Abstract
Introduction: The World Health Organization has declared carbapenem-resistant organisms a research and development priority. Although ceftazidime–avibactam was approved around a decade ago, there is still a lack of prospective data on the treatment of resistant pathogens with this agent. Methods: We conducted a prospective, observational, single-center, investigator-initiated study of patients treated with ceftazidime–avibactam for infections caused by carbapenem-resistant organisms. The primary outcome was clinical cure 14 days after the initiation of ceftazidime-avibactam treatment. Secondary outcomes, which were assessed on day 30, included microbiological failure, development of resistance, all-cause mortality, and length of stay in the intensive care unit. Results: A total of 50 patients were included in the study. At baseline, the median Charlson Comorbidity Index and Sequential Organ Failure Assessment Score were 5.5 and 7. Approximately three-quarters of the patients were treated in an intensive care unit and had undergone mechanical ventilation within the previous 7 days prior to the commencement of ceftazidime–avibactam treatment. Half of the patients were diagnosed with nosocomial pneumonia. Most infections were caused by Pseudomonas aeruginosa (48%) and Klebsiella pneumonia (28%). Clinical cure at day 14 was achieved in 59% of patients. Four deaths (9%) and two cases of microbiological failure (4%) were observed. The median length of stay in the intensive care unit was 14 days. There was no emergence of resistance to ceftazidime–avibactam. Discussion: Our study contributes to the growing body of evidence supporting the effectiveness of ceftazidime–avibactam in treating infections caused by carbapenem-resistant organisms. In this cohort of critically ill patients, our results in terms of both clinical success and survival are in the upper range compared to those from mainly retrospective and some prospective studies. Although the benefits of ceftazidime–avibactam have been demonstrated in this and other studies, it must be prescribed cautiously to ensure it remains effective.
1. Introduction
In 2019, the World Health Organization (WHO) identified antimicrobial resistance (AMR) as one of the top ten global public health threats facing humanity [1]. The WHO also defined priority pathogens for which the research and development of new antibiotics are important. In the 2024 update, priority was given to carbapenem-resistant Acinetobacter (A.) baumanii, carbapenem-resistant Enterobacterales, and third-generation cephalosporin-resistant Enterobacterales [2]. Carbapenem-resistant organisms (CROs) have traditionally been treated with combinations of agents with high toxicity (aminoglycosides, colistin), suboptimal pharmacokinetics (aminoglycosides, colistin, tigecycline), and/or known microbiological resistance (carbapenems) [3]. Fortunately, several new β-lactam and β-lactam/β-lactamase inhibitor antibiotics have been developed and approved in recent years, but availability has not always been guaranteed. Meropenem–vaborbactam was licensed by the European Medicines Agency (EMA) in 2018 but has only been available since the end of 2024 in Germany. Imipenem–cilastatin–relebactam and cefiderocol have been available since 2021 but their use has been limited due to their high costs and the view that they should only be used as a last resort. In 2022, ceftolozane–tazobactam was no longer available in all markets worldwide due to a recall. This leaves ceftazidime–avibactam (C-A) as the only β-lactam/β-lactamase inhibitor that has been consistently available for the treatment of CROs in Germany in recent years.
EMA has licensed C-A for the treatment of complicated intra-abdominal infections (cIAIs), complicated urinary tract infections (cUTIs), hospital-acquired pneumonia (HAP), bacteremia associated with any of the infections listed above, and infections caused by aerobic Gram-negative organisms with limited treatment options [4]. C-A has been extensively evaluated. A systematic review and meta-analysis of randomized controlled trials (RCT) evaluating C-A versus a comparator for the treatment of any infection found no significant difference between C-A and the comparator (mostly carbapenem) for 30-day all-cause mortality, late-term mortality and clinical response [5]. Several studies have also evaluated the use of C-A in treating CRO infections. However, these are largely retrospective [6,7] and are mostly limited to K. pneumoniae infections [3,6,8]. In addition, a recent systematic review and meta-analysis of patients with bloodstream infections or nosocomial pneumonia found lower all-cause mortality in patients with bacteremia and improved clinical cure rates in patients with bacteremia and nosocomial pneumonia. Of note, only two of the included studies were prospective [9]. We therefore undertook a prospective, real-life study to investigate this further.
2. Results
2.1. Inclusion
We screened 92 patients who had received at least one dose of C-A. Four of these patients were minors and were therefore not considered for study inclusion. Of the remaining 88 patients, 17 were receiving C-A for off-label indications, 16 had infections that were not caused by CRO, 1 patient was participating in an interventional study, 1 patient had been treated with C-A within the previous 4 weeks, and 3 patients declined to participate. Of the remaining 50 patients, 3 patients were discharged early and could not be evaluated further, and 1 patient received C-A for <3 days. Thus, 46 patients were included in the final analysis (Figure 1).
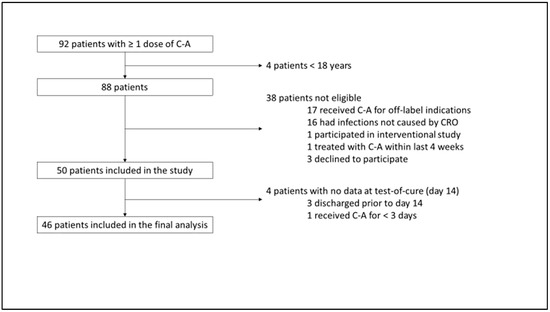
Figure 1.
Study tree. C-A = ceftazidime–avibactam; CRO = carbapenem-resistant organism.
2.2. Baseline Characteristics
Fifteen patients (30%) were female, the median age was 58 years and the median Charlson Comorbidity Index (CCI) was 5.5. The majority of patients (77%) were treated in an intensive care unit (ICU). More than 60% of patients had cardiac and pulmonary comorbidities. A substantial proportion of patients had undergone mechanical ventilation ≤ 7 days and/or surgery ≤ 30 days prior to the start of C-A (76 and 52%, respectively). Three patients were in septic shock. Renal replacement therapy on day 1 was administered in 16 patients (32%), and extracorporeal membrane oxygenation was administered in 2 (4%) patients. The median sequential organ failure assessment (SOFA) score at baseline was 7 (Table 1).

Table 1.
(a) Baseline characteristics; (b) clinical and laboratory parameters at baseline and test-of-cure.
2.3. Infection
The most common type of infection was nosocomial pneumonia, including ventilator-associated pneumonia (50%), followed by abdominal infections (28%, all of them complicated abdominal infections) and primary bloodstream infections (16%). CROs were primarily cultured from respiratory secretions including bronchoalveolar lavage (48%), from intraoperative specimens (30%), and from blood culture (22%). Most infections were caused by Pseudomonas (P.) aeruginosa and K. pneumoniae with a share of 48% and 28% of patients, respectively. One infection was caused by an intrinsically carbapenem-resistant organism (Stenotrophomonas (S.) maltophilia). Testing for carbapenemases was performed on all but one patient with Enterobacterales infections (in whom the pathogen had been isolated in a different hospital). OXA-48 and KPC were detected in 14 and 5 isolates, respectively. No carbapenemases were found in five CR Enterobacterales isolates (one isolate each showed combined porin loss and ESBL, and combined porin loss and AmpC; no resistance genes were detected in three isolates). The median (IQR) minimum inhibitory concentration (MIC) of C-A for P. aeruginosa was 4 (3.75–4.5); the median (IQR) MIC of C-A for all other CROs was 1 (1–2).
C-A was administered for a median duration of 13 days (assessed at day 30). Combination treatment (i.e., additional treatment with drugs to which the isolate was susceptible) was given to 23 (45%) patients. Partner drugs were administered intravenously only, nebulized only, or both intravenously and nebulized in 10, 8, and 5 patients, respectively. Polymicrobial infections were frequent. Additional pathogens were detected in 35 (69%) patients, most commonly Candida spp., E. faecium, and coagulase-negative staphylococci, occurring in 14, 11, and 10 patients, respectively. Multidrug therapy (i.e., additional treatment with drugs to which the isolate was not susceptible) was administered for 37 (80%) patients. Echinocandins were administered most frequently (17 patients), followed by linezolid, vancomycin, and metronidazole in 12, 10, and 8 patients, respectively (Table 2).

Table 2.
Infection characteristics and antimicrobial treatment.
2.4. Primary Outcome
Primary and secondary outcomes could be assessed in 46 patients. Clinical cure was achieved in 27 patients (59%). The reasons for failure were death in three (7%) patients, continued need for vasopressors in two (4%) patients, renewed detection of CROs from primarily sterile sites after ≥7 days of treatment in two (4%) patients, and no improvement in the oxygenation index in two (4%) patients. Additionally, the SOFA score was not improved in 10 (22%) patients (Table 3).

Table 3.
Outcomes.
2.5. Secondary Outcomes
The index isolate was detected in two patients from primarily sterile sites ≥ 7 days after the start of treatment. Both were patients with complicated abdominal infections in whom the infection focus could not be optimally sanitized. In addition, in five patients, index isolates were cultured from respiratory samples and were considered to be colonizers. None of the isolates, all of which had been susceptible in the first round, had become resistant to C-A in the second antimicrobial susceptibility test. The overall mortality at day 30 was 8.7%. The median length of stay (LOS) in the ICU (calculated on day 30) was 14 days (Table 3).
3. Discussion
In this prospective, single-center, observational study of patients infected with CRO, we demonstrate that 59% of patients achieved the composite primary outcome of clinical cure at day 14. Our cohort mostly comprised middle-aged patients (median age 58 years) with a high CCI (median 5.5). Around three-quarters of the patients had both been treated in the ICU and mechanically ventilated in the last seven days before study inclusion. The most common infections were nosocomial pneumonia and intra-abdominal infections (50 and 28%, respectively). The predominant carbapenem-resistant pathogens were P. aeruginosa (48%) and K. pneumoniae (28%). All-cause mortality at day 30 was low (9%).
While clinical cure by day 14 in just over half of the patients may seem like a modest result, we applied stringent criteria for this composite outcome based on the AIDA study by Paul et al., in which clinical failure at day 14 was observed in 79% of patients, albeit in a somewhat different patient population [10]. The median SOFA score on day 1 was 6 in their study, compared to 7 in ours. Importantly, however, the majority of infections (77%) were caused by A. baumanii with a high MIC for meropenem, and were treated with colistin alone or in combination with meropenem. Active β-lactam antibiotics were unavailable, which may explain the poorer clinical outcome. A multicenter retrospective study of 105 patients who were treated with C-A for infections caused by CROs observed clinical success at day 30 in 61% of patients [11], similar to our findings at day 14. The criteria for clinical success in that study were less rigorous than ours (e.g., unlike in our study, the researchers did not require stable vital signs, improvements in the SOFA score, or improvements in the oxygenation index).
The all-cause in-hospital mortality rate on day 30 in our study (4/46, 8.7%) is comparable to the results of a prospective study by van Duin et al., in which the treatment of infections due to CROs (almost exclusively K. pneumoniae) with colistin or C-A was investigated, and an all-cause in-hospital mortality rate of 8% (3/38) was similarly observed in patients treated with C-A [8]. To date, the largest prospective study—a national registry study from Greece—included patients infected mainly by KPC-producers and found a 28-day mortality rate of 20% [12]. Similar results to the latter study have been observed in retrospective investigations: a recent retrospective multicenter study of patients with carbapenem-resistant K. pneumoniae bloodstream infections found a 30-day mortality rate of 34%, which was reduced to 21% in patients who were appropriately treated with C-A [7]. The main differences compared to our study were that the aforementioned study had an exclusive focus on bacteremic K. pneumoniae infections and a multicenter retrospective design. Another observational retrospective study including 171 patients treated with C-A identified 30-day mortality in 22% of patients. All infections were due to Enterobacterales harboring OXA-48 [13]. We did not observe the development of resistance to C-A in our patients, although estimates of the emergence of resistance to C-A after exposure range from 10 to 20% [14,15]. However, resistance rates may be in the low single digits, as found in larger observational studies [11,12].
Approximately half of our patients had infections caused by carbapenem-resistant P. aeruginosa. Interestingly, a recent multicenter, retrospective, observational study compared ceftolozane–tazobactam with C-A in the treatment of multidrug-resistant P. aeruginosa (i.e., the isolate was non-susceptible to at least one agent in three or more antibiotic classes). Similar proportions of patients to those in our study were recruited from the ICU and were mechanically ventilated. The primary outcome of clinical success at day 30 was observed in 61% and 52% of patients who were treated with ceftozolane–tazobactam and C-A, respectively [16]. The emergence of resistance was common with both agents during the 90-day observation period (22%). Although we did not observe any resistance to C-A in our study, this was probably due to our shorter observation period of 30 days. Ceftolozane–tazobactam has been licensed in Germany since 2015. This drug is of great importance in the treatment of infections caused by multidrug-resistant P. aeruginosa. However, C-A has a broader spectrum of activity against Gram-negative bacteria harboring carbapenemases.
Our study has several limitations. Firstly, our study was small and single-center, so the findings may not be generalizable. Secondly, the study lacked a control group. We had originally planned to include matched historical controls but this was not feasible (see details in the methods section). Thirdly, the landscape of carbapenemases changes over time. OXA-48 has been the predominant carbapenemase in Enterobacterales in Germany and still is, according to a recent report from the German national reference center for Gram-negative bacteria [17]. However, in many areas, CROs susceptible to C-A have been replaced by organisms carrying metallo-β-lactamases. In Germany, this has particularly been reported for K. pneumoniae in patients with exposure in Ukraine [18]. C-A is the preferred treatment option for infections caused by OXA-48-producing CROs, whereas for pathogens carrying metallo-β-lactamases, preferred treatment options include C-A in combination with aztreonam or cefiderocol as monotherapy [14]. Alternatively, the recently approved combination of aztreonam and avibactam can be used to treat infections caused by aerobic Gram-negative pathogens for which there are limited treatment options [19].
The global burden of bacterial antimicrobial resistance is increasing. Among the Gram-negative bacteria, resistance to carbapenems has increased more than resistance to any other class of antibiotic, rising from 127,000 attributable deaths in 1990 to 216,000 in 2021. Forecasts indicate that antimicrobial resistance will lead to an even greater number of deaths in the future [20]. Carbapenem-resistant Enterobacterales have been declared a top priority for research and development by the WHO [2]. Antimicrobial stewardship (AMS) programs are critical in improving antibiotic selection and dosing and optimizing treatment duration to keep CROs in check [21]. It has been shown that AMS programs can lead to a sustained reduction in antibiotic use and a decreased incidence of nosocomial infections and CRO colonization [22]. Reserve antibiotics such as C-A are important therapeutic options for CRO-related infections. While the benefits of these antibiotics have been demonstrated in this and other studies, they must be prescribed cautiously to maintain their effectiveness.
4. Methods
Charité-Universitätsmedizin Berlin, Germany is a large tertiary care hospital with more than 3000 in-patient beds and 25 ICUs across three campuses. As of May 2025, the AMS team consisted of six physicians (one microbiologist and five infectious disease physicians) and two clinical pharmacists. This study was an investigator-initiated, single-center, prospective, observational study. The study was approved by the Ethics Committee of Charité-Universitätsmedizin Berlin (application number EA2/251/18). Patients were included if they were ≥18 years of age and had received ≥1 dose of C-A for a documented infection with a CRO. Patients were excluded if they were included in interventional trials, if they had been treated with C-A for ≥3 days within ≤4 weeks, or if C-A had been administered off-label (off-label use was not covered by the ethics committee vote). Only patients who had received C-A for a duration of ≥3 days were included in the final analysis.
We hypothesized that C-A is more effective than other antibiotics in treating CRO infections. We had originally planned to use a matched historical control group of patients with infections due to CROs who had been treated prior to the availability of C-A. However, patient recruitment was slow during the Coronavirus Disease 2019 (COVID-19) pandemic. In the meantime, a new version of our patient data management system was implemented and the data of historical controls could no longer be accessed.
4.1. Outcomes
The primary outcome was clinical cure 14 days after initiation of C-A. Clinical cure was defined as a composite of all of the following criteria: patient alive, systolic blood pressure ≥ 90 mmHg without vasopressors, improvement in the SOFA score (for a baseline SOFA score < 3, the score had to remain stable or decrease), and, for pneumonia improvement or the stability of the oxygenation index, microbiological cure (no detection of the index isolate from primarily sterile sites ≥ 7 days after start of treatment).
Secondary outcomes included microbiological failure (detection of the index isolate from primarily sterile sites ≥ 7 days after initiation of treatment), development of resistance to C-A, all-cause mortality at day 30, and LOS in the ICU.
Patients were followed if they received ≥3 days of treatment with C-A until 30 days after the initiation of therapy or until discharge, transfer to another hospital, death, or loss to follow-up, whichever occurred first.
4.2. Statistical Analysis
Univariate, descriptive analysis was used for data analysis. Patient characteristics are presented as absolute frequencies. Metric and ordinal numbers are presented with medians and interquartile ranges (IQR).
Retrospective studies have shown that clinical success with C-A in patients with CRO infections was at least 30% higher than that with other antibiotics. Based on these data, we initially calculated that we would need to include 38 patients to achieve 80% power and a two-sided significance of 5%. We anticipated losing 20% of the patients by the end of the follow-up period on day 30 after the start of treatment. Therefore, 46 patients and—with a matching ratio of 1:2—92 controls had to be included. However, the latter could not eventually be included.
Author Contributions
Conceptualization, F.P. and S.A.; methodology, F.P. and S.A.; software, F.P., A.T. and S.A.; validation, F.P., M.S.S. and R.L.; formal analysis, F.P. and R.L.; investigation, F.P., A.T. and S.A.; resources, F.P.; data curation, F.P., A.T. and S.A.; writing—original draft preparation, F.P.; writing—review and editing, F.P., R.L. and F.K.; visualization, F.P. and S.A.; supervision, L.E.S. and F.K.; project administration, F.P., L.E.S. and F.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the declaration of Helsinki, and approved by the Ethics Committee of Charité-Universitätsmedizin Berlin (application number EA2/251/18).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Ten Threats to Global Health in 2019. 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 13 September 2021).
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024. 2024. Available online: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1 (accessed on 4 October 2024).
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Potoski, B.A.; Marini, R.V.; Doi, Y.; Kreiswirth, B.N.; Clancy, C.J. Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia. Antimicrob. Agents Chemother. 2017, 61, e00883-17. [Google Scholar] [CrossRef] [PubMed]
- Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/zavicefta-epar-product-information_en.pdf (accessed on 10 September 2021).
- Sternbach, N.; Weissman, Y.L.; Avni, T.; Yahav, D. Efficacy and safety of ceftazidime/avibactam: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2018, 73, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Potoski, B.A.; Haidar, G.; Hao, B.; Doi, Y.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J.; Nguyen, M.H. Clinical Outcomes, Drug Toxicity, and Emergence of Ceftazidime-Avibactam Resistance Among Patients Treated for Carbapenem-Resistant Enterobacteriaceae Infections. Clin. Infect. Dis. 2016, 63, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Marelli, C.; Cattardico, G.; Fanelli, C.; Signori, A.; Di Meco, G.; Di Pilato, V.; Mikulska, M.; Mazzitelli, M.; Cattelan, A.M.; et al. Mortality in KPC-producing Klebsiella pneumoniae bloodstream infections: A changing landscape. J. Antimicrob. Chemother. 2023, 78, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Lok, J.J.; Earley, M.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Doi, Y.; et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin. Infect. Dis. 2018, 66, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Horcajada, J.P.; Kamat, S.; Irani, P.M.; Tawadrous, M.; Welte, T. Ceftazidime-Avibactam in the Treatment of Patients with Bacteremia or Nosocomial Pneumonia: A Systematic Review and Meta-analysis. Infect. Dis. Ther. 2024, 13, 1639–1664. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef]
- Ackley, R.; Roshdy, D.; Meredith, J.; Minor, S.; Anderson, W.E.; Capraro, G.A.; Polk, C. Meropenem-Vaborbactam versus Ceftazidime-Avibactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2020, 64, e02313-19. [Google Scholar] [CrossRef]
- Karaiskos, I.; Daikos, G.L.; Gkoufa, A.; Adamis, G.; Stefos, A.; Symbardi, S.; Chrysos, G.; Filiou, E.; Basoulis, D.; Mouloudi, E.; et al. Ceftazidime/Avibactam Registry Study, Ceftazidime/avibactam in the era of carbapenemase-producing Klebsiella pneumoniae: Experience from a national registry study. J. Antimicrob. Chemother. 2021, 76, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, H.; Alghamdi, A.; Alobaidallah, N.; Alfayez, A.; Almousa, R.; Albagli, R.; Shamas, N.; Farahat, F.; Mahmoud, E.; Bosaeed, M.; et al. Evaluation of ceftazidime/avibactam for treatment of carbapenemase-producing carbapenem-resistant Enterobacterales with OXA-48 and/or NDM genes with or without combination therapy. JAC Antimicrob. Resist. 2022, 4, dlac104. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2018, 62, e02497-17. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Abbo, L.M.; Ackley, R.; Aitken, S.L.; Albrecht, B.; Babiker, A.; Burgoon, R.; Cifuentes, R.; Claeys, K.C.; Curry, B.N.; et al. Effectiveness of ceftazidime-avibactam versus ceftolozane-tazobactam for multidrug-resistant Pseudomonas aeruginosa infections in the USA (CACTUS): A multicentre, retrospective, observational study. Lancet Infect. Dis. 2025, 25, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Pfennigwerth, N.; Cremanns, M.; Eisfeld, J.; Hans, J.; Anders, A.; Gatermann, S.G. Bericht des Nationalen Referenzzentrums für gramnegative Krankenhauserreger. Epid Bull. 2023, 27, 3–10. [Google Scholar]
- Sandfort, M.; Hans, J.B.; Fischer, M.A.; Reichert, F.; Cremanns, M.; Eisfeld, J.; Pfeifer, Y.; Heck, A.; Eckmanns, T.; Werner, G.; et al. Increase in NDM-1 and NDM-1/OXA-48-producing Klebsiella pneumoniae in Germany associated with the war in Ukraine, 2022. Euro. Surveill. 2022, 27, 2200926. [Google Scholar] [CrossRef] [PubMed]
- Emblaveo (Aztreonam/Avibactam). 2024. Available online: https://www.ema.europa.eu/en/documents/overview/emblaveo-epar-medicine-overview_en.pdf (accessed on 30 December 2024).
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Vasikasin, V.; Rawson, T.M.; Holmes, A.H.; Otter, J. Can precision antibiotic prescribing help prevent the spread of carbapenem-resistant organisms in the hospital setting? JAC Antimicrob. Resist. 2023, 5, dlad036. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vinau, T.; Penalva, G.; Garcia-Martinez, L.; Caston, J.J.; Munoz-Rosa, M.; Cano, A.; Recio, M.; Cisneros, J.M.; Perez-Nadales, E.; Aguirre, J.R.; et al. Impact of an Antimicrobial Stewardship Program on the Incidence of Carbapenem Resistant Gram-Negative Bacilli: An Interrupted Time-Series Analysis. Antibiotics 2021, 10, 586. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).