Abstract
Zirconia, with its excellent mechanical strength and esthetics, has a growing potential for applications in dentistry and orthopedics. However, in order for zirconia to have a high affinity with bone tissue, the bioactivity of the surface must be further increased. In order to increase the bioactivity of zirconia, research was conducted to make a porous support or to fill the porous structure with nano-hydroxyapatite (nHA). In this case, there is a risk that physically filled nHA could be released depending on the living environment. In this study, nHA and type I collagen were introduced to the zirconia surface by chemical covalent bonding to increase bioactivity and ensure safety in the body. The chemical reaction of the surface was confirmed by X-ray photoelectron spectroscopy (XPS), field emission scanning electron microscopy (FE-SEM) and Fourier transform infrared (FT-IR) spectroscopy. In addition, the biological activity was evaluated by examining the cytotoxicity and bone formation ability of the modified zirconia using osteoblasts. As a result, it was found that the bioactivity of the zirconia surface was greatly improved by immobilizing nHA and type I collagen.
1. Introduction
In the past few years, zirconia dental implants have emerged as an alternative to titanium implants due to their osseo-integration potential [1,2,3] and other beneficial properties such as transparency [4,5] that mimics the natural teeth surrounding zirconia. In previous studies, it has been reported that zirconia reduces bacterial adhesion and biofilm accumulation, which can lower the inflammatory response in adjacent implant peri-implant tissues [6,7,8]. Furthermore, recent in vitro and in vivo studies have demonstrated that zirconia implants have similar results for osseo-integration index when compared to titanium-based implants [9,10]. As such, zirconia is replacing the disadvantages of titanium, such as corrosion and inconsistent color with teeth [11,12], but in order for zirconia to coexist with bone tissue, its bioactivity needs to be further improved.
One of the methods for imparting compatibility with bone tissue to zirconia implants is collagen immobilization. This is because collagen is the main component of bones along with hydroxyapatite. Hsu et al. [13] prepared a rough zirconia surface containing hydroxyl groups serving as hydrogen bond donors or acceptors using sandblasting and alkali treatment. Subsequently, type I collagen was immobilized on the rough zirconia surface using procyanidin, a natural crosslinking agent, in order to improve the cellular response. They also studied how human bone marrow mesenchymal stem cells (hMSCs) respond to collagen-immobilized surfaces in cell adhesion, proliferation, differentiation and mineralization. On the other hand, a process for preparing a collagen-yttria-stabilized amorphous zirconia hybrid scaffold by introducing acetylacetone-inhibited zirconia precursor nanoparticles into a polyallylamine-coated collagen matrix has been reported [14]. This polyelectrolyte coating induces the condensation of precursors in the fibrils to amorphous zirconia, and then changes to yttria-stabilized zirconia through calcination. These studies gave birth to a new paradigm for the synthesis of non-natural collagen-based hybrid scaffolds under alcoholic mineralization conditions. They reported that yttria-stabilized zirconia could be hierarchically integrated into collagen scaffolds even under water-starved conditions through propanol-based mineralization media. The protocol they used provided evidence to induce yttria-stabilized zirconia (YSZ) nanoparticles within a collagen template, allowing deposition within the fibrin. Another way to impart compatibility with bone tissue to zirconia implants is to coat them with hydroxyapatite.
A study to make it suitable for use in dental implants by simply coating a sol-gel-derived hydroxyapatite (HA) layer on the surface of zirconia and sintering it at various temperatures has been reported [15]. Results from in vitro cellular experiments using progenitor bone cell lines showed that HA-coated zirconia surfaces act as preferred surfaces for cell adhesion and proliferation over bare zirconia surfaces. In in vivo animal experiments, the bone conductivity of HA-coated zirconia was dramatically improved, similar to that of Ti implants. These results suggest that the sol-gel-based HA-coated zirconia has a high potential for use as a dental implant material. Because hydroxyapatite has excellent biocompatibility and osseo-integration, research on porous hydroxyapatite has been very active. However, porous hydroxyapatite is mechanically weak and brittle, making it difficult to mold and inject. One way to solve this problem is to introduce a strong porous network with a hydroxyapatite coating. Lee et al. [16] prepared a zirconia ceramic to which porous zirconia and alumina were added by infiltrating the ceramic slurry into the expanded polystyrene bead molded body and calcining it at 1500 °C. A slurry of hydroxyapatite-borosilicate glass mixed powder was then coated on a porous ceramic and fired at 1200 °C. As a result, the compressive strength of the obtained porous ceramic was 5.3–36.8 MPa, and Young’s moduli was 0.30–2.25 GPa, which was comparable to the mechanical properties of cancellous bone.
Cho et al. [17] evaluated the in vitro bone formation potential after coating HA on zirconia using an aerosol deposition method. They used scanning electron microscopy and X-ray diffraction to confirm that the HA thin film was deposited on the zirconia in a shallow and regular fashion. Cells on HA-coated zirconia showed lower proliferation than cells on uncoated zirconia. Nevertheless, alkaline phosphatase (ALP), alizarin red S staining, and bone marker gene expression analysis showed that HA-coated zirconia showed a superior osteogenic response.
In this study, an attempt was made to convert bio-inertness, which is a disadvantage of zirconia implants, into bioactivity using biomimetic technology. That is, nano-hydroxyapatite (nHA), the main component of bone, was first introduced onto the zirconia surface by chemical bonding, and then type I collagen was chemically bonded to hydroxyapatite immobilized on zirconia. Confirmation of each reaction step was confirmed using X-ray photoelectron spectroscopy (XPS), field emission scanning electron microscopy (FE-SEM), confocal fluorescence spectroscopy, and Fourier transform infrared (FT-IR) spectroscopy. In addition, the biocompatibility of zirconia implants co-immobilized with nHA and type I collagen was evaluated through cell bioactivity and differentiation studies using osteoblasts.
2. Materials and Methods
2.1. Materials
Zirconia screws and discs were provided by Mecetech Co., Ltd. (Kwangju 61009, Korea) and were manufactured by kneading a mixture of organic binder and zirconia powder in a twin-screw kneader, followed by injection molding, binder removal, and sintering. A zirconia screw (diameter: 3.5 mm, length: 15.0 mm) was used to investigate the topology of the surface, and a zirconia disk (diameter: 14.0 mm, thickness: 15.0 mm) was used for cell experiments. A spherical hydroxyapatite with a size of 20–70 nm was purchased from Sigma–Aldrich company (St. Louis, MO, USA). For collagen immobilization on the zirconia implant surface, a concentrated solution obtained by precipitating pig-derived type I collagen with salt was used (Ubiosis Company, Seongnam-si, Korea). Succinic acid, aminopropyltriethoxysilane (APTES), 1-ethyl-3-(3-dimethylaminopropyldicarbodiimide hydrochloride) (EDC), N-hydroxysuccinimide (NHS), were purchased from Sigma–Aldrich Chemical Company, (St. Louis, MO, USA), and used without further purification. To investigate cell bioactivity, a Live/Dead cell assay kit (R37601, ThermoFisher, Waltham, MA, USA) composed of FITC and Texas red was used. In addition, alkaline phosphatase activity of osteoblasts was measured using alkaline phosphatase staining kit (StemTAGTM, CBA-306, Cell Biolabs Inc., San Diego, CA, USA), and calcium deposition by osteoblasts was investigated using alizarin red S (Millipore, Billerica, MA, USA). The cells were cultured in α-minimum essential medium (α-MEM, Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) and 1.0% penicillin G-streptomycin at 37 °C under a 5% CO2 atmosphere. The culture medium was changed every other day. Mouse pre-osteoblast cells (MC3T3-E1) were purchased from Korea cells bank (Seoul, Korea) and stored in liquid nitrogen before carrying out cells seeding experiments. A 10 × 10−3 mmol phosphate buffer saline (PBS) solution (pH 7.4) containing 87 × 10−3 mmol Na2HPO4, 14 × 10−3 mmol KH2PO4, 131 × 10−3 mmol NaCl and 27 × 10−3 mmol KCl was purchased from Sigma–Aldrich (St. Louis, MO, USA). All other chemicals and solvents used in experimental work were high purity reagents.
2.2. Surface Characterization
The surface morphology of the zirconia implant was investigated by recording a FE-SEM (400 Hitachi, Tokyo, Japan). To record the FE-SEM micrographs, the zirconia screw and disks were fixed on the holder using carbon tape and then sputter-coated with platinum. The platinum-coated zirconia samples were then examined by FE-SEM under a high vacuum. FITC labeled type I collagen bound to the zirconia surface was confirmed by confocal laser fluorescence microscopy. Whether succinic acid was reacted by chemical covalent bonding to the nHA surface was confirmed by FT-IR spectrum (FT-IR, Galaxy 7020A, Mattson, Fremont, CA, USA). Identification of nHA and type I collagen on the zirconia surface was performed using
XPS (ESCA LAB VIG microtech Mt 500/1, Etc, East Grinstead, UK). The progress of the surface reaction was evaluated using the binding energy and peak intensity of each element in the X-ray photoelectron spectrum.
2.3. Chemical Bonding of Succinic Acid to the Surface of nHA
It is known that hydroxyl groups exist on the surface of hydroxyapatite [18,19]. In this study, a carboxyl group was introduced to the nHA surface by reacting the hydroxyl group on the nHA with the carboxyl group of succinic acid [20]. Briefly, an excess amount of succinic acid was dissolved in distilled water. Then, the free carboxylic acid groups of succinic acid were activated using EDC (0.5 g; 0.25 wt%) and NHS (0.05 g, 0.25 wt%) and kept for 6 h at 20–25 °C with gentle stirring to activate the carboxylic acid groups. nHA was then added to the solution of activated succinic acid and kept for 6 h with constant gentle stirring (Figure 1). To remove unreacted succinic acid, the reactant was dialyzed using a 10-k membrane flask, excess water was removed using a rotary evaporator, and finally freeze-dried. Next, 10 Mg of the succinic acid-grafted HA (S-HA) was mixed with 1 g of KBr, sufficiently milled, and then formed into a disk shape and subjected to FT-IR measurement. The introduction of succinic acid to the nHA surface is expected to change the charge density on the nHA surface. Therefore, the change in surface charge was investigated by measuring the zeta potential of the surface-modified nHA using a particle size analyzer (Zetasizer Nano ZS, Malvern, Worcestershire, UK).
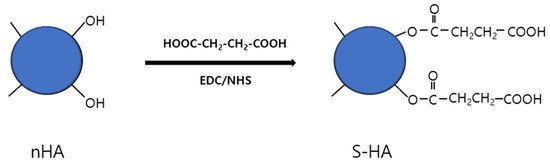
Figure 1.
Schematic diagram showing the chemical bonding of succinic acid (S) to the surface of nHA particles.
2.4. Chemical Bonding of nHA Particles to the Surface of Zirconia Implant
Before the surface reaction of the zirconia implant, the sample was washed as follows. Samples were immersed in 300 mL ethanol and sonicated for 30 min. Thereafter, the sample was immersed in distilled water containing 0.01% Tween 20, ultrasonically washed for 30 min, and finally washed with distilled water and dried. After mixing 240 mL ethanol and 10 mL APTES, the pH was adjusted to 4.5 with a small amount of acetic acid. After fastening the zirconia sample to the jig, 4% APTES solution was added and reacted in silicon oil bath at 90 °C for 2 h with gentle stirring under N2 ambient atmosphere. After the silanization reaction was completed, the zirconia sample was washed with an aqueous solution in an ultrasonic cleaner for 5 min to obtain a zirconia implant bound with a primary amine (ZrO2-NH2). ZrO2-NH2 was finally dried under reduced pressure at room temperature for 12 h. The presence of APTES on the zirconia surface was confirmed by measuring the XPS of the sample and observing nitrogen and silicon elements.
The linking reaction between ZrO2-NH2 and S-HA was performed as follows (Figure 2). First, ZrO2-NH2 was immersed in an aqueous solution of pH 8.5 so that the amino group on the surface did not become a salt. Then, 500 mg of S-HA was placed into a 50 mL conical tube, and 20 mL distilled water was added and ultrasonically dispersed for 1 min. Then, it was dispersed for 1 min using a homogenizer. This solution was placed in a 250 mL round flask and mixed with 200 mL of distilled water. After that, 0.25 g of EDC was added and stirred for 30 min. Subsequently, 0.25 g of NHS was added and reacted for 2 h to activate S-HA. After fastening ZrO2-NH2 to the jig, it was placed into a 500 mL reaction flask containing the activated S-HA solution, and reacted for 24 h at 1000 rpm under magnetic stirring to produce S-HA-grafted ZrO2 (ZrO2-HA). After the reaction, the sample was ultrasonically washed in distilled water for 30 s, dried, and then XPS analysis was performed to confirm the presence of nHA on the surface of ZrO2 implant.
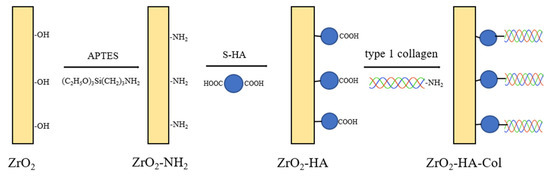
Figure 2.
Schematic diagram showing the process of chemically bonding nHA particles to the surface of zirconia and subsequently immobilizing type I collagen.
2.5. Chemical Bonding of Type I Collagen to the Surface of ZrO2-HA
A collagen solution was prepared by adding 10 μg of type I collagen to 200 mL of distilled water and stirring for 24 h. After activating ZrO2-HA using EDC/NHS for 2 h, it was reacted with a collagen solution for 48 h to prepare ZrO2-HA to which collagen was chemically bound (Figure 2). After the reaction, it was ultrasonically washed with distilled water for 30 s and then dried. Meanwhile, FITC-labeled collagen was reacted with ZrO2-HA, and introduction of collagen was confirmed using a confocal fluorescence microscopy.
2.6. Cell Viability of ZrO2-HA and ZrO2-HA-Col
Before proceeding with the bioactivity test of the zirconia implant, the surface-modified zirconia implant was entrusted to a specialized company (Solmedix Co., Ltd. Seoul, Korea) and EO gas sterilization of the sample was performed. To investigate the cellular activity of surface-modified zirconia (ZrO2, ZrO2-HA and ZrO2-HA-Col), the disks were mounted in 12-well culture dishes and Dulbecco’s 10% fetal bovine serum (FBS; Gibco, modified eagle medium (DMEM), Invitrogen, Carlsbad, CA, USA) was added. Then, 1 mL of MC3T3-E1 cell solution (1 × 104 cells/mL) was added to the surface-modified zirconia and incubated in a humidified atmosphere containing 5% CO2 at 37 °C for 1 and 3 days. After culture, osteoblasts were detached using 0.05% trypsin and suspended in PBS at a cell density of 1 × 105 to 1 × 106 cells/mL. Then, 200 µL of the cell suspension was mixed with 100 µL assay solution (90 µL FITC solution (1 mM in DMSO) and 9 µL Texas red (1.5 mM in H2O)) with 15 mL PBS and incubated for 30 min at a room temperature. Simultaneous monitoring of viable (green) and dead (red) cells was performed by examining cells with confocal fluorescence microscopy at 488 and 570 nm excitation, respectively [21].
2.7. Differentiation of Osteoblasts Cultured on the Surface-Modified Zirconia
To evaluate the osteogenic differentiation of MC3T3-E1 cells cultured on surface-modified ZrO2 discs, alkaline phosphatase (ALP) activity was measured [22] using alkaline phosphate staining kit (StemTAGTM, CBA-306, Cell Biolabs Inc., San Diego, CA, USA). To achieve this, sterile surface-modified ZrO2 discs were placed in a 12-well cell culture plate and MC3T3-E1 cells were plated onto it at a cell density of 1 × 105 cells/mL. After incubation for 21 days in a humidified atmosphere at 37 °C and 5% CO2, the culture medium was aspirated and washed with PBS containing 0.05% Tween-20 (PBS-T). Fixing solution (1 mL/well) was added to the cells and incubated for 2 min at room temperature. After removing the fixative, the cells were washed with PBS containing Tween-20. After aspirating the washing solution, 1 mL of the ALP staining solution (SBA-306) was added to each well. Plates were incubated at room temperature for 30 min protected from light. After removing the ALP staining solution and washing the cells stained with 2 mL of PBS-T twice, the stained cells were observed using an optical microscope (Nikon E 4500, Tokyo, Japan).
To measure the calcium-producing ability of MC3T3-E1 cultured for 21 days in surface-modified zirconia, calcium produced by cultured MC3T3-E1 cells was stained with alizarin red S (2003999, Nalgene Nunc Inc., Naperville, IL, USA) [22]. Briefly, MC3T3-E1 cells were cultured for 21 days on circular ZrO2 disks placed in 12-well culture plates at a cell density of 1 × 105 cells/mL in a humidified atmosphere of 5% CO2 at 37 °C. Then, the medium was carefully aspirated, the discs were washed twice with PBS solution, and then the adherent cells were fixed using 10% formaldehyde solution at room temperature for about 15 min. After fixation, the cells were rinsed 3 times (10 min each) with distilled water to remove residual formaldehyde. After removal of excess water, alizarin red staining of seeded cells was performed by adding 1 mL of alizarin red solution/well and incubating the samples for approximately 30 min. After staining, excess alizarin red was removed by gently washing with distilled water. Optical images of stained cells were captured using an optical microscope (Nikon E 4500, Tokyo, Japan).
3. Results
3.1. Chemical Bonding of Succinic Acid to the Surface of nHA
You et al. [23] investigated the pH change of the reaction solution by reacting the OH group of hydroxyapatite with tetraethylorthosilicate (TEOS) and APTES. From the result, it was confirmed that TEOS and APTES were not physically adsorbed to the OH group on the HA surface, but were crystallographically and chemically covalently bound to calcium and phosphate ions. In this study, after activating the carboxyl group of succinic acid [20], it reacted with the hydroxyl group located on the nHA surface. HA is positively and negatively charged along the 𝛼 and 𝑐 sides of the unit cell, respectively. The 𝛼 side tends to absorb more acidic proteins and the 𝑐 side tends to attract basic proteins [24,25]. In this study, the zeta potential values of nHA and S-HA nanoparticles were calculated from the electrophoretic mobility results and the Helmholtz-Smoluchowski equation [26]. As shown in Figure 3a, the error range of the first, second, and third measured zeta potential values was relatively large, ranging from 32 to 39 mV. This is thought to be because the aggregation state differs for each sample due to the aggregation phenomenon peculiar to nanoparticles [27]. On the other hand, after chemical bonding of succinic acid to the nHA surface, the error range of the zeta potential was very small regardless of the number of measurements, and the average value was about 10 mV. The decrease in the positive value of the S-HA potential and the low standard deviation are because anionic succinic acid was introduced to the surface to reduce the cationicity of HA by calcium, and the aggregation phenomenon was reduced due to the ionic repulsion of the S-HA particles [28].
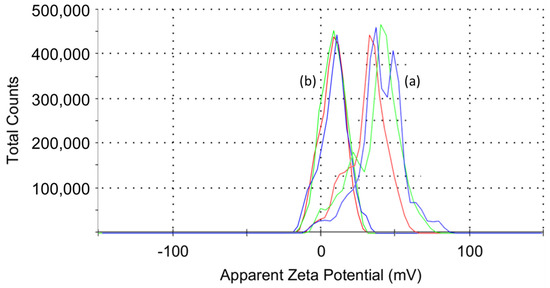
Figure 3.
Zeta potential values of (a) nHA and (b) S-HA nanoparticles. The zeta potential value of nHA was different each time it was measured due to aggregation in distilled water. On the other hand, S-HA had almost no aggregation, so the dispersing power was improved, and as a result, the same value was displayed every time it was measured.
Whether succinic acid was successfully introduced to the surface of the nHA particles can also be determined by infrared spectroscopy. Figure 4 shows the FT-IR spectra of nHA and S-HA. When the hydroxyl group of nHA and the carboxylic acid of succinic acid react, one ester group and one carboxyl group are formed [29]. In Figure 4a, stretching vibration caused by the carbonyl group of the ester was shown at 1700 cm−1. This indicates that succinic acid was successfully chemically bonded to the nHA surface.
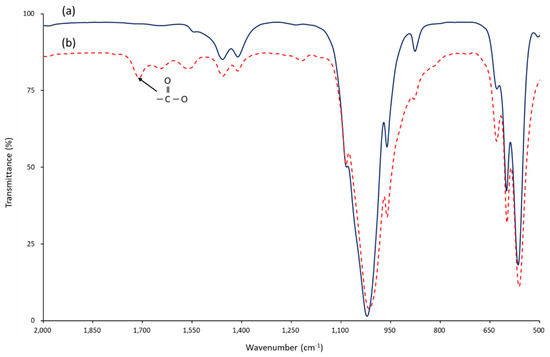
Figure 4.
FT−IR spectra of (a) nHA and (b) succinic acid-grafted nHA (S-HA) measured by KBr disc method. When succinic acid reacts with the hydroxyl group of nHA, an ester bond is formed and free carboxylic acid remains. The success of the surface reaction can be known from the presence of carbonyl stretching vibration (1700 cm−1) absorption in the IR spectrum.
3.2. Chemical Bonding of nHA to the Surface of ZrO2 Implant
Figure 5 shows the scanning electron micrograph of the surface of the zirconia implant (Figure 5a) and the nHA-bonded zirconia implant (Figure 5b). It can be seen that the surface of the zirconia implant obtained by injection molding is composed of granules with a diameter of 200–500 nm. After the surface reaction of nHA, it can be seen that HA particles with a diameter of about 20–70 nm are randomly bound to the zirconia surface. As a result of calculating the number of bound nHA particles per unit area for three samples, it was 21.7 + 2.4 ea/μm2.
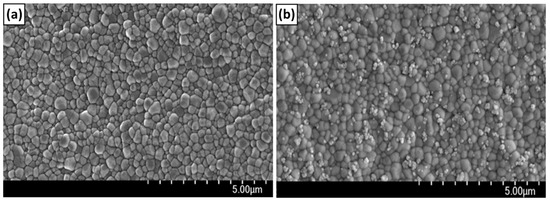
Figure 5.
Scanning electron microscopy images of ZrO2 disk (a) and ZrO2–HA disk (b). It can be seen that the zirconia disk (a) obtained by injection molding is composed of granules with a diameter of 200 to 500 nm. On the other hand, after the nHA reaction, single nHA and nHA clusters were bound to the zirconia surface in a dispersed state.
XPS is very useful when we want to check whether the surface reaction of a solid has progressed well. Figure 6 shows the XPS results of surface-modified zirconia implants. As aminopropyltriethoxysilane bound to zirconia (ZrO2-NH2), new peaks due to N1s and Si2s appeared at 399.5 eV and 133.8 eV, respectively, as shown in Figure 6b. In addition, the peak strength of Ca and P was significantly increased due to the introduction of nHA (Figure 6c).
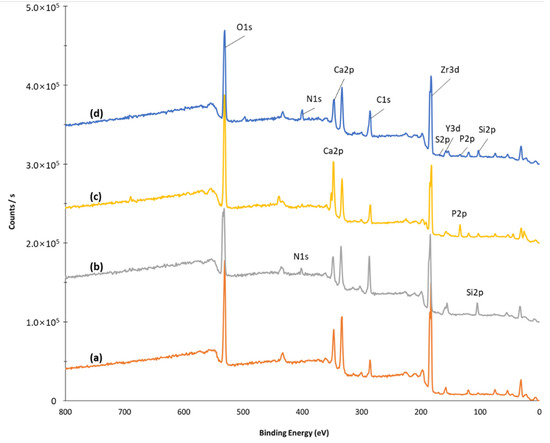
Figure 6.
X-ray photoelectron spectroscopy of ZrO2 (a), ZrO2-NH2 (b), ZrO2-HA (c), and ZrO2-HA-Col (d). After the APTES reaction, the Si peak appeared at 100 eV, after the HA reaction, the peaks due to P and Ca at 120 and 320 eV, and after the collagen reaction, the peak due to nitrogen appeared at 400 eV.
3.3. Chemical Bonding of Type I Collagen to the Surface of ZrO2 Implant
Type I collagen has poor solubility in aqueous solutions. Therefore, it is usually dissolved in 0.1% acetic acid aqueous solution and used for various reactions. In this study, by using a concentrated solution obtained by precipitating pig-derived type I collagen, it could be dissolved in a neutral aqueous solution excluding acetic acid [29]. Whether or not collagen is introduced to ZrO2-HA can be predicted through surface elemental analysis before and after collagen introduction. Figure 6 shows the XPS survey scan spectra of the surface-modified zirconia, and Table 1 shows the element ratio calculated from the area of each peak. Looking at Figure 6, in the case of ZrO2-HA before collagen introduction, peaks attributed to Ca2p at 347 eV and P2p at 134 eV appeared. On the other hand, after collagen introduction, the intensity of Ca2p and P2p peaks decreased, and the peak of 399.5 eV due to N1s increased significantly. This suggests that type I collagen was successfully introduced to ZrO2-HA.

Table 1.
Atomic percentages of surface modified ZrO2 implants calculated from the XPS survey scan spectra.
As shown in Figure 7b, it can be seen that nHA with a diameter of 20–70 nm is irregularly bonded to the surface of zirconia connected in granules of 200–500 nm. However, the appearance of nHA was not seen on the surface where collagen was bound to ZrO2-HA. This may be because the collagen molecules bound to the nHA and covered the image of the nanospheres. To confirm the presence of collagen, FITC-labeled type I collagen was reacted with ZrO2-HA and observed with a confocal fluorescence microscope. As shown in Figure 7d, it can be seen that the green image appears. From this, it can be seen that S-HA has an extra carboxyl group after reaction on the ZrO2 surface, and the collagen chemically reacts with these carboxyl groups to be immobilized.
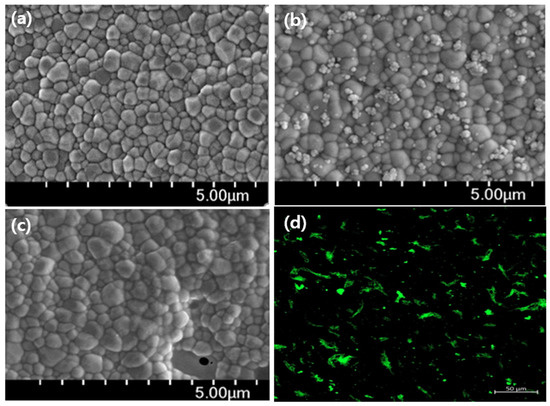
Figure 7.
Images of surface-modified step-by-step zirconia implants. SEM images of ZrO2 (a), ZrO2-HA (b), ZrO2-HA-Col (c), and confocal fluorescence image of ZrO2-HA-FITC-Col (d). It can be seen that nHA particles with a diameter of 20 to 70 nm are bound in ZrO2-HA. Once the collagen binds to the nHA, the image of the nHA particles disappears. The presence of collagen can be confirmed by confirming the green image by reacting the FITC-labeled collagen and then measuring it with a confocal fluorescence microscope.
3.4. Cytotoxicity of Osteoblasts Cultured on the Zirconia Implants Immobilized with nHA and Type I Collagen
Figure 8 shows the results of observation with a confocal fluorescence microscope after culturing osteoblasts in surface-modified zirconia for one day, staining them with FITC and Texas red. Texas red reacts with nucleic acids in dead cells whose membranes have been destroyed, giving them a red color [30]. In Figure 8, the red image did not appear at all. On the other hand, FITC is stained green in living cells. As shown on the right side of Figure 8, the number of cells attached to the ZrO2 implant was very small. However, more cells adhered to ZrO2-HA and ZrO2-HA-Col. On the other hand, when the incubation time was extended to 3 days, as shown in Figure 9, cells not only increased the number of adhesions on ZrO2-HA-Col but also spread well. Excellent adhesion and spreading of cells on ZrO2-HA-Col is due to the specific recognition between the integrin of the osteoblast membrane and the arginine-glycine-aspartic acid (RGD) sequence of immobilized collagen [29].
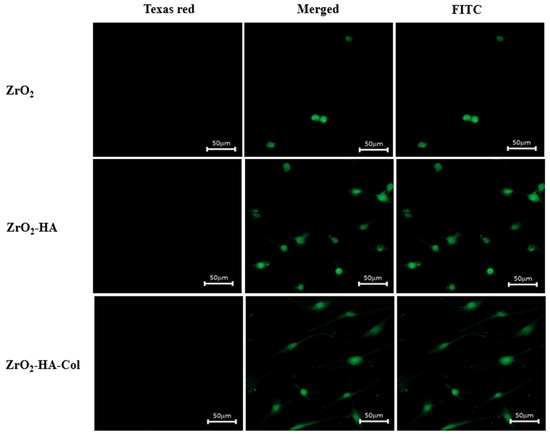
Figure 8.
Confocal fluorescence microscopy images of osteoblasts cultured for 1 day on the ZrO2, ZrO2-HA and ZrO2-HA-Col. In order to investigate the cytotoxicity by the scaffold, confocal fluorescence microscopy images were examined by staining with Texas red and FITC. Dead cells are stained by Texas red and appear red, while living cells are stained by FITC and appear green.
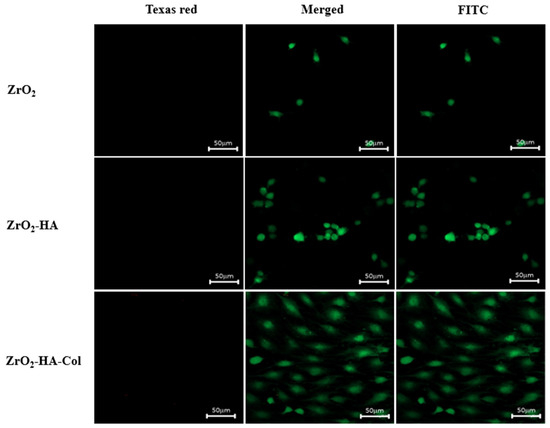
Figure 9.
Confocal fluorescence microscopy images of osteoblasts cultured for 3 days on the ZrO2, ZrO2-HA and ZrO2-HA-Col. Cells were stained with Texas red and FITC after culture.
3.5. Differentiation of Osteoblasts Cultured on the Zirconia Implants Immobilized with nHA and Type I Collagen
Figure 10 shows the results of culturing osteoblasts in surface-modified zirconia for 14 days and then staining them with alkaline phosphate staining kit. When ALP is stained, a purple image appears. As shown in Figure 10, almost no purple image was shown in ZrO2 and ZrO2-HA, and identifiable ALP was shown in ZrO2-HA-Col. The culture time of 14 days was considered insufficient to evaluate ALP activity, so the cell culture time was extended to 21 days.
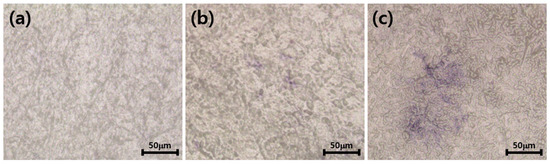
Figure 10.
Alkaline phosphatase activity of MC3T3-E1 cells cultured on ZrO2 (a), ZrO2-HA (b) and ZrO2-HA-Col (c). After culturing the cells for 14 days, the cells were stained with Fast Blue RR salt and naphthol AS MX phosphate alkaline solution, and a purple image showing ALP activity was observed under an optical microscope.
As shown in Figure 11, the amount of ALP synthesized by the cells cultured on the ZrO2-HA (b) and ZrO2-HA-Col (c) was apparently higher (blue spot in figure) than those synthesized on the ZrO2 (a). As ALP is one of the most exclusive proteins synthesized by osteoblasts, the presence of ALP in MC3T3-E1 osteoblasts, which were cultured on ZrO2-HA (b) and ZrO2-HA-Col (c) implants could be used as one of the markers to confirm the osteoblastic phenotype of the cells.
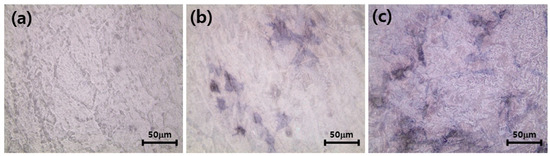
Figure 11.
Alkaline phosphatase activity of MC3T3-E1 cells cultured on ZrO2 (a), ZrO2-HA (b) and ZrO2-HA-Col (c). After culturing the cells for 21 days, the cells were stained with Fast Blue RR salt and naphthol AS MX phosphate alkaline solution, and a purple image showing ALP activity was observed under an optical microscope.
Alizarin red staining indicates the process of transformation of undifferentiated MC3T3-E1 cells to osteoblasts and their subsequent mineralization leading to bone formation. In vitro Alizarin red staining enables us to visualize the process of osteogenesis by producing a red color on calcium deposition. The intensity of alizarin red staining for MC3T3-E1 cells (red spots) inoculated on clean ZrO2 (Figure 12a), ZrO2-HA (Figure 12b) and ZrO2-HA-Col (Figure 12c) was compared. As a result, the osteogenic levels of cells cultured on ZrO2-HA (Figure 12b) and ZrO2-HA-Col (Figure 12c) were significantly higher than on ZrO2.
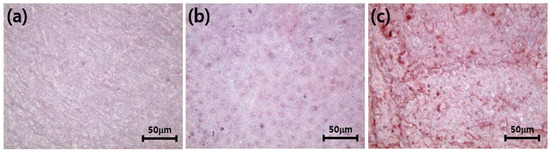
Figure 12.
Alizarin red staining of MC3T3-E1 cells cultured on pristine ZrO2 (a), ZrO2-HA (b) and ZrO2-HA-Col (c). Cells were cultured for 21 days in circular zirconia disks placed in wells of a 24-well culture plate. After removing the medium, the adhered cells were fixed with 10% formaldehyde aqueous solution. Thereafter, alizarin red staining was performed on the cultured cells. Digital images were captured with an optical microscope equipped with an advanced camera.
4. Discussion
It has been reported that zirconia can lower the inflammatory response of tissues around implants by reducing bacterial adhesion and biofilm accumulation [8]. According to a recent study, zirconia implants showed similar results in osseointegration index compared to titanium-based implants [10]. It is true that zirconia is replacing the disadvantages of titanium corrosion and tooth irregularity [12], but for zirconia to coexist with bone tissue, its bioactivity needs to be further improved. All-ceramic dental implants have received much attention because they can provide a solution to the potential immunological and esthetic damage observed with titanium implants [31]. In addition, it has been established that the inflammatory response and bone resorption induced by the released zirconia particles are much lower than the inflammatory response and bone resorption induced by titanium particles [17,32]. On the other hand, the zirconia surface is not easily etched by chemicals because it is strong, dense, and inert [33].
Macro, micro and nano scale physical, chemical and biological techniques have been used for surface modification to convert inactive zirconia to active zirconia. In particular, Chopra et al. [34] have extensively reported on nano-engineered zirconia-based implants that are highly bioactive and treatable from past to future prospects. In addition, Kohalet et al. [35] reported a long-term prospective clinical cohort study to determine the survival rate and marginal bone loss of an integrated transmucosal zirconia oral implant used for single tooth replacement. Porous zirconia scaffolds coated with HA can also be used as drug delivery systems to enhance bone response and ensure proper osseointegration [36]. Aboushelib and Shawky [37] evaluated the osteogenic ability of HA-filled porous zirconia scaffolds. As a result, the zirconia scaffolds filled with HA on the surface showed a significantly higher amount of new bone formation (33 ± 14%) than the scaffolds without HA (21 ± 11%).
Milling, grain grinding, high-temperature acid etching [38], laser micro-etching [39], salt extraction and sintering methods have been widely used for the manufacture of porous zirconia [40]. In addition, it has been known that the bioactivity can be increased to some extent by filling the prepared porous zirconia with HA. Porous zirconia filled with HA by simple physical diffusion has the potential to release HA particles within the body environment. Therefore, it is necessary to maintain safety so that the charged HA is not released. As a method to overcome this, a method of chemically covalently bonding nano-HA to a metal or ceramic surface has been reported. Park et al. [41] chemically reacted APTES to the titanium implant surface and subsequently bound albumin. After that, L-glutamic acid was chemically reacted with nano-HA, and this was reacted with the titanium surface. The resulting titanium-HA implant was found to exhibit better cell compatibility than the original titanium implant.
In this study, zirconia screws and discs were prepared by hot press injection molding using granular zirconia (diameter 200–500 nm). In order to increase the bioactivity of the obtained zirconia implant, nano HA (diameter 20–70 nm) was chemically bonded to the zirconia surface, and then type 1 collagen was chemically bonded thereto. Briefly, reactive amino groups were introduced by conjugating a silane coupling agent on the zirconia surface [41]. Thereafter, succinic acid [20] was introduced to the nHA surface and reacted with an amino group on the zirconia surface to prepare a zirconia implant chemically bonded to nHA. As shown in Figure 3, when succinic acid is introduced to the nHA surface, a carboxyl group is introduced and the zeta potential of the surface shifted from positive to negative. In addition, particle aggregation is suppressed due to the repulsive force of surface anions [42]. The carboxyl group introduced to the nHA surface could be easily identified through FT-IR (Figure 4). Whether S-HA was bound to the zirconia surface could be confirmed by observing the intensity of the binding energy values of Ca2p and P2p of XPS (Figure 6c). The nHA on the zirconia surface could also be directly confirmed by SEM (Figure 5b). Focusing on the fact that the main components of bone are hydroxyapatite and collagen, type I collagen was chemically bonded to the surface of zirconia in which nHA was introduced. When succinic acid reacts with the nHA surface, many free carboxylic acids (S-HA) remain on the nHA surface (Figure 2). In this study, type 1 collagen was chemically bound using the carboxyl group remaining on the nHA surface. It is difficult to identify the collagen introduced on the zirconia surface by SEM (Figure 7c). Therefore, after reacting ZrO2-HA with FITC-labeled collagen, a green image of the introduced collagen was confirmed with a confocal fluorescence microscope (Figure 7d). In addition, the elemental composition before and after the collagen reaction was observed using XPS. As a result, the successful introduction of collagen was confirmed from the decrease in the binding energy of Ca and P in HA and the increase in the binding energy due to N in collagen.
In a previous report [29], osteoblast adhesion experiments were performed on non-porous PEEK and nano-pore PEEK implants. As a result, it was found that osteoblasts adhered and spread very quickly on the nanopore surface. In this study, nHA and collagen were immobilized using the granulated zirconia surface as it is. As a result, there were very few adherent cells in ZrO2 after 1 day and 3 days. This means that the zirconia surface is inactive and the interaction with cells is very low. Adherent cells slightly increased on the surface where nHA was introduced to zirconia. The reason that the number of adherent cells in ZrO2-HA did not increase as expected may be due to the insufficient amount of nHA immobilization. On the other hand, in ZrO2-HA-Col introduced with collagen, the adhesion number of osteoblasts after 3 days was greatly increased, and the adhered cells spread well (Figure 12). Figure 11 and Figure 12 are images of cultured cells stained with FITC and Texas red. Texas red binds to dead cells, giving it a red color. As can be seen from the figure, it is judged that the surface-modified zirconia implant does not show toxicity because the red image is not seen at all.
Alkaline phosphatase (ALP) is considered an early marker of osteoblast differentiation. Because ALP is a by-product of osteoblast activity, an increase in ALP levels suggests active bone formation. Figure 10 shows an image of ALP expressed when osteoblasts are cultured on a zirconia scaffold for 21 days. As can be seen in Figure 10a, there were no images of ALPs marked in purple. However, purple images were clearly visible in HA-immobilized zirconia (ZrO2-HA (b)) and HA- and collagen-immobilized zirconia (ZrO2-HA-Col (c)), indicating that osteoblasts were well differentiated. Alizarin red S staining is a commonly used stain to identify calcium-bearing osteocytes in differentiated osteoblasts. Figure 11 shows the results of alizarin red S staining after culturing osteoblasts for 21 days. As a result, no red spots appear on ZrO2. This means that osteoblasts are not properly differentiated in zirconia. However, red spots were observed in ZrO2-HA and ZrO2-HA-Col. This is thought to be the result of the contribution of nHA and type I collagen introduced to the zirconia surface to the differentiation of osteoblasts. We were able to successfully introduce nHA and type I collagen to the zirconia surface through chemical covalent bonding. It was confirmed that nHA-immobilized zirconia (ZrO2-HA) and nHA- and type I collagen-immobilized zirconia (ZrO2-HA-Col) exhibit high bioactivity through in vitro osteoblastic cell experiments. Zirconia implants with improved bioactivity have a high potential to replace bones or teeth. Currently, animal experiments using rabbit mandibular teeth are in progress, and bone regeneration capacity and bone contact area will be investigated.
Author Contributions
Conceptualization, H.K.; methodology, Y.-H.L.; validation, N.-K.K.; Writing-original draft preparation, I.-K.K.; Project administration, H.K.; funding acquisition, H.K. and I.-K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Technology Development Program (S3005767) supported by the Ministry of SMEs and Startups (MSS, Korea).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
We would like to thank Mecetech Co., Ltd. (Kwangju 61009, Korea) for providing the zirconia discs and screws.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, such as the collection, analyses, or interpretation of data, writing of the manuscript, or the decision to publish the results.
References
- Kohal, R.J.; Weng, D.; Bachle, M.; Strub, J.R. Loaded custom-made zirconia and titanium implants show similar osseointegration: An animal experiment. J. Periodontol. 2004, 75, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin. Implant Dent. Relat. Res. 2005, 7, S13–S20. [Google Scholar] [CrossRef]
- Hoffmann, O.; Angelov, N.; Gallez, F.; Jung, R.E.; Weber, F.E. The zirconia implant-bone interface: A preliminary histologic evaluation in rabbits. Int. J. Oral Maxillofac. Implant. 2008, 23, 369–374. [Google Scholar]
- Ahmad, I. Yttrium-partially stabilized zirconium dioxide posts: An approach to restoring coronally compromised nonvital teeth. Int. J. Periodon. Restor. Dent. 1998, 18, 455–465. [Google Scholar]
- Jackson, M.C. Restoration of posterior implants using a new ceramic material. J. Dent. Technol. 1999, 16, 19–22. [Google Scholar]
- Scarano, A.; DiCarlo, F.; Quaranta, M.; Piattelli, A. Bone response to zirconia ceramic implants: An experimental study in rabbits. J. Oral Implantol. 2003, 29, 8–12. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Kohal, R.J.; Schwindling, F.S.; Bächle, M.; Spies, B.C. Peri-implant bone response to retrieved human zirconia oral implants after a 4-year loading period: A histologic and histomorphometric evaluation of 22 cases. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1622–1631. [Google Scholar] [CrossRef]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.-P.; Kiattavorncharoen, S.; Lauer, H.-C.; Meyer, U.; Kübler, N.R.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008, 4, 30. [Google Scholar] [CrossRef]
- Dubruille, J.H.; Viguier, E.; Le Naour, G.; Dubruille, M.T.; Auriol, M.; Charpentier, Y.L. Evaluation of combinations of titanium, zirconia, and alumina implants with 2 bone fillers in the dog. Int. J. Oral Maxillofac. Implant. 1999, 14, 271–277. [Google Scholar]
- Karoussis, I.K.; Salvi, G.E.; Heitz-Mayfield, L.J.A.; Bragger, U.; Hämmerle, C.H.F.; Lang, N.P. Long-term implant prognosis in patients with and without a history of chronic periodontitis: A 10-year prospective cohort study of the ITI dental implant system. Clin. Oral Implant. Res. 2003, 14, 329–339. [Google Scholar] [CrossRef]
- Mareci, D.; Trincă, L.C.; Căilean, D.; Souto, R.M. Corrosion resistance of Zr/Ti alloys with hydroxyapatite-zirconia-silver layer in simulated physiological solution containing proteins for biomaterial applications. Appl. Surf. Sci. 2016, 389, 1069–1075. [Google Scholar] [CrossRef]
- Hsu, C.M.; Sun, Y.S.; Huang, H.H. Enhanced cell response to zirconia surface immobilized with type I collagen. J. Dent. Res. 2019, 98, 556–563. [Google Scholar] [CrossRef]
- Zhou, B.; Niu, L.N.; Shi, W.; Zhang, W.; Arola, D.D.; Breschi, L.; Mao, J.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Synthesis of hierarchical mesoporous zirconia fiber by using collagen fiber as a template. J. Mater. Res. 2008, 23, 3263–3268. [Google Scholar]
- Kim, J.; Kang, I.G.; Cheon, K.H.; Lee, S.; Park, S.; Kim, H.E.; Han, C.M. Stable sol–gel hydroxyapatite coating on zirconia dental implant for improved osseointegration. J. Mater. Sci. Mater. Med. 2021, 32, 81. [Google Scholar] [CrossRef]
- Lee, J.; Eum, S.; Kim, J.; Jang, W.Y. Fabrication of hydroxyapatite-coated zirconia by room temperature spray process and microstructural change by heat-treatment. J. Korean Soc. Heat Treat. 2015, 28, 17–23. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, J.; Ryoo, H.; Kim, D.; Park, J.; Han, J. Osteogenic responses to zirconia with hydroxyapatite coating by aerosol deposition. J. Dent. Res. 2015, 94, 491–499. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D. Bioactive ceramics: Physical chemistry. In Comprehensive Biomaterials; Ducheyne, P., Healy, K., Hutmacher, D., Grainger, D.E., Kirkpatrick, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 187–221. [Google Scholar]
- Anwar, A.; Kanwal, Q.; Akbar, S.; Munawar, A.; Durrani, A.; Farooq, M.H. Synthesis and characterization of pure and nano-sized hydroxyapatite bioceramics. Nanotechnol. Rev. 2017, 6, 149–157. [Google Scholar] [CrossRef]
- Haider, A.; Gupta, K.C.; Kang, I.K. PLGA/nHA hybrid nanofiber scaffold as a nano-cargo carrier of insulin for accelerating bone tissue regeneration. Nanoscale Res. Lett. 2014, 9, 314. [Google Scholar] [CrossRef]
- Kimura, A.; Abe, H.; Tsuruta, S.; Chiba, S.; Fujii-Kuriyama, Y.; Sekiya, T.; Morita, R.; Yoshimura, A. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int. Immunol. 2013, 26, 209–220. [Google Scholar] [CrossRef]
- Kwon, G.; Kim, H.; Gupta, K.C.; Kang, I.-K. Enhanced Tissue Compatibility of Polyetheretherketone Disks by Dopamine-Mediated Protein Immobilization. Macromol. Res. 2018, 25, 128–138. [Google Scholar] [CrossRef]
- You, C.K.; Kim, S.M.; Ahn, M.W.; Kim, S.Y. Evaluation of hydroxyl groups on hydroxyapatite and calcium metaphosphate by grafting TEOS and APTES. Key Eng. Mater. 2007, 342–343, 677–680. [Google Scholar] [CrossRef]
- Uskokovic, V.; Uskokovi, D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. 2011, 96, 152–191. [Google Scholar] [CrossRef]
- Kandori, K.; Oda, S.; Fukusumi, M.; Morisada, Y. Synthesis of positively charged calcium hydroxyapatite nano-crystals and their adsorption behavior of proteins. Coll. Surf. 2009, 73, 140–145. [Google Scholar] [CrossRef]
- Park, H.M.; Lee, W.M. Helmholtz–Smoluchowski velocity for viscoelastic electroosmotic flows. J. Colloid Interface Sci. 2008, 317, 631–636. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Eftekhari, M.; Schwarzenberger, K.; Javadi, A.; Eckert, K. The influence of negatively charged silica nanoparticles on the surface properties of anionic surfactants: Electrostatic repulsion or the effect of ionic strength? Phys. Chem. Chem. Phys. 2020, 22, 2238–2248. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.H.; Kim, N.K.; Kang, I.-K. Immobilization of collagen on the surface of a PEEK implant with monolayer nanopores. Polymers 2022, 14, 1633. [Google Scholar] [CrossRef]
- Titus, J.A.; Haugland, R.; Sharrow, S.O.; Segal, D.M. Texas red, a hydrophilic, red-emitting flourophore for use with flourescein in dual parameter flow microfluorometric and fluorescence microscopic studies. J. Immunol. Methods 1982, 50, 193–204. [Google Scholar] [CrossRef]
- Kohal, R.J.; Knauf, M.; Larsson, B.; Sahlin, H.; Butz, F. One-piece zirconia oral implants: One-year results from a prospective cohort study. Single tooth replacement. J. Clin. Periodontol. 2012, 39, 590–597. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Flückiger, L.B.; Attin, T.; Schmidlin, P.R. Effect of titanium and zirconium oxide microparticles on pro-Inflammatory response in human macrophages under induced sterile inflammation: An in vitro study. Materials 2021, 14, 4166. [Google Scholar] [CrossRef]
- Smielak, B.; Klimek, L. Effect of hydrofluoric acid concentration and etching duration on select surface roughness parameters for zirconia. J. Prosth. Dent. 2015, 113, 596–602. [Google Scholar] [CrossRef]
- Chopra, D.; Jayasree, A.; Guo, T.; Gulati, K.; Ivanovski, S. Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioact. Mater. 2022, 13, 161–178. [Google Scholar] [CrossRef]
- Kohal, R.-J.; Spies, B.C.; Bauer, A.; Butz, F. One-piece zirconia oral implants for single-tooth replacement: Three-year results from a long-term prospective cohort study. J. Clin. Periodon. 2017, 45, 114–124. [Google Scholar] [CrossRef]
- Naleway, S.E.; Fickas, K.C.; Maker, Y.N.; Meyers, M.A.; McKittrick, J. Reproducibility of ZrO2-based freeze casting for biomaterials. Mater. Sci. Eng. C 2016, 61, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Aboushelib, M.N.; Shawky, R. Osteogenesis ability of CAD/CAM porous zirconia scaffolds enriched with nanohydroxyapatite particles. Int. J. Implant Dent. 2017, 3, 21. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, K.C.; Kim, N.-H.; Moon, H.S. Evaluation of zirconia sueface after strong acid etching and its effects on the shear bond strength of dental resin cement. Int. J. Dent. 2019, 2019, 3564275. [Google Scholar] [CrossRef]
- Turp, V.; Akgungor, G.; Sen, D.; Tuncelli, B. Evaluation of surface topography of zirconia ceramic after Er:YAG laser etching. Photomed. Laser. Surg. 2014, 32, 533–539. [Google Scholar] [CrossRef]
- An, S.-H.; Matsumoto, T.; Miyajima, H.; Nakahira, A.; Kim, K.-H.; Imazato, S. Porous zirconia/hydroxyapatite scaffolds for bone reconstruction. Dent. Mater. 2012, 28, 1221–1231. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, B.S.; Gupta, K.C.; Lee, D.Y.; Kang, I.-K. Hydroxyapatite nanorod-modified sand blasted titanium disk for endosseous dental implant applications. Tissue Eng. Regen. Med. 2018, 15, 601–614. [Google Scholar] [CrossRef]
- Liu, G.; Wu, C.; Zhang, X.; Liu, Y.; Meng, H.; Xu, J.; Xu, X.; Xu, Y. Surface functionalization of zirconium dioxide nano-adsorbents with 3-aminopropyltriethoxysilane and promoted adsorption activity for bovine serum albumin. Mater. Chem. Phys. 2016, 176, 129–135. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).