Abstract
This study evaluates a commercial polycyanoacrylate adhesive of medium viscosity regarding its suitability for the restoration of glass objects of cultural heritage in a museum environment (exhibition/storage). Loctite® Super Attak was investigated in terms of (a) its polymerisation rate and degree of conversion, using Infrared Spectroscopy FT-IR by monitoring the change of the C=C peak vs. C=O peak and (b) the alteration of the colour parameters of its films after its submission to UVC irradiation for several time intervals. It was confirmed that within 6 h, a thin-layered adhesive film acquires 80–85% of its polymerization in ambient conditions, while the reaction continues for up to 12–18 h in the conditions examined. The progress of the reaction is slower when the adhesive is in a protected environment. On the other hand, the effect of UVC rays on the glue is destructive and oxidative, provoking a yellow shade/colour from the first hours of exposure. The intensity of the yellowness becomes higher after 6 h of exposure, showing shifts in the absorption peaks of C–O/C=O groups of the initial IR spectrum and the augmentation of –OH absorptions. It was concluded that the adhesive is suitable for glass restoration, especially for instant, rapid, applications, under mild conditions of maintenance and exposure.
1. Introduction
The selection of a polymeric coating for the conservation or the restoration of a glass artifact is quite a complex matter, given the wide variety of commercially available adhesives or coatings. It is important to evaluate, first, the state of the surfaces and consider which materials would be required for the best conservative route. More specifically, the adhesives used for the conservation and restoration of glass objects must possess certain qualities before being considered for application, such as:
- (a)
- Satisfactory adhesion, either as a solution, emulsion, or as liquid pre-polymers. During the transition from liquid to gel or solid state, the contraction of the adhesive takes place and should not cause any physical damage to the object surface [1].
- (b)
- Reversibility upon request, usually by using solvents that dissolve linear polymers and swell the crosslinked ones. In the case of a lacquer coated on a smooth surface (glass or metal) total removal is possible; however, when the polymer is applied onto a porous material (paper or fabric) it seems impossible to remove the full amount of it.
- (c)
- Physical stability over time and towards external factors, since alterations, such as yellowing, brittleness, insolubility, contraction or expansion, fluidity, cracking, or dust absorption, are undesirable.
- (d)
- Certain acceptable aesthetics, provided that a conservation treatment should not alter the general appearance and interpretation of the object. (Interpretation means what the viewer, even the non-expert, perceives from the artifact, even if it is not visible to the eye, but can be perceived after scientific examination.) Generally, a conservation treatment must strengthen, reveal, and project the intention of the artist or manufacturer of the object and facilitate its interpretation for the world to see [2,3].
Consequently, the terms “conservation-grade” materials and materials of “museum-quality” indicate the appropriateness of the materials for inside-hall exhibitions. However, the term implies also that conservators can use these materials as safe ones for long-term preservation [4,5,6]. Therefore, a “conservation-grade/museum-quality” material is not something universal, as each material has its own properties, each conservation substrate is different, and each project requires a unique approach [7].
Glass maintenance involves (a) the prevention of further deterioration of an object with the appropriate surrounding storage (passive maintenance via controlled environmental conditions). These measures include control of the lighting levels, the stabilization of temperature and humidity, as well as the reduction of air pollutants in the atmosphere and the absence of microorganisms and insects in the area. (b) Active conservation, as the term implies, involves various levels of interference with the object, such as photography, X-rays, surface cleaning [8,9,10], anti-reflecting coating [11,12,13], and proper (re)packaging, additional repair (reconstruction of existing fragments), cosmetic actions, or restoration (partial or total replacement of other parts). Often, a primary restoration of glass objects may be necessary in order to ensure safe management during the subsequent steps, the reason being the fragility of the object, which makes it sensitive to physicochemical damage and tensions. Polycyanoacrylates are considered to be suitable adhesives for this initial step of treatment.
The use of polycyanoacrylates for the conservation of glass artifacts was recommended by Moncrieff in 1975 [14]. Cyanoacrylate adhesives are of low viscosity and are fast curing, and they are commonly used in applications where fast bonding is required at ambient conditions. They are characterised as “instant” adhesives for the bonding of similar or different surfaces and easy to handle (no primer or hardener required, manually coated or with simple equipment; they are ready-to-use products and do not require previous preparation, making them handy for in situ treatments). They are widely used in the automotive industry, home appliances, home repairs, and the furniture industry, and find some of the most unusual applications in cosmetics, for example, as instant-attach glue for false eye-lashes, and in medicine [15,16]. Cyanoacrylate adhesives, although rarely used in conservation compared to other polymeric materials, are recommended over epoxies and acrylics in some cases, as they are easier to apply and polymerise faster [5]. Additionally, the polymer can be easily modified and present additional properties that include impact resistance, environmental endurance, or surface insensitivity [17,18]. Given this information, it is certain that cyanoacrylate adhesives are proved very useful in specific conservation treatments.
A common monomer of this class of polymers is ethyl 2-cyanoacryalate [CH2=C(CN)COOC2H5], a colourless liquid with low viscosity and a slight odour in its pure form, and which is soluble in organic solvents. The polymerization process of cyanoacrylate adhesives occurs through anionic polymerization, producing addition polymers (the free-radical mechanism is also applicable, but the anionic one is easier to initiate). Highly electrophilic monomers, such as cyanoacrylates, require only weak nucleophile initiators (as H+ donors, strong bases are not strong enough for initiation). The presence of moisture [16] acts as an initiation source, since OH− is sufficient, while excessive moisture does not promote or harden the polymer. In anionic polymerization, initiation is normally very fast relative to propagation and all chains grow simultaneously. This leads to polymers with low polydispersity (or monodispersity) because the rate of inter-conversion between the different formulations is much faster than the polymerization [19]. Figure 1 shows in brief the polymerization reaction of ethyl 2-cyanoacryalate. In general, the curing conditions of cyanoacrylates, such as temperature, pH, and primers or impurities, determine the structure and properties of the final polymer. For example, in acidic conditions, no large molecular weights (MW) are observed at either 0 or 60 °C, while at neutral pH and 25 °C, the result is only polymers with high MW. Progress continues until the monomer is completely consumed or chain transfer or impurity termination occurs. Usually, some weak carboxylic acids, such as malonic, formic, oxalic, or cyanoacetic acid, are mentioned as terminators [20], and are formed by the hydrolysis of the monomer or have been formed and remained in various stages of production. Although water is the most common initiator for a cyanoacrylate adhesive, it is accepted that if the packaging contains up to thousands of ppm of water molecules, it causes the hydrolysis of monomers in combination with the presence of strong acid stabilizers [21,22].
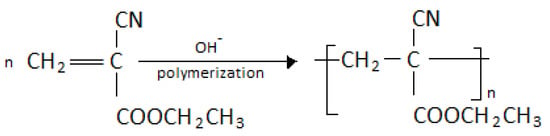
Figure 1.
Schematic reaction of ethyl 2-cyanoacrylate towards production of poly(ethyl 2-cyanoacrylate) PECA.
Eventually, all objects are subjected to some degree of deterioration, wear, or ageing, thus explaining the irreversible changes in the physical and chemical properties of the materials. The reason is that chemical interactions occur in the materials by itself, with other materials touching the object, or through corrosive interactions with nearby environments [23]. The results of some of the degradation processes are noticeable, while others are less visible, yet significant. In order to protect the objects in their care, conservators seek to minimise potentially harmful interactions by selecting appropriate materials for the storage and display of objects [3]. Solar radiation (including many types of rays), temperature change, humidity, oxygen, and pollutants act (often synergistically) during ageing. Solar energy is often responsible for the onset of the reactions leading to the degradation of materials. Consequently, yellowing, brittleness, and insolubility are due to the chemical reactions of the polymer that are caused by light (ultraviolet radiation), heating, and especially oxygen. All other alterations are due to the change inthe physical properties of the polymer (elasticity, refractive index, specific gravity, impact resistance, etc.). Despite the great fluctuations observed in climatic factors, in order to be able to examine the ageing of a material, experiments imitating ageing are usually performed. Aging experiments are performed either in a natural environment, called natural ageing experiments, or in the laboratory in devices that simulate exposure conditions, called artificial ageing experiments. Polymer tests in extreme conditions serve the investigation of regular use in prolonged times. Accelerated artificial ageing experiments have been performed for at least the last 80 years in order to detect, over a short period of time, the changes caused in materials due to natural ageing. The environment is quite complex, so the devices used for these experiments may simulate solar radiation, humidity, and temperature.
The aim of this work is to investigate the appropriateness of a commercially available cyanoacrylate adhesive of medium viscosity, a fast-curing adhesive specially for glass, in terms of (a) its rate of polymerization progress and (b) its tolerance against aggressive irradiation. In this way, interesting conclusions can be drawn regarding its suitability for glass restoration in affairs of cultural interest.
2. Materials and Methods
2.1. Polymerization Kinetics
Loctite® Super Attak Glass (3 g, Henkel, Henkel Hellas SA, Athens, Greece) is a commercial cyanoacrylate adhesive with ethyl cyanoacrylate (CAS# 7085-85-0) as the main monomer. It contains triethyl-o-acetylcitrate (C14H22O8, CAS# 77-89-4, 20–40%) as a plasticizer, hydroquinone (C6H6O2, CAS# 123-31-9, 0.01–0.1%) as an inhibitor to suppress free radical polymerization, and 2,2′-methylenebis(6-tert-butyl-4-methylphenol) (C23H32O2, CAS# 119-47-1, 0.1–1%) as the antioxidant ingredient. It is a colourless liquid with low viscosity and a slight odour in pure form. It has a density of d = 1.1 g/mL, a flash point at 80–93 °C, while it may contain n-butyl cyanoacrylate in small quantities [24]. Loctite® Super Attak Glass adhesive was supplied in several packages (IDH 2051823), used as received, and stored in a cool, dry, and shady place at ambient conditions. The chemical structure of the cyanoacrylate adhesive was studied via FT-IR. The spectra were collected using Spectrum One (Perkin Elmer, Waltham, MA, USA), while some drops were transferred between low density polyethylene (LDPE) films (carefully covered to avoid entrapped air bubbles) to avoid adhesion on the NaCl plates (round crystal window 25 mm × 4 mm, batch # 3110, Sigma-Aldrich, St. Louis, MO, USA), and placed inside the universal cell mount of Perkin Elmer (part # L1270986) as quickly as possible (~20 s after opening package). The resolution at 4 cm−1, 32 scans (~5 min scanning time), with a wavelength range of 4000–600 cm−1, was selected for overall characterization, and then the data were processed in the software Spectrum ver. 5.3.1. The evaluation/identification of the peaks of the spectrum followed (1617 cm−1 as changing peak and 1750 cm−1 as internal standard). Next, for the polymerization kinetics study, several spectra of restricted range 1900–1550 cm−1 were collected, in the same way, at certain short time intervals with 8 scans (~1.5 min scanning time) each time to observe the changes of the peaks until 6 h after initiation. Room conditions were stabilized at 25 ± 1 °C and 50–55% RH.
Several trials had been carried out using FT-IR, including bare adhesive films or ATR plates, but the aforementioned process proved to be more punctual, ensuring film smoothness and fast handling for repeated recordings.
2.2. Preparation of the Polycyanoacrylate Films
For the preparation of polycyanoacrylate films, improvised closed moulds were made in ambient conditions (25 ± 1 °C and 50–55% RH), after several trials being undertaken to obtain uniform films. The quality of the films acquired after each trial was evaluated visually and manually by the researcher/author experienced in restoration processes in terms of equal thickness, smooth surface, lack of entrapped air bubbles, and colour uniformity. Eventually, two glass plates (5 mm thick) covered with “in-house”-prepared PP film were inserted into a polyester mould with 3 cm × 5 cm × 0.7 cm of internal space between them. Afterwards, a quantity of the adhesive was poured into the mould gap and, after that, the second plate was placed and tightened with metal clamps to prevent the formation of air bubbles and secure the firmness of the film. More specifically, the plates were left in a horizontal position, with the help of clamps for 4 days. After the eased detachment, films were kept in a lightless environment (black sample bags), preventing any wear.
For the characterization of polycyanoacrylate films, the attenuated total reflectance (ATR) technique was used, as the films were quite thick, and the transmittance spectrum was not satisfactory. An HATR Sampling Accessory was engaged with a ZnSe plate (L120-5418 and L120-0313 correspondingly) at a 45° angle working at 32 scans, 4 cm−1 analysis, and at a wavelength range of 4000–600 cm−1. ATR was utilized for film characterization in a neat and in an oxidized form.
2.3. Ultraviolet Photooxidation
The photoaging process was performed in a closed oven of Gallenkap company (Cambridge, UK), OVR-400-010 G. In particular, the radiation emission of the apparatus is in the UVC region (λ = 253.7 nm) in room conditions (25 ± 1 °C and 50–55% RH). The oven provides 6 sockets (Panalight®, ref. # TL 2001/KPP 2001) for Hg-lamps (OSRAM®, Puritec HNS 8W, G5, G8T5/OF, RG3 HG, Italy) placed at a 10 cm distance from the shelf with the samples. Three adhesive films were transferred into the oven and observed regularly visually, colorimetrically, and spectroscopically at specific time intervals until 48 h of exposure.
2.4. Visual Observation of Aged Samples
In this experimental part, 8 strips of blue wool control fabric were placed fixed on an opaque cardboard “half-masked”, numbered as 1–8 (very low to very high light fastness, dimensions 1 cm × 6 cm). The woollen standards used were supplied by James H. Head Co., Ltd., Calderdale, UK (batch No. 000932). Blue wool standards were introduced along with the samples in the ageing device. The observation was conducted under standard conditions, in an observation chamber (Mulder’s VeriVide®), with the application of a D65 lamp, which mimics the average natural daylight, and anN lamp indicating the artificial light.
In addition to the blue wool standard observation, a visual observation of the samples also took place with the use of a greyscale contrast scale (the scale 5 to 1 indicates gradual contrast increase) as described in ISO 105-A02 (BS 1006 A02 1978 SDC Standard Methods 4th edition A02 of the British Standards Institution) in order to detect any colour changes in terms of contrast on the cyanoacrylic films [25]. The observation/comparison was made each time the sample was removed from the ageing chamber. In all cases, the same experienced experimenter made the naked-eye visual observations, standing in the same position, under the same conditions.
2.5. Colorimetry
Colour indexes and chemical changes in sample weathering were collected through spectrophotometer measurements. The MacBeth Color-eye® 3000 device made by Kollmorgen Instruments Corporation (Serial No. 33041773L, New York, NY, USA) was used accompanied with CroMatch, v.1.6.5b software and the CIELAB system of formulae. The light of observation was D65 illumination, and the angle of the standard observer was settled at 10°. As for apparatus status, it should be noticed that a UV component was included, the specular component was excluded, and the experiments were carried out through a small window (10 mm in diameter). The calibration was initially executed with a Macbeth Ref. Std. white plate (serial No. 33041773lL, prom No. H11773). The smoothest surface of each sample was fixed towards the observation window, while a white opaque background was placed behind the transparent polymeric film. The untreated film (0 h of exposure) was selected as a standard sample and the rest as weathered films for comparisons in each group. Colour indexes and parameters were recorded for all. It is important to mention that due to oxidation, the samples had to be handled with necessary care so as not to cause any cracks or further damage. The colour difference between the different stages of ageing was calculated according to the following equation:
3. Results
The characterization of the adhesive was carried out using FT-IR by recording several spectra; Figure 2 illustrates the adhesive directly at its application and after 6 h of curing at ambient conditions. In that way, the similarities and differences between the two states of the adhesive have been recorded. Here, t = 0 is the spectrum taken immediately after the package was opened, and this is compared to the spectrum taken after 6 h of polymerization between the PE covers. Table 1 demonstrates the major peak absorptions recorded. The two spectra seem alike, yet the disappearance of some peaks is the proof of the polymerization evolution.
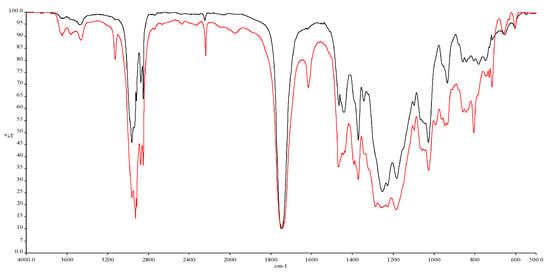
Figure 2.
IR spectra of the adhesive before (t = 0, red line) and after t = 6 h (black line) of polymerization.

Table 1.
Spectrum interpretation for Loctite® Super Attak Glass adhesive.
The cyanoacrylate adhesive is polymerised by anionic polymerisation, using water as a mild nucleophilic medium, due to its ability to attract e− from C of –COOR and –CN groups [26]. In most cases, the humidity of the atmosphere or the humidity adsorbed on the bonding surface is sufficient for the polymerization to begin within a few minutes. Specific peaks should be selected to better illustrate the polymerization progress. There are several options, depending on the chemistry of the macromolecule and on the experimental/operational tactic. In Figure 3, two summary spectral fragments show the peaks’ changes over time, for a period of 6 h of curing, in region 1950–1500 cm−1 (a) and 3300–2750 cm−1 (b). The stability of the peaks at 1750 and 2957 cm−1, correspondingly, and the gradual increase/decrease in the size of the neighbouring peaks is impressive, indicative of the careful operation during the experiments [27]. The large peaks work as internal standards for the credibility of the procedure, while the changing smaller peaks at 1617 and 3124 cm−1 describe the phenomenon.
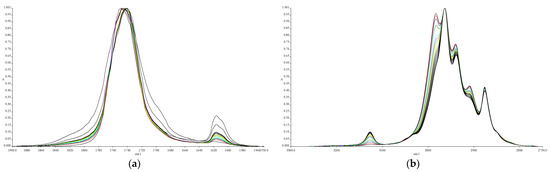
Figure 3.
Peaks’ changes in FT-IR spectra at1950–1500 cm−1 (a) and 3300–2750 cm−1 (b) regions, indicating the polymerisation progress of the adhesive.
Afterwards, it was decided that the progress of polymerization should be monitored via the quantification of the results of the peaks at 1617 and 1750 cm−1 because of their clear shaping and limits, which correspond to the changing vinyl bond C=C and the unchanged ester carbonyl C=O at specified short time intervals. The area was calculated for each peak using the IR software, for each time interval and for each sample recorded, and then the ratios were calculated. The equation applied to calculate the degree of conversion DC% is given as [28]:
where A denotes the peak area at each absorbance wavenumber immediately after adhesive was placed in IR (t = 0) and for each time interval t during polymerisation.
The above calculations can be performed by applying peak heights, too, since the C=C group becomes lower, while the overall interactions of the groups are better illustrated by area changes (due to slight peak shifts, conjunction effects with neighbouring bonds, etc.). In this way, Figure 4 was produced, demonstrating the total polymerization time required in order to conclude the curing of the adhesive, the rate of polymerization progress (Rp, %/min), and the DC% achieved at each time interval. The data refer to mean and standard deviation of n = 3 replicates. The results refer to the specific thickness of the adhesive and the particular experimental practice.
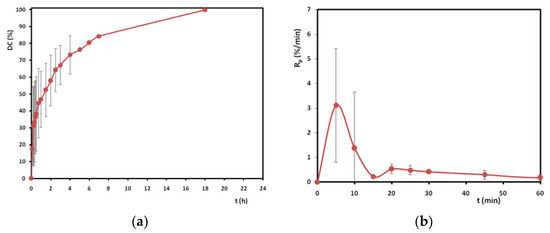
Figure 4.
DC% over time for the 1st day of curing (a) and rate of DC% increase for the 1st hour of curing (b).
Moving on to the second part of the study, the effect of UVC irradiation on the samples is seen in Figure 5, where the spectra are presented in full. During the ageing process, any molecular change caused by photodegradation was observed via the FT-IR, while the colour changes via reflectance spectrometry. More specifically, the spectra are taken in that case by ATR spectroscopy at selected photo-oxidation times, because neither the films’ thickness (~7 mm) nor the surfaces of the worn films allow the transmission mode to be in IR. By comparing the spectra in Figure 5 for three time-intervals of exposure, it has been observed that changes occur in both the intensity/width of the peaks and the baseline quality in the fingerprint area.
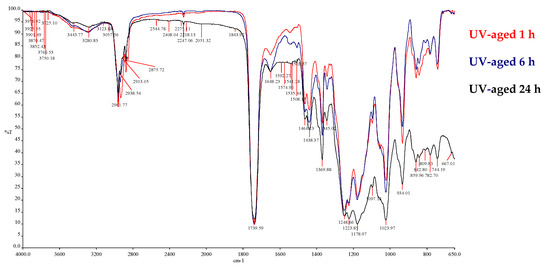
Figure 5.
Infrared spectra of polycyanoacrylate film after UV exposure for t = 1, 6, and 24 h.
The following step in analysing the aged films is spectrophotometry. The smoothest surface of each sample was fixed towards the observation window while a white opaque background was placed behind the transparent polycyanoacrylate film. The untreated film (0 h of exposure) was selected as the standard reference sample, while the rest were set as the exposed weathered films for comparisons in each group. The measurements of colour indexes, colour coordinates, and parameters were recorded for all exposed and standard reference samples. Table 2 summarizes the results obtained from the CIELAB co-ordinates L*, a*, b*, C*, h°, and ΔΕ* and the k/s reflectance values derived for all cases (mean ± st. dev., n = 3). The reflectance R presents its major values at λ = 400 nm. Figure 6 isolates the changes of a* and b* and demonstrates the great growth of the b* (yellowness) coordinate regarding colour changes.

Table 2.
Recordings and estimations of colorimetric parameters over several UV irradiation time intervals.
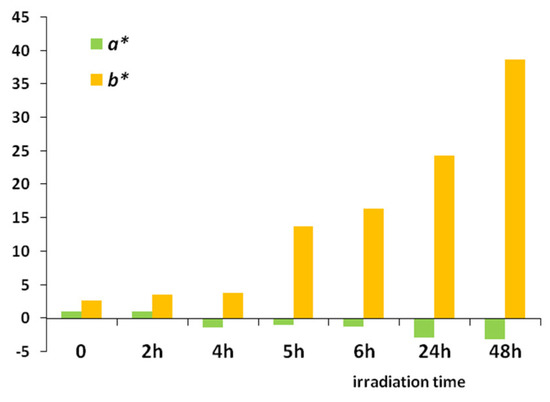
Figure 6.
Plot of a* and b* coordinates over the irradiation time in UV.
4. Discussion
Monitoring a specific IR peak for changes in polymerization and changes in material properties, especially in ancient artifacts [29,30,31], is not uncommon. From the main peaks shown in Figure 2 and indicated in Table 1, it is concluded that the peak at 3124 cm−1 is attributed to the vibrations of =CH2 group bonds. The peak diminishes with time (Figure 3b), since the unsaturated double bond is consumed during polymerization. The next noteworthy peak at 2237 cm−1corresponds to the –CN bond, often seen shifting to greater v and reducing in intensity [27]. This group does not participate in polymerization reactions, yet its absorption is affected by the nearby C=C bonds, and the conjugation phenomena are evident. The three unsaturated bonds, –CN, –COOR, and C=C, interact in terms of conjugation, and it is anticipated that the peak will reduce when curing proceeds. The strong and sharp C=O absorption attributed to the ester group at 1750 cm−1 remains unchanged over time. This helps the calculations in terms of credibility; the peak area is employed as an internal standard for each spectrum to minimize the accidental faults or the film’s external characteristics. The peak at 1617 cm−1, corresponding to C=C stretch vibration, varies with time and has been selected for study. It is well known that overlapping is a usual phenomenon in IR recordings, especially in the fingerprint region. Weaker bands may be covered by several peaks or shifted peaks, while the phenomenon unfolds as a result of inter- and intramolecular H bonds [32,33]. Moreover, in the initiation step, the –COOR and –CN groups significantly stabilise the anion formed at the α-carbon by delocalising the negative charge.
The chemistry of the main component of the adhesive, ethyl 2-cyanoacrylate, is identified in the IR findings. General monomer chemistry, synthesis, the modes of polymerization and the physical characteristics of the polymers have been studied [21]. In general, the polymerization behaviour of the product studied is in accordance with the behaviour of PECA, which is its main ingredient. The interesting property of polycyanoacrylatesis the variation in their properties when the R-group is altered in the ester (methylene groups or branched substitutes) and the possibility of cross-linking reactions for harder systems. The polymerization reactions are actually ambiguous depending on the concentration of the ions and the pH.
4.1. Polymerization Kinetics
By identifying a specific chemical group that is consumed in a chemical reaction, FT-IR can be used to monitor the kinetics and extent of reaction. In the case of cyanoacrylate adhesives, the C=C bond in the monomer is consumed during the polymerization. This bond absorbs infrared light at several wavelengths and is easily monitored. From the examination of the FT-IR absorption spectra, during the polymerization process of the adhesive (under controlled environmental and experimental conditions), a diminution of these peaks is observed in the spectrum zone. Those peaks are noticed at 3124 and 1617 cm−1 for the Loctite® adhesive. The choice of the right pair of peaks for IR calculations is an important decision that depends on the chemistry of the molecules, the form of the sample (viscous when uncured, thickens when cured), the quality of the instrument, etc. The reasoning for our method for handling the IR data is well described in the Results section. Figure 3 demonstrates the limits of the peaks and the reproducibility of the changes that allow us to choose either C=O at 1750 cm−1 or the methylene group at 2957 cm−1 as an internal standard. It is crucial for the absorption to be neighbouring, so the researcher takes a narrow fragment range of the spectrum in order to maximize the instrumental analysis and accuracy [28]. As seen in Equation (2), we have chosen the most definite pair of peaks, i.e., 1617 cm−1 for C=C and 1750 cm−1 for C=O. It should be underlined that (a) the normalization of spectral lines and a good quality of baseline are essential for correct quantification of IR results, (b) the lack of solvent in the chemistry of polycyanoacrylate glues facilitates the application of the method, and (c) no mathematical correction was incorporated for the processing of spectra lines, such as the deconvolution of interfering peaks, because the peaks chosen present clear start/finish limits.
Figure 4 illustrates the variation of the degree of conversion of the cyanoacrylate adhesive during curing. A rapid initial increase in polymerization was observed during the first 2 h, reaching 60% double bond conversion. Then, a slower reaction is evident for 2–8 h curing time, ending at around 85% conversion. Finally, the polymerization stops after 12–18 h (DC = 99.8 ± 0.14%) of curing. As for the polymerization rate, high values were measured during the first 15 min of curing, which verifies the label “instant adhesive” given to polycyanoacrylates, since the reaction is rapid once the glue is “out of the package”. Later, the curing rate is reduced. The rapidity of the phenomenon explains the high standard deviation of the values noticed during the first 10 min of the “violent” hardening. It should be underlined that, during the IR measurements, the polymer changes and hardening proceeds; hence, the outcome of a spectrum is the average of the polymer’s status in that particular time period. This is why, during the kinetic experiments, each IR measurement took a short time (~1.5 min, Section 2.1) to picture the polymer’s state, while the usual scan number is higher. On the other hand, it should be noted that although the product hardens within a few minutes, it takes several hours in order to achieve full polymerization under the investigated conditions. Once more, if the experimental conditions vary, for example, in terms of the film thickness, the moisture supply between the protective LDPE films [26,34], or the temperature, the final curing time may vary too. This is why the production of thicker films for the ageing experiments required 4 days for the total hardening of the adhesive (Section 2.2). All in all, the authors would advise the conservators to keep in mind that parameters such as temperature, RH%, surface topography, impurities, or O2 exposure do influence the anionic polymerization process [35]; thus, prolonging the hardening time compared to the one proposed by the manufacturer may prove useful for glass restorations with polycyanoacrylates.
4.2. UV Weathering
Photochemical alteration is the result of the absorption of a photon by a chemical group resulting in the breaking of a bond. This process is called photolysis or photolytic cleavage. Each chemical reaction that takes place in the polymer requires an amount of energy to break down the first chemical bond of the macromolecule, and the reactions continue intermolecularly via chain mechanisms. Therefore, polymers show many alterations during the ageing process. These are mainly due to the chemical reactions of the polymer macromolecular chains caused by light (any solar irradiation), heating, moisture circles, and especially the presence of air (oxidative conditions). More specifically, molecular changes are observed after the photo-oxidation of the polymer, where the carboxyl molecules are converted to hydroxyl, which affects the colour changes in the adhesive during photo-oxidation, causing the colouration (yellowing) of the films [6,36,37]. Changes in the properties of polymers, such as elasticity, refractive index, specific gravity, brittleness, colour change, insolubility, shrinkage, material flow, bonding, and retention of dust and dirt on the surface, are all consequences of chemical alterations.
The oxidation reactions that actually took place when the adhesive samples were placed in UV chamber were localized in the –CN and –COOR groups, which include electronegative heteroatoms and unsaturated bonds. The high electron charges found there facilitate the breaking of bonds, given the external energy provided. Thus, the formation of more C–O, C=O, –COOH, and N–O or nitro groups arises. Figure 5 provides a general picture of the spectra obtained after each time interval under UV irradiation. The absorbencies at 3443 and 3280 cm−1 in Figure 5 indicate the presence of –OH groups of various formations in the polymeric structure. No great alterations are noticed in the 3000–2800 cm−1 region, where the C–H bonds absorb stretch vibrations to become–CH3, –CH2 or –CH. The stability of the peak at 2247 cm−1 is impressive, corresponding to a –CN bond, shifted slightly to the right (relatively neatly to 2237 cm−1, as explained above) [38]. The peak at 1648 cm−1 possibly indicates new C=C bonds, formed due to scission reactions in the macro-chain structure. The literature states that the degradation of the polymer is predominantly an unzipping process [35]. More possibilities of conjugated double bonds exist after oxidation, such as –C=C–C=C–, –C=C–C=N–, –CO–C=C–, or –CO–C=N– [28]. At 1541 cm−1 this is likely to correspond to the –COO– asymmetric stretch vibrations of oxidised groups. The 1464–1438 cm−1 peak remains identical over UV irradiation, and the case is the same for the 1369 and 1345 cm−1 peaks. Below the 1248 cm−1 region (simple bonds C–O, C–C, C–H, and C–N) the peaks become wider over 24 h of exposure, but still the 6 h of irradiation time seems to retain a fingerprint spectrum of the polymer that is similar to the initial one.
As photo-oxidation progresses, it is observed that the coordinates L*, indicating the lightness of the sample, and a*, indicating the position of the colour over the green–red axis, decline slightly in the first few hours and more so after 24 h of irradiation. On the other hand, the coordinate b*, indicating the position of the colour over the yellow–blue axis, rises significantly, a fact also obvious to the naked eye. Thus, the colour differences calculated by Equation (1) for ΔΕ* follow a rising trend, reaching 25 after 6 h of explosion and 45 after 2 days under 254 nm. Table 2 also shows that the chroma coordinate C* (or saturation) becomes slightly lower as the exposure time increases, indicating that treated samples are becoming gently duller and less clear due to the resulting yellowing and intermolecular changes. The a* values show small variations towards the green shade, confirming that the main colour change is on the yellow scale, as the big change of the b* values of the treated samples is definite (Figure 6). As for h° (hue angle), 90° is the usual value for yellow samples; indeed, the values are around 90°, with an increasing trend (the theory indicates h + b* over yellowing). The L* values are high, as one would expect from bright, yellowish samples, and remain high with small fluctuations until 5 h of irradiation.
A difference of ΔE* > 5 is visible to the naked eye; thus, this value is achieved in less than 4 h of UV exposure in our case. The Feller system [39,40] could provide some information on the correspondence of those values over time with realistic use for any adhesive [41]. We should also notice that the wavelength chosen is highly corrosive (λ = 254 nm) and heavy ageing results in short times are expected; however, the power of the chamber is relatively low (48 W). Certainly, many parameters have to be set before projecting those experimental results to actual conservation times or safe storage periods for Loctite® adhesive. It seems that the tolerance of the adhesive towards weathering can be classified as “fair” [42].
The authors would like to mention that the spectrophotometer instrument (Section 2.5) provided, for sample placement, a hole of 1 cm diameter; thus, a big area of sampling was available to the photometer, with a risk of measuring uneven spots. In particular, after 6 h of irradiation, the specimens exiting the UV chamber were soft and elastic and were left to “calm” before observation. The specimens were placed onto microscope glass slides, providing a clear and smooth surface in case of detachment. The experiment was over (at 48 h) when it was understood that experimental errors and inconsistencies were possible due to the sticky adhesive.
The International Standard Organization (ISO) introduced a reference protocol for colour discolouration documentation after exposure to “museum conditions”. The colour stability documentation is created with a series of eight samples of different blue fabrics called the blue wool standard method (BS1006:1971). Each sample (of blue fabric) has the ability to discolour twice as much as the previous one, and this due to the durability of the dye, where it reacts to the environmental conditions during exposure (humidity, temperature, and radiation). This procedure belongs to the evaluation method of British Standard 1006:1971 “Method for the Determination of the Colour Fastness of Textiles to Light and Weathering” and is internationally certified by the International Organization for Standardization Recommendation (ISO) R105 [41]. Depending on the discolouration behaviour of the blue fabrics, the exposure conditions of the objects in the exhibition space are also evaluated and are categorised accordingly into three categories: Class A: excellent, for a period of one hundred years. Class B: moderate, for a duration of 20–100 years. Class C: unstable, for exposure for less than 20 years [41].
Apart from spectrophotometry, visual observations of the films were made throughout the UV ageing process under standard ambient/laboratory conditions by the same experimenter (Figure 7). As for Loctite® adhesive samples, they remained in good condition in terms of texture, without losing their elasticity, and remained easy to handle when in the UV chamber for a period of 48 h in total. The short time intervals brought slight changes only to the samples’ hue; however, it was noticed that after photooxidation of 3 h, the samples went through a total colour change from colourless (for the initial cyanoacrylate film) to yellow (Figure 7). The same observation holds for both average sunlight (D65 lamp) and artificial light in the observation chamber. It is worth noting that even with the colour change of the resin, the films remained clear and transparent. After 6 h of exposure, the film became more elastic and stickier (after being kept in a natural environment for a few minutes it turned more rigid). Regarding the colour changes to the blue woollen indicators, it was observed that their shade does not change, not even the first one, for the total 48 h of the experiments (Table 3).
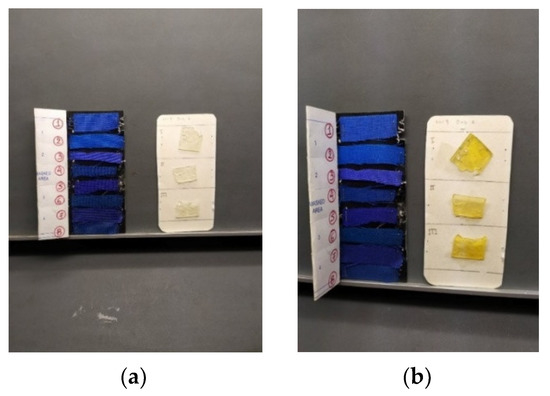
Figure 7.
Visual observation, under D65 lamp, of aged specimens after 1 h (a) and after 3 h (b) in the ageing chamber.

Table 3.
Observations of visual comparisons by experimenter during UV exposure.
In addition, the grey scale (approved and issued in collaboration with The Society of Dyers and Colourists) was employed by the experimenter to confirm and compare any visual discolouration between the photo-oxidised and non-photo-oxidised samples each time they were removed from the ageing chamber. The grey scale, numbered from 5 to 1, indicates gradual contrast increase from 5 (no contrast) to 1 (max contrast). The correlations of the contrast of the samples between exposed and non-exposed over the various time intervals was determined, and this was executed with a visual comparison of the samples with the grey scale, and a numerical rating was given [28]. It was observed that during the first few hours of photo-oxidation, there was a slight difference between them. This difference increased gradually and, after 6 h of photo-oxidation, the aged film had a strong contrast. Furthermore, it is worth noting that after 6 h of photooxidation, more chromatic/colour change cannot be perceived through visual observation.
Finally, it should be noted that the environmental conditions for glass art exposure in museums usually include 20 ± 2 °C temperature, 50 ± 10% RH, and λ = 300 nm for the lowest illumination, with display windows of high-silica and low-alkali glass, without a restriction in exhibition times. The experts may correlate the above results with the museum conditions in case they are interested in using the particular adhesive in the conservation/restoration process of glass artifacts, or take them into consideration for analogous cases of other polycyanoacrylate adhesives. Since the polymers ought to meet certain requirements, such as ease of application, stability after curing, removability, and lack of aesthetic effect on the object, some of these criteria have to be tested.
5. Conclusions
The aim of this research is the study of the commercial cyanoacrylate adhesive Loctite® Super Attak Glass (Henkel) in terms of suitability in the conservation of glass objects, and whether it could be classified as a “conservation-grade” material.
The chemical structure of the adhesive before and after curing was studied, along with the calculation of the degree of double bond conversion via FT-IR. It was concluded that the adhesive is correctly named as an “instantly curing” adhesive, since a high rate of polymerization progress takes place within the first 15 min. However, polymerization is not fulfilled within minutes; actually, 12–18 h are needed (under the conditions examined) for 100% hardening to take place. The ease of the adhesive handling is evident and verified in all experiments conducted.
Additionally, during the artificial ageing process under UVC irradiation, the adhesive showed little resistance to extreme weathering conditions, presenting discolouration (yellowing) and great ΔE* values. That leads to a fair behaviour over time, meaning that the adhesive is suitable for usual and not extreme storage and handling conditions, mainly for short period of times.
In conclusion, the data indicate that Loctite® Super Attak Glass appears to be a promising alternative to traditional adhesives used for the conservation of glass objects, given the advantage of instant curing. After the examination and testing of Loctite® Super Attak Glass, it has been confirmed that this cyanoacrylic adhesive meets some of the criteria. Its extensive use will undermine the restoration process over long periods of time under intense conditions, but it can respond satisfactorily under mild conditions in certain time periods.
Author Contributions
Conceptualization, D.S.A. and E.C.V.; methodology, E.C.V.; software, E.C.V., G.D.V. and P.D.M.; investigation, P.D.M. and E.C.V.; data curation, E.C.V.; writing—original draft preparation, E.C.V. and D.S.A.; writing—review and editing, D.S.A. and G.D.V.; visualization, E.C.V.; supervision, D.S.A.; project administration, D.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank N. Nikolaidis for the guidance regarding the colorimetric measurements and analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Green, L.R.; Thickett, D. Testing Materials for Use in the Storage and Display of Antiquities—A Revised Methodology. Stud. Conserv. 1995, 40, 145–152. [Google Scholar]
- Baek, S.-S.; Jang, S.-J.; Hwang, S.-H. Preparation and Adhesion Performance of Transparent Acrylic Pressure Sensitive Adhesives: Effects of Substituent Structure of Acrylate Monomer. Int. J. Adhes. Adhes. 2016, 64, 72–77. [Google Scholar] [CrossRef]
- Machalická, K.; Eliášová, M. Adhesive Joints in Glass Structures: Effects of Various Materials in the Connection, Thickness of the Adhesive Layer, and Ageing. Int. J. Adhes. Adhes. 2017, 72, 10–22. [Google Scholar] [CrossRef]
- Blank, S.D. Rubber in Museums. AICCM Bull. 1988, 14, 53–93. [Google Scholar] [CrossRef]
- Castro, E.; Teresa, M.; Carbó, D. An Appraisal of the Properties of Adhesives Suitable for the Restoration of Spanish Medieval Ceramics. In The Conservation of Glass and Ceramics: Research, Practice and Training; Tennent, N.H., Ed.; Science Publishers: London, UK, 1999; pp. 114–132. [Google Scholar]
- Down, J.L. A Literature Review of Cyanoacrylate Adhesives. Stud. Conserv. 2001, 46, 35–38. [Google Scholar] [CrossRef]
- O’Donnell, A.; Sturge, T. The Conservation of Leather Artefacts: Case Studies from the Leather Conservation Centre. J. Am. Inst. Conserv. 2001, 40, 163–166. [Google Scholar] [CrossRef]
- Dey, T.; Naughton, D. Cheap non-toxic non-corrosive method of glass cleaning evaluated by contact angle, AFM, and SEM-EDX measurements. Environ. Sci. Pollut. Res. 2017, 24, 13373–13383. [Google Scholar] [CrossRef]
- Koob, S.P. Cleaning glass: A many-faceted issue. Objects Specialty Group Postprints. 2004, 11, 60–70. [Google Scholar]
- Homola, T.; Matoušek, J.; Kormunda, M.; Wu, L.Y.L.; Černák, M. Plasma treatment of glass surfaces using diffuse coplanar surface barrier discharge in ambient air. Plasma Chem. Plasma Processing 2013, 33, 881–894. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Zhao, S.; Dong, W. Hydrophobic and optical properties of silica antireflective coating prepared via sol-gel method. Mater. Res. Express 2001, 8, 046403. [Google Scholar] [CrossRef]
- Dey, T.; Naughton, D. Nano-porous sol-gel derived hydrophobic glass coating for increased light transmittance through greenhouse. Mater. Res. Bull. 2019, 116, 126–130. [Google Scholar] [CrossRef]
- Löbmann, P. Sol-gel processing of MgF2 antireflective coatings. Nanomaterials 2018, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, P.; Borrelli, L.V. A Preliminary Note on the Use of Adhesives and Fillers in the Restoration of Ancient Materials with Special Reference to Glass. Stud. Conserv. 1975, 20, 201–205. [Google Scholar]
- Wartewig, S.; Neubert, R.H.H. Pharmaceutical Applications of Mid-IR and Raman Spectroscopy. Adv. Drug Deliv. Rev. 2005, 57, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kennedy, J.P. Polymerizability, Copolymerizability, and Properties of Cyanoacrylate. Polym. Adv. Technol. 2007, 18, 808–813. [Google Scholar] [CrossRef]
- Canale, A.J.; Goode, W.E.; Kinsinger, J.B.; Panchak, J.R.; Kelso, R.L.; Graham, R.K. Methyl α-cyanoacrylate. I. Free-radical Homopolymerization. J. Appl. Polym. Sci. 1960, 4, 231–236. [Google Scholar] [CrossRef]
- Han, M.G.; Kim, S. Controlled Degradation of Poly(ethyl cyanoacrylate-co-methyl methacrylate) (PECA-co-PMMA) Copolymers. Polymer 2009, 50, 1270–1280. [Google Scholar] [CrossRef]
- Baskaran, D.; Müller, A.H.E. Anionic Vinyl Polymerization. In Controlled and Living Polymerizations: From Mechanisms to Applications; Muller, A.H.E., Matyjaszewski, K., Eds.; WILEY-VCH Verlag GmbH & Co.: Weinheim, Germany, 2009; pp. 1–56. [Google Scholar]
- Whitaker, G.; Kincaid, B.J.; Raftery, D.P.; Van Hoof, N.; Regan, F.; Smyth, M.R.; Leonard, R.G. Potential of CE for the determination of inorganic and acidic anions in cyanoacrylate adhesives. Electrophoresis 2006, 27, 4532–4537. [Google Scholar] [CrossRef]
- Duffy, C.; Zetterlund, P.B.; Aldabbagh, F. Radical polymerization of alkyl 2-cyanoacrylates. Molecules 2018, 23, 465. [Google Scholar] [CrossRef]
- Estan-Cerezo, G.; Alonso, D.A.; Martín-Martínez, J.M. Structural and adhesion properties of poly (ethyl 2-cyanoacrylate) post-cured at different temperatures and times. J. Adhes. Sci. Technol. 2019, 33, 329–345. [Google Scholar] [CrossRef]
- Spathis, P.; Karagiannidou, E.; Magoula, A.-E. Influence of Titanium Dioxide Pigments on the Photodegradation of Paraloid Acrylic Resin. Stud. Conserv. 2003, 48, 57–64. [Google Scholar] [CrossRef]
- TDS Loctite® Super Attack Special Glass 3G. Henkel, ver 2018. Available online: https://dm.henkel-dam.com/is/content/henkel/tds (accessed on 10 June 2020).
- ISO Standards Regulation. Available online: https://www.iso.org/standard/3785.html (accessed on 12 February 2021).
- Jarikov, V.V.; Neckers, D.C. Anionic Photopolymerization of Methyl 2-Cyanoacrylate and Simultaneous Color Formation. Macromolecules 2000, 33, 7761–7764. [Google Scholar] [CrossRef]
- Tomlinson, S.K.; Ghita, O.R.; Hooper, R.M.; Evans, K.E. The Use of Near-Infrared Spectroscopy for the Cure Monitoring of an Ethyl Cyanoacrylate Adhesive. Vib. Spectrosc. 2006, 40, 133–141. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Vouvoudi, E.C.; Papadopoulos, G.D. Epoxy polymer Hxtal NYL-1™ used in restoration and conservation: Irradiation with short and long wavelengths and study of photo-oxidation by FT–IR spectroscopy. J. Cult. Herit. 2016, 18, 279–289. [Google Scholar] [CrossRef]
- Dey, T.; Carter, J.C.; Swift, K. SEM-EDX and FTIR analysis of archaeological ceramic potteries from southern Italy. Microscopy 2020, 69, 371–380. [Google Scholar] [CrossRef]
- Seetha, D.; Velraj, G. Determination of firing techniques of ancient artifacts by using FT-IR analysis. Chem. Sci. Rev. Lett. 2014, 2, 456–463. [Google Scholar]
- Thickett, D.; Pretzel, B. FTIR surface analysis for conservation. Herit. Sci. 2020, 8, 5. [Google Scholar] [CrossRef]
- López-Ballester, E.; Doménech-Carbó, M.T.; Gimeno-Adelantado, J.V.; Bosch-Reig, F. Study by FT-IR spectroscopy of ageing of adhesives used in restoration of archaeological glass objects. J. Mol. Struct. 1999, 482, 525–531. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, C.M.; Abidov, A.; Ravikumar, R.; Palanichamy, M.; Choi, E.Y.; Jang, H.T. Synthesis and Characterization of 1-octyl 2-cyano Acrylate for Wound Healing Applications. Int. J. Bio-Sci. Bio-Technol. 2016, 8, 339–350. [Google Scholar] [CrossRef][Green Version]
- Klemarczyk, P. Adhesion Studies of Mixtures of Ethyl Cyanoacrylate with a Difunctional Cyanoacrylate Monomer and with Other Electron-deficient Olefins. J. Adhes. 1999, 69, 293–306. [Google Scholar] [CrossRef]
- Han, M.G.; Kim, S.; Liu, S.X. Synthesis and degradation behavior of poly(ethyl cyanoacrylate). Polym. Degrad. Stab. 2008, 93, 1243–1251. [Google Scholar] [CrossRef]
- Flynn, J.H. Lifetime Prediction for Polymers from Thermal Analytical Experiments—Problems and How to Deal with Some of Them. Thermochim. Acta 1988, 134, 115–120. [Google Scholar] [CrossRef]
- Boxhammer, J. Shorter Test Times for Thermal- and Radiation-Induced Ageing of Polymer Materials. Polym. Test. 2001, 20, 719–724. [Google Scholar] [CrossRef]
- Pintus, V.; Wei, S.; Schreiner, M. UV ageing studies: Evaluation of lightfastness declarations of commercial acrylic paints. Anal. Bioanal. Chem. 2012, 402, 1567–1584. [Google Scholar] [CrossRef]
- Feller, R.L. Accelerating Aging—Photochemical and Thermal Aspects; The Getty Conservation Institute: Los Angeles, CA, USA, 1994. [Google Scholar]
- Feller, R.L. Further Studies on the International Blue-Wool Standards for the Exposure to Light. In Proceedings of the 5th Triennial Meeting, Zagreb, Croatia, 18 February 1978. [Google Scholar]
- Hattori, H.; Yoshizumi, K.; Crews, P.C. Wavelength Sensitivity of AATCC Blue Wool Lightfastness Standards under Light Radiation. Dye. Pigment. 2012, 92, 936–941. [Google Scholar] [CrossRef]
- Blum, M.-M.; John, H. Historical Perspective and Modern Applications of Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy (ATR-FTIR). Drug Test. Anal. 2011, 4, 298–302. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).