Abstract
Architectural exterior wall coatings require a balance of elasticity, stain resistance, and durability. Although nano-SiO2 enhances fracture resistance in elastic coatings, its limited hydrophobicity allows pollutant adhesion. Nano-TiO2 can photocatalytically degrade organics but is often encapsulated by the polymer matrix, reducing its effectiveness. This study introduces a SiO2-TiO2 composite topcoat applied via aqueous dispersion to overcome these limitations. Experimental results demonstrate that the composite coating significantly outperforms single-component modifications, improving stain resistance by 21.3% after 12 months of outdoor exposure. The surface remains brighter with markedly reduced pollutant accumulation. Mechanistically, SiO2 serves as an inert mesoporous carrier that improves the dispersion and photostability of TiO2, minimizing agglomeration and photocorrosion. Its inherent hardness and hydrophobicity reduce physical adsorption sites. Together, SiO2 and TiO2 create a nanoscale rough surface that enhances hydrophobicity through a lotus-like effect. Under UV irradiation, TiO2 generates radicals that decompose organic pollutants and inhibit microbial growth, enabling efficient self-cleaning with rainwater. This synergistic mechanism addresses the limitations of individual nanoparticles, successfully integrating elasticity with long-term anti-fouling and durability. This composite demonstrates a significant advancement in stain resistance and overall durability, offering potential applications in energy-efficient and environmentally sustainable building technologies.
1. Introduction
As the “outer garment” of buildings, architectural exterior wall coatings not only serve decorative and beautifying functions but also constitute a crucial component of the building protection system. Some studies have shown that high-quality exterior wall coatings can effectively block ultraviolet radiation [], inhibit microbial growth [], and achieve building energy efficiency by reflecting solar radiation []. Data from China’s Ministry of Housing and Urban-Rural Development indicate that high-performance exterior wall coatings can reduce building energy consumption by 8%–12%, demonstrating significant practical value in low-carbon environmental protection and technological innovation. In a certain region of China where the diurnal temperature variation is considerable, building exteriors endure intense thermal expansion and contraction year-round. Ordinary coatings develop surface micro-crack densities of up to 12–15 cracks/cm2 after three years of service []. Figure 1 illustrates the relationship between crack width and temperature, showing that higher temperatures result in narrower crack widths. To address this, elastic coating technology is widely adopted internationally, endowing the coating with deformation adaptability. According to the ASTM D6083 standard by the American Society for Testing and Materials, when the elongation at break exceeds 150%, it can effectively suppress temperature-induced cracking [].
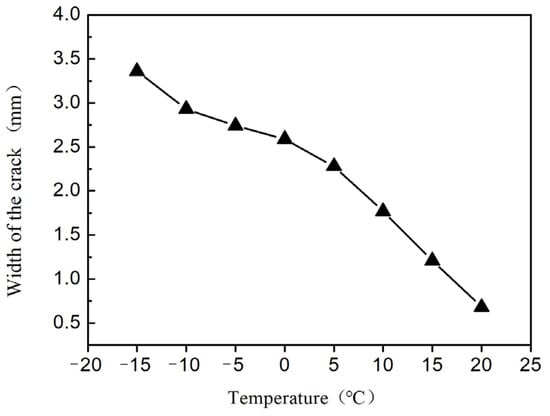
Figure 1.
The relationship between crack width and temperature.
Recent studies have focused on nano-SiO2 modification technology, whose three-dimensional network structure can increase the fracture elongation rate of coatings to 280% []. Therefore, elastic coatings incorporating SiO2 can effectively prevent the formation of microcracks (Figure 2). However, some research indicates that the addition of SiO2 does not significantly improve the stain resistance of coatings []. Korean scholar Kim [] analyzed this phenomenon and found that insufficient hydrophobic properties of the surface micro/nanostructure cause pollutants to easily form capillary adsorption at the SiO2 protrusions, leading to poor stain resistance.

Figure 2.
Comparison of walls with and without SiO2-added coating.
Based on this, the present study proposes an innovative solution of constructing a SiO2-TiO2 composite topcoat layer. Takami has confirmed that the photocatalytic properties of TiO2 can decompose organic pollutants under UV irradiation (degradation rate > 90%), while the mesoporous structure of SiO2 enhances the mobility of photogenerated carriers []. Research on synergistic effects by Matsuda from Toyohashi University of Technology demonstrated that SiO2-TiO2 coatings has good photocatalytic activity and stable physical properties []. Lei Miao et al. has constructed a SiO2-TiO2 nanocomposite coating, successfully combining high photocatalytic self-cleaning activity with good compatibility []. In addition, some researchers have focused on TiO2, SiO2 nano-, and composite structures [,]. Compared to laboratory tests, a more reliable method for evaluating the stain resistance of exterior wall coatings is to conduct natural exposure testing under sunlight. Currently, there is very limited research on SiO2-TiO2 composite topcoat layers under long-term natural weathering conditions. This paper aims to elucidate the synergistic reinforcement mechanism of SiO2-TiO2, which is particularly crucial for exterior wall coatings, providing new insights to overcome existing technological bottlenecks.
2. Experimental Details
2.1. Experimental Materials
The manufacturer of SiO2 is Nabaltec AG (Schwandorf, Germany), and the model is NEBOFILL 6001. The manufacturer of TiO2 is Nanjing Nano Innovation Materials Technology Co., Ltd. (Nanjing, China), and the model is Nacro NC-106. The basic formulation of the elastic coating is shown in Table 1.

Table 1.
Basic Formula of Coatings.
2.2. Materials and Methods
The reflectivity was measured using a C84-II reflectometer (Tianjin Weida Testing Machine Factory, Tianjin, China), tensile properties were tested with an electronic universal testing machine, stain resistance was evaluated with a custom-built device, and outdoor exposure was performed on a self-made rack.
2.2.1. Tensile Test Method
Stir and mix the coating thoroughly until uniform, then pour it into a steel film-casting mold in three steps for film preparation. First, pour the mixture into a 230 × 100 × 1.0 mm mold, allow it to form a film, and then remove the mold. Place a 235 × 105 × 1.2 mm mold on the formed film for the second casting, remove the mold after film formation, and finally place a 240 × 110 × 1.5 mm mold on the resulting film for the third casting. Each casting step is spaced 24 h apart. Place the prepared film in an environment maintained at a temperature of (23 ± 2) °C and relative humidity of (50 ± 5)% for 48 h of curing. After peeling off the film, place it in a drying oven at (80 ± 2) °C and maintain a constant temperature for 96 h. Finally, cut the film into dumbbell-shaped specimens using a cutting die, with specific dimensions as shown in Figure 3a.
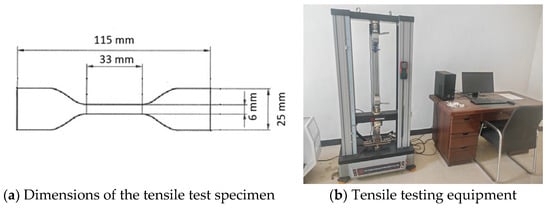
Figure 3.
Tensile testing equipment and dimensions of specimen.
First, the tensile properties of the coating film were measured under standard conditions. The specimen was mounted in the grips of the tensile testing machine, and the initial gauge length was recorded. The specimen was then stretched at a speed of 200 mm/min until a crack appeared. The gauge length at this point was recorded with an accuracy of 0.05 mm, and the maximum load encountered during the stretching process until fracture was also recorded. Next, the tensile properties of the coating film were measured at 0 °C and −10 °C, respectively. The specimens were mounted in the grips of the tensile testing machine at the respective temperatures (0 °C and −10 °C) and kept at these temperatures for 1 h. They were then stretched at a speed of 30 mm/min until a crack appeared. The gauge length at this point was recorded with an accuracy of 0.05 mm, and the maximum load during the stretching process until fracture was also recorded.
2.2.2. Stain Resistance Evaluation Method
Using 150 × 70 × 5 mm asbestos-free cement boards as the substrate, the coating was applied in two layers. After curing for 7 days under controlled conditions of temperature (23 ± 2) °C and relative humidity (50 ± 5)%, a self-made dirt resistance testing device (Figure 4) was employed. The coated test panels were placed on the sample rack of the flushing device, and 15 L of water were added to a 2 m-high water tank. The device was then activated to flush the coated panels for 1 min. The reflectance coefficient was measured before and after the test, and the difference was recorded to characterize the coating’s dirt resistance performance.
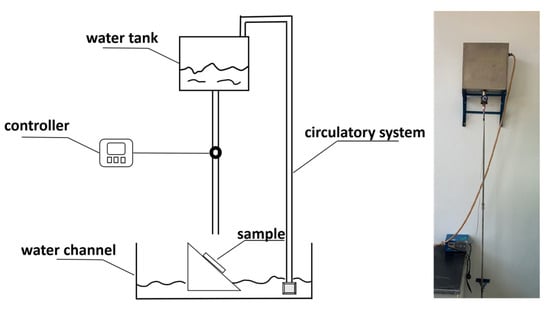
Figure 4.
The Self-made Stain Resistance Testing Equipment.
2.2.3. Solar Radiation Test
The preparation and curing of the test specimens for the exposure test are the same as those for the stain resistance test. The test panels are placed on a custom-made outdoor exposure rack (Figure 5) at a 45-degree angle to the horizontal and exposed to natural sunlight for 12 months. The location of the exposure test is in Xinyu City, Jiangxi Province, China, with an altitude of 500–1000 m. The local climate type is subtropical monsoon climate, characterized by sufficient sunshine and abundant precipitation. The average annual temperature range is 11.6–19.6 °C (The date is from “the China Meteorological Data Network” []). The reflectance coefficients before and after exposure are measured to obtain the stain resistance values at different stages. The outdoor exposure test samples involved in this paper were all conducted at the same location and exposure time was synchronized, so the influence of the environment on the samples is consistent, which can truly demonstrate the anti-fouling performance of the coating in outdoor environments. Therefore, for this paper, environmental conditions have almost no effect on the comparative results of the exposure test. In addition, environmental conditions such as sunlight direction, rainfall, or wind can have an impact on the stain resistance of coatings. Any single variable can affect the data values of the coating’s stain resistance performance. However, if different coatings are compared in the same environment, the impact of environmental changes on the regularity of experimental results is minimal.
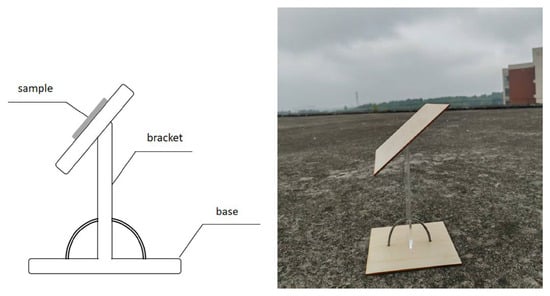
Figure 5.
Self-made Outdoor Exposure Test Rack.
3. Results
3.1. The Effect of SiO2 Addition Amount on Coating Performance
Nano-SiO2 was added to the elastic coating to sequentially improve the contact angle of the coating surface, achieving a spreading effect. Different proportions (0%, 5%, 10%) of nano-SiO2 were added and evenly mixed, and their effects on the elasticity and stain resistance of the elastic coating were tested. The results are shown in Figure 6, Figure 7 and Figure 8. As seen in Figure 6, as the proportion of nano-SiO2 increases, the tensile strength of the elastic coating improves, while the elongation rates under standard conditions, at 0 °C, and at −10 °C all decrease. The stain resistance also shows slight improvement.
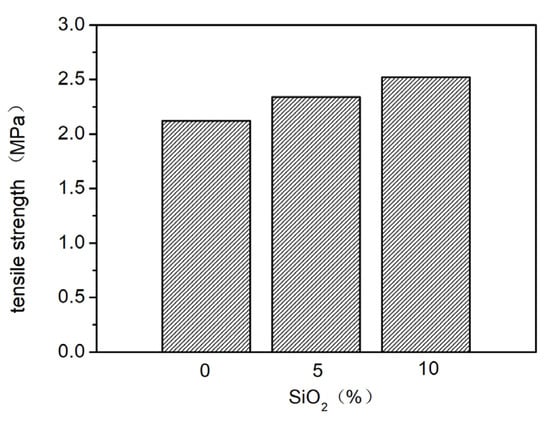
Figure 6.
The effect of adding different proportions of nano-SiO2 on tensile strength.
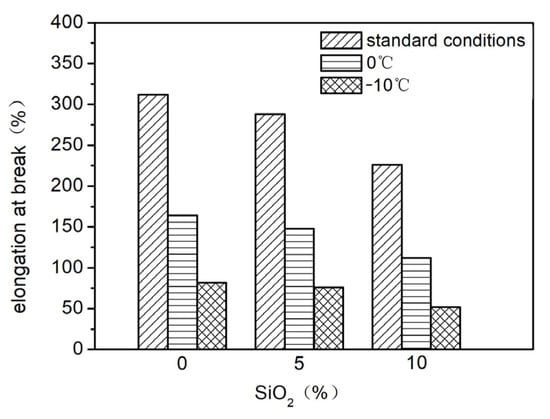
Figure 7.
The influence of incorporating varying ratios of nano-SiO2 on the fracture elongation rate.
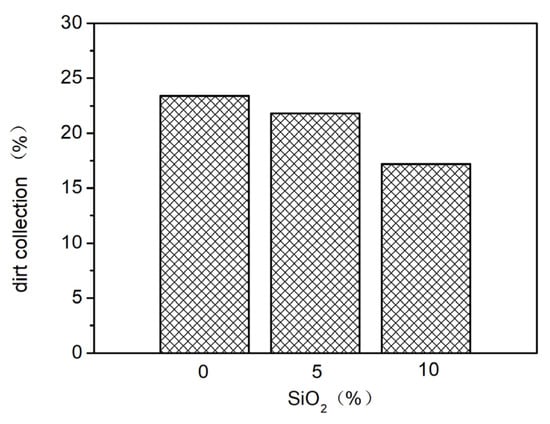
Figure 8.
The effect of adding different proportions of nano-SiO2 on stain resistance.
In addition to laboratory tests, outdoor exposure tests were also conducted. Paint samples were prepared using the roll-coating method to study the effects after 2 months and 12 months of outdoor exposure, with the results shown in Figure 9. As can be seen from Figure 9, the panels with added nano-SiO2 appeared cleaner, demonstrating significantly improved stain resistance. This is because the small particle size of nano-SiO2 allows it to fill the fine gaps within the coating film when compounded with the emulsion, enhancing the film’s compactness and hardness. Consequently, this improves the coating’s high-temperature anti-blocking properties and stain resistance.

Figure 9.
Outdoor exposure photo of elastic coating containing nano-SiO2.
3.2. The Effect of TiO2 Addition Amount on Coating Performance
Nano-TiO2 was added to the basic elastic coating at proportions of 0%, 3%, and 6%. The effects of different nano-TiO2 addition amounts on the elasticity (Figure 10 and Figure 11) and stain resistance (Figure 12) of the elastic coating were tested. The results show that the addition of nano-TiO2 did not significantly improve the elasticity or stain resistance of the elastic coating. This is because, during the film-forming process of the elastic coating, the film-forming substances in the elastic formulation encapsulated the nano-TiO2, leaving very little nano-TiO2 on the surface of the coating layer. As a result, it failed to deliver its intended effects.
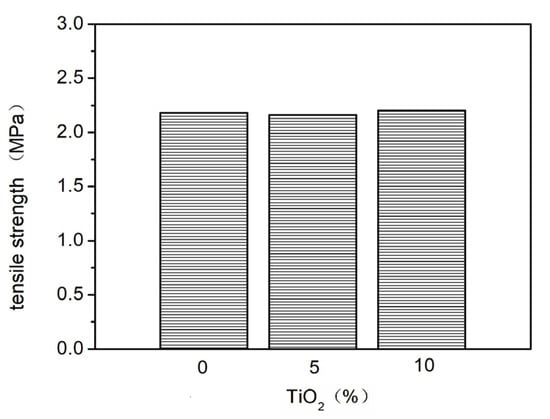
Figure 10.
The effect of adding different proportions of nano-TiO2 on tensile strength.
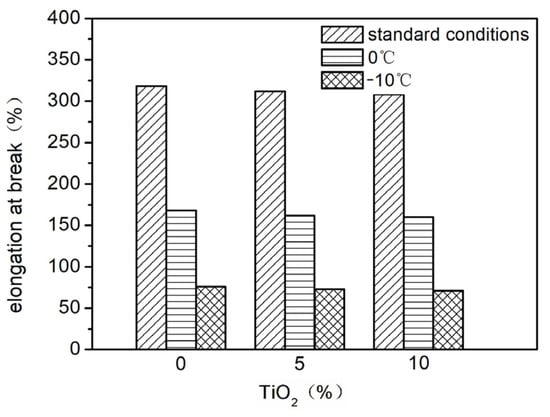
Figure 11.
The influence of incorporating varying ratios of nano-TiO2 on the fracture elongation rate.
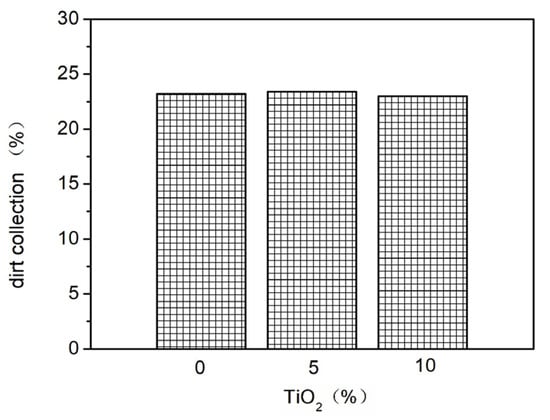
Figure 12.
The effect of adding different proportions of nano-TiO2 on stain resistance.
3.3. The Effect of SiO2-TiO2 Composite Surface Layer on the Stain-Resistant Performance of Coatings
The nano-SiO2 and TiO2 were diluted with water (water: nano-SiO2/nano-TiO2 = 10:1) and roll-coated onto a white painted surface for stain resistance testing. The results are shown in Figure 13. The stain resistance is calculated based on the difference in reflectivity. The difference between the initial reflection coefficient of the coating and the reflection coefficient after the stain resistance test is divided by the initial reflection coefficient of the coating to obtain the stain resistance of the coating. It can be observed that the addition of nano-SiO2 improved the stain resistance compared to the untreated sample, while the improvement from nano-TiO2 was not significant. The average stain resistance of ordinary paint is 23.4%, and the average stain resistance of SiO2-TiO2 composite coating is 18.4%. (23.4–18.4)/23.4 × 100% = 21.3%. Therefore, the stain resistance of the composite coating has been improved by 21.3%. However, the SiO2-TiO2 composite topcoat demonstrated the best stain resistance, outperforming the untreated sample by 21.3%. Similarly, outdoor exposure test results in Figure 14. Figure 14a–d show samples subjected to a 2-month outdoor sun exposure test, with Figure 14a being the ordinary paint, Figure 14b being the sample with added SiO2 in paint, Figure 14c being which with added TiO2, and Figure 14d being which with SiO2-TiO2 composite coating. Figure 14e–h show samples subjected to a 12-month outdoor sun exposure test, with Figure 14a being the ordinary paint, Figure 14b being the sample with added SiO2 in paint, Figure 14c being which with added TiO2, and Figure 14d being which with SiO2-TiO2 composite coating. It showed that the SiO2-TiO2 composite topcoat remained bright and clean even after 12 months of outdoor exposure, exhibiting significantly superior stain resistance.
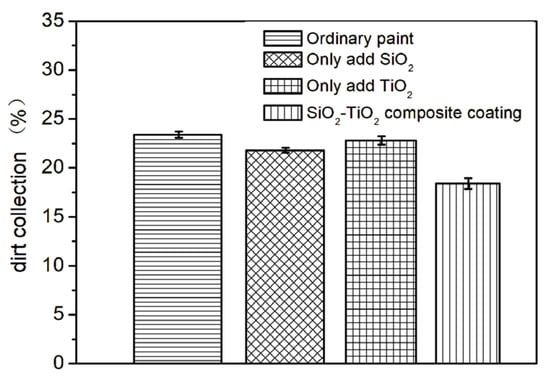
Figure 13.
The effect of SiO2-TiO2 composite coating on the stain resistance of paint.

Figure 14.
The exposure test results of the SiO2-TiO2 composite coating layer.
3.4. Stain Resistance Mechanism
Figure 15 illustrates the anti-fouling mechanism of the SiO2-TiO2 composite topcoat. TiO2 exhibits photocatalytic properties, generating electron–hole pairs under ultraviolet excitation, which produce hydroxyl radicals (•OH) and superoxide radicals (O2−). These radicals effectively decompose organic pollutants (such as oils and microbial metabolites) adhering to the coating surface, reducing chemical adsorption of stains []. The photocatalytic action of TiO2 also inhibits the attachment of microorganisms like algae and mold, preventing the accumulation of biological fouling []. Additionally, under light exposure, when the wavelength range of UV was 200–400 nm, the coating surface forms a uniform water film, allowing rainwater to wash away pollutants and achieve “self-cleaning,” as demonstrated in multiple studies. Incorporating TiO2 provides self-cleaning through two mechanisms. Its photocatalytic activity breaks down organic pollutants under UV light, while its photo-induced hydrophilicity allows water to sheet off, carrying away dirt [,]. However, adding TiO2 alone to exterior wall coatings does not significantly achieve these functions because the film-forming agents in elastic coatings encapsulate the nano-TiO2 during the curing process, leaving very little nano-TiO2 on the surface to exert anti-fouling effects. In contrast, diluting nano-SiO2 and nano-TiO2 with water and directly applying them as an outer layer over the elastic coating greatly enhances the coating’s stain resistance. Outdoor exposure tests show that the coating remains bright and clean even after 12 months of exposure. This improvement occurs because SiO2 acts as an inert carrier, enhancing the dispersion and stability of TiO2, preventing its deactivation due to photocorrosion or agglomeration, while also avoiding excessive catalytic degradation of the coating matrix []. The high hardness and wear resistance of SiO2 strengthen the coating’s mechanical properties, reducing surface scratches and microcracks, thereby minimizing anchor points for physical pollutant adsorption. Furthermore, the combination of SiO2 and TiO2 produces a nanoscale rough surface, which can cause water droplets to roll down and carry away pollutants. Composite coatings form a denser microstructure, reduce porosity, prevent pollutants from penetrating the coating, and reduce the actual contact area through nanoscale roughness. The synergy between SiO2 and TiO2 is not merely additive but achieves performance amplification through the following mechanisms: (a). Structural-Functional Integration: SiO2 provides a skeletal framework, while TiO2 imparts active functionality, forming an efficient interface at the nanoscale. (b). Stability-Activity Balance: SiO2 suppresses the photocorrosion (the phenomenon of decomposition or destruction caused by self-oxidation or reduction reactions under light exposure) of TiO2, extending its photocatalytic lifespan, while TiO2’s activity compensates for the functional limitations of SiO2. Thus, the SiO2-TiO2 composite topcoat significantly enhances the stain resistance of exterior wall coatings through multidimensional synergistic effects, including photocatalytic degradation, mechanical reinforcement, and chemical stability.
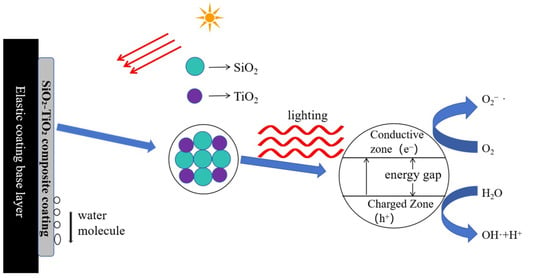
Figure 15.
The principle diagram of stain resistance for the SiO2-TiO2 composite coating.
4. Conclusions
- (1)
- The SiO2-TiO2 composite topcoat significantly enhances the stain resistance of the coating through synergistic effects. After diluting nano-SiO2 and nano-TiO2 with water, the photocatalytic action of TiO2 complements the mesoporous structure, high hardness, and hydrophobic properties of SiO2, working together to reduce pollutant adsorption and improve self-cleaning capabilities. Even after 12 months of outdoor exposure, the coating remains bright and new, demonstrating excellent stain resistance.
- (2)
- The addition of a single material has its limitations, while a composite strategy can break through technical bottlenecks. The sole incorporation of nano-SiO2 improves elongation at break and suppresses microcracks, but its enhancement of stain resistance is limited. On the other hand, the individual use of TiO2 hinders its photocatalytic activity due to film-forming encapsulation. However, the composite material method overcomes the performance drawbacks of single materials through integrated structural and functional design, achieving improvements in stain resistance and elasticity.
- (3)
- The anti-fouling mechanism of composite coatings involves a multi-dimensional synergistic process. TiO2 photocatalytically degrades organic pollutants, while SiO2 enhances mechanical strength and creates a hydrophobic surface to reduce physical adsorption. The combination of these materials forms a nanoscale rough structure that decreases porosity and prevents pollutant penetration. Additionally, SiO2 acts as an inert carrier, inhibiting TiO2 agglomeration and photocorrosion, thereby extending the coating’s service life. This study provides theoretical and technical support for the development of high-performance exterior wall coatings.
Author Contributions
Writing—original draft preparation, L.-J.D.; writing—review and editing, H.-K.P. and C.-D.L.; resources, S.-P.C.; Investigation, Y.-C.M.; data curation, J.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Jiangxi (20232BAB204105, 20224BAB214041), Xinyu University Education Reform Project (XJJG-24-2), and Undergraduate Course Construction Project for Cooperation between Universities and Enterprises “Civil Engineering Materials”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Maury-Ramírez, A.; Flores-Colen, I.; Kanematsu, H. Advanced Coatings for Buildings. Coatings 2020, 10, 728. [Google Scholar] [CrossRef]
- Hou, Y.X.; Wu, J.R. A research on Exterior Wall Coating Based on Environmental Protection and Automatic Spraying Technology. Int. Symp. Mater. Appl. Eng. (SMAE) 2016, 67, 04014. [Google Scholar] [CrossRef]
- Bettenhausen, C.A. Can cool coatings combat climate change? Paints that reduce the demand for cooling and heating are winning investment and attention from the construction industry. Chem. Eng. News 2023, 101, 30–35. [Google Scholar] [CrossRef]
- Wang, S. Preparation of Photocatalytic Coating Materials and Application in Prefabricated Buildings. Mater. Trans. 2025, 66, 66–75. [Google Scholar] [CrossRef]
- ASTM D6083-20; Standard Specification for Liquid-Applied Elastomeric Waterproofing Membranes with Integral Wearing Surfaces. ASTM International: West Conshohocken, PA, USA, 2020. Available online: https://www.antpedia.com/standard/1252340274.html (accessed on 10 March 2023).
- Gobara, M. Effects of TiO2/SiO2reinforced nanoparticles on the mechanical properties of green hybrid coating. Int. Lett. Chem. Phys. Astron. 2015, 47, 56. [Google Scholar] [CrossRef]
- Nozari, B.; Montazer, M.; Rad, M.M. Stable ZnO/SiO2 nano coating on polyester for anti-bacterial, self-cleaning and flame retardant applications. Mater. Chem. Phys. 2021, 267, 124674. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, P.K.; Lee, Y.B.; Kim, T.-S.; Jo, M.-S.; Lee, S.-Y.; Min, H.-W.; Yoon, J.-B. An experimental and numerical study on adhesion force at the nanoscale. Nanoscale Adv. 2024, 6, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Takami, K.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Preparation and reflectivity of self-organized nanograded SiO2/TiO2/PMMA thin films. J. Ceram. Soc. Jpn. 2004, 112, 138–142. [Google Scholar] [CrossRef]
- Matsuda, A. Functionalities and modification of sol–gel derived SiO2–TiO2 systems for advanced coatings and powders. J. Ceram. Soc. Jpn. 2022, 130, 143–162. [Google Scholar] [CrossRef]
- Miao, L.; Su, L.F.; Sakae, T.; Fisher, C.A.J.; Zhao, L.L.; Liang, Q.; Xu, G. Cost-effective nanoporous SiO2–TiO2 coatings on glass substrates with antireflective and self-cleaning properties. Appl. Energy 2013, 112, 1198–1205. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of poly(Titanium oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Borlaf, M.; Candelario, V.M.; Lopez-Sanchez, J.; Li, Y.; Valero-Saiz, M.; Cepa-Lopez, V.; Mas-Balleste, R.; Moreno, R. Digital light processing of SiO2-TiO2 materials by combining ceramic slurries and metal precursors. J. Eur. Ceram. Soc. 2025, 45, 117628. [Google Scholar] [CrossRef]
- Available online: https://www.nmc.cn (accessed on 30 December 2024).
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Katz, A.; McDonagh, A.; Tijing, L.; Shon, H.K. Fouling and Inactivation of Titanium Dioxide-Based Photocatalytic Systems. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1880–1915. [Google Scholar] [CrossRef]
- Armakovic, S.J.; Savanovic, M.M.; Siljegovic, M.V.; Kisic, M.; Scepanovic, M.; Grujic-Brojcin, M.; Simic, N.; Gavanski, L.; Armakovic, S. Self-Cleaning and Charge Transport Properties of Foils Coated with Acrylic Paint Containing TiO2 Nanoparticles. Inorganics 2024, 12, 35. [Google Scholar] [CrossRef]
- Sallehudin, M.E.; Affandi, N.D.N.; Harun, A.M.; Alam, M.K.; Indrie, L. Morphological Structures and Self-Cleaning Properties of Nano-TiO2 Coated Cotton Yarn at Different Washing Cycles. Nanomaterials 2023, 13, 31. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.J.; Ding, H.; Chen, Y. Quantum dots TiO2 loaded amorphous SiO2 composite photocatalysts: Significant performance enhancement and the effect of SiO2 surface hydroxyl groups. J. Alloys Compd. 2023, 960, 170700. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).