Trends in ‘Watch’ and ‘Reserve’ Antibiotic Use in Primary Care in Kazakhstan: The Imperative for Enhancing Stewardship Strategies
Abstract
1. Introduction
2. Results
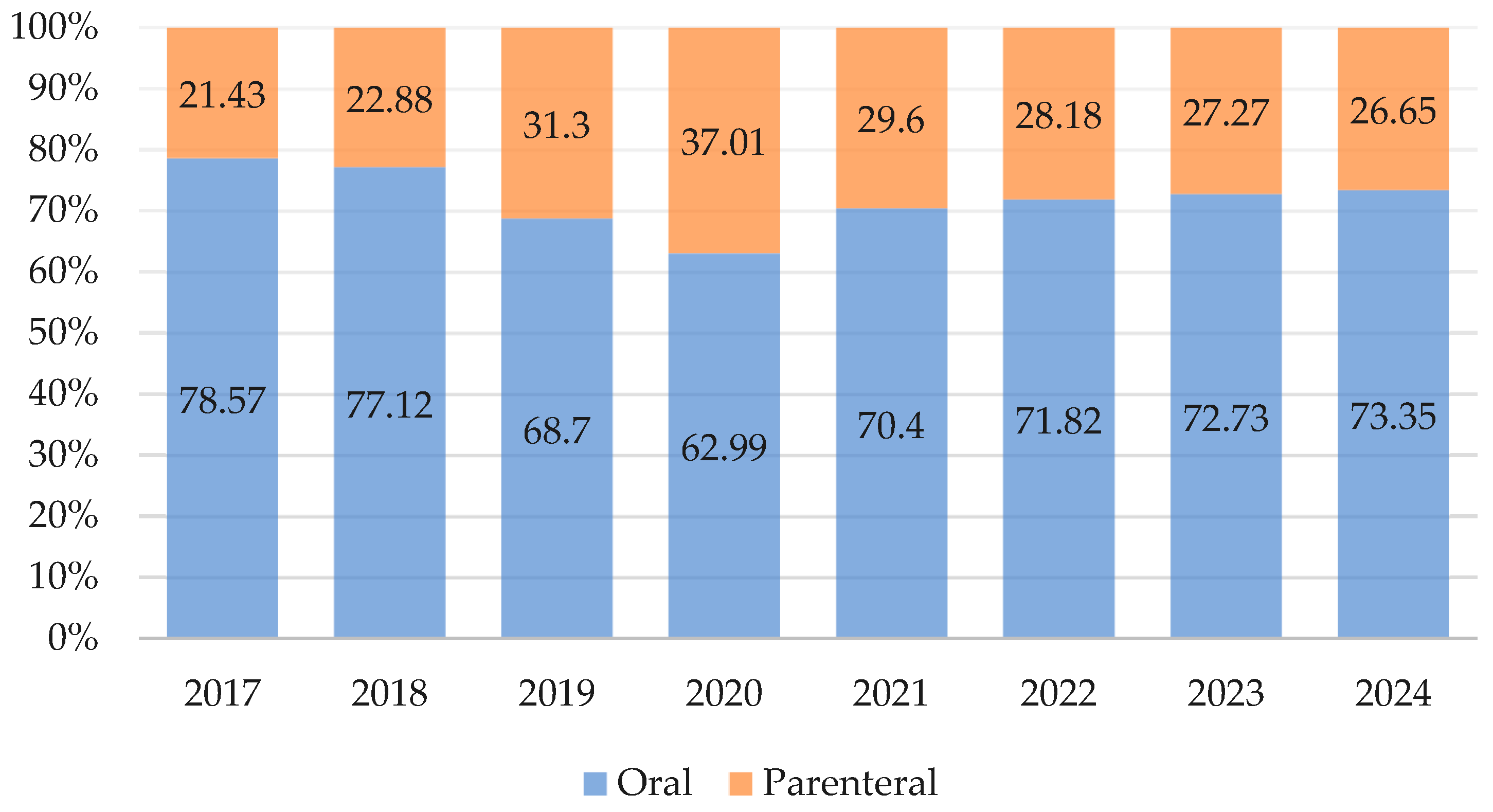
2.1. Antibiotic Consumption in the Primary Care, 2017–2024
2.2. Antibiotic Consumption and Primary Care Provider Rates, 2017–2024
3. Discussion
3.1. Antibiotic Consumption in the Primary Care Sector
3.2. Antibiotic Consumption and Primary Care Providers
3.3. Study Limitations
4. Materials and Methods
4.1. Study Design
4.2. Data Sources
4.3. Data Extraction and Processing
4.4. AWaRe Classification
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAPS | Average Annual Percent Change |
| AMR | Antimicrobial Resistance |
| AMS | Antimicrobial Stewardship |
| ATC | Anatomical Therapeutic Chemical Classification |
| AWaRe | Access, Watch, Reserve |
| DDD | Defined Daily Doses |
| DID | Defined Daily Doses per 1000 Inhabitants per Day |
| GLASS-AMC | Global Antimicrobial Resistance and Use Surveillance System |
| GP | General Practitioner |
| LMICs | Low- and Middle-Income Countries |
| MoH | Ministry of Health |
| OTC | Over-the-Counter |
| SPSS | Statistical Package for the Social Sciences |
| WHO | World Health Organization |
| 95% CI | 95% Confidence Interval |
References
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Sharma, S.; Chauhan, A.; Ranjan, A.; Mathkor, D.M.; Haque, S.; Ramniwas, S.; Tuli, H.S.; Jindal, T.; Yadav, V. Emerging challenges in antimicrobial resistance: Implications for pathogenic microorganisms, novel antibiotics, and their impact on sustainability. Front. Microbiol. 2024, 15, 1403168. [Google Scholar] [CrossRef]
- Keck, J.M.; Schultz, J.; Viteri, A. “Dusting Off the Cobwebs”: Rethinking How We Use New Antibiotics. Antibiotics 2025, 14, 862. [Google Scholar] [CrossRef]
- Zay, Y.K.; Win, P.T.N.; Bielicki, J.; Lambiris, M.; Fink, G. Association Between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2253806. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Latest Surveilance Data on Antimicrobial Consumption. Available online: https://qap.ecdc.europa.eu/public/extensions/AMC2_Dashboard/AMC2_Dashboard.html#consumption-distribution-tab (accessed on 28 August 2025).
- World Health Organization Regional Office for Europe. Antimicrobial Medicines Consumption (AMC) Network. AMC Data 2022; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2024; ISBN 9789289061346. [Google Scholar]
- World Health Organization Western Pacific Region. Antimicrobial Consumption in the WHO Western Pacific Region: Early Implementation of the Western Pacific Regional Antimicrobial Consumption Surveillance System (WPRACSS); World Health Organization Regional Office for Western Pacific Region: Manila, Philippines, 2021; ISBN 9789290619543. [Google Scholar]
- Semenova, Y.; Akhmetova, K.; Semenov, D.; Makalkina, L.; Surov, V.; Pivina, L.; Turgambayeva, A.; Belikhina, T.; Maukayeva, S.; Goremykina, M.; et al. Social Determinants of Health and Antibiotic Consumption. Antibiotics 2025, 14, 513. [Google Scholar] [CrossRef]
- Ashiru-Oredope, D.; Budd, E.L.; Bhattacharya, A.; Din, N.; McNulty, C.A.; Micallef, C.; Ladenheim, D.; Beech, E.; Murdan, S.; Hopkins, S.; et al. Implementation of Antimicrobial Stewardship Interventions Recommended by National Toolkits in Primary and Secondary Healthcare Sectors in England: TARGET and Start Smart Then Focus. J. Antimicrob. Chemother. 2016, 71, 1408–1414. [Google Scholar] [CrossRef]
- Anello, P.; Vianello, S.; Baldo, V.; Frasson, E.; Gallo, U.; Rampazzo, R.; Marchiori, M.; Carraro, M.; Marchiori, S.; Pigozzo, M.; et al. Antimicrobial Stewardship and Infection Prevention and Control in the Veneto Region, Northeastern Italy: Governance Models, Resources, and Key Challenges Across Hospital and Community Settings—Findings from the ARCO Project. Microorganisms 2025, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Nashwan, A.J.; Barakat, M.; Niaz, F.; Tariq, S.; Ahmed, S.K. Antimicrobial Resistance: Stewardship and One Health in the Eastern Mediterranean Region. Cureus 2024, 16, e58478. [Google Scholar] [CrossRef] [PubMed]
- Truppa, C.; Alonso, B.; Clezy, K.; Deglise, C.; Dromer, C.; Garelli, S.; Jimenez, C.; Kanapathipillai, R.; Khalife, M.; Repetto, E. Antimicrobial stewardship in primary health care programs in humanitarian settings: The time to act is now. Antimicrob. Resist. Infect. Control 2023, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lambert, H.; Zhao, L.; Liu, R.; Shen, X.; Wang, D.; Cabral, C. Antibiotic Stewardship in Retail Pharmacies and the Access-Excess Challenge in China: A Policy Review. Antibiotics 2022, 11, 141. [Google Scholar] [CrossRef]
- Lim, A.H.; Ab Rahman, N.; Nasarudin, S.N.S.; Velvanathan, T.; Fong, M.C.C.; Mohamad Yahaya, A.H.; Sivasampu, S. A comparison between antibiotic utilisation in public and private community healthcare in Malaysia. BMC Public Health 2024, 24, 79. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Castagna, T.; Pantolini, B.; Campanardi, M.C.; Roperti, M.; Grotto, A.; Fattori, M.; Dal Maso, L.; Carrara, F.; Zambarbieri, G.; et al. The challenge of antimicrobial resistance (AMR): Current status and future prospects. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 9603–9615. [Google Scholar] [CrossRef]
- Glushkova, N.; Semenova, Y.; Sarria-Santamera, A. Editorial: Public health challenges in post-Soviet countries during and beyond COVID-19. Front. Public Health 2023, 11, 1290910. [Google Scholar] [CrossRef] [PubMed]
- Semenova, Y.; Kassym, L.; Kussainova, A.; Aimurziyeva, A.; Makalkina, L.; Avdeyev, A.; Yessmagambetova, A.; Smagul, M.; Aubakirova, B.; Akhmetova, Z.; et al. Knowledge, Attitudes, and Practices towards Antibiotics, Antimicrobial Resistance, and Antibiotic Consumption in the Population of Kazakhstan. Antibiotics 2024, 13, 718. [Google Scholar] [CrossRef]
- Semenova, Y.; Yergaliyeva, A.; Aimurziyeva, A.; Manatova, A.; Kuntuganova, A.; Makalkina, L.; Aldiyarova, N.; Semenov, D.; Lim, L. A Nationwide Evaluation of Antibiotic Consumption in Kazakhstan from 2019 to 2023. Antibiotics 2024, 13, 1123. [Google Scholar] [CrossRef]
- Klein, E.Y.; Impalli, I.; Poleon, S.; Denoel, P.; Cipriano, M.; Van Boeckel, T.P.; Pecetta, S.; Bloom, D.E.; Nandi, A. Global trends in antibiotic consumption during 2016–2023 and future projections through 2030. Proc. Natl. Acad. Sci. USA 2024, 121, e2411919121. [Google Scholar] [CrossRef]
- National Center for Public Health of the Ministry of Health of the Kazakhstan Republic. Roadmap: On Measures to Contain Antimicrobial Resistance in the Kazakhstan Republic for 2023–2027. Available online: https://hls.kz/ru/piik-ru (accessed on 22 September 2025).
- Kazakh Telegraph Agency. The Minister of Health of the Republic of Kazakhstan Reminded About Responsibility for over-the-Counter Sale of Medicines. Available online: https://kaztag.kz/ru/news/ministr-zdravookhraneniya-rk-napomnil-ob-otvetstvennosti-za-bezretsepturnyy-otpusk-lekarstv (accessed on 30 August 2025).
- Kassym, L.; Kussainova, A.; Semenov, D.; Aimurziyeva, A.; Uzbekova, S.; Semenova, Y. National trends in Azithromycin consumption during 2017-2023 in Kazakhstan: Impact of the COVID-19 pandemic and the imperative for enhanced clinical guidelines. Sci. Rep. 2025, 15, 6309. [Google Scholar] [CrossRef]
- Kazakhstan Wants to Create a Unified Healthcare Information System. Available online: https://www.7152.kz/news/3832384/edinuu-informacionnuu-sistemu-zdravoohranenia-hotat-sozdat-v-kazahstane (accessed on 30 August 2025).
- Cresswell, K.; Hinder, S.; Sheikh, A.; Pontefract, S.; Watson, N.W.; Price, D.; Heed, A.; Coleman, J.; Ennis, H.; Beggs, J.; et al. ePrescribing-Based Antimicrobial Stewardship Practices in an English National Health Service Hospital: Qualitative Interview Study Among Medical Prescribers and Pharmacists. JMIR Form. Res. 2023, 7, e37863. [Google Scholar] [CrossRef]
- Zanichelli, V.; Sharland, M.; Cappello, B.; Moja, L.; Getahun, H.; Pessoa-Silva, C.; Sati, H.; van Weezenbeek, C.; Balkhy, H.; Simão, M.; et al. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book and Prevention of Antimicrobial Resistance. Bull. World Health Organ. 2023, 101, 290–296. [Google Scholar] [CrossRef]
- World Health Organization. Antibiotic Use: Target ≥70% of Total Antibiotic Use Being Access Group Antibiotics (70% Access target). Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/5767 (accessed on 30 August 2025).
- WHO Essential Medicines List Antibiotic Book. Available online: https://www.who.int/publications/m/item/the-who-essential-medicines-list-antibiotic-book-infographics (accessed on 30 August 2025).
- National Registry of Medicines and Medical Devices. Available online: https://register.ndda.kz/#/reestr (accessed on 30 August 2025).
- National Research Center of Healthcare Development Named after S. Kairbekova, Ministry of Healthcare. Standards of Care. Available online: https://nrchd.kz/clinical-protocols (accessed on 30 August 2025).
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Sevestre, J.; Mailhe, M.; Doudier, B.; Aubry, C.; Amrane, S.; et al. Clinical and Microbiological Effect of a Combination of Hydroxychloroquine and Azithromycin in 80 COVID-19 Patients with at Least a Six-Day Follow Up: A Pilot Observational Study. Travel Med. Infect. Dis. 2020, 34, 101663. [Google Scholar] [CrossRef]
- Yousafzai, A.D.K.; Bangash, A.H.; Asghar, S.Y.; Abbas, S.M.M.; Khawaja, H.F.; Zehra, S.; Khan, A.U.; Kamil, M.; Ayesha, N.; Khan, A.K.; et al. Clinical Efficacy of Azithromycin for COVID-19 Management: A Systematic Meta-Analysis of Meta-Analyses. Heart Lung 2023, 60, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The Resistance Mechanisms of Bacteria against Ciprofloxacin and New Approaches for Enhancing the Efficacy of This Antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef]
- Robertson, J.; Vlahović-Palčevski, V.; Iwamoto, K.; Högberg, L.D.; Godman, B.; Monnet, D.L.; Garner, S.; Weist, K.; ESAC-Net Study Group; WHO Europe AMC Network Study Group. Variations in the Consumption of Antimicrobial Medicines in the European Region, 2014–2018: Findings and Implications from ESAC-Net and WHO Europe. Front. Pharmacol. 2021, 12, 639207. [Google Scholar] [CrossRef]
- Kosherova, Z.; Zhazykhbayeva, D.; Aimurziyeva, A.; Bayesheva, D.; Semenova, Y. The Implementation of Antimicrobial Consumption Surveillance and Stewardship in Human Healthcare in Post-Soviet States: A Systematic Review. Antibiotics 2025, 14, 749. [Google Scholar] [CrossRef]
- Perry, T. (Ed.) Therapeutics Letter; Letter 155, Oral vs IV Antibiotics; Therapeutics Initiative: Vancouver, BC, Canada, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK615102 (accessed on 30 August 2025).
- Shurenova, M.; Kurakbayev, K.; Abildaev, T.; Tazhiyeva, A. Primary Healthcare Services’ Accessibility and Quality under Compulsory Social Health Insurance in Kazakhstan. Front. Public Health 2024, 12, 1418367. [Google Scholar] [CrossRef]
- World Health Organization. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book. Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 31 August 2025).
- Machowska, A.; Stålsby Lundborg, C. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2019, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Al Masud, A.; Walpola, R.L.; Sarker, M.; Kabir, A.; Asaduzzaman, M.; Islam, M.S.; Mostafa, A.T.; Akhtar, Z.; Barua, M.; Seale, H. Understanding antibiotic purchasing practices in community pharmacies: A potential driver of emerging antimicrobial resistance. Explor. Res. Clin. Soc. Pharm. 2024, 15, 100485. [Google Scholar] [CrossRef] [PubMed]
- Kasse, G.E.; Humphries, J.; Cosh, S.M.; Islam, M.S. Factors contributing to the variation in antibiotic prescribing among primary health care physicians: A systematic review. BMC Prim. Care 2024, 25, 8. [Google Scholar] [CrossRef]
- Vi-ORTIS. Market Research Company. Available online: https://base.viortis.kz/Account/LogOn?ReturnUrl=%2f (accessed on 31 August 2025).
- National Research Center of Healthcare Development named after S. Kairbekova, Ministry of Healthcare. Statistical Compilation “Population Health in the Republic of Kazakhstan and the Activities of Healthcare Organizations”. Available online: https://nrchd.kz/medical-statistics/analytics (accessed on 31 August 2025).
- Agency for Strategic Planning and Reforms of the Republic of Kazakhstan. Bureau of National Statistics. Statistical Collections. Available online: http://stat.gov.kz/edition/publication/collection (accessed on 12 May 2025).
- World Health Organization. The ATC/DDD Methodology. Available online: https://www.who.int/tools/atc-ddd-toolkit/methodology (accessed on 31 August 2025).
- Norwegian Institute of Public Health. ATC/DDD Index. Available online: https://atcddd.fhi.no/atc_ddd_index/?code=J&showdescription=yes (accessed on 31 August 2025).
- World Health Organization. GLASS Manual on the Management of Antimicrobial Consumption Data; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001019-2. [Google Scholar]
- World Health Organization. 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. 2019. Available online: https://www.who.int/publications/i/item/WHOEMPIAU2019.11 (accessed on 18 September 2025).
- World Health Organization. AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 31 August 2025).
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]

| Pharmacological Group | Year | 0 AAPC (95% * CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | ||
| Tetracyclines | 0.67 | 0.58 | 0.54 | 0.50 | 0.51 | 0.47 | 0.61 | 0.73 | 0.52 (−5.37–6.78) |
| Amphenicols | 0.33 | 0.33 | 0.32 | 0.28 | 0.28 | 0.25 | 0.27 | 0.42 | −0.14 (−6.64–6.81) |
| Penicillins | 1.68 | 1.62 | 1.75 | 1.23 | 1.04 | 0.90 | 1.30 | 1.53 | −4.55 (−12.45–4.06) |
| Beta-lactams | 0.50 | 0.45 | 0.37 | 0.34 | 0.50 | 0.67 | 0.83 | 1.13 | 13.97 (2.77–26.40) |
| Cephalosporins | 1.66 | 1.74 | 1.52 | 2.02 | 1.71 | 1.67 | 1.77 | 2.81 | 4.77 (−1.55–11.49) |
| Carbapenems | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 37.29 (−2.88–94.06) |
| Sulfonamides and their combinations | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −26.61 (−71.45–88.65) |
| Macrolides | 0.69 | 0.79 | 0.67 | 0.67 | 0.60 | 0.47 | 0.36 | 0.52 | −8.23 (−13.65–−2.47) |
| Lincosamides | 0.28 | 0.28 | 0.28 | 0.26 | 0.24 | 0.25 | 0.31 | 0.43 | 3.88 (−2.83–11.05) |
| Aminoglycosides | 0.62 | 0.70 | 0.74 | 1.34 | 1.30 | 1.27 | 1.27 | 2.17 | 17.16 (9.38–25.49) |
| Fluoroquinolones | 1.78 | 1.66 | 1.50 | 1.45 | 1.21 | 1.32 | 1.37 | 1.90 | −1.34 (−7.26–4.97) |
| Quinolones | 0.20 | 0.20 | 0.20 | 0.38 | 0.35 | 0.29 | 0.32 | 0.47 | 11.64 (3.48–20.46) |
| Combinations of antibacterials | 0.08 | 0.08 | 0.03 | 0.02 | 0.03 | 0.02 | 0.03 | 0.03 | −14.17 (−25.84–−0.67) |
| Glycopeptides | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | −4.58 (−60.16–128.53) |
| Polymyxins | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 30.75 (5.00–62.82) |
| Imidazoles | 0.16 | 0.20 | 0.18 | 0.20 | 0.14 | 0.16 | 0.19 | 0.31 | 4.47 (−4.05–13.76) |
| Nitrofuran derivatives | 0.61 | 0.58 | 0.51 | 0.44 | 0.46 | 0.47 | 0.47 | 0.81 | 0.78 (−7.26–9.52) |
| Aminocyclitols | 0.15 | 0.13 | 0.15 | 0.13 | 0.13 | 0.12 | 0.07 | 0.16 | −3.45 (−12.08–6.04) |
| Total | 9.41 | 9.34 | 8.76 | 9.28 | 8.50 | 8.33 | 9.17 | 13.43 | 2.58 (−2.96–8.43) |
| Substance and * AWaRe Category | Rank (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | ||
| Azithromycin | Watch | 6 (6.10) | 4 (7.08) | 4 (8.08) | 1 (14.09) | 1 (14.95) | 1 (14.96) | 1 (13.55) | 1 (15.62) |
| Ciprofloxacin | 1 (12.51) | 1 (12.05) | 2 (12.40) | 3 (11.41) | 2 (11.91) | 2 (12.39) | 2 (12.55) | 2 (12.03) | |
| Amoxicillin | Access | 2 (10.92) | 2 (11.26) | 1 (14.32) | 4 (10.77) | 4 (8.13) | 5 (7.57) | 3 (11.03) | 3 (8.97) |
| Ceftriaxone | Watch | 3 (8.10) | 3 (8.66) | 3 (8.30) | 2 (12.57) | 3 (9.92) | 3 (9.52) | 5 (8.28) | 4 (8.64) |
| Amoxicillin and 0 BLI | Access | 7 (5.26) | 7 (4.78) | 8 (4.26) | 8 (3.64) | 5 (5.75) | 4 (8.00) | 4 (9.01) | 5 (8.45) |
| Cefazolin | 5 (6.26) | 5 (6.27) | 7 (4.92) | 5 (5.48) | 7 (4.61) | 6 (4.81) | 8 (3.88) | 6 (5.07) | |
| Doxycycline | 8 (5.15) | 9 (4.22) | 5 (5.35) | 6 (4.56) | 6 (4.70) | 7 (4.59) | 6 (5.08) | 8 (4.91) | |
| Ampicillin | 4 (6.36) | 6 (5.10) | 6 (5.03) | 18 (2.13) | 10 (3.84) | 14 (2.82) | 13 (2.86) | 16 (2.14) | |
| Cefuroxime | Watch | 15 (2.21) | 14 (2.37) | 15 (2.51) | 14 (2.30) | 9 (3.85) | 8 (3.82) | 7 (4.28) | 7 (4.92) |
| Levofloxacin | 16 (2.15) | 16 (2.17) | 16 (2.21) | 7 (4.04) | 8 (4.11) | 9 (3.46) | 9 (3.5) | 10 (3.49) | |
| Nitrofurantoin | Access | 12 (3.50) | 12 (3.27) | 11 (3.12) | 15 (2.23) | 11 (3.43) | 10 (3.41) | 11 (2.94) | 9 (3.54) |
| Sulfamethoxazole and α TMP | 10 (4.05) | 8 (4.33) | 10 (3.41) | 9 (3.38) | 13 (3.19) | 11 (3.16) | 14 (2.75) | 13 (2.69) | |
| Chloramphenicol | 11 (3.54) | 11 (3.49) | 9 (3.62) | 10 (3.4) | 12 (3.33) | 12 (2.97) | 12 (2.92) | 12 (3.09) | |
| Clarithromycin | Watch | 14 (2.46) | 15 (2.36) | 14 (2.63) | 13 (2.36) | 14 (2.75) | 13 (2.94) | 10 (3.41) | 11 (3.23) |
| Furazidin | Access | 13 (3.01) | 13 (2.93) | 13 (2.70) | 12 (2.48) | 16 (1.96) | 15 (2.19) | 15 (2.20) | 14 (2.46) |
| Gentamicin | 9 (4.30) | 10 (3.94) | 12 (3.06) | 11 (2.55) | 26 (0.31) | 23 (0.65) | 19 (0.87) | 40 (0.01) | |
| Metronidazole | 18 (1.71) | 17 (2.11) | 18 (2.04) | 16 (2.15) | 17 (1.67) | 16 (1.92) | 16 (2.08) | 15 (2.31) | |
| Tetracycline | 17 (1.95) | 18 (1.95) | 21 (0.85) | 21 (0.87) | 18 (1.33) | 18 (1.05) | 17 (1.56) | 22 (0.09) | |
| Nitroxoline | ∞ UC | 19 (1.28) | 20 (1.13) | 19 (1.39) | 19 (1.20) | 19 (1.18) | 20 (1.04) | 21 (0.64) | 19 (0.75) |
| Norfloxacin | Watch | 20 (1.09) | 22 (0.91) | 20 (0.90) | 20 (0.87) | 20 (1.12) | 19 (1.05) | 23 (0.61) | 18 (1.03) |
| Cefixime | 27 (0.57) | 23 (0.86) | 23 (0.84) | 25 (0.55) | 21 (0.70) | 21 (1.02) | 18 (1.36) | 17 (1.36) | |
| Ofloxacin | 22 (0.84) | 25 (0.73) | 25 (0.60) | 23 (0.62) | 22 (0.69) | 22 (0.69) | 20 (0.74) | 20 (0.71) | |
| Erythromycin | 21 (1.04) | 21 (1.00) | 22 (0.85) | 24 (0.55) | 25 (0.47) | 25 (0.40) | 26 (0.35) | 25 (0.07) | |
| Year | “Access” Group Antibiotics, % (95% CI) | “Watch” Group Antibiotics, % (95% CI) | “Reserve” Group Antibiotics, % (95% CI) | “Unclassified” Group Antibiotics, % (95% CI) |
|---|---|---|---|---|
| 2025 | 42.91 (33.78–52.05) | 56.25 (47.06–65.44) | 0.001 (−0.001–0.004) | 0.89 (0.20–1.58) |
| 2026 | 41.07 (28.16–53.99) | 58.15 (45.15–71.14) | 0.001 (−0.001–0.004) | 0.89 (−0.09–1.87) |
| 2027 | 39.23 (23.41–55.05) | 60.04 (44.13–75.96) | 0.001 (−0.001–0.004) | 0.89 (−0.31–2.09) |
| 2028 | 37.39 (19.13–55.66) | 61.94 (43.56–80.32) | 0.001 (−0.001–0.004) | 0.89 (−0.50–2.27) |
| 2029 | 35.55 (15.13–55.98) | 63.84 (43.29–84.38) | 0.001 (−0.001–0.004) | 0.89 (−0.66–2.44) |
| 2030 | 33.71 (11.34–56.09) | 65.73 (43.22–88.24) | 0.001 (−0.001–0.004) | 0.89 (−0.81–2.58) |
| Model parameters | ARIMA (0.1.0) p = 0.240 | ARIMA (0.1.0) p = 0.230 | ARIMA (0.0.0) p = 0.011 | Simple p = 0.028 |
| Substance (0 ATC5 Code) | Primary Care Professionals | |||||
|---|---|---|---|---|---|---|
| * GPs | Internists and Pediatricians | Pharmacists | ||||
| r | p Value | r | p Value | r | p Value | |
| Cefpodoxime (J01DD13) | 0.941 | <0.001 | −0.934 | 0.001 | - | - |
| Ceftaroline-fosamil (J01DI02) | - | - | - | - | −0.740 | 0.036 |
| Erythromycin (J01FA01) | −0.911 | 0.002 | 0.967 | <0.001 | - | - |
| Spiramycin (J01FA02) | 0.703 | 0.052 | ||||
| Midecamycin (J01FA03) | - | - | 0.807 | 0.015 | - | - |
| Roxithromycin (J01FA06) | −0.841 | 0.009 | 0.901 | 0.002 | ||
| Josamycin (J01FA07) | - | - | 0.792 | 0.019 | - | - |
| Streptomycin (J01GA01) | −0.723 | 0.043 | 0.726 | 0.041 | - | - |
| Kanamycin (J01GB04) | −0.711 | 0.048 | - | - | ||
| Pefloxacin (J01MA03) | −0.929 | 0.001 | 0.928 | 0.002 | - | - |
| Moxifloxacin (J01MA14) | 0.888 | 0.003 | −0.881 | 0.004 | - | - |
| Pipemidic acid (J01MB04) | −0.944 | <0.001 | 0.912 | 0.002 | - | - |
| Vancomycin (J01XA01) | - | - | - | - | −0.766 | 0.027 |
| Colistin (J01XB01) | −0.700 | 0.053 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetova, K.; Makalkina, L.; Pivina, L.; Lim, L.; Aukenov, N.; Boranbayev, K.; Stukas, R.; Belikhina, T.; Aldiyarova, N.; Turgambayeva, A.; et al. Trends in ‘Watch’ and ‘Reserve’ Antibiotic Use in Primary Care in Kazakhstan: The Imperative for Enhancing Stewardship Strategies. Antibiotics 2025, 14, 963. https://doi.org/10.3390/antibiotics14100963
Akhmetova K, Makalkina L, Pivina L, Lim L, Aukenov N, Boranbayev K, Stukas R, Belikhina T, Aldiyarova N, Turgambayeva A, et al. Trends in ‘Watch’ and ‘Reserve’ Antibiotic Use in Primary Care in Kazakhstan: The Imperative for Enhancing Stewardship Strategies. Antibiotics. 2025; 14(10):963. https://doi.org/10.3390/antibiotics14100963
Chicago/Turabian StyleAkhmetova, Kamila, Larissa Makalkina, Lyudmila Pivina, Lisa Lim, Nurlan Aukenov, Kuandyk Boranbayev, Rimantas Stukas, Tatiana Belikhina, Nurgul Aldiyarova, Assiya Turgambayeva, and et al. 2025. "Trends in ‘Watch’ and ‘Reserve’ Antibiotic Use in Primary Care in Kazakhstan: The Imperative for Enhancing Stewardship Strategies" Antibiotics 14, no. 10: 963. https://doi.org/10.3390/antibiotics14100963
APA StyleAkhmetova, K., Makalkina, L., Pivina, L., Lim, L., Aukenov, N., Boranbayev, K., Stukas, R., Belikhina, T., Aldiyarova, N., Turgambayeva, A., & Semenova, Y. (2025). Trends in ‘Watch’ and ‘Reserve’ Antibiotic Use in Primary Care in Kazakhstan: The Imperative for Enhancing Stewardship Strategies. Antibiotics, 14(10), 963. https://doi.org/10.3390/antibiotics14100963









