Discovery and Quantification of Microplastic Generation in the Recycling of Coated Paper-Based Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Rhodamine-B-Tagged Latex Preparation and Application
2.2. Physical and Mechanical Testing
2.3. Recycling
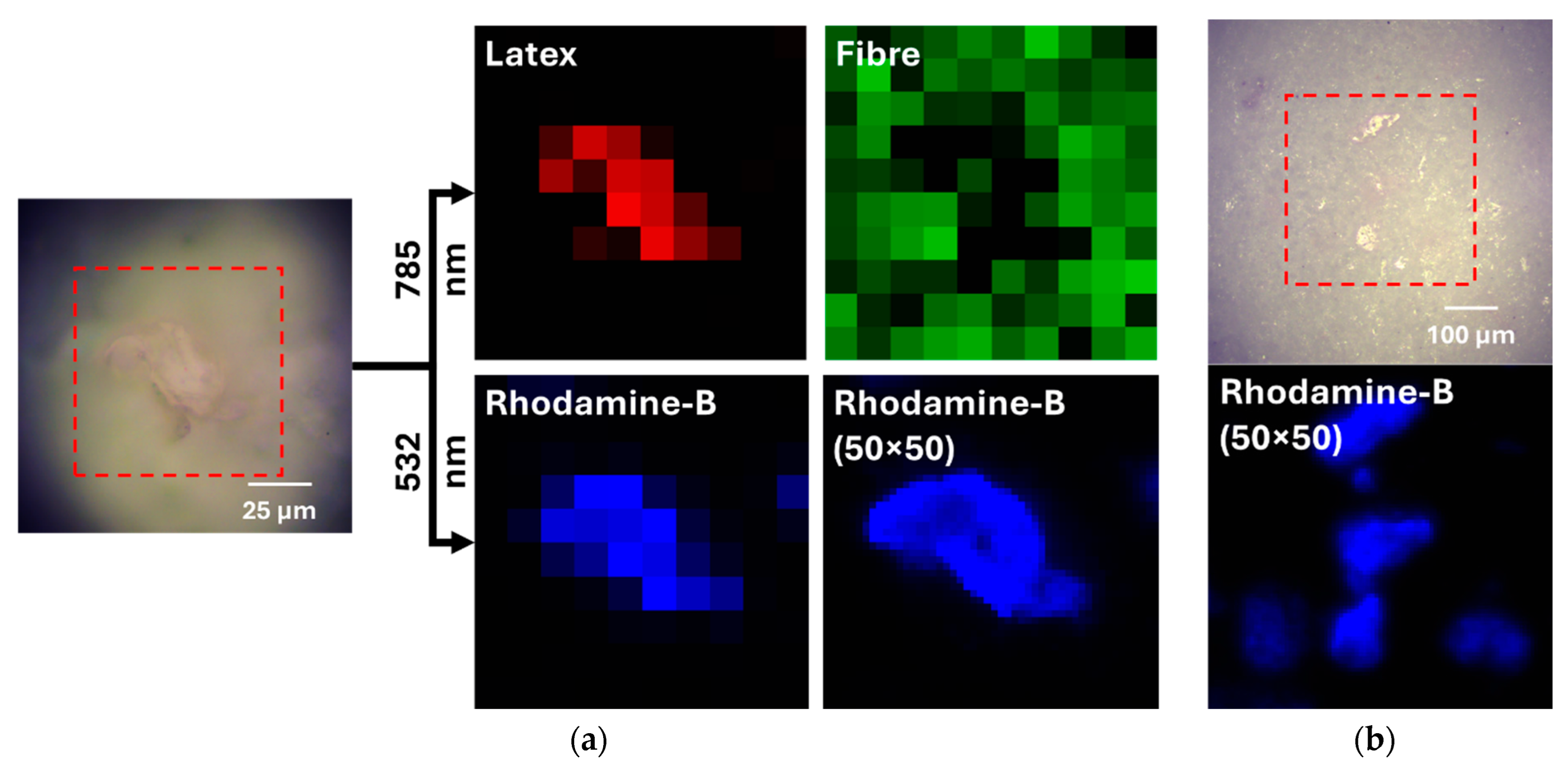
2.4. Raman Spectroscopy and Fluorescence Mapping
2.5. Microscopy
3. Results
3.1. Material Preparation
3.2. Coated and Uncoated Substrate Characterisation
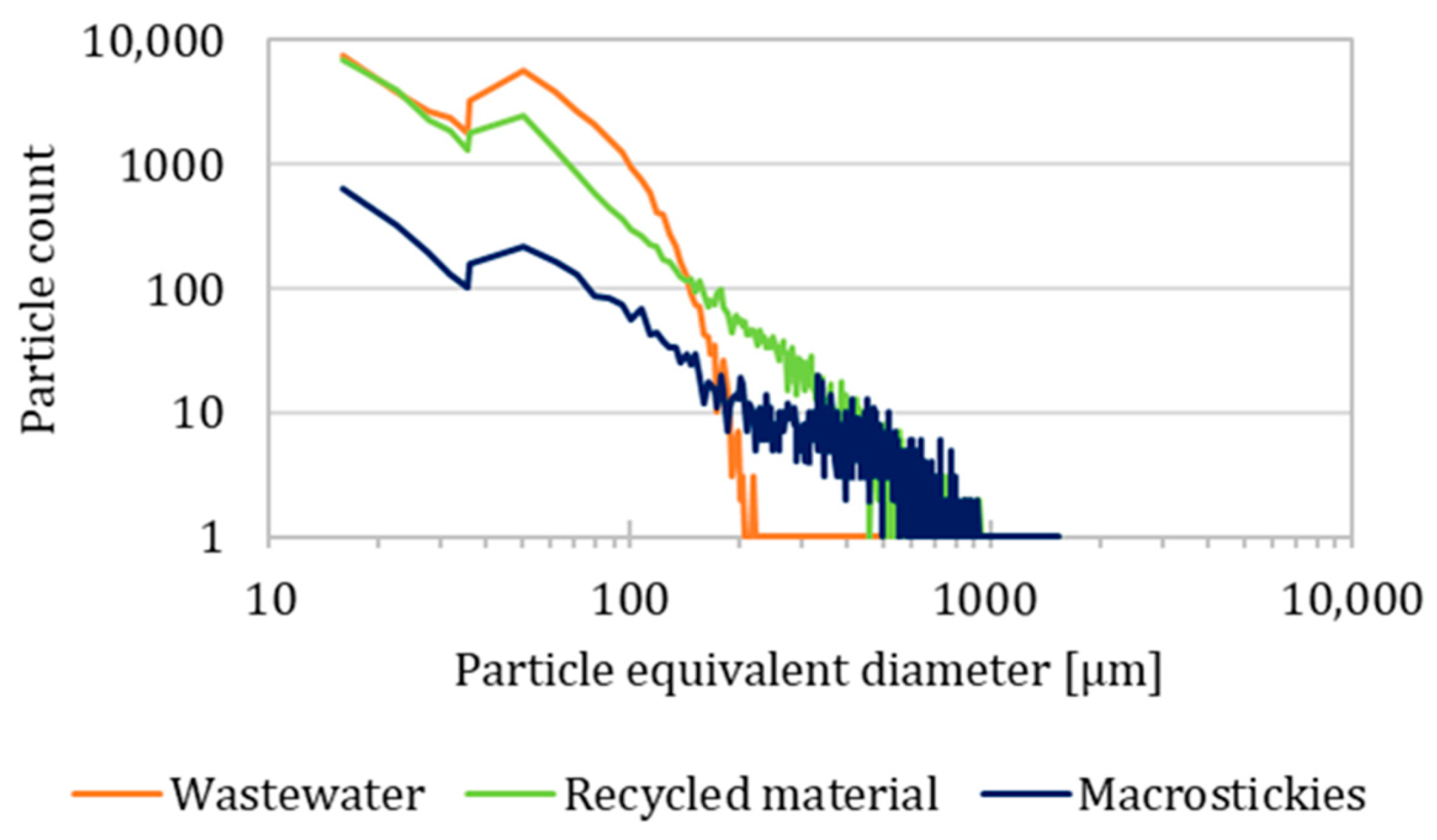
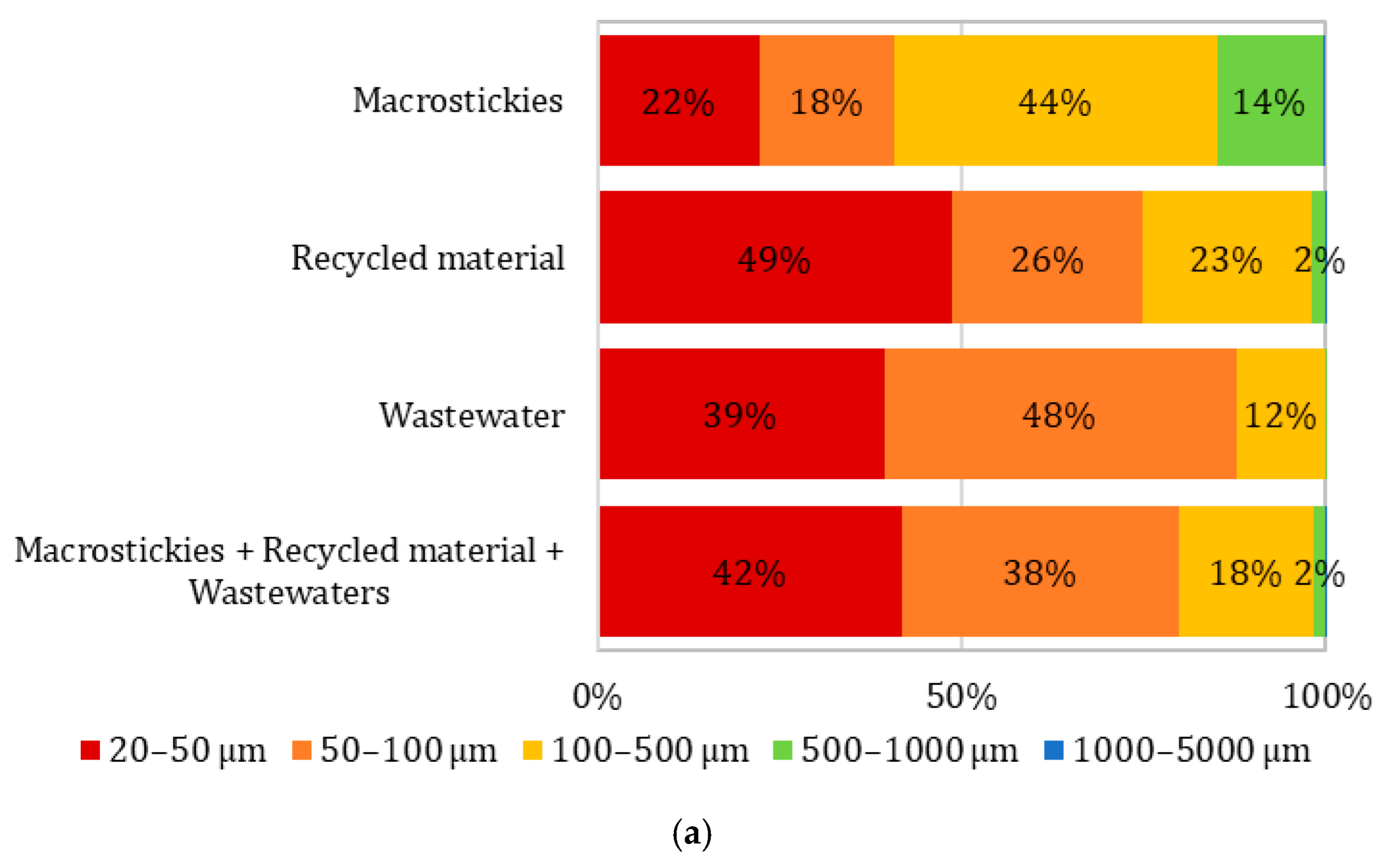
3.3. Recycling
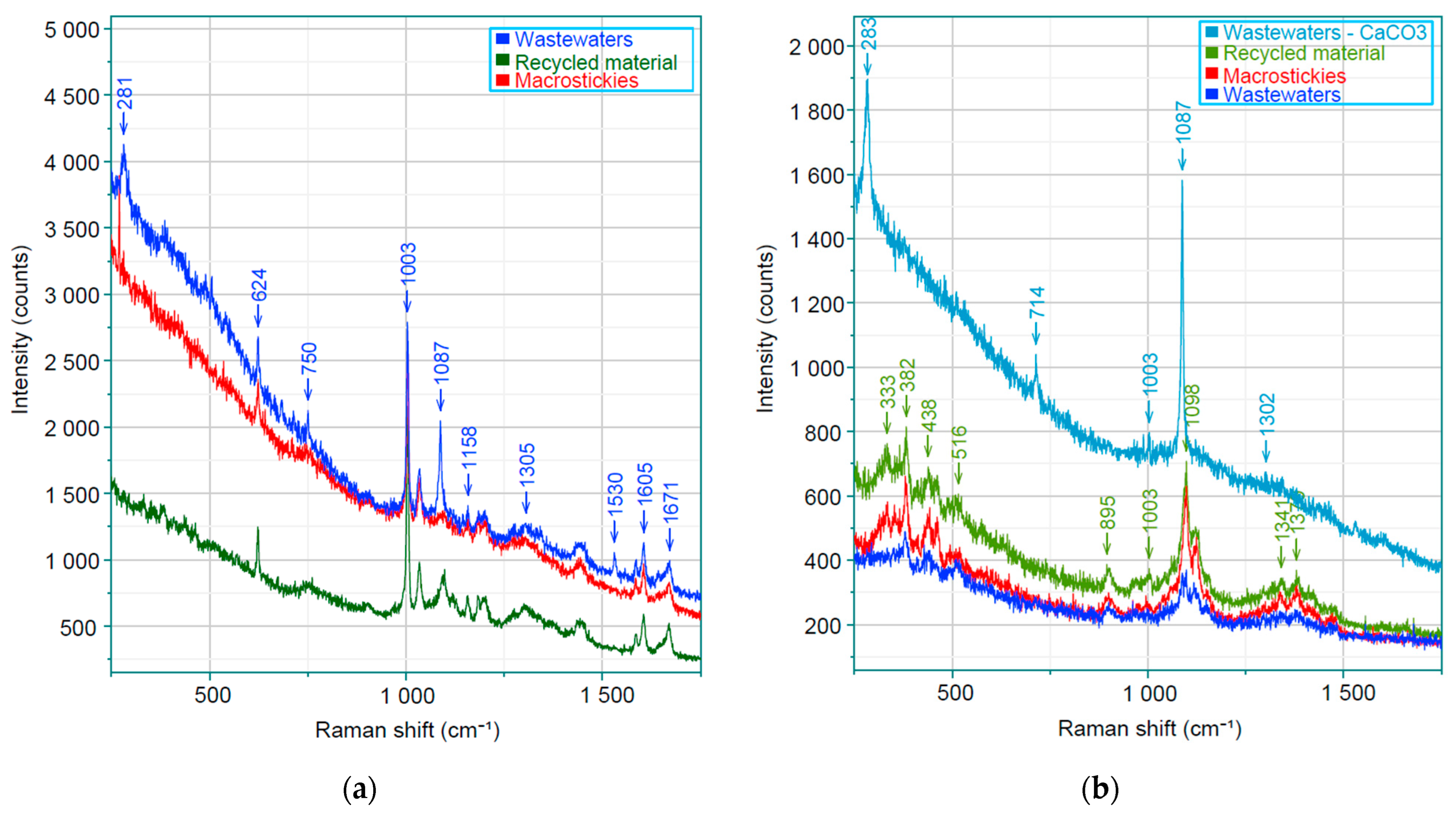
3.4. Raman Spectroscopy
3.5. Visible Light Imaging
3.6. Fluorescence Microscopy
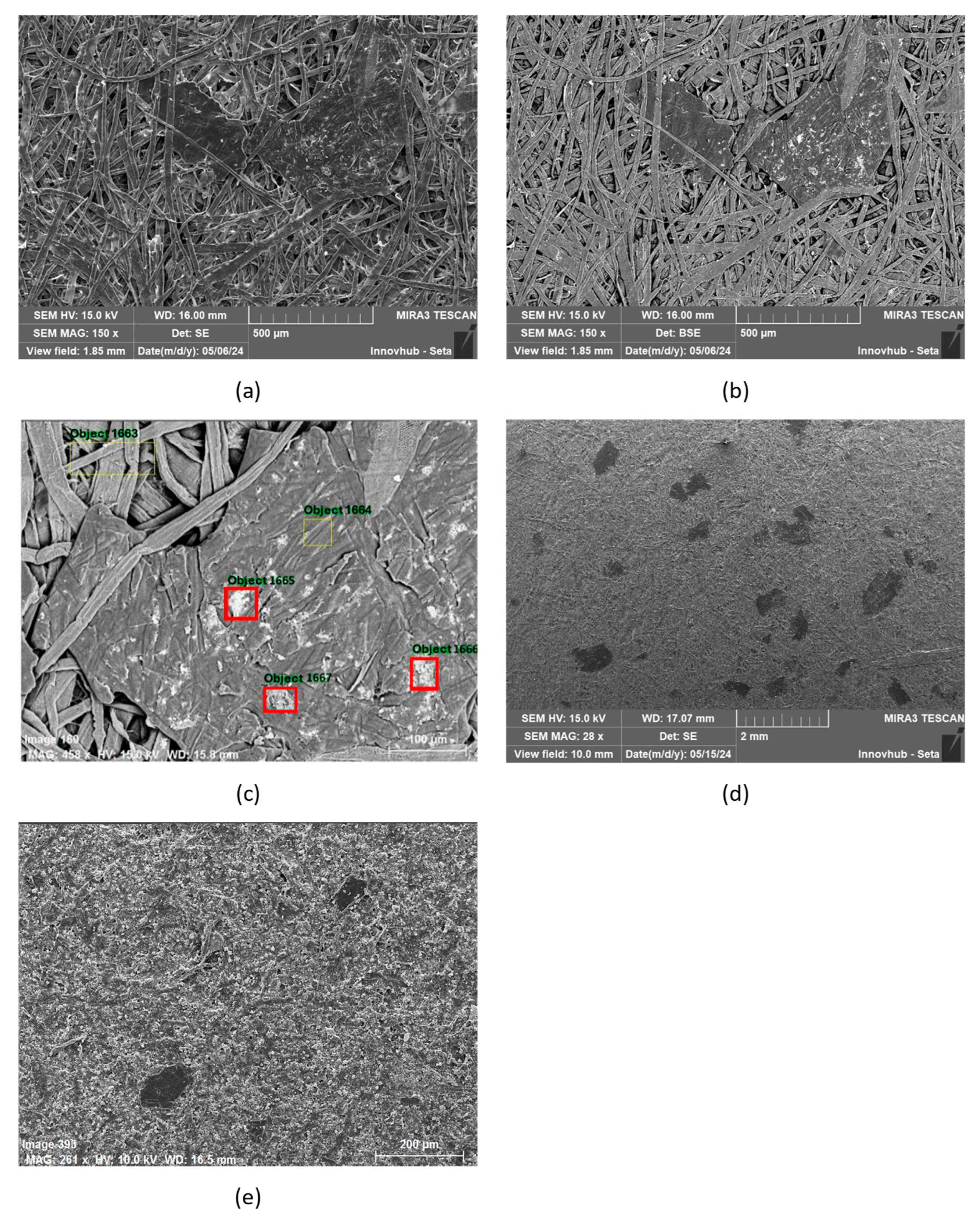
3.7. SEM
4. Discussion
5. Conclusions
- (1)
- Rhodamine-B can tag styrene–butadiene latex. No residual rhodamine-B remained untagged for concentrations of 0.03 wt%, as observed with Raman spectroscopy analysis.
- (2)
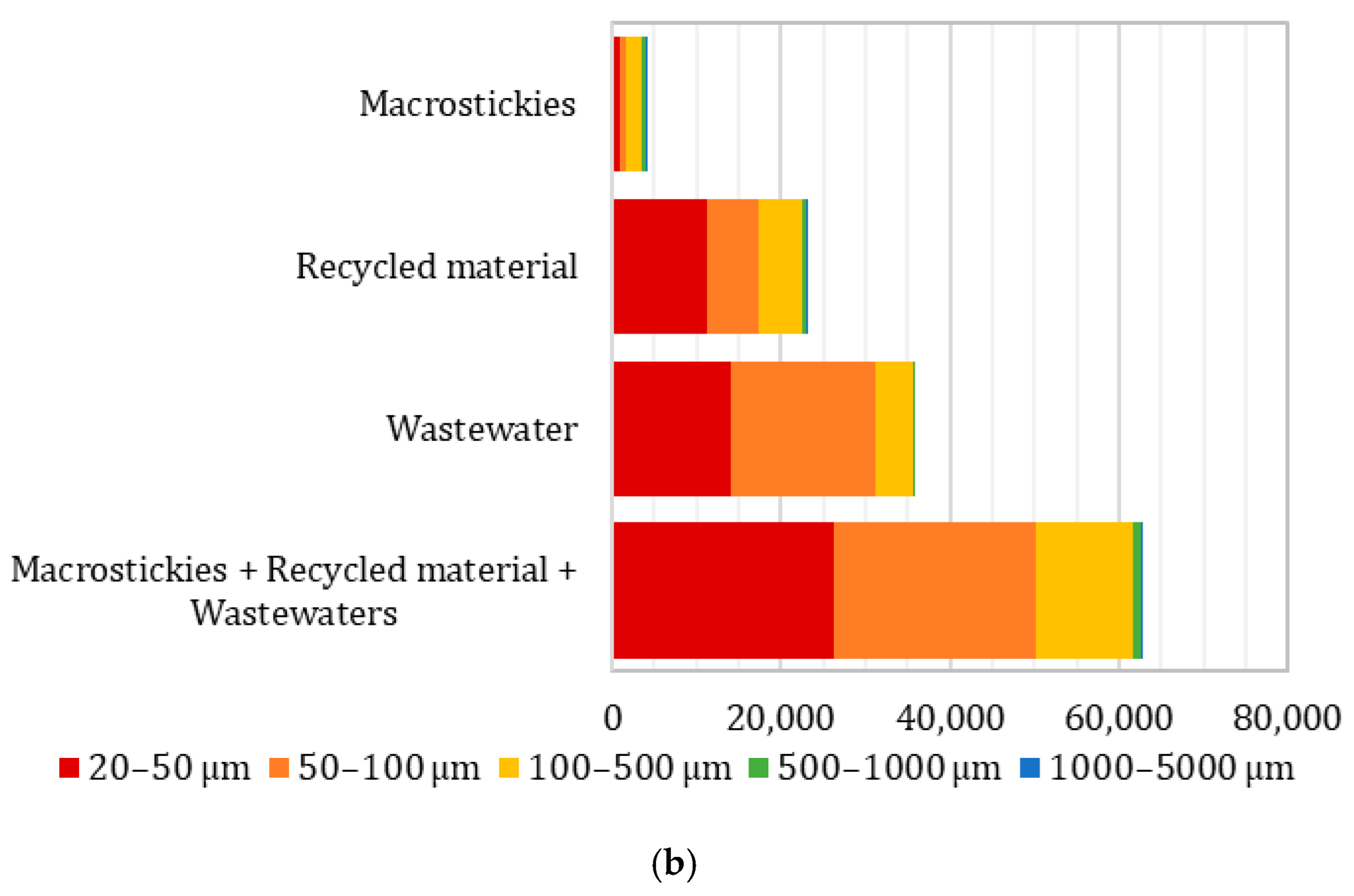
- Secondary microplastics were found in the macrosticky filter, recycled material, and wastewater filter. The particle number increased and average size reduced from the macrostickies to wastewater filter, up to an average of 51 µm in the wastewater.
- (3)
- High-resolution scanning under visible radiation proved adequate for broad area investigation; however, fractions below 20 µm require further investigation due to limits on the adopted equipment.
- (4)
- Raman spectroscopy and UV microscopy possess higher resolution and allow the measurement of particles partially covered by cellulose fibres (in the recycled material or in the wastewater filter) or in proximity to the surface.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.M.; Sultan, M.B.; Alam, M. Microplastics and adsorbed micropollutants as emerging contaminants in landfill: A mini review. Curr. Opin. Environ. Sci. Health 2023, 31, 100420. [Google Scholar] [CrossRef]
- Ha, J.; Yeo, M.-K. The environmental effects of microplastics on aquatic ecosystems. Mol. Cell. Toxicol. 2018, 14, 353–359. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Misra, M. Microplastics in ecosystems: Their implications and mitigation pathways. Environ. Sci. Adv. 2022, 1, 9–29. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Arthur, C.; Baker, J.E.; Bamford, H.A. Summary of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris. In Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 9–11 September 2008; National Oceanic and Atmospheric Administration: Tacoma, WA, USA, 2009; pp. 7–17. [Google Scholar]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, A.; Xu, J.-L. Microplastics in waste management systems: A review of analytical methods, challenges and prospects. Waste Manag. 2023, 171, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Golwala, H.; Zhang, X.; Iskander, S.M.; Smith, A.L. Solid waste: An overlooked source of microplastics to the environment. Sci. Total Environ. 2021, 769, 144581. [Google Scholar] [CrossRef]
- Samanta, P.; Dey, S.; Kundu, D.; Dutta, D.; Jambulkar, R.; Mishra, R.; Ghosh, A.R.; Kumar, S. An insight on sampling, identification, quantification and characteristics of microplastics in solid wastes. Trends Environ. Anal. Chem. 2022, 36, e00181. [Google Scholar] [CrossRef]
- Packaging Waste Statistics. Eurostat. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Packaging_waste_statistics (accessed on 14 March 2025).
- Global Waste Generation. Statista. Available online: https://www.statista.com/study/58790/global-waste-generation/ (accessed on 3 September 2024).
- Guo, Y.; Xia, X.; Ruan, J.; Wang, Y.; Zhang, J.; LeBlanc, G.A.; An, L. Ignored microplastic sources from plastic bottle recycling. Sci. Total Environ. 2022, 838, 156038. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Recent approaches and advanced wastewater treatment technologies for mitigating emerging microplastics contamination—A critical review. Sci. Total Environ. 2023, 858, 159681. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; MacDonald, A.; Allen, S.; Allen, D. The potential for a plastic recycling facility to release microplastic pollution and possible filtration remediation effectiveness. J. Hazard. Mater. Adv. 2023, 10, 100309. [Google Scholar] [CrossRef]
- Kankanige, D.; Babel, S. Contamination by ≥6.5 μm-sized microplastics and their removability in a conventional water treatment plant (WTP) in Thailand. J. Water Process Eng. 2021, 40, 101765. [Google Scholar] [CrossRef]
- Marinelli, A.; Diamanti, M.V.; Pedeferri, M.P.; Del Curto, B. Kaolin-Filled Styrene-Butadiene-Based Dispersion Coatings for Paper-Based Packaging: Effect on Water, Moisture, and Grease Barrier Properties. Coatings 2023, 13, 195. [Google Scholar] [CrossRef]
- Bakker, S.; Bosveld, L.; Metselaar, G.A.; Esteves, A.C.C.; Schenning, A.P.H.J. Understanding and Improving the Oil and Water Barrier Performance of a Waterborne Coating on Paperboard. ACS Appl. Polym. Mater. 2022, 4, 6148–6155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Cheng, Y.; Zhang, L.; Shen, W. Superhydrophobic surfaces with dual-scale roughness and water vapor-barrier property for sustainable liquid packaging applications. Cellulose 2022, 29, 9777–9790. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crops Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Thitsartarn, W.; Jinkarn, T. Water resistance improvement of paperboard by coating formulations based on nanoscale pigments. J. Coat. Technol. Res. 2020, 17, 1609–1617. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Imasha, H.U.E.; Babel, S. Microplastics contamination in the green mussels (Perna viridis) cultured for human consumption in Thailand. Reg. Stud. Mar. Sci. 2023, 67, 103203. [Google Scholar] [CrossRef]
- Idowu, G.A.; Olanipekun, O.O.; Adelodun, A.A.; Gbadamosi, O.K.; Adu, B.W.; Aiyesanmi, A.F. Meso- and micro-plastics contamination of water, sediments and fish species in coastal communities of Ondo State, Nigeria. Reg. Stud. Mar. Sci. 2024, 77, 103727. [Google Scholar] [CrossRef]
- Chi, K.; He, J.; Lin, W.-S.; Bokhari, S.M.Q.; Catchmark, J.M. Electrostatically Complexed Natural Polysaccharides as Aqueous Barrier Coatings for Sustainable and Recyclable Fiber-Based Packaging. ACS Appl. Mater. Interfaces 2023, 15, 12248–12260. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Schmid, M.; Nerney, O.M.; Wildner, J.; Smykala, L.; Lazzeri, A.; Cinelli, P. Processing and validation of whey-protein-coated films and laminates at semi-industrial scale as novel recyclable food packaging materials with excellent barrier properties. Adv. Mater. Sci. Eng. 2013, 2013, 496207. [Google Scholar] [CrossRef]
- Bakker, S.; Kloos, J.; Metselaar, G.A.; Catarina, A.; Esteves, A.C.C.; Schenning, P.H.J. About Gas Barrier Performance and Recyclability of Waterborne Coatings on Paperboard. Coatings 2022, 12, 1841. [Google Scholar] [CrossRef]
- Lee, T.J.; Yoon, C.; Ryu, J.Y. A new potential paper resource; recyclability of paper cups coated with water-soluble polyacrylate-based polymer. Nord. Pulp Pap. Res. J. 2017, 32, 155–161. [Google Scholar] [CrossRef]
- UNI 11743:2019; Carta e Cartone—Determinazione dei Parametri di Riciclabilità di Materiali e Prodotti a Prevalenza Cellulosica. UNI: Milano MI, Italy, 2019. Available online: https://store.uni.com/uni-11743-2019 (accessed on 2 July 2019).
- Cepi. Harmonised European Laboratory Test Method to Generate Parameters Enabling the Assessment of the Recyclability of Paper and Board Products in Standard Paper and Board Recycling Mills. 2022. Available online: https://www.cepi.org/cepi-recyclability-test-method-version-2/ (accessed on 3 August 2023).
- Toczyłowska-Mamińska, R. Limits and perspectives of pulp and paper industry wastewater treatment—A review. Renew. Sustain. Energy Rev. 2017, 78, 764–772. [Google Scholar] [CrossRef]
- Allender, B.; Covey, G.; Shore, D. Low-effluent Recycled Paper Mills. Appita Technol. Innov. Manuf. Environ. 2010, 63, 186–194. [Google Scholar]
- ASTM D8333-20; Standard Practice for Preparation of Water Samples with High, Medium, or Low Suspended Solids for Identification and Quantification of Microplastic Particles and Fibers Using Raman Spectroscopy, IR Spectroscopy, or Pyrolysis-GC/MS. Available online: https://store.astm.org/d8333-20.html (accessed on 20 October 2025).
- BSI PD CEN ISO/TR 21960:2020; Plastics—Environmental Aspects—State of Knowledge and Methodologies. Available online: https://bsol.bsigroup.com/Bibliographic/BibliographicInfoData/000000000030379025 (accessed on 20 October 2025).
- Tong, H.; Jiang, Q.; Zhong, X.; Hu, X. Rhodamine B dye staining for visualizing microplastics in laboratory-based studies. Environ. Sci. Pollut. Res. 2021, 28, 4209–4215. [Google Scholar] [CrossRef]
- Purington, E.; Blakeley, A.R.; Bousfield, D.; Gramlich, W.M. Visualization of latex and starch in paper coatings by tagging with fluorescent dyes. Nord. Pulp Pap. Res. J. 2017, 32, 395–406. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef]
- Kwon, S.; Oh, K.; Shin, S.J.; Lee, H.L. Effects of hydroxyethyl methacrylate comonomer in styrene/acrylate latex on coating structure and printability. Prog. Org. Coat. 2020, 147, 105862. [Google Scholar] [CrossRef]
- Kugge, C.; Johnson, B. Improved barrier properties of double dispersion coated liner. Prog. Org. Coat. 2008, 62, 430–435. [Google Scholar] [CrossRef]
- Mesic, B.B.; Cairns, M.; Järnstrom, L.; Le Guen, M.J.; Parr, R. Film formation and barrier performance of latex based coating: Impact of drying temperature in a flexographic process. Prog. Org. Coat. 2019, 129, 43–51. [Google Scholar] [CrossRef]
- Aticelca. Sistema di Valutazione 501:2023—Valutazione del Livello di Riciclabilità di Materiali e Prodotti a Prevalenza Cellulosica Sulla Base Della Norma UNI 11743:2019. 2023. Available online: https://www.aticelca.it/1/wp-content/uploads/2024/11/Sistema-di-valutazione-501-2023.pdf (accessed on 12 December 2024).
- Sears, W.M.; Hunt, J.L.; Stevens, J.R. Raman scattering from polymerizing styrene. I. Vibrational mode analysis. J. Chem. Phys. 1981, 75, 1589–1598. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Mediavilla-Martinez, I.; Sanz-Velasco, C.; Prieto, A.C.; Pinto, J. Pigments identification and analysis of the state of conservation of the decorative elements of the castle of Coca (XV-XVI AC, Segovia, Spain) by Raman Spectroscopy. J. Phys. Conf. Ser. 2022, 2204, 012010. [Google Scholar] [CrossRef]
- Kukkola, A.T.; Senior, G.; Maes, T.; Silburn, B.; Bakir, A.; Kröger, S.; Mayes, A.G. A large-scale study of microplastic abundance in sediment cores from the UK continental shelf and slope. Mar. Pollut. Bull. 2022, 178, 113554. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Desender, M.; Wilkinson, T.; Van Hoytema, N.; Amos, R.; Airahui, S.; Graham, J.; Maes, T. Occurrence and abundance of meso and microplastics in sediment, surface waters, and marine biota from the South Pacific region. Mar. Pollut. Bull. 2020, 160, 111572. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, F.; Kersten, A.; Schabel, S.; Kerpen, J. Microplastics in German paper mills’ wastewater and process water treatment plants: Investigation of sources, removal rates, and emissions. Water Res. 2025, 271, 123016. [Google Scholar] [CrossRef]
- Yli-Rantala, E.; Pham, T.; Sarlin, E.; Kokko, M. Extraction and analysis of microplastics in wastewater sludges of a multi-product pulp and paper mill. Environ. Pollut. 2024, 363, 125251. [Google Scholar] [CrossRef]
- Colakoglu, E.B.; Uyanik, I.; Elbir, H.; Sahinkaya, E.; Yurtsever, A. A novel gravity-driven dynamic membrane filtration reactor for microplastic removal from plastic recycling facility wastewater. J. Environ. Chem. Eng. 2025, 13, 115793. [Google Scholar] [CrossRef]
- Regulation (EU) 2025/40 of the European Parliament and of the Council of 19 December 2024 on Packaging and Packaging Waste, Amending Regulation (EU) 2019/1020 and Directive (EU) 2019/904, and Repealing Directive 94/62/EC. European Parliament and Council. Available online: https://eur-lex.europa.eu/eli/reg/2025/40/oj/eng (accessed on 27 January 2025).
- Ta, A.T.; Pupuang, P.; Babel, S.; Wang, L.P. Investigation of microplastic contamination in blood cockles and green mussels from selected aquaculture farms and markets in Thailand. Chemosphere 2022, 303, 134918. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Germaine, K.; Kakouli-Duarte, T.; Cleary, J. Assessment of microplastics in Irish river sediment. Heliyon 2022, 8, e09853. [Google Scholar] [CrossRef]
- Marinelli, A.; Papile, F.; Del Curto, B. Coated Paper-Based Packaging Waste: Investigation on Sensorial Properties Affecting the Material Class Perception. Sustainability 2023, 15, 16474. [Google Scholar] [CrossRef]
- Marinelli, A.; Lyytikäinen, J.; Tanninen, P.; Del Curto, B.; Leminen, V. Barrier, converting, and tray-forming properties of paperboard packaging materials coated with waterborne dispersions. Packag. Technol. Sci. 2023, 37, 149–165. [Google Scholar] [CrossRef]
- Marinelli, A.; Profaizer, M.; Diamanti, M.V.; Pedeferri, M.; Del Curto, B. Heat-Seal Ability and Fold Cracking Resistance of Kaolin-Filled Styrene-Butadiene-Based Aqueous Dispersions for Paper-Based Packaging. Coatings 2023, 13, 975. [Google Scholar] [CrossRef]
- Devisetti, S.; Lempsink, G.; Malla, P.B. Use of kaolin clay in aqueous barrier coating applications. TAPPI J. 2023, 22, 685–697. [Google Scholar] [CrossRef]
- Ovaska, S.-S.; Sami-Seppo, K.; Lyytikäinen, J.; Hirn, U.; Backfolk, K. Determination of Repulpability of Talc-Filled Biopolymer Dispersion Coatings and Optimization of Repulped Reject for Improved Material Efficiency by Tailoring Coatings. In Proceedings of the Tappi PaperCon 2017 Proceedings, Minneapolis, MN, USA, 23–26 April 2017; pp. 36–48. [Google Scholar]
| Parameters | Uncoated Substrate | Coated Substrate |
|---|---|---|
| Coarse Rejects [%] | <0.1 | <0.1 |
| Area of Macrostickies < 2000 µm [mm2/kg] | 250 | 272,740 |
| Fiber Flakes [%] | 0.2 | 3.8 |
| Recycled sheets—Adhesion test | Not detectable | Not detectable |
| Recycled sheets—Optical inhomogeneity | Level 1 | Level 2 1 |
| Assessment of recyclability according to Aticelca 501:2023 evaluation system | LEVEL A+ | NOT RECYCLABLE with paper |
| Sample | Area (%) |
|---|---|
| Macrostickies—Not inked | 5.67 ± 4.98 |
| Macrostickies—Inked | 4.28 ± 4.04 |
| Recycled material sheet | 10.01 ± 2.81 |
| Filtered waters | 4.59 ± 0.95 |
| Technique | Pros | Cons |
|---|---|---|
| Visible scanning imaging |
|
|
| Fluorescence microscopy |
|
|
| Raman spectroscopy |
|
|
| SEM-EDX |
|
|
| Ref. | Focus | Sample Preparation | Analysis Techniques | MP Dimensions |
|---|---|---|---|---|
| This work | Tracking MPs generated at lab scale during recycling process | Coating tagging with RhB | Visible light imaging, Raman spectroscopy and fluorescence mapping/microscopy | Mainly <100 μm; Lower limit: 15 μm |
| [48] | Outbound wastewater analysis of paper mills in Germany | Oxidative treatment + density separation | μ-Raman spectroscopy | Mainly <100 μm; Fraction >500 μm was negligible Lower limit: 20 μm |
| [49] | Sludges from a multi-purpose paper mill in Finland | Chemical-enzymatic digestion + density separation | Raman spectroscopy | Mainly <200 μm Lower limit: 20 μm |
| [17] | Outbound wastewater analysis of plastic recycling facilities in the United Kingdom | Digestion + Nile Red tagging | Fluorescence microscopy | Mainly <10 μm Lower limit: 2.6 μm |
| [50] | Outbound wastewater and sludge analysis of plastic recycling facilities in Türkiye | Density separation | Optic microscope | Mainly <250 μm Lower limit: 45 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinelli, A.; Baracani, S.; Bussini, D.; Boschi, A.; Lucotti, A.; Paterlini, L.; Diamanti, M.V.; Del Curto, B. Discovery and Quantification of Microplastic Generation in the Recycling of Coated Paper-Based Packaging. Coatings 2025, 15, 1284. https://doi.org/10.3390/coatings15111284
Marinelli A, Baracani S, Bussini D, Boschi A, Lucotti A, Paterlini L, Diamanti MV, Del Curto B. Discovery and Quantification of Microplastic Generation in the Recycling of Coated Paper-Based Packaging. Coatings. 2025; 15(11):1284. https://doi.org/10.3390/coatings15111284
Chicago/Turabian StyleMarinelli, Andrea, Sara Baracani, Daniele Bussini, Alessandra Boschi, Andrea Lucotti, Luca Paterlini, Maria Vittoria Diamanti, and Barbara Del Curto. 2025. "Discovery and Quantification of Microplastic Generation in the Recycling of Coated Paper-Based Packaging" Coatings 15, no. 11: 1284. https://doi.org/10.3390/coatings15111284
APA StyleMarinelli, A., Baracani, S., Bussini, D., Boschi, A., Lucotti, A., Paterlini, L., Diamanti, M. V., & Del Curto, B. (2025). Discovery and Quantification of Microplastic Generation in the Recycling of Coated Paper-Based Packaging. Coatings, 15(11), 1284. https://doi.org/10.3390/coatings15111284









