Influences of SiO2 Additions on the Structures and Thermal Properties of AlTaO4 Ceramics as EBC Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Structural Identification
2.3. Measurements of Thermal Properties
3. Results and Discussion
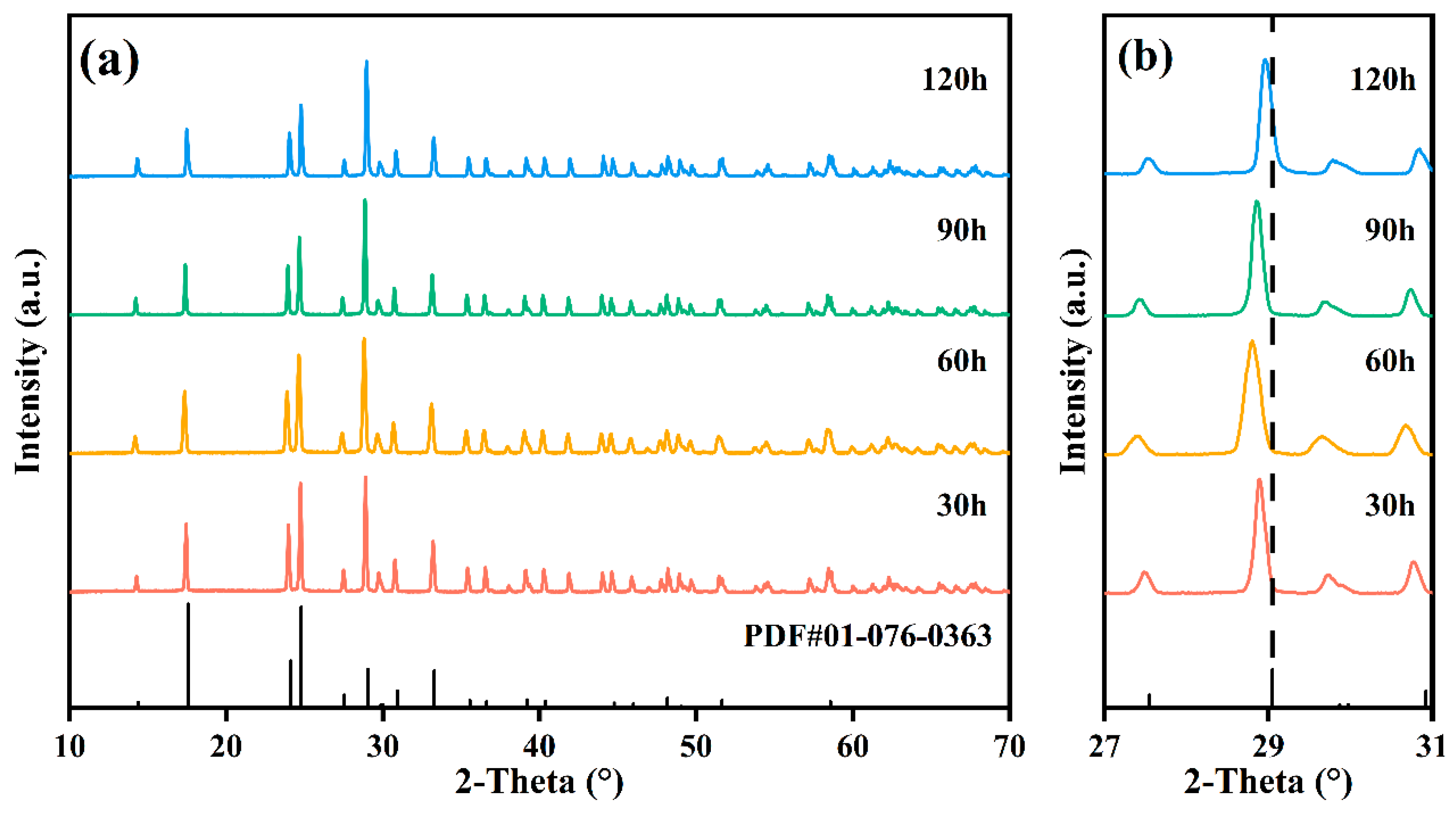
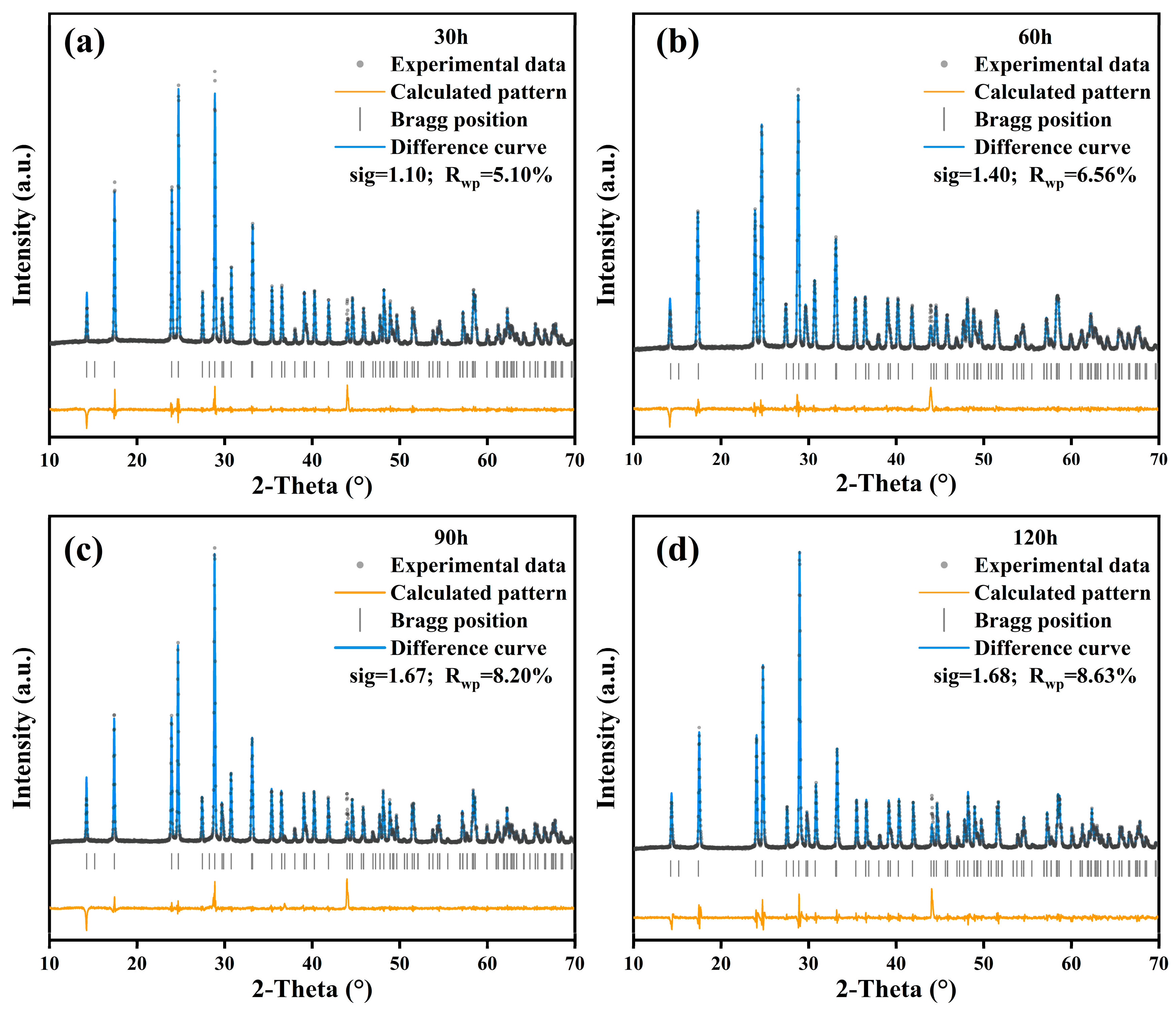
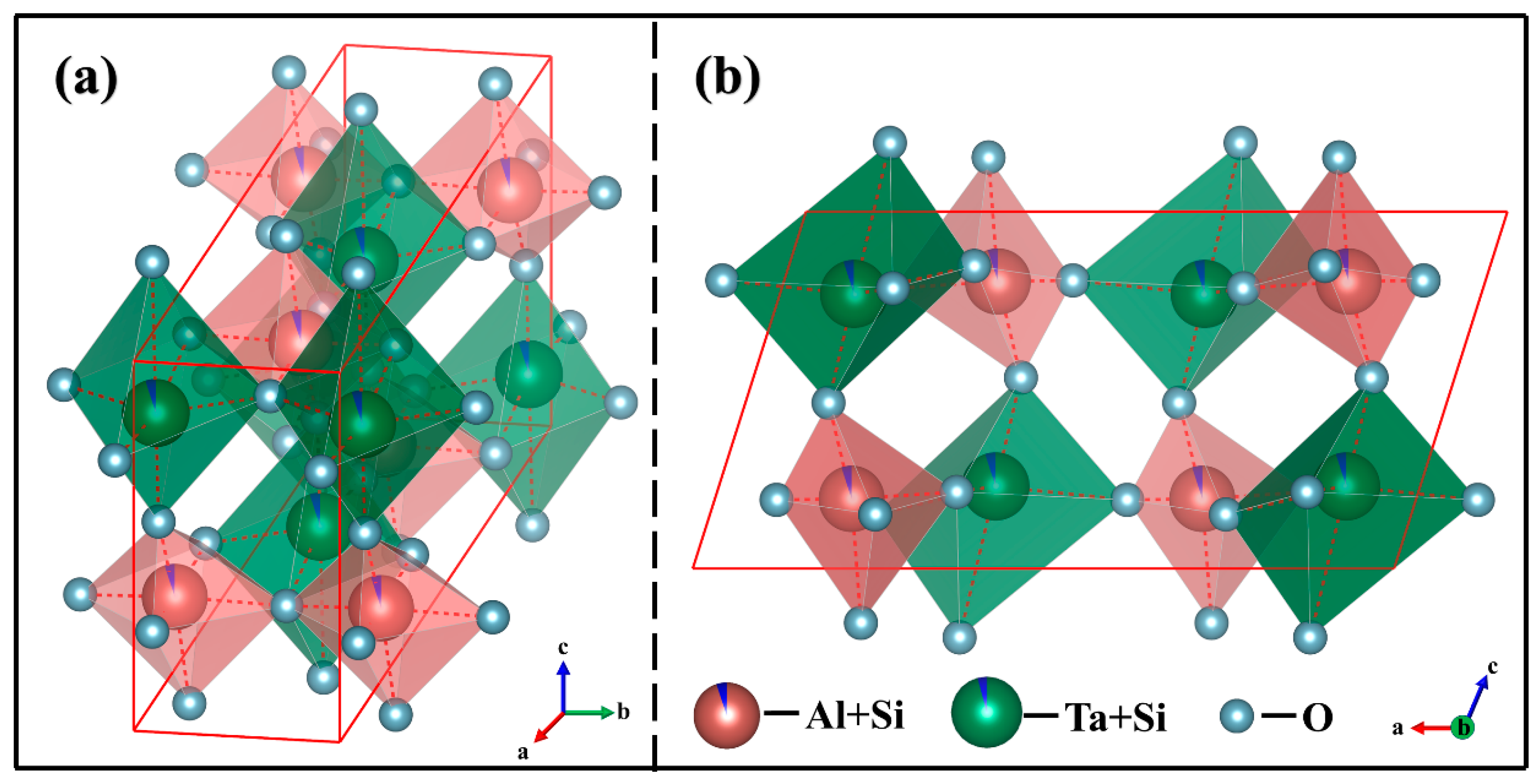
3.1. Crystal Structure
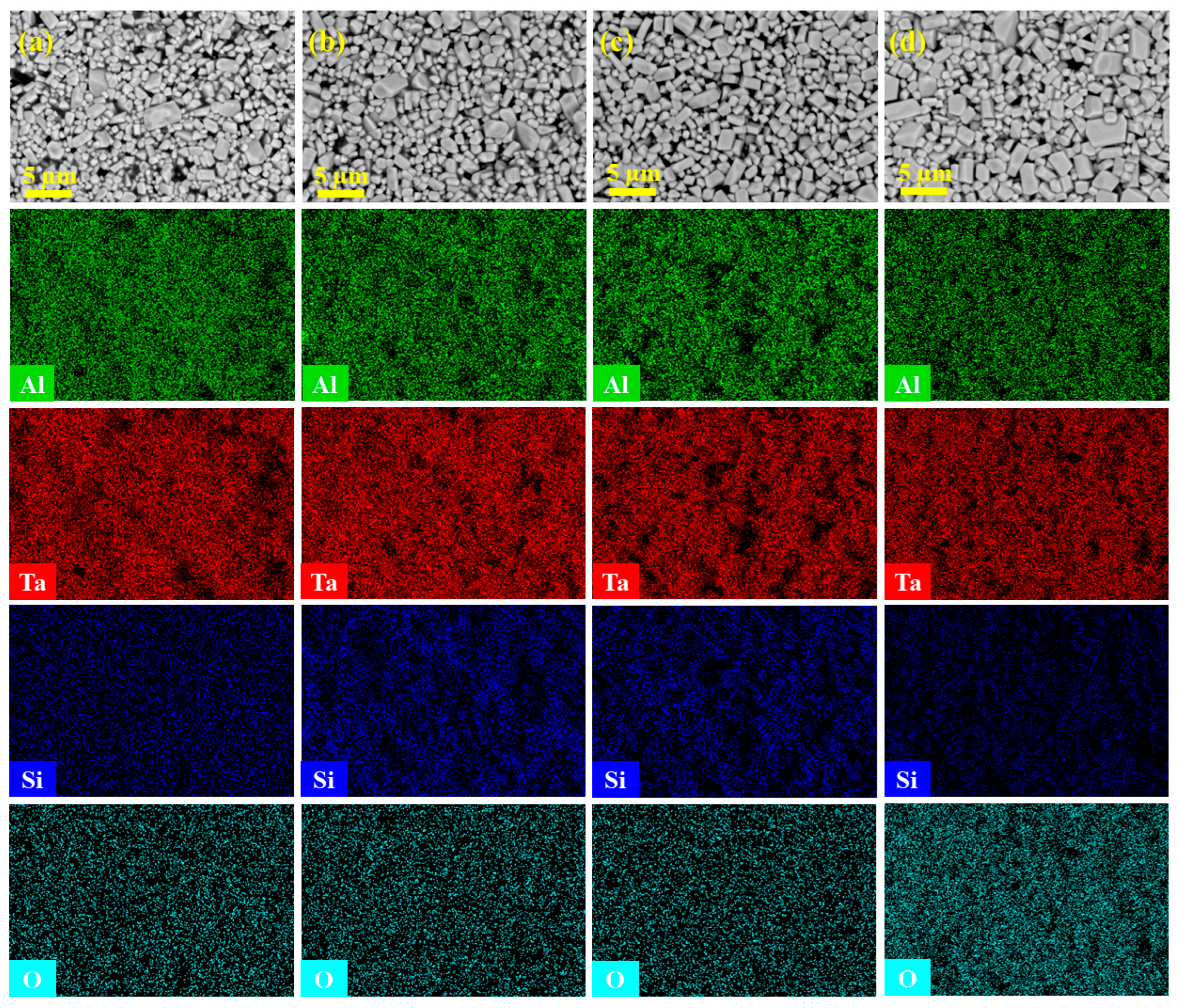
3.2. Microstructures and Elemental Distributions
3.3. Raman Spectra
3.4. Thermal Properties
4. Conclusions
- (1)
- The SiO2-AlTaO4 samples heat-treated at 1400 °C for 30–120 h maintained the monoclinic phase, and Si occupied the interstitial sites to form an interstitial solid solution at 30–60 h, where the Si atom migrated to lattice sites to form a substitutional solid solution at 90–120 h. AlTaO4 exhibited excellent high-temperature stability, and the addition of SiO2 did not alter its crystal structure.
- (2)
- SEM and EDS mapping revealed the uniform distribution of Si, Al, Ta, and O elements in each sample, without obvious segregation. The grain sizes were on the micron scale, gradually increasing in size and becoming more regularly arranged with extended heat treatment time. The XRD, SEM, and EDS results synergistically indicated the good phase stability of AlTaO4.
- (3)
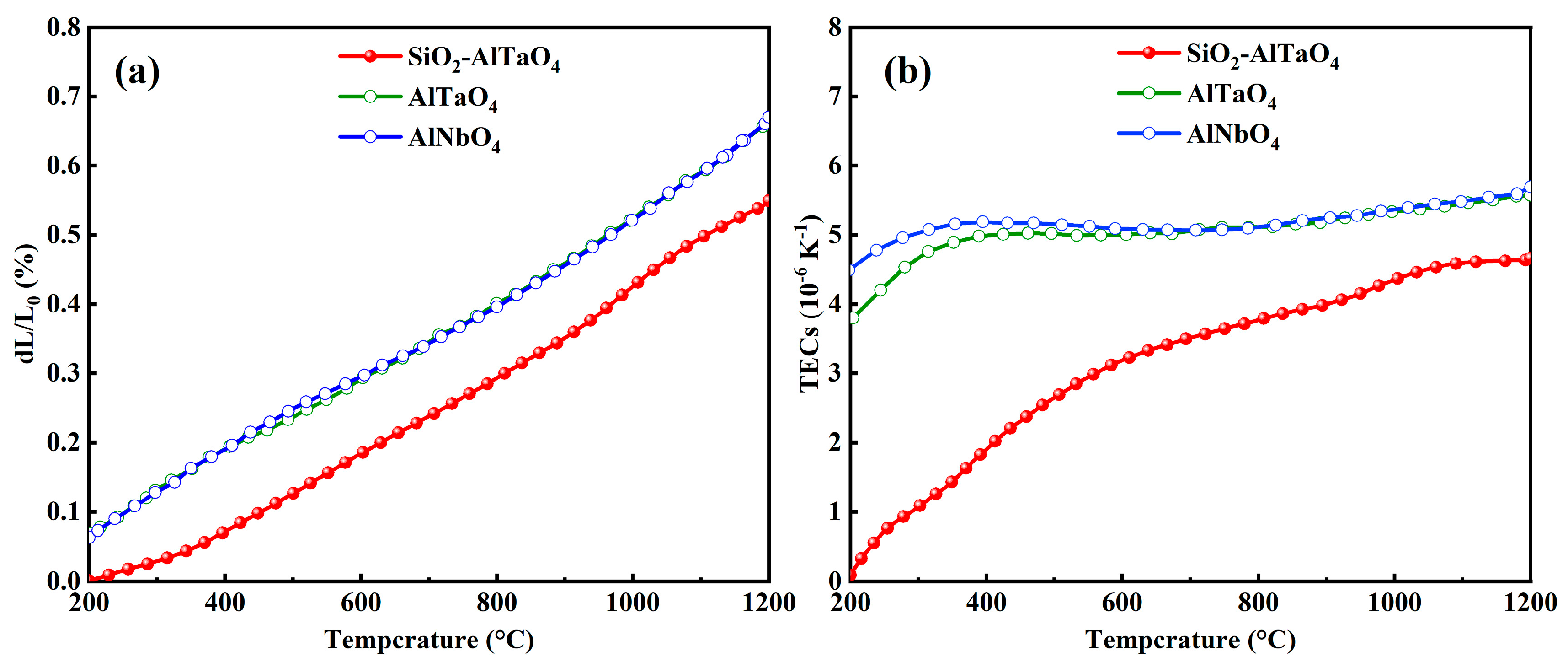
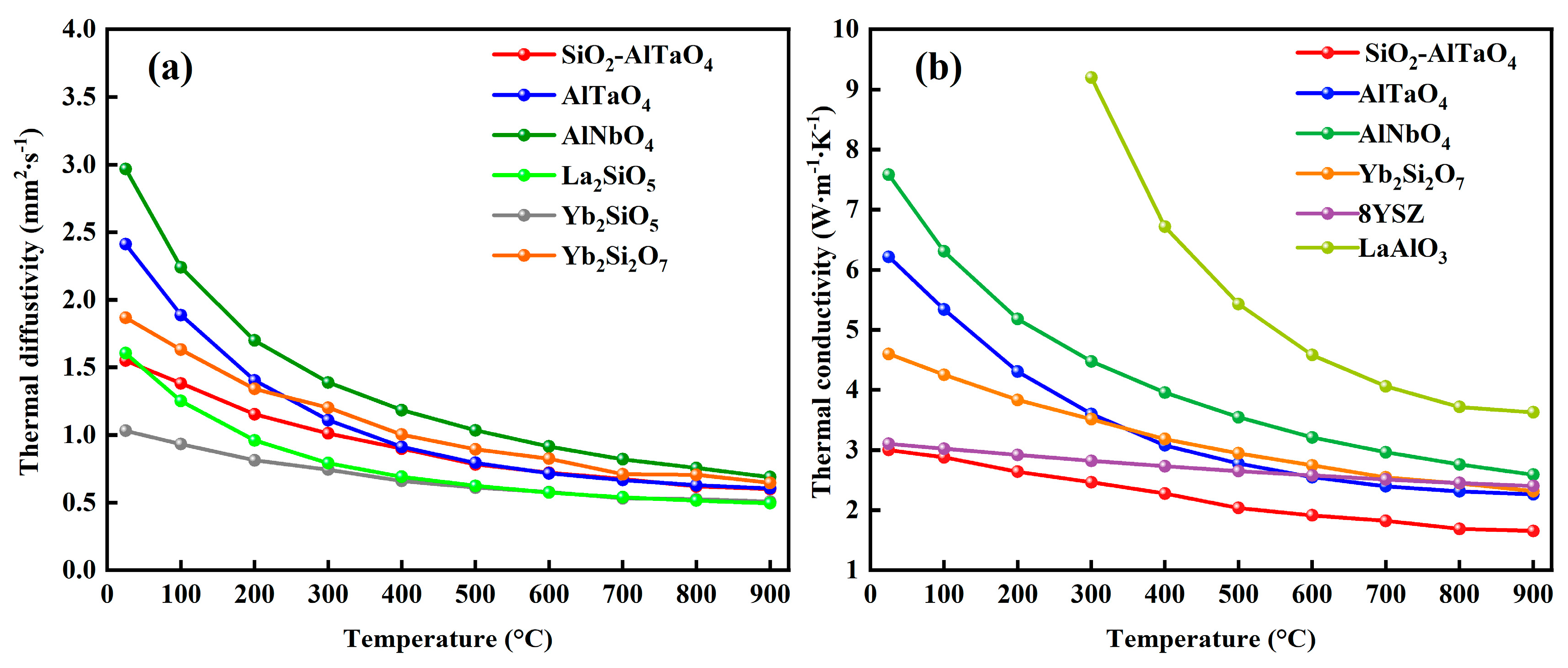
- The TECs of SiO2-AlTaO4 were approximately 4.65 × 10−6 K−1 at 1200 °C, which were lower than those of AlTaO4 and AlNbO4. The SiO2 additions could reduce the TECs of AlTaO4, thereby reducing the thermal stress between AlTaO4 and the Si bond coat. The thermal conductivity at 900 °C was 26.9% lower than that of AlTaO4, and the lowest value reached 1.65 W·m−1·K−1 as the phonon scattering strength was increased. Accordingly, the reaction between SiO2 and AlTaO4 could reduce the TECs and thermal conductivity, which were good for the EBC applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, S.; Wang, Q.Y.; Luo, J.; Chen, R.K. Advanced materials for high-temperature thermal transport. Adv. Funct. Mater. 2020, 30, 1904815. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Chen, L.; Li, B.H.; Feng, J. Rare-earth tantalates for next-generation thermal barrier coatings. Prog. Mater Sci. 2024, 144, 101265. [Google Scholar] [CrossRef]
- Lee, K.N. Current status of environmental barrier coatings for Si-Based ceramics. Surf. Coat. Technol. 2000, 133–134, 1–7. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, Z.K.; Wang, J.M.; Zeng, Y.; Song, W.L.; Xiong, X.; Li, X.C.; Li, T.Q.; Wang, Y.C. Insight into the effect of Ti substitutions on the static oxidation behavior of (Hf,Ti)C at 2500 °C. Adv. Powder Mater. 2024, 3, 100168. [Google Scholar] [CrossRef]
- Sciti, D.; Vinci, A.; Zoli, L.; Galizia, P.; Failla, S.; Mungiguerra, S.; Di Martino, G.D.; Cecere, A.; Savino, R. Propulsion tests on ultra-high-temperature ceramic matrix composites for reusable rocket nozzles. J. Adv. Ceram. 2023, 12, 1345–1360. [Google Scholar]
- Guo, L.X.; Huang, S.W.; Li, W.; Lv, J.S.; Sun, J. Theoretical design and experimental verification of high-entropy carbide ablative resistant coating. Adv. Powder Mater. 2024, 3, 100213. [Google Scholar] [CrossRef]
- He, X.; Yuan, X.H.; Xu, H.; Song, P.; Yu, X.; Li, C.; Huang, T.H.; Li, Q.L.; Lü, K.Y.; Feng, J.; et al. Analysis of structure and microhardness of Al2O3-40 wt.% TiO2/NiCoCrAl gradient coating with in-situ needle-like phase reinforcement after high-temperature treatment. Ceram. Int. 2019, 45, 14546–14554. [Google Scholar] [CrossRef]
- Madar, R. Silicon carbide in contention. Nature 2004, 430, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Kedir, N.; Garcia, E.; Kirk, C.; Gao, J.L.; Guo, Z.R.; Zhai, X.D.; Sun, T.; Fezzaa, K.; Sampath, S.; Chen, W.W. Impact damage of narrow silicon carbide (SiC) ceramics with and without environmental barrier coatings (EBCs) by various foreign object debris (FOD) simulants. Surf. Coat. Technol. 2021, 407, 126779. [Google Scholar] [CrossRef]
- Zou, B.L.; Khan, Z.S.; Fan, X.Z.; Huang, W.Z.; Gu, L.J.; Wang, Y.; Xu, J.Y.; Tao, S.Y.; Yang, K.Y.; Ma, H.M.; et al. A new double layer oxidation resistant coating based on Er2SiO5/LaMgAl11O19 deposited on C/SiC composites by atmospheric plasma spraying. Surf. Coat. Technol. 2013, 219, 101–108. [Google Scholar]
- Zhang, S.C.; Wu, L.H.; Sun, X.K.; Sun, H.R.; Ai, B.; Chen, Y.F. Ultra-low thermal conductivity multilayer composites for insulation in low pressure environments. Adv. Ceram. 2023, 44, 442–450. [Google Scholar]
- Liu, X.F.; He, J.F.; Li, Y.G.; Li, H.; Lei, W.; Jia, Q.L.; Zhang, S.W.; Zhang, H.J. Foam-gelcasting preparation of porous SiC ceramic for high-temperature thermal insulation and infrared stealth. Rare Met. 2023, 42, 3829–3838. [Google Scholar]
- Zhang, J.X.; Zhang, J.; Ye, X.L.; Ma, X.M.; Liu, R.; Sun, Q.L.; Zhou, Y.L. Ultralight and compressive SiC nanowires aerogel for high-temperature thermal insulation. Rare Met. 2023, 42, 3354–3363. [Google Scholar]
- Wright, A.J.; Mock, C.; Sharobem, T.; Sotiropoulos, N.; Dambra, C.; Keyes, B.; Ghoshal, A. Integrated microstructural and chemical approach for improving CMAS resistance in thermal and environmental barrier coatings. Coatings 2025, 15, 680. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, X.; He, D.Y. Research progress of CMAS corrosion and protection method for thermal barrier coatings in aero-engines. China Surf. Eng. 2023, 36, 1–13. [Google Scholar]
- Bryce, K.; Majee, B.P.; Huang, L.P.; Lian, J. A systematic study of thermomechanical properties and calcium-magnesium-aluminosilicate (CMAS) corrosion of multicomponent rare-earth phosphates. J. Adv. Ceram. 2024, 13, 1807–1822. [Google Scholar]
- Zhang, L.Y.; Luo, K.R.; Chen, L.; Wang, J.K.; Li, B.H.; Feng, J. A4Ta2O9 (A = Ca, Mg) tantalate as novel thermal barrier coatings materials with superior calcium-magnesium-alumino-silicate corrosion resistance. J. Eur. Ceram. Soc. 2025, 45, 117090. [Google Scholar] [CrossRef]
- Naraparaju, R.; Schulz, U.; Mechnich, P.; Döbber, P.; Seidel, F. Degradation study of 7wt.% yttria stabilised zirconia (7YSZ) thermal barrier coatings on aero-engine combustion chamber parts due to infiltration by different CaO-MgO-Al2O3-SiO2 variants. Surf. Coat. Technol. 2014, 260, 73–81. [Google Scholar] [CrossRef]
- Niu, Z.B.; Li, D.X.; Jia, D.C.; Yang, Z.H.; Lin, K.P.; Riedel, R.; Colombo, P.; Zhou, Y. Oxidation behavior of amorphous and nanocrystalline SiBCN ceramics—Kinetic consideration and microstructure. Adv. Powder Mater. 2024, 3, 100163. [Google Scholar] [CrossRef]
- Richards, B.T.; Wadley, H.N.G. Plasma spray deposition of tri-layer environmental barrier coatings. J. Eur. Ceram. Soc. 2014, 34, 3069–3083. [Google Scholar] [CrossRef]
- An, K.; Wang, Y.Q.; Sui, Y.; Qing, Y.Q.; Tong, W.; Wang, X.Z.; Liu, C.S. Recent advances of cerium compounds in functional coatings: Principle, strategies and applications. J. Rare Earths 2025, 43, 227–245. [Google Scholar] [CrossRef]
- Xie, Y.S.; Peng, Y.; Deng, Z.Z.; Zhu, Z.; Cheng, Y.; Ma, D.H.; Zhu, L.Y.; Zhang, X.H. Synergistic regulation of temperature resistance and thermal insulation performance of zirconia-based ceramic fibers. Rare Met. 2023, 42, 4189–4200. [Google Scholar] [CrossRef]
- Yang, D.L.; Liu, J.L.; Zhang, J.G.; Liang, X.H.; Zhang, X.F. In situ high-Temperature tensile fracture mechanism of PS-PVD EBCs. Coatings 2022, 12, 655. [Google Scholar] [CrossRef]
- Deijkers, J.A.; Wadley, H.N.G. A duplex bond coat approach to environmental barrier coating systems. Acta Mater. 2021, 217, 117167. [Google Scholar] [CrossRef]
- Cao, G.; Wang, S.Q.; Ding, Z.Y.; Wang, Y.H.; Liu, Z.G.; Ouyang, J.H.; Wang, Y.M.; Wang, Y.J. High temperature corrosion behavior and the inhibition of apatite formation evolving from corrosion of (Y1−xYbx)2SiO5 in water vapor environment. Appl. Surf. Sci. 2022, 601, 154284. [Google Scholar]
- Dong, S.J.; Lü, K.Y.; Wang, Y.H.; Zeng, J.Y.; Yuan, J.Y.; Jiang, J.N.; Deng, L.H.; Cao, X.Q. High-temperature corrosion of HfSiO4 environmental barrier coatings exposed to water vapor/oxygen atmosphere and molten calcium magnesium aluminosilicate. Corros. Sci. 2022, 197, 110081. [Google Scholar]
- Xiang, H.M.; Feng, Z.H.; Li, Z.P.; Zhou, Y.C. Crystal structure, mechanical and thermal properties of Yb4Al2O9: A combination of experimental and theoretical investigations. J. Eur. Ceram. Soc. 2017, 37, 2491–2499. [Google Scholar] [CrossRef]
- Morán-Ruiz, A.; Vidal, K.; Larrañaga, A.; Arriortua, M.I. Characterization of Ln4Al2O9 (Ln = Y, Sm, Eu, Gd, Tb) rare-earth aluminates as novel high-temperature barrier materials. Ceram. Int. 2018, 44, 8761–8767. [Google Scholar] [CrossRef]
- Bryce, K.; Shih, Y.-T.; Huang, L.; Lian, J. Calcium-magnesium-aluminosilicate (CMAS) corrosion resistance of high entropy rare-earth phosphate (Lu0.2Yb0.2Er0.2Y0.2Gd0.2)PO4: A novel environmental barrier coating candidate. J. Eur. Ceram. Soc. 2023, 43, 6461–6472. [Google Scholar] [CrossRef]
- Zhang, P.X.; Wang, E.H.; Guo, C.Y.; Yang, T.; Hou, X.M. High-entropy rare earth phosphates (REPO4, RE = Ho, Tm, Yb, Lu, Dy, Er and Y) with excellent comprehensive properties. J. Eur. Ceram. Soc. 2024, 44, 1873–1879. [Google Scholar] [CrossRef]
- Karaulov, A.G.; Zoz, E.I. Phase formation in the ZrO2-HfO2-Gd2O3 and ZrO2-HfO2-Yb2O3 systems. Refract. Ind. Ceram 1999, 40, 479–483. [Google Scholar] [CrossRef]
- Mikuśkiewicz, M.; Migas, D.; Moskal, G. Synthesis and thermal properties of zirconate, hafnate and cerate of samarium. Surf. Coat. Technol. 2018, 354, 66–75. [Google Scholar] [CrossRef]
- Lopato, L.M.; Red’Ko, V.P.; Gerasimyuk, G.I.; Shevchenko, A.V. ChemInform abstract: Synthesis and properties of the compounds M4Zr3O12 and M4Hf3O12 (M: Rare earth element). ChemInform 1991, 22. [Google Scholar] [CrossRef]
- Mikuśkiewicz, M.; Moskal, G.; Stopyra, M.; Korol, J. Thermal diffusivity and conductivity of Pr, Eu and Ho zirconates. J. Therm. Anal. Calorim. 2025, 150, 1195–1204. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, J.; Wang, H.C.; Wu, J.Z.; Dong, H.; Zhai, B.X.; He, J.; Gao, F. Thermophysical properties of Yb3+-doped nonstoichiometric gadolinium zirconate ceramics. Ceram. Int. 2024, 50, 47813–47823. [Google Scholar] [CrossRef]
- Fan, Y.; Bai, Y.L.; Li, Q.; Lu, Z.Y.; Chen, D.; Liu, Y.C.; Li, W.X.; Liu, B. Compositional design and phase formation capability of high-entropy rare-earth disilicates from machine learning and decision fusion. npj Comput. Mater. 2024, 10, 95. [Google Scholar] [CrossRef]
- Herweyer, L.A.; Opila, E.J. High-temperature Na2SO4 interaction with air plasma sprayed Yb2Si2O7 + Si EBC system: Topcoat behavior. J. Am. Ceram. Soc. 2021, 104, 6496–6507. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.Y.; Wu, P.; Feng, J. Thermal expansion performance and intrinsic lattice thermal conductivity of ferroelastic RETaO4 ceramics. J. Am. Ceram. Soc. 2019, 102, 4809–4821. [Google Scholar] [CrossRef]
- Ogawa, T.; Matsudaira, T.; Yokoe, D.; Kawai, E.; Kawashima, N.; Fisher, C.A.J.; Habu, Y.; Kato, T.; Kitaoka, S. Spontaneously formed nanostructures in double perovskite rare-earth tantalates for thermal barrier coatings. Acta Mater. 2021, 216, 117152. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.Y.; Zheng, X.D.; Feng, J. Characteristics of ferroelastic domains and thermal transport limits in HfO2 alloying YTaO4 ceramics. Acta Mater. 2023, 251, 118870. [Google Scholar] [CrossRef]
- Tian, L.J.; Peng, F.; Song, X.M.; Zheng, W.; Liu, Z.W.; Huang, Y.L.; Zeng, Y. Enhancing the thermodynamic properties of rare-earth niobates through high-entropy and composite engineering. Ceram. Int. 2024, 50, 19488–19501. [Google Scholar] [CrossRef]
- Chen, L.; Luo, K.R.; Li, B.H.; Hu, M.Y.; Feng, J. Mechanical property enhancements and amorphous thermal transports of ordered weberite-type RE3Nb/TaO7 high-entropy oxides. J. Adv. Ceram. 2023, 12, 399–413. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Li, C.R.; Shi, D.D.; Pan, W. Phase transformation inhibition and improved thermo-mechanical properties of rare earth niobates for thermal barrier coatings. Ceram. Int. 2024, 50, 29799–29805. [Google Scholar] [CrossRef]
- Gan, M.D.; Lai, L.P.; Wang, J.K.; Wang, J.; Chen, L.; He, J.J.; Feng, J.; Chong, X. Suppressing the oxygen-ionic conductivity and promoting the phase stability of the high-entropy rare earth niobates via Ta substitution. J. Mater. Sci. Technol. 2025, 209, 79–94. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.T.; Hu, M.Y.; Zhang, L.Y.; Wang, J.K.; Zhang, Z.B.; Liang, X.B.; Guo, J.; Feng, J. Achieved limit thermal conductivity and enhancements of mechanical properties in fluorite RE3NbO7 via entropy engineering. Appl. Phys. Lett. 2021, 118, 071905. [Google Scholar] [CrossRef]
- Song, G.D.; Yang, J.P.; Dong, Y.J.; Cui, C. Failure mechanism of plasma sprayed physical vapor deposition (PS-PVD) thermal barrier coating under high temperature and high velocity aviation kerosene gas thermal shock. Corros. Prot. 2022, 43, 20–25. [Google Scholar]
- Wei, L.L.; Guo, L.; Li, M.Z.; Guo, H.B. Calcium-magnesium-alumina-silicate (CMAS) resistant Ba2REAlO5 (RE = Yb, Er, Dy) ceramics for thermal barrier coatings. J. Eur. Ceram. Soc. 2017, 37, 4991–5000. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Sun, J.; Liu, G.H.; Han, Y.; Liu, W.; Li, Y.; Wang, W.; Liu, X.Y.; Zhang, P.; Pan, W.; et al. High toughness and CMAS resistance of REAlO3/RE2Zr2O7 (RE = La, Nd, Sm, Eu, Gd, and Dy) composites with eutectic composition for thermal barrier coatings. J. Adv. Ceram. 2024, 13, 800–809. [Google Scholar] [CrossRef]
- Li, H.Y.; Jia, Z.J.; Zhang, L.; Tian, Y.; Liu, X.G.; Bao, Y.W.; Wan, D.T. A review on the development of prestressed ceramics. Adv. Ceram. 2024, 45, 100–109. [Google Scholar]
- Chen, L.; Hu, M.Y.; Feng, J. Mechanical properties, thermal expansion performance and intrinsic lattice thermal conductivity of AlMO4 (M = Ta, Nb) ceramics for high-temperature applications. Ceram. Int. 2019, 45, 6616–6623. [Google Scholar] [CrossRef]
- Aladdin Reagents. Available online: https://www.aladdin-e.com/zh_cn/ (accessed on 3 October 2025).
- Bai, Y.; Han, Z.H.; Li, H.Q.; Xu, C.; Xu, Y.L.; Ding, C.H.; Yang, J.F. Structure-property differences between supersonic and conventional atmospheric plasma sprayed zirconia thermal barrier coatings. Surf. Coat. Technol. 2011, 205, 3833–3839. [Google Scholar] [CrossRef]
- Liu, R.X.; Liang, W.P.; Miao, Q.; Zhao, H.; Zhang, X.F.; Zhang, M.; Yan, R.X.; Ramasubramanian, B.; Ramakrishna, S. Revealing the oxidation growth mechanism and crack evolution law of novel Si-HfO2/Yb2Si2O7/Yb2SiO5 environmental barrier coatings during thermal cycling. J. Adv. Ceram. 2024, 13, 1677–1696. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.J.; Li, J.H.; Yang, G.J. Suppressing the phase-transition-induced cracking of SiO2 TGOs by lattice solid solution. J. Eur. Ceram. Soc. 2023, 43, 3201–3215. [Google Scholar] [CrossRef]
- Opila, E.J. Oxidation and volatilization of silica formers in water vapor. J. Am. Ceram. Soc. 2003, 86, 1238–1248. [Google Scholar] [CrossRef]
- Parker, C.G.; Opila, E.J. Stability of the Y2O3-SiO2 system in high-temperature, high-velocity water vapor. J. Am. Ceram. Soc. 2020, 103, 2715–2726. [Google Scholar] [CrossRef]
- Schlichting, K.W.; Padture, N.P.; Klemens, P.G. Thermal conductivity of dense and porous yttria-stabilized zirconia. J. Mater. Sci. 2001, 36, 3003–3010. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. B 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation: A quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570. [Google Scholar] [CrossRef]
- Tian, Z.L.; Lin, C.F.; Zheng, L.Y.; Sun, L.C.; Li, J.L.; Wang, J.Y. Defect-mediated multiple-enhancement of phonon scattering and decrement of thermal conductivity in (YxYb1-x)2SiO5 solid solution. Acta Mater. 2018, 144, 292–304. [Google Scholar] [CrossRef]
- Du, Z.J.; Shan, Z.T.; Qiao, A.; Tao, H.Z.; Yue, Y.Z. Tantalum-lanthanum mixing effect on structural and mechanical properties of aluminate glasses. J. Non-Cryst. Solids. 2025, 650, 123340. [Google Scholar] [CrossRef]
- Xie, M.X.; Xiang, C.Y.; Zeng, X.L.; Zhang, X.S.; Zhang, L.J.; Xiong, J.T. Optimization of the Raman spectroscopic method for crystal orientation and residual stress of aluminum oxide. J. Mater. Res. Technol. 2024, 33, 8265–8276. [Google Scholar] [CrossRef]
- Spitsberg, I.; Steibel, J. Thermal and environmental barrier coatings for SiC/SiC CMCs in aircraft engine applications. Int. J. Appl. Ceram. Technol. 2004, 1, 291–301. [Google Scholar] [CrossRef]
- Ning, D.; Huang, H.J.; Chao, R.X.; Lin, S.; Bi, Y.T. Lattice distortion, mechanical and thermodynamic properties of (TiZrHf)C and (TiZrHf)N ceramics. Appl. Phys. A 2023, 129, 720. [Google Scholar]
- Zhang, H.; Su, J.B.; Duo, S.W.; Zhou, X.; Yuan, J.Y.; Dong, S.J.; Yang, X.; Zeng, J.Y.; Jiang, J.N.; Deng, L.H.; et al. Thermal and mechanical properties of Ta2O5 doped La2Ce2O7 thermal barrier coatings prepared by atmospheric plasma spraying. J. Eur. Ceram. Soc. 2019, 39, 2379–2388. [Google Scholar] [CrossRef]
- Zhu, S.S.; Zhu, J.P.; Ye, S.B.; Yang, K.J.; Li, M.L.; Wang, H.L.; He, J.L. High-entropy rare earth titanates with low thermal conductivity designed by lattice distortion. J. Am. Ceram. Soc. 2023, 106, 6279–6291. [Google Scholar] [CrossRef]
- Tian, Z.L.; Zhang, J.; Zhang, T.Y.; Ren, X.M.; Hu, W.P.; Zheng, L.Y.; Wang, J.Y. Towards thermal barrier coating application for rare earth silicates RE2SiO5 (RE = La, Nd, Sm, Eu, and Gd). J. Eur. Ceram. Soc. 2019, 39, 1463–1476. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, P.; Huang, M.Z.; Han, Y.; Xu, N.; Li, Y.; Zhang, Z.J.; Pan, W.; Wan, C.L. Effect of lattice distortion in high-entropy RE2Si2O7 and RE2SiO5 (RE = Ho, Er, Y, Yb, and Sc) on their thermal conductivity: Experimental and molecular dynamic simulation study. J. Eur. Ceram. Soc. 2023, 43, 6407–6415. [Google Scholar] [CrossRef]
- Moskal, G.; Jasik, A.; Mikuśkiewicz, M.; Jucha, S. Thermal resistance determination of Sm2Zr2O7 + 8YSZ composite type of TBC. Appl. Surf. Sci. 2020, 515, 145998. [Google Scholar] [CrossRef]
- Vourdas, N.; Marathoniti, E.; Pandis, P.K.; Argirusis, C.; Sourkouni, G.; Legros, C.; Mirza, S.; Stathopoulos, V.N. Evaluation of LaAlO3 as top coat material for thermal barrier coatings. Trans. Nonferrous Met. Soc. China 2018, 28, 1582–1592. [Google Scholar] [CrossRef]


| Sample | a (Å) | b (Å) | c (Å) | β (°) | V (Å3) | ρ (g·cm−3) | Porosity |
|---|---|---|---|---|---|---|---|
| 30 h | 12.135 | 3.776 | 6.449 | 107.730 | 281.469 | 6.238 | 30.3% |
| 60 h | 12.136 | 3.779 | 6.449 | 107.710 | 281.747 | 6.232 | 36.1% |
| 90 h | 12.135 | 3.777 | 6.449 | 107.720 | 281.559 | 6.236 | 39.7% |
| 120 h | 12.148 | 3.766 | 6.458 | 107.705 | 281.455 | 6.238 | 38.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Zhang, L.; Chen, L.; Wang, J.; Gao, Z.; Feng, J. Influences of SiO2 Additions on the Structures and Thermal Properties of AlTaO4 Ceramics as EBC Materials. Coatings 2025, 15, 1204. https://doi.org/10.3390/coatings15101204
Wu B, Zhang L, Chen L, Wang J, Gao Z, Feng J. Influences of SiO2 Additions on the Structures and Thermal Properties of AlTaO4 Ceramics as EBC Materials. Coatings. 2025; 15(10):1204. https://doi.org/10.3390/coatings15101204
Chicago/Turabian StyleWu, Bingyan, Luyang Zhang, Lin Chen, Jiankun Wang, Zipeng Gao, and Jing Feng. 2025. "Influences of SiO2 Additions on the Structures and Thermal Properties of AlTaO4 Ceramics as EBC Materials" Coatings 15, no. 10: 1204. https://doi.org/10.3390/coatings15101204
APA StyleWu, B., Zhang, L., Chen, L., Wang, J., Gao, Z., & Feng, J. (2025). Influences of SiO2 Additions on the Structures and Thermal Properties of AlTaO4 Ceramics as EBC Materials. Coatings, 15(10), 1204. https://doi.org/10.3390/coatings15101204






