A Nosocomial Outbreak of Burkholderia cepacia complex Linked to Contaminated Intravenous Medications in a Tertiary Care Hospital
Abstract
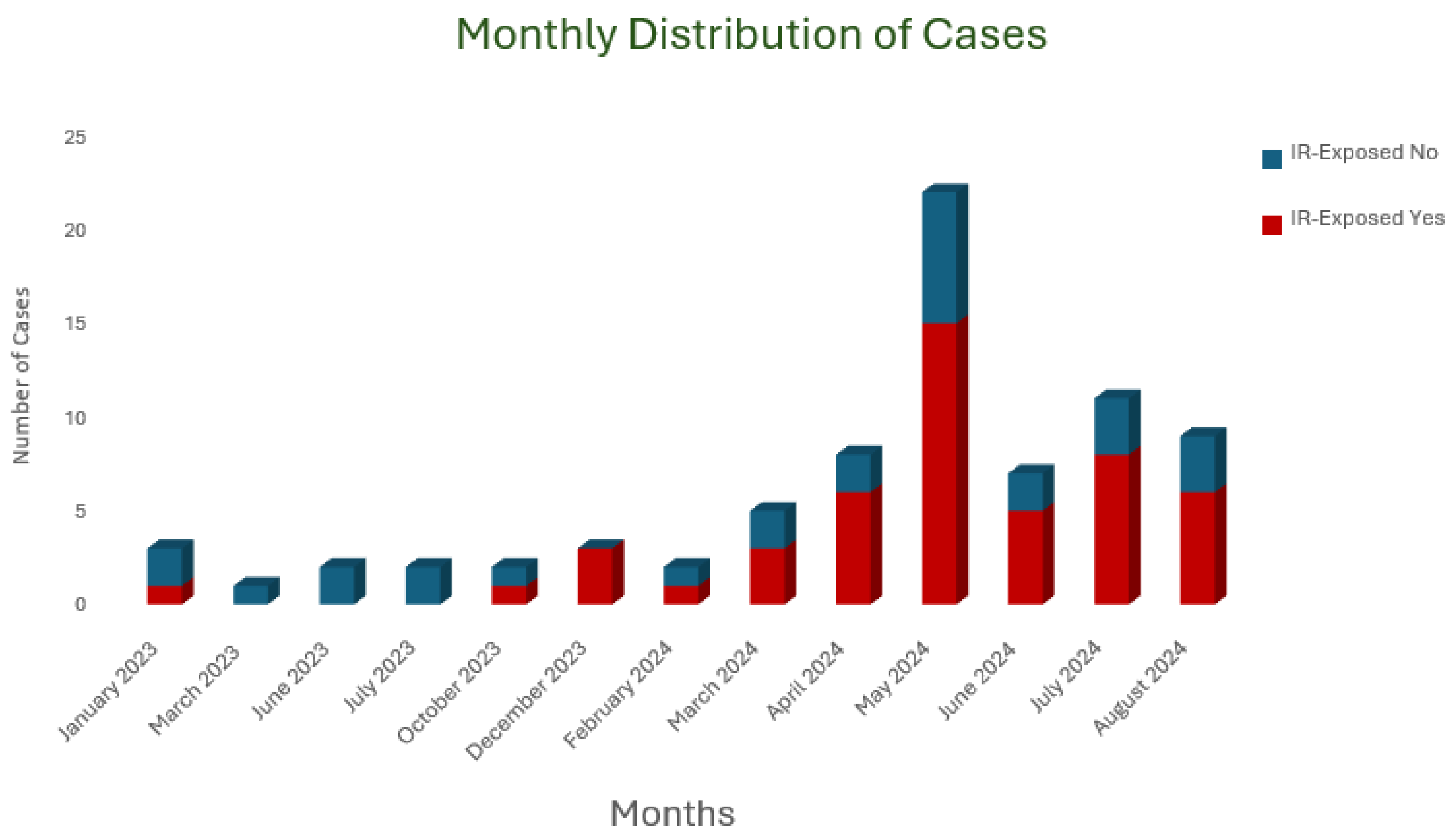
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Study Population
4.3. Data Collection
4.4. Bacterial Identification
4.5. Antimicrobial Susceptibility Testing
4.6. Data and Statistical Analyses
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, Y.; Zhou, J.; Zhou, J.; Hu, M.; Zhang, Q.; Kong, N.; Ren, H.; Liang, L.; Yue, J. Genome-based classification of Burkholderia cepacia complex provides new insight into its taxonomic status. Biol. Direct 2020, 15, 6. [Google Scholar] [CrossRef]
- Govan, J.; Balendreau, J.; Vandamme, P. Burkholderia cepacia—Friend and foe. ASM News 2000, 66, 124–125. [Google Scholar]
- Kenna, D.T.D.; Lilley, D.; Coward, A.; Martin, K.; Perry, C.; Pike, R.; Hill, R.; Turton, J.F. Prevalence of Burkholderia species, including members of Burkholderia cepacia complex, among UK cystic and non-cystic fibrosis patients. J. Med. Microbiol. 2017, 66, 490–501. [Google Scholar] [CrossRef]
- Tunccan, O.G.; Dizbay, M.; Sezer, B.E.; Aktas, F.; Arman, D. Nosocomial Burkholderia cepacia infections in a Turkish university hospital: A five-year surveillance. J. Infect. Dev. Ctries. 2009, 3, 273–277. [Google Scholar] [CrossRef]
- Caraher, E.; Reynolds, G.; Murphy, P.; McClean, S.; Callaghan, M. Comparison of antibiotic susceptibility of Burkholderia cepacia complex organisms when grown planktonically or as biofilm in vitro. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Wiener-Well, Y.; Segonds, C.; Mazuz, B.; Kopuit, P.; Assous, M.V. Successful outbreak investigation of Burkholderia cepacia complex bacteremia in intensive care patients. Am. J. Infect. Control 2014, 42, 580–581. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.L.; Berger, F.K.; Feldner, S.K.; Karliova, I.; Haber, M.; Mellmann, A.; Schäfers, H.-J.; Gärtner, B. Outbreak of Burkholderia cepacia complex infections associated with contaminated octenidine mouthwash solution, Germany, August to September 2018. Eurosurveillance 2018, 23, 1800540. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.-P.; Tsai, W.-C.; Liang, C.-Y.; Lin, Y.-S.; Huang, J.-W.; Chang, C.-Y.; Tyan, Y.-C.; Lu, P.-L.; Eberl, L. The contribution of antibiotic resistance mechanisms in clinical burkholderia cepacia complex isolates: An emphasis on efflux pump activity. PLoS ONE 2014, 9, e104986. [Google Scholar] [CrossRef]
- El Chakhtoura, N.G.; Saade, E.; Wilson, B.M.; Perez, F.; Papp-Wallace, K.M.; A Bonomo, R. A 17-Year Nationwide Study of Burkholderia cepacia Complex Bloodstream Infections Among Patients in the United States Veterans Health Administration. Clin. Infect. Dis. 2017, 65, 1253–1259. [Google Scholar] [CrossRef]
- Boszczowski, I.; Prado, G.V.B.D.; Dalben, M.F.; Telles, R.C.P.; Freire, M.P.; Guimaraes, T.; Oliveira, M.S.; Rosa, J.F.; Soares, R.E.; Llacer, P.E.D.; et al. Polyclonal outbreak of bloodstream infections caused by Burkholderia cepacia complex in hematology and bone marrow transplant outpatient units. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 71–76. [Google Scholar] [CrossRef]
- Rhodes, K.A.; Schweizer, H.P. Antibiotic resistance in Burkholderia species. Drug Resist. Updat. 2016, 28, 82–90. [Google Scholar] [CrossRef]
- Al Zunitan, M.; Aldawood, F.; El-Saed, A.; Azzam, M.; Yassine, K.A.; Alshammari, L.; Alshamrani, M. Two consecutive outbreaks caused by chlorhexidine mouthwash contaminated with Burkholderia contaminans in a two-hospital tertiary care system. J. Hosp. Infect. 2023, 142, 96–104. [Google Scholar] [CrossRef]
- Bharara, T.; Chakravarti, A.; Sharma, M.; Agarwal, P. Investigation of Burkholderia cepacia complex bacteremia outbreak in a neonatal intensive care unit: A case series. J. Med. Case Rep. 2020, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Marquez, L.; Jones, K.N.; Whaley, E.M.; Koy, T.H.; Revell, P.A.; Taylor, R.S.; Bernhardt, M.B.; Wagner, J.L.; Dunn, J.J.; LiPuma, J.J.; et al. An Outbreak of Burkholderia cepacian Complex Infections Associated with Contaminated Liquid Docusate. Infect. Control Hosp. Epidemiol. 2017, 38, 567–573. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Park, K.-H.; Moon, C.; Kim, D.Y.; Lee, M.S.; Kim, T.; Choo, E.J.; Chong, Y.P.; Kim, S.-H.; Kim, Y.S.; et al. Management and outcomes of Burkholderia cepacia complex bacteremia in patients without cystic fibrosis: A retrospective observational study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2057–2064. [Google Scholar] [CrossRef]
- Bressler, A.M.; Kaye, K.S.; LiPuma, J.J.; Alexander, B.D.; Moore, C.M.; Reller, L.B.; Woods, C.W. Risk factors for Burkholderia cepacian complex bacteremia among intensive care unit patients without cystic fibrosis: A case-control study. Infect. Control Hosp. Epidemiol. 2007, 28, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, X.; Chen, L.; Zhu, B.; Yan, W.; Ma, X. Burkholderia cepacia infection in children without cystic fibrosis: A clinical analysis of 50 cases. Front. Pediatr. 2023, 11, 1115877. [Google Scholar] [CrossRef]
- Lee, J.K. Two outbreaks of Burkholderia cepacia nosocomial infection in a neonatal intensive care unit. J. Paediatr. Child Health 2008, 44, 62–66. [Google Scholar] [CrossRef]
- Dolan, S.A.; Dowell, E.; LiPuma, J.J.; Valdez, S.; Chan, K.; James, J.F. An outbreak of Burkholderia cepacia complex associated with intrinsically contaminated nasal spray. Infect. Control Hosp. Epidemiol. 2011, 32, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Glowicz, J.; Crist, M.; Gould, C.; Moulton-Meissner, H.; Noble-Wang, J.; de Man, T.J.; Perry, K.A.; Miller, Z.; Yang, W.C.; Langille, S.; et al. A multistate investigation of health care–associated Burkholderia cepacia complex infections related to liquid docusate sodium contamination, January-October 2016. Am. J. Infect. Control 2018, 46, 649–655. [Google Scholar] [CrossRef]
- Bovolenta, E.; Zanon, M.P.; Mondino, S.; Manfrin, V.; Rassu, M.; Diquigiovanni, A.; Zenere, A.; Bertoncello, C.; Cazzaro, R.; Direction, V.R.M.-H.M.; et al. Management of an Epidemic Outbreak of Burkholderia Cepacia in A Hospital in The North of Italy. J. Food Nutr. 2023, 2, 1. [Google Scholar] [CrossRef]
- Paraskevopoulos, D.K.d.S.; Camargo, C.H.; Kodato, P.K.; Yamada, A.Y.; Almodovar, A.A.B.; Hilinski, E.G.; de Paula, A.I.; Irineu, E.F.; Barrio, S.R.; Fonseca, C.L.; et al. A Burkholderia contaminans outbreak in an intensive care unit associated with contaminated bath solution: Control and microbiological findings. Am. J. Infect. Control 2025, 53, 308–313. [Google Scholar] [CrossRef]
- Ormsby, J.; Wagner, T.; Gupta, R.; Millson, T.; Phillips, B. Safe injection, infusion, medication vial, and point-of-care testing practices in health care (2025). Am. J. Infect. Control, 2025; in press. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines on Core Components of Infection Prevention and Control Programmes at the National and Acute Health Care Facility Level; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/publications/i/item/9789241549929 (accessed on 24 July 2025).
- Centers for Disease Control and Prevention (CDC). Core Infection Prevention and Control Practices for Safe Healthcare Delivery in All Settings; U.S. Department of Health & Human Services: Atlanta, GA, USA, 2024. Available online: https://www.cdc.gov/infection-control/hcp/core-practices/index.html (accessed on 25 July 2025).
- Ibrahim, T.; Abdallah, T.A.; Abdallah, A.; Qazi, R.; Alimam, A.; Mohammad, H.; Eltayeb, F.; Daghfal, J.; Ali, M.; Hadi, H.A. Epidemiology, microbiological, clinical characteristics, and outcome of Burkholderia cepacia complex infections in non-cystic fibrosis adult patients from Qatar. IJID Reg. 2024, 11, 100355. [Google Scholar] [CrossRef]
- Nazik, S.; Topal, B.; Şahin, A.R.; Ateş, S. Nosocomial Burkholderia cepacia infection in a tertiary hospital; Five-year surveillance: A retrospective cross-sectional study. J. Surg. Med. 2019, 3, 121–123. [Google Scholar] [CrossRef]
- Cheesbrough, M. District Laboratory Practice in Tropical Countries. Part 2; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Ransom, E.M.; Alipour, Z.; Wallace, M.A.; Burnham, C.-A.D.; Simner, P.J. Evaluation of Optimal Blood Culture Incubation Time To Maximize Clinically Relevant Results from a Contemporary Blood Culture Instrument and Media System. J. Clin. Microbiol. 2021, 59, e02459-20. [Google Scholar] [CrossRef] [PubMed]
- Baselski, V.; Klutts, J.S.; Gilligan, P.H. Quantitative Cultures of Bronchoscopically Obtained Specimens Should Be Performed for Optimal Management of Ventilator-Associated Pneumonia. J. Clin. Microbiol. 2013, 51, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, A.; Mukhtar, A.; El-Adawy, A.; Elazizi, H.; Lotfy, A.; Nassar, H.; Ghaith, D. Ventilator associated pneumonia caused by extensive-drug resistant Acinetobacter species: Colistin is the remaining choice. Egypt. J. Anaesth. 2016, 32, 409–413. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 15.0. 2025. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_15.0_Breakpoint_Tables.pdf (accessed on 25 July 2025).
| Material Type | Frequency (n) | Percent (%) | ||||
|---|---|---|---|---|---|---|
| Burkholderia cepacia | ||||||
| Blood | 59 | 76.6 | ||||
| Endotracheal Aspirate | 2 | 2.6 | ||||
| Sputum | 5 | 6.5 | ||||
| BAL | 1 | 1.3 | ||||
| Urine | 7 | 9.11 | ||||
| Tissue/Wound/Fluid | 3 | 3.9 | ||||
| Total | 77 | 100 | ||||
| Burkholderia gladioli | Endotracheal Aspirate | 2 | 50 | |||
| BAL | 1 | 25 | ||||
| Urine | 1 | 25 | ||||
| Total | 4 | 100 | ||||
| Burkholderia cenocepacia | Urine | 1 | 100 | |||
| Antimicrobial Susceptibility n (%) | ||||||
| CAZ | MEM | TXM-STX | TET | CHL | ||
| Burkholderia cepacia | S | 3 (4.1) | 66 (90.4) | 2 (2.7) | 0 (0) | 0 (0) |
| I | 68 (93.2) | 0 | 68 (93.2) | 0 (0) | 58 (95.1) | |
| R | 2 (2.7) | 7 (9.6) | 3 (4.1) | 68 (100) | 3 (4.9) | |
| Total | 73 (94.8) | 73 (94.8) | 73 (94.8) | 68 (88.3) | 61 (79.2) | |
| Missing | 4 (5.2) | 4 (5.2) | 4 (5.2) | 9 (11.7) | 16 (20.8) | |
| Burkholderia cenocepacia | R | R | I | R | missing | |
| Burkholderia gladioli | S | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 0 (0) |
| I | 1 | 0 | 3 (100) | 0 | 4 (100) | |
| R | 2 | 0 | 0 | 4 (100) | 0 | |
| Total | 3 (75) | 3 (75) | 3 (75) | 4 (100) | 4 (100) | |
| Missing | 1 (25) | 1 (25) | 1 (25) | 0 (0) | 0 (0) | |
| No. (%) of Cases (n = 77) | No. (%) of Controls (n = 77) | p-Value | OR (%95CI) | |
|---|---|---|---|---|
| Presence of risk factors | ||||
| Urinary catheter | 54 (70.1) | 49 (63.6) | 0.494 | 1.34 (0.68–2.63) |
| Endotracheal intubation | 24 (31.2) | 23 (29.9) | >0.9 | 1.06 (0.54–2.11) |
| CVC | 40 (51.9) | 12 (15.6) | <0.001 | 5.86 (2.74–12.53) |
| PVC | 72 (93.5) | 76 (98.7) | 0.209 | 0.19 (0.02–1.66) |
| PAC | 25 (32.5) | 12 (15.6) | 0.023 | 2.60 (1.20–5.68) |
| Nasogastric tube | 30 (39.0) | 21 (27.3) | 0.17 | 1.70 (0.86–3.36) |
| Chest tube | 8 (10.4) | 2 (2.6) | 0.098 | 4.35 (0.89–21.19) |
| History of transfusion | 28 (36.4) | 13 (16.9) | 0.01 | 2.81 (1.32–5.99) |
| Hemodialysis | 4 (5.2) | 2 (2.6) | 0.681 | 2.06 (0.37–11.56) |
| Recent surgery | 41 (53.2) | 28 (36.4) | 0.051 | 1.99 (1.05–3.78) |
| Surgical drain | 15 (19.5) | 10 (13) | 0.382 | 1.62 (0.68–3.88) |
| Total parenteral nutrition | 7 (9.1) | 7 (9.1) | >0.9 | 1 (0.33–3.00) |
| Interventional radiology procedures | 49 (63.6) | 10 (13) | <0.001 | 11.73 (5.21–26.37) |
| Recent ICU admission | 47 (61) | 47 (61) | >0.9 | 1 (0.52–1.91) |
| Presence of pressure ulcers | 3 (3.9) | 1 (1.3) | 0.62 | 3.08 (0.31–30.3) |
| Altered consciousness | 30 (39) | 19 (24.7) | 0.083 | 1.95 (0.98–3.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakoc Parlayan, H.N.; Aksoy, F.; Ozdemir, M.N.; Ozkaya, E.; Yilmaz, G. A Nosocomial Outbreak of Burkholderia cepacia complex Linked to Contaminated Intravenous Medications in a Tertiary Care Hospital. Antibiotics 2025, 14, 774. https://doi.org/10.3390/antibiotics14080774
Karakoc Parlayan HN, Aksoy F, Ozdemir MN, Ozkaya E, Yilmaz G. A Nosocomial Outbreak of Burkholderia cepacia complex Linked to Contaminated Intravenous Medications in a Tertiary Care Hospital. Antibiotics. 2025; 14(8):774. https://doi.org/10.3390/antibiotics14080774
Chicago/Turabian StyleKarakoc Parlayan, Hanife Nur, Firdevs Aksoy, Masite Nur Ozdemir, Esra Ozkaya, and Gurdal Yilmaz. 2025. "A Nosocomial Outbreak of Burkholderia cepacia complex Linked to Contaminated Intravenous Medications in a Tertiary Care Hospital" Antibiotics 14, no. 8: 774. https://doi.org/10.3390/antibiotics14080774
APA StyleKarakoc Parlayan, H. N., Aksoy, F., Ozdemir, M. N., Ozkaya, E., & Yilmaz, G. (2025). A Nosocomial Outbreak of Burkholderia cepacia complex Linked to Contaminated Intravenous Medications in a Tertiary Care Hospital. Antibiotics, 14(8), 774. https://doi.org/10.3390/antibiotics14080774





