Abstract
Polyaniline (PANI), as a classical conducting polymer, has attracted significant attention in the field of energy storage due to its low cost, facile synthesis, environmental stability, and unique dual electronic/ionic conductivity. Particularly, one-dimensional (1D) nanostructures of PANI, such as nanowires and nanorods, exhibit superior electrochemical performance and cycling stability, attributed to their high surface area and efficient charge transport pathways. This review provides a comprehensive summary of recent advances in 1D PANI-based anode materials for lithium-ion, sodium-ion, and other types of rechargeable batteries. The specific capacity, rate performance, and long-term cycling behavior of these materials are discussed in detail. Moreover, strategies for performance enhancement through combination with carbon materials, metal oxides, and silicon, as well as chemical doping and structural modification, are systematically reviewed. Key challenges including electrochemical stability, structural durability, and large-scale fabrication are analyzed. Finally, the future directions in structural design, composite engineering, and commercialization of 1D PANI anode materials are outlined. This review aims to provide insight and guidance for the further development and practical application of PANI-based energy storage systems.
1. Introduction
The growing global demand for sustainable energy solutions, coupled with mounting environmental concerns, has intensified research into advanced energy storage technologies [1,2,3,4]. Among these, rechargeable batteries play a central role, with their performance closely tied to the properties of electrode materials [5]. Traditional anode materials, such as graphite, offer stable cycling and well-established processing techniques but suffer from limited theoretical capacity, hindering their ability to meet increasing energy density requirements [6,7]. Consequently, the exploration of alternative high-capacity and structurally stable anode materials has become a critical research focus.
Polyaniline (PANI), a prototypical conducting polymer, has garnered significant attention since the 1980s due to its low cost, facile synthesis, environmental stability, and distinctive mixed ionic/electronic conductivity [8,9]. The electrical properties and morphology of PANI can be finely tuned by controlling the polymerization conditions, doping agents, and synthetic templates, making it a versatile platform for electrode material design [10,11]. These features have made PANI an attractive candidate for a variety of energy storage applications.
Particularly, the development of one-dimensional (1D) PANI nanostructures, including nanowires, nanofibers, and nanorods, has demonstrated substantial advantages in electrochemical energy storage [12,13]. Owing to their high surface area and aligned pathways for charge transport, 1D architectures enable improved ion diffusion and electron conduction, thereby enhancing rate capability and cycling performance [14,15]. These benefits have positioned 1D PANI materials as promising alternatives or complements to conventional anode materials.
Recent studies have highlighted the efficacy of 1D PANI structures in various battery systems, including lithium-ion, sodium-ion, and zinc-ion batteries [16,17,18]. Their integration into anode designs has been shown to improve specific capacity, mitigate mechanical degradation from volume changes, and stabilize long-term cycling [19]. For example, in situ polymerization of PANI on silicon nanowires creates a continuous conductive framework that accommodates volume expansion and maintains electrode integrity over extended cycling [20].
Despite these advancements, practical challenges remain, such as conductivity fading, poor structural robustness under long-term operation, and scalability issues associated with synthesis [13,21]. To address these limitations, composite strategies involving carbon nanotubes, graphene, metal oxides, and silicon, as well as molecular-level modifications through doping or surface engineering, have been actively pursued [22,23].
This review provides a comprehensive overview of recent advances in 1D PANI-based anode materials for rechargeable batteries. Emphasis is placed on their structural design, electrochemical performance, composite strategies, and the mechanistic insights behind performance enhancements. Key technical barriers and future directions toward practical implementation are also discussed.
2. Polyaniline (PANI) Fundamental Principles and One-Dimensional Nanostructures
2.1. Chemical Structure and Properties of Polyaniline
Polyaniline (PANI) is a conductive polymer with excellent conductivity, adjustable chemical properties, and good environmental stability [24,25]. It is formed by the oxidation polymerization of aniline monomers and has three distinct oxidation states: leucoemeraldine, emeraldine, and pernigraniline. The conversion between these oxidation states determines PANI’s electrochemical behavior and conductivity. Among its three main oxidation states, the emeraldine form is usually the most electrochemically active, exhibiting higher conductivity. Specifically, the different oxidation states of PANI are as follows: Pernigraniline Base (PB), the most oxidized form with fewer electron transfers and thus lower conductivity; Emeraldine Base (EB), which has strong electrochemical activity and higher conductivity; Emeraldine Salt (ES), which is formed when PANI is doped with an acid, further enhancing conductivity; and finally, Leucoemeraldine Base (LEB), the reduced form with the lowest conductivity [26,27]. Figure 1 illustrates the chemical structures of the three main oxidation states of PANI. The reversible transitions among these states are fundamental to its functional applications, as they directly govern the material’s conductivity and electrochemical behavior [28].
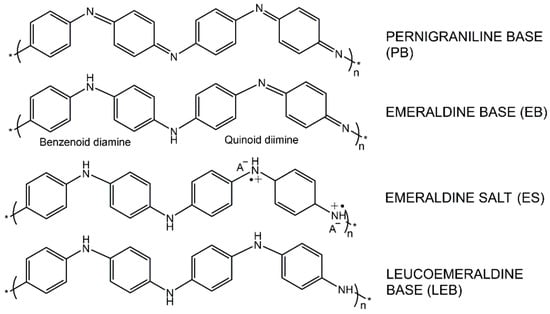
Figure 1.
Chemical structure diagram of Polyaniline (PANI) in different oxidation states. Including Pernigraniline Base (PB), Emeraldine Base (EB), Emeraldine Salt (ES), and Leucoemeraldine Base (LEB) [28]. * indicates the repeating unit in the polymer chain
The chemical structure of PANI consists of aniline units (C6H5NH), which are connected by conjugated chains, providing PANI with high electronic conductivity, especially in the emeraldine oxidation state, where PANI shows the highest conductivity. The conductivity of PANI can be adjusted by doping, with dopants (such as hydrochloric acid, phosphoric acid, and terephthalic acid) altering PANI’s electronic structure and oxidation state, thereby modulating its conductivity. For example, studies have shown that doping PANI with hydrochloric acid significantly increases conductivity as the concentration of hydrochloric acid ranges from 0.2 M to 1.25 M [29]. In another study, PANI’s conductivity reached its highest value of 7.95 × 10−2 S/cm after a two-step treatment (first with water, then with an acid solution) [30]. Moreover, phosphoric acid-doped PANI also exhibited enhanced conductivity. Studies using chemical oxidation methods to synthesize phosphoric acid-doped PANI, with phosphoric acid concentrations ranging from 10% v/v to 30% v/v, showed that conductivity improved, and the conductivity further increased with higher phosphoric acid concentration [31]. In another study, PANI composites doped with terephthalic acid demonstrated higher conductivity, especially when used in conjunction with hydrochloric acid, which further enhanced PANI’s conductivity and stability [32]. These examples highlight the important role dopants play in regulating the conductivity of PANI, and how different types and concentrations of dopants can significantly influence its electrical properties, providing broad prospects for PANI in applications such as solar cells, sensors, batteries, and membrane materials.
The conductivity mechanism of PANI mainly depends on the electron movement within its conjugated chains. In the doped state, the electron cloud of the PANI chain can move freely, thus improving its conductivity and electrochemical performance. The reversible doping and dedoping process allows PANI to perform well in many electrochemical applications such as supercapacitors, batteries, and sensors.
2.2. Electrochemical Behavior of PANI
The electrochemical performance of polyaniline (PANI) is crucial for energy storage and sensor applications. The charge and discharge processes of PANI involve redox reactions, making it an ideal electrode material. The charge–discharge mechanism of PANI typically accompanies electron transfer and protonation processes. In electrochemical applications, the oxidation states of PANI can transition from leucoemeraldine to emeraldine, and then to pernigraniline, while during discharge, the reverse reaction occurs. This process involves not only the transfer of electrons but also the transfer of protons, which gives PANI a high charge storage capacity.
Polyaniline (PANI) is a conductive polymer that holds significant potential in energy storage and sensor applications due to its excellent electrochemical performance. The charge and discharge process of PANI involves redox reactions, where PANI undergoes oxidation state transitions from leucoemeraldine to emeraldine and then to pernigraniline during charging, and the reverse occurs during discharging. This process involves electron transfer as well as proton transfer, resulting in a high charge storage capacity for PANI. However, despite the good stability of PANI in electrochemical environments, it may degrade during long-term use, especially under high-frequency charge–discharge cycles. To address this issue and improve cycling stability, researchers often employ doping and composite strategies. For example, Mažeikienė and Malinauskas investigated the electrochemical degradation kinetics of PANI films in 0.5 M sulfuric acid solution, with electrode potential ranging from 0.3 V to 1.0 V [33]. At lower potentials (0.3–0.6 V), the degradation of PANI follows a first-order rate constant, ranging from 4 × 10−5 to 5 × 10−5 s−1, corresponding to a half-life of 4–5 h. When the potential increases to 0.9–1.0 V, the degradation rate significantly increases, with the maximum degradation rate constant for PANI-PSS at ≈2.5 × 10−3 s−1, and for PANI-ITS at ≈1.2 × 10−3 s−1, due to the faster degradation of pernigraniline compared to emeraldine. To improve stability, Olena Okhay and other researchers [34] composited PANI with graphene and reduced graphene oxide (RGO) for supercapacitor electrodes. Pure PANI electrodes, due to aggregation during preparation, resulted in lower specific capacitance and poor cycling stability, while G/PANI composites exhibited improved electrochemical performance through a synergistic effect. The ratio of graphene to PANI, the type of oxidizing agent, and the polymerization conditions significantly affect the morphology and performance of the composite electrodes. Furthermore, PANI composites with acid-treated multi-walled carbon nanotubes (a-MWNT) showed better cycling stability than pure PANI, as a-MWNT effectively suppressed the morphological changes of PANI and enhanced its electrochemical accessibility [35]. Heat treatment further improved the stability of the composites under open-circuit conditions, although this effect gradually weakened with increasing cycles. Overall, while PANI shows promising electrochemical performance, doping and composite strategies significantly enhance its stability, especially under high-frequency charge–discharge cycles, thus improving its durability in energy storage and sensor applications.
The electrochemical behavior of PANI is significantly influenced by doping substances. The doping process not only enhances its conductivity but also improves its electrochemical stability. By adjusting the type and concentration of dopants, the redox reaction rate of PANI can be controlled, thereby improving its electrochemical performance in devices such as supercapacitors and lithium-ion batteries. For example, studies have shown that doping PANI with hydrochloric acid (HCl) and sulfuric acid (H2SO4) significantly enhances its specific capacitance. In 1 M KOH electrolyte, PANI doped with 1 M HCl (PH1) showed higher specific capacitance (167.75 F/g) than PANI doped with 1 M H2SO4 (PS), which exhibited 155.75 F/g. Furthermore, PH1 demonstrated a specific capacitance of 256 F/g at 6 M KOH concentration (at a current density of 1 A/g). X-ray diffraction (XRD) analysis confirmed the semi-crystalline nature of the doped PANI, and the lattice structure shifted to a higher angle, indicating enhanced charge storage capacity and stability. Field-emission scanning electron microscopy (FESEM) further revealed that PANI doped with 2 M HCl exhibited aggregation, while PANI doped with 1 M HCl and H2SO4 showed good nanofiber structures [36].
Moreover, PANI doped with lithium salts (such as LiPF6 and LiClO4) has been used as the positive electrode material for lithium batteries. After 10 charge–discharge cycles, PANI doped with LiPF6 exhibited an initial discharge capacity of 125 mAh/g and a stable reversible capacity of 114 mAh/g, whereas PANI doped with LiClO4 showed 112 mAh/g and 81 mAh/g, respectively. When using polymer electrolytes, PANI doped with LiPF6 exhibited better cycling performance and stability [37]. Another study showed that PANI doped with lithium chloride (LiCl) exhibited a specific capacitance of 395 F/g, and when using constant current charge–discharge curves in 1 M H2SO4 electrolyte, it showed a specific capacitance of 471 F/g. After 1000 cycles, it demonstrated 100% Coulombic efficiency and high energy density (65.42 W h/kg) and power density (178.57 W/kg) [38]. Additionally, PANI doped with transition metals such as silver (Ag+) and iron (Fe3+) showed significant performance improvements. The surface area and conductivity of the doped PANI increased threefold, along with enhanced specific capacitance, energy density, and power density. Electron paramagnetic resonance (EPR) analysis showed that the defect structure in the doped PANI electrode material changed, further confirming the optimization effect of doping strategies on the electrochemical performance of PANI. Figure 2 displays the electrochemical characteristics of iron-doped PANI (PANI:Fe) composites, with the left side showing the scanning electron microscope (SEM) image of PANI:Fe, which exhibits its nanofiber structure, aiding in its electrochemical performance [39]. The right side presents the impedance spectra of different PANI:Fe samples (S1–S6), showing the electrochemical behavior at various doping concentrations. Particularly, the S3 sample exhibited lower electrochemical impedance and better conductivity, suggesting its potential application in batteries or supercapacitors. Therefore, doping with different ions or lithium salts, optimizing electrolyte concentrations, and selecting appropriate electrolytes can significantly improve the electrochemical performance and stability of PANI, providing important support for its application in supercapacitors, lithium batteries, and other electrochemical devices.
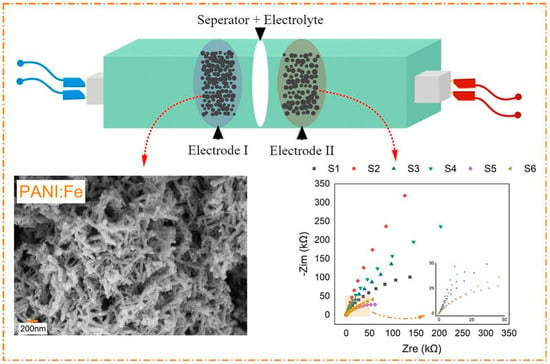
Figure 2.
Electrochemical characteristics of iron-doped polyaniline (PANI:Fe) composites. The left side shows the SEM image of PANI:Fe, displaying its nanofiber structure. The right side shows the impedance spectra of different samples (S1–S6), demonstrating the effect of doping concentration on the electrochemical performance, where the S3 sample shows lower electrochemical impedance and better conductivity, indicating its superior performance in batteries or supercapacitors [39].
2.3. One-Dimensional Polyaniline Nanostructures
In recent years, one-dimensional (1D) nanostructures of PANI, such as nanorods, nanowires, and nanotubes, have attracted significant attention in energy storage, sensor, and catalysis fields due to their unique morphology and excellent electrochemical performance. Compared to traditional two-dimensional and three-dimensional structures, 1D nanostructures significantly enhance the electrochemical performance of PANI due to their higher specific surface area and shorter electron/ion transport pathways, especially during fast charge–discharge processes. For example, crosslinked polyaniline nanorods (CPANI), prepared by chemical oxidative copolymerization, exhibited a conductivity of 33.3 S cm−1, significantly higher than that of pure PANI (4.26 S cm−1). CPANI showed a maximum specific capacitance of 455.1 F g−1 in 1 M H2SO4 electrolyte at a scan rate of 1 mV s−1, much higher than PANI’s 286.7 F g−1, and after 1300 cycles, the capacitance retention of CPANI electrodes improved significantly, showing excellent electrochemical stability [40]. In addition, MnO2 nanowires/polyaniline (MnO2NW/PANI) composite materials also demonstrated excellent electrochemical performance. The composite material exhibited a specific capacitance of 306.7 F g−1 at a current density of 0.5 A g−1, an increase of 28.4% compared to pure MnO2 (238.9 F g−1). The composite also showed excellent cycling stability, maintaining 88.7% capacity after 5000 cycles [41]. Furthermore, different 1D nanostructures of PANI, such as nanotubes and nanofibers, formed during the wet chemical oxidation process, also exhibited excellent electrochemical performance. PANI nanotubes, with diameters ranging from 250 to 1500 nm, demonstrated high charge transfer capability, making them promising for supercapacitor applications. Through different doping and composite strategies, the electrochemical performance of PANI has been further improved [14]. For example, when PANI was combined with 3-aminopropyltriethoxysilane (APTEs)-titanium nanowires (TNW), the specific capacitance in 1 M H2SO4 electrolyte reached 315.16 mF cm−2, significantly higher than pure PANI-TNW (271.67 mF cm−2), and the capacitance retention rate after 1000 cycles was 86.8%. The excellent performance of this composite material is attributed to the anchoring effect of APTEs, which promotes the formation of a compact structure between TNW and PANI nanoparticles, maintaining good stability during rapid charge–discharge processes [42].
One-dimensional nanostructures provide more active sites and facilitate the rapid transport of electrons and ions, which helps increase capacitance and charging speed. For example, the smaller diameter and longer length of PANI nanostructures give them a higher specific surface area and shorter ion diffusion paths, thus enhancing charge storage capacity and cycling stability. Additionally, the electronic transport properties of PANI are optimized in the 1D structure, allowing PANI to perform excellently in applications such as batteries and supercapacitors. Studies have shown that simple hydrothermal methods for synthesizing PANI nanowires (PANI NWs) at different temperatures significantly improve their electrochemical performance. For example, PANI nanowires synthesized at 80 °C in 0.5 M H2SO4 solution exhibited a specific capacitance of 540.0 F/g at a current density of 0.5 A/g, and after 1000 charge–discharge cycles, they maintained 82% of their initial capacitance, indicating excellent charge–discharge performance and cycling stability [43]. Another study demonstrated the electrochemical performance of a vertically aligned mesoporous silicon film (VMSF) grown on an ITO electrode using an electrochemical-assisted self-assembly (EASA) method, with PANI nanofibers (PANI/VMSF/ITO composite electrode) grown on the template (Figure 3). This composite electrode exhibited significantly improved electrochemical performance, with a specific capacitance of 3.00 mF/cm2 (at a current density of 20 μA/cm2), a 152% increase compared to pure PANI grown directly on the ITO substrate (PANI/ITO). The improvement in performance was primarily attributed to the optimized charge transfer efficiency and electrochemical reaction kinetics, stemming from the good alignment of the PANI nanostructures [44]. Furthermore, reducing electrode materials to the nanoscale is an effective strategy to overcome the low energy density problem of supercapacitors. Nanowires, as one type of 1D nanostructure, have gained widespread attention due to their unique structure, which provides efficient ion pathways, enhances the utilization of electroactive materials, and allows for the construction of various special architectures, thus offering more channels for electron and ion transport and improving charge transfer efficiency at the electrode/electrolyte interface. These studies further validate the important role of 1D nanostructures in enhancing the electrochemical performance of PANI and provide strong support for its application in supercapacitors.
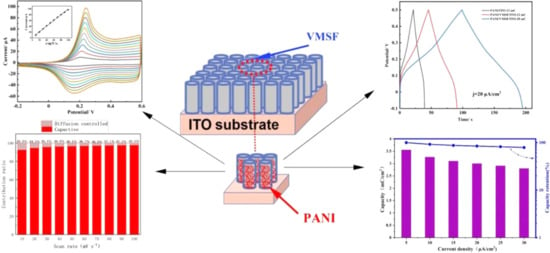
Figure 3.
Electrochemical performance of PANI nanofiber/mesoporous silicon composite electrodes prepared via electrochemical-assisted self-assembly (EASA). The upper-left corner shows the cyclic voltammetry (CV) curve, displaying the voltage response of the PANI/VMSF/ITO composite electrode at different current densities [44].
2.4. Synthesis Methods of One-Dimensional PANI Nanostructures
To optimize the one-dimensional (1D) nanostructures of PANI, researchers have developed various synthesis methods, including chemical oxidative polymerization, electrospinning, template-assisted synthesis, and hydrothermal methods. Chemical oxidative polymerization is the most common method for synthesizing 1D PANI nanostructures. By using oxidizing agents such as ammonium persulfate (APS), aniline monomers polymerize in solution to form PANI. This method is low-cost and easy to operate, but it requires precise control of reaction conditions (such as temperature, time, and solvent) to obtain uniform nanostructures. Previous studies have shown that using dodecylbenzenesulfonic acid (DBSA) and hydrochloric acid (HCl) as dopants, with ammonium persulfate (APS) as an oxidizing agent, PANI nanotubes with diameters of 350–650 nm and lengths of tens of micrometers can be obtained, as well as nanofibers with diameters of 120–160 nm. The structural uniformity of the nanotubes is nearly 100%, with a yield of up to 110% [45]. Another study utilized vanadic acid as an oxidizing agent and synthesized 1D PANI nanostructures without templates or structural directing agents. The results showed that the HCl concentration significantly affected the morphology: at low acid concentrations, nanorods with diameters of 10–20 nm and lengths of 50–60 nm were formed, while at higher acid concentrations, nanostructures with reduced diameters (40–100 nm) and smoother surfaces were obtained [46]. Additionally, interface polymerization has been used to prepare 1D PANI nanostructures, such as in a stable oil/water emulsion system with MgCO3 and CaCO3 particles as emulsifiers, producing nanofibers with an average diameter of 33 nm and nanotubes with an average outer diameter of 28 nm, with significantly shortened polymerization times [47]. Another important study pointed out that by altering the terminal groups of the surfactants used as additives, the dimensions of PANI can be effectively controlled. Specifically, using surfactants such as sodium dodecylbenzenesulfonate (SDBS), sodium dodecyl sulfate (SDS), and sodium dodecyl laurate (SLS), micelle soft templates were formed, successfully synthesizing PANI fiber plates, branched structures, and rod-like structures. The study showed that the rod-like structure of PANI had a higher specific surface area and rougher surface, which allowed more ions to enter its interior and contact active sites, making its specific capacitance (192 F/g) significantly higher than that of fiber plates (55 F/g) and branched structures (64 F/g), especially at a current density of 3.0 A/g. By adjusting the substituent groups of the additive molecules, the size of PANI can be altered, significantly affecting its electrochemical performance [48]. These studies demonstrate that by reasonably selecting synthesis methods and adjusting reaction conditions, PANI’s nanostructures can be optimized, greatly improving its electrochemical performance, especially in supercapacitor applications. In addition, Huang et al. [49] reported the synthesis of highly ordered PANI nanorod arrays in aqueous solution using hydrophilic Allura Red AC (ARAC) as a structure-directing agent and APS as the oxidant. As shown in Figure 4, the resulting nanorods (80–400 nm in diameter, 8–15 μm in length) form well-aligned arrays that promote efficient charge transport and provide abundant electroactive sites, further confirming that micellar template control is an effective strategy for constructing oriented 1D PANI nanostructures.

Figure 4.
Scanning electron microscope (SEM) image of ordered PANI nanorod arrays synthesized in an aqueous medium using Allura Red AC (ARAC) as the structure-directing agent and ammonium persulfate (APS) as the oxidant [49].
Electrospinning is a method that stretches a polymer solution into fibers using an electric field, suitable for preparing one-dimensional nanomaterials with high specific surface area. This method can effectively enhance the electrochemical performance of PANI, especially in supercapacitor applications. For example, Silas K et al. (2016) [50] used a single-step electrospinning method to fabricate high-purity PANI nanofibers, achieving nanofibers containing 93 wt% PANI. To meet the electrospinning requirements, the researchers mixed ultrahigh molecular weight polyethylene oxide (PEO) with PANI to provide sufficient chain entanglement. To further improve the conductivity and stability of the electrodes, they added a small amount of carbon nanotubes (CNTs) to the PANI/PEO solution and successfully prepared PANI/CNT/PEO nanofibers (12 wt% CNTs). Scanning electron microscopy (SEM) and BET surface area analysis indicated that the prepared nanofibers had excellent external morphology. Transmission electron microscopy (TEM), X-ray diffraction (XRD), and Fourier-transform infrared spectroscopy (FT-IR) analysis showed that PANI and PANI-CNT electrodes exhibited specific capacitances of 308 F/g and 385 F/g at a current density of 0.5 A/g, respectively. After 1000 charge–discharge cycles, the capacitance retention of PANI and PANI-CNT electrodes was 70% and 81.4%, respectively. The excellent electrochemical performance of these electrodes is attributed to the nonwoven, interconnected nanofiber network, which can efficiently conduct electricity and promote electrolyte penetration, ensuring fast ion transport [50].
In addition, El-Refaie Kenawy et al. [51] also synthesized hollow PANI nanofibers with controllable wall thickness using in situ polymerization of aniline, with electrospun polyetherimide fiber membranes as templates. At a current density of 1 A/g, the hollow PANI nanofibers exhibited a specific capacitance of 601 F/g, indicating that their hollow structure, thin wall thickness, and ordered pore structure significantly promoted ion diffusion and enhanced electroactive utilization. Additionally, Miao et al. [52] fabricated PAN-PPh/Fe3O4 composite electrode materials via electrospinning, which retained about 78.49% of the initial specific capacitance after 3000 charge–discharge cycles, demonstrating good cycling stability. These studies show that electrospinning, as an effective method for preparing high-surface-area one-dimensional nanomaterials, can significantly improve the electrochemical performance of PANI, particularly in supercapacitor applications.
Template-assisted synthesis is another important strategy. By using hard templates (such as anodized aluminum oxide) or soft templates (such as polymer micelles), the size and morphology of PANI can be precisely controlled to generate high-quality one-dimensional PANI nanostructures. For example, David Pahovnik et al. [53] polymerized aniline in an ionic liquid (ILs) solution containing imidazole, pyridine, and quaternary ammonium cations, studying the effects of IL type and aniline/IL molar ratio on the morphology and performance of PANI. The results showed that ILs significantly controlled the morphology of PANI, forming nanowires or complex two-dimensional and three-dimensional structures. In contrast, without ILs, PANI exhibited aggregated particle-like structures. UV-Vis, IR, and Raman spectroscopy analysis revealed that the ILs did not alter the chemical structure of PANI but affected the assembly of PANI during the polymerization process. Dynamic light scattering (DLS) showed that ILs and their mixtures with oxidizing agents and aniline formed ordered micellar structures in 1 M HCl, which likely acted as soft templates to guide the growth of PANI chains or polymerize aniline molecules into specific nanostructures.
Another study used sucrose octaacetate as an in situ seed and soft template for the oxidation polymerization of high-crystallinity PANI nanostructures [54]. The study demonstrated that by adjusting the concentration of sucrose octaacetate, different morphologies of PANI nanofibers and nanorods were obtained. As the concentration of sucrose octaacetate increased, the generated PANI structures exhibited irregular morphologies, such as particulate and scaffold-like aggregates. Fourier-transform infrared spectroscopy (FTIR), UV-Vis, and scanning electron microscopy (SEM) analyses showed that the presence of sucrose octaacetate only changed the morphology of PANI, without affecting its molecular structure. When using 2 g of sucrose octaacetate, the polymerized PANI exhibited higher specific capacitance, stronger conductivity, better thermal stability, and higher crystallinity and ordered structure.
Additionally, electrochemical polymerization was used with hexagonal packed, oriented mesoporous silicon films as hard templates to successfully control the vertical alignment and ordered growth of PANI nanofibers [55]. Using a 2 nm pore diameter template, nearly single PANI chains were generated. The study differentiated the stages of polymerization (induction, growth, and overgrowth) through chronoamperometric experiments and films of different thicknesses (100–200 nm). The study also showed that mesoporous silicon films as hard templates not only effectively generated isolated PANI nanofibers but also improved the reversibility of PANI and enhanced the electroactive surface area.
CAO et al. [10] also provided a simple one-step method to synthesize PANI nanostructures, using the organic dye ethyl orange (EO) as a soft template to synthesize PANI nanorods and significantly enhance their conductivity to 13.5 S/cm. The addition of EO improved the dispersibility of PANI, making it well-dispersed in water and stable in various organic solvents. Additionally, the EO-PANI coating exhibited significantly enhanced electrochemical stability and corrosion resistance in 3.5 wt% NaCl solution, with higher impedance and corrosion protection performance.
CAO et al. [56] used methyl orange (MO) as a soft template for electrospinning to synthesize PANI microtubes. MO effectively self-assembled into sheet-like and branched supramolecular aggregates and acted as a soft template to guide the synthesis of PANI microtubes. Ultimately, these PANI microtubes exhibited excellent conductivity and good dispersibility, showing great potential in conductive filler applications in polyurethane resin. By combining PANI microtubes with silver sheets, the prepared composite material exhibited lower resistance, demonstrating its great potential in enhancing the conductivity of resins. Figure 5 shows the process of synthesizing PANI microtubes using methyl orange (MO) as a soft template, where oxidation polymerization occurs, and MO interacts with aniline molecules, ultimately forming hollow PANI microtubes. This process diagram clearly shows the polymerization process, template function, and the structure of the resulting PANI microtubes.
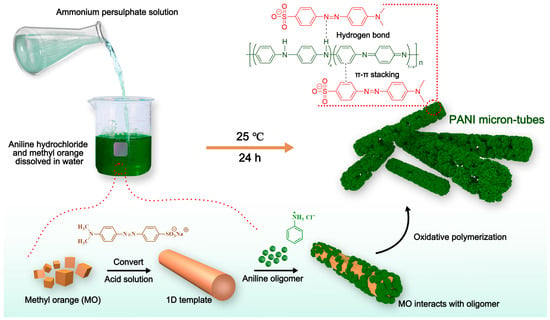
Figure 5.
Schematic of PANI microtubes synthesized with methyl orange (MO) as a soft template. MO self-assembles into a one-dimensional soft template in acidic solution, and aniline monomers polymerize on the template surface under the action of ammonium persulfate (APS) oxidizing agent, ultimately forming hollow PANI microtubes. The interaction between MO and PANI oligomers through hydrogen bonds and π–π stacking stabilizes the formation of microtubes. This method effectively improves the crystallinity and conductivity of PANI, and the resulting PANI microtubes exhibit excellent dispersibility and electrochemical performance [56].
Table 1 further summarizes and compares these synthesis methods, highlighting their main features, the morphology of the resulting PANI nanostructures, and their electrochemical performance.

Table 1.
Synthesis Methods, Morphology, and Electrochemical Performance of PANI Nanostructures.
3. Modification and Composite of Polyaniline One-Dimensional Materials
3.1. Composite with Carbon-Based Materials and Its Application in Batteries
One-dimensional polyaniline (PANI) materials have gained widespread application in energy storage devices such as batteries due to their excellent conductivity and electrochemical stability. However, the theoretical capacity of PANI itself is relatively low, limiting its use in high-energy-density batteries. Therefore, the composite of PANI with carbon-based materials (such as graphene, carbon nanotubes, etc.) has become an important strategy to enhance its performance. The combination with carbon-based materials significantly improves PANI’s conductivity, structural stability, and electrochemical performance. These carbon-based materials not only provide support and enhance PANI’s conductivity but also further improve the performance of the composite material by providing more active reaction sites and higher specific surface area. Therefore, the composite of PANI with carbon-based materials is not only an important method to improve the performance of PANI one-dimensional materials in batteries but also an effective approach to forming high-performance PANI one-dimensional materials.
For example, one study proposed a scheme to composite modified polyaniline (iPANI) with reduced graphene oxide (rGO) and carbon nanotubes (CNTs). This composite material serves as a double-interface engineering strategy in lithium-sulfur batteries (LSBs), effectively solving issues such as polysulfide shuttle, slow oxygen reduction kinetics, and lithium dendrite growth [57]. The study shows that iPANI@rGO-CNTs composite material not only effectively catalyzes the conversion of sulfur species but also significantly improves the battery’s cycle life and rate capability, demonstrating the great potential of PANI one-dimensional materials in batteries after the composite. Another study also proposed a similar scheme with iPANI, rGO, and CNTs for lithium-sulfur batteries, demonstrating the effectiveness of this composite in addressing key battery issues. Figure 6 shows the SEM and HR-TEM images of the iPANI@rGO-CNTs composite material, further validating its excellent electrochemical performance and potential in battery applications. Figure 6a,b display the overall structure of the material, while Figure 6c,d reveal higher resolution details, showing the uniform distribution of PANI on the rGO-CNTs substrate with good structural properties. Elemental analysis (Figure 6f) shows the distribution of elements such as C, N, O, and P, further supporting the successful synthesis of the iPANI@rGO-CNTs composite material. Raman spectroscopy (Figure 6g) and FT-IR (Figure 6h) analysis further verify the material’s structural features and the changes in functional groups. Finally, Figure 6i shows the difference in amino group content between iPANI@rGO-CNTs and PANI@rGO-CNTs, indicating the role these groups play in electrochemical reactions.
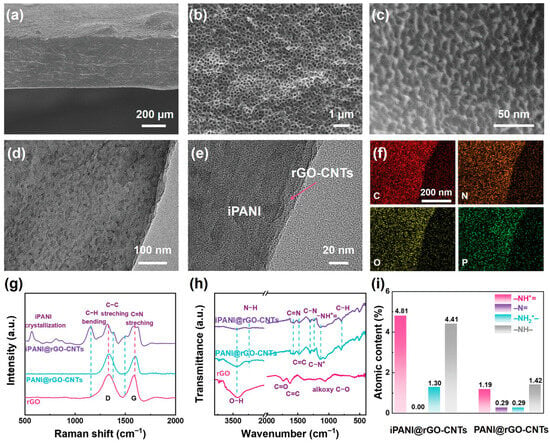
Figure 6.
Characterization of the iPANI@rGO-CNTs Composite Material. (a,b) show the overall scanning electron microscope (SEM) images of the material, displaying its surface structure. (c,d) are high-resolution transmission electron microscope (HR-TEM) images, revealing the uniform distribution of polyaniline (PANI) one-dimensional nanomaterials on reduced graphene oxide (rGO) and carbon nanotubes (CNTs). (e) shows the clear interface between iPANI and rGO-CNTs, indicating the intimate contact and tight integration between the two components. (f) is the elemental distribution map, showing the distribution of C, N, O, and P elements, confirming the composite structure of the material. (g,h) represent Raman spectra and Fourier-transform infrared (FT-IR) spectra, characterizing the structural features and functional group changes of the iPANI@rGO-CNTs composite material. Finally, (i) shows the difference in amino group content between iPANI@rGO-CNTs and PANI@rGO-CNTs, further indicating the formation of the polyaniline one-dimensional material and the enhancement of its electrochemical performance [57].
In one study, black phosphorus (BP) was combined with carbon nanotubes (CNTs) and polyaniline (PANI) to form a PANI/(BP–CNT) composite material. Figure 7a shows that the PANI/(BP–CNT) composite material exhibits an initial discharge capacity of 2127.9 mAh g−1, and after 100 charge–discharge cycles, it retains a reversible capacity of 1150.7 mAh g−1, significantly outperforming the pure black phosphorus (BP) material. This indicates that PANI’s expansion characteristics in the composite effectively improve the material’s stability, while carbon nanotubes (CNTs) protect the edge structure of BP, preventing structural collapse and enhancing the composite’s conductivity [58].
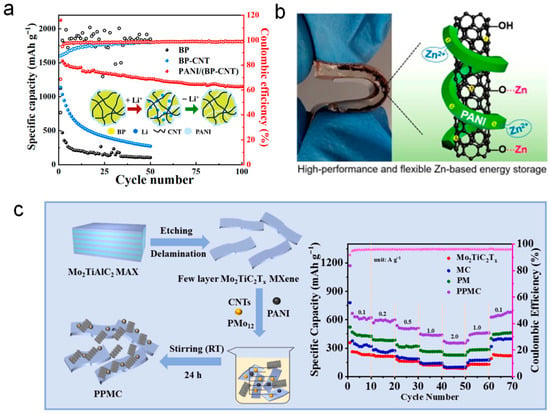
Figure 7.
Electrochemical performance of PANI-based 1D composites with carbon materials. (a) Discharge profiles of PANI/(BP–CNT) composite showing a high initial capacity of 2127.9 mAh g−1 and 1150.7 mAh g−1 after 100 cycles [58]. (b) Flexible f-CNT/PANI composite in Zn-based EES, exhibiting high capacity, good rate capability, and long-term stability [59]. (c) POM@PANI/Mo2TiC2Tx/CNTs composite delivering 621 mAh g−1 and 445 mAh g−1 at 1 A g−1 with excellent cycling stability [60].
In zinc-based electrochemical energy storage (EES) systems, functionalized carbon nanotubes (f-CNT) were combined with PANI to form an efficient composite material, which was used as the cathode material for zinc-based batteries (Figure 7b). The f-CNT/PANI composite effectively combines the capacitive energy storage mechanism of carbon nanotubes with the redox reaction mechanism of PANI, exhibiting ultra-high capacity, good rate performance, and long-term cycling stability [59]. This demonstrates the outstanding electrochemical performance of PANI one-dimensional materials when combined with carbon-based materials in zinc-based batteries. Figure 7b shows the flexible physical image and related mechanism of the composite.
Additionally, MXenes (two-dimensional transition metal carbides) are a material with high surface area and metallic conductivity, which have also been used in the POM@PANI/Mo2TiC2Tx/CNTs quaternary composite material [60]. This composite material exhibits a high lithium storage capacity of 621 mAh g−1 and retains 445 mAh g−1 at 1 A g−1, showing excellent electrochemical performance. Figure 7c demonstrates the superior performance of this composite material in the battery, particularly its ability to maintain a high specific capacity under high current density, with good cycling stability. The unique three-dimensional structure and synergistic effects of the composite significantly improve the material’s reactivity and stability, providing a new direction for the application of PANI one-dimensional materials in high-performance batteries.
MXene/PANI composites have demonstrated outstanding performance across multiple fields, further reinforcing their potential as high-performance energy storage materials. For instance, Pr2CuO4/MXene/MWCNT and PANI composites synthesized via a simple hydrothermal and in situ polymerization process have been successfully applied in symmetric supercapacitors [61]. The optimized PC2 composite (Pr2CuO4/MXene/MWCNT/PANI 50:50) exhibited remarkable electrochemical properties, with a specific capacitance of 2611.47 Fg−1 and an energy density of 58.032 Whkg−1, while the synergistic interactions effectively prevented MXene restacking and enhanced rate capability. Similarly, MXene/PANI-based fiber-shaped supercapacitors (FSSCs) showed excellent electrochemical characteristics, achieving a high areal capacitance of 510 mF cm−2 and an energy density of 15.71 μWh cm−2, highlighting their promise for wearable electronics [62]. Furthermore, MXene/PANI composites have also been successfully applied in electromagnetic interference (EMI) shielding. The fabricated composite fabrics exhibited an EMI shielding efficiency of 31.87 dB, along with outstanding washing resistance and bending stability, offering a practical route toward multifunctional wearable fabrics [63]. These studies collectively demonstrate that MXene/PANI composites possess broad application prospects in electrochemical energy storage and EMI protection, complementing the earlier discussion on high-performance batteries and capacitors.
Finally, in the application of supercapacitors, the PANI/MWNT composite material demonstrated excellent electrochemical performance. One study used polyamidoamine (PAMAM)-modified MWNT/PANI composite materials, observing that the PANI nanowires were arranged in an ordered manner on the MWNTs. This composite material exhibited higher specific capacitance and longer cycling life compared to MWNT/PANI, proving that covalently connecting PANI to MWNTs can improve the stability and conductivity of the battery [64].
3.2. Composite with Metal Oxides, Silicon, and Other High-Capacity Materials
Polyaniline (PANI) one-dimensional materials, due to their excellent conductivity and electrochemical stability, are widely used in energy storage devices such as batteries. However, the theoretical capacity of PANI itself is relatively low, limiting its application in high-energy-density batteries. Therefore, the composite of PANI one-dimensional materials with high-capacity materials (such as SnO2, MnO2, silicon, etc.) has become an important strategy to enhance its performance. By combining with these high-capacity materials, PANI can effectively address the low conductivity and severe volume expansion issues of metal oxides, forming a flexible conductive network, thus significantly improving the conductivity, structural stability, and electrochemical performance of the composite material.
For example, in one study, metal-assisted chemical etching combined with in situ polymerization was used to prepare a PANI/silicon one-dimensional nanowire array composite for lithium-ion battery anodes [65]. This composite material showed good electrochemical performance, specifically, the PANI/(Si-NW) composite material exhibited an initial discharge capacity of 2 mAh cm−2, and after 346 charge–discharge cycles, it still maintained a stable specific capacity. At high current densities (2 mA cm−2), it was able to maintain this capacity, significantly improving the cycle stability and rate performance. The introduction of PANI effectively alleviated the volume expansion problem of silicon, further enhancing the stability of the composite electrode. Figure 8a shows the preparation process of the PANI/silicon one-dimensional nanowire composite material, which maintains stable specific capacity after 346 charge–discharge cycles.
A core–shell SnO2@PANI nanorod array fabricated by hydrothermal synthesis followed by electrodeposition exhibits excellent cycling and rate performance (Figure 8b) [66]. The conductive PANI shell provides efficient electron pathways and serves as a mechanical buffer to mitigate the volume change of SnO2; its pseudocapacitive contribution further increases charge storage. Consequently, the composite delivers a reversible capacity of 506 mAh g−1 after 100 cycles with a low capacity fade of 0.579% per cycle, much lower than pristine SnO2 (1.151%) and PANI–SnO2 nanorods (1.150%); even at 3000 mA g−1 it maintains 660 mAh g−1, demonstrating outstanding rate capability and cycling stability. The introduction of the PANI shell not only alleviated the volume expansion of SnO2 but also improved the conductivity and stability of the composite material [66]. The enhanced electrochemical performance of PANI/SnO2 composites can be attributed to the synergistic effects between PANI and SnO2. The conductivity of PANI improves the overall conductivity of SnO2, facilitating better electron transport during charge and discharge cycles, thereby enhancing capacity and rate performance. The PANI shell also acts as a mechanical buffer, alleviating the volume expansion of SnO2 and preventing structural degradation, which improves cycling stability. Additionally, the pseudocapacitive nature of PANI further increases charge storage, enhancing the specific capacity and stability of the composite [67,68]. These factors collectively enable the PANI/SnO2 composite to exhibit higher reversible capacity, excellent rate capability, and superior cycling stability compared to pure SnO2 or other composites without PANI.
In addition, an innovative study prepared a three-dimensional graphite foam (GF)@SnO2 nanorod array (NRAs)@PANI composite structure. This composite material combines the good conductivity of graphite foam, the lithium-ion transport channels provided by SnO2 nanorods, and the conductivity of PANI. After 50 charge–discharge cycles, the composite material showed a high discharge capacity of 540 mAh/g, and at a high current density of 3 A g−1, it maintained a specific capacity of 414 mAh/g. The high capacity and excellent electrochemical stability of this composite material are attributed to the synergistic effects of the conductive network provided by PANI and the lithium-ion transport channels of SnO2 [66].
A study proposed a simple and environmentally friendly strategy, using a low-temperature synthesis method to successfully create a uniform polyaniline (PANi) spiky/BiOCl chip (BPB) heterostructure without surfactant assistance for the first time. Bi2S3 nanowires served as a sacrificial template and provided the Bi source for BiOCl, promoting the simultaneous formation of HCl-doped PANi conductive arrays and BiOCl chips supported by Bi2S3 nanowires [69]. This composite material demonstrated excellent electrochemical performance as a supercapacitor electrode material, especially in neutral media, exhibiting higher specific capacitance, better rate performance, and lower charge transfer impedance compared to pure PANi nanofibers. Its superior performance is attributed to the unique hierarchical nanostructure of BPB and the synergistic effect between BiOCl and PANi chains, effectively demonstrating the strategy of combining PANI with high-capacity materials to enhance electrochemical performance. Figure 8c shows the low-temperature synthesis and electrochemical performance of the PANI spiky/BiOCl chip heterostructure (BPB). The BPB composite material, synthesized by low-temperature methods, exhibited high specific capacitance, good rate performance, and low charge transfer impedance in neutral media. Its excellent performance is due to the hierarchical nanostructure of the BPB composite material and the synergistic effect between BiOCl and PANI chains [70].
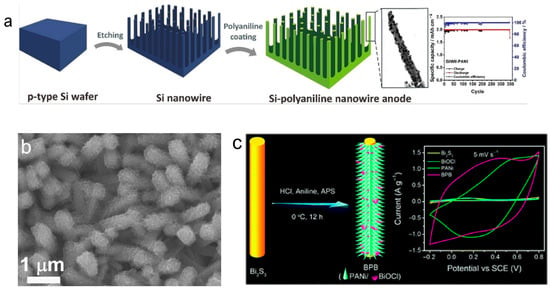
Figure 8.
Electrochemical performance of PANI one-dimensional materials combined with high-capacity materials. The figure shows the preparation and performance of three different composite materials: (a) Preparation and electrochemical performance of PANI/silicon one-dimensional nanowire array composite material, showing stable specific capacity after 346 charge–discharge cycles and good electrochemical performance at a high current density of 2 mA cm−2 [65]. (b) SEM image of the surface morphology of a SnO2@PANI core–shell nanorod array (scale bar: 1 μm) [66]. (c) Low-temperature synthesized PANI spiky/BiOCl chip heterostructure (BPB), exhibiting excellent electrochemical performance, including high specific capacitance, better rate performance, and low charge transfer impedance in neutral media. All composite materials demonstrate the advantage of significantly enhancing electrochemical performance by combining with high-capacity materials [70].
4. Electrochemical Performance of One-Dimensional PANI-Based Negative Electrode Materials
This section focuses on the electrochemical performance of one-dimensional polyaniline (PANI) materials in various energy storage systems, including lithium-ion batteries (LIBs), sodium-ion batteries (SIBs), other types of rechargeable batteries, and supercapacitors. Unlike the composite strategies discussed in the previous section, this section specifically analyzes the electrochemical behavior, performance metrics, and practical application of these materials.
4.1. Electrochemical Behavior of One-Dimensional PANI in Energy Storage Systems
One-dimensional PANI materials, with their unique structural features (such as high specific surface area and efficient electron/ion transport channels), have demonstrated enormous potential in various energy storage devices. In lithium-ion batteries, PANI composites with high-capacity materials (such as silicon, metal oxides, etc.) fully utilize its advantages in accommodating volume expansion and enhancing conductivity.
In lithium-ion batteries (LIBs), one-dimensional polyaniline (PANI) materials, when used as negative electrode materials, show significant improvements in electrochemical performance. Especially when PANI is combined with high-capacity materials like silicon (Si) nanowires (Si-NW), PANI effectively mitigates the volume expansion issues of silicon during charge and discharge cycles, significantly improving capacity retention and cycling stability. For example, after composite with silicon nanowires, PANI not only enhanced the electronic conductivity but also improved the structural stability of the electrode, helping the composite material retain high specific capacity and excellent cycling stability after multiple charge/discharge cycles. This shows that one-dimensional PANI materials not only improve the performance of batteries but also significantly extend their cycling life, solving the bottleneck issues of traditional lithium-ion battery negative electrodes. The enhanced performance of PANI/Si composites is due to several factors. PANI improves the conductivity of silicon, facilitating better electron transport and enhancing capacity. It also mitigates silicon’s volume expansion during cycling by providing mechanical support, thus preventing structural degradation and improving cycling stability. Additionally, PANI’s pseudocapacitive behavior increases charge storage, further boosting the composite’s capacity and stability. These combined effects allow PANI/Si composites to offer higher reversible capacity, better rate performance, and superior cycling stability compared to pure silicon.
To further enhance the electrochemical performance of PANI, researchers have also explored other composite materials, especially in lithium battery applications. For example, PANI/polyimide (PANI/PI) composite materials were successfully prepared with a hierarchical three-dimensional micro/nano structure through electrospinning and in situ polymerization methods [71]. The composite material exhibited a small average pore size (1.730 μm) and a narrow pore size distribution (1.552–1.882 μm). In lithium batteries, the PANI/PI composite separator, compared to traditional polyolefin separators, showed excellent thermal stability (up to 180 °C), higher porosity (84%), and greater electrolyte absorption (619%). These advantages allowed batteries with PANI/PI composite separators to not only display lower interfacial resistance but also exhibit enhanced capacity (133 mAh g−1 at 0.2C) and better rate performance (41.4% at 10C). Particularly, after 500 charge/discharge cycles, it retained 89.3% of its capacity, showcasing the great potential of one-dimensional PANI materials in improving lithium battery performance (as shown in Figure 9). The enhanced electrochemical performance of the PANI/PI composite separator is attributed to several key factors: the hierarchical three-dimensional structure improves electrolyte infiltration and ion transport, while PANI’s high conductivity and pseudocapacitive behavior enhance charge storage and rate performance. Additionally, the PI polymer’s mechanical stability and the composite’s excellent thermal stability (up to 180 °C) ensure reliable operation under harsh conditions. The high porosity of the composite further increases electrolyte absorption, ultimately contributing to better battery capacity, cycling stability, and overall performance.
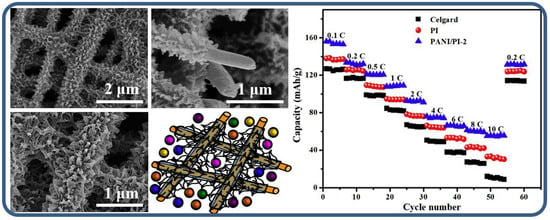
Figure 9.
Electrochemical performance of PANI/PI composite materials. The scanning electron microscope (SEM) images show the surface structure of PANI/PI composite materials. The discharge curves of the battery at different C-rates demonstrate the excellent capacity and stability of the PANI/PI composite materials at 0.2C and 10C rates, especially after 500 charge/discharge cycles, where it retained 89.3% of its capacity. The capacity retention of PANI/PI composite materials after 500 charge/discharge cycles indicates excellent cycling stability and good rate performance, particularly in high-rate applications [71].
Additionally, PANI nanowire arrays, as a new type of electrode material, have also demonstrated excellent electrochemical performance in lithium batteries [72]. A study used Na5V12O32 nanowires as a sacrificial template to prepare PANI nanowire arrays for the first time. After 100 charge/discharge cycles, the nanowire arrays were able to deliver a high discharge capacity of 119.79 mAh g−1 within a voltage range of 2.0 V to 4.0 V, with good capacity retention. This indicates that PANI nanowire arrays in lithium battery applications can not only maintain high capacity but also ensure long-term stability and high efficiency, further proving the promising application of one-dimensional PANI materials in lithium batteries.
Moreover, PANI-coated β-AgVO3 nanowires have also shown significant electrochemical performance improvement in lithium batteries. Using a simple soaking-drying method, a PANI coating was successfully applied to β-AgVO3 nanowires, significantly improving their capacity and cycling stability. Compared to bare β-AgVO3 nanowires, the PANI coating not only improved electrode conductivity but also effectively enhanced electrochemical stability, reducing charge transfer resistance [73]. This demonstrates that PANI, as a coating material, can effectively enhance lithium battery electrode performance, especially in terms of improving capacity retention and reducing charge transfer resistance, showing great application potential.
These studies clearly demonstrate that combining one-dimensional PANI materials with high-capacity materials (such as silicon, metal oxides, silver vanadate, etc.) significantly improves the electrochemical performance of lithium-ion batteries by enhancing conductivity and structural stability. Whether through composites with silicon, metal oxides, or PANI coatings, one-dimensional PANI materials in lithium batteries show broad prospects, particularly in enhancing capacity, rate performance, and cycling stability. Therefore, one-dimensional PANI materials, especially when combined with other high-capacity materials, demonstrate significant application potential and competitiveness, providing new solutions for high energy density, long-life lithium-ion batteries.
For SIBs, the larger ionic radius of Na+ (1.02 Å) compared to Li+ (0.76 Å) leads to more severe structural stress and volume fluctuations during insertion/extraction, posing greater challenges for anode stability. The flexible backbone and pseudocapacitive behavior of PANI make it especially suitable for accommodating such stress and ensuring rapid charge transport. A representative study demonstrated that MnO2 nanowires modified with PANI exhibited significantly enhanced electrochemical performance [74]. Specifically, MnO2/PANI nanowire arrays assembled on carbon cloth showed improved conductivity, structural stability, and reversibility during Na+ storage. The composite electrode delivered a capacity of ~200 mAh·g−1 after 60 cycles at 0.1 A·g−1, and still maintained 182 mAh·g−1 after 200 cycles, outperforming pristine MnO2 nanowires. These results highlight that PANI nanostructures can act as flexible conductive frameworks to buffer the volume expansion of Na+ insertion and improve the cycling durability of SIB anodes.
Beyond LIBs and SIBs, PANI 1D structures and their composites have also demonstrated great potential in other emerging battery systems. In lithium–sulfur (Li–S) batteries, the polysulfide shuttle effect is a major factor limiting cycling stability. To address this, researchers synthesized PANI-encapsulated amorphous V2O5·nH2O nanowires (VOH@PANI) via in situ oxidative polymerization and integrated them into polypropylene separators to construct VOH@PANI-modified functional separators [75]. The amorphous V2O5 nanowires provided strong polysulfide adsorption, catalytic activity, and ionic conductivity, while the PANI coating further enhanced conductivity and flexibility. Benefiting from this synergistic effect, the VOH@PANI separator effectively suppressed polysulfide shuttling and significantly improved the cycling stability of Li–S cells. In aluminum–selenium (Al–Se) batteries, the dissolution of selenium (Se) and intermediate species (Se2Cl2) in AlCl3-based electrolytes typically leads to structural collapse and capacity fading. To overcome this, a three-dimensional hierarchical Se@PANI@graphene (Se@PANI@G) composite cathode was constructed, where Se nanowires were encapsulated with a 15 nm-thick PANI shell and further embedded into graphene nanosheets [76]. This unique architecture provided high conductivity, robust encapsulation, and structural integrity, enabling the Al–Se battery to deliver an initial capacity of 445.5 mAh·g−1 at 200 mA·g−1 and to retain 164.0 mAh·g−1 after 160 cycles. Similarly, aqueous zinc-ion batteries, valued for their safety and low cost, face sluggish ion diffusion and poor rate performance due to the large hydrated Zn2+ ions. PANI nanorod arrays synthesized by chemical oxidative polymerization were demonstrated as cathode materials with enhanced ion accessibility and pseudocapacitive contribution [77]. They achieved a discharge capacity of 178.4 mAh·g−1 at 0.1 A·g−1, while maintaining 84.6% and 63.8% of capacity at 1 A·g−1 and 10 A·g−1, respectively, along with excellent cycling stability of 95.2% retention after 10,000 cycles. Further enhancement was realized in MnO2@PANI nanorod arrays, which combined the high theoretical capacity of MnO2 with the conductive and flexible framework of PANI, delivering 293.7 mAh·g−1 at 0.1 A·g−1 and superior rate performance, with 51% capacity retention under a tenfold increase in current density, compared to only 35% for MnO2@PANI films [78]. Collectively, these examples highlight the multifaceted roles of 1D PANI in next-generation batteries, functioning not only as a conductive polymer but also as a structural stabilizer, protective shell, and ion transport facilitator, thereby broadening its applicability across diverse electrochemical energy storage systems. These findings demonstrate that PANI can serve not only as a conductive polymer but also as a functional protective layer that mitigates dissolution and enhances long-term cycling.
In summary, one-dimensional PANI materials have exhibited remarkable versatility as anode components in a range of battery systems. In LIBs, they improve capacity and stabilize high-capacity anodes such as silicon; in SIBs, they mitigate large-ion-induced volume stress while maintaining fast charge transport; and in Li–S and Al–Se batteries, they act as multifunctional interlayers and encapsulation shells to suppress shuttle or dissolution effects. Moving forward, combining PANI nanostructures with carbonaceous materials, transition-metal compounds, and emerging two-dimensional materials will be an important strategy to further optimize their electrochemical properties and accelerate the development of high-performance next-generation rechargeable batteries.
4.2. Performance Parameters
The electrochemical performance of one-dimensional PANI-based negative electrode materials is typically evaluated through several key parameters:
Specific Capacity is a crucial indicator of the charge storage capability of electrode materials and directly affects the energy density of batteries [79]. For one-dimensional PANI-based negative materials, although PANI itself has a low specific capacity, the specific capacity increases significantly when it is combined with high-capacity materials such as silicon (Si) or tin oxide (SnO2). For example, PANI-SnO2 composite materials deliver a high reversible capacity of 506 mAh/g after 100 charge–discharge cycles, and at a high current density (such as 3000 mA/g), the specific capacity remains at 660 mAh/g. This indicates that PANI not only enhances the specific capacity but also maintains a high capacity at high current densities, effectively improving the energy density of the battery. Further research shows that one-dimensional PANI materials can significantly improve the specific capacity of lithium batteries [80]. For instance, a study demonstrated the electrochemical performance of a TiO2@CC@PANI core–shell structure electrode, which showed a specific capacity of 297.7 mAh/g at a current density of 100 mA/g after 100 charge–discharge cycles, compared to only 30.8 mAh/g for a pure TiO2@CC electrode. This study shows that by constructing PANI-based composite materials, the specific capacity of the battery can be significantly increased and better support high-current density applications.
Long-term cycling stability is an important parameter for evaluating the performance retention of electrode materials after multiple charge–discharge cycles. The cycling stability of one-dimensional PANI composite materials is significantly better than that of pure PANI materials. For example, in Anode-Free Lithium Metal Batteries (AFLMBs), a simple method of constructing an HCl-doped polyaniline modification layer on Cu foil (HPC) was developed to address the issues of dendrite growth and poor cycle stability [11]. The modified PANI layer promotes uniform lithium deposition and suppresses dendrite formation, resulting in a reduced overpotential of 20.6 mV and prolonged cycle life over 150 cycles. This approach achieved 63.81% capacity retention after 100 cycles in an anode-free full cell (N/P = 0) and 74.01% capacity retention in an anode-less cell (N/P = 1.6), demonstrating significant improvements in stability and performance for AFLMBs. This example underscores how PANI one-dimensional materials, through modification, can enhance the cycling stability and efficiency of energy storage systems, providing valuable insights for next-generation battery technology.
Rate capability reflects the ability of the electrode material to maintain reasonable capacity at high charge–discharge rates [81]. One-dimensional PANI materials, due to their efficient ion and electron transport pathways in the composite system, maintain a high specific capacity at high charge–discharge rates, giving them a significant advantage in fast-charging applications. In one study, a PANI-coated multi-walled carbon nanotube (MWCNT) core/sulfur shell composite material was prepared by rapid in situ chemical oxidation polymerization [82]. The study found that the PANI-S/MWCNT composite electrode had an initial discharge capacity of 1334.4 mAh/g, and after 80 charge–discharge cycles, the remaining capacity was 932.4 mAh/g, showing high cycling stability [68]. In contrast, the S/MWCNT composite material without a PANI coating had poorer capacity and cycling stability. The PANI coating effectively enhanced the electrode conductivity and shortened the lithium-ion diffusion path, thus improving the rate performance of the battery. Electrochemical impedance spectroscopy (EIS) analysis revealed that the addition of PANI not only reduced the interface resistance of the battery but also increased the lithium-ion diffusion rate, significantly improving the electrochemical performance of the PANI-S/MWCNT composite material at high charge–discharge rates. This result indicates that PANI not only increases the specific capacity of the electrode material but also effectively improves its capacity retention during long-term charge–discharge processes, demonstrating the great application potential of one-dimensional PANI materials in batteries.
To synthesize the above case studies and quantify trends discussed in Section 4.1 and Section 4.2, we compile a side-by-side summary of representative 1D PANI systems across battery chemistries and supercapacitors. This comparative view highlights how PANI’s roles—conductive scaffold, elastic buffer, and pseudocapacitive contributor—translate into gains in specific capacity/capacitance, rate capability, and durability when interfaced with high-capacity hosts (Si, SnO2, BP) or conductive frameworks (CNTs, MXenes). Key takeaways are the consistently improved high-rate retention and cycling stability enabled by PANI’s fast redox kinetics and mechanical compliance, with the largest absolute capacity gains observed when PANI is paired with conversion/alloying hosts (Table 2).

Table 2.
Comparison of PANI-based Materials for Energy Storage Systems.
4.3. Electrochemical Mechanism and Stability
The stability of one-dimensional PANI materials in electrochemical applications is crucial for their efficient performance as electrode materials. Their electrochemical stability is mainly influenced by factors such as redox mechanisms, structural integrity, and improved conductivity. These factors work together to determine the performance retention of PANI materials during long-term charge–discharge cycles [83].
The charge storage mechanism of PANI involves reversible redox reactions. During charge and discharge, the three main oxidation states of PANI—leucoemeraldine, emeraldine, and pernigraniline—participate in the transfer of electrons and protons, giving PANI high electrochemical activity [84]. In particular, the emeraldine oxidation state typically exhibits strong conductivity and electrochemical activity, making PANI a good candidate for batteries and supercapacitors, where the redox reactions contribute to its high capacitance and good electrochemical performance. By adjusting the oxidation state of PANI, its electrochemical performance can be further optimized, thereby enhancing its efficiency in battery applications [85].
Although PANI has good electrochemical stability, its volume expansion during high-frequency charge–discharge cycles can cause mechanical degradation of the electrode, affecting its long-term stability [86]. This issue is more pronounced in metal oxides or high-capacity materials. To improve the structural stability of PANI, researchers typically use composite strategies with materials such as carbon nanotubes (CNTs) and graphene [87]. This composite approach helps PANI maintain the integrity of its structure during charge and discharge, preventing morphological changes. Carbon nanotubes and graphene provide a good conductive network and structural support, making PANI more stable during electrochemical cycling, significantly improving its cycle life and stability. This structural optimization allows PANI to maintain good electrochemical performance over longer cycles.
In addition to structural enhancements, the main degradation pathways of one-dimensional PANI materials include hydrolytic degradation, oxidative degradation, and structural deformation. Hydrolytic degradation occurs when PANI is exposed to aqueous electrolytes for prolonged periods, leading to the formation of oligomers that reduce conductivity and capacitance retention [88]. Oxidative degradation happens during cycling, especially at higher potentials, causing oxidation and the formation of over-oxidized species and head-to-head structures, which undermine PANI’s stability [89]. Structural deformation is due to the inherent instability of PANI’s molecular structure, leading to mechanical stress and deformation [90]. To mitigate these degradation processes, strategies such as using short-chain aniline derivatives (e.g., aniline trimers) to maintain electrode microstructure, cross-linking with triphenylamine to prevent over-oxidation, and applying protective coatings to shield PANI from environmental degradation have been developed [90,91]. These approaches improve the long-term stability and performance of PANI-based electrodes in energy storage applications.
The conductivity of PANI is a key factor in its efficient performance as an electrode material. Although pure PANI has good tunable electrochemical properties, its conductivity in its basic form is low, limiting its performance in high-rate applications [92]. To enhance PANI’s conductivity, researchers have adopted doping and composite strategies. For example, by combining PANI with carbon-based materials (such as graphene, carbon nanotubes) or metal oxides (such as SnO2, MnO2), the conductivity of PANI can be significantly improved. These composite materials not only improve PANI’s conductivity but also enhance its rate performance and capacity retention at high current densities [93,94]. The composite PANI materials better facilitate electron and ion transport, improving battery performance under high charge–discharge rates, and meeting the demands of modern high-energy-density batteries for fast charging and long cycle life.
One-dimensional polyaniline (PANI) materials primarily exhibit pseudocapacitive characteristics in electrochemical energy storage, where charge storage is achieved through fast reversible Faradaic redox reactions, protonation/deprotonation, and redox transitions between the leucoemeraldine, emeraldine, and pernigraniline states, rather than through diffusion-limited intercalation processes [95,96]. This behavior is supported by various diagnostic methods commonly used for PANI and its one-dimensional structures, including power law analysis from cyclic voltammetry (b-value around 0.8–1.0) [97], Dunn’s decomposition method (which shows that most of the current contribution comes from the surface-controlled k1v component), and the small charge transfer resistance and weak peak separation in cyclic voltammetry/impedance plots, further confirming the fast redox reactions in the emeraldine state [98]. Additionally, in situ and operando spectroscopic techniques such as FTIR, XPS, UV-Vis, and Raman spectroscopy support the pseudocapacitive charge storage mechanism by monitoring real-time changes in oxidation states/protonation and correlating these with conductivity and ion transport dynamics [99]. Structurally, the one-dimensional morphology shortens the ion/electron transport path and increases the electroactive surface area, enhancing the pseudocapacitive contribution. This results in excellent high-rate performance for PANI nanowire/nanorod arrays in lithium-ion and zinc-based batteries. However, under certain conditions, such as thick films, low doping levels, slow anion/proton transport, or when PANI is combined with battery-type main materials (e.g., Si, SnO2, MnO2), diffusion-controlled components may become significant. In such composite materials, PANI provides fast pseudocapacitive charge storage, while the inorganic phase contributes diffusion-limited intercalation/conversion.
Recent advances have introduced powerful in situ and operando characterization techniques to elucidate the structural evolution and electrochemical mechanisms of PANI-based electrodes during real-time operation. Hong et al. [100] utilized in situ FTIR and XPS to monitor protonation/deprotonation and oxidation-state transitions of PANI under cycling. These techniques revealed dynamic shifts among leucoemeraldine, emeraldine, and pernigraniline states, directly correlating spectral features with conductivity changes and ion transport kinetics, thus clarifying the molecular origin of its pseudocapacitive behavior. Similarly, Goldoni et al. [101] investigated the in situ electropolymerization of PANI on pencil graphite electrodes through electrochemical measurements coupled with UV–Vis and impedance spectroscopy as well as DFT simulation, which clarified the potential-controlled nucleation and chain growth processes leading to uniform nanostructure formation and enhanced charge-transfer efficiency. Furthermore, Zoric et al. provided a comprehensive overview of in situ and operando spectroscopic approaches [99]—covering UV–Vis, infrared (IR), Raman, X-ray absorption spectroscopy (XAS), and X-ray photoelectron spectroscopy (XPS)—used to investigate redox processes in energy-storage and conversion materials. Their review emphasized that these techniques enable real-time monitoring of fundamental reaction pathways and structural transformations and highlighted emerging directions such as improving temporal/spatial resolution, integrating multiple spectroscopies into a single experiment (multispectroscopic approach), and incorporating machine-learning-assisted data interpretation.
In conclusion, the electrochemical stability of one-dimensional PANI materials is determined by the synergistic effects of redox reversibility, structural integrity, and electrical conductivity. Reversible transitions among leucoemeraldine, emeraldine, and pernigraniline states underpin their high pseudocapacitive activity, while composite strategies with conductive components such as CNTs, graphene, and metal oxides effectively mitigate structural degradation and enhance ion/electron transport. Moreover, recent in situ and operando spectroscopic studies have revealed real-time redox dynamics and structural evolution during cycling, providing molecular-level insights for performance optimization. As a result, 1D PANI and its composites exhibit excellent rate capability and cycling durability, making them promising electrode materials for high-energy, long-life rechargeable batteries and supercapacitors.
4.4. Comparison of 1D PANI with Other Conducting Polymers and Carbon-Based Materials
One-dimensional polyaniline (PANI) materials have garnered attention in energy storage applications due to their tunable electrochemical properties, high surface area, and pseudocapacitive behavior. However, when compared to other conducting polymers such as PEDOT (poly(3,4-ethylenedioxythiophene)) and PPy (polypyrrole), as well as carbon-based materials like graphene and carbon nanotubes (CNTs), PANI exhibits certain limitations in conductivity and mechanical stability. PANI’s conductivity, while good, is generally lower than that of PEDOT and PPy, which are known for their high conductivity when doped and good electrochemical stability under cycling [102,103,104]. Carbon-based materials such as graphene and CNTs, on the other hand, provide superior conductivity and mechanical strength due to their unique structures and excellent electron transport properties [105,106].
Electrochemically, PANI shows good performance due to its reversible redox transitions, especially in applications requiring pseudocapacitance [107]. However, graphene and CNTs, while exhibiting lower capacitance on their own, significantly enhance electrochemical performance when combined with PANI. The high surface area and unique structural properties of graphene and CNTs provide enhanced ion and electron transport, making them ideal for improving the rate capability and cycling stability of PANI-based electrodes. Furthermore, PANI’s susceptibility to volume expansion during cycling, which can lead to mechanical degradation, is mitigated by composite strategies using carbon-based materials, thus enhancing the overall stability and longevity of PANI-based systems.
While PANI alone shows promise, combining it with other materials results in synergistic effects. For example, combining PANI with CNTs or graphene not only improves its conductivity but also reduces mechanical degradation, offering a more stable structure during cycling. This synergy leads to enhanced electrochemical performance, making PANI-carbon composites suitable for high-performance energy storage systems, especially in supercapacitors and batteries.
Overall, 1D PANI’s main advantages lie in its pseudocapacitive behavior and flexibility in electrochemical applications, but it can benefit from the superior conductivity and mechanical properties of carbon-based materials. The combination of PANI with conducting polymers and carbon-based materials provides an excellent strategy to optimize performance, ensuring better cycling stability, high rate performance, and long-term reliability for energy storage devices.
5. Challenges and Future Perspectives
While one-dimensional polyaniline (PANI) materials show significant promise for energy storage applications, several practical challenges must be addressed to ensure their large-scale manufacturability, long-term stability, and cost-effectiveness. One of the primary concerns is scalability. The synthesis of high-quality, uniform PANI nanostructures at a large scale remains a challenge due to the complexities involved in controlling the morphology and achieving reproducibility in industrial settings. Methods such as electrochemical polymerization and template-assisted synthesis have been explored to address this issue. Additionally, the processing techniques must be refined to enable the cost-effective production of PANI-based materials without compromising their performance.
Regarding long-term stability, although PANI exhibits excellent electrochemical stability during initial cycles, its performance can degrade over time due to volume expansion, structural deformation, and chemical instability. This issue is particularly critical when scaling up for commercial applications. To address this, ongoing research focuses on improving the structural integrity of PANI through composite strategies with materials like carbon nanotubes and graphene, which have shown promise in enhancing both mechanical strength and cycling stability. Studies have shown that combining PANI with carbon-based materials improves the overall structural stability and prevents morphological changes during repeated charge/discharge cycles. Additionally, researchers are exploring hybrid materials such as PANI/MXene composites that exhibit enhanced conductivity and stability under high-rate cycling.
From an environmental and cost perspective, the synthesis of PANI involves the use of potentially harmful chemicals and solvents, which may limit its widespread adoption in commercial applications. Moreover, the cost of raw materials and production processes, including the doping and composite engineering required to enhance PANI’s performance, can be prohibitive for large-scale implementation. Therefore, cost-reduction strategies, such as using more abundant or environmentally friendly dopants and optimizing synthesis routes, will be essential for making PANI-based anodes commercially viable. Green synthesis routes for PANI, utilizing eco-friendly solvents or renewable feedstocks, have been explored to reduce environmental impact. Furthermore, incorporating low-cost dopants like bio-derived molecules or metals could further reduce the cost of large-scale production.
In summary, addressing these practical aspects will be key to realizing the full potential of PANI-based materials for large-scale energy storage applications. Future research should focus on optimizing the scalability of synthesis, improving long-term stability, and reducing the environmental and cost impacts of PANI production. With continued innovation, particularly through AI-assisted material design and advanced composite strategies, PANI-based anodes could play a significant role in the next generation of sustainable, high-performance energy storage systems.
6. Conclusions
In conclusion, one-dimensional polyaniline (PANI) materials have emerged as promising candidates for energy storage applications, particularly in rechargeable batteries such as lithium-ion, sodium-ion, and other emerging systems. The unique properties of PANI, including its high conductivity, environmental stability, and tunable electrochemical characteristics, make it an ideal material for improving the performance of energy storage devices. The development of 1D PANI nanostructures such as nanowires, nanorods, and nanofibers has been particularly advantageous, providing enhanced surface area and more efficient pathways for electron and ion transport.
The electrochemical performance of 1D PANI-based anode materials, especially when combined with high-capacity materials like silicon, metal oxides, and carbon-based materials, shows significant improvements in terms of specific capacity, cycling stability, and rate capability. The ability of PANI to accommodate volume changes during cycling and its ability to mitigate mechanical degradation are key benefits for enhancing the long-term stability of electrodes in various battery systems.
While PANI-based materials demonstrate substantial promise, challenges such as conductivity degradation, poor structural integrity under long-term cycling, and scalability of synthesis processes remain. However, strategies such as composite engineering with carbon nanotubes, graphene, metal oxides, and silicon, as well as doping and surface modifications, offer effective solutions to address these issues. These approaches improve the mechanical strength, conductivity, and electrochemical stability of PANI materials, making them more viable for practical applications in large-scale energy storage systems.
Looking forward, AI-assisted material design is expected to play a transformative role in accelerating the discovery and optimization of PANI-based composites [108,109]. By integrating machine learning and data-driven modeling, researchers can efficiently predict and optimize structure–property relationships, guide the selection of dopants, morphologies, and synthesis parameters, and ultimately achieve significant improvements in conductivity, capacity, and cycling life. The integration of AI with high-throughput experimentation and in situ characterization will further accelerate material innovation, enabling the transition from laboratory research to industrial-scale production [110].
Furthermore, ongoing advances in composite design, doping strategies, and the incorporation of emerging materials—such as MXenes and transition-metal compounds—will continue to expand the functional versatility of PANI systems. As evidenced by recent innovations in electrochromic energy storage, flexible sensors, and wearable electronics, the combination of AI-driven design principles with 1D PANI structures opens new avenues for developing high-performance, durable, and commercially viable energy storage devices. These developments offer valuable insights for enhancing the functionality and scalability of PANI-based materials [111,112,113,114]. The combination of these materials with 1D PANI structures will likely open new avenues for the development of high-performance, long-lasting, and commercially viable energy storage devices. Therefore, the continued exploration and optimization of 1D PANI-based materials hold great promise for the next generation of rechargeable batteries and energy storage systems.
Author Contributions
G.L. contributed to the conceptualization, literature review, and manuscript drafting of the review article. H.Z. was responsible for analyzing and summarizing the electrochemical performance and application sections of one-dimensional PANI materials. G.C. supervised the entire work, provided critical feedback, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the State Key Laboratory of Precision Welding & Joining of Materials and Structures (MSWJ-24M11).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.
Acknowledgments
The authors would like to acknowledge the support from the State Key Laboratory of Precision Welding & Joining of Materials and Structures (MSWJ-24M11) for funding and resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jafarizadeh, H.; Yamini, E.; Zolfaghari, S.M.; Esmaeilion, F.; Assad, M.E.H.; Soltani, M. Navigating challenges in large-scale renewable energy storage: Barriers, solutions, and innovations. Energy Rep. 2024, 12, 2179–2192. [Google Scholar] [CrossRef]
- Njema, G.G.; Ouma, R.B.O.; Kibet, J.K. A review on the recent advances in battery development and energy storage technologies. J. Renew. Energy 2024, 2024, 2329261. [Google Scholar] [CrossRef]
- Fu, H.; Tian, J.; Chin, C.-L.; Liu, H.; Yuan, J.; Tang, S.; Mai, R.; Wu, X. Axial compression behavior of GFRP-steel composite tube confined seawater sea-sand concrete intermediate long columns. Eng. Struct. 2025, 333, 120157. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Lv, L.; Yu, Z.; Yao, W.; Cheng, H.; Niu, W.; Wang, J.; Zhang, J.; Qi, H. Design and Verification of a Wearable Micro-Capacitance Test System for POC Biosensing. IEEE Trans. Instrum. Meas. 2025, 74, 2007411. [Google Scholar]
- Li, L.; Zhang, Q.; He, B.; Pan, R.; Wang, Z.; Chen, M.; Wang, Z.; Yin, K.; Yao, Y.; Wei, L. Advanced multifunctional aqueous rechargeable batteries design: From materials and devices to systems. Adv. Mater. 2022, 34, 2104327. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhou, Y.; Huang, J.; Feng, L.; Yu, P.; Zhang, Q.; Yang, W.; Wang, Y. Rethinking the electrode multiscale microstructures: A review on structuring strategies toward battery manufacturing genome. Adv. Energy Mater. 2023, 13, 2301385. [Google Scholar] [CrossRef]
- Cheng, Z.; Jiang, H.; Zhang, X.; Cheng, F.; Wu, M.; Zhang, H. Fundamental understanding and facing challenges in structural design of porous Si-based anodes for lithium-ion batteries. Adv. Funct. Mater. 2023, 33, 2301109. [Google Scholar] [CrossRef]
- Sun, F.; Jiang, H.; Wang, H.; Zhong, Y.; Xu, Y.; Xing, Y.; Yu, M.; Feng, L.-W.; Tang, Z.; Liu, J. Soft fiber electronics based on semiconducting polymer. Chem. Rev. 2023, 123, 4693–4763. [Google Scholar] [CrossRef]
- Mishra, P.K.; Sharma, H.K.; Gupta, R.; Manglik, M.; Brajpuriya, R. A critical review on recent progress on nanostructured polyaniline (PANI) based sensors for various toxic gases: Challenges, applications, and future prospects. Microchem. J. 2025, 208, 112369. [Google Scholar] [CrossRef]
- Cao, G.; Wu, A.; Wang, Z.; Zhong, S.; Cao, Z.; Zhang, H. Enhancing the conductivity and dispersion properties of polyaniline using ethyl orange. Colloids Surf. A Physicochem. Eng. Asp. 2025, 704, 135489. [Google Scholar] [CrossRef]
- Li, N.; Kang, G.; Huang, S.; Biao, J.; Liu, Y.; Kang, F.; Cao, Y. Protonated Polyaniline-Modified Copper Current Collector for Anode-Free Lithium Metal Battery. ACS Appl. Mater. Interfaces 2025, 17, 36627–36638. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, D. One-Dimensional Nanostructured Polyaniline: Syntheses, Morphology Controlling, Formation Mechanisms, New Features, and Applications. Adv. Polym. Technol. 2013, 32, E323–E368. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Q. Controlled synthesis and energy applications of one-dimensional conducting polymer nanostructures: An overview. Adv. Energy Mater. 2012, 2, 179–218. [Google Scholar] [CrossRef]
- Sun, Q.; Park, M.-C.; Deng, Y. Studies on one-dimensional polyaniline (PANI) nanostructures and the morphological evolution. Mater. Chem. Phys. 2008, 110, 276–279. [Google Scholar] [CrossRef]
- Machín, A.; Fontánez, K.; Arango, J.C.; Ortiz, D.; De León, J.; Pinilla, S.; Nicolosi, V.; Petrescu, F.I.; Morant, C.; Márquez, F. One-dimensional (1D) nanostructured materials for energy applications. Materials 2021, 14, 2609. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wang, Z.-Q.; Chen, Z.; Yang, Z.-L.; Tang, Z.-L.; Luo, H.-Y.; Huang, Y.; Li, X.; Xu, W. One dimensional and coaxial polyaniline@ tin dioxide@ multi-wall carbon nanotube as advanced conductive additive free anode for lithium ion battery. Chem. Eng. J. 2018, 334, 162–171. [Google Scholar] [CrossRef]
- Saji, V.S.; Kim, Y.-S.; Kim, T.-H.; Cho, J.; Song, H.-K. One-dimensional (1D) nanostructured and nanocomposited LiFePO 4: Its perspective advantages for cathode materials of lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 19226–19237. [Google Scholar] [CrossRef]
- Cong, Z.; Guo, W.; Zhang, P.; Sha, W.; Guo, Z.; Chang, C.; Xu, F.; Gang, X.; Hu, W.; Pu, X. Wearable antifreezing fiber-shaped Zn/PANI batteries with suppressed Zn dendrites and operation in sweat electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 17608–17617. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, C.; Gao, L.; Lin, M.-F.; Eh, A.L.-S.; Chen, J.; Li, S. Recent advances in electrospun nanostructured electrodes in zinc-ion batteries. Batteries 2024, 10, 22. [Google Scholar] [CrossRef]
- Liu, H.; Chen, N.; Umar, A.; Li, H.; Li, S.; Bai, P.; Frans de Rooij, N.; Wang, Y.; Zhou, G. In situ construction of the coral-like polyaniline on the aligned silicon nanowire arrays for silicon substrate on-chip supercapacitors. ACS Appl. Energy Mater. 2020, 3, 11792–11802. [Google Scholar] [CrossRef]
- Tahir, M.S.; Kainat, I.; Ghazanfar, H.; Seo, Y.S. Flexible electrodes for high-performance energy storage: Materials, conductivity optimization, and scalable fabrication. Nanoscale 2025, 17, 18016–18048. [Google Scholar] [CrossRef]
- Sabet, M. Advanced functionalization strategies for carbon nanotube polymer composites: Achieving superior dispersion and compatibility. Polym. Plast. Technol. Mater. 2025, 64, 465–494. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, H.; Pan, Z.; Wu, Z.; Chin, C.-L.; Ma, C.-K. Behaviour of Corroded Circular Steel Tube Strengthened with External FRP Tube Grouting Under Eccentric Loading: Numerical Study; Elsevier: Amsterdam, The Netherlands, 2023; p. 104810. [Google Scholar]
- Rahman, S.U.; Dan, X.; Farooq, S.; Sajid, M.; Tao, F.-Y.; Kitchamsetti, N.; Liu, C.; Xu, W.-J.; Zhang, J.-P. Tailoring polyaniline with dual dopant engineering as a high efficiency cathode material for aqueous zinc ion batteries. J. Colloid Interface Sci. 2025, 700, 138600. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, L.; Chen, L.; Liu, X. Recent progress and challenges of flexible supercapacitor-sensor integrated systems based on conductive polymers. J. Mater. Chem. C 2025, 13, 16320–16349. [Google Scholar] [CrossRef]
- Barta, P.; Kugler, T.; Salaneck, W.R.; Monkman, A.P.; Libert, J.; Lazzaroni, R.; Brédas, J.L. Electornic structure of emeraldine and pernigraniline base: A joint theoretical and experimental study. Synth. Met. 1998, 93, 83–87. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Manohar, S.K.; Masters, J.G.; Sun, Y.; Weiss, H.; Epstein, A.J. Polyaniline: Synthesis and properties of pernigraniline base. Synth. Met. 1991, 41, 621–626. [Google Scholar] [CrossRef]
- Zujovic, Z.; Kilmartin, P.A.; Travas-Sejdic, J. The applications of solid-state NMR to conducting polymers. The special case on polyaniline. Molecules 2020, 25, 444. [Google Scholar] [CrossRef]
- Toroń, B.; Das, T.K.; Kozioł, M.; Szperlich, P.; Kępińska, M. Impact of hydrochloric acid doping on polyaniline conductivity and piezoelectric performance in polyaniline/bismuth oxyiodide nanocomposites. Compos. Part B Eng. 2025, 289, 111960. [Google Scholar] [CrossRef]
- Shawky, N.A.; Abdallah, S.M.A.; Sorour, M.H.; Abouelata, A.M.A.; Abdel-Fatah, M.A. Enhancement of Conductivity and Productivity of Polyaniline Emeraldine Salt Using Diverse Treatment Solutions. Egypt. J. Chem. 2025, 68, 1–13. [Google Scholar] [CrossRef]
- Roslan, N.C.; Aizamddin, M.F.; Omar, S.N.I.; Jani, N.A.; Halim, M.I.A.; Ariffin, Z.Z.; Mahat, M.M. Morphological and conductivity studies of polyaniline fabric doped phosphoric acid. Malays. J. Anal. Sci 2020, 24, 698–706. [Google Scholar]
- Liu, R.; Yao, Q.; Liu, L.; Meng, F.; Wang, F. Studies of different acid doped polyaniline incorporated into epoxy organic coatings on the Mg alloy. Prog. Org. Coat. 2022, 166, 106774. [Google Scholar] [CrossRef]
- Mažeikien, R.; Malinauskas, A. Electrochemical stability of polyaniline. Eur. Polym. J. 2002, 38, 1947–1952. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Synergetic Effect of Polyaniline and Graphene in Their Composite Supercapacitor Electrodes: Impact of Components and Parameters of Chemical Oxidative Polymerization. Nanomaterials 2022, 12, 2531. [Google Scholar] [CrossRef]
- Rauhala, T.; Davodi, F.; Sainio, J.; Sorsa, O.; Kallio, T. On the stability of polyaniline/carbon nanotube composites as binder-free positive electrodes for electrochemical energy storage. Electrochim. Acta 2020, 336, 135735. [Google Scholar] [CrossRef]
- Acharya, A.D.; Thakur, Y.S. Tuning electrochemical performance of polyaniline-based supercapacitors by inclusion of protonic acid and electrolyte concentration. J. Indian Chem. Soc. 2025, 102, 101836. [Google Scholar] [CrossRef]
- Manuel, J.; Kim, J.-K.; Matic, A.; Jacobsson, P.; Chauhan, G.S.; Ha, J.K.; Cho, K.-K.; Ahn, J.-H. Electrochemical properties of lithium polymer batteries with doped polyaniline as cathode material. Mater. Res. Bull. 2012, 47, 2815–2818. [Google Scholar] [CrossRef]
- Dominic, J.; David, T.; Vanaja, A.; Muralidharan, G.; Maheswari, N.; Kumar, K.K.S. Supercapacitor performance study of lithium chloride doped polyaniline. Appl. Surf. Sci. 2018, 460, 40–47. [Google Scholar] [CrossRef]
- Güngör, A.; Bakan-Misirlioglu, F.; Genç Alturk, R.; Erdem, E. Elevating supercapacitor performance: Enhancing electrochemical efficiency with transition metal-doped polyaniline electrode. J. Energy Storage 2024, 76, 110143. [Google Scholar] [CrossRef]
- Wang, X.; Deng, J.; Duan, X.; Liu, D.; Guo, J.; Liu, P. Crosslinked polyaniline nanorods with improved electrochemical performance as electrode material for supercapacitors. J. Mater. Chem. A 2014, 2, 12323–12329. [Google Scholar] [CrossRef]
- Du, X.; Liu, S.; Xu, Q.; Shi, X. Synthesis and Electrochemical Performance of MnO2 Nanowires/Polyaniline Composite as Supercapacitor Electrode Material. J. Electron. Mater. 2023, 52. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, L.; Wang, X.; Cui, J.; Lin, Z.; Ngai, S.; Vogel, F.; Wang, H.; Li, W.; Li, S.; et al. Improvement of electrochemical performance of titania nanowires for supercapacitor electrodes by in-situ growth of polyaniline nanoparticles. Ceram. Int. 2022, 48, 1731–1739. [Google Scholar] [CrossRef]
- Chu, J.; Li, X.; Li, Q.; Ma, J.; Wu, B.; Wang, X.; Zhang, R.; Gong, M.; Xiong, S. Hydrothermal synthesis of PANI nanowires for high-performance supercapacitor. High Perform. Polym. 2020, 32, 258–267. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, K.; Ma, J.; Liu, Y.; Liang, X.; Xuan, H.; Han, P. Preparation and Electrochemical Properties of Polyaniline Nanostructures Using Vertically Aligned Mesochannels as Confinement. ChemElectroChem 2022, 9, e202200110. [Google Scholar] [CrossRef]
- Yin, H.; Yang, J. Synthesis of high-performance one-dimensional polyaniline nanostructures using dodecylbenzene sulfonic acid as soft template. Mater. Lett. 2011, 65, 850–853. [Google Scholar] [CrossRef]
- Li, G.; Jiang, L.; Peng, H. One-Dimensional Polyaniline Nanostructures with Controllable Surfaces and Diameters Using Vanadic Acid as the Oxidant. Macromolecules 2007, 40, 7890–7894. [Google Scholar] [CrossRef]
- He, Y. One-dimensional polyaniline nanostructures synthesized by interfacial polymerization in a solids-stabilized emulsion. Appl. Surf. Sci. 2006, 252, 2115–2118. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, M.; Yin, X.; Shao, Q.; Lu, N.; Feng, Y.; Lu, Y.; Wujcik, E.K.; Mai, X.; Wang, C.; et al. Tuning polyaniline nanostructures via end group substitutions and their morphology dependent electrochemical performances. Polymer 2018, 156, 128–135. [Google Scholar] [CrossRef]
- Xia, H.; Narayanan, J.; Cheng, D.; Xiao, C.; Liu, X.; Chan, H.S.O. Formation of Ordered Arrays of Oriented Polyaniline Nanoparticle Nanorods. J. Phys. Chem. B 2005, 109, 12677–12684. [Google Scholar] [CrossRef] [PubMed]
- Simotwo, S.K.; DelRe, C.; Kalra, V. Supercapacitor Electrodes Based on High-Purity Electrospun Polyaniline and Polyaniline–Carbon Nanotube Nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 21261–21269. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Moharram, Y.I.; Abouharga, F.S.; Elfiky, M. Electrospun polyacrylonitrile-polyphenyl/magnetite nanofiber electrode for enhanced capacitance of supercapacitor. Sci. Rep. 2025, 15, 14885. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Fan, W.; Chen, D.; Liu, T. High-Performance Supercapacitors Based on Hollow Polyaniline Nanofibers by Electrospinning. ACS Appl. Mater. Interfaces 2013, 5, 4423–4428. [Google Scholar] [CrossRef]
- Pahovnik, D.; Žagar, E.; Kogej, K.; Vohlídal, J.; Žigon, M. Polyaniline nanostructures prepared in acidic aqueous solutions of ionic liquids acting as soft templates. Eur. Polym. J. 2013, 49, 1381–1390. [Google Scholar] [CrossRef]
- Qiu, H.; Qi, S.; Wang, D.; Wang, J.; Wu, X. Synthesis of polyaniline nanostructures via soft template of sucrose octaacetate. Synth. Met. 2010, 160, 1179–1183. [Google Scholar] [CrossRef]
- Gamero-Quijano, A.; Karman, C.; Vilà, N.; Herzog, G.; Walcarius, A. Vertically Aligned and Ordered One-Dimensional Mesoscale Polyaniline. Langmuir 2017, 33, 4224–4234. [Google Scholar] [CrossRef]
- Cao, G.; Xu, J.; Cai, S.; Chen, Y.; Zhou, D.; Zhang, H.; Jiang, C.; Zhang, G.; Tian, Y. Highly conductive and dispersible polyaniline microtubes controlled by methyl orange. ACS Appl. Polym. Mater. 2022, 5, 593–601. [Google Scholar] [CrossRef]
- Li, M.; Chen, H.; Guo, C.; Qian, S.; Li, H.; Wu, Z.; Xing, C.; Xue, P.; Zhang, S. Interfacial Engineering on Cathode and Anode with Iminated Polyaniline@ rGO-CNTs for Robust and High-Rate Full Lithium–Sulfur Batteries. Adv. Energy Mater. 2023, 13, 2300646. [Google Scholar]
- Yuan, S.; Sun, G.; Hong, J.; Jin, H. Black Phosphorus/PANI/Carbon Nanotube Composite as a Lithium-Ion Battery Anode. ACS Appl. Nano Mater. 2024, 7, 7766–7772. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Xie, S.; Zhou, Y.; Rong, J.; Dong, L. Zinc-based energy storage with functionalized carbon nanotube/polyaniline nanocomposite cathodes. Chem. Eng. J. 2022, 427, 131799. [Google Scholar] [CrossRef]
- Xu, C.; Yang, X.; Hu, C.; Zhang, J.; Yang, L.; Yin, S. One pot synthesis of polyoxometalate@polyaniline/MXene/CNTs quaternary composites with a 3D structure as efficient electrode materials for Li-ion batteries applications. Compos. Commun. 2024, 45, 101814. [Google Scholar] [CrossRef]
- Shahid, A.; Kayani, Z.N.; Sahar, M.; Shahid, E.; Riaz, S.; Naseem, S. Pr2CuO4/MWCNT/MXene/PANI: A novel quaternary composite with tunable properties for potential applications in optoelectronics and energy storage devices. J. Alloys Compd. 2025, 1010, 177264. [Google Scholar] [CrossRef]
- Tang, J.; Zheng, X.; Ding, B.; Zou, L.; Wang, P.; Li, C.; Hong, X.; Wang, Z. MXene/PANI composite fiber-based asymmetric supercapacitors for self-powered energy storage system. Mater. Lett. 2024, 355, 135494. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Fang, Y.; Wu, Z.; Dong, J.; Zhao, X.; Teng, C. Flexible and efficient MXene/PANI/aramid fabrics with high interface durability for wearable electromagnetic wave shielding. Compos. Commun. 2024, 45, 101776. [Google Scholar] [CrossRef]
- Jin, L.; Jiang, Y.; Zhang, M.; Li, H.; Xiao, L.; Li, M.; Ao, Y. Oriented polyaniline nanowire arrays grown on dendrimer (PAMAM) functionalized multiwalled carbon nanotubes as supercapacitor electrode materials. Sci. Rep. 2018, 8, 6268. [Google Scholar] [CrossRef]
- Eldona, C.; Hanif Hawari, N.; Haidar Hamid, F.; Dempwolf, W.; Iskandar, F.; Peiner, E.; Suryo Wasisto, H.; Sumboja, A. A free-standing polyaniline/silicon nanowire forest as the anode for lithium-ion batteries. Chem. Asian J. 2022, 17, e202200946. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, K.; Niu, C.; Zhang, L.; Cai, Z.; Han, C.; He, L.; Shen, T.; Yan, M.; Qu, L. Heterogeneous branched core–shell SnO2–PANI nanorod arrays with mechanical integrity and three dimentional electron transport for lithium batteries. Nano Energy 2014, 8, 196–204. [Google Scholar]
- Baboukani, A.R.; Khakpour, I.; Adelowo, E.; Drozd, V.; Shang, W.; Wang, C. High-performance red phosphorus-sulfurized polyacrylonitrile composite by electrostatic spray deposition for lithium-ion batteries. Electrochim. Acta 2020, 345, 136227. [Google Scholar]
- Krithika, S.; Balavijayalakshmi, J. Investigation of structural, morphological and electrochemical properties of MoS2/PANI/SnO2 nanocomposites for energy storage applications. Inorg. Chem. Commun. 2023, 148, 110324. [Google Scholar]
- Wang, Q.; Hui, J.; Li, J.; Cai, Y.; Yin, S.; Wang, F.; Su, B. Photodegradation of methyl orange with PANI-modified BiOCl photocatalyst under visible light irradiation. Appl. Surf. Sci. 2013, 283, 577–583. [Google Scholar] [CrossRef]
- Nie, G.; Lu, X.; Wang, W.; Chi, M.; Jiang, Y.; Wang, C. One-dimensional polyaniline thorn/BiOCl chip heterostructures: Self-sacrificial template-induced synthesis and electrochemical performance. Mater. Chem. Front. 2017, 1, 859–866. [Google Scholar] [CrossRef]
- Ye, W.; Zhu, J.; Liao, X.; Jiang, S.; Li, Y.; Fang, H.; Hou, H. Hierarchical three-dimensional micro/nano-architecture of polyaniline nanowires wrapped-on polyimide nanofibers for high performance lithium-ion battery separators. J. Power Sources 2015, 299, 417–424. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Hua, K.; Li, S.; Fang, D.; Luo, Z.; Bao, R.; Fan, X.; Yi, J. Vertically aligned polyaniline nanowire arrays for lithium-ion battery. Colloid Polym. Sci. 2018, 296, 1395–1400. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, S.; Liu, S.; Ren, L.; Wang, S.; Fu, J. Preparation of polyaniline-coated β-AgVO3 nanowires and their application in lithium-ion battery. Mater. Lett. 2013, 110, 168–171. [Google Scholar] [CrossRef]
- Ma, D.; Yin, X.; Li, X.; Qin, X.; Qi, M. Study on the Performance of Aniline Electrodeposited on MnO2 Nanowire as an Anode for Sodium-Ion Batteries. Polymers 2024, 16, 1856. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, G.; Xiao, L.; Li, P.; Li, W.; Xu, Q.; Xu, J. Polyaniline Encapsulated Amorphous V2O5 Nanowire-Modified Multi-Functional Separators for Lithium—Sulfur Batteries. Small Methods 2021, 5, 2001056. [Google Scholar] [CrossRef]
- Lei, H.; Tu, J.; Li, S.; Huang, Z.; Luo, Y.; Yu, Z.; Jiao, S. Graphene-encapsulated selenium@ polyaniline nanowires with three-dimensional hierarchical architecture for high-capacity aluminum–selenium batteries. J. Mater. Chem. A 2022, 10, 15146–15154. [Google Scholar]
- Fu, X.; Zhang, W.; Lan, B.; Wen, J.; Zhang, S.; Luo, P.; Zhang, R.; Hu, S.; Liu, Q. Polyaniline Nanorod Arrays as a Cathode Material for High-Rate Zinc-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 12360–12367. [Google Scholar] [CrossRef]
- Wen, J.; Hu, Z.; Song, R.; Huang, R.; Liu, Q.; Zhang, R.; Hu, S.; Fu, X. MnO2@ PANI nanorod arrays for high-performance aqueous zinc-ion batteries. J. Alloys Compd. 2024, 976, 173209. [Google Scholar] [CrossRef]
- Mansour, F.R.; Bedair, A.; de Souza, F.M.; Gupta, R.K. Battery Efficiency and Performance Scoring Index (BEPSI) as an innovative evaluation and comparison tool. Chem. Eng. J. 2025, 519, 164937. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Liu, Z.; Li, H.; Qi, M.; Mu, X.; Cui, J. Self-standing TiO2@ CC@ PANI core–shell nanowires as flexibles lithium-ion battery anodes. J. Mater. Sci. 2024, 59, 20657–20670. [Google Scholar]
- Abavi-Torghabeh, N.; Kalantarian, M.M.; Dehkhoda, S.; Sadeghian, Z. A systematic evaluation of charge-discharge behaviors, performance, and rate-capability of Al-ion batteries. Electrochim. Acta 2023, 437, 141508. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.; Li, L.; Zhao, T.; Chen, R. Improvement of Rate and Cycle Performence by Rapid Polyaniline Coating of a MWCNT/Sulfur Cathode. J. Phys. Chem. C 2011, 115, 24411–24417. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L. Research progress on applications of polyaniline (PANI) for electrochemical energy storage and conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef]
- Majeed, A.H.; Mohammed, L.A.; Hammoodi, O.G.; Sehgal, S.; Alheety, M.A.; Saxena, K.K.; Dadoosh, S.A.; Mohammed, I.K.; Jasim, M.M.; Salmaan, N.U. A review on polyaniline: Synthesis, properties, nanocomposites, and electrochemical applications. Int. J. Polym. Sci. 2022, 2022, 9047554. [Google Scholar] [CrossRef]
- Yoon, S.-B.; Yoon, E.-H.; Kim, K.-B. Electrochemical properties of leucoemeraldine, emeraldine, and pernigraniline forms of polyaniline/multi-wall carbon nanotube nanocomposites for supercapacitor applications. J. Power Sources 2011, 196, 10791–10797. [Google Scholar]
- Sekar, P.; Anothumakkool, B.; Vijayakumar, V.; Lohgaonkar, A.; Kurungot, S. Unravelling the mechanism of electrochemical degradation of PANI in supercapacitors: Achieving a feasible solution. ChemElectroChem 2016, 3, 933–942. [Google Scholar] [CrossRef]
- Tundwal, A.; Kumar, H.; Binoj, B.J.; Sharma, R.; Kumar, G.; Kumari, R.; Dhayal, A.; Yadav, A.; Singh, D.; Kumar, P. Developments in conducting polymer-, metal oxide-, and carbon nanotube-based composite electrode materials for supercapacitors: A review. RSC Adv. 2024, 14, 9406–9439. [Google Scholar] [CrossRef]
- Chang, X.; Yang, Z.; Huang, A.; Katsuyama, Y.; Lin, C.-W.; El-Kady, M.F.; Wang, C.; Kaner, R.B. Understanding the Degradation Mechanisms of Conducting Polymer Supercapacitors. Macromol. Rapid Commun. 2024, 45, 2300237. [Google Scholar] [CrossRef]
- Nunes, W.G.; Pires, B.M.; Thaines, E.H.N.S.; Pereira, G.M.A.; da Silva, L.M.; Freitas, R.G.; Zanin, H. Operando Raman spectroelectrochemical study of polyaniline degradation: A joint experimental and theoretical analysis. J. Energy Storage 2022, 55, 105770. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Tang, S.; Zheng, R.; Jia, C. Ultrahigh cycling stability and wide infrared modulation of electrochromic devices based on electrodeposited triphenylamine cross-linked polyaniline derivatives. J. Mater. Chem. A 2024, 12, 32104–32116. [Google Scholar] [CrossRef]
- Yoon, Y.; Shin, S.; Shin, M.W. Study on the Structural Degradation of the Polyaniline(PANi) Coated LiNi0.90Co0.85Mn0.15o Particles. ECS Meet. Abstr. 2022, MA2022-02, 303. [Google Scholar] [CrossRef]
- Sumdani, M.G.; Islam, M.R.; Yahaya, A.N.A.; Safie, S.I. Recent advancements in synthesis, properties, and applications of conductive polymers for electrochemical energy storage devices: A review. Polym. Eng. Sci. 2022, 62, 269–303. [Google Scholar] [CrossRef]
- Shawky, N.A.; Abdallah, S.M.; Sorour, M.H.; Abouelata, A.M.A.; Abdel-Fatah, M.A. Electrochemical Polymerization of Polyaniline: A Comprehensive Review of Synthesis Conditions, Nanocomposites, and Industrial Applications. Cureus J. 2025, 2, 1–18. [Google Scholar] [CrossRef]
- Gupta, R. A review of functionalized nanomaterials for supercapacitor and hybrid capacitor technologies. Discov. Electron. 2024, 1, 24. [Google Scholar] [CrossRef]
- Mahdavi, H.; Shahalizade, T. Investigation of the pseudocapacitive properties of polyaniline nanostructures obtained from scalable chemical oxidative synthesis routes. Ionics 2019, 25, 1331–1340. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, E.; Wang, Z.; Feng, T.; Xie, A. Redox-Active Anthraquinone-1-Sulfonic Acid Sodium Salt-Loaded Polyaniline for Dual-Functional Electrochromic Supercapacitors. Gels 2025, 11, 568. [Google Scholar] [CrossRef]
- Cevher, D. Electroactive Polymers for Next-Generation Energy Storage and Conductive Composite Materials: A Focus on Pani and Pedot-Based Conjugated Polymers. Ph.D. Thesis, Middle East Technical University, Ankara, Türkiye, 2024. [Google Scholar]
- Patil, A.; Kamble, G.; Tawade, A.; Sharma, K.; Kim, J.; Dalavi, D. One-dimensional polyaniline nanoworms: Binder-free electrodes for next-generation supercapacitors. Chem. Pap. 2025, 1–10. [Google Scholar] [CrossRef]
- Zoric, M.R.; Fabbri, E.; Herranz, J.; Schmidt, T.J. In Situ and Operando Spectroscopic Techniques for Electrochemical Energy Storage and Conversion Applications. J. Phys. Chem. C 2024, 128, 19055–19070. [Google Scholar] [CrossRef]
- Hong, Y.; Jia, K.; Zhang, Y.; Li, Z.; Jia, J.; Chen, J.; Liang, Q.; Sun, H.; Gao, Q.; Zhou, D.; et al. Energetic and durable all-polymer aqueous battery for sustainable, flexible power. Nat. Commun. 2024, 15, 9539. [Google Scholar] [CrossRef] [PubMed]
- Goldoni, R.; Thomaz, D.V.; Ottolini, M.; Di Giulio, S.; Di Giulio, T. Characterization of In situ electrosynthesis of polyaniline on pencil graphite electrodes through electrochemical, spectroscopical and computational methods. J. Mater. Sci. 2024, 59, 10287–10308. [Google Scholar] [CrossRef]
- Liu, E.; Liu, C.; Zhu, Z.; Shi, H.; Jiang, Q.; Jiang, F.; Xu, J.; Xiong, J.; Hu, Y. Enhanced thermoelectric performance of PEDOT:PSS films by solvent thermal treatment. J. Polym. Res. 2015, 22, 240. [Google Scholar] [CrossRef]
- Cao, G.; Cai, S.; Chen, Y.; Zhou, D.; Zhang, H.; Tian, Y. Facile synthesis of highly conductive and dispersible PEDOT particles. Polymer 2022, 252, 124952. [Google Scholar] [CrossRef]
- Cao, G.; Cui, H.; Wang, L.; Wang, T.; Tian, Y. Highly conductive and highly dispersed polythiophene nanoparticles for fabricating high-performance conductive adhesives. ACS Appl. Electron. Mater. 2020, 2, 2750–2759. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Mmuoegbulam, A.O.; Okezie, O.; Durumin Iya, N.I.; Mohammed, S.A.E.; James, P.H.; Muhammad, A.B.; Unimke, A.A.; Alim, S.A.; Yahaya, S.M. Recent progress in carbon-based nanomaterials: Critical review. J. Nanoparticle Res. 2024, 26, 106. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, F.; Liu, X.; Lu, D.; Liu, T.; Li, Y.; Wu, Z. Sandwich-structure CNT-graphene film with covalent bond for high-performance electromagnetic shielding and thermal management. Carbon 2024, 228, 119420. [Google Scholar]
- Gharahcheshmeh, M.H.; Chowdhury, K. Fabrication methods, pseudocapacitance characteristics, and integration of conjugated conducting polymers in electrochemical energy storage devices. Energy Adv. 2024, 3, 2668–2703. [Google Scholar] [CrossRef]
- Hussain, T.; Chandio, I.; Ali, A.; Hyder, A.; Memon, A.A.; Yang, J.; Thebo, K.H. Recent developments of artificial intelligence in MXene-based devices: From synthesis to applications. Nanoscale 2024, 16, 17723–17760. [Google Scholar] [CrossRef]
- Boublia, A.; Guezzout, Z.; Haddaoui, N.; Badawi, M.; Darwish, A.S.; Lemaoui, T.; Banat, F.; Yadav, K.K.; Jeon, B.-H.; Elboughdiri, N. Enhancing precision in PANI/Gr nanocomposite design: Robust machine learning models, outlier resilience, and molecular input insights for superior electrical conductivity and gas sensing performance. J. Mater. Chem. A 2024, 12, 2209–2236. [Google Scholar]
- Cheetham, A.K.; Seshadri, R. Artificial Intelligence Driving Materials Discovery? Perspective on the Article: Scaling Deep Learning for Materials Discovery. Chem. Mater. 2024, 36, 3490–3495. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, M.; Sun, F.; Sun, Q.; Cao, G.; Wu, X.; Ling, H.; Su, F.; Tian, Y.; Liu, Y.J. High-Efficiency and High-Capacity Aqueous Electrochromic Energy Storage Devices Enabled by Decoupled Titanium Oxide/Viologen Derivative Hybrid Materials. Research 2025, 8, 909. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Hu, Z.; Yang, Z.; Zhang, J.; Wu, J.J.; Cheng, C.; Wang, C.; Zheng, L. Capacitive aptasensor coupled with microfluidic enrichment for real-time detection of trace SARS-CoV-2 nucleocapsid protein. Anal. Chem. 2022, 94, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yan, L.; Hou, Z.; Lin, J.; Zhao, Y.; Ji, Z.; Wang, Y. Error Analysis Strategy for Long-term Correlated Network Systems: Generalized Nonlinear Stochastic Processes and Dual-Layer Filtering Architecture. IEEE Internet Things J. 2025, 12, 33731–33745. [Google Scholar] [CrossRef]
- Fu, H.; Tian, J.; Chen, S.; Chin, C.-L.; Ma, C.-K. Axial compressive performance of CFRP-steel composite tube confined seawater sea-sand concrete intermediate slender columns. Constr. Build. Mater. 2024, 441, 137399. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).