Abstract
Burkholderia is a metabolically versatile genus of Gram-negative bacteria that inhabits niches ranging from soil and water to plants and clinical environments. This review provides an integrated examination of Burkholderia species, focusing on their dual roles as both pathogens and beneficial microorganisms. Key pathogenic species, such as members of the Burkholderia cepacia complex and the Burkholderia pseudomallei group, pose significant threats to human, animal, and plant health due to their intrinsic antibiotic resistance and diverse virulence factors. Conversely, several environmental and plant-associated Burkholderia species promote plant growth, enhance nutrient uptake, and serve as biocontrol agents, supporting sustainable agriculture. We synthesize current knowledge across taxonomy, genomics, pathogenicity, beneficial interactions, and secondary metabolite biosynthesis—including the prolific production of antibiotics, toxins, and volatile organic compounds with pharmaceutical and agricultural potential. Advances in high-throughput genomics are revealing substantial genetic diversity, genome plasticity, and mechanisms underlying both pathogenicity and beneficial traits. Clarifying this dual nature and identifying strategies to mitigate risks will guide the safe and effective exploitation of Burkholderia in medicine, agriculture, and biotechnology.
1. Introduction
The genus Burkholderia comprises a diverse group of Gram-negative bacteria, characterized by their metabolic versatility and ability to colonize a wide range of ecological niches, including soil, water, and host-associated environments [1,2]. Notably, the genus includes both pathogenic species, which pose significant health risks, and beneficial species, which contribute to sustainable agriculture and biotechnology [3,4]. This duality has made Burkholderia a subject of considerable interest in medical, agricultural, and biotechnological research.
Several Burkholderia species are recognized as significant human, animal, and plant pathogens. Notably, members of the Burkholderia cepacia complex (Bcc) are major opportunistic pathogens in individuals with cystic fibrosis (CF) and other immunocompromised conditions, often leading to chronic lung infections [5,6]. Burkholderia pseudomallei and Burkholderia mallei, the causative agents of melioidosis and glanders, respectively, pose serious public health and veterinary concerns due to their high virulence and intrinsic antibiotic resistance [7,8,9]. Furthermore, certain species, such as Burkholderia gladioli, cause diseases in crops and contribute to significant agricultural losses [10]. Comparative genomic and functional studies have shown that pathogenic Burkholderia sensu stricto lineages typically harbor suites of virulence determinants—such as specialized secretion systems, capsular polysaccharides, and intracellular survival factors—that enable host invasion, immune evasion, and tissue damage [11,12,13].
In contrast, some other Burkholderia species contribute positively to ecosystem functioning and sustainable agriculture. Many environmental and plant-associated members of Burkholderia sensu lato, including strains now reclassified as Paraburkholderia and Caballeronia, generally do not harbor the virulence gene repertoires characteristic of clinical pathogens and instead encode traits linked to rhizosphere competence, nutrient acquisition, and symbiosis, such as nitrogen fixation and modulation of plant immunity [14,15,16,17]. Burkholderia phytofirmans (reclassified as Paraburkholderia phytofirmans) promotes plant growth by aiding nutrient uptake and inducing systemic resistance against pathogens [18]. Additionally, certain species can fix nitrogen, thereby improving soil fertility and supporting sustainable farming practices [19]. At the cellular level, pathogenic Burkholderia often invade and persist within host cells and/or cause extensive tissue damage, as shown for B. pseudomallei and members of Bcc in experimental and clinical studies [3,12,13,20]. In contrast, beneficial endophytes such as P. phytofirmans PsJN colonize root and shoot tissues without visible disease symptoms and can prime induced systemic resistance in their plant hosts [18,21].
The genus also produces a wide range of bioactive secondary metabolites, including siderophores, antibiotics, toxins, and volatile organic compounds (VOCs), which are important for drug discovery, crop protection, and ecological interactions. Some species have been studied as biocontrol agents because of their ability to suppress plant pathogens through antagonistic interactions or the production of secondary metabolites [14,15]. The genomic complexity and metabolic diversity of Burkholderia underpin both their adaptability and their capacity for innovation in metabolite production, environmental resilience, and symbiosis. However, the presence of multidrug resistance, the capacity for horizontal gene transfer, and the potential for pathogenicity highlight the need for careful assessment and management in both clinical and environmental contexts. Recent advances in comparative genomics, genome editing, and regulatory-network engineering are beginning to reveal and harness the genus’s hidden metabolic capacity.
Given the remarkable versatility of Burkholderia, as both a threat and a resource, a comprehensive understanding of its taxonomy, genomic features, pathogenic mechanisms, beneficial traits, and application potential is essential. Our literature survey primarily focused on publications from approximately 2000 to early 2025, while also incorporating earlier foundational studies that are essential for context. This review provides an integrated analysis of these aspects. By bridging knowledge from medical, agricultural, and biotechnological perspectives, we aim to clarify the risks and opportunities presented by Burkholderia and to guide future research toward safe and effective utilization of this complex genus.
2. Ecological Diversity of Burkholderia Species
Burkholderia is a genus in the Burkholderiaceae family within the order Burkholderiales, class Betaproteobacteria, and phylum Proteobacteria [16]. This genus was proposed by Yabuuchi et al. in 1992 after separation from Pseudomonas based on molecular and phenotypic characteristics [22]. Since then, Burkholderia has undergone taxonomic revisions, resulting in the reclassification of certain species into new genera such as Paraburkholderia, Caballeronia, Robbsia, Mycetohabitans, and Trinickia based on phylogenetic and functional distinctions [17,23,24].
Currently, the Burkholderia sensu lato comprises over 100 recognized species, broadly divided into two major groups based on their ecological roles and pathogenicity: (1) Pathogenic Burkholderia species, such as Bcc, B. pseudomallei, and B. mallei [11,20] and (2) environmental or plant-beneficial species such as P. phytofirmans and Burkholderia tropica (reclassified as Paraburkholderia tropica) [18]. The Burkholderia species included in this review are summarized in Figure 1.
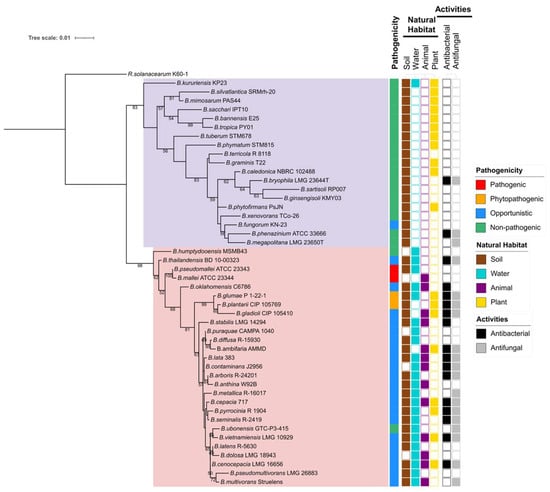
Figure 1.
Maximum-likelihood phylogenetic tree based on 16S rRNA gene sequences of Burkholderia species, with Ralstonia solanacearum included as an outgroup. Sequence alignment and phylogenetic reconstruction were performed in MEGA 12 [25] using the Tamura–Nei evolutionary model. The resulting tree was visualized and annotated using iTOL (https://itol.embl.de/ (accessed on 13 October 2025)). Displayed features, including pathogenicity, natural habitat, and biological activities, are species-specific rather than strain-specific. White boxes in the activity column indicate that information is unavailable. Bootstrap support values (>50%) are shown at the corresponding branch nodes. Background shading: lavender/purple represents Paraburkholderia; pink/rose represents Burkholderia.
3. Genomic Features of Burkholderia Species
Genomes of Burkholderia species are highly complex and diverse, which explains their metabolic versatility and ability to adapt to various environments. Typically, these bacteria possess multiple chromosomes (commonly two or three) with genome sizes varying from 6 to 9 megabase pairs [26,27]. This multipartite genome structure contributes to their genetic plasticity and adaptability [28]. The GC content of Burkholderia genomes is relatively high (≈65–68%), and elevated GC in prokaryotes has been associated with DNA double-strand break repair-linked processes [29,30,31].
Burkholderia species are known for extensive horizontal gene transfer, which contributes to their antibiotic resistance, virulence, and metabolic diversity [32,33]. Pathogenic species harbor multiple virulence factors, including type III secretion system (T3SS) and type VI secretion system (T6SS), extracellular polysaccharide biosynthesis, quorum sensing (QS), and biofilm formation genes, all contributing to host infection, immune evasion, persistence, and antibiotic resistance [12,13,34,35]. Furthermore, many Burkholderia species have gene clusters encoding non-ribosomal peptide synthetases (NRPS) and polyketide synthases (PKS), which are responsible for producing bioactive compounds with antimicrobial properties, making them valuable for drug discovery [36]. Burkholderia genomes contain numerous genes associated with stress response, efflux pumps, and degradation of complex organic compounds, enabling them to survive in diverse environments, including contaminated soils and host tissues [37,38,39].
Comparative genomic studies have highlighted significant genetic diversity within Burkholderia sensu lato, with distinct evolutionary trajectories for pathogenic and environmental lineages. For example, the genomes of host-adapted pathogens such as B. pseudomallei and B. mallei show signs of genome reduction, pseudogene accumulation, and loss of certain environmental functions consistent with long-term adaptation to animal hosts [26,40], whereas environmental species like Burkholderia xenovorans (reclassified as Paraburkholderia xenovorans) retain large, multireplicon genomes with extensive catabolic pathways for degrading xenobiotic compounds and supporting broad metabolic versatility [37]. These contrasting patterns illustrate divergent genome-size and content trajectories for pathogenic versus environmental members of Burkholderia sensu lato.
Whole-genome sequencing and phylogenomic analyses have also clarified the evolutionary split between pathogenic Burkholderia and environmental Paraburkholderia lineages. In these analyses, clinically important and phytopathogenic Burkholderia sensu stricto (including the Bcc and the B. pseudomallei/B. mallei group) form a clade distinct from predominantly non-pathogenic, plant-associated species that have been reclassified into Paraburkholderia and related genera [17,23]. This phylogenetic division parallels the genomic trends described above: host-restricted pathogens generally possess more compact, reduced genomes, whereas Paraburkholderia species typically maintain larger genomes enriched in genes for plant association, nitrogen fixation, and other symbiotic or rhizosphere functions [17,23].
4. Pathogenicity of Burkholderia Species
Several Burkholderia species are significant pathogens, causing severe infections in humans, animals, and plants. The pathogenicity of Burkholderia is linked to its ability to survive in diverse environments, evade host immune responses, and resist antibiotics. At the genetic level, these pathogenic lineages carry characteristic virulence loci, including T3SS and T6SS and high-affinity siderophore biosynthesis with transport genes. Examples include T3SS-1 and T6SS-1 in B. pseudomallei and the ornibactin biosynthesis and transport genes in Bcc species; collectively, these systems promote intracellular survival, immune evasion, and systemic infection [12,13,41,42]. The primary pathogenic species can be grouped as follows:
4.1. Human and Animal Pathogens
The Bcc consists of at least 20 closely related species, including Burkholderia cepacia, Burkholderia cenocepacia, and Burkholderia multivorans, which are opportunistic pathogens primarily affecting individuals with CF and other immunocompromised conditions [11,43,44]. Some infections progress to cepacia syndrome, an acute, rapidly progressive necrotizing pneumonia characterized by high fever and bacteremia and associated with high mortality [45,46,47].
B. pseudomallei, commonly found in soil and water, is the causative agent of melioidosis, a severe infectious disease endemic to Southeast Asia and northern Australia [20]. Melioidosis presents with a range of symptoms, including pneumonia, abscess formation, sepsis, and neurological complications, and can be fatal; mortality is ~10% in settings with rapid diagnosis, optimal therapy, and intensive care, but is ≥40% in many endemic regions where such resources are limited [20,48].
B. mallei is a host-adapted pathogen responsible for glanders, primarily affecting horses, donkeys, and mules, though humans can also be infected. In equines, glanders is characterized by ulcerating nodular lesions of the skin and mucous membranes with generalized signs such as fever and cough [49]. In humans, glanders presents as pulmonary, cutaneous, or septicemic infections, with a high fatality rate if untreated. B. mallei has been classified as a biodefense concern due to its prior use as a biological weapon and its high case-fatality rate [50].
4.2. Plant Pathogens
B. gladioli is a plant-associated bacterium with a broad host range, including rice, orchidaceous plants, gladiolus, onions, and mushrooms [51,52]. The species comprises multiple pathovars (pv.); pv. gladioli, pv. alliicola, pv. agaricicola, and pv. cocovenenans. Pathovars pv. gladioli, pv. alliicola, and pv. agaricicola are linked to soft-rot diseases of gladiolus, onion bulbs, and mushrooms, respectively, with pv. agaricicola recognized as an important mushroom pathogen due to its potential to cause major crop losses. In contrast, pv. cocovenenans is distinguished by its association with toxic food spoilage [52]. In rice, B. gladioli is associated with bacterial grain rot and leaf-sheath browning. It has also been isolated from healthy rice plants and rice nursery soil, supporting its ability to persist in agricultural environments [51].
Burkholderia glumae is an important phytopathogen of rice that causes bacterial panicle blight and seedling rot, leading to significant economic losses [53,54,55]. Disease symptoms commonly include grain discoloration, spikelet sterility (failure of grain filling), and seedling rot, which severely reduce crop yields [53,55].
4.3. Treatment and Control
Treatment and control of infections caused by Burkholderia species pose significant clinical and agricultural challenges due to their inherent multidrug resistance and diverse pathogenic strategies. For infections caused by the Bcc, therapy is typically individualized and combination-based according to in vitro susceptibility testing. Commonly used agents include ceftazidime, trimethoprim-sulfamethoxazole (TMP-SMX), meropenem or levofloxacin [56], but in vitro resistance of Bcc species to these drugs is frequent, underscoring the need for susceptibility-guided regimens [57,58]. Emerging approaches to address resistance include the use of ceftazidime-avibactam [59] and, in highly resistant cases, a combination of ceftazidime-avibactam, ciprofloxacin, meropenem, minocycline, sulfadiazine, and tobramycin [60]. Bacteriophage therapy is under active investigation as an adjunct or alternative approach [61]. Strict infection control measures remain essential, especially for CF patients and immunocompromised individuals.
Management of melioidosis involves intensive intravenous antibiotic therapy (e.g., ceftazidime, meropenem, or imipenem) for at least 2 weeks, depending on severity, followed by oral eradication therapy (3 to >6 months) with TMP-SMX as a first-line; doxycycline or amoxicillin/clavulanic acid as alternatives [20,48]. Public health measures such as reducing exposure to contaminated soil and water, wearing protective clothing, and raising public awareness are essential in endemic regions.
For glanders, antibiotic therapy typically includes doxycycline, ceftazidime, or TMP-SMX [49,50]. Control relies heavily on stringent biosecurity measures, including early detection, quarantine, and culling of infected animals to prevent transmission [49,50]. Given its potential as a bioterrorism agent, robust surveillance and adherence to biosecurity protocols are vital [50].
Control of B. gladioli–induced plant diseases relies primarily on integrated cultural practices: plant only scab-free corms, avoid replanting in previously affected soils, implement multi-year rotations (≈3 years), improve drainage and sanitation, and manage bulb mites that create infection courts [62,63]. Because copper and streptomycin resistance is common in B. gladioli, bactericides have limited and inconsistent efficacy and should not be relied on as primary control measures [64]. As a biological option, prophylactic bacteriophage treatments—for example, Burkholderia phages KS12 and AH2—have been shown to prevent or reduce B. gladioli–associated tissue destruction in a quantitative ex planta model, offering a promising eco-friendly adjunct to cultural controls [65].
Disruption of the bacterial proton motive force—by genetic manipulation or by chemical means such as sodium bicarbonate—significantly reduces the virulence of B. glumae in rice [66]. Additionally, biological control strategies show promise: seed/seedling treatments using Cytobacillus firmus JBRS159 in combination with silicon (SiO2 nanoparticles or K2SiO3) suppressed bacterial seedling rot of rice plants [67]. A flagella-dependent jumbo phage (S13) requires B. glumae flagella for attachment and infection, lysing flagellated cells and selecting for nonflagellated survivors with reduced virulence—an effect that protects rice seedlings [68]. These advances provide practical solutions towards integrated and sustainable management of B. glumae-associated rice diseases.
5. Environmental and Beneficial Burkholderia Species
In contrast to pathogenic members of Burkholderia sensu stricto, some members of Burkholderia sensu lato (including Paraburkholderia) play beneficial roles in agriculture and environmental sustainability. Notably, P. phytofirmans and P. tropica have been recognized for their plant growth-promoting and biocontrol properties.
P. phytofirmans, particularly the strain PsJN, is a well-studied endophytic bacterium capable of colonizing diverse plant hosts (e.g., grapevine, tomato, and potato). It promotes plant growth through traits including phytohormone-associated pathways (notably indole-3-acetic acid), 1-aminocyclopropane-1-carboxylate deaminase activity, and siderophore production. PsJN can also enhance protection against phytopathogens by inducing plant-mediated systemic resistance and improve tolerance to abiotic stresses such as drought and cold [21].
P. tropica is a plant-associated diazotrophic bacterium isolated from the rhizosphere and endophytic compartments of crops, including maize, sugarcane, and teosinte. This species exhibits nitrogenase activity under microaerobic conditions in nitrogen-free media, supporting its capacity for biological nitrogen fixation [69].
These beneficial functions are supported by characteristic gene modules in plant-associated Paraburkholderia. Diazotrophic taxa such as P. tropica and the legume symbiont Paraburkholderia phymatum harbor conserved nitrogen-fixation genes (e.g., nifHDK and associated nif genes), while P. phymatum also carries nod gene sets (e.g., nodABCD) linked to nodulation [15,17]. Together, these genetic modules underpin rhizosphere/endophytic competence and biological nitrogen fixation that can enhance plant nutrient status and stress resilience [15,17,21].
6. Secondary Metabolites Produced by Burkholderia Species
Burkholderia species are prolific producers of diverse secondary metabolites, which have significant implications for both ecology and medicine. These metabolites contribute to the genus’s ability to thrive in diverse environments, suppress competitors, interact with plant hosts, and, in some cases, cause disease. An overview of secondary metabolites discussed in this review and their producing species is summarized in Table 1.

Table 1.
Overview of secondary metabolites produced by Burkholderia species, summarizing their biosynthetic system, mode of action, prospective applications, corresponding producing strains, and references.
6.1. Siderophores
Siderophores produced by Burkholderia species are critical for iron acquisition, especially under iron-limiting conditions encountered in natural and host-associated environments [70]. These compounds not only support microbial survival and competitiveness but also contribute to pathogenicity and ecological adaptability.
6.1.1. Cepaciachelin
Cepaciachelin is notably produced by B. cepacia and B. ambifaria. By sequestering iron from the environment, cepaciachelin enables producing strains to thrive in niches where iron availability is limited, thereby contributing to their ecological success and pathogenic potential [70,71,128]. In clinical settings, particularly among immunocompromised individuals or those with CF, such mechanisms may contribute to the persistence and virulence of these bacteria [32].
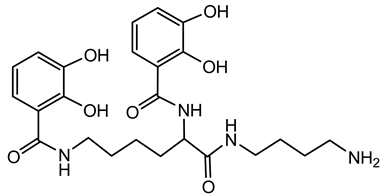
6.1.2. Ornibactin
Ornibactin is produced by various Burkholderia species, notably B. cenocepacia and B. contaminans. These bacteria utilize ornibactin to acquire iron from the iron-limited environment, such as that within a host organism [41,72,73]. Mutants deficient in ornibactin production exhibit impaired growth under iron-limited conditions and reduced virulence in animal models. For instance, B. cepacia mutants lacking ornibactin biosynthesis were less effective in establishing infections in murine respiratory models [73].
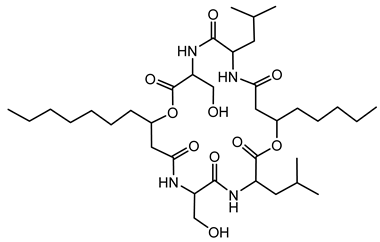
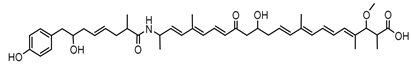
Ornibactin is a linear hydroxamate-hydroxycarboxylate siderophore, composed of a tetrapeptide backbone: L-Orn1(Nδ-OH, Nδ-acyl)-D-threo-Asp(β-OH)-L-Ser-L-Orn4(Nδ-OH, Nδ-formyl)-1,4-diaminobutane. The terminal ornithine residues are modified with hydroxyl and acyl groups, with the acyl chains varying in length (e.g., 3-hydroxybutanoic acid, 3-hydroxyhexanoic acid, and 3-hydroxyoctanoic acid), leading to different ornibactin variants such as ornibactin-C4, ornibactin-C6, and ornibactin-C8 [73].
The biosynthesis of ornibactin is mediated by NRPSs, specifically OrbI and OrbJ. These enzymes sequentially assemble the ornibactin molecule by incorporating and modifying amino acid substrates. The process is regulated by the extracytoplasmic function sigma factor OrbS, which controls the expression of ornibactin biosynthesis and transport genes in response to iron availability [42].
Recent studies have explored the potential of ornibactin in diagnostic imaging. A radiolabeled form of ornibactin complexed with gallium-68, [68Ga]Ga-ornibactin, has been developed for positron emission tomography imaging to detect Burkholderia infections. This approach demonstrated specific accumulation of the radiotracer at infection sites in animal models, suggesting its utility in diagnosing and monitoring infections caused by the Bcc [129].
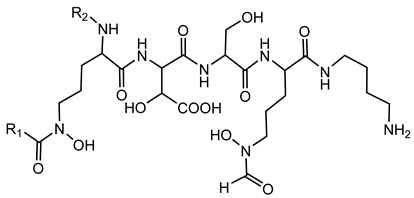
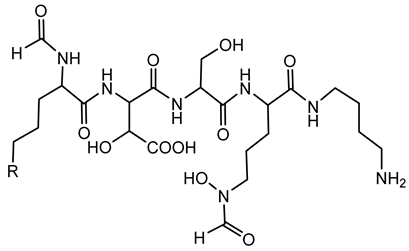
6.1.3. Malleobactin
Malleobactin is notably produced by B. pseudomallei, B. mallei, and B. thailandensis [28,75]. The biosynthesis of malleobactin is directed by the mba gene cluster, which encodes NRPSs responsible for assembling the siderophore. Notably, the NRPS involved in malleobactin production exhibits a rare flexibility, yielding diverse peptide backbones and resulting in various malleobactin congeners [75]. Malleobactin is characterized by its unusual peptide backbone that includes the rare 2-amino-5-nitropentanoic acid, which was reported for the first time as a natural product building block in this siderophore [76].
In pathogenic Burkholderia species, malleobactin plays a crucial role in iron acquisition, facilitating survival and proliferation within iron-restricted environments like the human host. However, studies have shown that while malleobactin contributes to iron uptake, it is not solely responsible for virulence. For instance, B. pseudomallei strains deficient in malleobactin production remain virulent in murine models, suggesting the presence of alternative iron acquisition systems that compensate for the loss of malleobactin [130].
The expression of malleobactin biosynthesis genes is tightly regulated in response to iron availability. Under iron-limiting conditions, the extracytoplasmic function sigma factor MbaS upregulates the transcription of genes involved in malleobactin production and transport. This regulatory mechanism ensures that siderophore production is induced when iron is scarce, optimizing bacterial survival strategies [74].
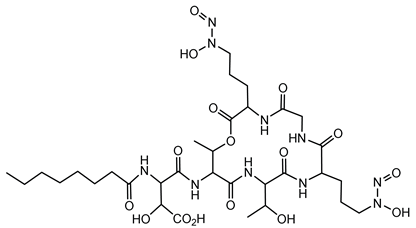
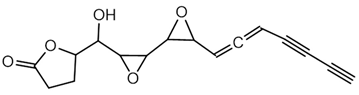
6.1.4. Bolagladins
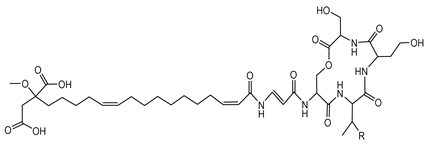
Bolagladins are a class of lipodepsipeptides produced by B. gladioli isolates BCC0238 and BCC1622 from the lungs of CF patients. These metabolites exhibit distinctive structural features, including a citrate-derived fatty acid and a rare dehydro-β-alanine residue [77]. The biosynthesis of bolagladins is orchestrated by a cryptic NRPS gene cluster. Through a combination of bioinformatics analyses and genetic experiments, researchers elucidated the mechanisms responsible for incorporating the distinctive citrate-derived fatty acid and the dehydro-β-alanine residue into the bolagladin structure [77].
6.1.5. Gramibactin
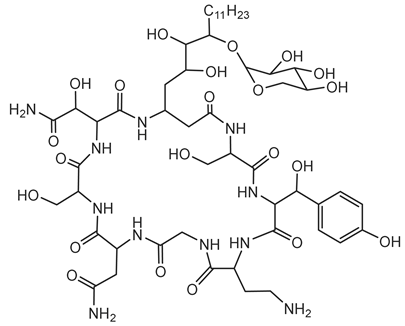
Gramibactin is a novel class of diazeniumdiolate (N-nitroso-N-hydroxylamine) siderophore produced by certain Burkholderia species, notably P. graminis, a bacterium associated with cereal rhizospheres [78].
Structurally, gramibactin is a lipodepsipeptide composed of six amino acids, including two D-graminine (Gra) residues, along with glycine, D-allo-threonine, L-threonine, and D-threo-β-hydroxyaspartic acid. The presence of the rare diazeniumdiolate moiety in graminine is pivotal for its iron-binding capability. Biosynthetically, graminine originates from L-arginine, with the N-N bond in the diazeniumdiolate formed between the Nδ and Nω atoms of the guanidinium group in L-arginine [131]. The diazeniumdiolate groups in gramibactin exhibit a strong affinity for ferric ions, facilitating efficient iron sequestration. This high-affinity binding is crucial for the survival of P. graminis in iron-limited environments, such as the rhizosphere [79].
Beyond iron acquisition, gramibactin’s diazeniumdiolate moiety can release nitric oxide (NO), a signaling molecule involved in various plant physiological processes. This NO release may enhance plant growth and stress tolerance, suggesting a symbiotic relationship between P. graminis and its host plants [80]. The dual functionality of gramibactin, as an iron chelator and NO donor, opens avenues for its application in agriculture, particularly as a biofertilizer to improve plant health and yield. Additionally, understanding its biosynthesis could lead to the development of novel compounds with therapeutic or industrial relevance.
6.2. Antibiotics
Burkholderia species produce a wide array of antimicrobial substances as a competitive strategy to survive and thrive in complex microbial communities. At subinhibitory concentrations, some antibiotics may act as signaling molecules affecting the inter- and intraspecies communication. Many antibiotics produced by Burkholderia species exhibit antagonistic activity against drug-resistant nosocomial bacterial pathogens through novel modes of action, highlighting their potential use in the medical sector. However, additional preclinical and clinical studies are needed to assess their efficacy and safety.
6.2.1. Gladiolin
Gladiolin is a macrolide antibiotic initially discovered from B. gladioli BCC0238 through genome mining and metabolomic approaches. Its structure features a large cyclic lactone ring with a complex pattern of unsaturated and oxygenated functionalities [81].
Gladiolin biosynthesis occurs via modular type-I PKS pathways. This biosynthetic gene cluster (BGC) encodes a trans-AT PKS system; an atypical feature compared with the more common cis-AT systems found in other bacterial macrolide producers [81,132]. Gladiolin exhibits potent activity against Gram-positive bacteria, notably drug-resistant Mycobacterium tuberculosis. Gladiolin acts by selectively targeting bacterial RNA polymerase, disrupting transcription, thereby inhibiting bacterial growth [81]. This mode of action differs from many conventional antibiotics, offering promise for overcoming resistance mechanisms prevalent among multidrug-resistant pathogens.
6.2.2. Enacyloxin
Enacyloxins are polyketide antibiotics, exhibiting significant antimicrobial activity. They are produced by certain Burkholderia species, notably B. ambifaria, through an unusual hybrid modular PKS pathway, highlighting the bacterium’s capacity to produce diverse bioactive compounds [83]. Among its congeners, enacyloxin IIa is notable for its potent activity against multidrug-resistant pathogens and represents a promising candidate for new antimicrobial development [82,83].
6.2.3. Gladiostatin
Gladiostatin, also known as gladiofungin, was initially discovered in B. gladioli strains BCC0238 and BCC1622. It is a glutarimide-containing polyketide with a terminal 2-acyl-4-hydroxy-3-methylbutenolide moiety, a structural feature that differentiates gladiostatin from the other glutarimide antibiotics previously described from Streptomyces species [85,133]. The biosynthesis of gladiostatin is orchestrated by a trans-AT PKS pathway. Notably, an AfsA-like domain at the C-terminus of the PKS plays a crucial role by catalyzing the condensation of 3-ketothioesters with dihydroxyacetone phosphate, providing a noncanonical polyketide chain-release mechanism that installs the butanolide side chain [85,86]. This compound displays both antifungal and antiproliferative activity against human cancer cell lines [85,86].
6.2.4. Icosalide
Icosalide is a distinctive lipopeptidiolide antibiotic produced by certain Burkholderia species. Originally, icosalide A1 was isolated from the fungus Aureobasidium sp. MSX 59166. However, further investigations uncovered that the true producers were B. gladioli strains associated with the fungal cultures. This finding highlighted the intricate relationships between fungi and their bacterial symbionts [87,88]. Icosalide A1 is characterized by a 20-membered lipopeptidiolide ring, comprising two serine and two leucine residues, along with 3-hydroxy fatty acid chains of varying lengths. The presence of a D-leucine residue in icosalide A1 distinguishes it from its analogs, icosalide A2 and icosalide B, which contain only L-amino acids [87].
The biosynthesis of icosalide is orchestrated by an NRPS encoded by the icoA gene. This NRPS exhibits an unusual architecture, featuring two chain-initiating condensation (C_I) domains. One C_I domain is located at the N-terminus of module 1, a common feature in lipopeptide assembly, while the other is embedded within module 3. This architecture enables the initiation of two separate lipopeptide chains, which are subsequently joined to form the asymmetric diolide structure of icosalide [88].
Icosalide A1 exhibits antimicrobial properties, particularly against Gram-positive bacteria such as Streptococcus pyogenes. The incorporation of a D-leucine residue is crucial for its antibacterial activity, as analogs lacking this residue do not display similar efficacy. Additionally, icosalide A1 has been shown to inhibit swarming motility in certain bacterial species, suggesting a role in modulating microbial behavior [87].
6.2.5. Burkholdines
Burkholdines were first isolated from B. ambifaria strain 2.2N. This bacterium was found to produce two primary variants: burkholdine 1229 (Bk-1229) and burkholdine 1097 (Bk-1097). Both compounds demonstrated significant antifungal activity against a range of fungal pathogens, with potencies surpassing that of amphotericin B, a commonly used antifungal agent [89,134].
These NRPS-derived compounds are characterized by their cyclic octapeptide structure, incorporating nonproteinogenic amino acids such as β-hydroxytyrosine and β-hydroxyasparagine. Additionally, they contain a characteristic fatty acyl amino acid moiety that contributes to their amphipathic nature, which is crucial for their interaction with fungal cell membranes [90].
6.2.6. Pyrrolnitrin
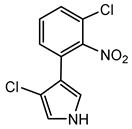
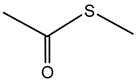
Pyrrolnitrin is a halogenated phenylpyrrole antibiotic initially isolated from Pseudomonas pyrrocinia. It has since been identified in several other bacterial genera, including Burkholderia, notably B. cepacia. Pyrrolnitrin is characterized by its chlorinated pyrrole structure, specifically 3-chloro-4-(3-chloro-2-nitrophenyl)-1H-pyrrole. In Burkholderia species, the biosynthesis of pyrrolnitrin involves the conversion of tryptophan through a series of enzymatic steps, leading to the formation of this bioactive compound [94].
Pyrrolnitrin exhibits potent antifungal activity against a wide range of filamentous fungi and yeasts. Additionally, it shows efficacy against certain Gram-positive bacteria, including Streptomyces species. The compound functions by inhibiting the electron transport chain in target organisms and disrupting cellular respiration, leading to cell death [91,94].
Pyrrolnitrin’s agricultural relevance is demonstrated by its biocontrol efficacy, as shown in B. cepacia strain 5.5B, which suppresses Rhizoctonia solani-induced stem rot in poinsettia [135]. In addition, B. cepacia strain K87 has been shown to produce novel oxidized derivatives of pyrrolnitrin, contributing to the discovery of structurally diverse antifungal agents with potentially improved activities [136].
6.2.7. Cepacin
Cepacin A and B are acetylenic antibiotics, containing a series of conjugated triple bonds in their carbon backbone. Cepacin exhibits significant antifungal activity, effectively inhibiting the growth of various phytopathogenic fungi [95]. In biological assays, B. ambifaria strains producing cepacin were able to suppress damping-off diseases caused by Globisporangium ultimum (formerly Pythium ultimum) in peas, showcasing its effectiveness in real-world agricultural scenarios [137].
6.2.8. Thailandenes
Thailandenes are a class of polyene natural products produced by B. thailandensis. The biosynthetic pathways responsible for thailandene production involve a series of enzymatic reactions that assemble these complex molecules from simpler precursors [96].
While the full spectrum of thailandene biological activities is still under investigation, preliminary studies suggest that these compounds may possess antimicrobial properties. Their polyene structures are known to interact with microbial membranes, potentially leading to antibacterial effects.
6.2.9. Thailandamide
Thailandamide is also a polyene natural antibiotic produced by B. thailandensis. It inhibits fatty acid biosynthesis by targeting acetyl-CoA carboxylase (AccA), an early, essential enzyme in the pathway [98]. Consistent with this target, thailandamide shows strong antibacterial activity against Gram-positive and cell wall-weakened Gram-negative bacteria [138].
Thailandamide biosynthetic genes co-occur with a duplicate, resistant accA allele (accA2), which provides producer self-resistance [98]. This self-resistance mechanism is critical for the survival of B. thailandensis when producing thailandamide. The biosynthesis of thailandamide is tightly regulated by QS, with QS mutants exhibiting altered secondary-metabolite profiles [99,139]. In addition, LysR-type regulator (LTTR) ScmR broadly influences secondary metabolism in B. thailandensis, linking thailandamide to a larger regulatory network [140].
6.2.10. Bactobolin
Bactobolin is an antibiotic produced by B. thailandensis whose biosynthesis is coordinated by an N-acyl homoserine lactone QS system in response to cell density, and the corresponding BGC has been well characterized in B. thailandensis strain E264 [100,141].
Bactobolin inhibits translation, and resistance to bactobolin can be conferred by mutations in ribosomal protein L2, supporting the ribosome as its primary target [101]. This mode of action is distinct from many classical antibiotics, highlighting bactobolin’s potential as a lead compound for antibiotic development [101].
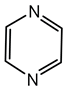
6.2.11. 4-Hydroxy-3-methyl-2-alkylquinolines (HMAQs)
HMAQs are specialized secondary metabolites produced primarily by species within the B. pseudomallei group, including B. thailandensis, B. pseudomallei, and closely related species [102,141]. These molecules share structural similarities with 4-hydroxy-2-alkylquinolines produced by Pseudomonas aeruginosa, but instead possess a methyl substitution at the 3-position of their quinoline ring, distinguishing their chemical properties and biological activities [102,141].
The biosynthetic pathway responsible for HMAQ production in Burkholderia involves the hmqABCDEFG operon, analogous to the pqs operon found in P. aeruginosa. However, the hmq operon is specifically adapted within Burkholderia species to yield methylated quinoline derivatives [102]. This operon has been well characterized in B. thailandensis, B. pseudomallei, and B. ambifaria, demonstrating its conserved presence among diverse members of this genus [102].
HMAQs exhibit notable antimicrobial activity, particularly against Gram-positive bacteria such as Staphylococcus aureus, as well as certain fungal species [142]. The antimicrobial activity of HMAQs and their N-oxide derivatives underscores their ecological function, enabling Burkholderia to compete effectively within soil, plant-associated environments, and potentially clinical niches by suppressing microbial competitors [141].
Apart from antimicrobial activity, HMAQs are also involved in complex QS regulatory networks within Burkholderia species. Specifically, studies on B. thailandensis strains have demonstrated that HMAQs contribute significantly to the regulation of genes associated with biofilm formation, motility, and stress responses [140,143,144], emphasizing their role in bacterial survival and environmental adaptation.
6.2.12. Occidiofungin
Occidiofungin is a cyclic glycopeptide exhibiting potent antifungal activity, produced primarily by the bacterium B. contaminans strain MS14. It shows significant activity against a wide range of fungal pathogens, including Candida species and the mold Aspergillus flavus [145,146]. Structurally, occidiofungin consists of unusual amino acid residues and glycosylation, contributing to its distinctive biochemical properties and antifungal activity [104]. Its potent antifungal efficacy arises from a novel mechanism of action involving binding to actin, a key cytoskeletal protein that disrupts actin polymerization, ultimately leading to fungal cell death [107].
The biosynthesis of occidiofungin involves several genes within a well-characterized BGC. Genetic studies revealed that the cluster includes genes encoding NRPSs, regulatory elements, and specific enzymes such as the xylosyltransferase encoded by the ocfC gene, which is essential for occidiofungin’s glycosylation [105,147]. Variants of occidiofungin, designated as OCF-E through OCF-J, have been identified and characterized, expanding the known structural diversity and biological potential of this metabolite [108].
6.3. Toxins
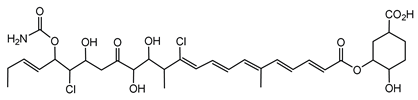
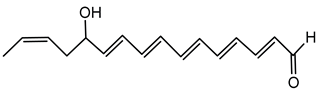
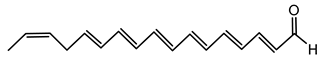
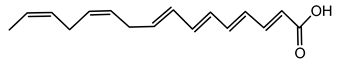
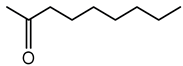
6.3.1. Bongkrekic Acid
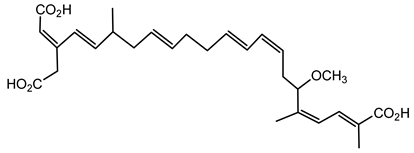
Bongkrekic acid is produced by B. gladioli pv. cocovenenans, a strain historically associated with severe food poisoning outbreaks, particularly in Southeast Asia. It is a respiratory toxin that inhibits mitochondrial adenine nucleotide translocase, a key protein responsible for the exchange of ADP and ATP across the inner mitochondrial membrane. This inhibition disrupts cellular energy homeostasis, leading to rapid bioenergetic failure and potentially fatal outcomes. The compound was first identified following fatal poisonings linked to consumption of fermented coconut-based foods, notably “tempe bongkrèk”—a traditional Indonesian food. Hence, the toxin was named “bongkrekic acid” [148,149,150]. Over the past decade, bongkrekic acid poisoning has been documented in a large outbreak in Mozambique linked to a traditional African beverage, in multiple foodborne incidents in China, and, more recently, in the first confirmed cases reported in North America and Taiwan, underscoring its emergence as a global food-safety threat [150,151,152,153,154,155].
Structurally, bongkrekic acid is a highly unsaturated tricarboxylic acid. It is extremely stable, heat-resistant, and unaffected by typical cooking processes, significantly complicating control measures once contamination occurs [156]. Human exposure to bongkrekic acid typically occurs through ingestion of contaminated food products. Symptoms manifest rapidly, often within hours of ingestion, and include severe vomiting, nausea, dizziness, headaches, epigastric discomfort, jaundice, and neurological symptoms such as seizures and unconsciousness. Reported mortality rates vary widely, with values ranging from 30% to 100% depending on the outbreak [150,156]. Due to its potency and stability, bongkrekic acid poisoning remains a serious public health concern, particularly in regions where traditional fermentation practices are prevalent.
The bongkrekic acid produced by B. gladioli pv. cocovenenans is best known for its role in severe food poisoning outbreaks, while its broader ecological role remains poorly understood [110]. Detecting bongkrekic acid contamination is crucial for preventing foodborne outbreaks. Analytical methods such as HPLC and mass spectrometry have been employed successfully to identify and quantify bongkrekic acid in contaminated food samples [157].
Despite its toxicity, the potent inhibitory action of bongkrekic acid on mitochondrial function has found niche applications in biochemical research, specifically for studying mitochondrial ATP transport and energy metabolism in cellular models. It serves as an important research tool for exploring the mitochondrial membrane function and bioenergetics in both physiological and pathological contexts [158].
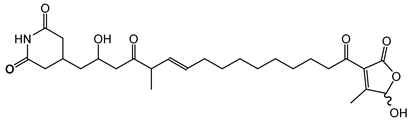
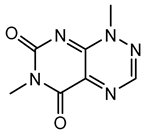
6.3.2. Toxoflavin
Toxoflavin acts as a phytotoxin, contributing to plant diseases such as rice grain rot and wilt in various crops [111]. Toxoflavin is a potent secondary metabolite originally isolated in 1934 from the bacterium B. gladioli, initially identified as Pseudomonas cocovenenans. It is a yellow-colored azapteridine compound recognized primarily for its phytotoxicity, contributing to severe agricultural diseases and posing challenges in crop production, especially rice cultivation [112,159]. B. gladioli, along with other related species, synthesizes toxoflavin as a significant virulence factor to infect host plants and compete within ecological niches [159].
The biosynthesis of toxoflavin is mediated by a dedicated gene cluster identified in the genomes of toxin-producing Burkholderia species, particularly B. gladioli and B. glumae. This cluster encodes several enzymes involved in a pathway starting from glycine and guanosine triphosphate. The biosynthetic mechanism involves multiple enzymatic steps, including cyclization, methylation, and oxidation reactions, ultimately yielding toxoflavin. Key enzymes characterized in this pathway include methyltransferases and oxidases, which together shape the chemical structure of the final compound [160,161].
Toxoflavin primarily exerts its biological effects through oxidative stress, mediated by the generation of reactive oxygen species, such as hydrogen peroxide and superoxide radicals. Its redox-active nature enables toxoflavin to participate in redox cycling reactions, disrupting cellular redox homeostasis. In plants, toxoflavin causes extensive damage to tissues, leading to symptoms such as necrosis, chlorosis, and wilting. The compound’s potent phytotoxicity is prominently involved in diseases such as rice grain rot and bacterial wilt in numerous crops [111,114]. This significant phytotoxicity poses a considerable threat to agriculture, particularly in rice-producing regions. Effective management strategies include developing disease-resistant crop varieties, employing good agricultural practices, seed sterilization, soil fumigation, and biological control strategies utilizing antagonistic microorganisms to suppress pathogen populations and detoxify soils contaminated with toxoflavin [115,162].
Beyond its role as a phytotoxin, toxoflavin has also been explored as a bioactive scaffold for therapeutic and agrochemical development, including anticancer, antimicrobial, and herbicidal leads. Genome mining and synthetic biology approaches are being used to re-engineer toxoflavin biosynthesis to generate derivatives with altered activity and reduced toxicity, paving the way for potential biotechnological applications [162].
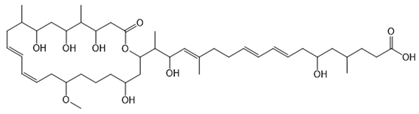
6.3.3. Malleilactone
Malleilactone is a polyketide-derived secondary metabolite produced by species within the B. pseudomallei group, including B. pseudomallei and B. thailandensis. This compound is recognized as a key virulence factor, contributing to the pathogenicity of B. pseudomallei, the causative agent of melioidosis [116,117]. The biosynthesis of malleilactone is directed by a PKS gene cluster, which is conserved among B. pseudomallei group pathogens [116].
Malleilactone production is tightly regulated by QS systems and can also be induced by exposure to certain antibiotics [117,163]. Specifically, the orphan LuxR-family regulator MalR in B. thailandensis can activate malleilactone biosynthesis independently of acyl-homoserine lactones, demonstrating the complex regulation of this cytotoxin [163]. These findings highlight the importance of malleilactone both in microbial competition and in the ability of B. pseudomallei group bacteria to cause disease.
6.3.4. Thailanstatin
Thailanstatins are a group of natural products produced by B. thailandensis MSMB43. They exhibit potent antiproliferative activities through inhibition of pre-mRNA splicing. These compounds are structurally related to FR901464, a known splicing inhibitor, but they possess enhanced stability, making them promising candidates for anticancer drug development [118]. Thailanstatins are characterized by a hybrid polyketide-nonribosomal peptide structure. Compared with FR901464, they lack the C1 hydroxyl group associated with the hemiketal center and instead possess an additional carboxyl moiety at C17; together these differences render thailanstatins significantly more stable than FR901464 under physiologically relevant conditions [118,119].
Thailanstatins bind to the SF3b subcomplex of the U2 small nuclear ribonucleoprotein within the spliceosome. In vitro assays demonstrated that thailanstatins inhibit pre-mRNA splicing with half-maximal inhibitory concentrations in the single to sub-micromolar range. Cell culture assays indicated potent antiproliferative activities against various human cancer cell lines, with half-maximal growth inhibitory concentrations in the single nanomolar range [118]. Efforts have been made to improve the production yields of thailanstatins through metabolic engineering of the biosynthetic pathway in B. thailandensis, facilitating further preclinical studies [164,165].
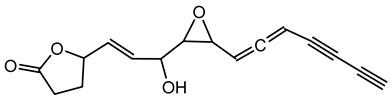
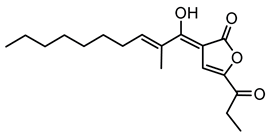
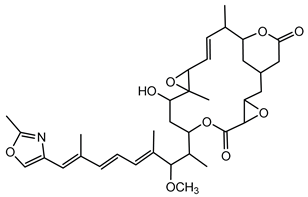
6.3.5. Rhizoxin
Rhizoxin is a potent antimitotic macrolide initially attributed to the fungus Rhizopus microsporus, the causative agent of rice seedling blight. However, subsequent research unveiled that the true producers of rhizoxin are endosymbiotic bacteria residing within the fungal cytosol, specifically B. rhizoxinica (reclassified as Mycetohabitans rhizoxinica) [17,166]. The symbiosis between B. rhizoxinica and Rhizopus microsporus is integral to the biology and virulence of the fungal host, because the bacterium supplies rhizoxin, which R. microsporus uses as a virulence factor in rice seedling blight. Additionally, B. rhizoxinica is essential for the fungal host’s vegetative spore formation, highlighting a mutualistic relationship where the bacterium provides chemical weaponry while the fungus provides protection [122,167,168].
Structurally, rhizoxin contains a 16-membered macrolactone ring linked to an oxazole moiety via a long unsaturated aliphatic chain. This structure enables rhizoxin to bind β-tubulin, resulting in inhibition of microtubule assembly and cell cycle arrest [169]. The BGC responsible for rhizoxin production encodes a hybrid PKS and NRPS assembly line, facilitating the incorporation of various building blocks into the rhizoxin molecule [120,122].
Because of its antimitotic properties, rhizoxin has been investigated as a potential anticancer agent. Phase II clinical trials in patients with advanced breast cancer and melanoma demonstrated that rhizoxin could be safely administered intravenously at a dose of 2.0 mg/m2 every 3 weeks. However, its therapeutic efficacy was limited, with minimal antitumor activity observed in both patient groups. This outcome, along with issues related to drug stability and toxicity, has hindered its further clinical development [121].
6.4. Other Metabolites
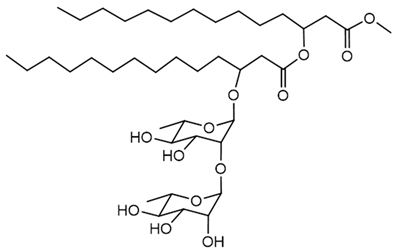
Rhamnolipids
Rhamnolipids are glycolipid biosurfactants produced by several Burkholderia species, with structures and properties that make them valuable for various industrial and environmental applications. The genetic regulation of rhamnolipid biosynthesis in Burkholderia involves rhl gene clusters, most notably rhlA, rhlB, and rhlC. In B. thailandensis, two identical rhl gene clusters have been identified, and these clusters encode enzymes responsible for rhamnolipid biosynthesis. The regulation of these genes is closely linked to QS systems and environmental conditions, indicating a tightly controlled genetic mechanism that responds to both cell density and external factors [127]. In B. glumae, the rhamnolipid biosynthetic pathway is also under the control of rhlA, rhlB, and rhlC homologs, and the production profile can differ from that of other species, reflecting differences in genetic regulation and metabolic potential [125].
The purity and composition of rhamnolipid preparations can be influenced by the cultivation medium, carbon source, and process conditions, which have been optimized in studies with B. glumae and Burkholderia kururiensis to maximize yield and tailor the congener profile to specific applications [125,170,171]. Burkholderia plantarii DSM 9509T was found to produce rhamnolipids under various culture conditions, with yields and congener patterns distinct from those of Pseudomonas species. The study highlighted that although the total yield may be lower, the specific structural forms of rhamnolipids produced by B. plantarii can have characteristic physicochemical properties that are of interest for specialized applications [172].
The ongoing interest in green technologies has further increased the demand for microbial biosurfactants, including those from Burkholderia, for environmentally friendly applications [173]. Nevertheless, safety assessments are crucial since some Burkholderia species are considered opportunistic pathogens, and the use of wild-type or genetically modified strains in large-scale production requires careful risk evaluation.
7. Volatile Organic Compounds (VOCs) Produced by Burkholderia Species
VOCs are small molecules characterized by high vapor pressure and low boiling points, enabling them to evaporate quickly and diffuse readily through air and soil [174]. Burkholderia species synthesize a broad spectrum of VOCs with important ecological, biotechnological, and agricultural functions. These VOCs include sulfur-containing compounds, alcohols, ketones, pyrazines, hydrocarbons, and aromatic compounds, which play roles in microbial interactions, plant growth, pathogen inhibition, and communication. A summary of VOCs is provided in Table 2; and selected examples are detailed below.

Table 2.
VOCs emitted by various Burkholderia species. The table summarizes their chemical structure, biological activities, and associated references.
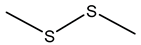
7.1. Dimethyl Disulfide (DMDS)
Dimethyl disulfide (DMDS), characterized by a strong garlic-like odor, exhibits potent antimicrobial, antifungal, and nematicidal properties, making it a promising agent in agriculture for biocontrol purposes. DMDS production has been prominently observed in B. gladioli, B. ambifaria, and various strains within the Bcc [175,176,182].
DMDS production in Burkholderia species is typically associated with the catabolism of sulfur-containing amino acids, particularly methionine and cysteine. Enzymatic processes involving methionine-γ-lyase or cysteine desulfhydrase result in volatile sulfur-containing by-products, with DMDS as a prominent end product. This metabolic pathway allows the bacteria to detoxify excess sulfur and regulate intracellular sulfur pools [182,187].
B. ambifaria H8 was shown to inhibit Fusarium graminearum and protect maize plants against stalk rot through DMDS production [175]. B. gladioli strain BBB-01 has demonstrated fungicidal activity through DMDS and other VOCs [176]. DMDS also exhibits significant toxicity toward root-knot nematode Meloidogyne incognita [183,184]. Beyond antifungal and nematicidal properties, DMDS has demonstrated antibacterial and insecticidal activities. Its sulfurous nature disrupts microbial metabolism, inhibits pathogenic bacterial growth, and deters insect herbivory [188].
Given its broad-spectrum biological activities, DMDS offers a potential alternative to conventional chemical fumigants. In fact, DMDS has been registered and commercialized as a soil fumigant under the trade name Paladin® EC, manufactured by Arkema Inc., and is approved for use by the U.S. Environmental Protection Agency [189,190]. Its formulation provides a practical, field-deployable alternative to methyl bromide, offering strong biocidal activity while breaking down rapidly into non-toxic sulfur compounds.
While promising, the DMDS application has considerations. Its strong odor may restrict use in some settings, and inhalation at high concentrations may pose risks to humans. Therefore, formulation and application strategies must be optimized to balance efficacy and safety.
7.2. Dimethyl Sulfide (DMS)
Dimethyl sulfide (DMS) is characterized by its distinctive odor, often associated with cooked cabbage or marine environments. It plays a significant role in the global sulfur cycle and has implications for climate regulation due to its involvement in cloud formation [176,191]. Certain Burkholderia species have been identified as producers of DMS through the catabolism of dimethylsulfoniopropionate, a compatible solute synthesized by various marine algae and some angiosperms [191,192].
The production of DMS by Burkholderia species holds important ecological significance across various environments. In the atmosphere, DMS plays a role in climate regulation by oxidizing into sulfate aerosols that contribute to cloud condensation nuclei, thereby influencing cloud formation and potentially affecting global temperature and precipitation patterns [176,192]. In terrestrial and plant-associated ecosystems, DMS produced by root-associated Burkholderia may participate in plant-microbe interactions, potentially serving as a chemical signal or modulator of microbial community dynamics. The presence and expression of volatile sulfur compound biosynthesis genes can even help distinguish species such as B. pseudomallei from closely related non-pathogenic species [181].
7.3. S-Methyl Thioacetate (SMT)
S-methyl thioacetate (SMT) is characterized by its simple thioester structure and is known for a distinctive sulfurous odor reminiscent of cabbage or garlic. B. pyrrocinia CNUC9, isolated from the maize rhizosphere, emits a spectrum of VOCs, including SMT, DMDS, and 2-undecanone. These emissions have been linked to enhanced germination and survival rates of Arabidopsis thaliana under salt stress conditions. Furthermore, exposure to the emission has been shown to alter root architecture and increase leaf area in A. thaliana [185]. These findings suggest a multifaceted function for SMT, with contributions to plant resilience and growth promotion under abiotic stress.
Production of SMT by B. gladioli was also reported. In terms of biocontrol, SMT exhibits significant fumigant toxicity against nematodes Caenorhabditis elegans and M. incognita. Specifically, the lethal concentration (LC50) was determined to be 1.43 μg/cm3 of air for C. elegans. In parallel comparison, SMT showed stronger fumigant toxicity against M. incognita than DMDS [183]. The utilization of SMT-producing bacteria, such as Bacillus aryabhattai [193], or the direct application of synthesized SMT could provide an eco-friendly alternative to conventional nematicides [183].
7.4. 2,5-Dimethylfuran
2,5-Dimethylfuran, characterized by its aromatic odor, occurs naturally in a variety of environmental contexts. In the context of Burkholderia species, 2,5-dimethylfuran has been identified as a significant VOC when B. gladioli strain BBB-01 is cultivated in potato extract-glucose medium [186].
2,5-Dimethylfuran exhibits inhibitory activity against several phytopathogenic fungi, including Magnaporthe oryzae, Gibberella fujikuroi, Sarocladium oryzae, Phellinus noxius, and Colletotrichum fructicola, as well as the human pathogen Candida albicans [186]. This broad-spectrum antifungal activity underscores its potential role in biological control and plant protection strategies.
7.5. 2-Undecanone
2-Undecanone is a medium-chain methyl ketone detected in the headspace of B. pyrrocinia CNUC9. Functionally, pure 2-undecanone showed dose-dependent effects on plants: low doses promoted Arabidopsis thaliana root and shoot growth, while high doses inhibited growth—demonstrating that even a minor VOC can have bioactivity [185]. Additionally, 2-undecanone has been identified as an antifungal VOC produced by Burkholderia. In B. ambifaria, the compound reduced the growth of Rhizoctonia solani and Alternaria alternata at higher doses [182].
7.6. Methyl Salicylate
Methyl salicylate is a VOC known for its characteristic wintergreen aroma. While it is predominantly produced by various plant species, certain bacterial species, including members of the Burkholderia genus, have also been identified to synthesize this compound.
Research indicates that B. cenocepacia strain ETR-B22 emits methyl salicylate among many aromatic VOCs, including methyl anthranilate, methyl benzoate, benzyl propionate, benzyl acetate, 3,5-di-tert-butylphenol, allyl benzyl ether, and benzyl benzoate, all of which have demonstrated inhibitory effects against several fungal plant pathogens [178,187].
In plants, methyl salicylate serves as a signaling molecule involved in systemic acquired resistance, a key defense mechanism against pathogens. It also plays a role in inter-plant communication, alerting neighboring plants to potential threats [194,195]. The production of methyl salicylate by Burkholderia species highlights their potential as biological control agents in agriculture. By inhibiting fungal pathogens, these bacteria could reduce the reliance on chemical fungicides, promoting more sustainable farming practices.
8. Discussion and Perspectives
The genus Burkholderia stands at a critical crossroads of risk and opportunity. While many species are recognized for their roles as plant and human pathogens, recent advances in genomics, synthetic biology, and biotechnology are rapidly transforming our approach to these bacteria. Increasingly, researchers are looking beyond the risks to explore Burkholderia as a treasure trove of biotechnologically valuable metabolites and ecological functions. Many environmental and plant-associated species act as plant growth promoters, nutrient mobilizers, and potent biocontrol agents. In fact, the phylogeny illustrated in Figure 1 reveals a clear clade-level asymmetry: the Burkholderia sensu stricto clade concentrates species with both antibacterial and antifungal activities, and some of these occur across multiple habitats, including soil, water, plants, and animals. This ecological profile is typical of metabolite-rich generalists. By contrast, Paraburkholderia skews toward plant-associated lifestyles and a sparser secondary metabolite footprint. Species spanning multiple habitats and showing dual antibacterial and antifungal phenotypes should be prioritized as “secondary metabolite generalists,” because ecological breadth and dual activity correlate with metabolite richness and competitive fitness. From a metabolite-discovery perspective, this pattern suggests that Burkholderia sensu stricto should be prioritized as primary sources of potent chemistry under appropriate containment, while plant-beneficial Paraburkholderia and related environmental lineages provide complementary, lower-risk reservoirs of bioactive traits for agricultural applications.
Compared with classic natural-product workhorses such as Streptomyces and Bacillus, Burkholderia occupies a distinctive but more constrained niche in practical applications. Streptomyces spp. have historically dominated antibiotic discovery and are generally regarded as low-risk soil saprophytes, while many Bacillus spp. combine metabolite production with spore-based formulations that are straightforward to stabilize and register as biopesticides. By contrast, Burkholderia sensu stricto species have large, multireplicon genomes with rich, often cryptic BGC repertoires and frequently produce structurally unusual metabolites with potent antibacterial or antifungal activity [3,4,37,128]. This combination of genomic complexity and chemical diversity makes them particularly attractive for the discovery of new antimicrobial scaffolds. At the same time, some members of this group are opportunistic or frank pathogens with intrinsic multidrug resistance, so Burkholderia-based biotechnology must balance metabolic novelty and ecological competitiveness against heightened regulatory scrutiny, careful strain selection (e.g., favoring Paraburkholderia and other strictly environmental lineages for field applications), and the need to attenuate or avoid virulence- and resistance-associated traits.
The capacity of these bacteria to colonize the rhizosphere and compete with harmful microbes makes them attractive candidates for next-generation biocontrol products. For example, cepacin-producing B. ambifaria strains suppress pea damping-off caused by Globisporangium ultimum [95,137], DMDS-producing B. ambifaria H8 can control maize stalk rot [175], illustrating how Burkholderia-derived antibiotics and VOCs can function as seed or soil treatments within integrated disease management strategies. However, deploying Burkholderia in biotechnology also carries non-trivial biosafety concerns: members of the Bcc are opportunistic, often multidrug-resistant, pathogens of people with CF and other underlying conditions, sometimes associated with poor clinical outcomes, while B. pseudomallei and B. mallei cause severe, difficult-to-treat infections that require prolonged antimicrobial therapy [3,5,7,20,43]. Their high intrinsic resistance, diverse virulence factors, and broad ecological range demand rigorous risk assessment, regulatory compliance, and long-term biosafety testing for any translational application [196,197]. To achieve this, removing or attenuating pathogenicity determinants could be crucial, for example, by deleting secretion systems or toxin/virulence metabolite pathways. In Burkholderia models, such targeted edits consistently reduce virulence, including T3SS bscN mutants in B. cepacia [198], loss of the T6SS effector TecA in B. cenocepacia [199], toxoflavin-deficient B. glumae [53], and disruption of the malleilactone cluster in the B. pseudomallei group [116,117]. In practical terms, this means that translational applications should rely on carefully characterized, low-risk species and strains, and should be designed to minimize environmental and clinical exposure, especially in contexts involving vulnerable patient groups or the food chain.
Unlocking the full metabolic potential of Burkholderia requires activation of cryptic or silent BGCs [200], which often remain unexpressed under standard laboratory conditions. Advanced genome editing and recombineering technologies, such as targeted mutagenesis (host-based homologous recombination, phage-based recombineering, CRISPR-Cas systems), untargeted mutagenesis (transposon-based approaches), and heterologous expression in surrogate hosts (including Burkholderia chassis), provide practical means to implement both attenuation and activation strategies without compromising desired traits [201,202,203,204]. For example, LTTRs play a pivotal role in modulating the expression of these silent clusters [140]. By engineering LTTRs or manipulating their regulatory networks, researchers have succeeded in activating previously inaccessible BGCs, leading to the discovery of novel antibiotics and other bioactive metabolites. Combining LTTR modulation with genome mining, promoter engineering, and advanced omics approaches offers a promising strategy for expanding Burkholderia’s arsenal of valuable natural products for agricultural and pharmaceutical use. Given the increasing availability of complete genome sequences, functional genomics tools, and efficient transformation protocols, Burkholderia is now highly amenable to such integrated activation and attenuation strategies.
Looking forward, the integration of high-throughput genome mining, synthetic biology, and systems biology approaches will further accelerate the discovery and optimization of Burkholderia-derived bioactive compounds for the agriculture and medicine sectors. Metabolic engineering could also be used to boost the yields of key antifungal metabolites or VOCs. For instance, the antifungal glycopeptide occidiofungin from B. contaminans has shown potent, broad-spectrum activity against human and plant-pathogenic fungi and is being investigated for both clinical and crop protection applications [104,145,146], while biosynthetic and media engineering of Burkholderia spp. has enabled improved production of spliceostatin/thailanstatin-type pre-mRNA splicing inhibitors as anticancer leads [118,164,165]. In bioindustrial and environmental contexts, rhamnolipid biosurfactants from Burkholderia spp. are being explored for green formulations and bioremediation of persistent pollutants [124,173], while aromatic-degrading Paraburkholderia strains such as P. xenovorans LB400 are used for the breakdown of persistent organic pollutants [37]. Beneficial species are already being tested as biofertilizers, nitrogen fixers, and bioremediators, but coordinated efforts linking molecular insights with field-level trials and risk mitigation will be essential to realize these applications responsibly. In summary, the future of Burkholderia research lies not only in managing its risks but also in unlocking its vast biotechnological promise. The rational design of safe, effective, and application-tailored strains, coupled with stringent biosafety frameworks, could position Burkholderia as a cornerstone of next-generation sustainable agriculture, biotechnology, and medicine.
Author Contributions
Conceptualization, A.D. and M.M.; methodology, A.D.; software, A.D.; validation, A.D., A.A. and M.M.; formal analysis, A.D.; investigation, A.D. and A.A.; resources, M.M.; data curation, A.D.; writing—original draft preparation, A.D.; writing—review and editing, A.D., A.A. and M.M.; visualization, A.D. and A.A.; supervision, M.M.; project administration, M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science and Technology Council, Taiwan, ROC [grant number: NSTC 114-2313-B-005-001].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Coenye, T.; Vandamme, P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 2003, 5, 719–729. [Google Scholar] [CrossRef]
- Eberl, L.; Vandamme, P. Members of the genus Burkholderia: Good and bad guys. F1000Research 2016, 5, 1007. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Urban, T.A.; Goldberg, J.B. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 2005, 3, 144–156. [Google Scholar] [CrossRef]
- Depoorter, E.; Bull, M.J.; Peeters, C.; Coenye, T.; Vandamme, P.; Mahenthiralingam, E. Burkholderia: An update on taxonomy and biotechnological potential as antibiotic producers. Appl. Microbiol. Biotechnol. 2016, 100, 5215–5229. [Google Scholar] [CrossRef]
- Lipuma, J.J. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010, 23, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Drevinek, P.; Mahenthiralingam, E. Burkholderia cenocepacia in cystic fibrosis: Epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 2010, 16, 821–830. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Currie, B.J.; Peacock, S.J. Melioidosis. N. Engl. J. Med. 2012, 367, 1035–1044. [Google Scholar] [CrossRef]
- Dance, D. Treatment and prophylaxis of melioidosis. Int. J. Antimicrob. Agents 2014, 43, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, G.C.; Estes, D.M.; Torres, A.G. Glanders: Off to the races with Burkholderia mallei. FEMS Microbiol. Lett. 2007, 277, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Dutta, B.; Kvitko, B. Onion-pathogenic species: Role and regulation of characterized virulence determinants. Plant Pathol. 2024, 73, 2281–2297. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Baldwin, A.; Dowson, C.G. Burkholderia cepacia complex bacteria: Opportunistic pathogens with important natural biology. J. Appl. Microbiol. 2008, 104, 1539–1551. [Google Scholar] [CrossRef]
- Burtnick, M.N.; Brett, P.J.; Harding, S.V.; Ngugi, S.A.; Ribot, W.J.; Chantratita, N.; Scorpio, A.; Milne, T.S.; Dean, R.E.; Fritz, D.L.; et al. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect. Immun. 2011, 79, 1512–1525. [Google Scholar] [CrossRef]
- D’Cruze, T.; Gong, L.; Treerat, P.; Ramm, G.; Boyce, J.D.; Prescott, M.; Adler, B.; Devenish, R.J. Role for the Burkholderia pseudomallei type three secretion system cluster 1 bpscN gene in virulence. Infect. Immun. 2011, 79, 3659–3664. [Google Scholar] [CrossRef]
- Compant, S.; Nowak, J.; Coenye, T.; Clement, C.; Ait Barka, E. Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol. Rev. 2008, 32, 607–626. [Google Scholar] [CrossRef]
- Suarez-Moreno, Z.R.; Caballero-Mellado, J.; Coutinho, B.G.; Mendonca-Previato, L.; James, E.K.; Venturi, V. Common features of environmental and potentially beneficial plant-associated Burkholderia. Microb. Ecol. 2012, 63, 249–266. [Google Scholar] [CrossRef]
- Sawana, A.; Adeolu, M.; Gupta, R.S. Molecular signatures and phylogenomic analysis of the genus Burkholderia: Proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Front. Genet. 2014, 5, 429. [Google Scholar] [CrossRef] [PubMed]
- Estrada-de Los Santos, P.; Palmer, M.; Chavez-Ramirez, B.; Beukes, C.; Steenkamp, E.T.; Briscoe, L.; Khan, N.; Maluk, M.; Lafos, M.; Humm, E.; et al. Whole Genome Analyses Suggests that Burkholderia sensu lato Contains Two Additional Novel Genera (Mycetohabitans gen. nov., and Trinickia gen. nov.): Implications for the Evolution of Diazotrophy and Nodulation in the Burkholderiaceae. Genes 2018, 9, 389. [Google Scholar] [CrossRef]
- Sessitsch, A.; Coenye, T.; Sturz, A.V.; Vandamme, P.; Barka, E.A.; Salles, J.F.; Van Elsas, J.D.; Faure, D.; Reiter, B.; Glick, B.R.; et al. Burkholderia phytofirmans sp. nov., a novel plant-associated bacterium with plant-beneficial properties. Int. J. Syst. Evol. Microbiol. 2005, 55, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Estrada-De Los Santos, P.; Bustillos-Cristales, R.; Caballero-Mellado, J. Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Appl. Environ. Microbiol. 2001, 67, 2790–2798. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Virk, H.S.; Torres, A.G.; Currie, B.J.; Peacock, S.J.; Dance, D.A.B.; Limmathurotsakul, D. Melioidosis. Nat. Rev. Dis. Primers. 2018, 4, 17107. [Google Scholar] [CrossRef]
- Esmaeel, Q.; Miotto, L.; Rondeau, M.; Leclere, V.; Clement, C.; Jacquard, C.; Sanchez, L.; Barka, E.A. Paraburkholderia phytofirmans PsJN-Plants Interaction: From Perception to the Induced Mechanisms. Front. Microbiol. 2018, 9, 2093. [Google Scholar] [CrossRef]
- Yabuuchi, E.; Kosako, Y.; Oyaizu, H.; Yano, I.; Hotta, H.; Hashimoto, Y.; Ezaki, T.; Arakawa, M. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immunol. 1992, 36, 1251–1275. [Google Scholar] [CrossRef] [PubMed]
- Dobritsa, A.P.; Samadpour, M. Transfer of eleven species of the genus Burkholderia to the genus Paraburkholderia and proposal of Caballeronia gen. nov. to accommodate twelve species of the genera Burkholderia and Paraburkholderia. Int. J. Syst. Evol. Microbiol. 2016, 66, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Santos, L.; Castro, D.B.A.; Ferreira-Tonin, M.; Correa, D.B.A.; Weir, B.S.; Park, D.; Ottoboni, L.M.M.; Neto, J.R.; Destefano, S.A.L. Reassessment of the taxonomic position of Burkholderia andropogonis and description of Robbsia andropogonis gen. nov., comb. nov. Antonie Van. Leeuwenhoek 2017, 110, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Losada, L.; Ronning, C.M.; DeShazer, D.; Woods, D.; Fedorova, N.; Kim, H.S.; Shabalina, S.A.; Pearson, T.R.; Brinkac, L.; Tan, P.; et al. Continuing evolution of Burkholderia mallei through genome reduction and large-scale rearrangements. Genome Biol. Evol. 2010, 2, 102–116. [Google Scholar] [CrossRef]
- Nierman, W.C.; DeShazer, D.; Kim, H.S.; Tettelin, H.; Nelson, K.E.; Feldblyum, T.; Ulrich, R.L.; Ronning, C.M.; Brinkac, L.M.; Daugherty, S.C.; et al. Structural flexibility in the Burkholderia mallei genome. Proc. Natl. Acad. Sci. USA 2004, 101, 14246–14251. [Google Scholar] [CrossRef]
- Holden, M.T.; Titball, R.W.; Peacock, S.J.; Cerdeno-Tarraga, A.M.; Atkins, T.; Crossman, L.C.; Pitt, T.; Churcher, C.; Mungall, K.; Bentley, S.D.; et al. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. USA 2004, 101, 14240–14245. [Google Scholar] [CrossRef]
- Weilharter, A.; Mitter, B.; Shin, M.V.; Chain, P.S.; Nowak, J.; Sessitsch, A. Complete genome sequence of the plant growth-promoting endophyte Burkholderia phytofirmans strain PsJN. J. Bacteriol. 2011, 193, 3383–3384. [Google Scholar] [CrossRef]
- Mullins, A.J.; Mahenthiralingam, E. The Hidden Genomic Diversity, Specialized Metabolite Capacity, and Revised Taxonomy of Burkholderia Sensu Lato. Front. Microbiol. 2021, 12, 726847. [Google Scholar] [CrossRef]
- Weissman, J.L.; Fagan, W.F.; Johnson, P.L.F. Linking high GC content to the repair of double strand breaks in prokaryotic genomes. PLoS Genet. 2019, 15, e1008493. [Google Scholar] [CrossRef]
- Holden, M.T.; Seth-Smith, H.M.; Crossman, L.C.; Sebaihia, M.; Bentley, S.D.; Cerdeno-Tarraga, A.M.; Thomson, N.R.; Bason, N.; Quail, M.A.; Sharp, S.; et al. The genome of Burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J. Bacteriol. 2009, 191, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Tuanyok, A.; Leadem, B.R.; Auerbach, R.K.; Beckstrom-Sternberg, S.M.; Beckstrom-Sternberg, J.S.; Mayo, M.; Wuthiekanun, V.; Brettin, T.S.; Nierman, W.C.; Peacock, S.J.; et al. Genomic islands from five strains of Burkholderia pseudomallei. BMC Genom. 2008, 9, 566. [Google Scholar] [CrossRef]
- Bylund, J.; Burgess, L.A.; Cescutti, P.; Ernst, R.K.; Speert, D.P. Exopolysaccharides from Burkholderia cenocepacia inhibit neutrophil chemotaxis and scavenge reactive oxygen species. J. Biol. Chem. 2006, 281, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Suppiger, A.; Schmid, N.; Aguilar, C.; Pessi, G.; Eberl, L. Two quorum sensing systems control biofilm formation and virulence in members of the Burkholderia cepacia complex. Virulence 2013, 4, 400–409. [Google Scholar] [CrossRef]
- Kunakom, S.; Eustaquio, A.S. Burkholderia as a Source of Natural Products. J. Nat. Prod. 2019, 82, 2018–2037. [Google Scholar] [CrossRef]
- Chain, P.S.; Denef, V.J.; Konstantinidis, K.T.; Vergez, L.M.; Agullo, L.; Reyes, V.L.; Hauser, L.; Cordova, M.; Gomez, L.; Gonzalez, M.; et al. Burkholderia xenovorans LB400 harbors a multi-replicon, 9.73-Mbp genome shaped for versatility. Proc. Natl. Acad. Sci. USA 2006, 103, 15280–15287. [Google Scholar] [CrossRef]
- Podnecky, N.L.; Rhodes, K.A.; Schweizer, H.P. Efflux pump-mediated drug resistance in Burkholderia. Front. Microbiol. 2015, 6, 305. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Sass, A.; Mahenthiralingam, E.; Nelis, H.; Coenye, T. Transcriptional response of Burkholderia cenocepacia J2315 sessile cells to treatments with high doses of hydrogen peroxide and sodium hypochlorite. BMC Genom. 2010, 11, 90. [Google Scholar] [CrossRef]
- Nandi, T.; Ong, C.; Singh, A.P.; Boddey, J.; Atkins, T.; Sarkar-Tyson, M.; Essex-Lopresti, A.E.; Chua, H.H.; Pearson, T.; Kreisberg, J.F.; et al. A genomic survey of positive selection in Burkholderia pseudomallei provides insights into the evolution of accidental virulence. PLoS Pathog. 2010, 6, e1000845. [Google Scholar] [CrossRef]
- Visser, M.B.; Majumdar, S.; Hani, E.; Sokol, P.A. Importance of the ornibactin and pyochelin siderophore transport systems in Burkholderia cenocepacia lung infections. Infect. Immun. 2004, 72, 2850–2857. [Google Scholar] [CrossRef]
- Agnoli, K.; Lowe, C.A.; Farmer, K.L.; Husnain, S.I.; Thomas, M.S. The ornibactin biosynthesis and transport genes of Burkholderia cenocepacia are regulated by an extracytoplasmic function sigma factor which is a part of the Fur regulon. J. Bacteriol. 2006, 188, 3631–3644. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Chiarelli, L.R.; Trespidi, G.; Mentasti, M.; Riccardi, G.; Buroni, S. Burkholderia cenocepacia Infections in Cystic Fibrosis Patients: Drug Resistance and Therapeutic Approaches. Front. Microbiol. 2017, 8, 1592. [Google Scholar] [CrossRef]
- Rhodes, K.A.; Schweizer, H.P. Antibiotic resistance in Burkholderia species. Drug. Resist. Updat. 2016, 28, 82–90. [Google Scholar] [CrossRef]
- Somayaji, R.; Yau, Y.C.W.; Tullis, E.; LiPuma, J.J.; Ratjen, F.; Waters, V. Clinical Outcomes Associated with Burkholderia cepacia Complex Infection in Patients with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2020, 17, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Dodd, M.E.; Govan, J.R.; Barcus, V.; Doherty, C.J.; Morris, J.; Webb, A.K. Burkholderia cenocepacia and Burkholderia multivorans: Influence on survival in cystic fibrosis. Thorax 2004, 59, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Dacco, V.; Alicandro, G.; Consales, A.; Rosazza, C.; Sciarrabba, C.S.; Cariani, L.; Colombo, C. Cepacia syndrome in cystic fibrosis: A systematic review of the literature and possible new perspectives in treatment. Pediatr. Pulmonol. 2023, 58, 1337–1343. [Google Scholar] [CrossRef]
- CDC. Clinical Overview of Melioidosis. Available online: https://www.cdc.gov/melioidosis/hcp/clinical-overview/index.html (accessed on 30 September 2025).
- Khan, I.; Wieler, L.H.; Melzer, F.; Elschner, M.C.; Muhammad, G.; Ali, S.; Sprague, L.D.; Neubauer, H.; Saqib, M. Glanders in animals: A review on epidemiology, clinical presentation, diagnosis and countermeasures. Transbound. Emerg. Dis. 2013, 60, 204–221. [Google Scholar] [CrossRef]
- Van Zandt, K.E.; Greer, M.T.; Gelhaus, H.C. Glanders: An overview of infection in humans. Orphanet J. Rare Dis. 2013, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Ura, H.; Furuya, N.; Iiyama, K.; Hidaka, M.; Tsuchiya, K.; Matsuyama, N. Burkholderia gladioli associated with symptoms of bacterial grain rot and seedling blight of rice plants. J. Gen. Plant. Pathol. 2006, 72, 98–103. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of Metabolic Activity, Beneficial and Pathogenic Aspects of Burkholderia Spp. Metabolites 2021, 11, 321. [Google Scholar] [CrossRef]
- Ham, J.H.; Melanson, R.A.; Rush, M.C. Burkholderia glumae: Next major pathogen of rice? Mol. Plant. Pathol. 2011, 12, 329–339. [Google Scholar] [CrossRef]
- Naughton, L.M.; An, S.Q.; Hwang, I.; Chou, S.H.; He, Y.Q.; Tang, J.L.; Ryan, R.P.; Dow, J.M. Functional and genomic insights into the pathogenesis of Burkholderia species to rice. Environ. Microbiol. 2016, 18, 780–790. [Google Scholar] [CrossRef]
- Ortega, L.; Rojas, C.M. Bacterial Panicle Blight and Burkholderia glumae: From Pathogen Biology to Disease Control. Phytopathology 2021, 111, 772–778. [Google Scholar] [CrossRef]
- Shi, H.; Chen, X.; Chen, L.; Zhu, B.; Yan, W.; Ma, X. Burkholderia cepacia infection in children without cystic fibrosis: A clinical analysis of 50 cases. Front. Pediatr. 2023, 11, 1115877. [Google Scholar] [CrossRef]
- El Chakhtoura, N.G.; Saade, E.; Wilson, B.M.; Perez, F.; Papp-Wallace, K.M.; Bonomo, R.A. A 17-Year Nationwide Study of Burkholderia cepacia Complex Bloodstream Infections Among Patients in the United States Veterans Health Administration. Clin. Infect. Dis. 2017, 65, 1253–1259. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Becka, S.A.; Zeiser, E.T.; Ohuchi, N.; Mojica, M.F.; Gatta, J.A.; Falleni, M.; Tosi, D.; Borghi, E.; Winkler, M.L.; et al. Overcoming an Extremely Drug Resistant (XDR) Pathogen: Avibactam Restores Susceptibility to Ceftazidime for Burkholderia cepacia Complex Isolates from Cystic Fibrosis Patients. ACS Infect. Dis. 2017, 3, 502–511. [Google Scholar] [CrossRef]
- Tamma, P.D.; Fan, Y.; Bergman, Y.; Sick-Samuels, A.C.; Hsu, A.J.; Timp, W.; Simner, P.J.; Prokesch, B.C.; Greenberg, D.E. Successful Treatment of Persistent Burkholderia cepacia Complex Bacteremia with Ceftazidime-Avibactam. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Cassir, N.; Coiffard, B.; Hadjadj, L.; Bermudez, J.; Okdah, L.; Ailhaud, L.; Baron, S.A.; Reynaud-Gaubert, M.; D’Journo, X.B.; Hraiech, S.; et al. Multi-target combination of antibiotics as salvage therapy for severe infection caused by pan-resistant Burkholderia cenocepacia following lung transplantation. Transpl. Rep. 2025, 10, 100170. [Google Scholar] [CrossRef]
- Canning, J.S.; Laucirica, D.R.; Ling, K.M.; Nicol, M.P.; Stick, S.M.; Kicic, A. Phage therapy to treat cystic fibrosis Burkholderia cepacia complex lung infections: Perspectives and challenges. Front. Microbiol. 2024, 15, 1476041. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, J. UC IPM Pest Management Guidelines: Floriculture and Ornamental Nurseries; UC ANR Publication 3392; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2021. [Google Scholar]
- Pacific Northwest Pest Management Handbooks. Gladiolus-Bacterial Scab. Available online: https://pnwhandbooks.org/plantdisease/host-disease/gladiolus-bacterial-scab (accessed on 30 March 2025).
- Keith, L.M.; Sewake, K.T.; Zee, F.T. Isolation and Characterization of Burkholderia gladioli from Orchids in Hawaii. Plant. Dis. 2005, 89, 1273–1278. [Google Scholar] [CrossRef]
- Lauman, P.; Dennis, J.J. Prophylactic phage biocontrol prevents Burkholderia gladioli infection in a quantitative ex planta model of bacterial virulence. Appl. Environ. Microbiol. 2024, 90, e0131724. [Google Scholar] [CrossRef]
- Iqbal, A.; Panta, P.R.; Ontoy, J.; Bruno, J.; Ham, J.H.; Doerrler, W.T. Chemical or Genetic Alteration of Proton Motive Force Results in Loss of Virulence of Burkholderia glumae, the Cause of Rice Bacterial Panicle Blight. Appl. Environ. Microbiol. 2021, 87, e0091521. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.A.; Dutta, S.; Lee, Y.H. Biocontrol of bacterial seedling rot of rice plants using combination of Cytobacillus firmus JBRS159 and silicon. PLoS ONE 2023, 18, e0290049. [Google Scholar] [CrossRef]
- Supina, B.S.I.; McCutcheon, J.G.; Peskett, S.R.; Stothard, P.; Dennis, J.J. A flagella-dependent Burkholderia jumbo phage controls rice seedling rot and steers Burkholderia glumae toward reduced virulence in rice seedlings. mBio 2025, 16, e0281424. [Google Scholar] [CrossRef] [PubMed]
- Reis, V.M.; Santos, P.E.L.; Tenorio-Salgado, S.; Vogel, J.; Stoffels, M.; Guyon, S.; Mavingui, P.; Baldani, V.L.D.; Schmid, M.; Baldani, J.I.; et al. Burkholderia tropica sp. nov., a novel nitrogen-fixing, plant-associated bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 2155–2162. [Google Scholar] [CrossRef]
- Barelmann, I.; Meyer, J.-M.; Taraz, K.; Budzikiewicz, H. Cepaciachelin, a new catecholate siderophore from Burkholderia (Pseudomonas) cepacia. Z. Für Naturforschung C 1996, 51, 627–630. [Google Scholar] [CrossRef]
- Esmaeel, Q.; Pupin, M.; Kieu, N.P.; Chataigne, G.; Bechet, M.; Deravel, J.; Krier, F.; Hofte, M.; Jacques, P.; Leclere, V. Burkholderia genome mining for nonribosomal peptide synthetases reveals a great potential for novel siderophores and lipopeptides synthesis. Microbiologyopen 2016, 5, 512–526. [Google Scholar] [CrossRef]
- Deng, P.; Foxfire, A.; Xu, J.; Baird, S.M.; Jia, J.; Delgado, K.H.; Shin, R.; Smith, L.; Lu, S.E. The Siderophore Product Ornibactin Is Required for the Bactericidal Activity of Burkholderia contaminans MS14. Appl. Environ. Microbiol. 2017, 83, e00051-17. [Google Scholar] [CrossRef]
- Sokol, P.A.; Darling, P.; Woods, D.E.; Mahenthiralingam, E.; Kooi, C. Role of ornibactin biosynthesis in the virulence of Burkholderia cepacia: Characterization of pvdA, the gene encoding L-ornithine N(5)-oxygenase. Infect. Immun. 1999, 67, 4443–4455. [Google Scholar] [CrossRef] [PubMed]
- Alice, A.F.; Lopez, C.S.; Lowe, C.A.; Ledesma, M.A.; Crosa, J.H. Genetic and transcriptional analysis of the siderophore malleobactin biosynthesis and transport genes in the human pathogen Burkholderia pseudomallei K96243. J. Bacteriol. 2006, 188, 1551–1566. [Google Scholar] [CrossRef]
- Franke, J.; Ishida, K.; Hertweck, C. Plasticity of the malleobactin pathway and its impact on siderophore action in human pathogenic bacteria. Chemistry 2015, 21, 8010–8014. [Google Scholar] [CrossRef]
- Franke, J.; Ishida, K.; Ishida-Ito, M.; Hertweck, C. Nitro versus hydroxamate in siderophores of pathogenic bacteria: Effect of missing hydroxylamine protection in malleobactin biosynthesis. Angew. Chem. Int. Ed. Engl. 2013, 52, 8271–8275. [Google Scholar] [CrossRef] [PubMed]
- Dashti, Y.; Nakou, I.T.; Mullins, A.J.; Webster, G.; Jian, X.; Mahenthiralingam, E.; Challis, G.L. Discovery and Biosynthesis of Bolagladins: Unusual Lipodepsipeptides from Burkholderia gladioli Clinical Isolates*. Angew. Chem. Int. Ed. Engl. 2020, 59, 21553–21561. [Google Scholar] [CrossRef] [PubMed]
- Hermenau, R.; Ishida, K.; Gama, S.; Hoffmann, B.; Pfeifer-Leeg, M.; Plass, W.; Mohr, J.F.; Wichard, T.; Saluz, H.P.; Hertweck, C. Gramibactin is a bacterial siderophore with a diazeniumdiolate ligand system. Nat. Chem. Biol. 2018, 14, 841–843. [Google Scholar] [CrossRef]
- Gama, S.; Hermenau, R.; Frontauria, M.; Milea, D.; Sammartano, S.; Hertweck, C.; Plass, W. Iron Coordination Properties of Gramibactin as Model for the New Class of Diazeniumdiolate Based Siderophores. Chemistry 2021, 27, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Hermenau, R.; Mehl, J.L.; Ishida, K.; Dose, B.; Pidot, S.J.; Stinear, T.P.; Hertweck, C. Genomics-Driven Discovery of NO-Donating Diazeniumdiolate Siderophores in Diverse Plant-Associated Bacteria. Angew. Chem. Int. Ed. Engl. 2019, 58, 13024–13029. [Google Scholar] [CrossRef]
- Song, L.; Jenner, M.; Masschelein, J.; Jones, C.; Bull, M.J.; Harris, S.R.; Hartkoorn, R.C.; Vocat, A.; Romero-Canelon, I.; Coupland, P.; et al. Discovery and Biosynthesis of Gladiolin: A Burkholderia gladioli Antibiotic with Promising Activity against Mycobacterium tuberculosis. J. Am. Chem. Soc. 2017, 139, 7974–7981. [Google Scholar] [CrossRef]
- Heath, N.L.; Rowlands, R.S.; Webster, G.; Mahenthiralingam, E.; Beeton, M.L. Antimicrobial activity of enacyloxin IIa and gladiolin against the urogenital pathogens Neisseria gonorrhoeae and Ureaplasma spp. J. Appl. Microbiol. 2021, 130, 1546–1551. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Song, L.; Sass, A.; White, J.; Wilmot, C.; Marchbank, A.; Boaisha, O.; Paine, J.; Knight, D.; Challis, G.L. Enacyloxins are products of an unusual hybrid modular polyketide synthase encoded by a cryptic Burkholderia ambifaria Genomic Island. Chem. Biol. 2011, 18, 665–677. [Google Scholar] [CrossRef]
- Jones, C.; Webster, G.; Mullins, A.J.; Jenner, M.; Bull, M.J.; Dashti, Y.; Spilker, T.; Parkhill, J.; Connor, T.R.; LiPuma, J.J.; et al. Kill and cure: Genomic phylogeny and bioactivity of Burkholderia gladioli bacteria capable of pathogenic and beneficial lifestyles. Microb. Genom. 2021, 7, 000515. [Google Scholar] [CrossRef]
- Nakou, I.T.; Jenner, M.; Dashti, Y.; Romero-Canelon, I.; Masschelein, J.; Mahenthiralingam, E.; Challis, G.L. Genomics-Driven Discovery of a Novel Glutarimide Antibiotic from Burkholderia gladioli Reveals an Unusual Polyketide Synthase Chain Release Mechanism. Angew. Chem. Int. Ed. Engl. 2020, 59, 23145–23153. [Google Scholar] [CrossRef]
- Niehs, S.P.; Kumpfmuller, J.; Dose, B.; Little, R.F.; Ishida, K.; Florez, L.V.; Kaltenpoth, M.; Hertweck, C. Insect-Associated Bacteria Assemble the Antifungal Butenolide Gladiofungin by Non-Canonical Polyketide Chain Termination. Angew. Chem. Int. Ed. Engl. 2020, 59, 23122–23126. [Google Scholar] [CrossRef]
- Dose, B.; Niehs, S.P.; Scherlach, K.; Florez, L.V.; Kaltenpoth, M.; Hertweck, C. Unexpected Bacterial Origin of the Antibiotic Icosalide: Two-Tailed Depsipeptide Assembly in Multifarious Burkholderia Symbionts. ACS Chem. Biol. 2018, 13, 2414–2420. [Google Scholar] [CrossRef]
- Jenner, M.; Jian, X.; Dashti, Y.; Masschelein, J.; Hobson, C.; Roberts, D.M.; Jones, C.; Harris, S.; Parkhill, J.; Raja, H.A.; et al. An unusual Burkholderia gladioli double chain-initiating nonribosomal peptide synthetase assembles ‘fungal’ icosalide antibiotics. Chem. Sci. 2019, 10, 5489–5494. [Google Scholar] [CrossRef]
- Tawfik, K.A.; Jeffs, P.; Bray, B.; Dubay, G.; Falkinham, J.O.; Mesbah, M.; Youssef, D.; Khalifa, S.; Schmidt, E.W. Burkholdines 1097 and 1229, potent antifungal peptides from Burkholderia ambifaria 2.2N. Org. Lett. 2010, 12, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Konno, H.; Sasaki, M.; Sano, H.; Osawa, K.; Nosaka, K.; Yano, S. The Hydrophobicity and Antifungal Potentiation of Burkholdine Analogues. Molecules 2022, 27, 1191. [Google Scholar] [CrossRef] [PubMed]
- el-Banna, N.; Winkelmann, G. Pyrrolnitrin from Burkholderia cepacia: Antibiotic activity against fungi and novel activities against streptomycetes. J. Appl. Microbiol. 1998, 85, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Blom, J.F.; Pernthaler, J.; Berg, G.; Baldwin, A.; Mahenthiralingam, E.; Eberl, L. Production of the antifungal compound pyrrolnitrin is quorum sensing-regulated in members of the Burkholderia cepacia complex. Environ. Microbiol. 2009, 11, 1422–1437. [Google Scholar] [CrossRef]
- Zaman, N.R.; Chowdhury, U.F.; Reza, R.N.; Chowdhury, F.T.; Sarker, M.; Hossain, M.M.; Akbor, M.A.; Amin, A.; Islam, M.R.; Khan, H. Plant growth promoting endophyte Burkholderia contaminans NZ antagonizes phytopathogen Macrophomina phaseolina through melanin synthesis and pyrrolnitrin inhibition. PLoS ONE 2021, 16, e0257863. [Google Scholar] [CrossRef]
- Pawar, S.; Chaudhari, A.; Prabha, R.; Shukla, R.; Singh, D.P. Microbial Pyrrolnitrin: Natural Metabolite with Immense Practical Utility. Biomolecules 2019, 9, 443. [Google Scholar] [CrossRef]
- Mullins, A.J.; Murray, J.A.H.; Bull, M.J.; Jenner, M.; Jones, C.; Webster, G.; Green, A.E.; Neill, D.R.; Connor, T.R.; Parkhill, J.; et al. Genome mining identifies cepacin as a plant-protective metabolite of the biopesticidal bacterium Burkholderia ambifaria. Nat. Microbiol. 2019, 4, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Moon, K.; Miller, C.; Rose, J.; Xu, F.; Ebmeier, C.C.; Jacobsen, J.R.; Mao, D.; Old, W.M.; DeShazer, D.; et al. Thailandenes, Cryptic Polyene Natural Products Isolated from Burkholderia thailandensis Using Phenotype-Guided Transposon Mutagenesis. ACS Chem. Biol. 2020, 15, 1195–1203. [Google Scholar] [CrossRef]
- Nguyen, T.; Ishida, K.; Jenke-Kodama, H.; Dittmann, E.; Gurgui, C.; Hochmuth, T.; Taudien, S.; Platzer, M.; Hertweck, C.; Piel, J. Exploiting the mosaic structure of trans-acyltransferase polyketide synthases for natural product discovery and pathway dissection. Nat. Biotechnol. 2008, 26, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, C.E.; Lin, Z.; Schmidt, E.W.; Hughes, K.T.; Liou, T.G. Thailandamide, a Fatty Acid Synthesis Antibiotic That Is Coexpressed with a Resistant Target Gene. Antimicrob. Agents Chemother. 2018, 62, e00463-18. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Lincke, T.; Behnken, S.; Hertweck, C. Induced biosynthesis of cryptic polyketide metabolites in a Burkholderia thailandensis quorum sensing mutant. J. Am. Chem. Soc. 2010, 132, 13966–13968. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R.; Chandler, J.R.; Blodgett, J.A.; Lima, P.S.; Duerkop, B.A.; Oinuma, K.; Greenberg, E.P.; Clardy, J. Quorum-sensing-regulated bactobolin production by Burkholderia thailandensis E264. Org. Lett. 2010, 12, 716–719. [Google Scholar] [CrossRef]
- Chandler, J.R.; Truong, T.T.; Silva, P.M.; Seyedsayamdost, M.R.; Carr, G.; Radey, M.; Jacobs, M.A.; Sims, E.H.; Clardy, J.; Greenberg, E.P. Bactobolin resistance is conferred by mutations in the L2 ribosomal protein. mBio 2012, 3, e00499-12. [Google Scholar] [CrossRef]
- Vial, L.; Lepine, F.; Milot, S.; Groleau, M.C.; Dekimpe, V.; Woods, D.E.; Deziel, E. Burkholderia pseudomallei, B. thailandensis, and B. ambifaria produce 4-hydroxy-2-alkylquinoline analogues with a methyl group at the 3 position that is required for quorum-sensing regulation. J. Bacteriol. 2008, 190, 5339–5352. [Google Scholar] [CrossRef]
- Wu, Y.; Seyedsayamdost, M.R. Synergy and Target Promiscuity Drive Structural Divergence in Bacterial Alkylquinolone Biosynthesis. Cell Chem. Biol. 2017, 24, 1437–1444 e1433. [Google Scholar] [CrossRef]
- Lu, S.E.; Novak, J.; Austin, F.W.; Gu, G.; Ellis, D.; Kirk, M.; Wilson-Stanford, S.; Tonelli, M.; Smith, L. Occidiofungin, a unique antifungal glycopeptide produced by a strain of Burkholderia contaminans. Biochemistry 2009, 48, 8312–8321. [Google Scholar] [CrossRef]
- Gu, G.; Smith, L.; Liu, A.; Lu, S.E. Genetic and biochemical map for the biosynthesis of occidiofungin, an antifungal produced by Burkholderia contaminans strain MS14. Appl. Environ. Microbiol. 2011, 77, 6189–6198. [Google Scholar] [CrossRef]
- Wang, X.Q.; Liu, A.X.; Guerrero, A.; Liu, J.; Yu, X.Q.; Deng, P.; Ma, L.; Baird, S.M.; Smith, L.; Li, X.D.; et al. Occidiofungin is an important component responsible for the antifungal activity of Burkholderia pyrrocinia strain Lyc2. J. Appl. Microbiol. 2016, 120, 607–618. [Google Scholar] [CrossRef]
- Hansanant, N.; Smith, L. Occidiofungin: Actin Binding as a Novel Mechanism of Action in an Antifungal Agent. Antibiot. (Basel) 2022, 11, 1143. [Google Scholar] [CrossRef]
- Hansanant, N.; Cao, K.; Tenorio, A.; Joseph, T.; Ju, M.; McNally, N.; Kummari, E.; Williams, M.; Cothrell, A.; Buhrow, A.R.; et al. Previously Uncharacterized Variants, OCF-E-OCF-J, of the Antifungal Occidiofungin Produced by Burkholderia contaminans MS14. J. Nat. Prod. 2024, 87, 186–194. [Google Scholar] [CrossRef]
- Moebius, N.; Ross, C.; Scherlach, K.; Rohm, B.; Roth, M.; Hertweck, C. Biosynthesis of the respiratory toxin bongkrekic acid in the pathogenic bacterium Burkholderia gladioli. Chem. Biol. 2012, 19, 1164–1174. [Google Scholar] [CrossRef]
- Rohm, B.; Scherlach, K.; Hertweck, C. Biosynthesis of the mitochondrial adenine nucleotide translocase (ATPase) inhibitor bongkrekic acid in Burkholderia gladioli. Org. Biomol. Chem. 2010, 8, 1520–1522. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, J.; Kim, S.; Kang, Y.; Nagamatsu, T.; Hwang, I. Toxoflavin Produced by Burkholderia glumae Causing Rice Grain Rot Is Responsible for Inducing Bacterial Wilt in Many Field Crops. Plant Dis. 2003, 87, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Wang, R.; Wang, Q.; Lu, L. Toxoflavin Produced by Burkholderia gladioli from Lycoris aurea Is a New Broad-Spectrum Fungicide. Appl. Environ. Microbiol. 2019, 85, e00106-19. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F.; Sawada, H.; Azegami, K.; Tsuchiya, K. Molecular characterization of the tox operon involved in toxoflavin biosynthesis of Burkholderia glumae. J. Gen. Plant. Pathol. 2004, 70, 97–107. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.G.; Kang, Y.; Jang, J.Y.; Jog, G.J.; Lim, J.Y.; Kim, S.; Suga, H.; Nagamatsu, T.; Hwang, I. Quorum sensing and the LysR-type transcriptional activator ToxR regulate toxoflavin biosynthesis and transport in Burkholderia glumae. Mol. Microbiol. 2004, 54, 921–934. [Google Scholar] [CrossRef]
- Kim, N.; Lee, D.; Lee, S.B.; Lim, G.H.; Kim, S.W.; Kim, T.J.; Park, D.S.; Seo, Y.S. Understanding Burkholderia glumae BGR1 Virulence through the Application of Toxoflavin-Degrading Enzyme, TxeA. Plants 2023, 12, 3934. [Google Scholar] [CrossRef] [PubMed]
- Biggins, J.B.; Ternei, M.A.; Brady, S.F. Malleilactone, a polyketide synthase-derived virulence factor encoded by the cryptic secondary metabolome of Burkholderia pseudomallei group pathogens. J. Am. Chem. Soc. 2012, 134, 13192–13195. [Google Scholar] [CrossRef] [PubMed]
- Klaus, J.R.; Deay, J.; Neuenswander, B.; Hursh, W.; Gao, Z.; Bouddhara, T.; Williams, T.D.; Douglas, J.; Monize, K.; Martins, P.; et al. Malleilactone Is a Burkholderia pseudomallei Virulence Factor Regulated by Antibiotics and Quorum Sensing. J. Bacteriol. 2018, 200, e00008-18. [Google Scholar] [CrossRef]
- Liu, X.; Biswas, S.; Berg, M.G.; Antapli, C.M.; Xie, F.; Wang, Q.; Tang, M.C.; Tang, G.L.; Zhang, L.; Dreyfuss, G.; et al. Genomics-guided discovery of thailanstatins A, B, and C As pre-mRNA splicing inhibitors and antiproliferative agents from Burkholderia thailandensis MSMB43. J. Nat. Prod. 2013, 76, 685–693. [Google Scholar] [CrossRef]
- Eustaquio, A.S.; Janso, J.E.; Ratnayake, A.S.; O’Donnell, C.J.; Koehn, F.E. Spliceostatin hemiketal biosynthesis in Burkholderia spp. is catalyzed by an iron/alpha-ketoglutarate-dependent dioxygenase. Proc. Natl. Acad. Sci. USA 2014, 111, E3376–E3385. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P.; Hertweck, C. A gene cluster encoding rhizoxin biosynthesis in “Burkholderia rhizoxina”, the bacterial endosymbiont of the fungus Rhizopus microsporus. Chembiochem 2007, 8, 41–45. [Google Scholar] [CrossRef]
- Hanauske, A.R.; Catimel, G.; Aamdal, S.; ten Bokkel Huinink, W.; Paridaens, R.; Pavlidis, N.; Kaye, S.B.; te Velde, A.; Wanders, J.; Verweij, J. Phase II clinical trials with rhizoxin in breast cancer and melanoma. The EORTC Early Clinical Trials Group. Br. J. Cancer 1996, 73, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Lackner, G.; Moebius, N.; Partida-Martinez, L.P.; Boland, S.; Hertweck, C. Evolution of an endofungal lifestyle: Deductions from the Burkholderia rhizoxinica genome. BMC Genom. 2011, 12, 210. [Google Scholar] [CrossRef]
- Petrova, Y.D.; Mahenthiralingam, E. Discovery, mode of action and secretion of Burkholderia sensu lato key antimicrobial specialised metabolites. Cell Surf. 2022, 8, 100081. [Google Scholar] [CrossRef]
- Funston, S.J.; Tsaousi, K.; Rudden, M.; Smyth, T.J.; Stevenson, P.S.; Marchant, R.; Banat, I.M. Characterising rhamnolipid production in Burkholderia thailandensis E264, a non-pathogenic producer. Appl. Microbiol. Biotechnol. 2016, 100, 7945–7956. [Google Scholar] [CrossRef]
- Costa, S.G.; Deziel, E.; Lepine, F. Characterization of rhamnolipid production by Burkholderia glumae. Lett. Appl. Microbiol. 2011, 53, 620–627. [Google Scholar] [CrossRef]
- Elshikh, M.; Moya-Ramirez, I.; Moens, H.; Roelants, S.; Soetaert, W.; Marchant, R.; Banat, I.M. Rhamnolipids and lactonic sophorolipids: Natural antimicrobial surfactants for oral hygiene. J. Appl. Microbiol. 2017, 123, 1111–1123. [Google Scholar] [CrossRef]
- Dubeau, D.; Deziel, E.; Woods, D.E.; Lepine, F. Burkholderia thailandensis harbors two identical rhl gene clusters responsible for the biosynthesis of rhamnolipids. BMC Microbiol. 2009, 9, 263. [Google Scholar] [CrossRef]
- Foxfire, A.; Buhrow, A.R.; Orugunty, R.S.; Smith, L. Drug discovery through the isolation of natural products from Burkholderia. Expert. Opin. Drug. Discov. 2021, 16, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Bendova, K.; Raclavsky, V.; Novotny, R.; Luptakova, D.; Popper, M.; Novy, Z.; Hajduch, M.; Petrik, M. [(68)Ga]Ga-Ornibactin for Burkholderia cepacia complex Infection Imaging Using Positron Emission Tomography. J. Med. Chem. 2023, 66, 7584–7593. [Google Scholar] [CrossRef]
- Kvitko, B.H.; Goodyear, A.; Propst, K.L.; Dow, S.W.; Schweizer, H.P. Burkholderia pseudomallei known siderophores and hemin uptake are dispensable for lethal murine melioidosis. PLoS Negl. Trop. Dis. 2012, 6, e1715. [Google Scholar] [CrossRef]
- Makris, C.; Carmichael, J.R.; Zhou, H.; Butler, A. C-Diazeniumdiolate Graminine in the Siderophore Gramibactin Is Photoreactive and Originates from Arginine. ACS Chem. Biol. 2022, 17, 3140–3147. [Google Scholar] [CrossRef]
- Jian, X.; Pang, F.; Hobson, C.; Jenner, M.; Alkhalaf, L.M.; Challis, G.L. Antibiotic Skeletal Diversification via Differential Enoylreductase Recruitment and Module Iteration in trans-Acyltransferase Polyketide Synthases. J. Am. Chem. Soc. 2024, 146, 6114–6124. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bai, X.; Sun, T.; Wang, X.; Zhang, Y.; Bian, X.; Zhou, H. The Genomic-Driven Discovery of Glutarimide-Containing Derivatives from Burkholderia gladioli. Molecules 2023, 28, 6937. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Falkinham, J.O., 3rd; Tawfik, K.A.; Jeffs, P.; Bray, B.; Dubay, G.; Cox, J.E.; Schmidt, E.W. Burkholdines from Burkholderia ambifaria: Antifungal agents and possible virulence factors. J. Nat. Prod. 2012, 75, 1518–1523. [Google Scholar] [CrossRef]
- Hwang, J.; Chilton, W.S.; Benson, D.M. Pyrrolnitrin production by Burkholderia cepacia and biocontrol of Rhizoctonia stem rot of poinsettia. Biol. Control 2002, 25, 56–63. [Google Scholar] [CrossRef]
- Sultan, Z.; Park, K.; Lee, S.Y.; Park, J.K.; Varughese, T.; Moon, S.S. Novel oxidized derivatives of antifungal pyrrolnitrin from the bacterium Burkholderia cepacia K87. J. Antibiot. 2008, 61, 420–425. [Google Scholar] [CrossRef]
- Webster, G.; Mullins, A.J.; Petrova, Y.D.; Mahenthiralingam, E. Polyyne-producing Burkholderia suppress Globisporangium ultimum damping-off disease of Pisum sativum (pea). Front. Microbiol. 2023, 14, 1240206. [Google Scholar] [CrossRef]
- Wu, Y.; Seyedsayamdost, M.R. The Polyene Natural Product Thailandamide A Inhibits Fatty Acid Biosynthesis in Gram-Positive and Gram-Negative Bacteria. Biochemistry 2018, 57, 4247–4251. [Google Scholar] [CrossRef] [PubMed]
- Majerczyk, C.; Brittnacher, M.; Jacobs, M.; Armour, C.D.; Radey, M.; Schneider, E.; Phattarasokul, S.; Bunt, R.; Greenberg, E.P. Global analysis of the Burkholderia thailandensis quorum sensing-controlled regulon. J. Bacteriol. 2014, 196, 1412–1424. [Google Scholar] [CrossRef]
- Mao, D.; Bushin, L.B.; Moon, K.; Wu, Y.; Seyedsayamdost, M.R. Discovery of scmR as a global regulator of secondary metabolism and virulence in Burkholderia thailandensis E264. Proc. Natl. Acad. Sci. USA 2017, 114, E2920–E2928. [Google Scholar] [CrossRef] [PubMed]
- Klaus, J.R.; Coulon, P.M.L.; Koirala, P.; Seyedsayamdost, M.R.; Deziel, E.; Chandler, J.R. Secondary metabolites from the Burkholderia pseudomallei complex: Structure, ecology, and evolution. J. Ind. Microbiol. Biotechnol. 2020, 47, 877–887. [Google Scholar] [CrossRef]
- Piochon, M.; Coulon, P.M.L.; Caulet, A.; Groleau, M.C.; Deziel, E.; Gauthier, C. Synthesis and Antimicrobial Activity of Burkholderia-Related 4-Hydroxy-3-methyl-2-alkenylquinolines (HMAQs) and Their N-Oxide Counterparts. J. Nat. Prod. 2020, 83, 2145–2154. [Google Scholar] [CrossRef]
- Le Guillouzer, S.; Groleau, M.C.; Mauffrey, F.; Deziel, E. ScmR, a Global Regulator of Gene Expression, Quorum Sensing, pH Homeostasis, and Virulence in Burkholderia thailandensis. J. Bacteriol. 2020, 202, e00776-19. [Google Scholar] [CrossRef]
- Williams, M.D.; Sweeney, T.R.; Trieu, S.; Orugunty, R.; Barbour, A.; Younesi, F.; Glogauer, M.; Hansanant, N.; Shin, R.; Lu, S.E.; et al. Antibiofilm properties of 4-hydroxy-3-methyl-2-alkenylquinoline, a novel Burkholderia-derived alkaloid. mSphere 2025, 10, e0108124. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Gosai, J.; Emrick, C.; Heintz, R.; Romans, L.; Gordon, D.; Lu, S.E.; Austin, F.; Smith, L. Occidiofungin’s chemical stability and in vitro potency against Candida species. Antimicrob. Agents Chemother. 2012, 56, 765–769. [Google Scholar] [CrossRef]
- Jia, J.; Ford, E.; Hobbs, S.M.; Baird, S.M.; Lu, S.E. Occidiofungin Is the Key Metabolite for Antifungal Activity of the Endophytic Bacterium Burkholderia sp. MS455 Against Aspergillus flavus. Phytopathology 2022, 112, 481–491. [Google Scholar] [CrossRef]
- Chen, K.C.; Ravichandran, A.; Guerrero, A.; Deng, P.; Baird, S.M.; Smith, L.; Lu, S.E. The Burkholderia contaminans MS14 ocfC gene encodes a xylosyltransferase for production of the antifungal occidiofungin. Appl. Environ. Microbiol. 2013, 79, 2899–2905. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, J.L.; Hsueh, P.R. Burkholderia gladioli and bongkrekic acid: An under-recognized foodborne poisoning outbreak. J. Infect. 2024, 89, 106182. [Google Scholar] [CrossRef]
- Han, D.; Chen, J.; Chen, W.; Wang, Y. Bongkrekic Acid and Burkholderia gladioli pathovar cocovenenans: Formidable Foe and Ascending Threat to Food Safety. Foods 2023, 12, 3926. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Chen, L.; Liu, Z.; Liang, J.; Shi, M.; Gao, F.; Song, Y.; Chen, J.; Fu, P. Epidemiology of foodborne bongkrekic acid poisoning outbreaks in China, 2010 to 2020. PLoS ONE 2023, 18, e0279957. [Google Scholar] [CrossRef]
- Rivera Blanco, L.E.; Kuai, D.; Titelbaum, N.; Fiza, B.; Reehl, D.; Hassan, Z.; Dbouk, N.; Krotulski, A.J.; Logan, B.K.; Walton, S.E.; et al. Death from bongkrekic acid toxicity: First report in North America. Toxicol. Commun. 2024, 8. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, R.; Liang, Q.; Song, L.; Huang, J.; Lang, N.; Zhou, J. A Foodborne Bongkrekic Acid Poisoning Incident—Heilongjiang Province, 2020. China CDC Wkly. 2020, 2, 975–978. [Google Scholar] [CrossRef]
- Su, Y.J. The first time devastating food poisoning happened in Taiwan—Bongkrekic acid poisoning. Taiwan. J. Obstet. Gynecol. 2024, 63, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Gudo, E.S.; Cook, K.; Kasper, A.M.; Vergara, A.; Salomao, C.; Oliveira, F.; Ismael, H.; Saeze, C.; Mosse, C.; Fernandes, Q.; et al. Description of a Mass Poisoning in a Rural District in Mozambique: The First Documented Bongkrekic Acid Poisoning in Africa. Clin. Infect. Dis. 2018, 66, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Falconer, T.M.; Kern, S.E.; Brzezinski, J.L.; Turner, J.A.; Boyd, B.L.; Litzau, J.J. Identification of the potent toxin bongkrekic acid in a traditional African beverage linked to a fatal outbreak. Forensic. Sci. Int. 2017, 270, e5–e11. [Google Scholar] [CrossRef]
- Anwar, M.; Kasper, A.; Steck, A.R.; Schier, J.G. Bongkrekic Acid-a Review of a Lesser-Known Mitochondrial Toxin. J. Med. Toxicol. 2017, 13, 173–179. [Google Scholar] [CrossRef]
- Zhou, B.; Li, H.L.; Ma, J.; Dong, F.; Yu, Y. Fast determination of bongkrekic acid in plasma by high performance liquid chromatography-tandem mass spectrometry. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing. Za Zhi 2022, 40, 219–221. [Google Scholar] [CrossRef]
- Ruprecht, J.J.; King, M.S.; Zogg, T.; Aleksandrova, A.A.; Pardon, E.; Crichton, P.G.; Steyaert, J.; Kunji, E.R.S. The Molecular Mechanism of Transport by the Mitochondrial ADP/ATP Carrier. Cell 2019, 176, 435–447 e415. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.; Kim, S.; Park, I.; Seo, Y.S. Differential regulation of toxoflavin production and its role in the enhanced virulence of Burkholderia gladioli. Mol. Plant. Pathol. 2016, 17, 65–76. [Google Scholar] [CrossRef]
- Fenwick, M.K.; Almabruk, K.H.; Ealick, S.E.; Begley, T.P.; Philmus, B. Biochemical Characterization and Structural Basis of Reactivity and Regioselectivity Differences between Burkholderia thailandensis and Burkholderia glumae 1,6-Didesmethyltoxoflavin N-Methyltransferase. Biochemistry 2017, 56, 3934–3944. [Google Scholar] [CrossRef]
- Song, K.; Li, W.; Zhao, Z.; Li, H.; Liu, Y.; Zhao, G.; He, H.Y.; Du, Y.L. Heterologous Reconstitution of Toxoflavin Biosynthesis Reveals Key Pathway Intermediates and a Cofactor-Independent Oxidase. Org. Lett. 2023, 25, 2918–2922. [Google Scholar] [CrossRef]
- Choi, J.E.; Nguyen, C.M.; Lee, B.; Park, J.H.; Oh, J.Y.; Choi, J.S.; Kim, J.C.; Song, J.K. Isolation and characterization of a novel metagenomic enzyme capable of degrading bacterial phytotoxin toxoflavin. PLoS ONE 2018, 13, e0183893. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.T.; Seyedsayamdost, M.; Greenberg, E.P.; Chandler, J.R. A Burkholderia thailandensis Acyl-Homoserine Lactone-Independent Orphan LuxR Homolog That Activates Production of the Cytotoxin Malleilactone. J. Bacteriol. 2015, 197, 3456–3462. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, H.; Biswas, S.; Cheng, Y.Q. Improved production of cytotoxic thailanstatins A and D through metabolic engineering of Burkholderia thailandensis MSMB43 and pilot scale fermentation. Synth. Syst. Biotechnol. 2016, 1, 34–38. [Google Scholar] [CrossRef]
- Eustaquio, A.S.; Chang, L.P.; Steele, G.L.; CJ, O.D.; Koehn, F.E. Biosynthetic engineering and fermentation media development leads to gram-scale production of spliceostatin natural products in Burkholderia sp. Metab. Eng. 2016, 33, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Partida-Martinez, L.P.; Hertweck, C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 2005, 437, 884–888. [Google Scholar] [CrossRef]
- Lackner, G.; Moebius, N.; Hertweck, C. Endofungal bacterium controls its host by an hrp type III secretion system. ISME J. 2011, 5, 252–261. [Google Scholar] [CrossRef]
- Lackner, G.; Moebius, N.; Partida-Martinez, L.; Hertweck, C. Complete genome sequence of Burkholderia rhizoxinica, an Endosymbiont of Rhizopus microsporus. J. Bacteriol. 2011, 193, 783–784. [Google Scholar] [CrossRef]
- Scherlach, K.; Partida-Martinez, L.P.; Dahse, H.M.; Hertweck, C. Antimitotic rhizoxin derivatives from a cultured bacterial endosymbiont of the rice pathogenic fungus Rhizopus microsporus. J. Am. Chem. Soc. 2006, 128, 11529–11536. [Google Scholar] [CrossRef] [PubMed]
- Nickzad, A.; Guertin, C.; Déziel, E. Culture Medium Optimization for Production of Rhamnolipids by Burkholderia glumae. Colloids Interfaces 2018, 2, 49. [Google Scholar] [CrossRef]
- Tavares, L.F.; Silva, P.M.; Junqueira, M.; Mariano, D.C.; Nogueira, F.C.; Domont, G.B.; Freire, D.M.; Neves, B.C. Characterization of rhamnolipids produced by wild-type and engineered Burkholderia kururiensis. Appl. Microbiol. Biotechnol. 2013, 97, 1909–1921. [Google Scholar] [CrossRef]
- Hörmann, B.; Müller, M.M.; Syldatk, C.; Hausmann, R. Rhamnolipid production by Burkholderia plantarii DSM 9509T. Eur. J. Lipid Sci. Technol. 2010, 112, 674–680. [Google Scholar] [CrossRef]
- Kabeil, S.S.A.; Darwish, A.M.G.; Abdelgalil, S.A.; Shamseldin, A.; Salah, A.; Taha, H.; Bashir, S.I.; Hafez, E.E.; El-Enshasy, H.A. Rhamnolipids bio-production and miscellaneous applications towards green technologies: A literature review. PeerJ 2025, 13, e18981. [Google Scholar] [CrossRef]
- David, E.; Niculescu, V.C. Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials. Int. J. Environ. Res. Public. Health 2021, 18, 13147. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, J.; Chen, A.J.; Wang, L.; Jiang, X.; Gong, A.; Liu, W.; Wu, H. Burkholderia ambifaria H8 as an effective biocontrol strain against maize stalk rot via producing volatile dimethyl disulfide. Pest. Manag. Sci. 2024, 80, 4125–4136. [Google Scholar] [CrossRef]
- Teng, Z.J.; Qin, Q.L.; Zhang, W.; Li, J.; Fu, H.H.; Wang, P.; Lan, M.; Luo, G.; He, J.; McMinn, A.; et al. Biogeographic traits of dimethyl sulfide and dimethylsulfoniopropionate cycling in polar oceans. Microbiome 2021, 9, 207. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, P.; Bai, B.; Bai, F.; Jin, T.; Ren, J. Volatile Organic Compounds of Endophytic Burkholderia pyrrocinia Strain JK-SH007 Promote Disease Resistance in Poplar. Plant Dis. 2020, 104, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Xiang, W.; Cao, K.X.; Lu, X.; Yao, S.C.; Hung, D.; Huang, R.S.; Li, L.B. Characterization of Volatile Organic Compounds Emitted from Endophytic Burkholderia cenocepacia ETR-B22 by SPME-GC-MS and Their Inhibitory Activity against Various Plant Fungal Pathogens. Molecules 2020, 25, 3765. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Jiang, Y.; Cui, S.; Hu, J.; Wei, Y.; Li, J.; Wu, Y. Volatile Organic Compounds Produced by Co-Culture of Burkholderia vietnamiensis B418 with Trichoderma harzianum T11-W Exhibits Improved Antagonistic Activities against Fungal Phytopathogens. Int. J. Mol. Sci. 2024, 25, 11097. [Google Scholar] [CrossRef]
- Papazlatani, C.; Wagner, A.; Chen, Z.; Zweers, H.; de Boer, W.; Garbeva, P. Enhancement of production of pathogen-suppressing volatiles using amino acids. Curr. Res. Microb. Sci. 2025, 8, 100385. [Google Scholar] [CrossRef] [PubMed]
- Inglis, T.J.; Hahne, D.R.; Merritt, A.J.; Clarke, M.W. Volatile-sulfur-compound profile distinguishes Burkholderia pseudomallei from Burkholderia thailandensis. J. Clin. Microbiol. 2015, 53, 1009–1011. [Google Scholar] [CrossRef]
- Groenhagen, U.; Baumgartner, R.; Bailly, A.; Gardiner, A.; Eberl, L.; Schulz, S.; Weisskopf, L. Production of bioactive volatiles by different Burkholderia ambifaria strains. J. Chem. Ecol. 2013, 39, 892–906. [Google Scholar] [CrossRef]
- Diyapoglu, A.; Chang, T.H.; Chang, P.L.; Yen, J.H.; Chiang, H.I.; Meng, M. Fumigant Activity of Bacterial Volatile Organic Compounds against the Nematodes Caenorhabditis elegans and Meloidogyne incognita. Molecules 2022, 27, 4714. [Google Scholar] [CrossRef]
- Diyapoglu, A.; Oner, M.; Meng, M. Application Potential of Bacterial Volatile Organic Compounds in the Control of Root-Knot Nematodes. Molecules 2022, 27, 4355. [Google Scholar] [CrossRef]
- Luo, H.; Riu, M.; Ryu, C.M.; Yu, J.M. Volatile organic compounds emitted by Burkholderia pyrrocinia CNUC9 trigger induced systemic salt tolerance in Arabidopsis thaliana. Front. Microbiol. 2022, 13, 1050901. [Google Scholar] [CrossRef]
- Lin, Y.T.; Lee, C.C.; Leu, W.M.; Wu, J.J.; Huang, Y.C.; Meng, M. Fungicidal Activity of Volatile Organic Compounds Emitted by Burkholderia gladioli Strain BBB-01. Molecules 2021, 26, 745. [Google Scholar] [CrossRef]
- Rodriguez-Cisneros, M.; Morales-Ruiz, L.M.; Salazar-Gomez, A.; Rojas-Rojas, F.U.; Estrada-de Los Santos, P. Compilation of the Antimicrobial Compounds Produced by Burkholderia Sensu Stricto. Molecules 2023, 28, 1646. [Google Scholar] [CrossRef] [PubMed]
- Dugravot, S.; Grolleau, F.; Macherel, D.; Rochetaing, A.; Hue, B.; Stankiewicz, M.; Huignard, J.; Lapied, B. Dimethyl disulfide exerts insecticidal neurotoxicity through mitochondrial dysfunction and activation of insect K(ATP) channels. J. Neurophysiol. 2003, 90, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Inc., A. Paladin® EC Soil Fumigant, Active Ingredient Dimethyl Disulfide (DMDS). Available online: https://www.arkema.com/usa/en/product-range/thiochemicals/dmds-for-agricultural-soil-fumig/ (accessed on 6 May 2025).
- Agency, U.S.E.P. Soil fumigant labels—Dimethyl disulfide (DMDS). Available online: https://www.epa.gov/soil-fumigants/soil-fumigant-labels-dimethyl-disulfide-dmds (accessed on 31 December 2024).
- Carrion, O.; Curson, A.R.J.; Kumaresan, D.; Fu, Y.; Lang, A.S.; Mercade, E.; Todd, J.D. A novel pathway producing dimethylsulphide in bacteria is widespread in soil environments. Nat. Commun. 2015, 6, 6579. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.W.; Todd, J.D.; Sun, L.; Nikolaidou-Katsaridou, M.N.; Curson, A.R.; Rogers, R. Molecular diversity of bacterial production of the climate-changing gas, dimethyl sulphide, a molecule that impinges on local and global symbioses. J. Exp. Bot. 2008, 59, 1059–1067. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Huang, D.; Cheng, W.; Shao, Z.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Volatile Organic Compounds from Bacillus aryabhattai MCCC 1K02966 with Multiple Modes against Meloidogyne incognita. Molecules 2021, 27, 103. [Google Scholar] [CrossRef]
- Gondor, O.K.; Pal, M.; Janda, T.; Szalai, G. The role of methyl salicylate in plant growth under stress conditions. J. Plant. Physiol. 2022, 277, 153809. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Parke, J.L.; Gurian-Sherman, D. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu. Rev. Phytopathol. 2001, 39, 225–258. [Google Scholar] [CrossRef]
- Tavares, M.; Kozak, M.; Balola, A.; Sa-Correia, I. Burkholderia cepacia Complex Bacteria: A Feared Contamination Risk in Water-Based Pharmaceutical Products. Clin. Microbiol. Rev. 2020, 33, e00139-19. [Google Scholar] [CrossRef] [PubMed]
- Tomich, M.; Griffith, A.; Herfst, C.A.; Burns, J.L.; Mohr, C.D. Attenuated virulence of a Burkholderia cepacia type III secretion mutant in a murine model of infection. Infect. Immun. 2003, 71, 1405–1415. [Google Scholar] [CrossRef]
- Loeven, N.A.; Perault, A.I.; Cotter, P.A.; Hodges, C.A.; Schwartzman, J.D.; Hampton, T.H.; Bliska, J.B. The Burkholderia cenocepacia Type VI Secretion System Effector TecA Is a Virulence Factor in Mouse Models of Lung Infection. mBio 2021, 12, e0209821. [Google Scholar] [CrossRef]
- Rutledge, P.J.; Challis, G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Adaikpoh, B.I.; Fernandez, H.N.; Eustaquio, A.S. Biotechnology approaches for natural product discovery, engineering, and production based on Burkholderia bacteria. Curr. Opin. Biotechnol. 2022, 77, 102782. [Google Scholar] [CrossRef] [PubMed]
- Hemmerling, F.; Piel, J. Strategies to access biosynthetic novelty in bacterial genomes for drug discovery. Nat. Rev. Drug. Discov. 2022, 21, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Scoffone, V.C.; Trespidi, G.; Barbieri, G.; Irudal, S.; Israyilova, A.; Buroni, S. Methodological tools to study species of the genus Burkholderia. Appl. Microbiol. Biotechnol. 2021, 105, 9019–9034. [Google Scholar] [CrossRef]
- Covington, B.C.; Xu, F.; Seyedsayamdost, M.R. A Natural Product Chemist’s Guide to Unlocking Silent Biosynthetic Gene Clusters. Annu. Rev. Biochem. 2021, 90, 763–788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.