Assessing the Use of Aloe vera Gel Alone and in Combination with Lemongrass Essential Oil as a Coating Material for Strawberry Fruits: HPLC and EDX Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. A. vera Gel Extraction and HPLC Analysis of Phenolic Compounds
2.3. Extraction and Chemical Analysis of Lemongrass EO
2.4. Preparation of A. vera–Lemongrass EO Coating
2.5. Treatment Application and Analysis
2.6. Physical Parameters of Strawberry
2.6.1. Weight Loss (%)
2.6.2. Fruit Firmness
2.7. Color Fruit Samples
2.8. Physicochemical and Bioactive Constituents of Strawberry
2.8.1. Soluble Solid Content (SSC)
2.8.2. Titratable Acidity (TA), pH and Total Anthocyanin Measurements
2.8.3. Total Phenolic Content
2.8.4. Antioxidant Activity
2.8.5. Fruit Extraction and HPLC Analysis of Flavonoid Compounds
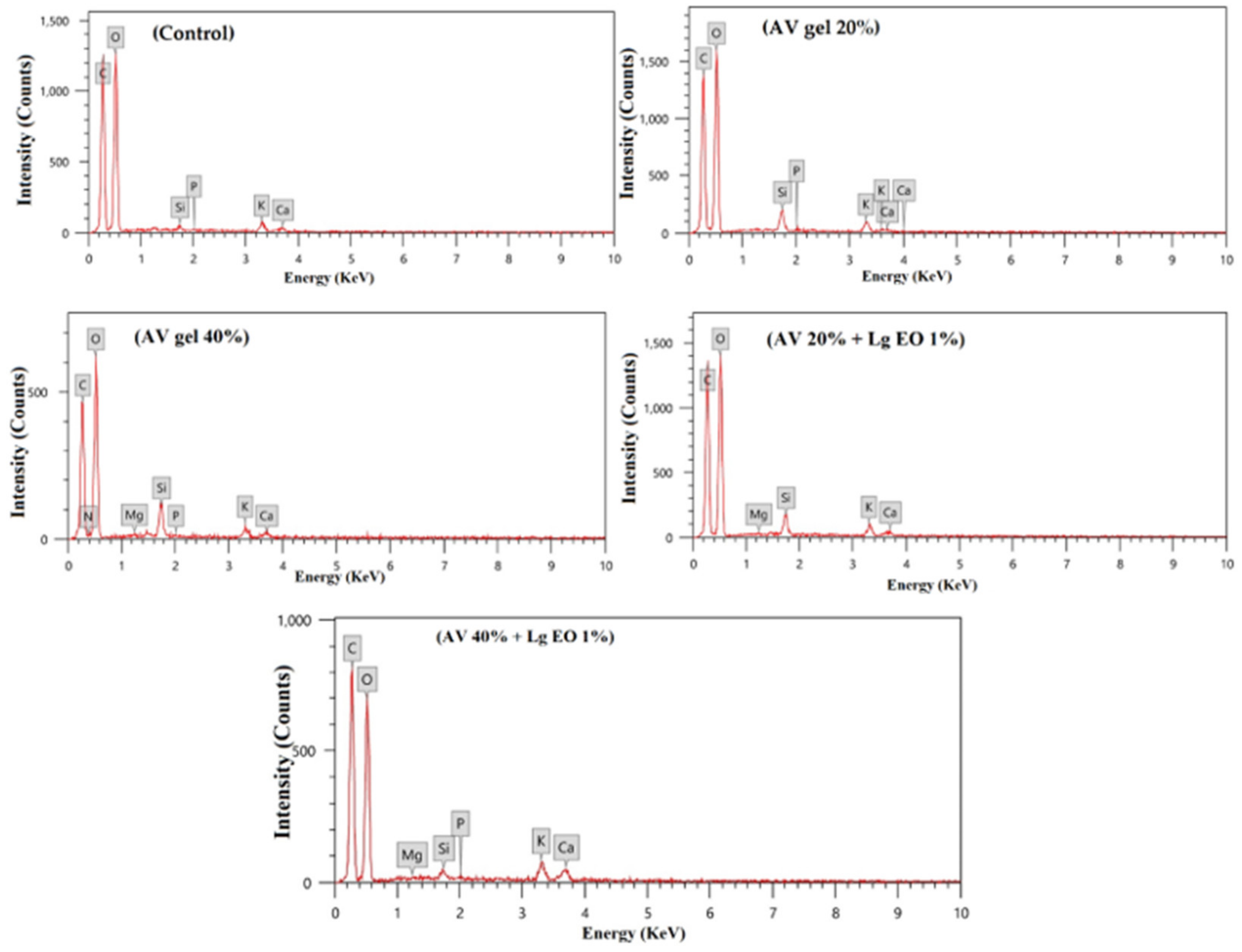
2.9. Elemental Analysis of Strawberry Fruit EDX Analysis
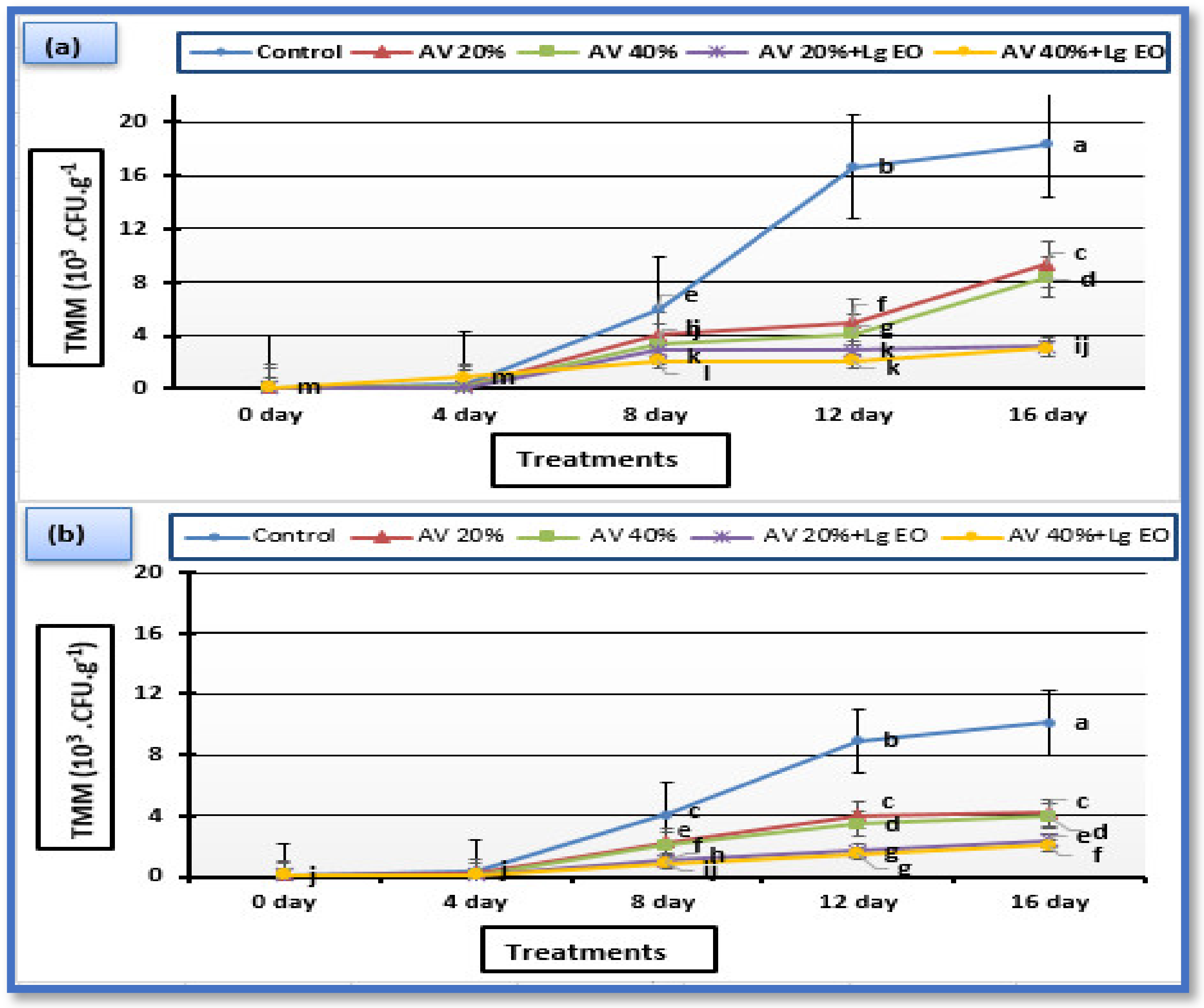
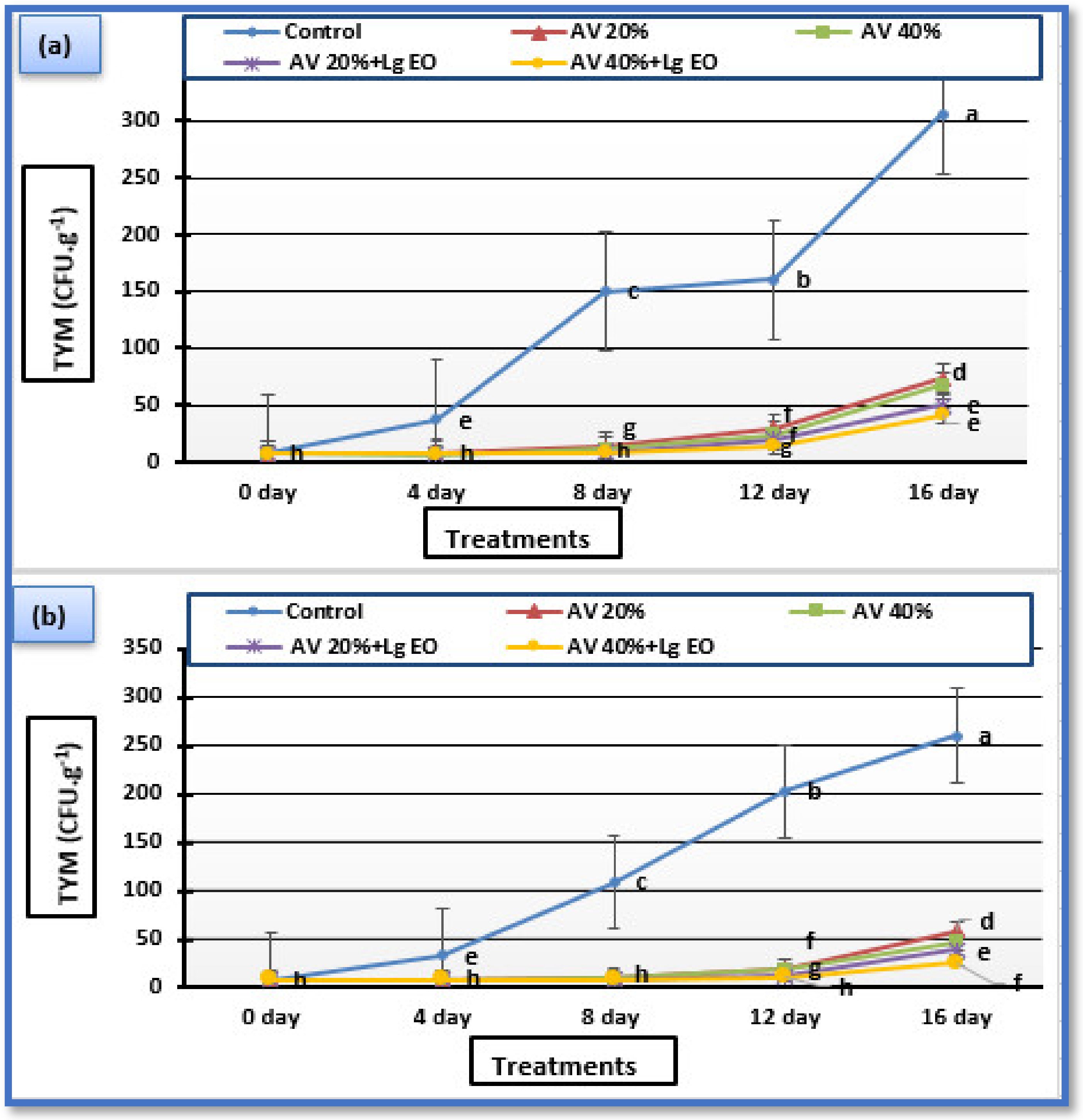
2.10. Microbiological Analysis
2.11. Statistical Analysis
3. Results and Discussion
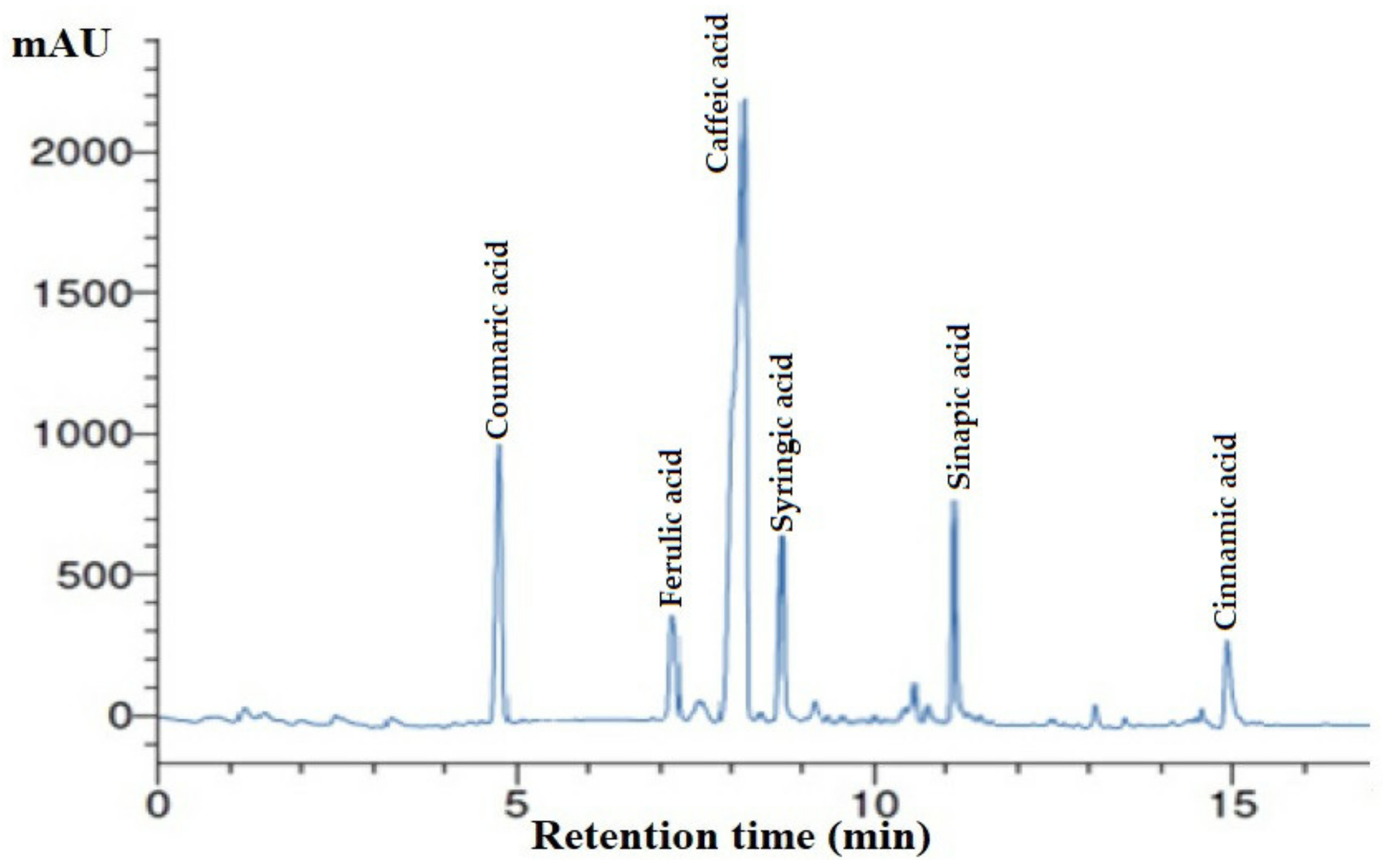
3.1. Phenolic Profile of A. vera Gel by HPLC
3.2. Chemical Constituents of Lemongrass Oil
3.3. Physical Parameters of Strawberry fruits
3.3.1. Weight Loss (%)
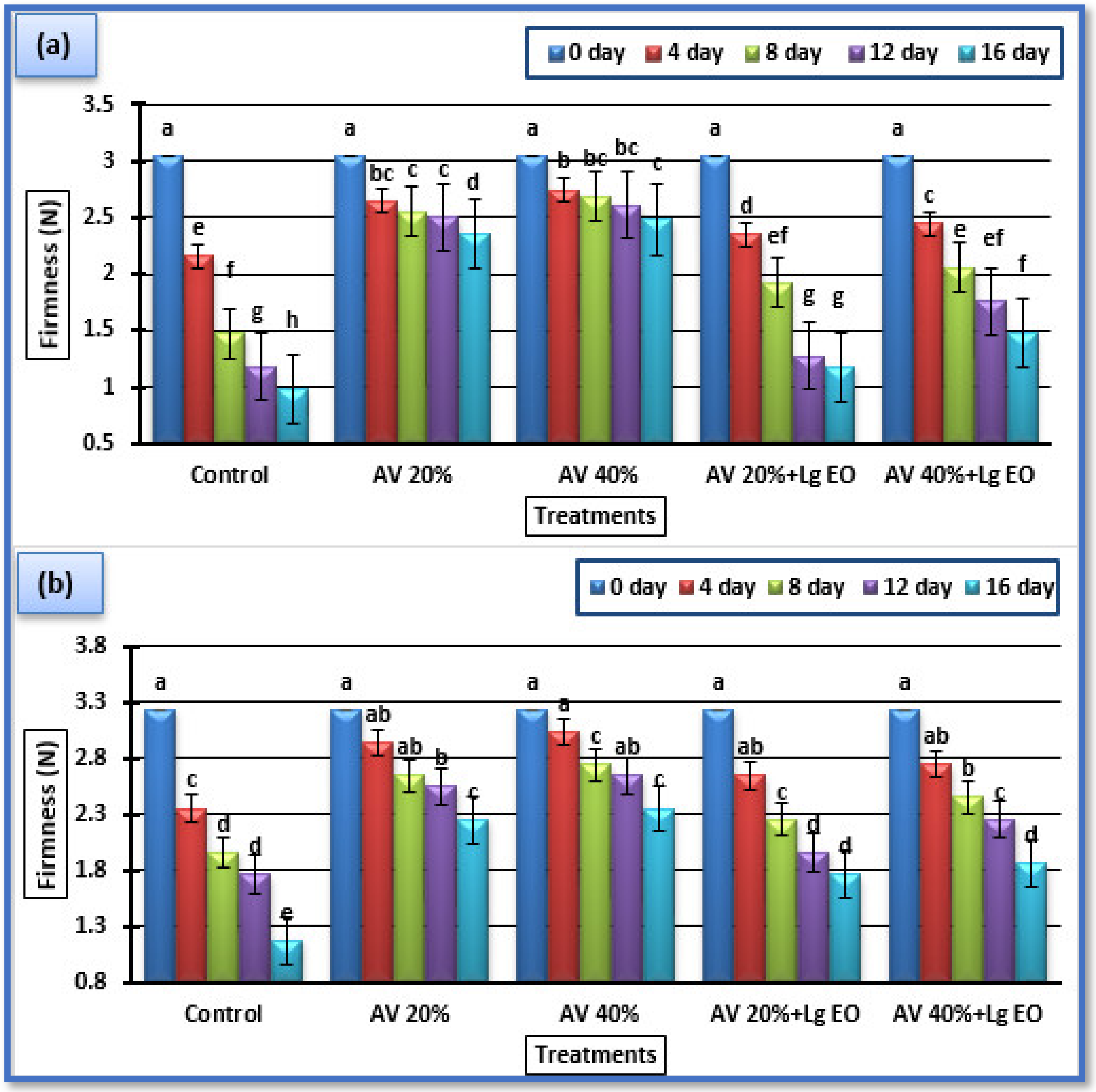
3.3.2. Fruit Firmness
3.4. Color Value
3.5. Physicochemical and Bioactive Constituents of Strawberry
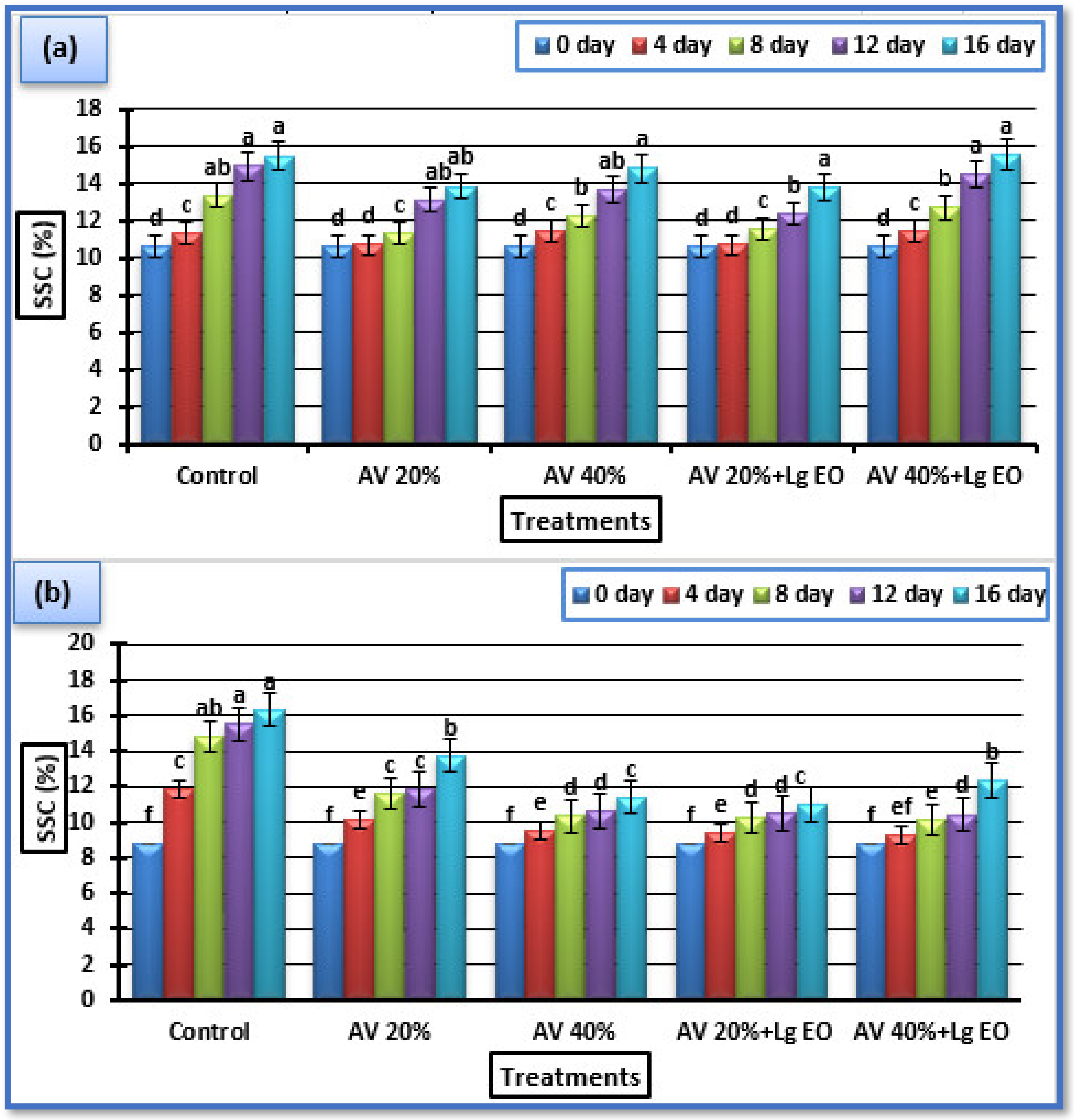
3.5.1. Soluble Solid Content (SSC)
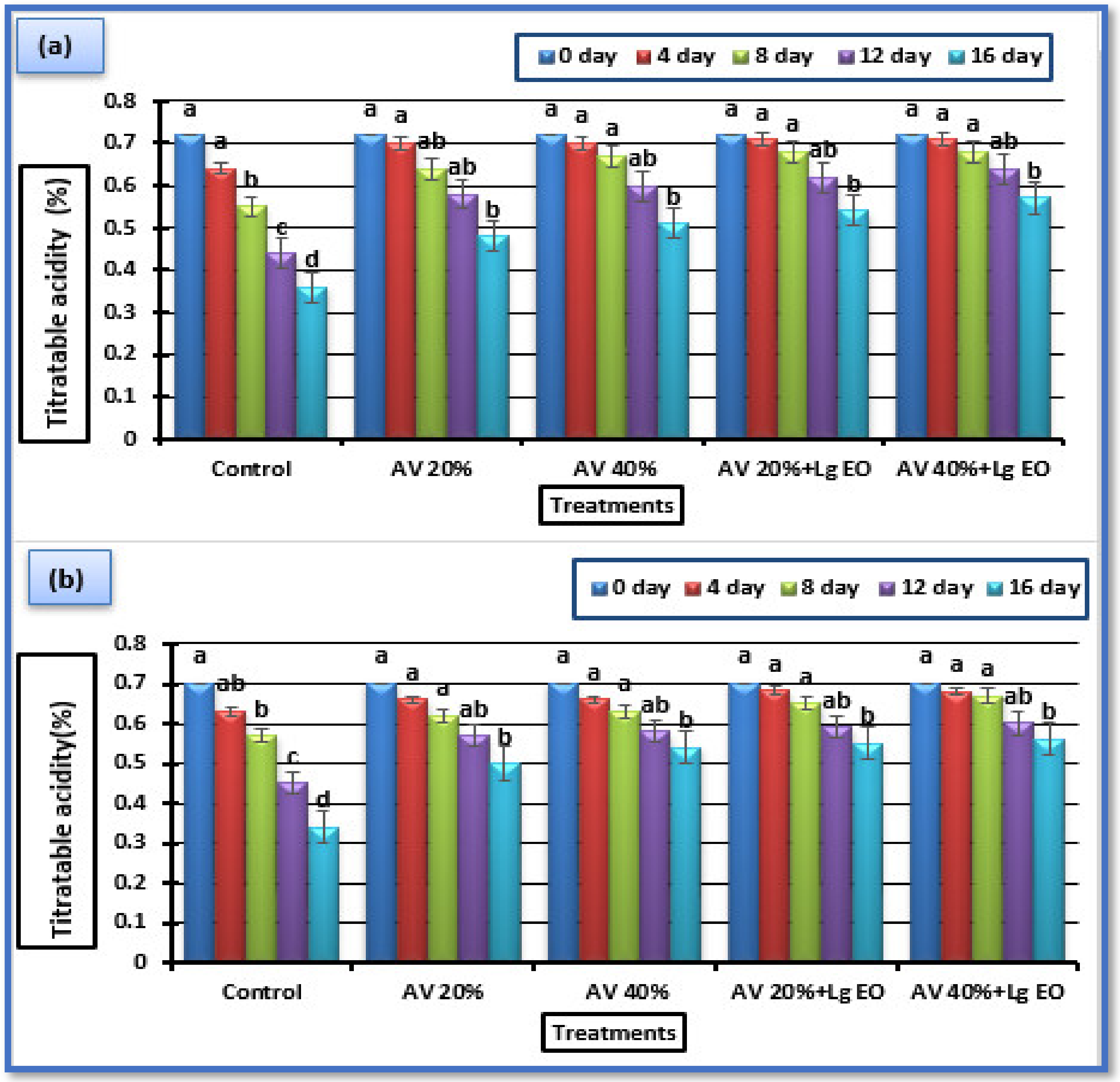
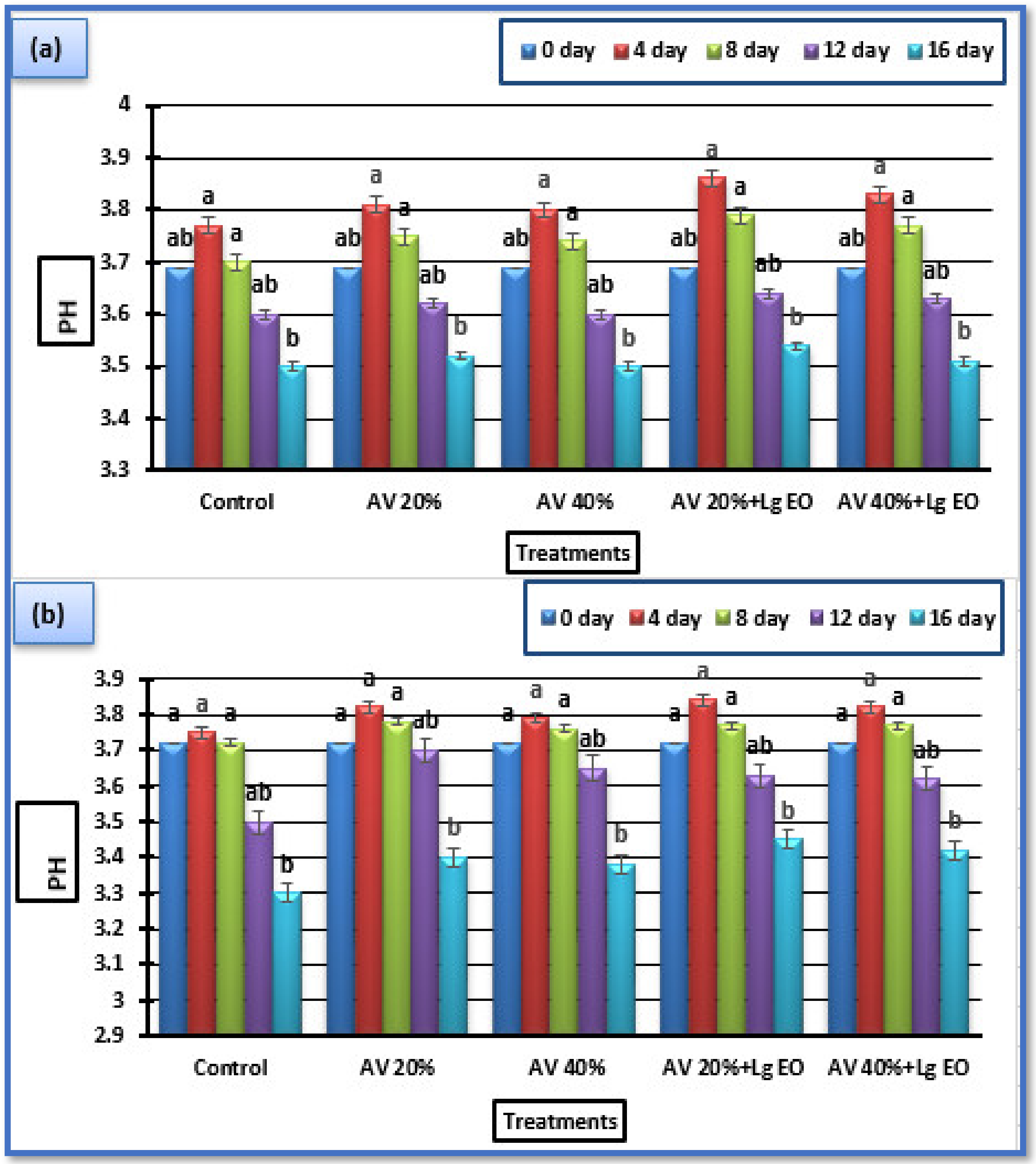
3.5.2. Titratable Acidity (TA) and pH
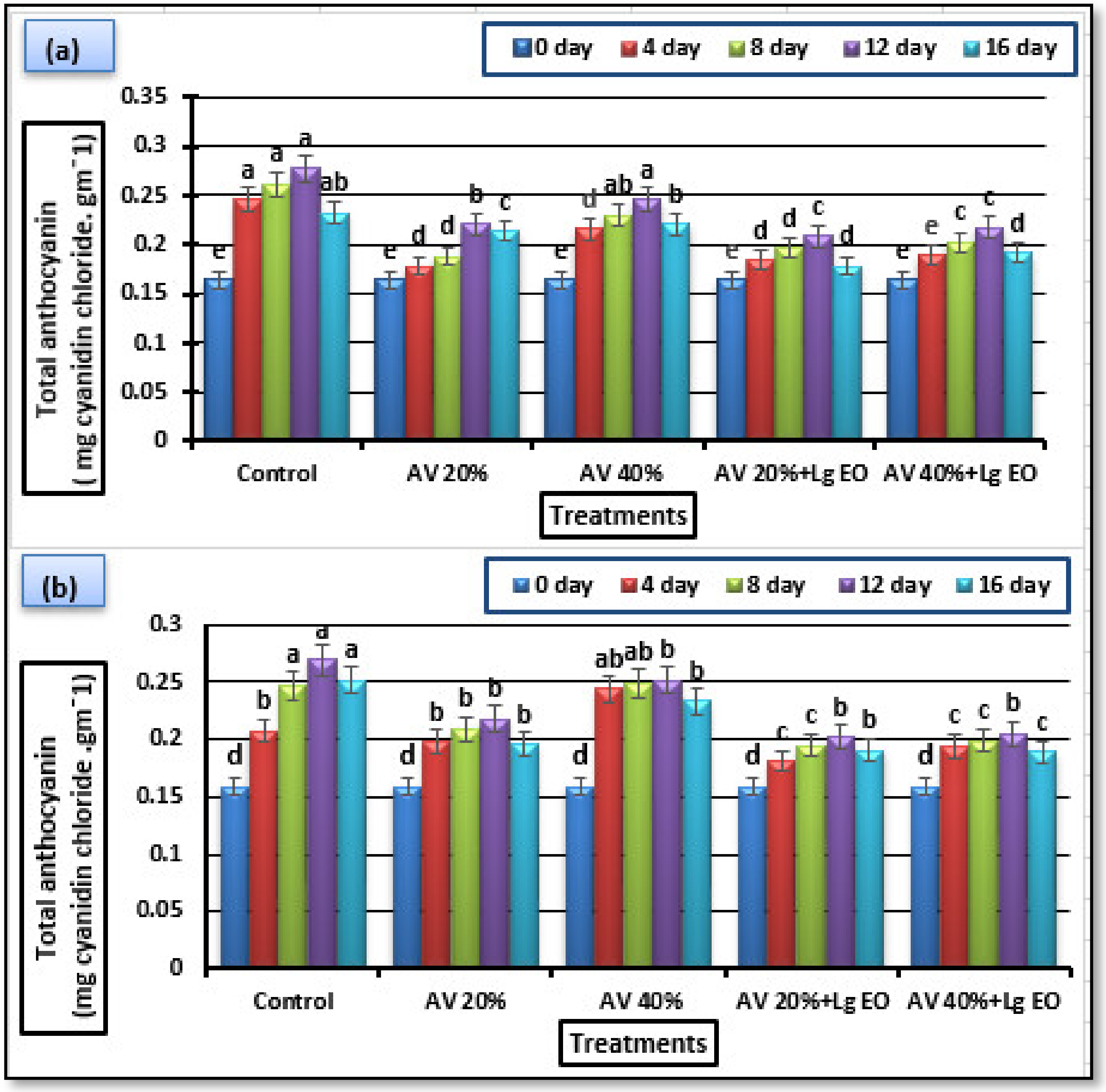
3.5.3. Total Anthocyanins
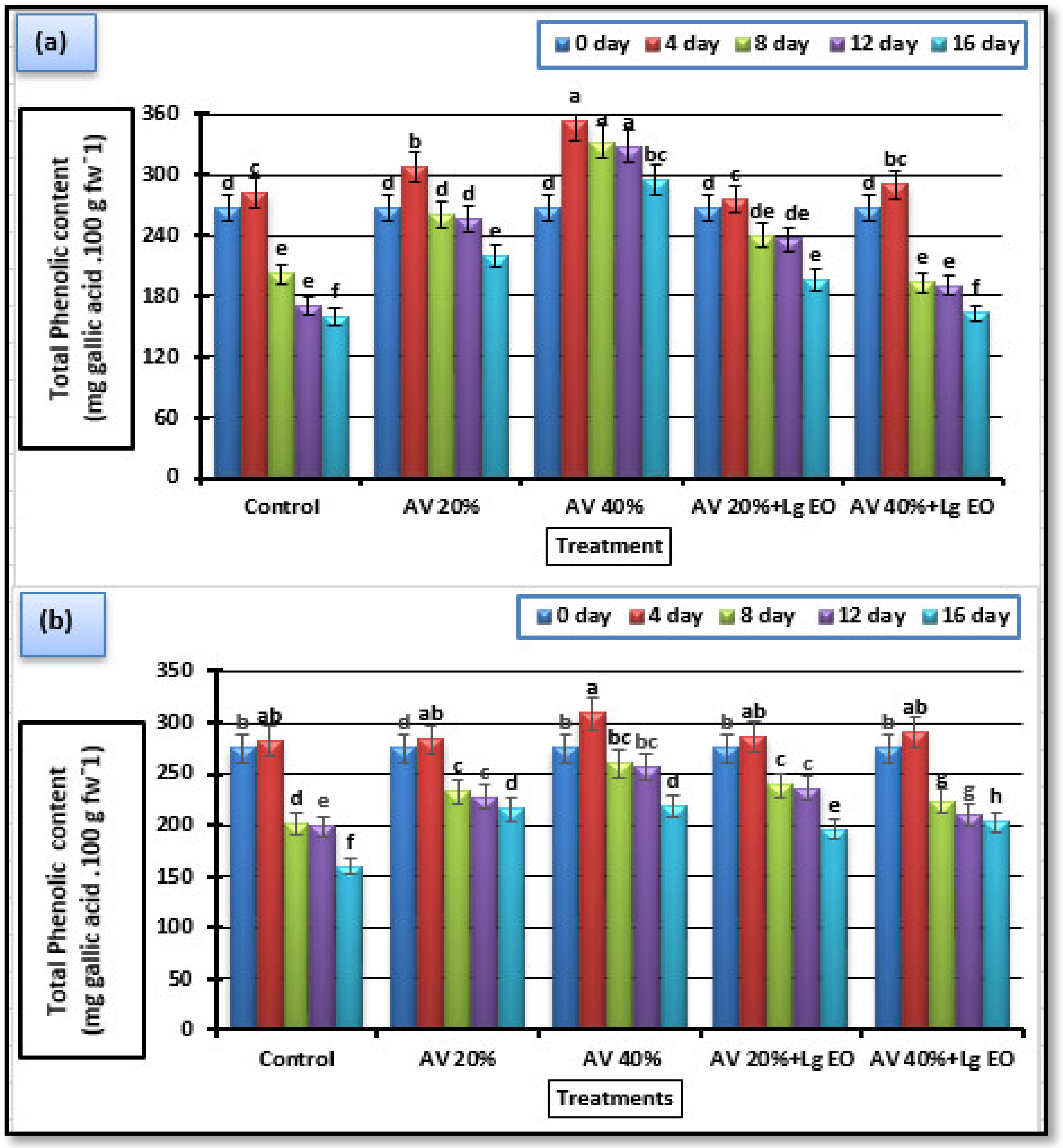
3.5.4. Total Phenolic Content
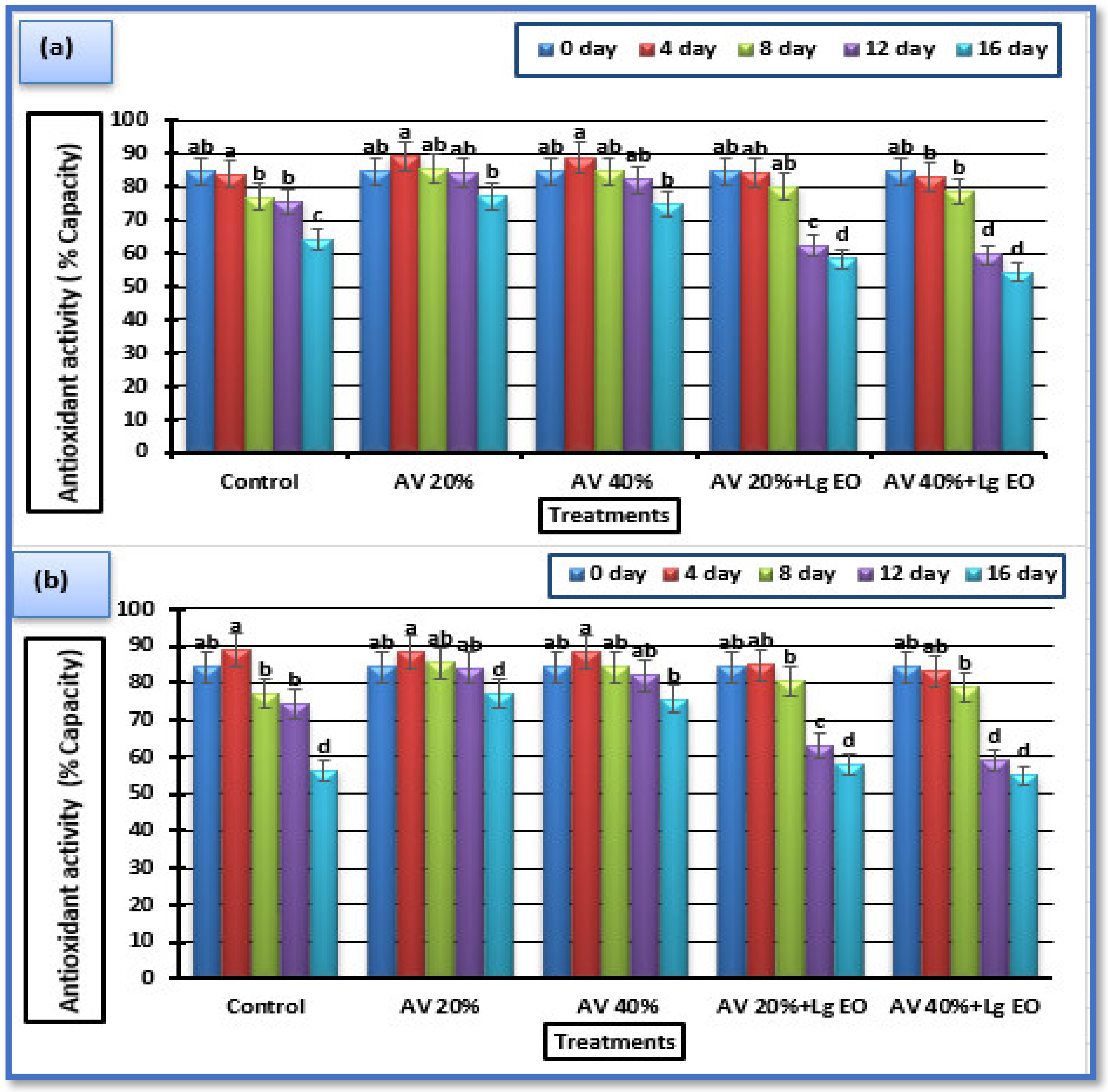
3.5.5. Antioxidant Activity
3.5.6. Fruit Extraction and HPLC Analysis of the Fruit’s Flavonoids
3.6. EDX Analysis for Elemental Composition of Strawberry Fruits
3.7. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahn, M.G.; Kim, D.S.; Ahn, S.R.; Sim, H.S.; Kim, S.; Kim, S.K. Characteristics and Trends of Strawberry Cultivars throughout the Cultivation Season in a Greenhouse. Horticulturae 2021, 7, 30. [Google Scholar] [CrossRef]
- Ilari, A.; Toscano, G.; Boakye-Yiadom, K.A.; Duca, D.; Foppa Pedretti, E. Life Cycle Assessment of Protected Strawberry Productions in Central Italy. Sustainability 2021, 13, 4879. [Google Scholar] [CrossRef]
- Mozafari, A.a.; Dedejani, S.; Ghaderi, N. Positive responses of strawberry (Fragaria × ananassa Duch.) explants to salicylic and iron nanoparticle application under salinity conditions. Plant Cell Tissue Organ Cult. 2018, 134, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Abu Salha, B.; Gedanken, A. Extending the Shelf Life of Strawberries by the Sonochemical Coating of their Surface with Nanoparticles of an Edible Anti-Bacterial Compound. Appl. Nano 2021, 2, 14–24. [Google Scholar] [CrossRef]
- Kumar, R.; Bakshi, P.; Singh, M.; Singh, A.; Vikas, V.; Srivatava, J.; Kumar, V.; Gupta, V. Organic production of strawberry: A review. Int. J. Chem. Stud. 2018, 6, 1231–1236. [Google Scholar]
- Eum, H.-L.; Han, S.-H.; Lee, E.-J. High-CO2 Treatment Prolongs the Postharvest Shelf Life of Strawberry Fruits by Reducing Decay and Cell Wall Degradation. Foods 2021, 10, 1649. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, V.; Garavaglia, L.; Giacalone, G. The Postharvest Quality of Fresh Sweet Cherries and Strawberries with an Active Packaging System. Foods 2019, 8, 335. [Google Scholar] [CrossRef] [Green Version]
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef] [PubMed]
- Ziv, C.; Fallik, E. Postharvest Storage Techniques and Quality Evaluation of Fruits and Vegetables for Reducing Food Loss. Agronomy 2021, 11, 1133. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic Acid Bacteria as Antibacterial Agents to Extend the Shelf Life of Fresh and Minimally Processed Fruits and Vegetables: Quality and Safety Aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a Biopolymer With Triple Action on Postharvest Decay of Fruit and Vegetables: Eliciting, Antimicrobial and Film-Forming Properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.; Xylia, P.; Chrysargyris, A. Sage Essential Oil Improves the Effectiveness of Aloe vera Gel on Postharvest Quality of Tomato Fruit. Agronomy 2019, 9, 635. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elkader, D.Y.; Salem, M.Z.M.; Komeil, D.A.; Al-Huqail, A.A.; Ali, H.M.; Salah, A.H.; Akrami, M.; Hassan, H.S. Post-Harvest Enhancing and Botrytis cinerea Control of Strawberry Fruits Using Low Cost and Eco-Friendly Natural Oils. Agronomy 2021, 11, 1246. [Google Scholar] [CrossRef]
- Farina, V.; Passafiume, R.; Tinebra, I.; Palazzolo, E.; Sortino, G. Use of Aloe vera Gel-Based Edible Coating with Natural Anti-Browning and Anti-Oxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’ Apple. Agronomy 2020, 10, 515. [Google Scholar] [CrossRef] [Green Version]
- Ghoora, M.D.; Srividya, N. Effect of Packaging and Coating Technique on Postharvest Quality and Shelf Life of Raphanus sativus L. and Hibiscus sabdariffa L. Microgreens. Foods 2020, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Amraie, M.; Salehi, M.; Mohseni, M.; Aloui, H. Effect of chitosan-based coatings enriched with savory and/or tarragon essential oils on postharvest maintenance of kumquat (Fortunella sp.) fruit. Food Sci. Nutr. 2019, 7, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, D.; Hamidi-Esfahani, Z.; Rahmati, E. Effect of edible coatings on the shelf-life of fresh strawberries: A comparative study using TOPSIS-Shannon entropy method. NFS J. 2021, 23, 17–23. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ. Effects of lemongrass oil application and modified atmosphere packaging on the postharvest life and quality of strawberry fruits. Sci. Hortic. 2019, 256, 108527. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Verma, K.; Kumar, D.; Nilofer; Lothe, N.B.; Kumar, A.; Chaudhary, A.; Kaur, P.; Singh, K.P.; Singh, A.K.; et al. Optimized irrigation regime and planting technique improve yields and economics in aloe vera [Aloe barbadensis (Miller)]. Ind. Crops Prod. 2021, 167, 113539. [Google Scholar] [CrossRef]
- Habeeb, F.; Shakir, E.; Bradbury, F.; Cameron, P.; Taravati, M.R.; Drummond, A.J.; Gray, A.I.; Ferro, V.A. Screening methods used to determine the anti-microbial properties of Aloe vera inner gel. Methods 2007, 42, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.J.; Navarro, D.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M. Characterisation of gels from different Aloe spp. as antifungal treatment: Potential crops for industrial applications. Ind. Crop. Prod. 2013, 42, 223–230. [Google Scholar] [CrossRef]
- Morillon, V.; Debeaufort, F.; Blond, G.; Capelle, M.; Voilley, A. Factors Affecting the Moisture Permeability of Lipid-Based Edible Films: A Review. Crit. Rev. Food Sci. Nutr. 2002, 42, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; Abrar, M. The therapeutic properties and applications of Aloe vera: A review. J. Herb. Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Nicolau-Lapeña, I.; Colàs-Medà, P.; Alegre, I.; Aguiló-Aguayo, I.; Muranyi, P.; Viñas, I. Aloe vera gel: An update on its use as a functional edible coating to preserve fruits and vegetables. Prog. Org. Coat. 2021, 151, 106007. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Hasan, M.U.; Riaz, R.; Malik, A.U.; Khan, A.S.; Anwar, R.; Rehman, R.N.U.; Ali, S. Potential of Aloe vera gel coating for storage life extension and quality conservation of fruits and vegetables: An overview. J. Food Biochem. 2021, 45, e13640. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Karakaya, O.; Yıldız, K.; Saracoglu, O. Effects of Aloe vera gel and MAP on bioactive compounds and quality attributes of cherry laurel fruit during cold storage. Sci. Hortic. 2019, 249, 31–37. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Nawaz, A.; Anjum, M.A.; Naz, S.; Ejaz, S.; Hussain, S. Aloe vera gel coating delays postharvest browning and maintains quality of harvested litchi fruit. Postharvest Biol. Technol. 2019, 157, 110960. [Google Scholar] [CrossRef]
- Marpudi, S.L.; Pushkala, R.; Srividya, N. Aloe vera gel coating for post-harvest quality maintenance of fresh fig fruits. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 878–887. [Google Scholar]
- Ding, P.; Lee, Y. Use of essential oils for prolonging postharvest life of fresh fruits and vegetables. Int. Food Res. J. 2019, 26, 363–366. [Google Scholar]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Effects of Essential Oil Vapour Treatment on the Postharvest Disease Control and Different Defence Responses in Two Mango (Mangifera indica L.) Cultivars. Food Bioprocess Technol. 2017, 10, 1131–1141. [Google Scholar] [CrossRef]
- Tawfeek, M.E.; Ali, H.M.; Akrami, M.; Salem, M.Z.M. Potential Insecticidal Activity of Four Essential Oils against the Rice Weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). BioResources 2021, 16, 7767–7783. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; Awad, M.; Amer, A.; Hassan, N.N.; Ibrahim, E.-D.S.; Ali, H.M.; Akrami, M.; Salem, M.Z.M. Insecticidal Activity of Lemongrass Essential Oil as an Eco-Friendly Agent against the Black Cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). Insects 2021, 12, 737. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.A.; El-Hefny, M.; Salem, M.Z.M.; Ali, H.M. The Biofungicide Activity of Some Plant Essential Oils for the Cleaner Production of Model Linen Fibers Similar to Those Used in Ancient Egyptian Mummification. Processes 2020, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Utama, I.M.S.; Yulianti, N.L.; Prastya, O.A.; Luther, G. Sesame and Lemon Grass Oils as Coating Materials to Reduce the Deterioration of Tomato Fruits during Storage. In Proceedings of the Indonesian Horticultural Society National Seminar, Malang, Indonesia, 6–9 November 2014; pp. 1–9. [Google Scholar]
- Helal, G.A.; Sarhan, M.M.; Shahla, A.N.K.A.; Ei-Khair, E.K.A. Antimicrobial Activity of Some Essential Oils Against Microorganisms Deteriorating Fruit Juices. Mycobiology 2006, 34, 219–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamgir, A.N.M. (Ed.) Secondary Metabolites: Secondary Metabolic Products Consisting of C and H; C, H, and O; N, S, and P Elements; and O/N Heterocycles. In Therapeutic Use of Medicinal Plants and Their Extracts: Volume 2: Phytochemistry and Bioactive Compounds; Springer International Publishing: Cham, Switzerland, 2018; pp. 165–309. [Google Scholar]
- El-Gioushy, S.F.; Baiea, M.H.M. Impact of gelatin, lemongrass oil and peppermint oil on storability and fruit quality of Samany date palm under cold storage. Bull. Natl. Res. Cent. 2020, 44, 14. [Google Scholar] [CrossRef]
- Trang, D.T.; Hoang, T.K.V.; Nguyen, T.T.M.; Van Cuong, P.; Dang, N.H.; Dang, H.D.; Nguyen Quang, T.; Dat, N.T. Essential Oils of Lemongrass Cymbopogon citratus Stapf Induces Apoptosis and Cell Cycle Arrest in A549 Lung Cancer Cells. BioMed Res. Int. 2020, 2020, 5924856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.H.; Tran, T.K.N.; Ngo, T.C.Q.; Pham, T.N.; Bach, L.G.; Phan, N.Q.A.; Le, T.H.N. Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil. Open Chem. 2021, 19, 820–829. [Google Scholar] [CrossRef]
- Whitaker, V.M.; Chandler, C.K.; Santos, B.M.; Peres, N.; Cecilia do Nascimento Nunes, M.; Plotto, A.; Sims, C.A. Winterstar™ (‘FL 05-107’) Strawberry. HortScience 2012, 47, 296–298. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Tiku, A.B. Immunomodulatory potential of acemannan (polysaccharide from Aloe vera) against radiation induced mortality in Swiss albino mice. Food Agric. Immunol. 2016, 27, 72–86. [Google Scholar] [CrossRef]
- El-Hefny, M.; Abo Elgat, W.A.A.; Al-Huqail, A.A.; Ali, H.M. Essential and Recovery Oils from Matricaria chamomilla Flowers as Environmentally Friendly Fungicides Against Four Fungi Isolated from Cultural Heritage Objects. Processes 2019, 7, 809. [Google Scholar] [CrossRef] [Green Version]
- Okla, M.K.; Alamri, S.A.; Salem, M.Z.M.; Ali, H.M.; Behiry, S.I.; Nasser, R.A.; Alaraidh, I.A.; Al-Ghtani, S.M.; Soufan, W. Yield, Phytochemical Constituents, and Antibacterial Activity of Essential Oils from the Leaves/Twigs, Branches, Branch Wood, and Branch Bark of Sour Orange (Citrus aurantium L.). Processes 2019, 7, 363. [Google Scholar] [CrossRef] [Green Version]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of Some Citrus Essential Oils on Post-Harvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Granato, D.; Masson, M.L. Instrumental color and sensory acceptance of soy-based emulsions: A response surface approach. Food Sci. Technol. 2010, 30, 1090–1096. [Google Scholar] [CrossRef] [Green Version]
- The Association of Official Analytical Chemists (A.O.A.C.). Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Fuleki, T.; Francis, F.J. Quantitative Methods for Anthocyanins. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- El-Hefny, M.; Ashmawy, N.A.; Salem, M.Z.M.; Salem, A.Z.M. Antibacterial activities of the phytochemicals-characterized extracts of Callistemon viminalis, Eucalyptus camaldulensis and Conyza dioscoridis against the growth of some phytopathogenic bacteria. Microb. Pathog. 2017, 113, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-S.; Thi, N.D. Effects of Extraction and Processing Methods on Antioxidant Compound Contents and Radical Scavenging Activities of Laver (Porphyra tenera). Prev. Nutr. Food Sci. 2014, 19, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.S.; Mohamed, A.A.; Feleafel, M.N.; Salem, M.Z.M.; Ali, H.M.; Akrami, M.; Abd-Elkader, D.Y. Natural Plant Extracts and Microbial Antagonists to Control Fungal Pathogens and Improve the Productivity of Zucchini (Cucurbita pepo L.) In Vitro and in Greenhouse. Horticulturae 2021, 7, 470. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Ali, H.M.; Akrami, M. Moringa oleifera seeds-removed ripened pods as alternative for papersheet production: Antimicrobial activity and their phytoconstituents profile using HPLC. Sci. Rep. 2021, 11, 19027. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Snedecor, W.; Cochran, G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- López, A.; De Tangil, M.S.; Vega-Orellana, O.; Ramírez, A.S.; Rico, M. Phenolic Constituents, Antioxidant and Preliminary Antimycoplasmic Activities of Leaf Skin and Flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules 2013, 18, 4942–4954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbandy, M.A.; Abed, S.; Gad, S.; Abdel-Fadeel, M. Aloe vera gel as a functional ingredient and natural preservative in mango nectar. World J. Dairy Food Sci. 2014, 9, 191–203. [Google Scholar] [CrossRef]
- Numan, I.N. Identification of Flavonoids and Phenolic Compound in Aloe vera gel by HPLC. Tikrit J. Pure Sci. 2018, 23, 91–94. [Google Scholar] [CrossRef]
- Do, D.N.; Nguyen, H.T.T.; Huynh, T.H.; Nguyen, N.P.; Luu, X.C. Chemical composition, antibacterial and antioxidant activities of lemongrass (Cymbopogon citratus) essential oil and its fractions obtained by vacuum distillation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1166, 012051. [Google Scholar] [CrossRef]
- Verma, R.K.; Verma, R.S.; Chauhan, A.; Bisht, A. Evaluation of essential oil yield and chemical composition of eight lemongrass (Cymbopogon spp.) cultivars under Himalayan region. J. Essent. Oil Res. 2015, 27, 197–203. [Google Scholar] [CrossRef]
- Shaikh, M.N.; Suryawanshi, Y.C.; Mokat, D.N. Volatile Profiling and Essential Oil Yield of Cymbopogon citratus (DC.) Stapf Treated with Rhizosphere Fungi and Some Important Fertilizers. J. Essent. Oil Bear. Plants 2019, 22, 477–483. [Google Scholar] [CrossRef]
- Tajidin, N.; Ahmad, S.; Rosenani, A.; Azimah, H.; Munirah, M. Chemical composition and citral content in lemongrass (Cymbopogon citratus) essential oil at three maturity stages. Afr. J. Biotechnol. 2012, 11, 2685–2693. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.A.; Asi, M.R.; Hameed, A.; Bourquin, L.D. Effect of Postharvest Application of Aloe vera Gel on Shelf Life, Activities of Anti-Oxidative Enzymes, and Quality of ‘Gola’ Guava Fruit. Foods 2020, 9, 1361. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, G.; Abbas, H.T.; Ali, I.; Waseem, M. Aloe vera gel enriched with garlic essential oil effectively controls anthracnose disease and maintains postharvest quality of banana fruit during storage. Hortic. Environ. Biotechnol. 2019, 60, 659–669. [Google Scholar] [CrossRef]
- Castillo, S.; Navarro, D.; Zapata, P.J.; Guillén, F.; Valero, D.; Serrano, M.; Martínez-Romero, D. Antifungal efficacy of Aloe vera in vitro and its use as a preharvest treatment to maintain postharvest table grape quality. Postharvest Biol. Technol. 2010, 57, 183–188. [Google Scholar] [CrossRef]
- Ravanfar, R.; Niakousari, M.; Maftoonazad, N. Postharvest sour cherry quality and safety maintenance by exposure to Hot- water or treatment with fresh Aloe vera gel. J. Food Sci. Technol. 2014, 51, 2872–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazrati, S.; Beyraghdar Kashkooli, A.; Habibzadeh, F.; Tahmasebi-Sarvestani, Z.; Sadeghi, A.R. Evaluation of Aloe vera Gel as an Alternative Edible Coating for Peach Fruits During Cold Storage Period. Gesunde Pflanz. 2017, 69, 131–137. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Garcia, O.R.; Gutierrez, R.; Luna, J.C.; Villa, C.C. Increasing shelf life of strawberries (Fragaria ssp) by using a banana starch-chitosan-Aloe vera gel composite edible coating. Int. J. Food Sci. Technol. 2020, 55, 92–98. [Google Scholar] [CrossRef]
- Mohammadi, L.; Ramezanian, A.; Tanaka, F.; Tanaka, F. Impact of Aloe vera gel coating enriched with basil (Ocimum basilicum L.) essential oil on postharvest quality of strawberry fruit. J. Food Meas. Charact. 2021, 15, 353–362. [Google Scholar] [CrossRef]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Nadim, Z.; Ahmadi, E.; Sarikhani, H.; Amiri Chayjan, R. Effect of Methylcellulose-Based Edible Coating on Strawberry Fruit’s Quality Maintenance During Storage. J. Food Process. Preserv. 2015, 39, 80–90. [Google Scholar] [CrossRef]
- Atress, A.S.H.; El-Mogy, M.; Aboul-Anean, H.; Alsanius, B. Improving strawberry fruit storability by edible coating as a carrier of thymol or calcium chloride. J. Hortic. Sci. Ornam. Plants 2010, 2, 88–97. [Google Scholar]
- Gol, N.B.; Patel, P.R.; Rao, T.V.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Dhital, R.; Mora, N.B.; Watson, D.G.; Kohli, P.; Choudhary, R. Efficacy of limonene nano coatings on post-harvest shelf life of strawberries. LWT Food Sci. Technol. 2018, 97, 124–134. [Google Scholar] [CrossRef]
- Hassanpour, H. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT Food Sci. Technol. 2015, 60, 495–501. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Mohd Adzahan, N. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1–7. [Google Scholar] [CrossRef]
- García-Alonso, M.; Rimbach, G.; Rivas-Gonzalo, J.C.; de Pascual-Teresa, S. Antioxidant and Cellular Activities of Anthocyanins and Their Corresponding Vitisins A Studies in Platelets, Monocytes, and Human Endothelial Cells. J. Agric. Food Chem. 2004, 52, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Stamminger, R. Impact of ultraviolet radiation treatments on the physicochemical properties, antioxidants, enzyme activity and microbial load in freshly prepared hand pressed strawberry juice. Food Sci. Technol. Int. 2014, 21, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Hashmi, M.S. Chitosan–Aloe vera gel coating delays postharvest decay of mango fruit. Hortic. Environ. Biotechnol. 2020, 61, 279–289. [Google Scholar] [CrossRef]
- Ehtesham Nia, A.; Taghipour, S.; Siahmansour, S. Pre-harvest application of chitosan and postharvest Aloe vera gel coating enhances quality of table grape (Vitis vinifera L. cv. ‘Yaghouti’) during postharvest period. Food Chem. 2021, 347, 129012. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, Y.; Xu, J. Free radical-scavenging activity of Aloe vera (Aloe barbadensis Miller) extracts by supercritical carbon dioxide extraction. Food Chem. 2005, 91, 85–90. [Google Scholar] [CrossRef]
- Hidayati, J.R.; Yudiati, E.; Pringgenies, D.; Oktaviyanti, D.T.; Kusuma, A.P. Comparative Study on Antioxidant Activities, Total Phenolic Compound and Pigment Contents of Tropical Spirulina platensis, Gracilaria arcuata and Ulva lactuca Extracted in Different Solvents Polarity. E3S Web Conf. 2020, 147, 03012. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Hannum, S.M. Potential Impact of Strawberries on Human Health: A Review of the Science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef]
- Co, H.; Markakis, P. Flavonoid Compounds in the Strawberry Fruit. J. Food Sci. 1968, 33, 281–283. [Google Scholar] [CrossRef]
- Sun, Y.; Asghari, M.; Zahedipour-Sheshgelani, P. Foliar Spray with 24-Epibrassinolide Enhanced Strawberry Fruit Quality, Phytochemical Content, and Postharvest Life. J. Plant Growth Regul. 2020, 39, 920–929. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Zamindar, N.; Paidari, S.; Ibrahim, S.A.; Mohammadi Nafchi, A. The synergistic effects of Aloe vera gel and modified atmosphere packaging on the quality of strawberry fruit. J. Food Process. Preserv. 2021, 45, e16003. [Google Scholar] [CrossRef]
- Arrubla Vélez, J.P.; Guerrero Álvarez, G.E.; Vargas Soto, M.C.; Cardona Hurtado, N.; Pinzón, M.I.; Villa, C.C. Aloe Vera Gel Edible Coating for Shelf Life and Antioxidant Proprieties Preservation of Andean Blackberry. Processes 2021, 9, 999. [Google Scholar] [CrossRef]
- Solís-Contreras, G.A.; Rodríguez-Guillermo, M.C.; de la Luz Reyes-Vega, M.; Aguilar, C.N.; Rebolloso-Padilla, O.N.; Corona-Flores, J.; de Abril Alexandra Soriano-Melgar, L.; Ruelas-Chacon, X. Extending Shelf-Life and Quality of Minimally Processed Golden Delicious Apples with Three Bioactive Coatings Combined with Cinnamon Essential Oil. Foods 2021, 10, 597. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Rasouli, M.; Koushesh Saba, M.; Ramezanian, A. Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Antara, N.S.; Paramita, D.; Duwipayana, A.A.; Gunam, I. Inhibitory activity of lemongrass essential oil against Eschericia coli, Staphylococcus aureus, and Vibrio cholera. In Proceedings of the Seminar Nasional Patpi 2013, Jember, Indonesia, 26–29 August 2013. [Google Scholar]
- Velkov, Z.; Balabanova, E.; Tadjer, A. Radical scavenging activity prediction of o-coumaric acid thioamide. J. Mol. Struct. THEOCHEM 2007, 821, 133–138. [Google Scholar] [CrossRef]

| Treatments | Concentration |
|---|---|
| 1 | Control |
| 2 | A. vera gel 20% (v/v) |
| 3 | A. vera gel 40% (v/v) |
| 4 | A. vera gel 20% + lemongrass EO 1% |
| 5 | A. vera gel 40% + lemongrass EO 1% |
| R.T. (min) | Compound | Concentration (mg/mL) |
|---|---|---|
| 5.00 | Coumaric acid | 22.4 |
| 7.01 | Ferulic acid | 8.22 |
| 8.00 | Caffeic acid | 30.77 |
| 9.00 | Syringic acid | 15.12 |
| 11.10 | Sinapic acid | 14.05 |
| 15.00 | Cinnamic acid | 7.14 |
| Chemical Compound | Percentage (%) | MF |
|---|---|---|
| Linalool | 1.77 | 861 |
| Isocitral | 0.97 | 853 |
| Isoneral | 6.67 | 943 |
| α-Citral (Neral) | 40.10 | 930 |
| β-Citral (Geranial) | 30.71 | 916 |
| Citral | 1.22 | 931 |
| Neryl acetal | 5.64 | 876 |
| γ-Dodecalactone | 10.24 | 912 |
| Geraniol acetate | 0.91 | 897 |
| 2-Tridecanone | 0.70 | 867 |
| Nizatidine | 0.32 | 979 |
| β-Caryophyllene epoxide | 0.36 | 917 |
| Selin-6-en-4β-ol | 0.40 | 892 |
| Experiment 1 | |||||
|---|---|---|---|---|---|
| Treatments | Days | L* | a* | b* | h° |
| Control | 0 | 43.83 ± 1.21 b | 43.64 ± 1.89 b | 21.05 + 2.87 b | 1.97 + 1.12 ab |
| 8 | 35.66 ± 2.24 d | 44.93 ± 2.13 b | 20.87 + 1.87 b | 1.57 + 0.87 d | |
| 16 | 33.70 ± 1.54 e | 45.45 ± 1.97 ab | 19.16 + 1.22 cd | 1.90 + 0.58 b | |
| A. vera gel 20% | 0 | 43.83 ± 1.21 b | 43.64 ± 1.89 b | 21.05 ± 2.87 b | 1.97 ± 1.12 ab |
| 8 | 39.40 ± 1.32 cd | 49.47 ± 2.54 a | 19.48 ± 2.01 cd | 1.37 ± 1.02 ef | |
| 16 | 35.61 ± 1.12 d | 44.63 ± 2.33 b | 17.07 ± 1.33 d | 1.57 ± 0.98 d | |
| A. vera gel 40% | 0 | 43.83 ± 1.21 b | 43.64 ± 1.89 b | 21.05 ± 2.87 b | 1.97 ± 1.12 ab |
| 8 | 38.40 ± 2.12 cd | 44.05 ± 2.01 b | 18.49 ± 1.21 cd | 1.45 ± 1.03 e | |
| 16 | 36.84 ± 1.34 d | 44.87 ± 2.44 b | 16.28 ± 1.32 d | 1.15 ± 0.45 f | |
| A. vera gel 20% + lemongrass EO 1% | 0 | 43.83 ± 1.21 b | 43.64 ± 1.89 b | 21.05 ± 2.87 b | 1.97 ± 1.12 ab |
| 8 | 37.80 ± 2.65 cd | 36.43 ± 2.12 c | 25.25 ± 1.56 ab | 2.03 ± 1.11 a | |
| 16 | 49.90 ± 1.67 a | 36.97 ± 2.03 c | 28.59 ± 1.12 a | 2.03 ± 1.01 a | |
| A. vera gel 20% + lemongrass EO 1% | 0 | 43.83 ± 1.21 b | 43.64 ± 1.89 b | 21.05 ± 2.87 b | 1.97 ± 1.12 ab |
| 8 | 44.05 ± 1.78 b | 35.14 ± 2.43 c | 22.42 ± 1.65 b | 1.84 ± 0.55 cd | |
| 16 | 46.80 ± 2.03 a | 34.57 ± 2.63 c | 25.93 ± 1.78 ab | 1.81 ± 0.98 cd | |
| Experimental 2 | |||||
|---|---|---|---|---|---|
| Treatments | Days | L* | a* | b* | h° |
| Control | 0 | 43.49 ± 2.44 c | 42.07 ± 2.61 b | 20.39 ± 1.77 b | 1.89 ± 0.31 c |
| 8 | 34.71 ± 2.03 e | 43.87 ± 2.11 b | 19.83 ± 1.34 c | 1.27 ± 0.61 e | |
| 16 | 33.23 ± 1.65 f | 42.69 ± 1.34 b | 18.66 ± 1.01 c | 2.07 ± 0.54 b | |
| A. vera gel 20% | 0 | 43.49 ± 2.44 c | 42.07 ± 2.61 b | 20.39 ± 1.77 b | 1.89 ± 0.31 c |
| 8 | 40.52 ± 2.23 d | 47.48 ± 2.43 a | 18.73 ± 1.22 c | 1.96 ± 1.21 bc | |
| 16 | 34.67 ± 2.01 e | 43.25 ± 1.87 b | 17.16 ± 1.02 d | 2.75 ± 1.32 a | |
| A. vera gel 40% | 0 | 43.49 ± 2.44 c | 42.07 ± 2.61 b | 20.39 ± 1.77 b | 1.89 ± 0.31 c |
| 8 | 39.94 ± 2.46 d | 46.71 ± 2.21 a | 18.49 ± 1.67 d | 1.54 ± 0.64 d | |
| 16 | 35.17 ± 1.78 e | 43.22 ± 1.44 b | 20.10 ± 1.25 b | 1.37 ± 0.67 e | |
| A. vera gel 20% + lemongrass EO 1% | 0 | 43.49 ± 2.44 c | 42.07 ± 2.61 b | 20.39 ± 1.77 b | 1.89 ± 0.31 c |
| 8 | 47.18 ± 2.67 a | 36.20 ± 1.98 c | 26.88 ± 1.06 ab | 1.08 ± 0.23 g | |
| 16 | 48.50 ± 2.43 a | 37.30 ± 1.66 c | 29.73 ± 1.33 a | 1.95 ± 0.35 bc | |
| A. vera gel 20% + lemongrass EO 1% | 0 | 43.49 ± 2.44 c | 42.07 ± 2.61 b | 20.39 ± 1.77 b | 1.89 ± 0.31 c |
| 8 | 45.37 ± 1.56 b | 35.86 ± 1.78 d | 26.99 ± 1.89 ab | 1.16 ± 0.33 f | |
| 16 | 47.08 ± 1.32 d | 34.19 ± 1.56 d | 27.19 ± 1.65 a | 1.86 ± 0.54 c | |
| R.T. (min) | Compound | Concentration of Flavonoid Compounds (μg/mL) in Strawberry Fruit as Treated by | |||||
|---|---|---|---|---|---|---|---|
| Initial (0 day) | Control | A. vera 20% | A. vera 40% | A. vera 20% + Lemongrass EO 1% | A. vera 40% + Lemongrass EO 1% | ||
| 4.6 | Rutin | 6.14 | 9.14 | ND | 7.13 | 7.14 | 16.25 |
| 5.2 | Naringin | 5.16 | 8.16 | ND | 6.19 | ND | 5.66 |
| 6.0 | Isorhamnetin | ND | ND | ND | ND | ND | 10.23 |
| 6.9 | Quercetin | 10.41 | 15.23 | 15.36 | 8.47 | 9.56 | 9.66 |
| 8.1 | Kaempferol | 5.17 | 6.17 | 6.15 | 20.47 | 22.17 | 11.43 |
| 9.0 | Luteolin | 7.13 | 7.46 | 14.66 | ND | 8.15 | ND |
| 10.0 | Hesperidin | 13.45 | 14.56 | 8.12 | ND | ND | 22.15 |
| 11.0 | 7-Hydroxyflavone | ND | ND | ND | 14.16 | 12.02 | 8.14 |
| 12.01 | Catechin | 8.14 | 9.52 | 20.56 | 11.78 | 16.11 | 1.13 |
| 14.6 | Genistein | ND | ND | ND | ND | ND | 3.52 |
| 15.0 | Chrysoeriol | 25.08 | ND | 4.21 | 17.44 | 7.66 | 15.04 |
| 15.2 | Myricetin | ND | ND | ND | 2.25 | ND | ND |
| Treatment | Element (Atom %) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Si | P | K | Ca | Mg | N | |
| Control | 42.36 ± 0.26 ad | 55.61 ± 0.21 ab | 0.25 ± 0.12 a | 0.14 ± 0.02 a | 1.33 ± 0.12 b | 0.31 ± 0.05 b | nd | nd |
| A. vera gel 20% | 41.30 ± 1.35 b | 56.10 ± 1.11 a | 0.49 ± 0.49 a | 0.17 ± 0.05 a | 1.54 ± 0.16 ab | 0.25 ± 0.02 b | 0.15 ± 0.08 ab | nd |
| A. vera gel 40% | 41.75 ± 1.12 b | 55.01 ± 0.17 ab | 1.04 ± 0.76 ab | 0.11 ± 0.01 ab | 1.10 ± 0.06 b | 0.38 ± 0.13 b | 0.19 ± 0.04 a | 0.40 ± 0.40 |
| A. vera gel 20% + lemongrass EO 1% | 43.61 ± 0.80 ad | 53.74 ± 0.56 b | 0.63 ± 0.41 b | 0.04 ± 0.01 ab | 1.48 ± 0.20 ab | 0.48 ± 0.03 b | 0.03 ± 0.03 bc | nd |
| A. vera gel 40% + lemongrass EO 1% | 45.06 ± 0.66 a | 51.54 ± 0.56 c | 0.15 ± 0.15 a | 0.17 ± 0.02 a | 1.80 ± 0.07 a | 1.23 ± 0.14 a | 0.05 ± 0.03 abc | nd |
| p-value | 0.0908 ns | 0.0030 | 0.6712 ns | 0.0652 ns | 0.0516 ns | 0.0001 | 0.0612 ns | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, H.S.; EL-Hefny, M.; Ghoneim, I.M.; El-Lahot, M.S.R.A.; Akrami, M.; Al-Huqail, A.A.; Ali, H.M.; Abd-Elkader, D.Y. Assessing the Use of Aloe vera Gel Alone and in Combination with Lemongrass Essential Oil as a Coating Material for Strawberry Fruits: HPLC and EDX Analyses. Coatings 2022, 12, 489. https://doi.org/10.3390/coatings12040489
Hassan HS, EL-Hefny M, Ghoneim IM, El-Lahot MSRA, Akrami M, Al-Huqail AA, Ali HM, Abd-Elkader DY. Assessing the Use of Aloe vera Gel Alone and in Combination with Lemongrass Essential Oil as a Coating Material for Strawberry Fruits: HPLC and EDX Analyses. Coatings. 2022; 12(4):489. https://doi.org/10.3390/coatings12040489
Chicago/Turabian StyleHassan, Hanaa S., Mervat EL-Hefny, Ibrahim M. Ghoneim, Mina S. R. Abd El-Lahot, Mohammad Akrami, Asma A. Al-Huqail, Hayssam M. Ali, and Doaa Y. Abd-Elkader. 2022. "Assessing the Use of Aloe vera Gel Alone and in Combination with Lemongrass Essential Oil as a Coating Material for Strawberry Fruits: HPLC and EDX Analyses" Coatings 12, no. 4: 489. https://doi.org/10.3390/coatings12040489
APA StyleHassan, H. S., EL-Hefny, M., Ghoneim, I. M., El-Lahot, M. S. R. A., Akrami, M., Al-Huqail, A. A., Ali, H. M., & Abd-Elkader, D. Y. (2022). Assessing the Use of Aloe vera Gel Alone and in Combination with Lemongrass Essential Oil as a Coating Material for Strawberry Fruits: HPLC and EDX Analyses. Coatings, 12(4), 489. https://doi.org/10.3390/coatings12040489








