Abstract
To reduce the cost of tools operated in extreme environments, we developed films with excellent corrosion/oxidation resistance. Two high-entropy nitride films, (AlCrSi0.3TiZr)N and (AlCr1.5Si0.3TiZr0.5)N, were deposited using reactive DC magnetron sputtering under different substrate biases. The films exhibited a maximum hardness of 32.5 GPa ((AlCrSi0.3TiZr)N) and 35.3 GPa ((AlCr1.5Si0.3TiZr0.5)N) when deposited at −150 V, corresponding to 27 and 142% increases compared to those deposited at 0 V. This indicates that the bias strengthened (AlCr1.5Si0.3TiZr0.5)N (higher Cr/Zr ratio) more significantly. The enhancement of the mechanical properties was highly correlated with the interstitial point defects and densification of the film microstructures. The corrosion resistance of the films deposited on 6061 Al alloy substrate under different biases was tested in 0.1 M H2SO4. (AlCrSi0.3TiZr)N and (AlCr1.5Si0.3TiZr0.5)N displayed the lowest corrosion currents of 0.75 and 0.19 μA/cm2 when deposited at −100 and −150 V, respectively. These values are two orders of magnitude lower than that of the uncoated substrate. The (AlCr1.5Si0.3TiZr0.5)N film showed better oxidation resistance than the (AlCrSi0.3TiZr)N film and remained partially oxidized after heat treatment at 1000 °C. The (AlCr1.5Si0.3TiZr0.5)N film deposited at −150 V exhibits excellent mechanical properties and corrosion/oxidation resistances, making it suitable for protecting tools operating in harsh environments.
1. Introduction
The corrosion, oxidation, and abrasion of materials operating in harsh environments are major sources of cost for various industrial applications. For example, pumps, valves, and molds are commonly operated at high temperatures and are subjected to corrosive and abrasive substances. Other applications, such as aircraft engines, automotive transmission components, marine propellers, and mining drills, are also exposed to the threats of material corrosion, oxidation, and abrasion [1,2]. The deterioration of components and tools poses safety concerns and causes economic losses due to maintenance costs. Consequently, the damage prevention and prolonged durability of components and tools have become major challenges in material science. A standard solution to these issues is to deposit protective coatings onto the surfaces of the components and tools. Thus, new, more effective anti-corrosion and anti-oxidation hard coatings are urgently needed.
Various techniques can be used to prepare protective coatings for components and tools, such as anodization, cold spraying, and paint baking [3]. However, coatings synthesized using these methods may be rough, porous, soft, and non-uniform, resulting in poorer mechanical properties and corrosion resistance. In contrast, coatings prepared via physical vapor deposition (PVD) exhibit good uniformity and excellent mechanical properties. Although PVD is a line-of-site process that cannot be applied to tools with complex geometries, the process is clean and environmentally friendly and does not release hazardous substances. Therefore, PVD is a promising approach for improving the mechanical properties and corrosion resistance of components and tools.
TiN is the most commonly used hard-coating material because of its high hardness and chemical inertness. However, it oxidizes at temperatures above 600 °C, which hinders its use in high-temperature applications [4]. To address this issue, alloying TiN with a third element, such as Al, can improve its oxidation resistance. This is because the formation of a dense Al2O3 layer at high temperatures retards the diffusion of O2 into the interior film [5,6]. Therefore, compositional modification has become an effective strategy for improving the performance of hard coatings and broadening their range of applications.
In 2004, the “high-entropy alloys” (HEAs) proposed by Yeh et al. [7] led to a breakthrough in the alloy design approach. HEAs contain at least five elements, each with a concentration between 5 and 35 at%. The high mixing entropy of such multicomponent systems significantly stabilizes the formation of a single phase, which explains the nomenclature of these alloys. Various HEAs have been studied for their superior mechanical properties, such as high strength, ductility, and corrosion and wear resistance, making them a promising field in the 21st century.
The concept of HEAs also extends to the field of surface coatings. Various high-entropy alloy [8], nitride [9,10,11], carbide [12], and oxynitride and carbonitride [13,14] films have been synthesized to fulfill different requirements. In particular, because of their distinctive compositions and excellent mechanical properties, high-entropy nitride films (HENFs) have been widely investigated to prevent corrosion, oxidation, and abrasion. For example, Shen et al. prepared (Al0.34Cr0.22Nb0.11Si0.11Ti0.22)50N50, which showed superior oxidation resistance compared to traditional nitrides [10]. Lo et al. deposited (AlCrNbSiTiMo)N at different substrate biases and demonstrated its outstanding anti-wear performance at high temperatures [11].
In this study, two non-equimolar HENFs were deposited via reactive DC magnetron sputtering (DCMS). Magnetron sputtering is the most commonly used PVD method because the stoichiometry of the target elements can be accurately maintained during deposition. Although the target used in DCMS is limited to electrical conductors, the advantage of DCMS includes its higher deposition rate compared to that of radio frequency (RF) magnetron sputtering or high-power impulse magnetron sputtering (HiPIMS) [15]. Additionally, the application of a substrate bias can refine the microstructures and strengthen the mechanical properties of thin films [16]. For example, Huang et al. deposited (AlCrNbSiTiV)N films and achieved a maximum hardness of 42 GPa by adjusting the substrate bias [17]. Zhang et al. synthesized (CrNbTiAlV)Nx films with a compact microstructure under a substrate bias of −126 V, and the film exhibited the lowest corrosion current of 0.013 uA/cm2 in 3.5 wt% NaCl solution [18]. Therefore, we also applied substrate bias in this study to deposit films with excellent mechanical properties and corrosion resistance.
In our group, the effect of N content and substrate bias on the corrosion resistance of equimolar (AlCrSiTiZr)100−xNx has been previously studied [9]. Although the addition of Si can facilitate the formation of a nanocomposite structure and the enhancement of mechanical properties [19], if the Si content is too high, the mechanical properties deteriorate instead because the amorphous region becomes too large at the expense of the crystalline size. Hence, according to a previous study [20], the optimum Si content in the compositional metal elements is approximately 7 at%. With these facts in mind, we designed our first target, AlCrSi0.3TiZr (or Al23.25Cr23.25Si7Ti23.25Zr23.25 in percentage).
Because Cr is cheaper than Zr, increasing the Cr content and decreasing the Zr content can lower the cost of the materials. In addition, Cr can form a dense oxide that improves the oxidation resistance of nitride films, whereas Zr forms a porous oxide that may have the opposite effect [21,22]. Other studies have shown that with an increasing amount of Cr and a decreasing amount of Zr, the oxidation resistance of the nitride film could be significantly improved [23,24]. Therefore, we proceeded to increase the Cr content and decrease the Zr content to improve the high-temperature applicability of the film. Consequently, we designed our second target, AlCr1.5Si0.3TiZr0.5 (or Al23.25Cr34.9Si7Ti23.25Zr11.6 in percentage). This ratio was chosen to maximize the Cr content while keeping each element within the range between 5 and 35 at%, in accordance with the definition of high-entropy alloys [25].
Because the main difference between these two targets is their “Cr/Zr” ratio, which is “one” in AlCrSi0.3TiZr and “three” in AlCr1.5Si0.3TiZr0.5, we denoted the nitride film of the former as “low-Cr/Zr film” and that of the latter as “high-Cr/Zr film” to avoid confusion.
The films were first deposited on Si (100) substrates at different substrate biases to characterize their chemical composition, crystal structures, microstructures, and mechanical properties. Subsequently, they were deposited on 6061 Al alloy and immersed in a 0.1 M H2SO4 solution to test the corrosion resistance. The H2SO4 solution was selected to simulate an industrial environment where SO2 gas might exist and dissolve in water to form H2SO4(aq) [26]. The films were also deposited on SiO2/Si substrates and annealed in air at different temperatures for two hours to examine their oxidation resistances. The result of this study demonstrated the enhancement extent of the substrate bias to HENFs with different Cr/Zr ratios, providing a design strategy for high-entropy hard coatings.
2. Materials and Methods
2.1. Target and Film Preparation
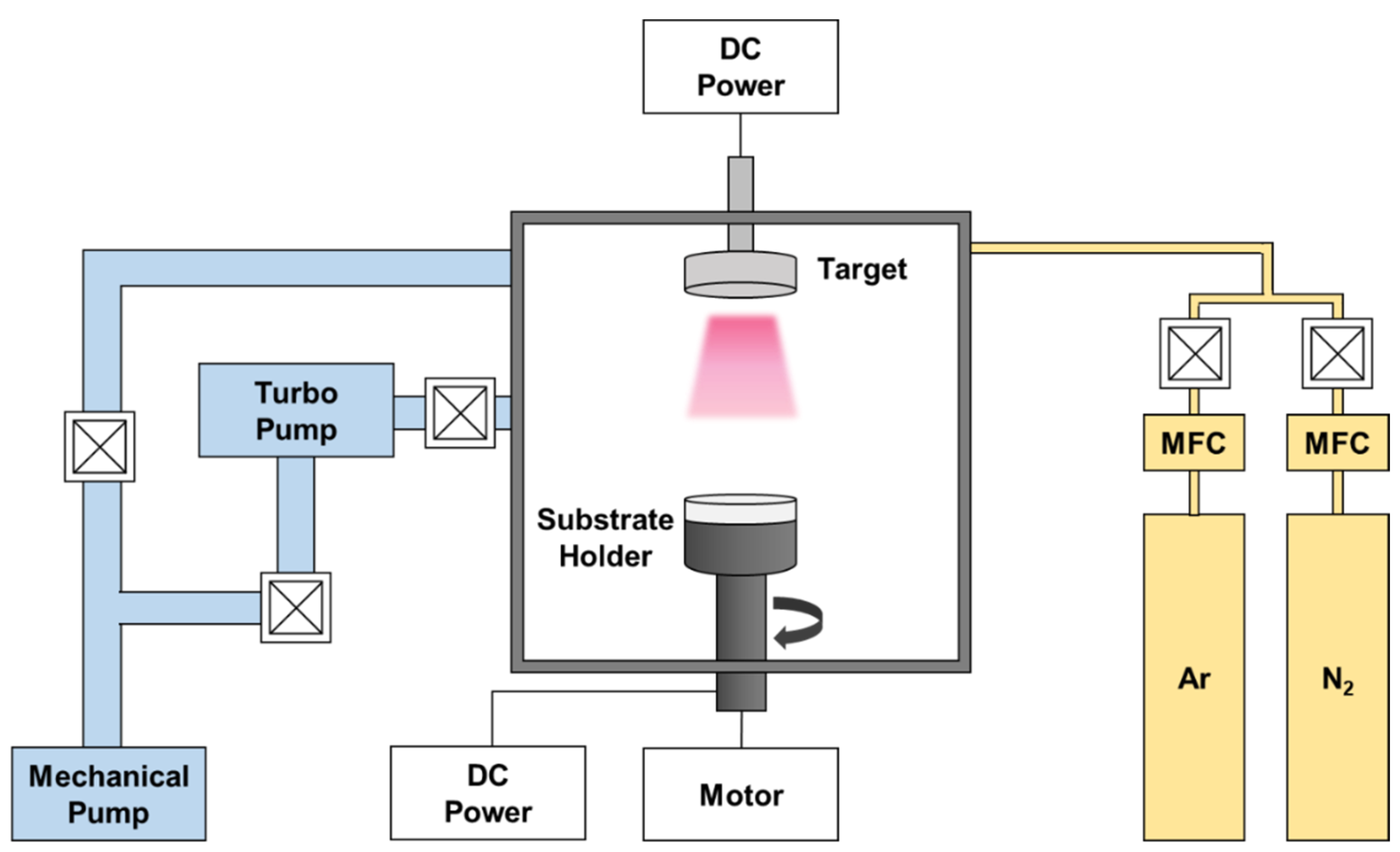
The AlCrSi0.3TiZr and AlCr1.5Si0.3TiZr0.5 targets were prepared by vacuum arc melting. The purity of all the raw elements (materials) was greater than 99.9%. After each arc-melting process, the as-cast ingot was flipped upside down and remelted. This cycle was repeated at least seven times to ensure alloy homogeneity. Subsequently, the alloy ingots were polished and machined into disc-shaped targets, each with a two-inch diameter. The films were deposited on various substrates by reactive DCMS; the detailed deposition parameters are listed in Table 1, and the schematic of the DCMS system is shown in Figure 1. Si (100) substrates were used for various film characterizations, and 6061 Al alloy was used to test the combined corrosion resistance of the films and substrates. SiO2/Si substrates were used for heat treatment because the pre-grown SiO2 with a thickness of about 300 nm can act as a diffusion barrier to prevent the interdiffusion of Si atoms and film atoms during heat treatment. All substrates were cleaned ultrasonically in the order of acetone, ethanol, and deionized water for 10 min each. Before deposition, the substrates were further cleaned with Ar plasma for 30 min under a bias of −500 V, and the target was pre-sputtered for 10 min to remove surface impurities. The deposition time was controlled so that each film thickness was at least 1 μm.

Table 1.
Film deposition parameters.
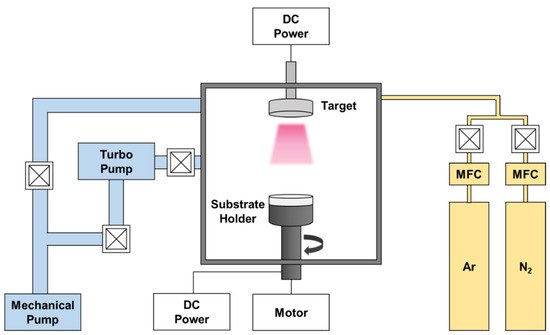
Figure 1.
Schematic of the DCMS system.
2.2. Film Characterization
Chemical compositions, crystal structures, hardness and Young’s modulus, and residual stress measurements of the films were all conducted using Si (100) substrates. The chemical compositions of the films were determined using an electron probe microanalyzer (JEOL JAX-8800, Peabody, MA, USA). The crystal structures of the films were characterized using a grazing incidence X-ray diffractometer (MAC Science MXP18, Tokyo, Japan) with Cu Kα radiation. The X-ray wavelength was 1.5405 Å, and the operational voltage and current were 40 kV and 150 mA, respectively. The incident angle was fixed at 1° with a scan speed of 4°/min. The lattice constant was determined using the Nelson–Riley extrapolation function [27]. At least three diffraction peaks were used for the linear regression of the lattice constant against , where is the Bragg angle. The intercept obtained from the linear regression was taken as the value of the lattice constant, and the standard error of the intercept was viewed as the measurement uncertainty. If fewer than three diffraction peaks were present, the lattice constant was calculated using the diffraction peak with the highest angle for higher precision. Based on the literature, its measurement uncertainty was estimated at approximately 0.001 nm [27]. The average grain size was estimated using the Scherrer equation [28].
where is the grain size, is a constant (assumed to be 0.9), is the X-ray wavelength, is the full width at half maximum (FWHM) of the peak, and is the Bragg angle. The surface and cross-sectional morphologies were obtained using field-emission scanning electron microscopy (FESEM; JEOL JSM-6500F, Tokyo, Japan). The hardness and Young’s moduli of the films were determined using a nanoindenter (NanoTest 600, Micro Materials, Wrexham, UK) with a Berkovich tip and an applied load of 5 mN. Before the test, air indentation was performed to calibrate the spring constant of the load cell spring. Then, the optic probe tip offset and area function were calibrated using a polycarbonate and a fused quartz substrate, respectively. Following the ISO 14577 standard [29], the uncertainty can be constrained within 5%. The penetration depth was less than a tenth of the film thickness to avoid the influence of the substrate. In addition, the contact depth was also controlled to be greater than 40 nm, below which the calibration value is unreliable due to the influence of the tip shape. The residual stress of the films was measured via the substrate curvature method using the Stoney equation [30].
where is the film stress, and are Young’s modulus and Poisson’s ratio of the substrate, respectively, is the substrate thickness, is the film thickness, and and are the average radii of curvature before and after deposition, respectively. The surface topography was analyzed using atomic force microscopy (AFM, NS3a controller with a D3100 stage, Veeco/Digital Instruments, Fullerton, CA, USA).
2.3. Corrosion and Oxidation Resistance Tests
The anti-corrosion properties of the films were tested using an electrochemical workstation (CHI Model 600 Series, CH Instruments, Austin, TX, USA) equipped with a three-electrode system. The sample, Ag/AgCl in 3 M KCl solution, and platinum were used as the working, reference, and auxiliary electrodes, respectively. A test solution of 0.1 M sulfuric acid was used. The solution was deoxygenated using N2 gas for 15 min before the polarization tests, and the N2 gas was continuously passed above the solution surface during the tests to avoid further oxygen dissolution. Potentiodynamic polarization curves were obtained by scanning from −0.5 to 1.5 V relative to the open circuit potential (OCP) at a scan rate of 1 mV/s. Before testing, the sample surface was treated by applying a voltage of −0.2 VSHE for 600 s and a waiting period of 300 s to stabilize the OCP. The corrosion potential (Ecorr) and corrosion current (icorr) were determined by extrapolating the linear region of the Tafel curves.
The oxidation resistance of the films was examined by annealing the samples in air at temperatures ranging from 600 to 1000 °C for 2 h. After the heat treatment, the samples were analyzed using grazing incidence X-ray diffractometry (GIXRD, MAC Science MXP18, Tokyo, Japan) and scanning electron microscopy (SEM, JEOL JSM-6500F, Tokyo, Japan) to determine the extent of oxidation.
3. Results and Discussion
3.1. Basic Characterization
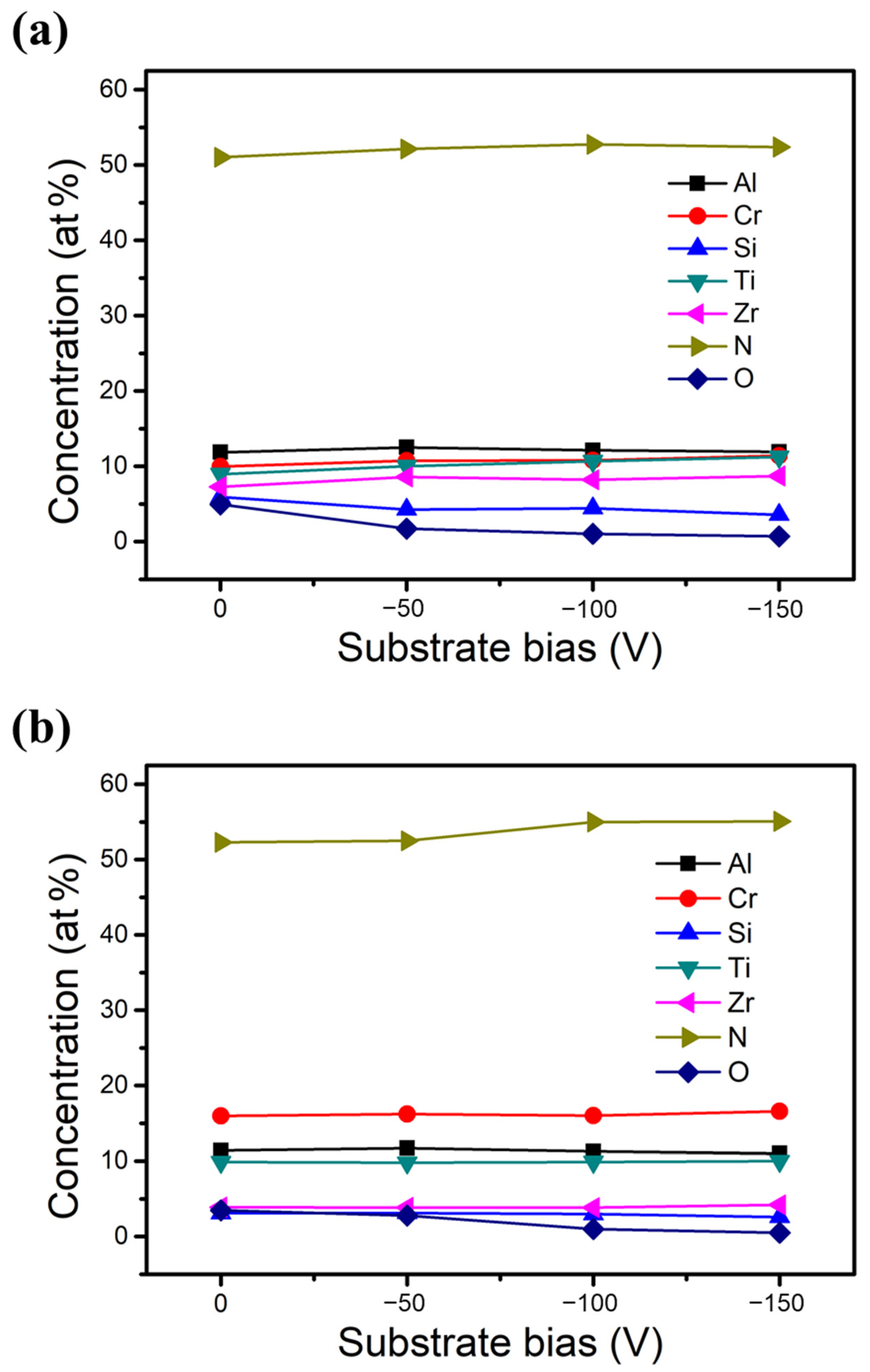
Figure 2 illustrates the chemical compositions of the two HENFs determined using EPMA with increasing substrate bias from 0 to −150 V. The nitrogen concentration for each HENF was between 51 at% and 55 at% under all substrate biases, and the target elements showed only a small variation. These results indicate that the two designed HENFs were slightly over-stoichiometric, regardless of the substrate bias. Additionally, a slight increase in the N concentration and a slight decrease in the O concentration were observed in both HENFs with increasing bias, suggesting that more N atoms were activated to bond with the target elements in the presence of the substrate bias. Regarding the other elements, the Si concentration decreased, whereas the Zr concentration increased with increasing bias. This trend can be ascribed to the re-sputtering effect after applying the bias. The re-sputtering effect occurs when energetic ions bombard the film atoms, causing the already deposited atoms to be sputtered again [31]. This effect becomes more pronounced at high bias, where the ions have higher energy of bombardment. In addition, light elements such as Si are more likely to be re-sputtered than larger and heavier atoms such as Zr, thereby leading to a decrease in the Si concentration and an increase in the Zr concentration at high bias [32]. Furthermore, the concentrations of Si and Zr in the low-Cr/Zr film varied more significantly than those in the high-Cr/Zr film. This is because the ions of the low-Cr/Zr film contained more heavy atoms (Zr) during deposition, increasing the energy of bombardment, thus intensifying the re-sputtering effect.
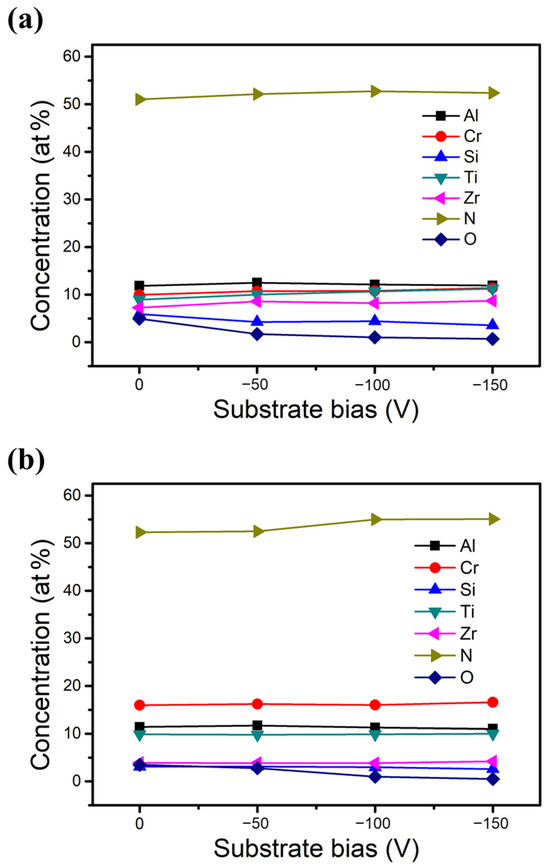
Figure 2.
Chemical compositions of the two deposited HENFs. Plots of concentration versus substrate bias of (a) (AlCrSi0.3TiZr)N and (b) (AlCr1.5Si0.3TiZr0.5)N.
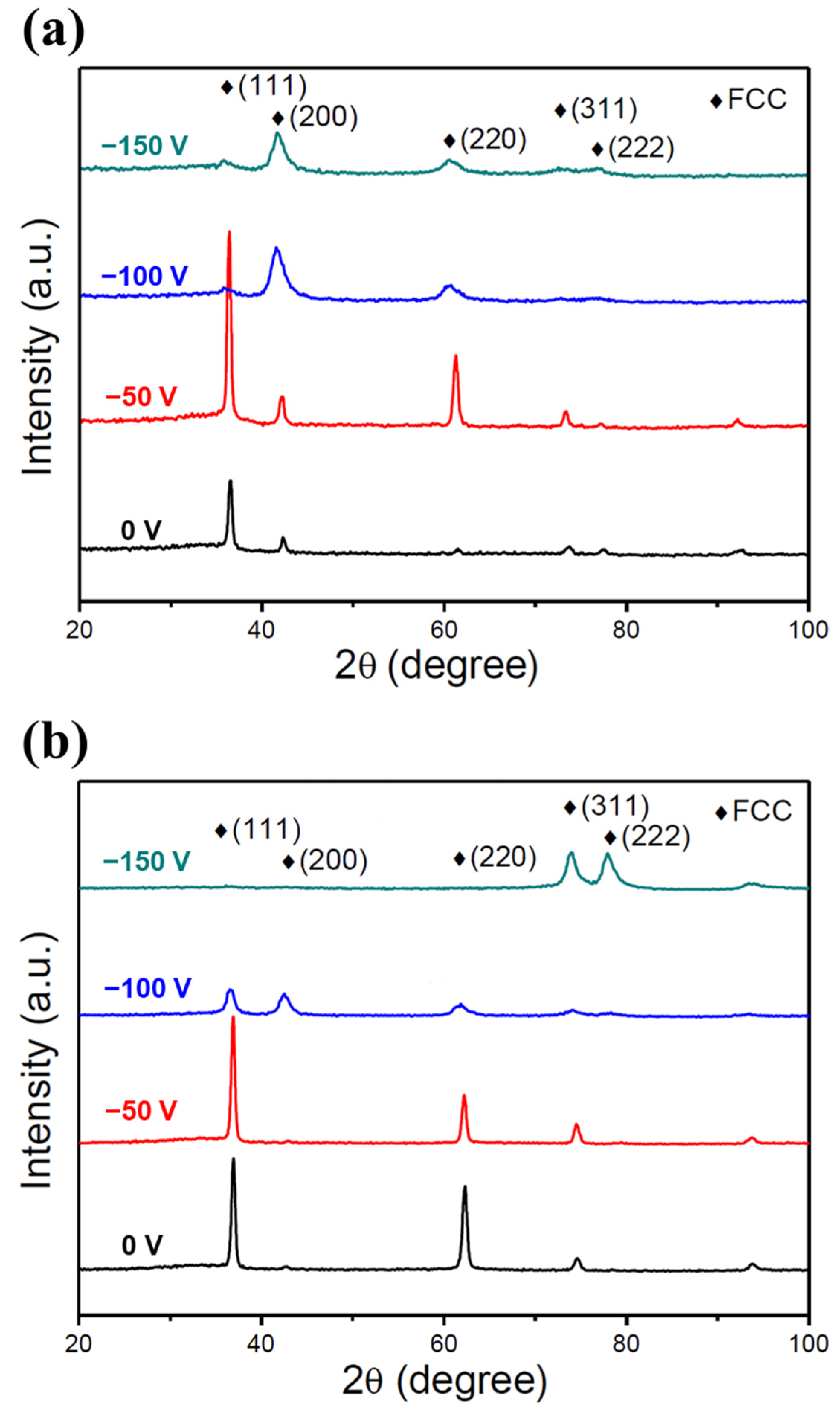
Figure 3a,b show the GIXRD patterns of the low- and high-Cr/Zr films deposited under different substrate biases. All of the films exhibited a single NaCl-type FCC (or B1) structure familiar to various previously reported HENFs [9,11,33]. The formation of a homogeneous single-phase structure is attributed to the high mixing entropy of the constituent elements. The binary nitrides of the constituent elements have different crystal structures. Specifically, CrN, TiN, and ZrN have an FCC structure, AlN an HCP structure, and Si3N4 an HCP or amorphous structure. Despite these differences, a high mixing entropy significantly reduces the mixing free energy and the limited kinetics during PVD (extremely high cooling rate). It thus stabilizes the solid solution phase of the FCC (B1) structure.
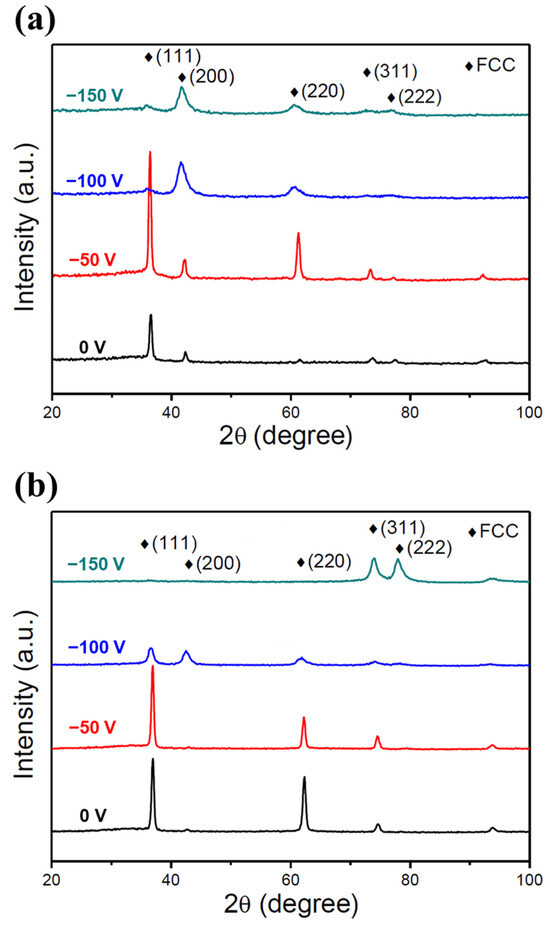
Figure 3.
GIXRD analysis of the two HENFs. GIXRD patterns of the (a) (AlCrSi0.3TiZr)N and (b) (AlCr1.5Si0.3TiZr0.5)N films deposited at different substrate biases.
The GIXRD patterns were also used to obtain the lattice constant and grain sizes of the films. Table 2 lists the lattice constants and grain sizes of all of the film samples calculated using Bragg’s and Scherrer’s equations. As the substrate bias increased, the lattice constant increased, whereas the grain size decreased. The increase in the lattice constant can be explained by the “ion-peening effect” [30]. Specifically, because the bombardment of energetic particles became more marked when the substrate bias increased, the number of point defects, e.g., Frenkel pairs and anti-Schottky defects, increased correspondingly [34]. These defects would make the unit cell expand to accommodate them, causing an increase in the lattice constant. This phenomenon would also create a strain field that made dislocation motion difficult, increasing the hardness of the films, as discussed in Section 3.2. The decrease in the grain size was ascribed to the increase in the nucleation sites with increasing substrate bias. It has been suggested that new embryos could nucleate on the defects induced by ion bombardment [35]. Hence, from a kinetics perspective, nucleation rather than grain growth could be the dominant process at high substrate bias, leading to a smaller grain size.

Table 2.
Lattice constants with standard errors (SEs) and grain sizes of the two HENFs derived from GIXRD analysis.
Further comparison of the two HENFs revealed that the low-Cr/Zr films had larger lattice constants and smaller grain sizes than the high-Cr/Zr films. The larger lattice constants of the former films could stem from their higher Zr contents because Zr has a larger atomic radius than Cr (Zr: 1.55 Å; Cr: 1.40 Å). The smaller grain sizes of the low-Cr/Zr films can also be attributed to the larger amount of Zr, which is strongly associated with the film morphology.
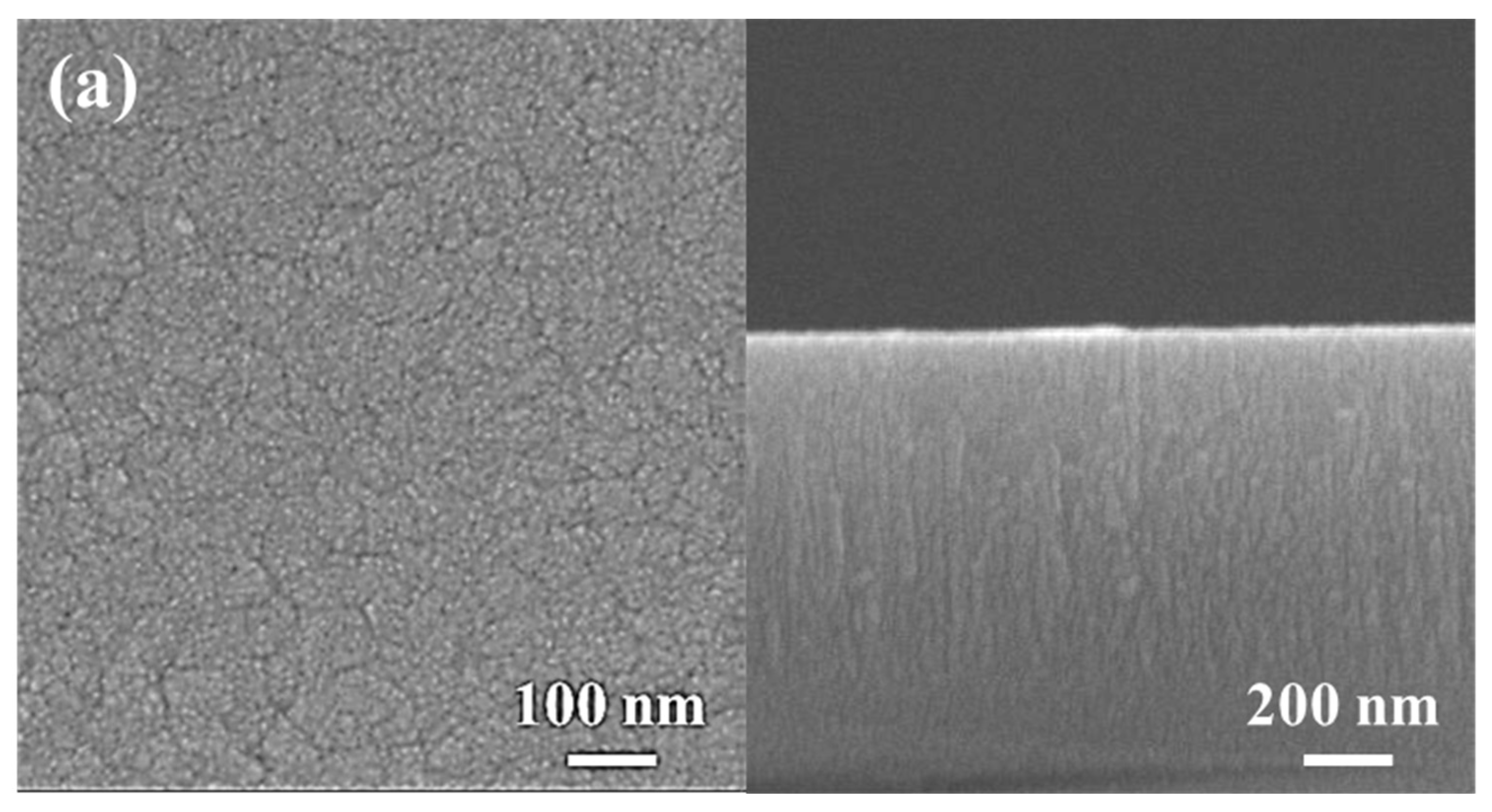
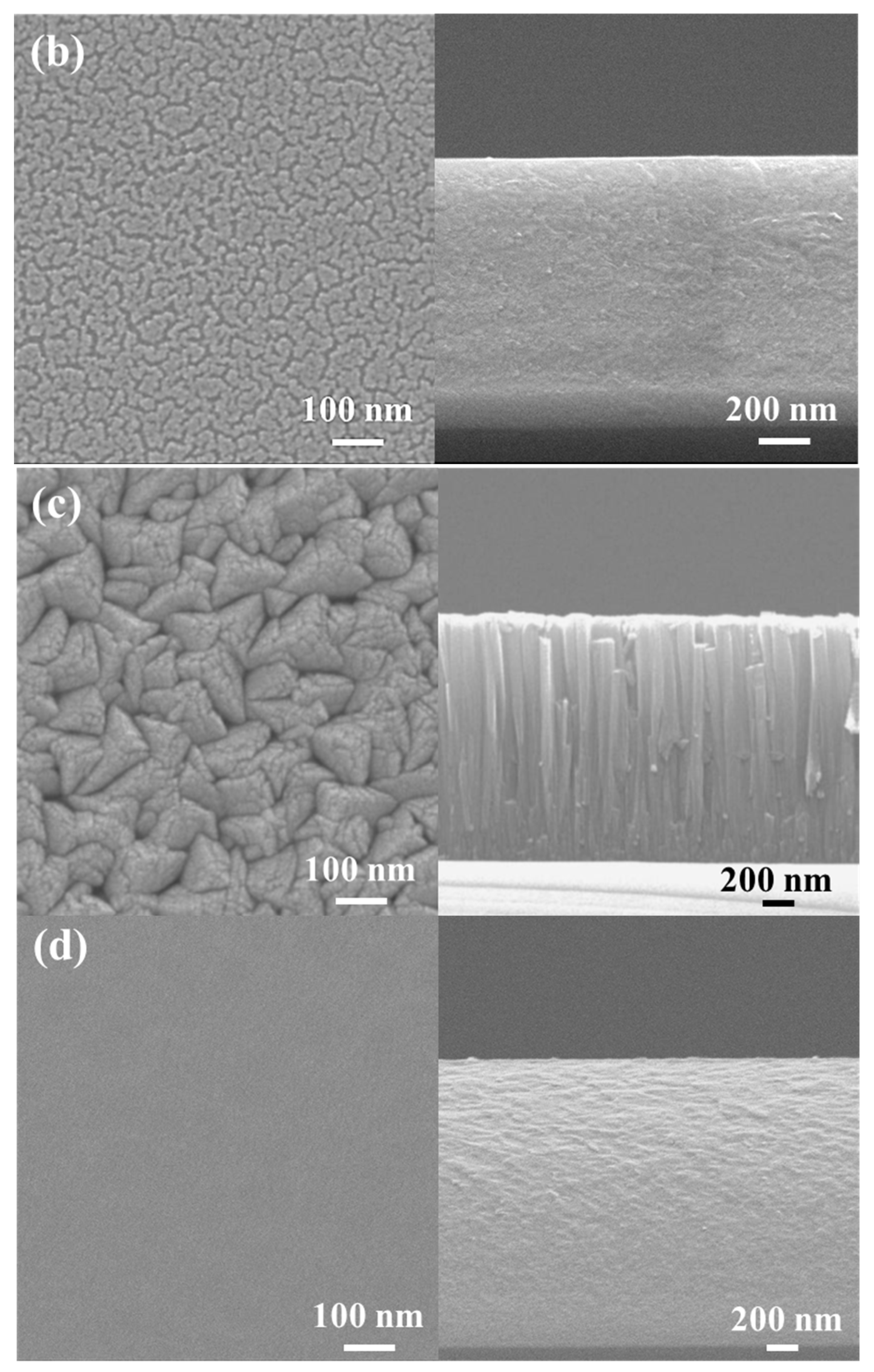
Figure 4 shows the surface and cross-sectional morphologies of the low-Cr/Zr film deposited at 0 and −150 V. In the absence of a substrate bias (0 V), the film surface was composed of clusters approximately 80 nm in size (Figure 4a), and a fine columnar structure can be seen on the film cross-section. After applying the bias (−150 V), the film surface became quite smooth and had interconnected clusters with a feature width of approximately 20 nm (Figure 4b). The cross-section also became dense and relatively smooth without any columnar features.
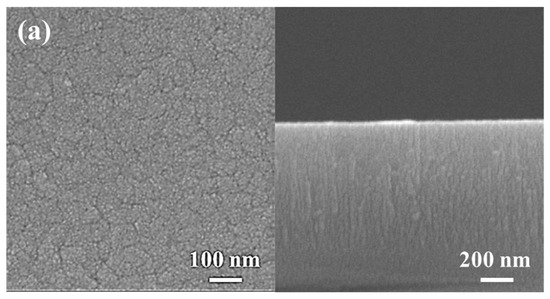
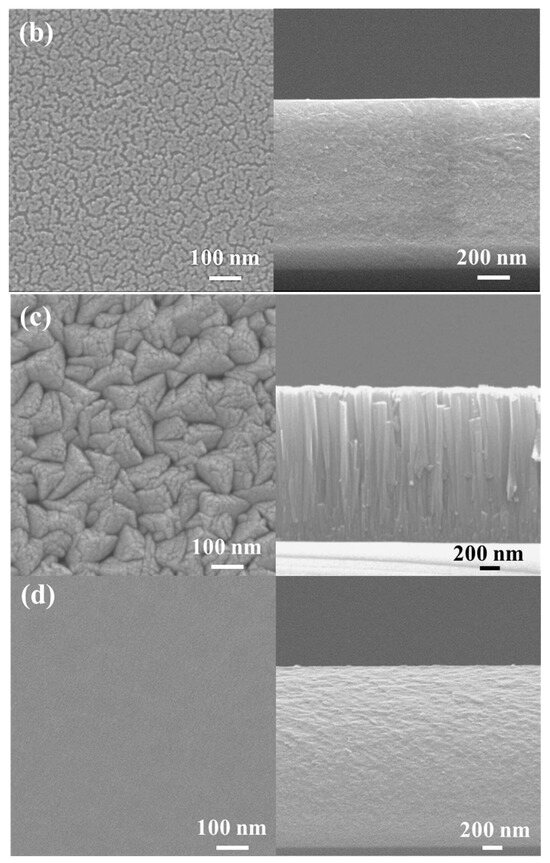
Figure 4.
SEM analysis of (AlCrSi0.3TiZr)N and (AlCr1.5Si0.3TiZr0.5)N. Plan-view (left) and cross-sectional (right) SEM images of the (AlCrSi0.3TiZr)N films at (a) 0 V and (b) −150 V and the (AlCr1.5Si0.3TiZr0.5)N films at (c) 0 V and (d) −150 V.
A similar transition was observed in the high-Cr/Zr film. Without a bias, the film surface comprised pyramid-like clusters approximately 100 nm in size (Figure 4c), and the cross-section was composed of a columnar structure approximately 100 nm in width. When a bias (−150 V) was applied, the surface became flat and featureless, and the columnar structure transformed into a compact structure with no columnar features (Figure 4d).
The transitions in microstructure and composition can be explained by the increased adatom mobility when a substrate bias is applied. High-energy ion bombardment can facilitate the diffusion of the atoms, thus filling the voids during film growth. In addition, the strong re-sputtering effect at high bias reduces the grain growth rate, resulting in fine equiaxed grains. Therefore, the film structure became more compact and denser, consistent with the structural zone model proposed by Messier et al. [16].
Comparing the morphologies of the two HENFs under no substrate bias, the low-Cr/Zr film (Figure 4a) has a fine columnar structure, whereas the high-Cr/Zr film has a defined large columnar structure (Figure 4c). This discrepancy can also be explained by the differences in adatom mobility. During deposition, a higher target element-to-Ar mass ratio (MMe/MAr) suggests a higher energy of the sputtered target atoms and backscattered Ar atoms [36]. These energetic atoms have higher mobility when they reach the substrate, resulting in the densification and renucleation of the film. The low-Cr/Zr film had a higher Zr content, and Zr had the largest mass among the target elements, indicating a higher MMe/MAr ratio. This also explains the finer and denser morphology of the low-Cr/Zr film and the smaller grain sizes calculated from the XRD analysis, as listed in Table 2. A similar morphological change was observed when Zr was added to a CrN film [37].
3.2. Mechanical Properties
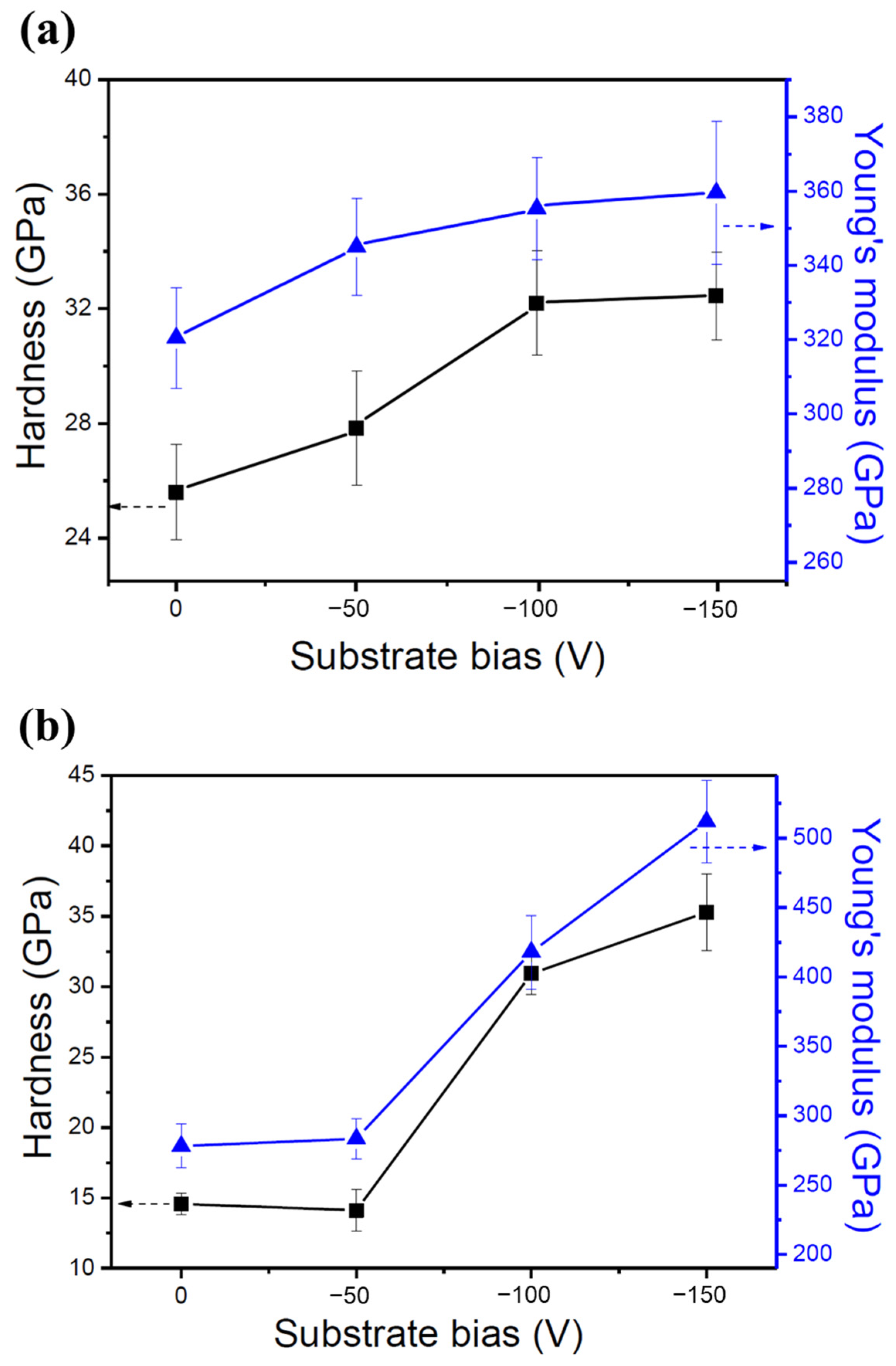
Figure 5 shows the hardness and Young’s moduli of the two HENFs under different substrate biases, and their values are listed in Table 3. The hardness of the low-Cr/Zr films was 25.6 GPa at 0 V and increased to 32.5 GPa at −150 V, with an increase in magnitude of 27%. For the high-Cr/Zr films, the hardness increased significantly from 14.6 GPa at 0 V to 35.3 GPa at −150 V, with an increase in magnitude of 142%. The Young’s moduli of the two HENFs also exhibited an increasing trend as the hardness increased. The increase in these two properties is consistent with the results of numerous reported studies [11,33,34]. The hardness difference between these two HENFs and the strengthening effects of the substrate bias can be associated with four main factors: dense microstructures, compositional differences, the Hall–Petch effect, and point defects.

Figure 5.
Mechanical properties of the two HENFs. Hardness and Young’s moduli of the (a) (AlCrSi0.3TiZr)N and (b) (AlCr1.5Si0.3TiZr0.5)N films at different substrate biases.

Table 3.
Hardness (H), Young’s moduli (E), and their standard deviations (SD) of the two HENFs at different biases.
The densification of the film structures under bias is displayed in Figure 3 and Figure 4. Because the voids in the films deteriorated their mechanical properties, the dense structure of the films implied fewer voids and higher hardness and Young’s moduli. The densification also originated from the compositional differences between the two HENFs. As explained in Section 3.1, the low-Cr/Zr film had a higher MMe/MAr ratio during deposition, indicating higher adatom mobility and, thus, a denser microstructure. This denser structure contributed to the higher hardness of the low-Cr/Zr film than that of the high-Cr/Zr film when both were deposited at 0 V.
The effect of the grain size on the hardness can be described by the Hall–Petch relationship.
where is an intrinsic parameter of the material, is the strengthening coefficient of the specific material, and is the average grain size. This relationship fits well in some related studies [17]. However, the hardness did not necessarily follow the grain-size change in this study. For example, the grain size of the (AlCrSi0.3TiZr)N film at −50 V was slightly larger than that at 0 V, whereas the hardness still increased at −50 V. This discrepancy indicates that the grain-size effect was not the dominant factor affecting the increase in hardness.
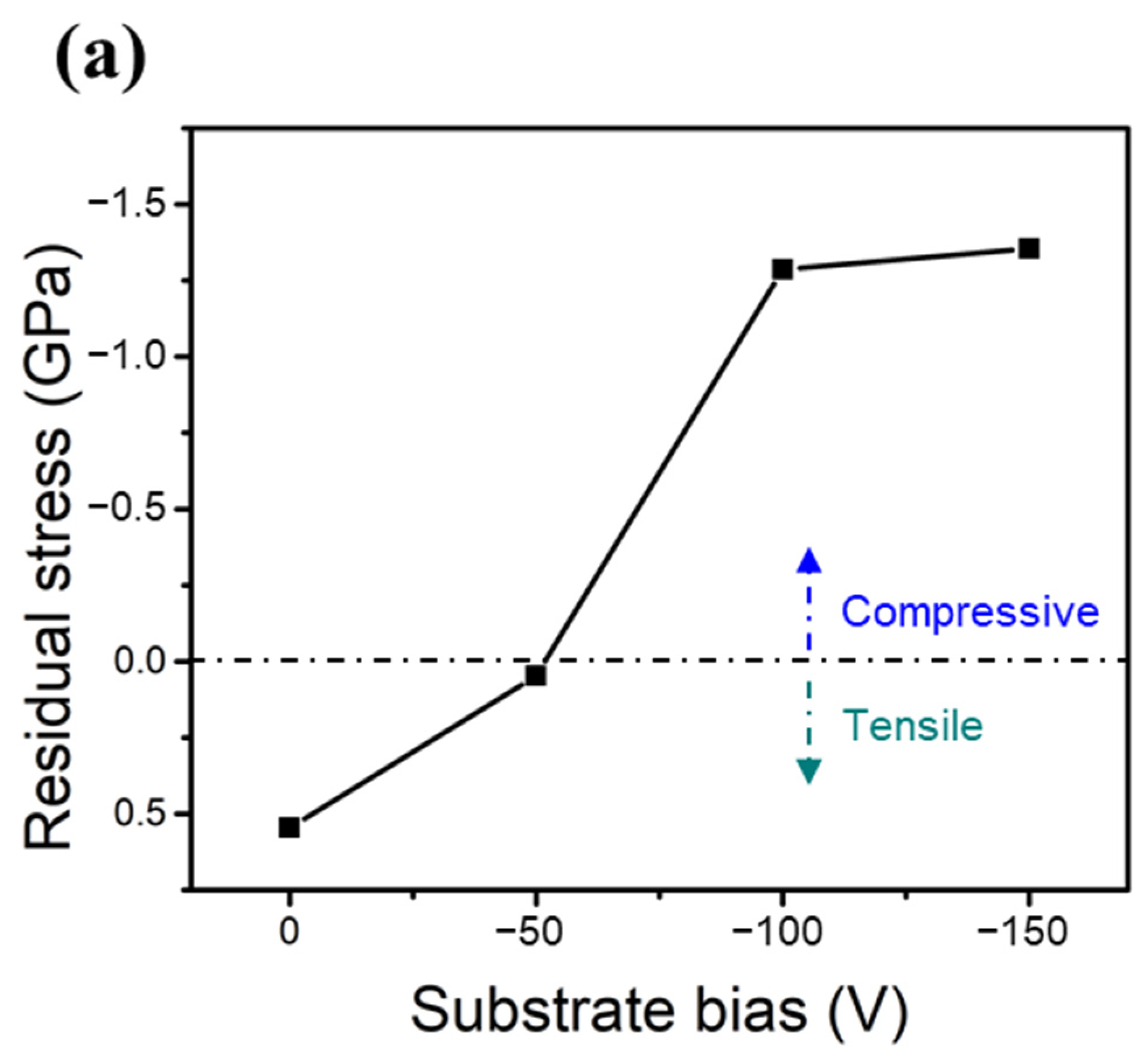
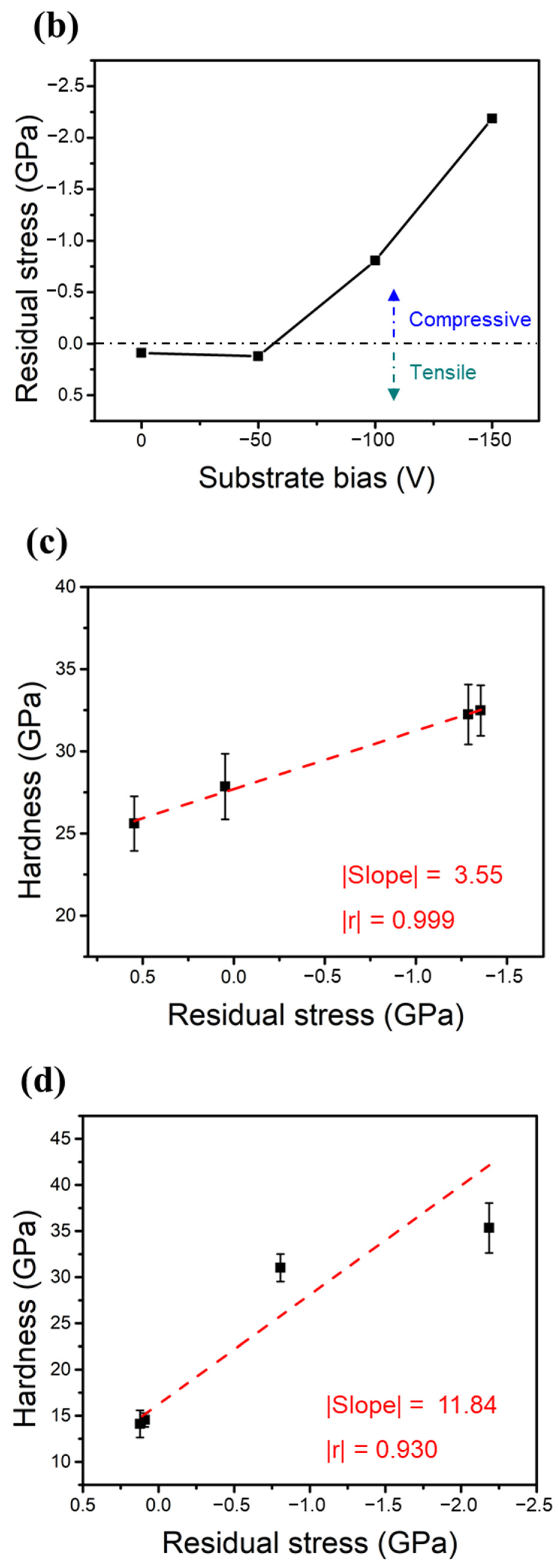
Figure 6a,b show the measured residual stresses of the two HENFs under different biases. The residual stress in the thin films can be assumed to have two components: thermal stress () and intrinsic stress () [38]. The total measured residual stress () can thus be expressed as
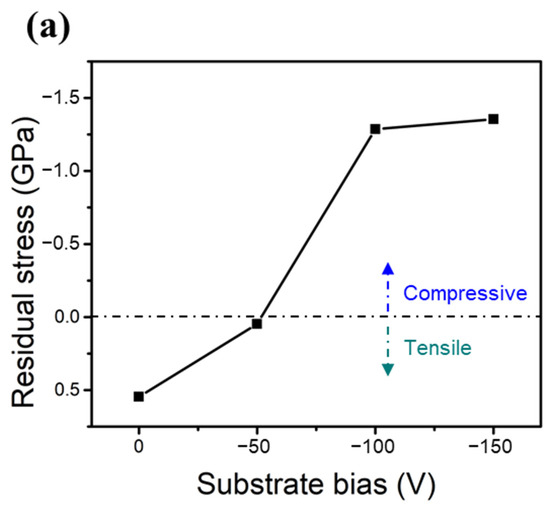
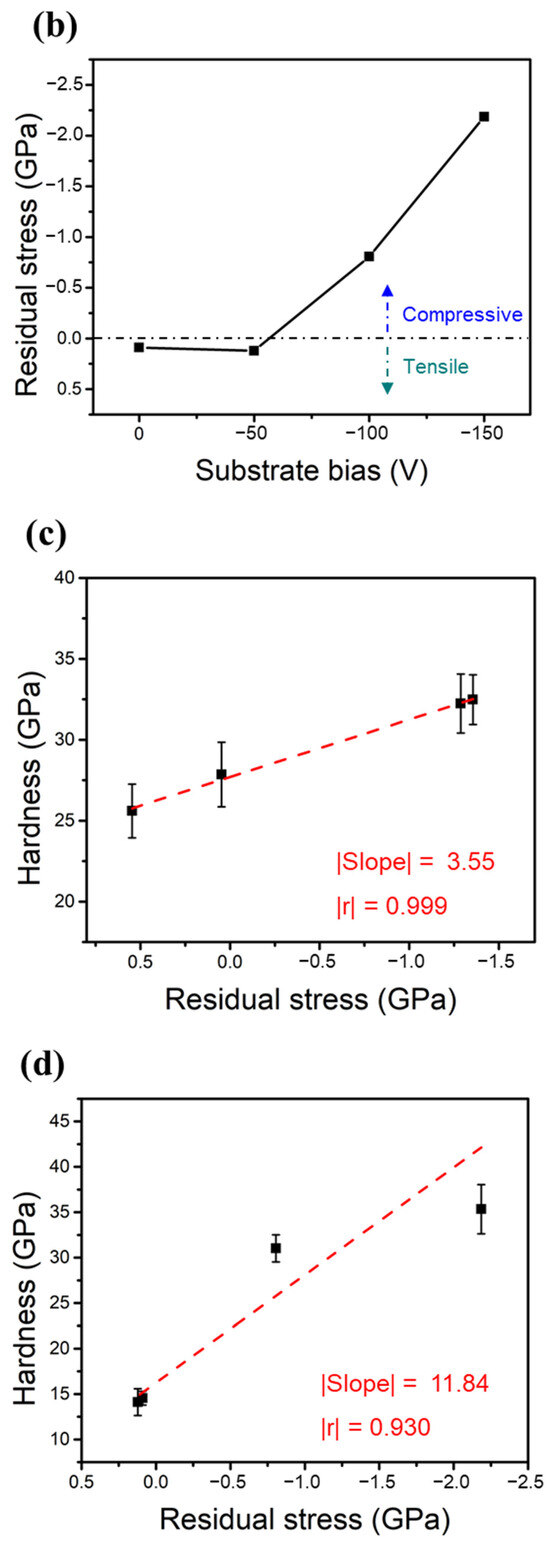
Figure 6.
Mechanical properties of the two HENFs. Residual stress of the (a) (AlCrSi0.3TiZr)N and (b) (AlCr1.5Si0.3TiZr0.5)N films at different substrate biases. (c,d) Correlation plots of the residual stress and hardness of the (AlCrSi0.3TiZr)N and (AlCr1.5Si0.3TiZr0.5)N films, respectively.
The thermal stress originates from the difference between the thermal expansion coefficients of the substrate and film, and the intrinsic stress stems from the point defects induced by the strong ion bombardment. The thermal expansion coefficient of an HENF can be calculated from its compositional binary nitrides using the rule of mixtures [33]. Because the chemical composition did not significantly change under different biases and all of the films were deposited at the same temperature, this thermal stress term did not vary significantly in the same HENF. Hence, we supposed that the change in the measured residual stress at different biases was mainly due to the intrinsic stress. As the bias increased, the ion bombardment became stronger; therefore, the compressive intrinsic stress increased accordingly (Figure 6a,b) due to an increased amount of excess interstitial atoms, which was also verified by the expansion of the lattice constant (Table 2). Furthermore, Figure 6c,d show plots of the residual stress versus hardness, with dashed lines fitted by linear regression. The absolute values of the Pearson correlation coefficient “r” in both figures exceeded 0.9, indicating a high correlation between these two quantities. Because the compressive intrinsic stress originated from the point defects caused by the ion-peening effect, this correlation suggests that the strain field generated by the point defects was responsible for the increase in hardness [39]. Additionally, there was a significant difference between the slopes of these two lines, implying that these two films had different sensitivities to residual stress or substrate bias. As stated previously, the low-Cr/Zr film had a higher MMe/MAr ratio and, thus, a higher energy during deposition. This originally strong energy may weaken the influence of the substrate bias, resulting in a lower value of the slope shown in Figure 6c relative to that shown in Figure 6d. As a result, compositional differences may also influence the strengthening extent of the substrate bias. In summary, the densification of the film structure and the compressive residual stress excess interstitial point defects caused by applying a substrate bias were the main factors that enhanced the hardness and Young’s moduli of the two HENFs.
We next compared the low-Cr/Zr film, (AlCrSi0.3TiZr)N, deposited at 0 V with its equimolar counterpart (AlCrSiTiZr)100−xNx studied by our group before [9]. Under near-identical deposition parameters, the non-equimolar designed (AlCrSi0.3TiZr)N film demonstrated a hardness of 25.6 GPa. This value is approximately 9 GPa greater than that of stoichiometric (AlCrSiTiZr)100−xNx (x ≈ 50 at RN = 30%), thus confirming that our design strategy, which reduces the Si content to 7 at%, can effectively increase the hardness.
3.3. Corrosion Resistance
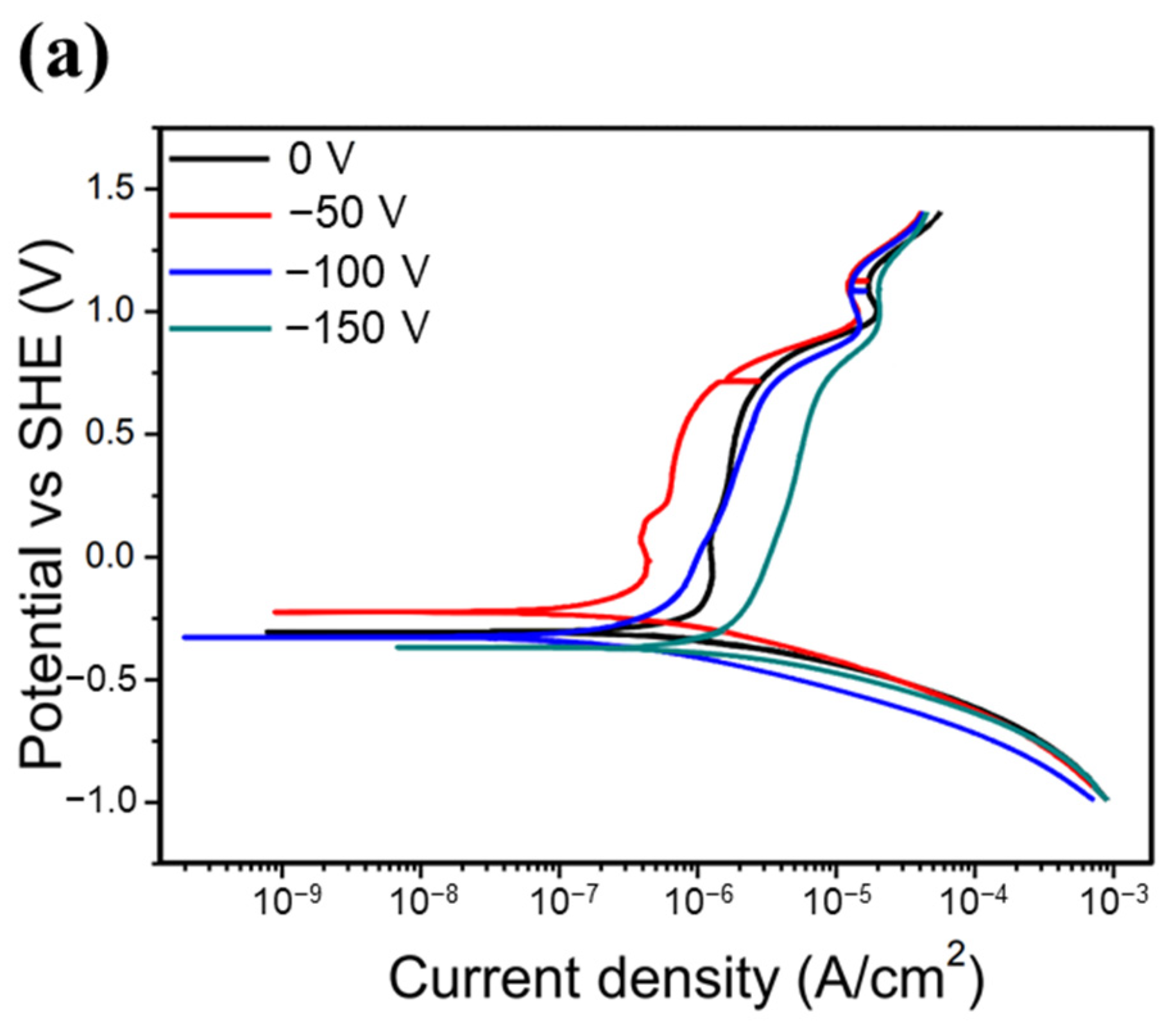
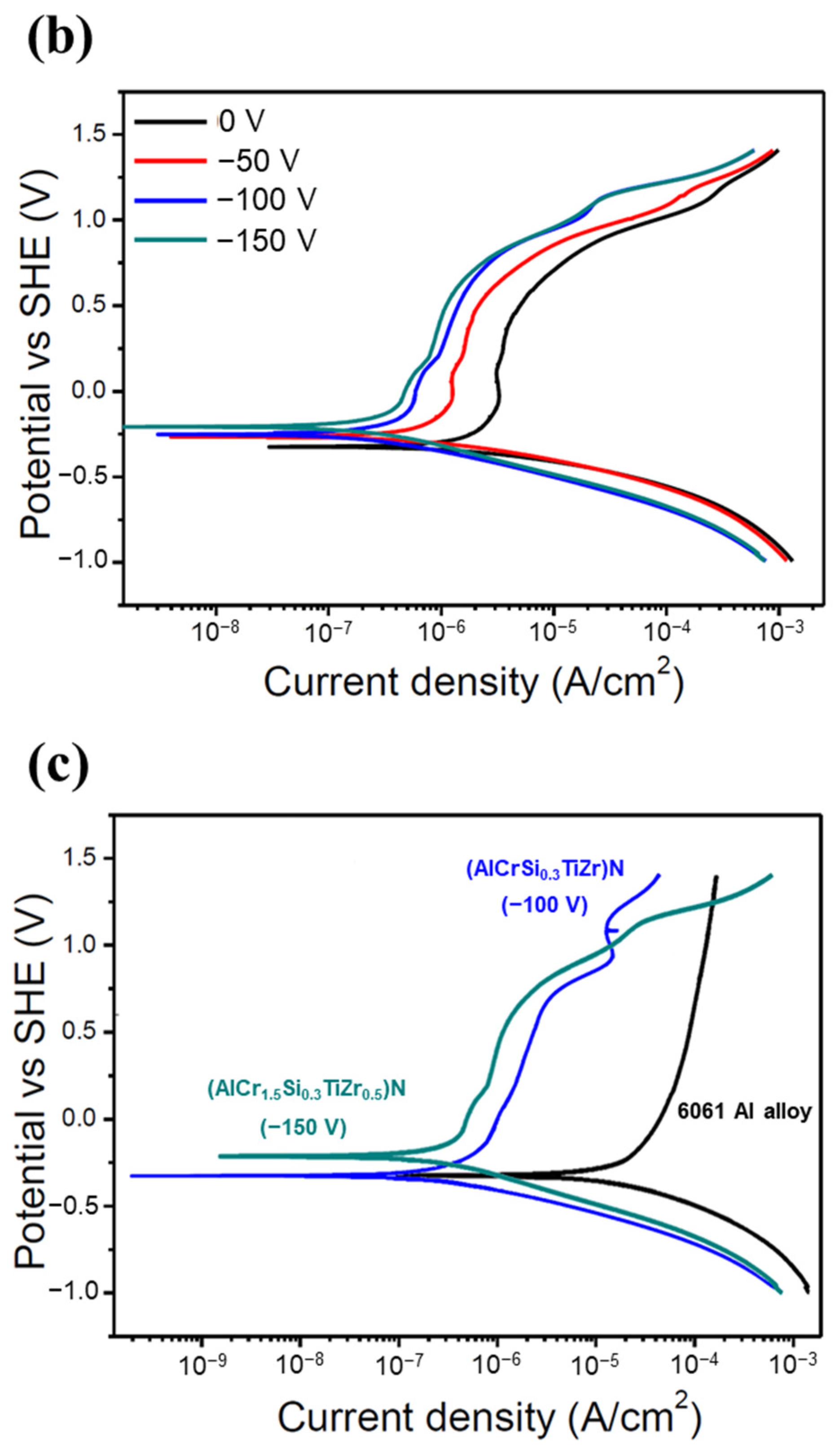
Figure 7 shows the potentiodynamic polarization curves of all of the HENF-coated samples and 6061 Al alloy substrates. The corresponding electrochemical parameters determined from the Tafel curves are listed in Table 4. Generally, a lower icorr value and a more positive Ecorr value indicate that the material is less prone to corrosion. For the low-Cr/Zr films, the icorr value decreased from 3.68 μA/cm2 at 0 V to 0.75 μA/cm2 at −100 V, but increased again to 3.03 μA/cm2 at −150 V. The Ecorr first became more positive at low bias and then more negative at high bias. On the other hand, the icorr of the high-Cr/Zr films decreased from 4.55 μA/cm2 to 0.19 μA/cm2 when the bias increased from 0 to −150 V; the Ecorr also changed to more positive values consistently with increasing bias. First, comparing both films deposited at 0 V, the low-Cr/Zr film presented a lower icorr and more positive Ecorr, indicating better corrosion resistance than that of the high-Cr/Zr film. This difference reflects the intrinsic corrosion resistances of the two HENFs without the influence of a bias. Because ZrN has been reported to have better corrosion resistance than CrN [40], its higher Zr content could explain the better corrosion resistance of the low-Cr/Zr film at 0 V. Furthermore, the increased corrosion resistance of both films with increasing bias can be attributed to microstructural changes. As shown in Figure 4, both films became denser at high bias, which prevented the corrosive ions from attacking the substrate material through the voids. Moreover, when the films became denser, the exposed surface area decreased, reducing the chances of reactions between the film atoms and the corrosive ions [41]. Therefore, the icorr value decreased as the bias increased. However, the icorr value of the low-Cr/Zr film increased again at −150 V, which could be explained by the increased surface roughness.
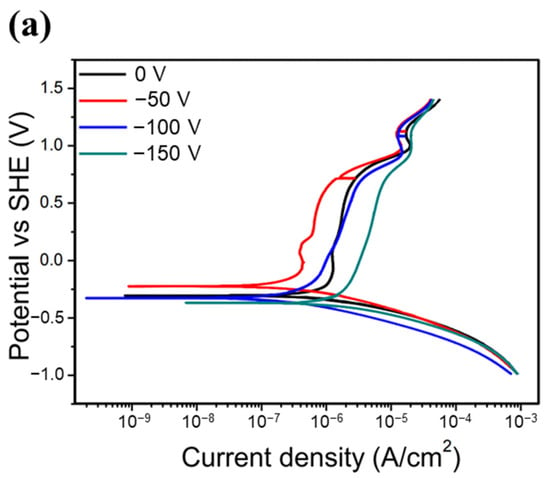
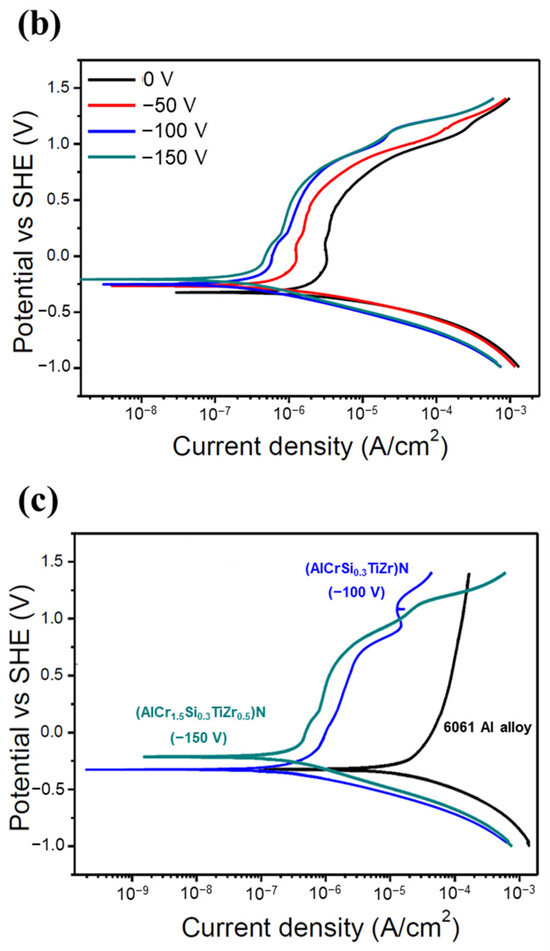
Figure 7.
Corrosion resistance of the HENFs. Potentiodynamic polarization curves of the (a) (AlCrSi0.3TiZr)N and (b) (AlCr1.5Si0.3TiZr0.5)N films deposited on 6061 Al alloy at different biases in 0.1 M H2SO4. (c) Combined comparison of the two films with the lowest corrosion current density (icorr) and 6061 Al alloy substrate.

Table 4.
Electrochemical parameters of the two HENFs deposited on 6061 Al alloy at different biases in 0.1 M H2SO4.
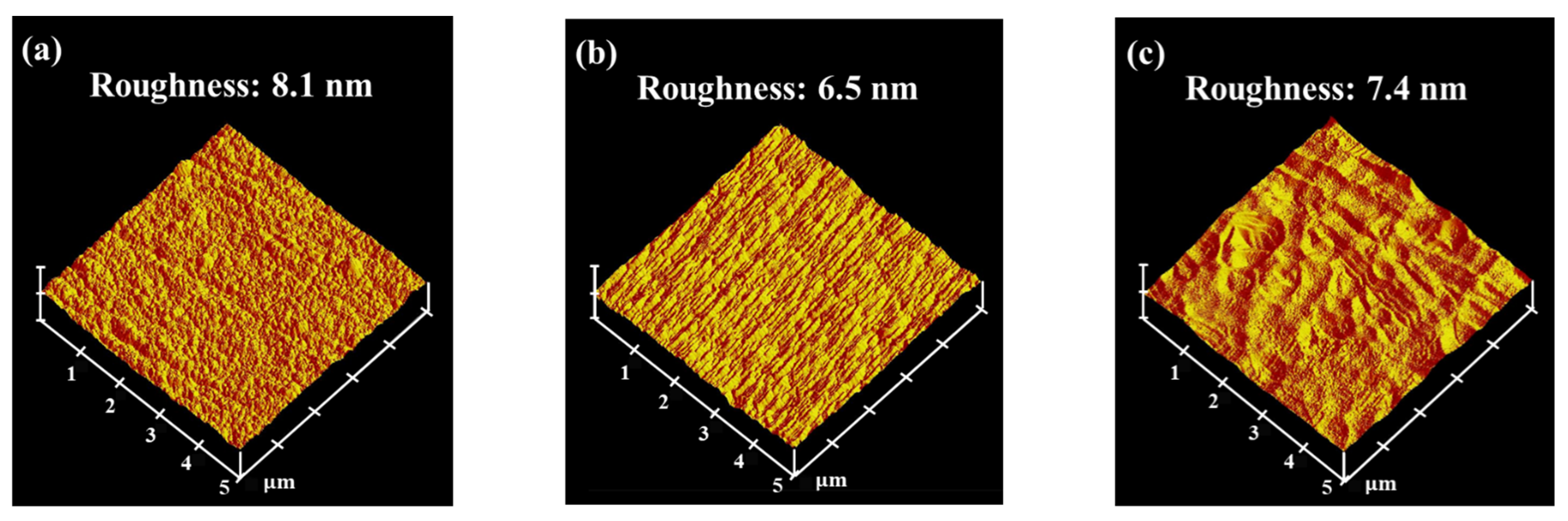
Figure 8 shows the surface topography and root mean square (RMS) roughness of the low-Cr/Zr films deposited at different biases. The RMS roughness first decreased as the bias increased from 0 to −50 V and increased again as the bias reached −150 V. The surface roughness is expected to decrease under a moderate bias, owing to the densification of the film structure. Nevertheless, the high-energy bombardment at a high bias could form a crater-like surface morphology (Figure 8c), thereby increasing the surface roughness. Li et al. proposed that a rougher surface exhibits a higher electronic work function fluctuation, making it more electrochemically active [42]. Similar phenomena were also observed in the (CrNbTiAlV)Nx system studied by Zhang et al. [18], where both the surface roughness and the icorr value increased at high bias.
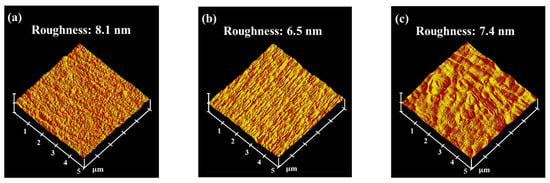
Figure 8.
Surface properties of (AlCrSi0.3TiZr)N. Surface topography and root mean square (RMS) roughness of the (AlCrSi0.3TiZr)N films deposited on 6061 Al alloy substrates at (a) 0 V, (b) −50 V, and (c) −150 V.
The larger amount of Zr ions during deposition may explain why only the low-Cr/Zr films displayed ion-induced damage at high bias. Specifically, because Zr is the heaviest element in this study, its higher momentum during the deposition process may result in stronger re-sputtering of the already deposited light atoms, increasing the surface roughness. Therefore, the corrosion resistance of both HENFs can be improved by applying a substrate bias, as long as the bias is not sufficiently high to increase the surface roughness.
In summary, according to Figure 7c and Table 4, the icorr values of the low- and high-Cr/Zr films at their optimum biases were 80% and 96% lower than those under no bias. Moreover, these icorr values were approximately one-hundredth of those of the uncoated sample, suggesting that the corrosion resistance of the alloy was significantly enhanced by the deposition of either of the two HENFs. In addition, the high-Cr/Zr film deposited at −150 V exhibited a lower icorr value and higher Ecorr value than did the low-Cr/Zr film deposited at −100 V, indicating a better strengthening effect on the corrosion resistance of the high-Cr/Zr film through the application of substrate bias.
3.4. Oxidation Resistance
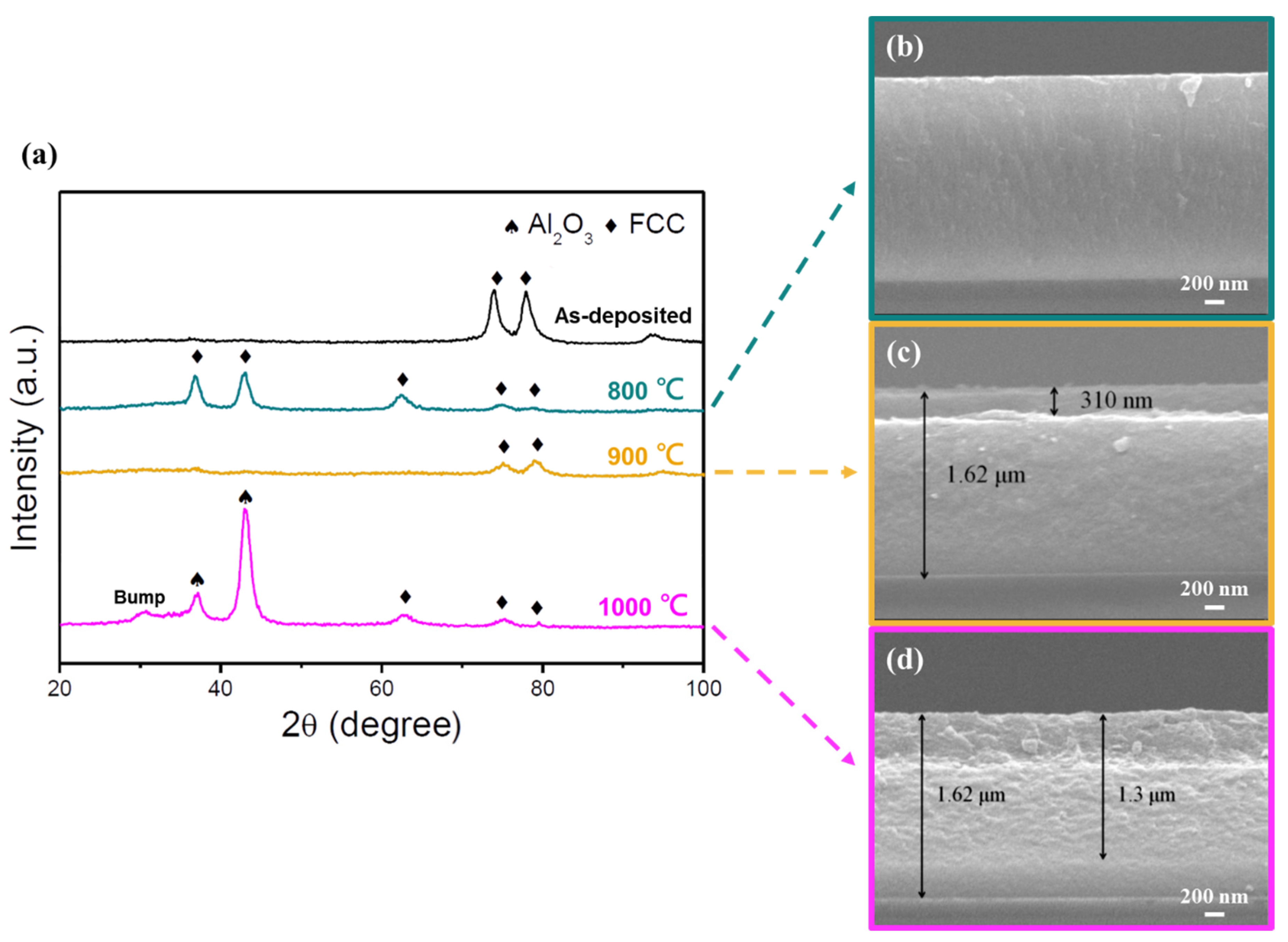
The oxidation behavior of multicomponent systems is complex and has been extensively studied [10,43,44]. Multilayered oxides are often observed because of the different diffusion abilities of the compositional elements and oxygen. To investigate the oxidation resistance of the two HENFs, both films deposited at −150 V were selected for heat treatment because of their dense microstructures and optimum mechanical properties. The GIXRD patterns and cross-sectional SEM images of the low- and high-Cr/Zr films after annealing are shown in Figure 9 and Figure 10, respectively.
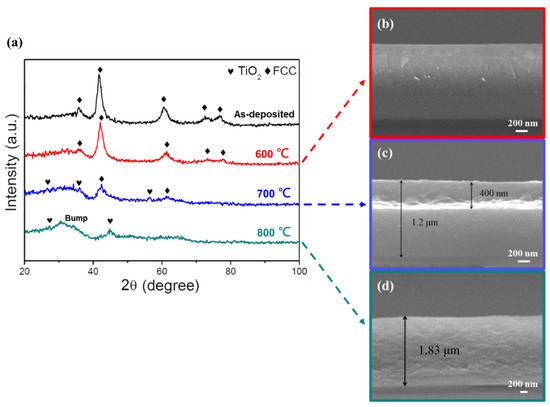
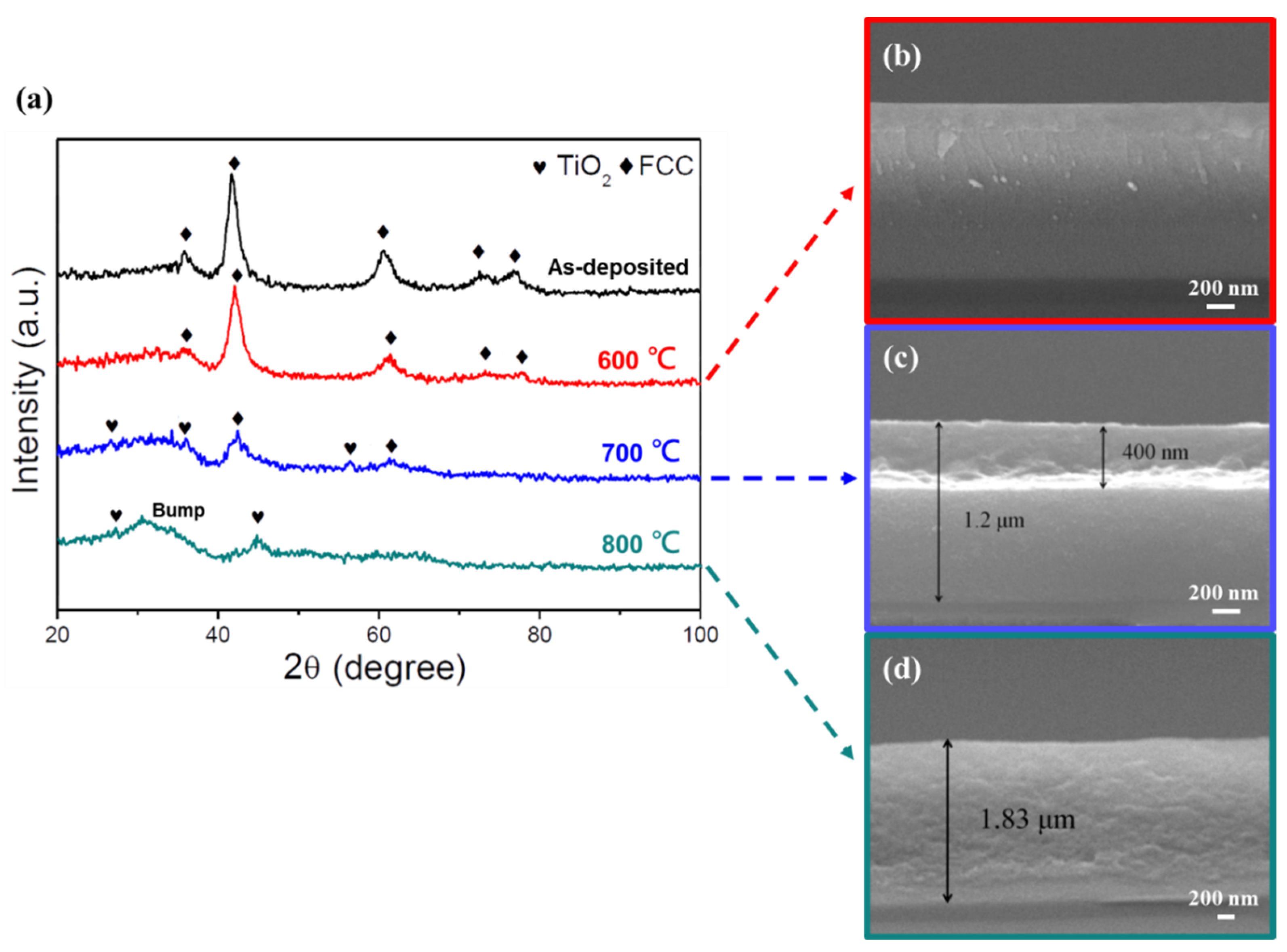
Figure 9.
Oxidation resistance test of the (AlCrSi0.3TiZr)N (−150 V) films. (a) GIXRD patterns of the as-deposited film and films after annealing in air for 2 h at different temperatures. (b–d) Cross-sectional SEM images of the films at annealing temperatures of 600, 700, and 800 °C, respectively.
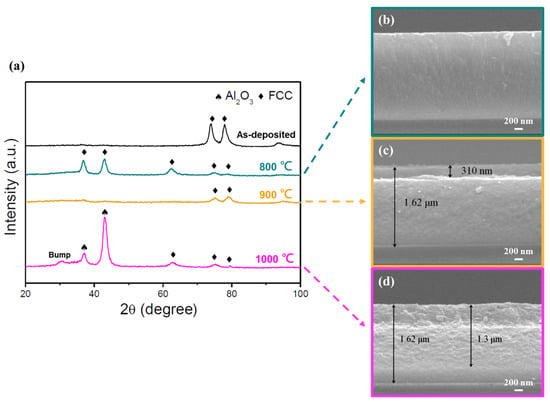
Figure 10.
Oxidation resistance test of the (AlCr1.5Si0.3TiZr0.5)N (−150 V) films. (a) GIXRD patterns of the as-deposited film and films after annealing in air for 2 h at different temperatures. (b–d) Cross-sectional SEM images of the films at annealing temperatures of 800, 900, and 1000 °C, respectively.
For the low-Cr/Zr film (Figure 9), the GIXRD pattern remained the same after heat treatment at 600 °C, and the corresponding cross-sectional image also showed an intact and featureless structure. At 700 °C, diffraction peaks corresponding to TiO2 (rutile) were detected, and the oxide layer thickness measured by SEM was 400 nm. At 800 °C, the nitride peaks disappeared, and the film observed by SEM was fully oxidized. A bump caused by the amorphous phase at low 2 θ values was also observed.
For the high-Cr/Zr film (Figure 10), the film structure remained the same after annealing at 800 °C, but the diffraction pattern was slightly different. Three new FCC peaks were observed, indicating that the recrystallization may occur at this temperature, leading to the detection of new peaks from different oriented grains. At 900 °C, the nitride diffraction peaks became weaker, but could still be identified; moreover, the measured oxide layer thickness was 310 nm. At 1000 °C, diffraction peaks corresponding to Al2O3 (corundum) and the amorphous bump at low 2 θ values appeared, but the nitride peaks were still observed. Furthermore, the corresponding SEM image shows that the oxide layer thickness reached 1.3 μm; however, the film was still not fully oxidized.
Both kinetics and thermodynamics should be considered to explain the oxidation behavior in HENFs [45]. Kinetically, TiO2 and ZrO2 have loose and porous structures, which could intensify the inward diffusion of oxygen and, thus, deteriorate the oxidation resistance of the film. In contrast, Al2O3 and Cr2O3 have dense structures that can prevent oxygen from diffusing into the film, which is beneficial for the film’s oxidation resistance. Although the low-Cr/Zr film contained Al and Cr, their concentrations did not provide adequate protection. Owing to the higher amount of Ti and Zr in the low-Cr/Zr film, the dense Al, Cr, and Si-rich oxide layer was affected and the oxygen diffusion intensified through the incorporation of TiO2 and ZrO2, as verified by the GIXRD pattern and SEM images in Figure 9. Consequently, the superior oxidation resistance of the high-Cr/Zr film compared to that of the low-Cr/Zr film may have resulted from the higher concentration of Cr and lower concentration of Zr, which delayed the oxidation reaction of the interior film. The superior oxidation resistance of the high-Cr/Zr film surpasses that of many traditional titanium-based coatings such as TiN, TiCN, TiAlN, and TiAlSiN [46].
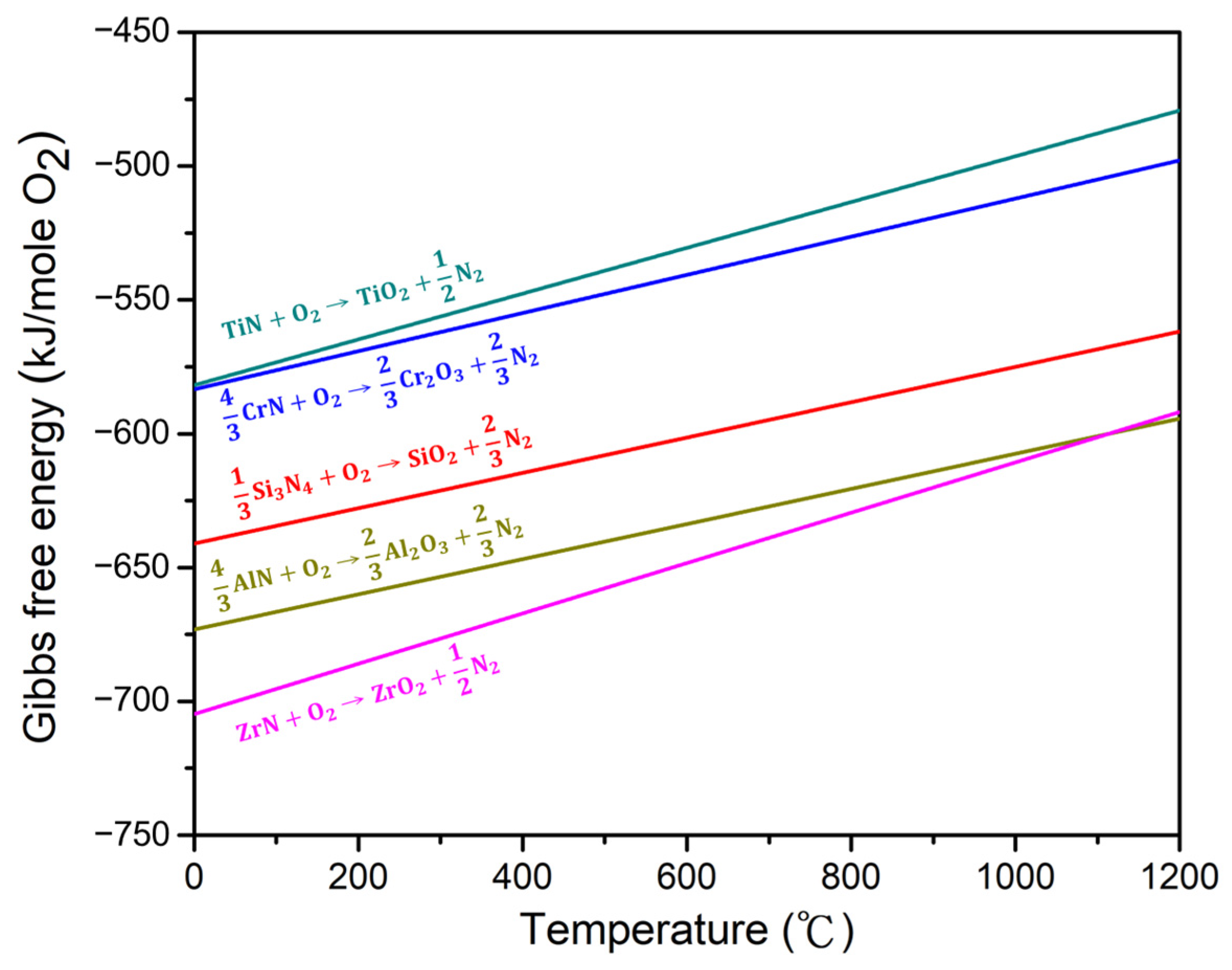
Only TiO2 peaks were detected below 1000 °C, and no distinct Cr2O3 and ZrO2 peaks were observed across any of the studied temperature ranges. Plots of the Gibbs free energy change of the compositional nitrides to oxides per mole of oxygen as a function of temperature are shown in Figure 11. Thermodynamically, ZrO2 exhibited the highest driving force for its formation, followed by, in descending order, Al2O3, SiO2, Cr2O3, and TiO2. However, Al2O3 diffraction peaks were only visible at 1000 °C, which could be ascribed to its higher crystallization temperature [44,47]. In addition, the presence of amorphous SiO2 increases the degree of amorphization and lowers the peak intensity, as confirmed by other Si-rich high-entropy nitride systems [10,44]. The absence of ZrO2 peaks may also be attributed to the SiO2, as can be seen from the amorphous bumps shown in Figure 9 and Figure 10. With regard to Cr2O3, the incorporation of Cr2O3 into the Al2O3 layer has been observed in some Al- and Cr-containing high-entropy nitride systems [10,43]; therefore, independent diffraction peaks of Cr2O3 could not be seen in this study. Moreover, Cr2O3 becomes unstable above 900 °C [48], which further contributes to the undetectability of its diffraction peaks above this temperature.
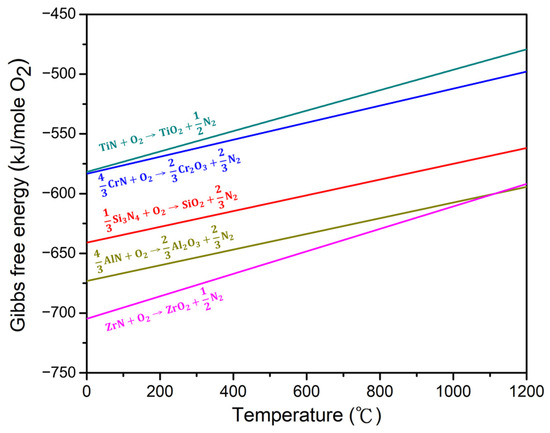
Figure 11.
Gibbs free energy changes per mole of oxygen of the nitride oxidation reactions as functions of temperature [49].
4. Conclusions
Two non-equimolar high-entropy nitride films, (AlCrSi0.3TiZr)N and (AlCr1.5Si0.3TiZr0.5)N, referred to as “low-Cr/Zr film” and “high-Cr/Zr film,” respectively, were deposited via reactive DCMS at different substrate biases. The main conclusions of this study are as follows:
- (1)
- Due to high mixing entropy and limited kinetics during PVD, both films had NaCl-type FCC structures, regardless of the substrate bias. The microstructures of both films became compact and featureless as the bias was increased to −150 V because the increased adatom mobility reduced the voids, and the enhanced re-sputtering effect refined the grains.
- (2)
- The hardness of both films reached maxima when the bias was increased to −150 V, of which the values were 32.5 GPa (low-Cr/Zr film) and 35.3 GPa (high-Cr/Zr film). The increases in the hardness and Young’s moduli aligned with the densification of the film structures. Also, the excess interstitial point defects generated by high energetic bombardment at high bias could have created the strain field that increased the film hardness, which can be verified by the increase in compressive residual stress.
- (3)
- The strengthening effect of the substrate bias was found to be different for the two studied films. The magnitude of hardness increase was 142% for the high-Cr/Zr film, but only 27% for the low-Cr/Zr film. The film with a higher MMe/MAr ratio may weaken the enhancement effect of the substrate bias due to its inherently high deposition energy.
- (4)
- The lowest icorr values of the two films were observed for the low-Cr/Zr film deposited at −100 V (0.75 μA/cm2) and the high-Cr/Zr film deposited at −150 V (0.19 μA/cm2), both of which were two orders of magnitude lower than the icorr value of the uncoated 6061 Al alloy. The strengthening effect of substrate bias on their corrosion resistance was mainly due to the densification of the film structures, reducing the surface area that would react with the corrosive ions and protecting the substrate from being attacked by those ions through voids.
- (5)
- The low-Cr/Zr film was fully oxidized after annealing in air at 800 °C for 2 h, while the high-Cr/Zr film remained partially unoxidized after the same annealing process at 1000 °C. This is because the sufficiently high amount of Cr formed a dense oxide that retarded the diffusion of oxygen into the interior film and delayed the oxidation reaction.
- (6)
- The high-Cr/Zr film deposited at −150 V exhibited good mechanical properties, corrosion resistance, and oxidation resistance, indicating its potential to protect tools operating under high-temperature and corrosive environments.
Author Contributions
Conceptualization, H.-Y.C. and J.-W.Y.; methodology, H.-Y.C. and J.-W.Y.; validation, H.-E.P., C.-Y.L. and J.-W.Y.; formal analysis, H.-Y.C.; investigation, H.-Y.C.; data curation, H.-E.P. and H.-Y.C.; writing—original draft preparation, H.-E.P.; writing—review and editing, C.-Y.L. and J.-W.Y.; visualization, H.-E.P. and H.-Y.C.; supervision, C.-Y.L. and J.-W.Y.; project administration, J.-W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the “High Entropy Materials Center” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) and the Project MOST 111-2634-F-007-008 by the National Science and Technology Council (NSTC) in Taiwan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We extend our gratitude to Su-Yueh Tsai from the Instrumentation Center at NTHU for the valuable assistance in conducting high-resolution EPMA analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schmitt, G.; Schütze, M.; Hays, G.F. Global Needs for Knowledge Dissemination, Research, and Development in Materials Deterioration and Corrosion Control; World Corrosion Organization: New York, NY, USA, 2009; Volume 38, p. 14. [Google Scholar]
- Mundhra, G.; Hariharan, V.S.; Murty, B.S. Design of a novel Al–Ti–Zr light-weight alloy: CALPHAD and experiments. J. Alloys Compd 2020, 835, 155304. [Google Scholar] [CrossRef]
- Dong, H. Surface Engineering of Light Alloys: Aluminium, Magnesium and Titanium Alloys; Woodhead Publishing: Sawston, UK, 2010. [Google Scholar]
- Chen, H.-Y.; Lu, F.-H. Oxidation behavior of titanium nitride films. J. Vac. Sci. Technol. A 2005, 23, 1006–1009. [Google Scholar] [CrossRef]
- Ikeda, T.; Satoh, H. Phase formation and characterization of hard coatings in the Ti–Al–N system prepared by the cathodic arc ion plating method. Thin Solid Film. 1991, 195, 99–110. [Google Scholar] [CrossRef]
- Huang, J.L.; Shew, B.Y. Effects of aluminum concentration on the oxidation behaviors of reactively sputtered TiAlN films. J. Am. Ceram. Soc. 1999, 82, 696–704. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W.; Gan, J.-Y. Diffusion barrier properties of AlMoNbSiTaTiVZr high-entropy alloy layer between copper and silicon. Thin Solid Film. 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Hsueh, H.-T.; Shen, W.-J.; Tsai, M.-H.; Yeh, J.-W. Effect of nitrogen content and substrate bias on mechanical and corrosion properties of high-entropy films (AlCrSiTiZr)100−xNx. Surf. Coat. Technol. 2012, 206, 4106–4112. [Google Scholar] [CrossRef]
- Shen, W.J.; Tsai, M.H.; Tsai, K.Y.; Juan, C.C.; Tsai, C.W.; Yeh, J.W.; Chang, Y.S. Superior oxidation resistance of (Al0. 34Cr0. 22Nb0. 11Si0. 11Ti0. 22)50N50 high-entropy nitride. J. Electrochem. Soc. 2013, 160, C531–C535. [Google Scholar] [CrossRef]
- Lo, W.-L.; Hsu, S.-Y.; Lin, Y.-C.; Tsai, S.-Y.; Lai, Y.-T.; Duh, J.-G. Improvement of high entropy alloy nitride coatings (AlCrNbSiTiMo) N on mechanical and high temperature tribological properties by tuning substrate bias. Surf. Coat. Technol. 2020, 401, 126247. [Google Scholar] [CrossRef]
- Malinovskis, P.; Fritze, S.; Riekehr, L.; von Fieandt, L.; Cedervall, J.; Rehnlund, D.; Nyholm, L.; Lewin, E.; Jansson, U. Synthesis and characterization of multicomponent (CrNbTaTiW)C films for increased hardness and corrosion resistance. Mater. Des. 2018, 149, 51–62. [Google Scholar] [CrossRef]
- Yu, R.-S.; Huang, R.-H.; Lee, C.-M.; Shieu, F.-S. Synthesis and characterization of multi-element oxynitride semiconductor film prepared by reactive sputtering deposition. Appl. Surf. Sci. 2012, 263, 58–61. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chien, C.-H.; Yeh, J.-W.; Lin, S.-J. Effects of the carbon-to-nitrogen ratio on the microstructure and properties of (CrNbSiTiZr)CxNy high-entropy carbonitride films. Mater. Chem. Phys. 2022, 277, 125374. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Messier, R.; Giri, A.P.; Roy, R.A. Revised structure zone model for thin film physical structure. J. Vac. Sci. Technol. A 1984, 2, 500–503. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W. Effects of substrate bias on structure and mechanical properties of (AlCrNbSiTiV)N coatings. J. Phys. D Appl. Phys. 2009, 42, 115401. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, X.; Zhou, H.; Wang, Y.; Sui, X.; Shi, Z.; Hao, J. Construction of a compact nanocrystal structure for (CrNbTiAlV) Nx high-entropy nitride films to improve the tribo-corrosion performance. Surf. Coat. Technol. 2022, 429, 127921. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.L.; Ong, S.E.; Sun, D.; Bui, X.L. Hard yet tough nanocomposite coatings–present status and future trends. Plasma Processes Polym. 2007, 4, 219–228. [Google Scholar] [CrossRef]
- Sandu, C.S.; Sanjinés, R.; Benkahoul, M.; Medjani, F.; Lévy, F. Formation of composite ternary nitride thin films by magnetron sputtering co-deposition. Surf. Coat. Technol. 2006, 201, 4083–4089. [Google Scholar] [CrossRef]
- Lee, D.B.; Kim, M.H.; Lee, Y.C.; Kwon, S.C. High temperature oxidation of TiCrN coatings deposited on a steel substrate by ion plating. Surf. Coat. Technol. 2001, 141, 232–239. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Kim, Y.J.; Han, J.G.; Lee, D.B. Oxidation of TiZrAlN nanocomposite thin films in air at temperatures between 500 and 700 °C. Thin Solid Film. 2009, 517, 5216–5218. [Google Scholar] [CrossRef]
- Li, W.; Chen, Q.; Polcar, T.; Serra, R.; Cavaleiro, A. Influence of Zr alloying on the mechanical properties, thermal stability and oxidation resistance of Cr–Al–N coatings. Appl. Surf. Sci. 2014, 317, 269–277. [Google Scholar] [CrossRef]
- Khamseh, S.; Araghi, H. A study of the oxidation behavior of CrN and CrZrN ceramic thin films prepared in a magnetron sputtering system. Ceram. Int. 2016, 42, 9988–9994. [Google Scholar] [CrossRef][Green Version]
- Tsai, M.-H.; Yeh, J.-W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Zhang, S.; Liu, M.; Zhou, K.; Mu, X. Properties degradation and atmospheric corrosion mechanism of 6061 aluminum alloy in industrial and marine atmosphere environments. Mater. Corros. 2017, 68, 529–535. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Publishing: Boston, MA, USA, 1956. [Google Scholar]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- ISO 14577-1:2015; Metallic materials–Instrumented indentation test for hardness and materials parameters–Part 1: Test Method. International Organization for Standardization: Geneva, Switzerland, 2015.
- Bull, S.; Jones, A.; McCabe, A. Residual stress in ion-assisted coatings. Surf. Coat. Technol. 1992, 54, 173–179. [Google Scholar] [CrossRef]
- Ohring, M. (Ed.) Chapter 4—Discharges, Plasmas, and Ion–Surface Interactions. In Materials Science of Thin Films, 2nd ed.; Academic Press: San Diego, CA, USA, 2002; pp. 145–202. [Google Scholar]
- Choi, S.R.; Park, I.-W.; Kim, S.H.; Kim, K.H. Effects of bias voltage and temperature on mechanical properties of Ti–Si–N coatings deposited by a hybrid system of arc ion plating and sputtering techniques. Thin Solid Film. 2004, 447, 371–376. [Google Scholar] [CrossRef]
- Shen, W.-J.; Tsai, M.-H.; Chang, Y.-S.; Yeh, J.-W. Effects of substrate bias on the structure and mechanical properties of (Al1. 5CrNb0. 5Si0. 5Ti)Nx coatings. Thin Solid Film. 2012, 520, 6183–6188. [Google Scholar] [CrossRef]
- Chang, H.-W.; Huang, P.-K.; Yeh, J.-W.; Davison, A.; Tsau, C.-H.; Yang, C.-C. Influence of substrate bias, deposition temperature and post-deposition annealing on the structure and properties of multi-principal-component (AlCrMoSiTi) N coatings. Surf. Coat. Technol. 2008, 202, 3360–3366. [Google Scholar] [CrossRef]
- Håkansson, G.; Sundgren, J.-E.; McIntyre, D.; Greene, J.; Münz, W.-D. Microstructure and physical properties of polycrstalline metastable Ti0.5Al0. 5N alloys grown by dc magnetron sputter deposition. Thin Solid Film. 1987, 153, 55–65. [Google Scholar] [CrossRef]
- Abadias, G.; Koutsokeras, L.E.; Siozios, A.; Patsalas, P. Stress, phase stability and oxidation resistance of ternary Ti–Me–N (Me= Zr, Ta) hard coatings. Thin Solid Film. 2013, 538, 56–70. [Google Scholar] [CrossRef]
- Kim, G.; Kim, B.; Lee, S.; Hahn, J. Structure and mechanical properties of Cr–Zr–N films synthesized by closed field unbalanced magnetron sputtering with vertical magnetron sources. Surf. Coat. Technol. 2005, 200, 1669–1675. [Google Scholar] [CrossRef]
- Oettel, H.; Wiedemann, R.; Preissler, S. Residual stresses in nitride hard coatings prepared by magnetron sputtering and arc evaporation. Surf. Coat. Technol. 1995, 74, 273–278. [Google Scholar] [CrossRef]
- Tung, H.-M.; Huang, J.-H.; Tsai, D.-G.; Ai, C.-F.; Yu, G.-P. Hardness and residual stress in nanocrystalline ZrN films: Effect of bias voltage and heat treatment. Mater. Sci. Eng. A 2009, 500, 104–108. [Google Scholar] [CrossRef]
- Tao, H.; Zhylinski, V.; Vereschaka, A.; Chayeuski, V.; Yuanming, H.; Milovich, F.; Sotova, C.; Seleznev, A.; Salychits, O. Comparison of the mechanical properties and corrosion resistance of the Cr-CrN, Ti-TiN, Zr-ZrN, and Mo-MoN coatings. Coatings 2023, 13, 750. [Google Scholar] [CrossRef]
- Aouadi, K.; Tlili, B.; Nouveau, C.; Besnard, A.; Chafra, M.; Souli, R. Influence of substrate bias voltage on corrosion and wear behavior of physical vapor deposition CrN coatings. J. Mater. Eng. Perform. 2019, 28, 2881–2891. [Google Scholar] [CrossRef]
- Li, W.; Li, D.Y. Influence of surface morphology on corrosion and electronic behavior. Acta Mater. 2006, 54, 445–452. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Tsai, C.-W.; Lin, S.-J.; Yeh, J.-W. Effects of silicon content on the structure and mechanical properties of (AlCrTaTiZr)–Six–N coatings by reactive RF magnetron sputtering. J. Phys. D Appl. Phys. 2011, 44, 205405. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Deng, M.-J.; Chang, Z.-C.; Kuo, B.-H.; Chen, E.-C.; Chang, S.-Y.; Shieu, F.-S. Oxidation resistance and characterization of (AlCrMoTaTi)-Six-N coating deposited via magnetron sputtering. J. Alloys Compd 2015, 647, 179–188. [Google Scholar] [CrossRef]
- Wang, J.-J.; Ouyang, F.-Y. Oxidation behavior of Al-Cr-Nb-Si-Zr high entropy nitride thin films at 850 °C. Corros. Sci. 2021, 187, 109467. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Tan, A.L.K.; Zeng, X.T. Oxidation and wear behaviors of Ti-based thin films. Surf. Coat. Technol. 2006, 201, 4094–4098. [Google Scholar] [CrossRef]
- Evans, P.G.; Chen, Y.; Tilka, J.A.; Babcock, S.E.; Kuech, T.F. Crystallization of amorphous complex oxides: New geometries and new compositions via solid phase epitaxy. Curr. Opin. Solid State Mater. Sci. 2018, 22, 229–242. [Google Scholar] [CrossRef]
- Caplan, D.; Cohen, M. The volatilization of chromium oxide. J. Electrochem. Soc. 1961, 108, 438. [Google Scholar] [CrossRef]
- Barin, I.; Platzki, G. Thermochemical Data of Pure Substances; Wiley Online Library: Hoboken, NJ, USA, 1989; Volume 304. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).