Abstract
This study aimed to determine the effect of edible coatings based on whey protein isolate and essential oils (lemon and lemongrass) on the colour, hardness, polyphenols and flavonoids content, structure, and sensory attributes of fresh-cut pears during storage at 4 °C. The optical and barrier properties of the edible films were also determined. Analysed films showed good transparency (Lightness 86.6–95.0) and excellent oxygen and carbon dioxide permeability, which were reduced due to the presence of lemon and lemongrass essential oils. Pears were coated by immersion in a solution containing 8% of whey protein isolate and the addition of lemon oil at 1.0% or lemongrass essential oil at 0.5%. Coating caused a reduction in colour changes, loss in hardness, polyphenols and flavonoids. The study showed that the highest efficiency was demonstrated by the whey protein isolate coatings without the addition of essential oils by preserving the colour and firmness of fresh-cut pears. For these samples, the highest sensory acceptability was also achieved.
1. Introduction
Nowadays, many studies have reported the importance of a balanced diet, which led to an increase in the consumption of fruits and vegetables, such as fresh-cut ones, and is related to the current dietary recommendations. However, the lifestyle of big cities and the few times available to prepare meals have demanded convenient foods. Fresh-cut fruits and vegetables are ready-to-eat products, which maintain the fresh and nutritional quality of the whole product. On the other hand, the peeling and cutting operations can accelerate the metabolic activities of plant tissue, making the minimally processed product more perishable than intact fruits and vegetables [1]. Therefore, new strategies and technologies to maintain quality and extend shelf life are required to make the marketing of these products viable. The use of edible films and coatings is an alternative to preserve the quality and freshness of minimally processed products and prolong their shelf life [2,3]. An edible coating is a thin layer of edible material, which is formed as a protective coating on foods and can be consumed together with those products. They are applied in liquid form onto the food surface, usually by immersing the product in a film-forming solution formed by the structural matrix. The edible film is a thin layer of edible materials that stand alone in nature in comparison to edible coatings which remain adhesive to the food surface [4,5].
Edible films and coatings based on proteins such as corn, wheat, soybeans, peanut, milk, or gelatin were found to possess good functional properties and be suitable for the coating of many fruits and vegetables. Most protein-based films and coatings perform well on hydrophilic surfaces, but in most cases, they exhibit poor resistance to water vapour diffusion. Protein-derived coatings are excellent barriers to carbon dioxide and oxygen but not to water vapour and have poor mechanical properties [6]. Whey protein-based edible materials are transparent and odourless, which show very low permeability to oxygen at low relative humidity conditions, which is the most advantage in perishable foods including whole or fresh-cut fruits and vegetables [7]. Therefore, whey protein-based edible films and coatings were a research area of great interest during recent decades [8].
Pears (Pyrus communis) are climacteric fruits whose ripening process is regulated by ethylene and can be characterized as a fruit with a relatively short shelf life. During the ripening of pears, different changes can be observed in firmness, colour, acidity, sugar content, and development of aroma flavour. According to the sensory research, the optimum quality for eating pears is characterized by a buttery texture, mixed with appropriate colour changes, a characteristic natural flavour associated with the content of sugars, acids, and volatile compounds [9]. Several authors have studied fresh-cut pears to prolong their shelf life by the application of different edible coatings [10,11,12]. Recently, investigations about plant active substances, such as extracts or essential oils, and their role as potential natural food additives have attracted increasing attention. The incorporation of these ingredients into edible coatings gives an opportunity to create active coatings which can help to maintain the food quality. Hashemi et al. [13] observed that fresh-cut pears were positively affected by the starch coating treatment formulated with A. capillus veneris extract, increasing their antioxidant status and decreasing bacterial and fungal contamination. Essential oils are a mixture of volatile fragrances isolated from fresh or dried plants by a distillation method with a water pair. They are characterized by an intense aroma through which they are used in perfumery compositions, food aromas, or applicable for medicinal purposes. The use of essential oils for food is often limited due to costs, intense odour, or possible toxicity [14]. However, the incorporation of essential oils in the formulation of edible films or coatings is promising to reduce the usage dose while maintaining their effects. Lemon essential oil, used as a food additive or favouring agent, is extracted from Citrus lemon and its main components are limonene, valencene and ocimene [15]. Lemongrass essential oil is a natural mixture of different compounds such as terpenes, alcohols, aldehydes, ketones, carboxylic acids, and others found to exhibit antimicrobial activity against different types of microorganisms [16] and can be used as an active ingredient in edible coatings. Kapetanakou et al. [17] observed the positive effect of using sodium alginate coatings incorporated with cinnamon essential oil (0.3% and 0.9% v/v) as a natural active compound on the fungal growth and ochratoxin A production in coated apple and pears. Nevertheless, there is little information in the literature about the effect of whey protein-based edible coatings on the quality attributes of fresh-cut pears during refrigerated storage. Therefore, the aim of the present study was to evaluate the effects of whey protein-based edible coatings incorporated with lemon and lemongrass essential oils on the quality of fresh-cut pears, including colour, firmness, polyphenols and flavonoids content, sensory analysis and microstructure observations. In addition, edible films were prepared from the same film-forming solutions and characterized by colour and gas barrier permeability (water vapour, oxygen, and carbon dioxide).
2. Materials and Methods
2.1. Materials
The whey protein isolate (WPI, ~95% protein) BiPRO was obtained from Davisco Foods International Inc. (La Sueur, MN, USA). Anhydrous glycerol (purity ≥ 99.5%) and salts were used to prepare saturated solutions for the determination of water vapour sorption isotherms, including lithium chloride, potassium acetate, magnesium chloride, potassium carbonate, magnesium nitrate, sodium nitrate, sodium chloride, sulfate ammonium, barium chloride, Folin’s phenol reagent and ethanol were supplied by Avantor Performance Materials Poland S.A. (Gliwice, Poland).
2.2. Preparation of Film-Forming Solutions
The aqueous coating solutions of 8% was obtained as a result of mixing the whey protein isolate (BiPRO, Davisco Inc., Le Sueur, MN, USA) with distilled water and heated at 80 °C for 30 min using RCT Basic Ikamag magnetic agitator (IKA—Werke GMBH & Co., Staufen, Germany) rotating at 250 rpm. Glycerol was added as a plasticizer in an amount of 50% in relation to the protein. The lemon essential oil was added to the film-forming solution (Pollena Aroma, Warsaw, Poland) at a concentration of 1% (w/w) and lemongrass essential oil (Essence sp. Z o.o., Nadarzyn, Poland) at a concentration of 0.5% (w/w). The mixtures were homogenized for 3 min at 24,000 rpm with the use of the IKA YellowLine DI25 Basic homogenizer (IKA—Werke GMBH & Co., Staufen, Germany). The solution without the addition of other substances was determined as a control. The density of the solutions was measured using portable density metre (Mettler Toledo, Columbus, OH, USA) at the room temperature and was in the range of 1.0296–1.0299 ± 0.0001 g·cm−3 for lemon and lemongrass essential oils containing formulations and 1.0302 ± 0.0001 g·cm−3 for the control solution.
2.3. Preparation of Films and Their Characterization
The solutions were poured on a Petri dish in a constant amount and dried at 25 °C and 50% of relative humidity for 24 h in the climate chamber model KBF 720 (Binder, Tuttlingen, Germany). Dried films were peeled off and conditioned at 25 °C and had a relative humidity of 50% prior to testing.
2.3.1. Thickness
Film thickness was measured with an electronic gauge (Metrison S.A., Mościska, Poland) with a precision of 1 μm. The final film thickness was 80 ± 5 μm.
2.3.2. Colour of Films
Colour measurement was performed in 10 repetitions using Minolta colorimeter (model CR-300, Konica Minolta, Tokyo, Japan) in the CIE L*a*b* system, where L* is the brightness, a* and b* are the trichromatic coordinates. The reference material was a standard white plate with colour parameters L* = 95.99, a* = −0.14, and b* = 2.04. The total colour difference between the film and the white standard was calculated according to the equation presented by Sobral et al. [18]:
where ΔE—total colour difference; L*, a*, b*—parameters for white standard; L, a, b—parameters for films.
2.3.3. Water Vapour Permeability
The water vapour permeability was performed in three repetitions based on the gravimetric method presented by Debeaufort et al. [19]. The samples were placed between two open rings and closed in special twist-off glass vessels with an open lid. Distilled water was used for a relative humidity of 100%. The samples were placed into a climatic chamber model KBF 720 (Binder, Tuttlingen, Germany) with a humidity of the relative environment 10% and at 23 ± 1 °C. Sample mass measurements were performed with an accuracy of 0.0001 g twice a day for 7 days. Linear regression was used to calculate the weighting of sampling over time, omitting the first measurements to stabilize the process conditions. The water vapour permeability was calculated and expressed in g·mm·m−2·d−1·kPa−1.
2.3.4. Oxygen Permeability
Oxygen permeability was measured at least in two repetitions according to the standard test method for oxygen gas transmission rate through plastic film and sheeting using a coulometric sensor based on ASTM F 1927-98 [20], using an OXTRAN 2/21 MH modular system (Mocon, Inc., Minneapolis, MN, USA). A film sample was placed on a stainless steel mask with an opening testing area of 5 cm2. One side of the sample was exposed to nitrogen gas flow and the other side was exposed to oxygen gas flow at 25 °C and relative humidity of 50%.
2.3.5. Carbon Dioxide Permeability
A Permatran-C 4/41 MC (Mocon, Inc., Minneapolis, MN, USA) permeation system with an infrared detector was used to measure carbon dioxide permeability according to the standard test method for the carbon dioxide gas transmission rate through barrier materials based on ASTM F 2476-05 [21]. A film sample was placed on a stainless steel mask with an opening testing area of 5 cm2. One side of the sample was exposed to nitrogen gas flow and the other side was exposed to carbon dioxide gas flow. The analysis was performed at least in two repetitions at 25 °C.
2.4. Fruit Sample Preparation and Characterization
2.4.1. Pear Characterization
The research materials were the Conference pear variety from the experimental fields of Warsaw University of Life Sciences. Until the start of the tests Pears were stored in the climate chamber model KBP 720 (Binder, Tuttlingen, Germany) at 4 °C and the relative humidity of 80%. Before starting testing, the pears were washed under running water and dried, then peeled off, cut into slices with a thickness of 1 cm with the usage of a slicer model CL50 Version D (Robot Coupe, Montpellier, France) and then into 2 cm diameter discs. Pears that were used in the experiment had extract at 12.2 °Bx, total acidity of 0.15 g/100 g (converted into citric acid), pH 4.77 and they contained 12.9% of dry matter.
2.4.2. Sample Coating
The pear discs were coated by immersion for 2 min in film-forming solutions at room temperature and then were dried on a filter paper for 1 min and subjected to further processes. The control test was an uncoated pear immersed in distilled water.
2.4.3. Sample Packing and Storage
Control and coated pears were packed with polyamide/polyethylene film PA / PE 70 T-FLEX 70 (Pakmar, Warsaw, Poland) and closed in an air atmosphere using the PP5.4 packaging machine (Tepro S.A, Koszalin, Poland). Fruit samples were stored in the climate chamber model KBF 720 (Binder, Tuttlingen, Germany) at 4 °C and the relative humidity of 80% environment without access to light for 28 days. After 7, 14, 21, and 28 days of storage at 4 °C, the colour, firmness, and content of polyphenols and flavonoids were measured. Sensory assessment was carried out for samples stored at 0, 7 and 14 days. The microstructure observations were made immediately after coating and after 28 days of storage at 4 °C.
2.4.4. Colour
The CIELAB colour parameters of the films were measured in ten repetitions using a colourimeter Model CR-300 (Konica Minolta, Tokyo, Japan). L*, a*, and b* values were obtained with the same intensity of lighting. The Browning Index (BI), which is used as an index of browning in sugar-containing food products, was also calculated [22]:
where:
2.4.5. Firmness
The firmness of the samples was tested using a TA-TX2i texturometer (Stable Micro Systems Ltd., Godalming, UK) with the TextureExpert software. The apple rings were subjected to a 5 mm penetration test with a 10 mm diameter pin at a speed of 1 mm s−1. The measurement was performed in 10 replications. The measure of firmness was the maximum force obtained from the dependence of the force on penetration time.
2.4.6. Total Polyphenol Content
The content of polyphenolic compounds was determined by the Folin–Ciocâlteu method [23] using a UV/VIS spectrophotometer (Helios Gamma, Thermo Electron, Waltham, MA, USA). The samples were grounded in an analytical mill (IKA A11 basic, IKA Werke GmbH & Co. KG, Staufen, Germany), weighed into glass beakers adding 80% ethanol solution. The Folin’s phenol reagent (the gallic acid equivalence) was used. The incubation was carried out in the darkness at 25 °C for an hour. The determination was performed in triplicate. The content of polyphenols was expressed as mg of gallic acid per g of dry matter.
2.4.7. Flavonoid Content
The content of flavonoids was determined according to the modified Lamaison method [24] using a UV/VIS spectrophotometer (Helios Gamma Thermo Electron, Waltham, MA, USA). The determination was performed in triplicate. The flavonoid content was expressed as mg of quercetin per g of dry matter.
2.4.8. Sensory Analysis
The sensory evaluation was carried out for samples directed after coating and stored for 7 and 14 days with the use of a 9-point rating scale among 30 people. The evaluators were students and staff of the Faculty of Food Sciences from the age group of 20–55. The assessed quality characteristics were: taste, smell, colour, hardness and general acceptance of the products.
2.4.9. Microstructure
The observations of the pear microstructure were performed using the FEI Quanda 200 scanning electron microscope (FEI, Brno, Czech Republic). The samples were observed at a magnification of ×100 and an intensity of 30 kV.
2.5. Statistical Analysis
Statistica 10.0 (StatSoft Inc., Tulsa, OK, USA) was used to analyse the resulting data. The analysis of variance (ANOVA) at a significance level of 0.05 was performed with Tukey’s post hoc test to detect significant differences in film properties.
3. Results and Discussion
3.1. Edible Films
All films obtained after the drying process were characterized by a continuous structure, without cracks and pores. The control films were transparent and slightly shiny, the films containing lemon oil were less transparent and slightly milky, and the films containing lemongrass oil were transparent and yellow. The essential oils gave both materials a distinct lemon scent, which corresponds with fresh-cut pears.
3.1.1. Colour
The colour of the films affects the appearance of the coated products, thus affecting the consumer’s acceptance. All tested films were visually transparent, characterized by high lightness (L* parameter) values in the range of 86.6–95.0 regardless of the essential oil addition (Table 1). The highest value was found for the control films and the lowest for films containing lemongrass oil, which were yellow in colour. Similar observations were observed by Socaciu et al. [25] for whey protein films prepared with the addition of tarragon essential oil. The degree of transparency of lipid-containing films can be related to the initial structure of film-forming emulsions, particle size distribution, the concentration of droplets and the microstructure of the films, which is created during solvent evaporation by the drying process. All films had negative a* (red–green) parameters and positive values of b* (yellow–blue) parameters. A significant reduction of the a* parameter was observed, from −0.88 to −2.41 and −2.48 for films containing lemongrass and lemon oils, respectively, which was lower than for control films (−0.88). The values of parameter b* increased from 5.36 to 11.5 for films with lemon oil and to 44.4 for films with lemongrass oil. The changes in colour parameters are due to the colour of pure essential oils which affects the film appearance. However, all analysed films displayed a fairly translucent appearance, which is a desire regarding application to fresh-cut fruits or vegetables where colour is a crucial parameter in quality assessment. According to Erdem, Dıblan, and Kaya [26], this observation can be attributed to higher polymer chain mobility and intermolecular spacing which could facilitate the permeability of the light through edible films. The incorporation of essential oils produced a statistically significant increase in the total colour difference (ΔE) between the film and white standard from 3.59 for control films to 9.87 for films with lemon oil and 43.5 for the film with lemongrass oil. This drastic increase in value can be explained by the yellowing of whey films due to the original colour of essential oils and the oil distribution in the film matrix, which affected the higher differences from the white standard. A similar tendency was observed for whey protein films with tarragon essential oil [25].

Table 1.
L*, a*, b* colour parameters and total colour difference (ΔE) of whey protein films with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils.
3.1.2. Water Vapour Permeabilityg·
The barrier properties are one of the most important attributes of a food packaging film due to the gas migration during storage. The addition of essential oils significantly reduced the water vapour permeability from 25.2 mm·m−2·d−1·kPa−1 for the control film to 22.8 and 18.2 mm·m−2·d−1·kPa−1, respectively, for the film with the addition of oil lemon and lemongrass (Table 2). The reduction of the water vapour permeability of the films is related to the increase in the hydrophobicity of the surface of films enriched with essential oils. Water vapour permeability (WVP) is an important parameter determining the degree of suitability of packaging material for food packaging. The migration of water vapour from the air to the food products, or the loss of moisture from the product to the environment, is a factor that affects the stability and quality of packaged food during the entire storage period. The use of coatings can help to prevent fruit dehydration [27]. Moraes et al. [28] analysed the films used for coating pears with the addition of 2% alginate, they obtained a water vapour permeability value of 19.83, while for coatings with the addition of 0.5% carrageenan, the water vapour permeability was 45.18 mm·m−2·d−1·kPa−1. Oms-Oliu et al. [10] showed that the water vapour permeability for pectin and alginate or gellan-coated pears was significantly higher than for uncoated fruit pieces. Similar observations were observed by Çakmak et al. [29] for whey protein films obtained with the addition of lemon and bergamot essential oils. Reduction in water vapour permeability values due to the oil incorporation may be explained by the generation of an interconnecting lipid network within the whey protein matrix, which caused higher hydrophobicity and ultimately reduced the absorption of water through the film [26]. Previously, similar trends were also reported in different studies for biopolymer films modified with the addition of different oils [30]. In addition, water vapour permeability could also be affected by film morphology and the presence of pores, which could increase the water vapour migration, because gas permeation contributes to the capillary mechanism [31].

Table 2.
Water vapour (WVP), oxygen (O2P) and carbon dioxide (CO2P) permeability of whey protein films with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils.
3.1.3. Oxygen Permeability
Edible films based on whey protein are characterized usually by an excellent oxygen barrier, because of their highly polar nature connected with the high degree of hydrogen bonding of highly polar proteins. Generally, it is well known that hydrogen bonding creates limited polymer chain motion, which results in relatively low gas permeability [26], which is lower than that of conventional, petroleum-based packaging films such as low-density polyethylene (LDPE) or high-density polyethylene (HDPE) [7]. A significant decrease in the oxygen permeability from 112.5 to 81.3–88.1 cm3·µm·m−2·d−1·kPa−1 was observed as a result of the presence of essential oils (Table 2). There were no significant differences (p < 0.05) between the values obtained for films containing lemon and lemongrass essential oils. In general, oxygen (nonpolar compound) solubility is higher in the lipid phase than hydrocolloids and the increase in oxygen permeability was previously observed for lipid-containing films [26]. However, the opposite phenomenon was observed in our study, suggesting that the low content of essential oils and their nature did not affect the oxygen permeability of the analysed films. It can be also connected with intermolecular connections between whey protein molecules and components of essentials oils resulting in lower oxygen permeability values. In addition, the homogenization process could result in a change in polymer structure in comparison with the control films. However, similar observations were observed by Çakmak et al. [29] for whey protein films obtained with the addition of lemon and bergamot essential oils. According to Oms-Oliu et al. [10], the addition of substances such as N-acetylcysteine and glutathione in film-forming solutions may reduce the oxygen exchange of coatings covering pear pieces. Xiao et al. [32] showed that storage increases the oxygen permeability of the coatings and maintains their integrity, which is a major factor in controlling the toasting rate. Sultan et al. [27] obtained low values of the oxygen transfer coefficient for chitosan-based coatings and for chitosan-based coatings and beeswax addition, with which pears were coated. Beeswax was a highly hydrophobic component that increased hydrophobicity. Sánchez et al. [33] coated “Rocha” pears with chitosan coating, which acted as a semi-permeable barrier that controlled gas exchange. In addition, the inclusion of ascorbic acid was an effective method to control the browning of pear slices.
3.1.4. Carbon Dioxide Permeability
In general, edible coatings lead to high carbon dioxide and low oxygen internal gas concentrations in the coated fruit by lowering respiration rates, which contributes to longer shelf life [34]. Carbon dioxide permeability values for the analysed films are shown in Table 2. It can be observed that the addition of essential oils reduced the carbon dioxide permeability from 16.6 for control films to 1.55 and 0.35 cm3·µm·m−2·d−1·kPa−1 for films containing lemon and lemongrass essential oils, respectively. The carbon dioxide permeability of biopolymer films depends strongly on the chemical composition of the polymer and other components, as well as the solubility of carbon dioxide in the lipid phase. In addition, the film microstructure and the presence of pores may affect the increase in gas permeation. In the previous study, Galus and Kadzińska [7] showed an increase in carbon dioxide permeability of whey protein isolate films due to the presence of almond and walnut oils at the concentration of 0.5% and 1.0%. However, plant essential oils play not only a role as a lipid component since different chemical compounds are present, therefore the permeation of carbon dioxide may vary. Since carbon dioxide permeability is crucial for the respiration of living tissues, such as fresh-cut pears, films characterized by higher permeation to carbon dioxide would be more appropriate [35]. However, there is little information in the literature about the gas barrier properties of edible films, especially for carbon dioxide permeability, therefore future studies are needed.
3.2. Coated Pears
3.2.1. Colour
The colour of the fruit is one of the main factors that can affect the acceptability of the product by the consumer. Therefore, it was crucial to determine the colour parameters during the coating process and then during storage. Figure 1 shows the changes in the L* (Lightness) parameter for the tested pears during storage, from 69.66 to 79.41. It was observed that pears coated with the whey protein isolate showed the highest values of the L* parameter during storage, compared to the control sample and coatings based on the isolate with the addition of essential oils. This means that the use of whey coatings was the most beneficial and inhibited the loss of lightness during storage. The greatest reduction in the L* parameter was observed for pears coated with whey coatings with essential oils, while the use of the whey coating inhibited the reduction in lightness during storage. After 28 days of storage, only fresh-cut pears coated with whey protein isolate had a higher L* value compared to uncoated samples. In addition, the use of whey coating inhibited the decrease in lightness during storage. From Figure 2, it can be seen that the lowest values of the browning index were obtained by pears coated with whey protein coatings. The use of a coating with lemongrass oil resulted in much higher browning index values related to the yellow colour of the oil. Moreover, this result indicates that the use of a coating with a large amount of lemon oil retained significantly higher values of the browning index. Browning is caused by an enzyme (polyphenol oxidase) that is found in many fruits. Sharma and Rao et al. [36] proved that the use of cinnamic acid delays the browning of fresh-cut pears. This delay is caused by the inhibitory effect of polyphenol oxidase activity. Xiao et al. [32] observed that pears coated with chitosan and rosemary showed an inhibitory effect on polyphenol oxidase. Similar observations were observed by Olivas et al. [37] who used the addition of ascorbic acid to delay the browning of pears after cutting. Ochoa-Velasco and Guerrero-Beltrán [12] also achieved a reduction in the lightness loss of cut pears through the use of a chitosan coating.
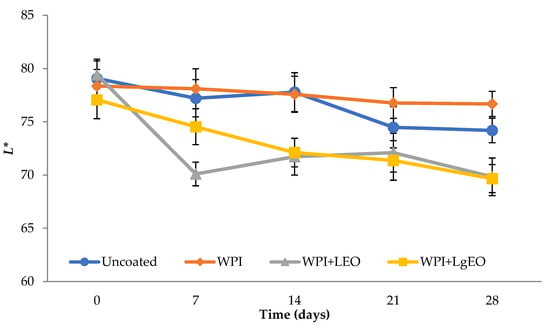
Figure 1.
Lightness (Parameter L*) of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during 28 days of storage at 4 °C.
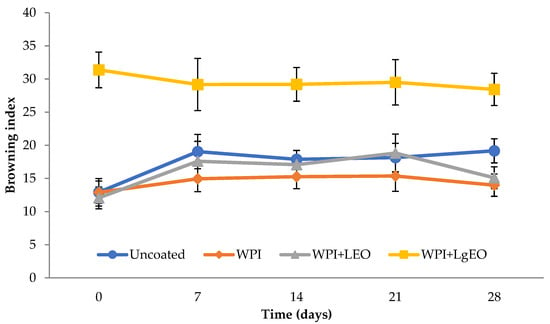
Figure 2.
Browning index of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during 28 days of storage at 4 °C.
Pears with the addition of lemongrass oil showed the largest (even linear) decrease in the L* parameter during storage, which were statistically significant (Table S1). The value of the a* parameter (red and green) takes negative values, from −4.39 to −0.19, which means an increased proportion of green. The parameter has the highest value in the case of the test with the addition of lemongrass oil, which indicates progressive colour changes. It was observed that the storage time significantly influenced the change of the parameter a*. In the first days of storage, the type of coating used did not affect the a* parameter, only the coatings with lemongrass oil showed significant differences. In the case of whey protein isolate with the addition of lemon oil, statistically significant differences were observed only for the last day of storage (28 days). The value of the b* parameter (yellow and blue) for all samples showed a positive value, from 9.94 to 23.93, which proves a high proportion of yellow. Similar to the case of the parameter a*, the highest value was observed for the coatings with the addition of lemongrass oil. It was observed that the type of coating used had significant statistical differences between the value of the parameter b* for the coatings with lemongrass oil and the other analysed samples. The differences in the values b* for the control sample, the coated sample with the whey protein isolate, as well as the sample with lemon oil added, were not statistically significant (Table S1). Only the sample of whey protein isolate with the addition of lemongrass oil differed from the others. Similar tendencies were observed for the browning index (Figure 2). The fresh-cut pears coated with a coating based on whey protein isolate with lemon essential oil showed the lowest values of the parameter, 12.07 and 15.10, at day 0 and at 28 days, respectively. Regarding days 7–21, the samples coated with whey protein isolate showed the lowest values, from 14.95 to 15.37. The highest values of the parameters were observed in the case of pears coated with a formulation with lemongrass oil, which was reduced during storage, from 31.37 to 28.43. The used oil showed a significant proportion of yellow colour and thus had an opposing effect on the browning index values. Additionally, comparing the coated samples with the control sample, it is clearly visible that the control sample improved the progressive browning to a much greater extent during storage. The increasing values of the browning index were accompanied by an increase in the colour parameters a* and b* and a decrease in the value of the L* parameter. Statistically significant differences were observed in the case of changes in the value of the browning index during storage for the control sample and for the whey sample with the addition of lemon oil. The coatings based on whey protein isolate and the coatings with the addition of lemongrass oil showed no significant changes in this parameter during storage (Table S1). In the case of apples coated with coatings based on whey protein isolate, the browning index also showed significantly lower values compared to the control (uncoated) sample.
3.2.2. Firmness
The analysed pears showed high firmness with values of 22–23 N (Figure 3) with a slight tendency to lose hardness during storage, which was the highest for the whey coatings with the addition of lemon oil. Loss of firmness of pears during storage can be caused by cell wall degradation and reduction in turgor pressure in the cells. Thus, this factor indicates the quality of fruits and their shelf life. Results showed that the firmness of the coated fruit was maintained during 28 days of storage at 4 °C. A significant decrease in firmness was observed only in the case of pears with the coatings containing lemon essential oil (Table S2). Similar values of pear hardness during storage were obtained by Oms-Oliu et al. [10], who showed that pears coated with alginate, pectin, or gellan gum did not significantly change fruit firmness. Sánchez et al. [33] observed that covering pears with a higher concentration of chitosan coating resulted in higher firmness values. Application of chitosan concentration of 1.5 g/L retained the original firmness of the pears for 10 days of storage. Xiao et al. [32] reported the positive effect of chitosan and carboxymethyl chitosan of stored pears for 10 days at 4 °C. Based on the statistical analysis, it was found that the storage time did not significantly affect the hardness of the samples, except for the control sample. The same tendency was also observed by Ochoa-Velasco and Guerrero-Beltrán [12] for the prickly pear fruit coated with chitosan with the addition of acetic acid, which maintained its hardness until the 12th day of storage, after that day the loss of this parameter gradually began. The authors explained it with the loss of the cell wall structure as well as the initiation of enzymatic reactions.
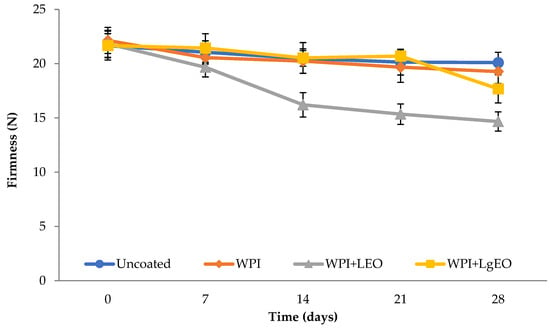
Figure 3.
Firmness of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during 28 days of storage at 4 °C.
3.2.3. Total Polyphenol Content
The content of polyphenols, expressed in mg gallic acid per g of dry matter in the uncoated and coated fresh-cut pears decreased during storage (Figure 4). Higher levels of polyphenols were observed in pears coated with whey coatings with the addition of essential oils, which can be connected with the character of plant essential oils naturally containing polyphenols.
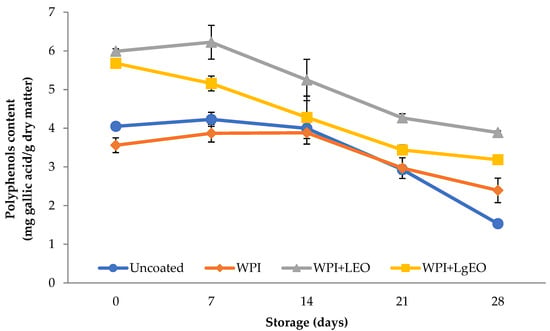
Figure 4.
Polyphenols content of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during 28 days of storage at 4 °C.
Higher values of polyphenols immediately after application of coatings and during storage were observed for pears coated with whey protein isolate with the addition of essential oils. Especially the addition of lemon oil caused a marked increase in the content of polyphenols in the coated samples. The highest decrease in polyphenol content was observed for the control sample, indicating that the whey coatings had a positive effect on fresh-cut pears during storage regarding polyphenol content. Statistical analysis confirmed that the storage time of coated and uncoated samples had a significant impact on the content of polyphenols. The differences in the values of polyphenols are statistically significant (p < 0.05) only after the first 7 days of storage for coatings with the addition of essential oils and after the first 14 days in the case of the control and whey samples. The type of coating used clearly affected the content of phenolic compounds (Table S3). On the other hand, in the study conducted by Oms-Oliu et al. [10], the total phenol content in pears was significantly higher in the samples containing the addition of N-acetylcysteine and glutathione than in the samples that had not been treated with the antioxidant. According to Sharma and Rao [36], the increased ability to act as an antioxidant may result from an increase in the total phenol content in coated pears of the “Rocha” variety. Comparing other fruit preservation processes, a high degree of loss of phenolic compounds is clearly visible. Rząca and Witrowa-Rajchert [38] investigated the effect of the convection drying process on the antioxidant activity of dried apples, the research showed that this process resulted in approx. 70% reduction in the antioxidant activity of phenolic compounds compared to the raw material. This was confirmed by Ścibisz and Mitek [39], who showed that the pretreatment of fruit, i.e., blanching and osmotic dehydration, decreased the antioxidant capacity in the case of high-bush blueberry fruits.
3.2.4. Flavanoids
The content of flavonoids expressed as mg of quercetin per g of dry matter of analysed fresh-cut pears significantly decreased during storage (Figure 5). The highest value of flavonoids was observed in the control sample when the lowest for samples coated with whey protein isolate coatings (Table S4). The highest content of flavonoids after 28 days of pear storage was observed for samples coated with whey protein-lemongrass essential oil formulation, 2.55 mg·g·d.m.−1, while the lowest for samples covered with whey protein isolate, 1.39 mg·g·d.m.−1. Flavonoids, being bioactive substances, contribute to high antioxidant and antimicrobial activity. Hashemi et al. [13] showed that the content of flavonoids in fresh-cut pears coated with starch-based formulations containing the extract of Adiantum capillus veneris leaf affected their extension of shelf life.
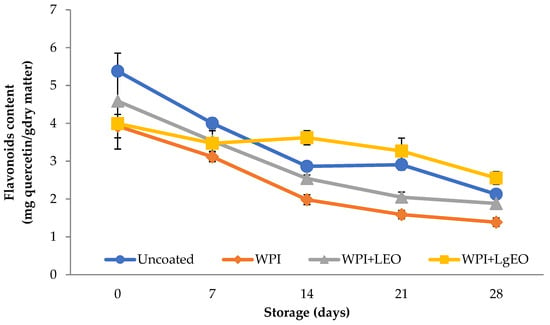
Figure 5.
Flavonoids content of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during 28 days of storage at 4 °C.
3.2.5. Atmosphere in Packaging (CO2)
The analysed samples were packed in the air atmosphere in polyamide/polyethylene film packages. During storage, the internal composition of the packaging atmosphere was analysed, and the content of oxygen and carbon dioxide was examined. On the basis of the obtained results, it was observed that the oxygen content was at a relatively low level of <0.1%, and a particularly low content was observed on the 2nd day of storage for the control sample and the coated whey protein isolates. With the storage time, the oxygen content decreased, after some time anaerobic conditions were obtained. Anaerobic conditions may favour the growth of anaerobic bacteria, thus deteriorating the quality of the product. Therefore, there is a need for further research to adapt the appropriate packaging conditions for coated pears to avoid anaerobic conditions.
However, low levels of oxygen in the packaging of coated pears were also obtained by Oms-Oliu et al. [10] and Del Nobile et al. [11], who also observed a significant increase in the concentration of carbon dioxide, which was also observed for all analysed fresh-cut pears (Figure 6). As reported in the literature, the rate of oxygen penetration through the layers based on the whey protein isolate is 325 to 1750 times lower than that of polyethylene film [40]. The high oxygen barrier creates conditions in which the coatings reduce the respiration rate of fruits and vegetables. Bai et al. [41] showed that the application of coatings on the apple surface reduced the partial pressure of oxygen in the tissue by a factor of 2 and the partial pressure of carbon dioxide by a factor of 4. Similar relationships were observed in the case of strawberries coated with a mixture of chitosan and oleic acid and grapes protected with Aloe vera-based coatings [42,43]. The statistical analysis shows that the differences in the content of carbon dioxide during 28 days of storage were statistically significant for all samples. At the same time, the type of coating layer significantly affected the carbon dioxide content (Table S5).
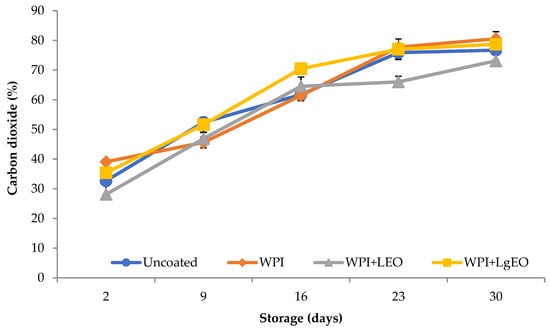
Figure 6.
Carbon dioxide content in packaging of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during 28 days of storage at 4 °C.
3.2.6. Sensory Analysis
The sensory evaluation illustrated the effect of coatings on the changes in pear quality characteristics during storage, which showed that the applied film-forming substances did not significantly affect the deterioration of the sensory properties of the raw material (Figure 7).
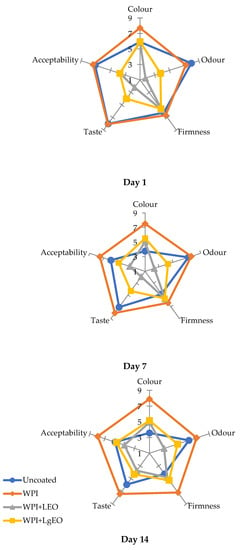
Figure 7.
Sensory attributes of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during storage at 4 °C.
The coating based on whey protein isolate obtained the best scores during the sensory analysis. The use of this coating did not adversely affect the quality characteristics of the pears (colour, taste, smell, and total rating), however, the fresh-cut pears coated with whey protein isolate obtained the highest overall rating compared to other samples (Table S6). The whey coatings obtained the best marks on days 7 and 14, when the control began to lose its sensory properties. However, the use of essential oils for the protein matrix resulted in negative assessments of the taste and aroma of pears, especially on days 1 and 7 of storage, probably due to the oil intensity, which is far from the original smell. Meanwhile, on day 14, trials with the addition of essential oils received higher scores, mainly due to the lower intensity of the lemon oil odour during storage. Colour was an important distinguishing feature of the attractiveness of the assessed samples. The colour criterion, i.e., maintains brightness and thus reduces enzymatic browning, which is a crucial criterium for sensory evaluation. The coatings based on the whey protein isolate received the highest marks for the colour discriminant on all days of analysis, i.e., on the 1st, 7th, and 14th day of storage at 4 °C. Another important indicator was the smell. For the odour criterion, the control, uncoated samples had the highest scores on the 1st day, but over time (7th and 14th days), the pears coated with whey protein isolates were rated the highest among all samples. Subsequent qualitative characteristics, i.e., hardness, taste, and overall rating were less important criteria for the sensory evaluation, but still clearly influenced the final evaluation of the product. The literature examples also confirm the advantages of using coatings based on whey protein isolate. Apples coated with the whey protein isolate received significantly higher scores during the sensory analysis compared to the uncoated test. As suggested in the literature, this is probably due to the antioxidant effect of amino acids such as cysteine or the higher oxygen barrier of protein coatings [44].
3.2.7. Microstructure
The surface microstructure of the pears was observed immediately after coating and after 28 days of storage, which is presented in Figure 8. The uncoated pears were characterized by a porous structure characteristic of the pear tissue, while the surfaces with the applied coatings showed few closed pores. Most of the coating mixture was inside the pores of the tissue.

Figure 8.
Scanning electron micrographs of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated (Magnification 100×).
The photographs of the surface of the pears after storage showed drying of the surface related to the disruption of the tissue and its drying during storage. The highest degree of dryness is visible for uncoated pears. The pears with whey protein coating showed a wetter surface and the drying of the material was slowed down, which can be attributed to the hydrophilic nature of protein [45]. However, all pears tested showed a low degree of dryness related to packaging use and non-occurring weight changes (inhibited water loss). The coated pears showed few closed pores because most of the film-forming solutions penetrated deep into the pores of the tissue. The dryness, related to the disruption of the tissue, and its progressive drying during storage, were also observed. The highest degree of this phenomenon was observed for the uncoated control. The coated pears showed a lower degree of drying, due to the maintenance of a higher degree of humidity and thus a slower drying process. Nevertheless, all tests showed a relatively low degree of dryness and high quality due to the refrigerated storage conditions and the use of appropriate packaging, which prevented weight loss.
4. Conclusions
The essential oils of lemon and lemongrass can be successfully used in the preparation of whey protein isolate edible coatings and films. They were characterized by a continuous structure, without cracks and pores, with a characteristic milky (film with lemon oil) and yellow colour (films with lemongrass oil) and a refreshing lemon scent. The results of the barrier properties showed a significant influence of the essential oils used on the reduction of the permeability of water vapour, oxygen, and carbon dioxide. All in all, it was evident that the incorporation of essential oils improved the gas barrier property of whey protein isolate films as shown by their low values compared to the control. The whey protein coatings significantly reduced the browning of pears during storage, except for the formulation with lemongrass essential oil, which showed significantly higher values related to the nature of the oils and their yellow colour. The firmness of the pears during storage was maintained, however, a tendency in the reduction of firmness was observed for samples coated with the formulation with lemon oil. The content of polyphenols and flavonoids in pears decreased during 28 days of storage. The sensory evaluation showed that the use of whey protein coatings did not significantly reduce the taste, smell, colour, and hardness of fresh-cut pears, however, the incorporation of essential oils into the protein matrix reduced the acceptability of pears. Analysis of the composition of the atmosphere in the pear packaging showed a very limited level of oxygen and increased production of carbon dioxide during storage. A change in the surface of the coated pear tissue compared to the uncoated pear was observed. After 28 days of storage, drying of the material was visible with greater intensity for uncoated samples. However, the analysed coatings can also find applications for other fresh-cut fruits, such as apples or pineapples, for maintaining the quality attributes, such as colour or texture. Nevertheless, future studies are required to determine the effects of the optimized whey protein coatings on the physiological responses, physicochemical properties, and antioxidant capacity of fresh-cut pears during refrigerated storage.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/coatings11070745/s1, Table S1: L*, a*, b* colour parameters and browning index (BI) of fresh-cut pears coated with whey 1protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during storage, Table S2: Firmness of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during storage, Table S3: Polyphenol content of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during storage, Table S4: Flavonoid content of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during storage, Table S5: Carbon dioxide content in packaging of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during storage, Table S6: Sensory attributes of fresh-cut pears coated with whey protein isolate coatings (WPI) incorporated with lemon (WPI + LEO) and lemongrass (WPI + LgEO) essential oils or uncoated samples during storage.
Author Contributions
Conceptualization, S.G.; methodology, S.G.; software, S.G.; validation, S.G.; investigation, S.G.; resources, S.G.; data curation, S.G.; writing—original draft preparation, S.G., M.M.; writing—review and editing, S.G., M.M., A.C., E.D., J.K., A.M. and H.K.; visualization, S.G.; supervision, S.G. and H.K.; project administration, S.G. and H.K.; funding acquisition, S.G. and H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed with funds under the WULS (Warsaw University of Life Sciences) Support System (decision no. SMPB 7/2020). The work was also co-financed by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Food Sciences of Warsaw University of Life Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available on request from the corresponding author.
Acknowledgments
The authors would like to thank Magdalena Kamińska for her help in conducting the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Galus, S. Development of Edible Coatings in the Preservation of Fruits and Vegetables: A Critical Discussion and Exhaustive in Polymers for Agri-Food Applications; Springer Nature: New York, NY, USA, 2019; pp. 377–390. [Google Scholar]
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and functionalized films/coatings—performances and perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, A.E.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advances in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Rossi-Márquez, G.; Helguera, M.; Briones, M.; Dávalos-Saucedo, C.A.; Di Pierro, P. Edible coating from enzymatically reticulated whey protein-pectin to improve shelf life of roasted peanuts. Coatings 2021, 11, 329. [Google Scholar] [CrossRef]
- Cruz, V.; Rojas, R.; Sausedo-Pompa, S.; Martínez, D.G.; Aguilera-Carbó, A.F.; Alvarez, O.B.; Rodríguez, R.; Ruiz, J.; Aguilar, C.N. Improvement of shelf life and sensory quality of pears using a specialized edible coating. J. Chem. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martın-Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Conte, A.; Scrocco, C.; Brescia, I. New strategies for minimally processed cactus pear packaging. Innov. Food Sci. Emerg. Technol. 2009, 10, 356–362. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Guerrero-Beltrán, J.A. Postharvest quality of peeled prickly pear fruit treated with acetic acid and chitosan. Postharvest Biol. Technol. 2014, 92, 139–145. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Zahabi, N.; Rezaee, Z.; Maherani, Z.; Boghori, P.; Keshavarz, Z. Evaluation of a starch-based edible film as carrier of adiantum capillus-veneris extract to improve the shelf life of fresh-cut pears. J. Food Saf. 2016, 36, 291–296. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Demircan, B.; Özdestan-Ocak, Ö. Effects of lemon essential oil and ethyl lauroyl arginate on the physico-chemical and mechanical properties of chitosan films for mackerel fillet coating application. J. Food Meas. Charact. 2021, 15, 1499–1508. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Opara, U.L.; Fawole, O.A. Optimization of gum arabic and starch-based edible coatings with lemongrass oil using response surface methodology for improving postharvest quality of whole “Wonderful” pomegranate fruit. Coatings 2021, 11, 442. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Nestora, S.; Evageliou, V.; Skandamis, P.N. Sodium alginate, cinnamon essential oil coated apples and pears: Variability of Aspergillus carbonarius growth and ochratoxin A production. Food Res. Int. 2019, 119, 876–885. [Google Scholar] [CrossRef]
- Sobral, P.J.; dos Santos, J.S.; Garcia, F.T. Effect of protein and plasticizer concentration in film forming solutions on physical properties of edible films based on muscle proteins of a Thai Tilapia. J. Food Eng. 2005, 70, 93–100. [Google Scholar] [CrossRef]
- Debeaufort, F.; Martin-Polo, M.; Voilley, A. Polarity and structure affect water vapor permeability of model edible films. J. Food Sci. 1993, 58, 428–434. [Google Scholar] [CrossRef]
- ASTM F 1927-98 Standard Test Method for Determination of Oxygen Gas Transmission Rate, Permeability, and Permeance at Controlled Relative Humidity through Barrier Materials a Coulometric Sensor; American Society for Testing and Materials: Philadelphia, PA, USA, 1999.
- ASTM F 2476-05 Test Method for the Determination of Carbon Dioxide Gas Transmission Rate (CO2TR) through Barrier Materials Using an Infrared Detector; American Society for Testing and Materials: Philadelphia, PA, USA, 2005.
- Buera, M.P.; Lozano, R.D.; Petriella, C. Definition of colour in the non-enzymatic browning process. Die Fabre. 1985, 32–33, 318–322. [Google Scholar]
- Sluis, A.; Dekker, M.; Skrede, G.; Jongen, W. Activity and concentration of polyphenolic antioxidants in apple juice. Effect of existing production methods. J. Agr. Food Chem. 2002, 50, 7211–7214. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Gressier, B.; Trotin, F.; Brunet, C.; Dine, T.; Luyckx, M.; Vaseur, J.; Cazin, M.; Cazin, J.-C.; Pinkas, M. Oxygen species skavering activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneim. Forsch. Drug Res. 1996, 46, 1086–1089. [Google Scholar]
- Socaciu, M.-I.; Fogarasi, M.; Semeniuc, C.A.; Socaci, S.A.; Rotar, M.A.; Mureşan, V.; Pop, O.L.; Vodnar, D.C. Formulation and characterization of antimicrobial edible films based on whey protein isolate and tarragon essential oil. Polymers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Erdem, B.G.; Dıblan, S.; Kaya, S. Development and structural assessment of whey protein isolate/sunflower seed oil biocomposite film. Food Bioproduct. Proces. 2019, 118, 270–280. [Google Scholar] [CrossRef]
- Sultan, M.; Hafez, O.M.; Saleh, M.A.; Youssef, A.M. Smart edible coating films based on chitosan and beeswax–pollen grains for the postharvest preservation of Le Conte pear. Royal. Soc. Chem. 2021, 11, 9572–9585. [Google Scholar]
- Moraes, K.S.; Fagundes, C.; Melo, M.C.; Andreani, P.; Monteiro, A.R. Conservation of Williams pear using edible coating with alginate and carrageenan. Ciênc. Tecnol. Aliment. 2012, 32, 679–684. [Google Scholar] [CrossRef] [Green Version]
- Çakmak, H.; Özselek, Y.; Turan, O.Y.; Firatligil, E.; Karbancioğlu-Güler, F. Whey protein isolate edible films incorporated with essential oils: Antimicrobial activity and barrier properties. Polym. Degrad. Stab. 2020, 179, 109285. [Google Scholar] [CrossRef]
- Valenzuela, C.; Abugoch, L.; Tapia, C. Quinoa protein-chitosan-sunflower oil edible film: Mechanical, barrier and structural properties. LWT Food Sci. Technol. 2013, 50, 531–537. [Google Scholar] [CrossRef]
- Srinivasa, P.; Ramesh, M.; Tharanathan, R. Effect of plasticizers and fatty acids on mechanical and permeability characteristics of chitosan films. Food Hydrocoll. 2007, 21, 1113–1122. [Google Scholar] [CrossRef]
- Xiao, C.; Zhu, L.; Luo, W.; Song, X.; Deng, Y. Combined action of pure oxygen pretreatment and chitosan coating incorporated with rosemary extracts on the quality of fresh-cut pears. Food Chem. 2010, 121, 1003–1009. [Google Scholar] [CrossRef]
- Sánchez, C.; Lidon, F.C.; Vivas, M.; Ramos, P.; Santos, M.; Barreiro, M.G. Effect of chitosan coating on quality and nutritional value of fresh-cut ‘Rocha’ pear. Emir. J. Food Agric. 2015, 27, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Mo, Y.; Li, Y.; Zhao, Y.; Zhang, G.; Hu, Y. Quality and internal characteristics of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua) treated with different kinds of coatings during storage. Postharvest Biol. Technol. 2008, 49, 171–179. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vincente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Rao, R.T.V. Xanthan gum based edible coating enriched with cinnamic acid prevents browning and extends the shelf-life of fresh-cut pears. LWT Food Sci. Technol. 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Rząca, M.; Witrowa-Rajchert, D. Radical scavenging activity of phenolic compounds contained in dried apple. Food. Sci. Technol. Qual. 2006, 2, 280–289. [Google Scholar]
- Ścibisz, I.; Mitek, M. Antioxidant activity and phenolic compound capacity in dried highbush blueberries (Vaccinium corymbosum L.). Food. Sci. Technol. Qual. 2006, 4, 68–76. [Google Scholar]
- Krochta, J.M.; McHugh, T.H. Permeability properties of edible films. In Edible Coatings and Films to Improve Food Quality; Technomic Publishing: Lancaster, PA, USA, 1994; pp. 139–187. [Google Scholar]
- Bai, J.; Alleyne, V.; Hgenmaier, R.D.; Mattheis, J.P.; Baldwin, E.A. Formulation of zein coatings for apples (Malus domestica Borkh). Postharvest Biol. Technol. 2003, 28, 259–268. [Google Scholar] [CrossRef]
- Chen, X.; Sun, D.; Xu, S. Preservation of kiwifruit coated with an edible film at ambient temperature. J. Food Eng. 2001, 50, 211–216. [Google Scholar]
- Albors, A.; Chiralt, A.; González-Martínez, C.; Vargas, M. Quality of cold-stored strawberries as affected by chitosan-oleic acid edible coatings. Postharvest Biol. Technol. 2006, 41, 164–171. [Google Scholar]
- Perez-Gago, M.B.; Serra, M.; Alonso, M.; Mateos, M.; del Rio, M.A. Effect of solid content and lipid content of whey protein isolate–beeswax edible coatings on color change of fresh-cut apples. J. Food Sci. 2003, 68, 2186–2191. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Effect of protein concentration on kinetics of water vapour adsorption by coatings prepared on the basis of whey protein isolate. Food. Sci. Technol. Qual. 2011, 6, 66–73. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).