesses shown in Figure 3 was determined using Equation (1) [13,27,41]. The value of H was obtained from the relationship , extracted from Figure 10. The Hurst exponents characterizing the morphology of the borided layers for the different temperatures and boron diffusion times are presented in Table 3. Therefore, the fluctuation associated with the borided layer thickness is characterized by the scaling of the standard deviation with respect to the window widths [41,42,43]. From Table 3, for s and different treatment temperatures, a self-affine behavior is observed with , , and , respectively. Similarly, self-affine behaviors are observed at the second, third, and fourth hours, with , , and , respectively. For s, the values are , , and . For s, the ranges are , , and .

Table 3.
Hurst exponents associated with the fluctuation function for .
In addition, the scaling interval is extended compared with the characterization of the AISI M2 steel interfaces reported by the authors [13], where different techniques were used to estimate the Hurst exponent. In that case, the Hurst exponent was established within the interval , which implies that their interfaces are more homogeneous compared with those of AISI 9254 steel.
4. Conclusions
- The Fe2B layers developed on the steel surface exhibited a saw-tooth morphology, and their thickness increased with the boriding temperature. The maximum boride layer thickness was recorded in the sample subjected to boriding at 1273.15 K, with a measurement of 69.35
- X-ray diffraction analysis revealed the formation of borides under all treatment conditions, specifically Fe2B, CrB, and Mn2B. The hardness of the boride layer formed on AISI 9254 steel showed an increase up to 1620 HV. Additionally, localized hardness reduction was observed in the transition regions.
- The adhesion tests showed HF1 and HF2 failure modes for the iron boride layer, indicating good adhesion to the AISI 9254 substrate. Therefore, the coatings obtained may have applications in various manufacturing areas.
- The activation energy was evaluated using the traditional Arrhenius-type diffusion model, resulting in an estimated value of 106 kJ .
- Within the characterization of the saw-tooth type growth fronts, their roughness was assessed. These fronts were characterized by Hurst exponents within the range associated with statistical persistence, meaning that they are governed by long-range memory processes, which implies more homogeneous growth fronts.
Author Contributions
Conceptualization, N.L.-P., M.A.D.-R. and S.M.-G. methodology, M.A.D.-R., N.L.-P., L.S.-F., E.D.G.-B., E.I.G.-O. and H.D.S.-C.; software, L.S.-F., E.D.G.-B. and E.I.G.-O.; validation, N.L.-P. and M.A.D.-R.; formal analysis, N.L.-P. and S.M.-G.; investigation, M.A.D.-R. and H.D.S.-C.; data curation, E.I.G.-O.; writing-original draft preparation, M.A.D.-R., S.M.-G., and E.I.G.-O.; writing—review and editing, N.L.-P., H.D.S.-C., L.S.-F. and E.D.G.-B.; project administration, N.L.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Laboratory for Surface Studies, Modification, and Application (LEMAS)-Polytechnic University of the Valley of Mexico in the project CIR/0022/2022 of the program “Researchers for Mexico” of SECIHTI.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Matuschka, A. Boronizing; Library of Congress: Philadelphia, PA, USA, 1980; p. 97. [Google Scholar]
- Fichtl, W. Boronizing and its practical applications. Mater. Des. 1981, 2, 276–286. [Google Scholar] [CrossRef]
- Prince, M.; Raj, G.S.; Kumar, D.Y.; Gopalakrishnan, P. Boriding of Steels: Improvement of Mechanical Properties-a Review. High Temp. Mater. Processes Int. Q. High-Technol. Plasma Processes 2022, 26, 43–89. [Google Scholar] [CrossRef]
- Dearnley, P.A.; Bell, T. Engineering the Surface with Boron Based Materials. Surf. Eng. 1985, 1, 203–217. [Google Scholar] [CrossRef]
- Campos-Silva, I.E.; Rodríguez-Castro, G.A. Boriding to improve the mechanical properties and corrosion resistance of steels. In Thermochemical Surface Engineering of Steels; Woodhead Publishing: Cambridge, UK, 2015; pp. 651–702. [Google Scholar] [CrossRef]
- Winter, K.M.; Kalucki, J.; Koshel, D. Process technologies for thermochemical surface engineering. In Thermochemical Surface Engineering of Steels; Woodhead Publishing: Cambridge, UK, 2015; pp. 141–206. [Google Scholar] [CrossRef]
- Murathan, Ö.F.; Davut, K.; Kilicli, V. Effect of austenitizing temperatures on the microstructure and mechanical properties of AISI 9254 steel. Mater. Test. 2021, 63, 48–54. [Google Scholar] [CrossRef]
- Charee, W.; Tangwarodomnukun, V. Experimental investigation and modeling of laser surface melting process for AISI 9254 commercially high silicon spring steel. Opt. Laser Technol. 2019, 115, 109–117. [Google Scholar] [CrossRef]
- Ortiz-Domínguez, M.; Morales-Robles, Á.; Gómez-Vargas, O.; Solis-Romero, J. Recycling of the Powder-pack Boriding Mixture: Microstructural Characterization of Fe2B Layers on ASTM A36 Steel. Microsc. Microanal. 2020, 26, 2220–2222. [Google Scholar] [CrossRef]
- Sinha, A. Metals Handbook, Volume 4: Heat Treating—Boriding (Boronizing) of Steels; ASM International: Materials Park, OH, USA, 1991; Volume 4, pp. 978–999. [Google Scholar]
- Perrusquia, N.L.; Ruiz, M.D.; Bustos, E.G.; Torres, C.R.; Miguel, S.; Domínguez, V.O. Microstructural Characterization on AISI 4140 Steel Boriding by New and Reused Dehydrated Boron Paste. Microsc. Microanal. 2020, 26, 1462–1463. [Google Scholar] [CrossRef]
- Kulka, M. Current Trends in Boriding; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Balankin, A.S.; Sierra, A.H.; López-Perrusquia, N.; Escobar-Galindo, R.; Morales-Matamoros, D. Characterization of rough interfaces obtained by boriding. Appl. Surf. Sci. 2008, 255, 2596–2602. [Google Scholar] [CrossRef]
- Morales, O.; Campos, I.; Martínez, J.; Tejeida, R.; Balankin, A. Self-Affine Patterns of Boride Layers. Mater. Sci. Forum 2007, 553, 27–32. [Google Scholar] [CrossRef]
- Campos, I.; Ramírez, G.; VillaVelázquez, C.; Figueroa, U.; Rodríguez, G. Study of microcracks morphology produced by Vickers indentation on AISI 1045 borided steels. Mater. Sci. Eng. A 2008, 475, 285–292. [Google Scholar] [CrossRef]
- Olivares-Luna, M.; Rosales-Lopez, J.L.; Castillo-Vela, L.E.; Chaparro-Pérez, K.D.; Delgado-Brito, A.M.; Mejía-Caballero, I.; Campos-Silva, I. Insights on the pulsed-DC powder-pack boriding process: The role of the electric charge on the growth of the boride layer and the semiconductor behavior of the boriding media. Surf. Coatings Technol. 2024, 480, 130588. [Google Scholar] [CrossRef]
- Orihel, P.; Ptačinová, J.; Gogola, P.; Keddam, M.; Jurči, P. Pack-boriding of Sleipner steel: Microstructure analysis and kinetics modeling. Mater. Test. 2024, 66, 43–55. [Google Scholar] [CrossRef]
- Ortiz-Domínguez, M.; Morales-Robles, Á.J.; Gómez-Vargas, O.A.; de Jesús Cruz-Victoria, T. Analysis of Diffusion Coefficients of Iron Monoboride and Diiron Boride Coating Formed on the Surface of AISI 420 Steel by Two Different Models: Experiments and Modelling. Materials 2023, 16, 4801. [Google Scholar] [CrossRef] [PubMed]
- İpek Ayvaz, S. Growth Kinetics and Microstructure of Iron Boride Layers on AISI 1050 Steel. Met. Sci. Heat Treat. 2024, 65, 751–757. [Google Scholar] [CrossRef]
- Ortiz-Domínguez, M.; Keddam, M. Modelling boron diffusion for Fe2B layer formation: Comparative kinetics analysis in pack-boronized AISI 4147 steel. Mater. Test. 2023, 65, 1539–1550. [Google Scholar] [CrossRef]
- Ortiz-Dominguez, M.; Keddam, M. Diffusion Kinetics and Characterization of Fe2B Coatings Grown Thermochemically on Steel ASTM A709. Met. Sci. Heat Treat. 2024, 65, 538–546. [Google Scholar] [CrossRef]
- Wahl, G. Boronizing, a method for the production of hard surfaces for extreme wear. In Durferrit-Technical Information; Durferrit GmbH: Mannheim, Germany, 1975; pp. 785–789. [Google Scholar]
- Ruiz, M.A.D.; Perrusquia, N.L.; Huerta, D.S.; Miguel, C.R.T.S.; Calderón, G.M.U.; Moreno, E.A.C.; Suarez, J.V.C. Growth kinetics of boride coatings formed at the surface AISI M2 during dehydrated paste pack boriding. Thin Solid Film. 2015, 596, 147–154. [Google Scholar] [CrossRef]
- VDI 3198:1992-08; Beschichten von Werkzeugen der Kaltmassivumformung; CVD- und PVD-Verfahren. DIN: Berlin, Germany, 1992.
- Balankin, A.S.; Otamendi, E.G.; Samayoa, D.; Patiño, J.; Rodríguez, M.A. Depinning and creeplike motion of wetting fronts in weakly vibrated granular media. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2012, 85, 036313. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. Fractals and Scaling in Finance; Springer: New York, NY, USA, 1997. [Google Scholar] [CrossRef]
- Peitgen, H.O.; Jürgens, H.; Saupe, D. Chaos and Fractals; Springer: New York, NY, USA, 1992. [Google Scholar] [CrossRef]
- Xie, F.; juan Wang, X.; wei Pan, J. Accelerate pack boriding with reused boriding media by simultaneously employing Al and alternating current field. Vacuum 2017, 141, 166–169. [Google Scholar] [CrossRef]
- Sezgin, C.T.; Hayat, F. The effects of boriding process on tribological properties and corrosive behavior of a novel high manganese steel. J. Mater. Process. Technol. 2022, 300, 117421. [Google Scholar] [CrossRef]
- Neccaroglu, V.; Karademir, I.; Unal, O. Effects of pack boriding temperature on wear and corrosion performance of high-strength armor steel. Emerg. Mater. Res. 2025, 14, 83–97. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Sánchez-Fuentes, Y.; Linares-Duarte, L.A.; Balderas-López, J.A.; Miranda-Hernández, J.G.; Tadeo-Rosas, R.; Proa-Coronado, C.; Hernández-Sánchez, E. Effect of boriding time on the effective thermal diffusivity of the borided AISI 1018 steel. Sci. Rep. 2025, 15, 19125. [Google Scholar] [CrossRef]
- Góral, M.; Kościelniak, B.; Ochał, K.; Kubaszek, T.; Jopek, J.; Drajewicz, M. The Structure of Boride Diffusion Coatings Produced on Selected Grades of Structural Steels. Solid State Phenom. 2024, 355, 95–100. [Google Scholar] [CrossRef]
- Omar, N.; Hassan, R.; Masripan, N. Kinetic study of boronized AISI 304 ball bearing using new and used boronizing powder. Proc. Mech. Eng. Res. Day 2017, 2017, 338–339. [Google Scholar]
- Fuentes, L.S.; Perrusquia, N.L.; Espinosa, M.C.E.; Máximo, D.V.M.; de la Mora Ramírez, T.; Domínguez, V.H.O.; Ruiz, M.A.D. Adhesion Characterization on AISI 9254 steel boriding. Microsc. Microanal. 2024, 30, 1273–1275. [Google Scholar] [CrossRef]
- Erdemir, A.; Eryillmaz, O.; Sista, V. Ultra-Fast Boriding for Improved Efficiency and Reduced Emissions in Materials Processing Industries; Argonne National Lab.: Argonne, IL, USA, 2012. [Google Scholar] [CrossRef]
- Daas, A.; Allaoui, L.A.; Zidelmel, S.; Allaoui, O. Paste Borided Layers Produced on XC38 Steel Using a New Activator. Mater. Perform. Charact. 2020, 9, 392–399. [Google Scholar] [CrossRef]
- Keddam, M.; Bouarour, B.; Abdellah, Z.N.; Chegroune, R. The effective diffusion coefficient of boron in the Fe2B layers formed on the iron substrate. MATEC Web Conf. 2013, 3, 01012. [Google Scholar] [CrossRef]
- Keddam, M.; Ortiz-Domínguez, M.; Elias-Espinosa, M.; Damián-Mejía, O.; Arenas-Flores, A.; Gómez-Vargas, O.A.; Abreu-Quijano, M.; Aldana-González, J.I.; Zuno-Silva, J. Growth Kinetics of the Fe2B Coating on AISI H13 Steel. Trans. Indian Inst. Met. 2015, 68, 433–442. [Google Scholar] [CrossRef]
- Chegroune, R.; Keddam, M.; Abdellah, Z.; Ulker, S.; Taktak, S.; Gunes, I. Characterization and kinetics of plasma-paste-borided AISI 316 steel. Mater. Technol. 2016, 50, 263–268. [Google Scholar] [CrossRef]
- Balankin, A.S. Dynamic scaling approach to study time series fluctuations. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2007, 76, 056120. [Google Scholar] [CrossRef]
- Barabási, A.L.; Vicsek, T. Multifractality of self-affine fractals. Phys. Rev. A 1991, 44, 2730–2733. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. Harold Edwin Hurst. In Statisticians of the Centuries; Springer: New York, NY, USA, 2001; pp. 335–338. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).