Drug Susceptibility Testing and Synergistic Antibacterial Activity of Curcumin with Antibiotics against Enterotoxigenic Escherichia coli
Abstract
1. Introduction
2. Results
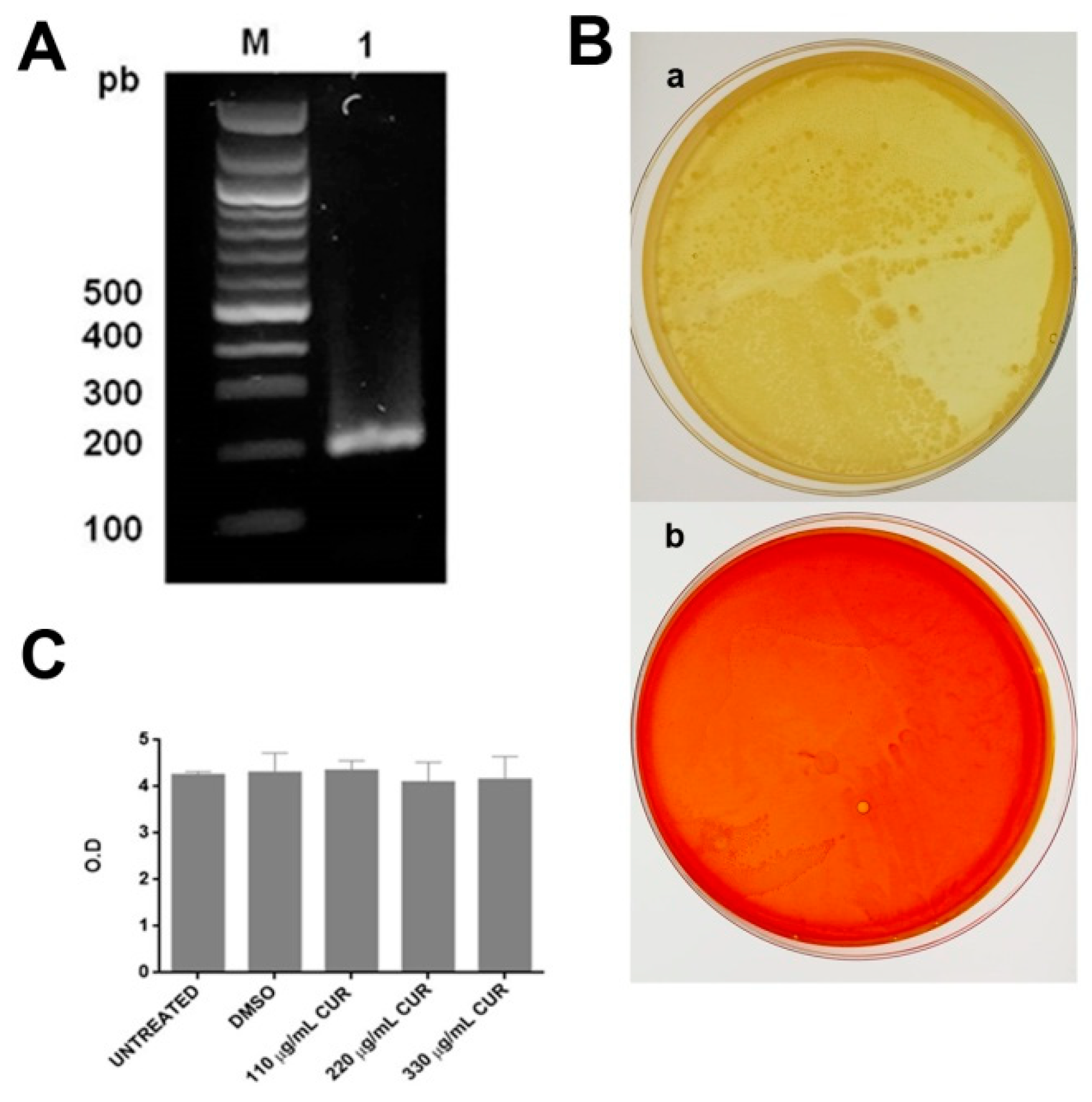
2.1. Curcumin Did not Affect the Growth of Enterotoxigenic E. coli
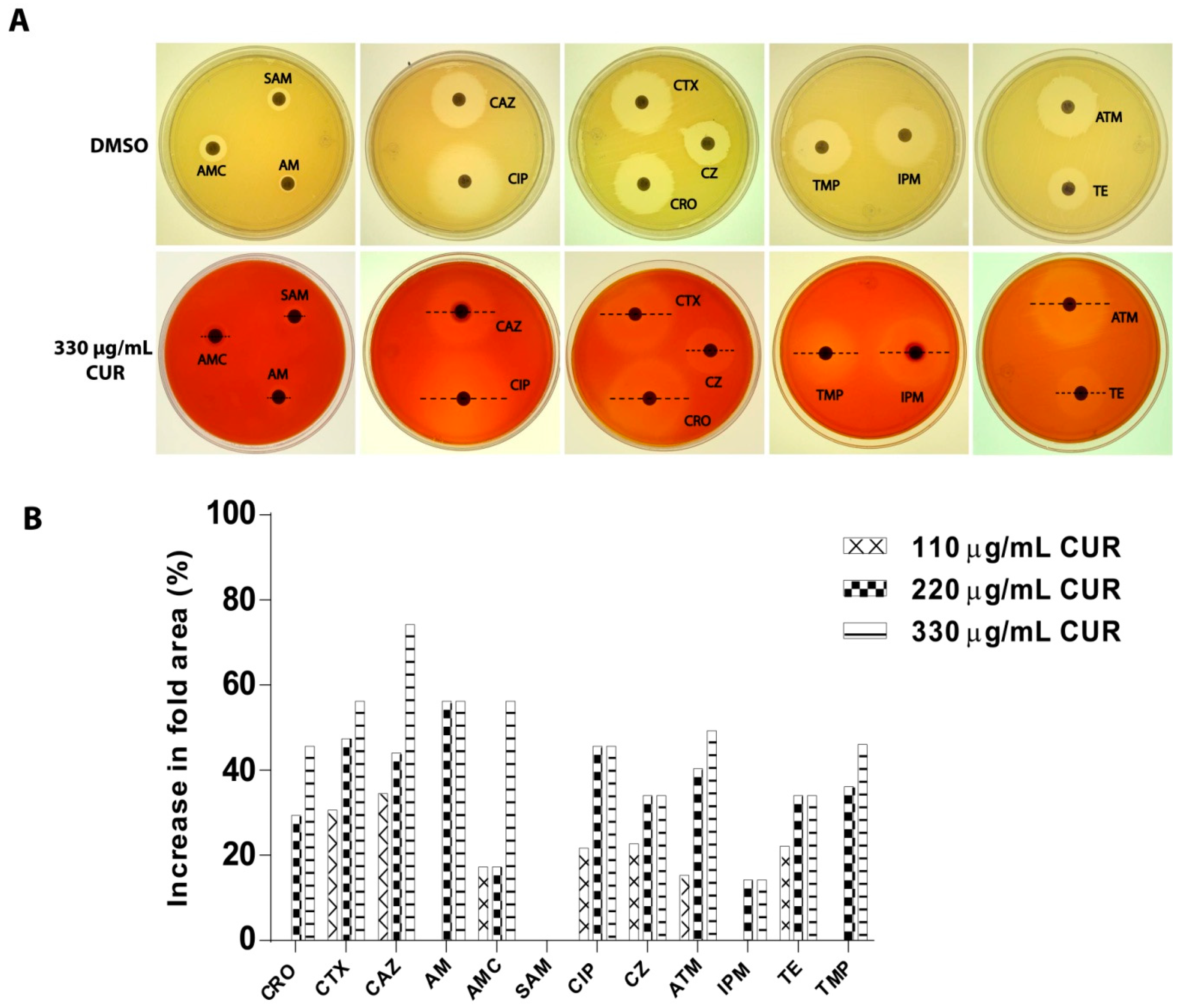
2.2. Susceptibility of Enterotoxigenic E. coli
2.3. Synergistic Effect of Curcumin
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain
4.2. Maintenance and Preservation of Microorganisms
4.3. Identification of the Presence of the Gene of the Heat-labile Toxin in Enterotoxigenic E. coli by PCR
4.3.1. Extraction of Genomic DNA
4.3.2. Expression of LT Gene
4.4. Antibacterial Effect of Curcumin
4.4.1. Preparation of Curcumin Stocks
4.4.2. Killing Assays
4.5. Determination of Bacterial Susceptibility to Antibiotics
4.6. Synergistic Effect of Curcumin on a Strain of Enterotoxigenic E. coli
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lanata, C.F.; Fischer-Walker, C.L.; Olascoaga, A.C.; Torres, C.X.; Aryee, M.J.; Black, R.E.; Child Health Epidemiology Reference Group of the World Health Organization; UNICEF. Global causes of diarrheal disease mortality in children <5 years of age: A systematic review. PLoS ONE 2013, 8, e72788. [Google Scholar] [CrossRef] [PubMed]
- Canizalez-Roman, A.; Flores-Villaseñor, H.M.; Gonzalez-Nuñez, E.; Velazquez-Roman, J.; Vidal, J.E.; Muro-Amador, S.; Alapizco-Castro, G.; Díaz-Quiñonez, J.A.; León-Sicairos, N. Surveillance of diarrheagenic Escherichia coli strains isolated from diarrhea cases from children, adults and elderly at northwest of Mexico. Front Microbiol. 2016, 7, 1924. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Estrada-Garcia, T. Epidemiology of diarrheagenic Escherichia coli pathotypes in Mexico, past and present. In Pathogenic Escherichia coli in Latin America; Tores, G.A., Ed.; Bentham Science Publishers: Rio de Janeiro, Brazil, 2010; pp. 191–208. [Google Scholar]
- Qadri, F.; Svennerholm, A.M.; Faruque, A.S.; Sack, R.B. Enterotoxigenic Escherichia coli in developing countries: Epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 2005, 18, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Okoh, A.I.; Osode, A.N. Enterotoxigenic Escherichia coli (ETEC): A recurring decimal in infants’ and travelers’ diarrhea. Rev. Environ. Health 2008, 23, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, S.W.; Warren, C.A.; Guerrant, R. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology 2009, 136, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L. Travelers’ diarrhea: Antimicrobial therapy and chemoprevention. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 191–198. [Google Scholar] [CrossRef]
- Tagajdid, M.R.; Boumhil, L.; Iken, M.; Adnaoui, M. Resistance to fluoroquinolones and third generation cephalosporin of Escherichia coli isolated from urines. Med. Mal. Infect. 2010, 40, 70–73. [Google Scholar] [CrossRef]
- Estrada-Garcia, T.; Cerna, J.F.; Paheco-Gil, L.; Velázquez, R.F.; Ochoa, T.J.; Torres, J.; DuPont, H.L. Drug-resistant diarrheogenic Escherichia coli, Mexico. Emerg. Infect. Dis. 2005, 11, 1306–1308. [Google Scholar] [CrossRef]
- Camins, B.C.; Marschall, J.; DeVader, S.R.; Maker, D.E.; Hoffman, M.W.; Fraser, V.J. The clinical impact of fluoroquinolone resistance in patients with E coli bacteremia. J. Hosp. Med. 2011, 6, 344–349. [Google Scholar] [CrossRef]
- Liang, W.J.; Liu, H.Y.; Duan, G.C.; Zhao, Y.X.; Chen, S.Y.; Yang, H.Y.; Xi, Y.L. Emergence and mechanism of carbapenem-resistant Escherichia coli in Henan, China, 2014. J. Infect. Public Health 2018, 11, 347–351. [Google Scholar] [CrossRef]
- World Health Organization. Antibiotic-Resistant “Priority Pathogens”; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- de Paula Aguiar, D.; Brunetto Moreira Moscardini, M.; Rezende Morais, E.; Graciano de Paula, R.; Ferreira, P.M.; Afonso, A.; Belo, S.; Tomie Ouchida, A.; Curti, C.; Cunha, W.R.; et al. Curcumin generates oxidative stress and induces apoptosis in adult Schistosoma mansoni worms. PLoS ONE 2016, 11, e0167135. [Google Scholar] [CrossRef]
- Gutierrez-Gutierrez, F.; Palomo-Ligas, L.; Hernández-Hernández, J.M.; Pérez-Rangel, A.; Aguayo-Ortiz, R.; Hernández-Campos, A.; Castillo, R.; González-Pozos, S.; Cortés-Zárate, R.; et al. Curcumin alters the cytoskeleton and microtubule organization on trophozoites of Giardia lamblia. Acta Trop. 2017, 172, 113–121. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar]
- Zhang, Y.; Zeng, Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4 /MyD88/NF-kappaB signal pathway. Drug Dev. Res. 2019. [Google Scholar] [CrossRef]
- Teow, S.Y.; Liew, K.; Ali, S.A.; Khoo, A.S.; Peh, S.C. Antibacterial action of curcumin against Staphylococcus aureus: A brief review. J. Trop. Med. 2016, 2016, 2853045. [Google Scholar] [CrossRef]
- Hu, P.; Huang, P.; Chen, M.W. Curcumin reduces Streptococcus mutans biofilm formation by inhibiting sortase A activity. Arch. Oral. Biol. 2013, 58, 1343–1348. [Google Scholar] [CrossRef]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef]
- García-Ariza, L.; Sierra-Acevedo, O.-M.J.; Padilla-Sanabria, L. Biological activity of three curcuminoids from Curcuma longa L. (turmeric) grown un Quíndio, Colombia. Rev. Cubana Plant Med. 2017, 22, 1–14. [Google Scholar]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef]
- Sasidharan, N.K.; Sreekala, S.R.; Jacob, J.; Nambisan, B. In vitro synergistic effect of curcumin in combination with third generation cephalosporins against bacteria associated with infectious diarrhea. Biomed. Res. Int. 2014, 2014, 561456. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.; Kali, A.; Bhuvaneshwar, D.; Charles, P.M.V.; Srinivasaiah Seetha, K. Antibacterial synergy of curcumin with antibiotics against biofilm producing clinical bacterial isolates. J. Basic Clin. Pharm. 2016, 7, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Ajjampur, S.S.; Chidambaram, D.; Chandrabose, G.; Thangaraj, B.; Sarkar, R.; Samuel, P.; Rajan, D.P.; Kang, G. Pathotypes of diarrheagenic Escherichia coli in children attending a tertiary care hospital in South India. Diagn. Microbiol. Infect. Dis. 2010, 68, 117–122. [Google Scholar] [CrossRef]
- Mave, V.; Chandanwale, A.; Kagal, A.; Khadse, S.; Kadam, D.; Bharadwaj, R.; Dohe, V.; Robinson, M.L.; Kinikar, A.; Joshi, S.; et al. High burden of antimicrobial resistance and mortality among adults and children with community-onset bacterial infections in India. J. Infect. Dis. 2017, 215, 1312–1320. [Google Scholar] [CrossRef]
- GBD Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- GBD Diarrhoeal Disease Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef]
- Hong, K.S.; Kim, J.S. Rifaximin for the treatment of acute infectious diarrhea. Therap. Adv. Gastroenterol. 2011, 4, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Deokule, J.S.; Garg, P.; Bhattacharya, S.K.; Nandy, R.K.; Balakrish Nair, G.; Yamasaki, S.; Takeda, Y.; Ramamurthy, T. Concomitant infection of enterotoxigenic Escherichia coli in an outbreak of cholera caused by Vibrio cholerae O1 and O139 in Ahmedabad, India. J. Clin. Microbiol. 2001, 39, 3241–3246. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S.; Deilami, M.K.; Zandi, K.; Fouladvand, M.; Ramedani, E.; Asayeshm, G. Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr. J. Biotechnol. 2008, 7, 3832–3835. [Google Scholar]
- Ejim, L.; Farha, M.A.; Falconer, S.B.; Wildenhain, J.; Coombes, B.K.; Tyers, M.; Brown, E.D.; Wright, G.D. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat. Chem. Biol. 2011, 7, 348–350. [Google Scholar] [CrossRef]
- Roudashti, S.; Zeighami, H.; Mirshahabi, H.; Bahari, S.; Soltani, A.; Haghi, F. Synergistic activity of sub-inhibitory concentrations of curcumin with ceftazidime and ciprofloxacin against Pseudomonas aeruginosa quorum sensing related genes and virulence traits. World J. Microbiol. Biotechnol. 2017, 33, 50. [Google Scholar] [CrossRef] [PubMed]
- Teow, S.Y.; Ali, S.A. Synergistic antibacterial activity of Curcumin with antibiotics against Staphylococcus aureus. Pak. J. Pharm. Sci. 2015, 28, 2109–2114. [Google Scholar] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 5th ed.; National Committee for Clinical Laboratory Standards: Villanova, PA, USA, 2000; Volume 20. [Google Scholar]
| Antibiotic | Concentration (μg) | Inhibition Halo (mm) | ||
|---|---|---|---|---|
| Sensitive (S) | Intermediate (I) | Resistant (R) | ||
| Ceftriaxone (CRO) | 30 | ≥23 | 20–22 | ≤ 19 |
| Cefotaxime (CTX) | 30 | ≥26 | 23–55 | ≤ 22 |
| Ceftazidime (CAZ) | 30 | ≥21 | 18–20 | ≤ 17 |
| Ampicillin (AM) | 10 | ≥17 | 14–16 | ≤ 13 |
| Amoxicillin/Clavulanic acid (AMC) | 20/10 | ≥18 | 14–17 | ≤ 13 |
| Ampicillin/Sulbactam (SAM) | 10/10 | ≥15 | 12–14 | ≤ 11 |
| Ciprofloxacin (CIP) | 5 | ≥31 | 21–30 | ≤ 20 |
| Cefazolin (CZ) | 30 | ≥23 | 20- 22 | ≤ 19 |
| Aztreonam (ATM) | 30 | ≥21 | 18–20 | ≤ 17 |
| Imipinem (IPM) | 10 | ≥23 | 20–22 | ≤ 19 |
| Tetracycline (TE) | 30 | ≥15 | 12–14 | ≤ 11 |
| Trimethoprim (TMP) | 5 | ≥16 | 11–15 | ≤ 10 |
| Antibiotics | Only Antibiotic |
|---|---|
| Ceftriaxone | 29 ± 1.0 (S) |
| Cefotaxime | 28 ± 1.2(S) |
| Ceftazidime | 25 ± 1.2 (S) |
| Ampicillin | 8 ± 1.0 (R) |
| Amoxicillin/ Clavulanic acid | 12 ± 1.0 (R) |
| Ampicillin/Sulbactam | 11 ± 1.0 (R) |
| Ciprofloxacin | 29 ± 1.0 (I) |
| Cefazolin | 19 ± 1.2 (R) |
| Aztreonam | 27 ± 1.0 (S) |
| Imipinem | 29 ± 1.2 (S) |
| Tetracycline | 19 ± 1.0 (S) |
| Trimethomprim | 24 ± 0.6 (S) |
| Antibiotics | Antibiotic + DMSO A | Antibiotic + 110 µg/mL CUR B | Antibiotic + 220 µg/mL CUR B | Antibiotic + 330 µg/mL CUR B |
|---|---|---|---|---|
| Ceftriaxone | 29 ± 1.0 (S) | 29 ± 1.0 (S) | 33 ± 1.2 (S) | 35 ± 1.0 (S) |
| Cefotaxime | 28 ± 1.0 (S) | 32 ± 0.6 (S) | 34 ± 1.0 (S) | 35 ± 1.0 (S) |
| Ceftazidime | 25 ± 1.2 (S) | 29 ± 1.0 (S) | 30 ± 1.0 (S) | 33 ± 0.6 (S) |
| Ampicillin | 8 ± 1.0 (R) | 8 ± 0.0 (R) | 10 ± 0.6(R) | 10 ± 0.0 (R) |
| Amoxicillin/Clavulanic acid | 12 ± 1.2 (R) | 13 ± 1.0 (R) | 13 ± 1.0 (R) | 15 ± 1.0 (I) |
| Ampicillin/Sulbactam | 11 ± 0.0 (R) | 11 ± 0.6 (R) | 11 ± 1.0 (R) | 11 ± 1.0 (R) |
| Ciprofloxacin | 29 ± 1.0 (I) | 32 ± 1.0 (S) | 35 ± 1.0 (S) | 35 ± 1.2 (S) |
| Cefazolin | 19 ± 1.2 (R) | 21 ± 1.0 (I) | 22 ± 1.0 (I) | 22 ± 0.6 (I) |
| Aztreonam | 27 ± 0.0 (S) | 29 ± 0.6 (S) | 32 ± 0.6 (S) | 33 ± 1.0 (S) |
| Imipinem | 29 ± 0.6 (S) | 29 ± 1.2 (S) | 31 ± 0.6 (S) | 31 ± 0.0 (S) |
| Tetracycline | 19 ± 1.0 (S) | 21 ± 1.0 (S) | 22 ± 0.6 (S) | 22 ± 0.6 (S) |
| Trimethomprim | 24 ± 0.6 (S) | 24 ± 1.0 (S) | 28 ± 1.0 (S) | 29 ± 1.0 (S) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itzia Azucena, R.-C.; José Roberto, C.-L.; Martin, Z.-R.; Rafael, C.-Z.; Leonardo, H.-H.; Gabriela, T.-P.; Araceli, C.-R. Drug Susceptibility Testing and Synergistic Antibacterial Activity of Curcumin with Antibiotics against Enterotoxigenic Escherichia coli. Antibiotics 2019, 8, 43. https://doi.org/10.3390/antibiotics8020043
Itzia Azucena R-C, José Roberto C-L, Martin Z-R, Rafael C-Z, Leonardo H-H, Gabriela T-P, Araceli C-R. Drug Susceptibility Testing and Synergistic Antibacterial Activity of Curcumin with Antibiotics against Enterotoxigenic Escherichia coli. Antibiotics. 2019; 8(2):43. https://doi.org/10.3390/antibiotics8020043
Chicago/Turabian StyleItzia Azucena, Rangel-Castañeda, Cruz-Lozano José Roberto, Zermeño-Ruiz Martin, Cortes-Zarate Rafael, Hernández-Hernández Leonardo, Tapia-Pastrana Gabriela, and Castillo-Romero Araceli. 2019. "Drug Susceptibility Testing and Synergistic Antibacterial Activity of Curcumin with Antibiotics against Enterotoxigenic Escherichia coli" Antibiotics 8, no. 2: 43. https://doi.org/10.3390/antibiotics8020043
APA StyleItzia Azucena, R.-C., José Roberto, C.-L., Martin, Z.-R., Rafael, C.-Z., Leonardo, H.-H., Gabriela, T.-P., & Araceli, C.-R. (2019). Drug Susceptibility Testing and Synergistic Antibacterial Activity of Curcumin with Antibiotics against Enterotoxigenic Escherichia coli. Antibiotics, 8(2), 43. https://doi.org/10.3390/antibiotics8020043




