The N-Terminal Extension of the Mycobacterium avium Rel Protein Is a Dual Regulator of the Bifunctional Enzyme and Represents a Novel Target
Abstract
1. Introduction

2. Results and Discussion
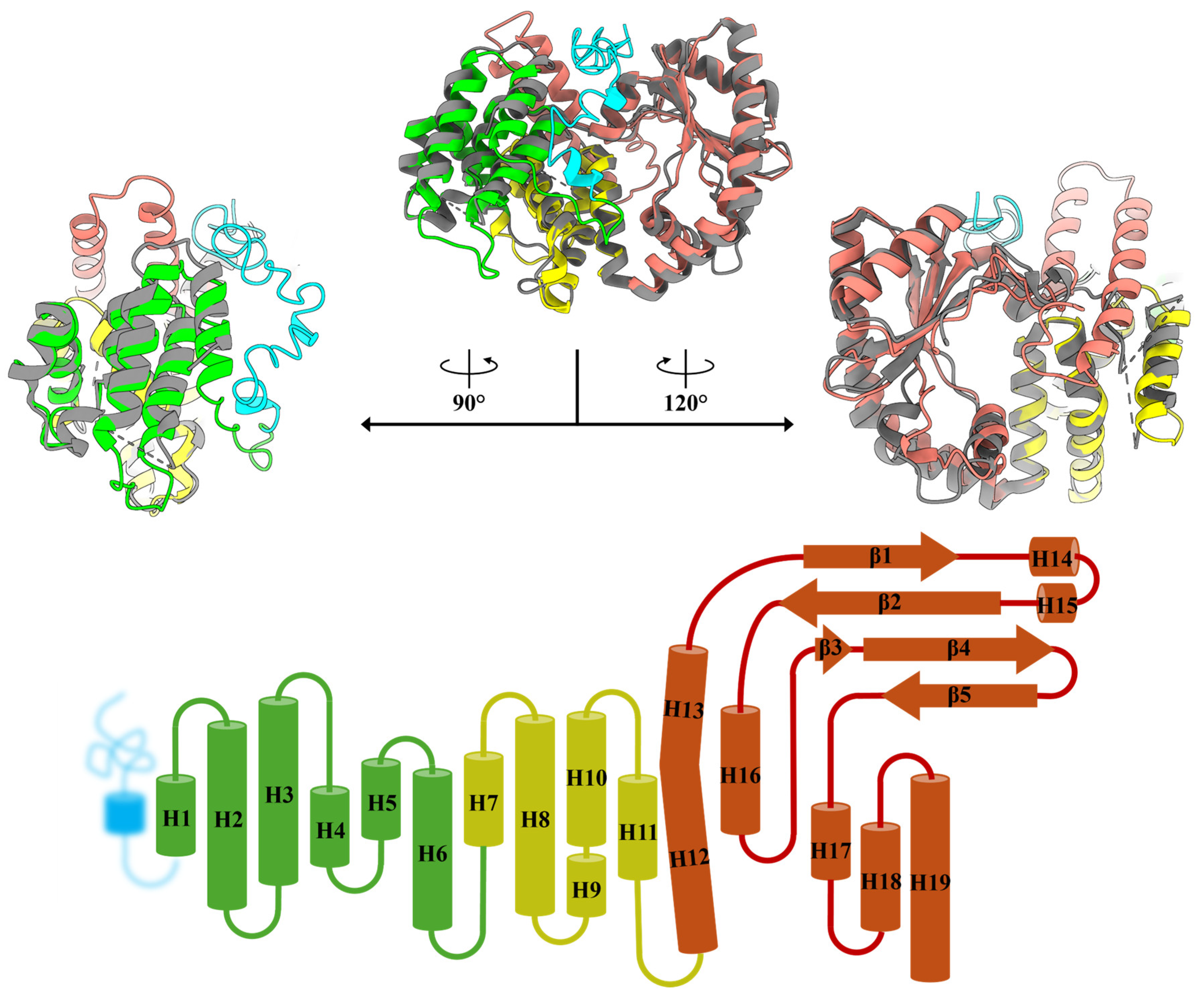
2.1. Construction of MavRel1–444 Prediction Model and Identification of Probable NTD Binding Motif
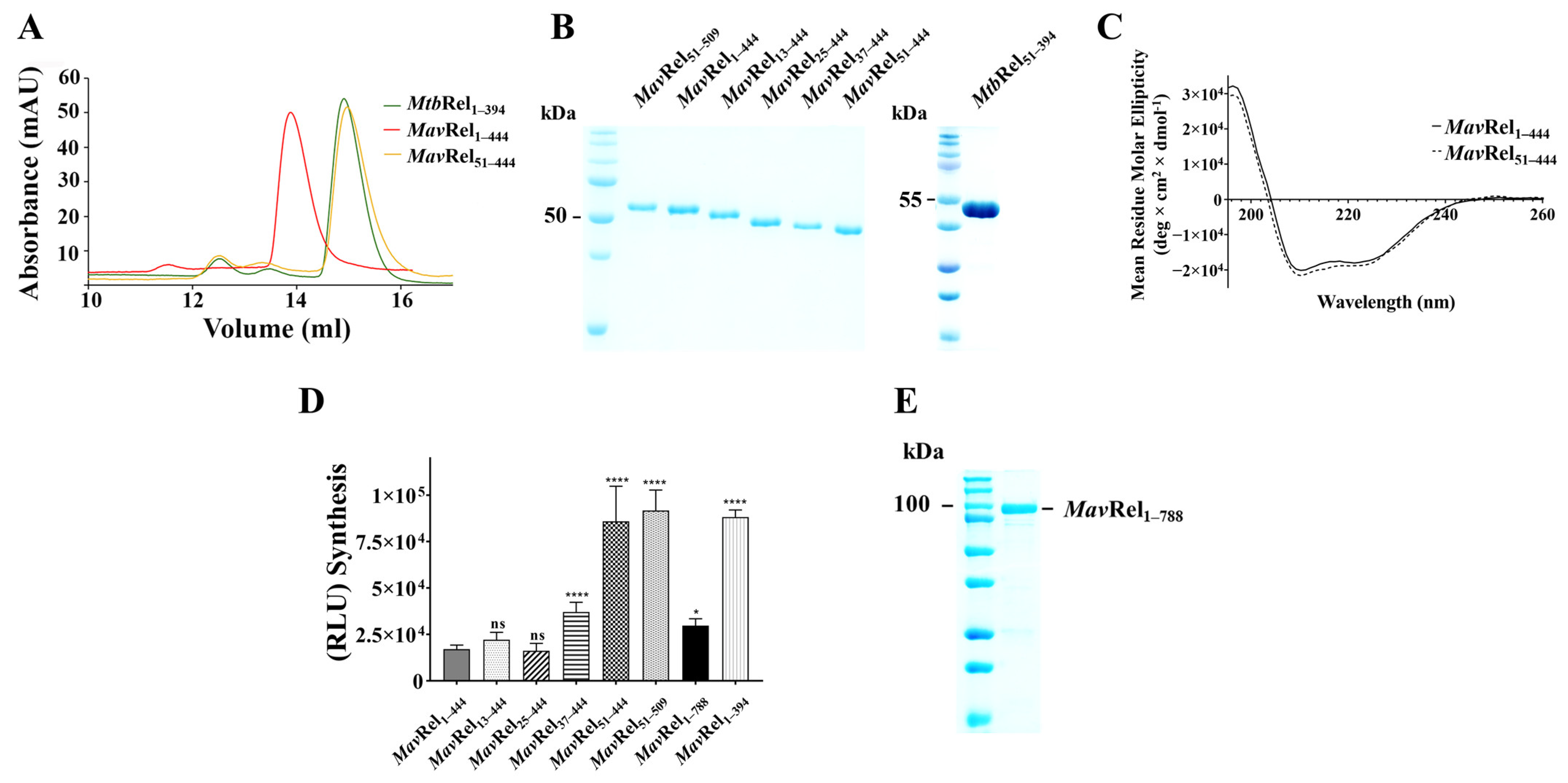
2.2. Expression and Isolation of MavRel NTD and Its Truncates
2.3. The N-Terminal Extension of MavRel1–444 Affects Synthetase Activity
2.4. MavRel Is Critical for the Balancing of Currencies of Life
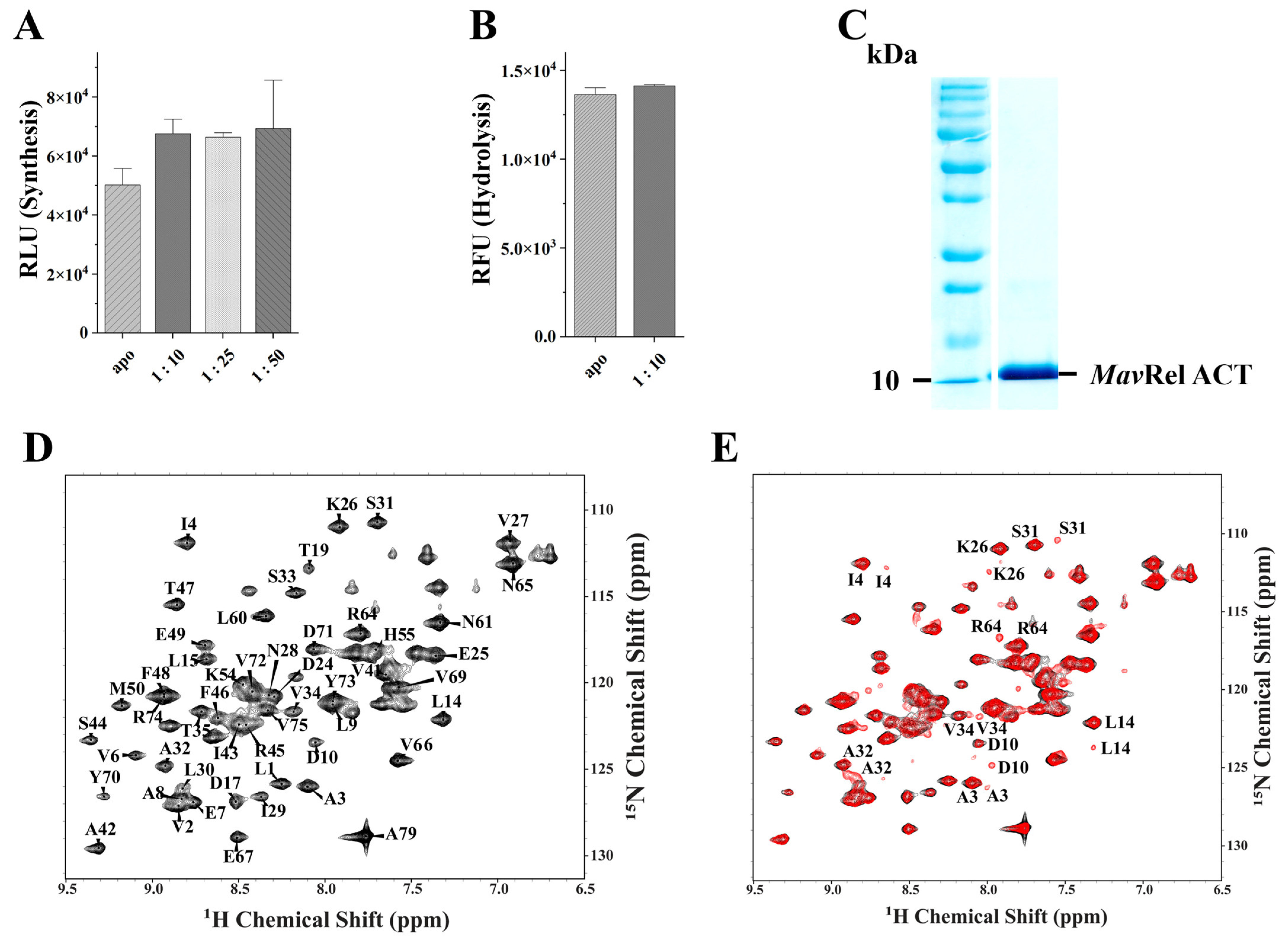
2.5. TGS Domain Does Not Affect Mav’s Bifunctional NTD Domain
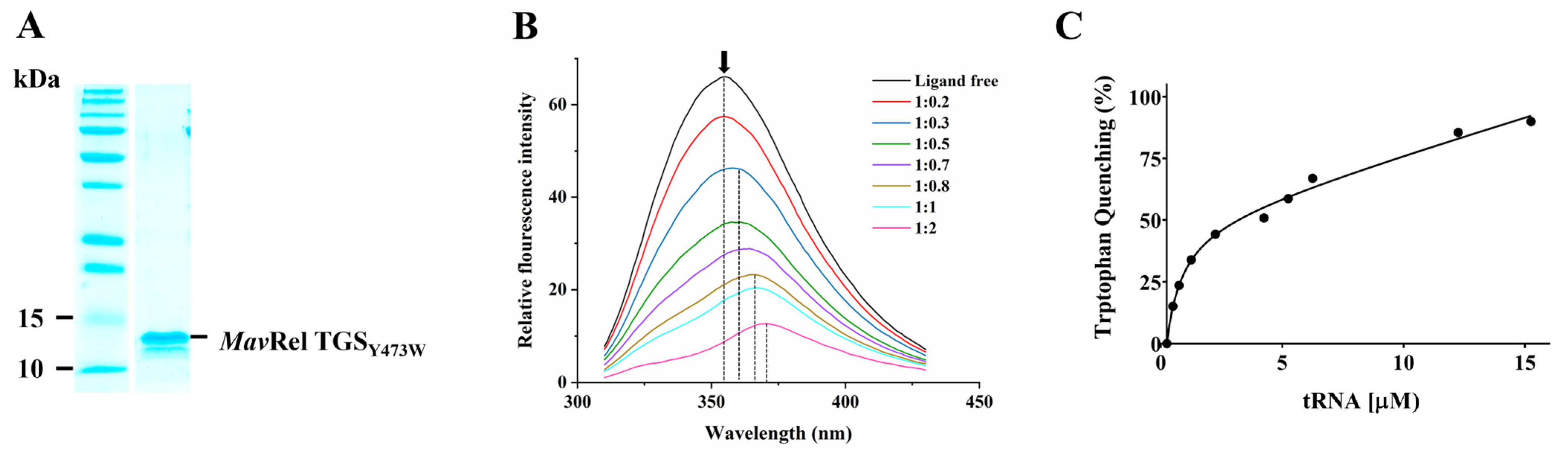
2.6. TGS Domain Binds to the tRNA
2.7. MavRel ACT Interacts with Valine
3. Conclusions
4. Materials and Methods
4.1. Structure Prediction and Modeling
4.2. Generation of MavRel WT, NTD Constructs, Y473W Substitutions, and TGS/ACT-Domain Isolates
4.3. Expression and Purification of MavRel WT and Its Mutants
4.4. CD Spectroscopy
4.5. Synthetase Activity Assay
4.6. Hydrolysis Assay
4.7. Tryptophan Fluorescence Quenching Spectroscopy
4.8. NMR Spectroscopy Data Acquisition and Backbone Assignment
4.9. NMR Titration of MavRel ACT with Valine
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tortoli, E.; Rindi, L.; Garcia, M.J.; Chiaradonna, P.; Dei, R.; Garzelli, C.; Kroppenstedt, R.M.; Lari, N.; Mattei, R.; Mariottini, A.; et al. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54 Pt 4, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, B.; Schafle, D.; Kalin, N.; Widmer, T.; Sander, P. Drug susceptibility distributions of Mycobacterium chimaera and other non-tuberculous mycobacteria. Antimicrob. Agents Chemother. 2023, 65, e02131-20. [Google Scholar]
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Marras, T.K.; Nelson, P.; Peci, A.; Richard-Greenblatt, M.; Brode, S.; Sullivan, A.; Jamieson, F.B.; Kus, J.V. Pulmonary Nontuberculous Mycobacteria, Ontario, Canada, 2020. Emerg. Infect. Dis. 2023, 29, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Jeong, B.H.; Jeon, K.; Lee, N.Y.; Lee, K.S.; Woo, S.Y.; Shin, S.J.; Kwon, O.J. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest 2012, 142, 1482–1488. [Google Scholar] [CrossRef]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium avium and Mycobacterium abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef]
- Nishiuchi, Y.; Iwamoto, T.; Maruyama, F. Infection Sources of a Common Non-tuberculous Mycobacterial Pathogen, Mycobacterium avium Complex. Front. Med. 2017, 4, 27. [Google Scholar] [CrossRef]
- Struelens, M.J.; Plachouras, D. Mycobacterium chimaera infections associated with heater-cooler units (HCU): Closing another loophole in patient safety. Euro Surveill. 2016, 21, 30397. [Google Scholar] [CrossRef]
- Busatto, C.; Vianna, J.S.; da Silva, L.V.J.; Ramis, I.B.; da Silva, P.E.A. Mycobacterium avium: An overview. Tuberculosis 2019, 114, 127–134. [Google Scholar] [CrossRef]
- van Ingen, J. Microbiological diagnosis of nontuberculous mycobacterial pulmonary disease. Clin. Chest Med. 2015, 36, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Dulberger, C.L.; Rubin, E.J.; Boutte, C.C. The mycobacterial cell envelope—A moving target. Nat. Rev. Microbiol. 2020, 18, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.; Young, L.S.; Bermudez, L.E. A subinhibitory concentration of clarithromycin inhibits Mycobacterium avium biofilm formation. Antimicrob. Agents Chemother. 2004, 48, 4907–4910. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xie, J. Comparative genomics of Mycobacterium tuberculosis drug efflux pumps and their transcriptional regulators. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 163–180. [Google Scholar] [CrossRef]
- Renvoise, A.; Brossier, F.; Galati, E.; Veziris, N.; Sougakoff, W.; Aubry, A.; Robert, J.; Cambau, E.; Jarlier, V.; Bernard, C. Assessing primary and secondary resistance to clarithromycin and amikacin in infections due to Mycobacterium avium complex. Antimicrob. Agents Chemother. 2015, 59, 7153–7155. [Google Scholar] [CrossRef]
- Zhang, Y.; Yew, W.W. Mechanisms of drug resistance in Mycobacterium tuberculosis: Update 2015. Int. J. Tuberc. Lung Dis. 2015, 19, 1276–1289. [Google Scholar] [CrossRef]
- Egelund, E.F.; Fennelly, K.P.; Peloquin, C.A. Medications and monitoring in nontuberculous mycobacteria infections. Clin. Chest Med. 2015, 36, 55–66. [Google Scholar] [CrossRef]
- Koh, W.J.; Jeong, B.H.; Jeon, K.; Lee, S.Y.; Shin, S.J. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2012, 186, 797–802. [Google Scholar] [CrossRef]
- Xu, H.B.; Jiang, R.H.; Li, L. Treatment outcomes for Mycobacterium avium complex: A systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 347–358. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kim, S.; Hong, Y.; Lee, S.D.; Kim, W.S.; Kim, D.S.; Shim, T.S.; Jo, K.W. Risk factors for recurrence after successful treatment of Mycobacterium avium complex lung disease. Antimicrob. Agents Chemother. 2015, 59, 2972–2977. [Google Scholar] [CrossRef]
- Charrier, E.S.A.; Dassonville-Klimpt, A.; Andrejak, C.; Sonnet, P. M. avium Complex Pulmonary Infections: Therapeutic Obstacles and Progress in Drug Development. Pharmaceuticals 2025, 18, 891. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabala, X.; Fernandez, I.S.; Kelley, A.C.; Carton, D.G.; Ramakrishnan, V.; Valle, M. The ribosome triggers the stringent response by RelA via a highly distorted tRNA. EMBO Rep. 2013, 14, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Singal, B.; Gruber, A.; Wong, D.M.K.; Ragunathan, P.; Gruber, G. Atomic structure of the regulatory TGS domain of Rel protein from Mycobacterium tuberculosis and its interaction with deacylated tRNA. FEBS Lett. 2021, 595, 3006–3018. [Google Scholar] [CrossRef]
- Jain, V.; Saleem-Batcha, R.; China, A.; Chatterji, D. Molecular dissection of the mycobacterial stringent response protein Rel. Protein Sci. 2006, 15, 1449–1464. [Google Scholar] [CrossRef]
- Avarbock, D.; Avarbock, A.; Rubin, H. Differential regulation of opposing RelMtb activities by the aminoacylation state of a tRNA·ribosome·mRNA·RelMtb complex. Biochemistry 2000, 39, 11640–11648. [Google Scholar] [CrossRef]
- Wang, J.D.; Sanders, G.M.; Grossman, A.D. Nutritional control of elongation of DNA replication by (p)ppGpp. Cell 2007, 128, 865–875. [Google Scholar] [CrossRef]
- Durfee, T.; Hansen, A.M.; Zhi, H.; Blattner, F.R.; Jin, D.J. Transcription profiling of the stringent response in Escherichia coli. J. Bacteriol. 2008, 190, 1084–1096. [Google Scholar] [CrossRef]
- Mitkevich, V.A.; Ermakov, A.; Kulikova, A.A.; Tankov, S.; Shyp, V.; Soosaar, A.; Tenson, T.; Makarov, A.A.; Ehrenberg, M.; Hauryliuk, V. Thermodynamic characterization of ppGpp binding to EF-G or IF2 and of initiator tRNA binding to free IF2 in the presence of GDP, GTP, or ppGpp. J. Mol. Biol. 2010, 402, 838–846. [Google Scholar] [CrossRef]
- Gropp, M.; Strausz, Y.; Gross, M.; Glaser, G. Regulation of Escherichia coli RelA requires oligomerization of the C-terminal domain. J. Bacteriol. 2001, 183, 570–579. [Google Scholar] [CrossRef]
- Mechold, U.; Murphy, H.; Brown, L.; Cashel, M. Intramolecular regulation of the opposing (p)ppGpp catalytic activities of Rel(Seq), the Rel/Spo enzyme from Streptococcus equisimilis. J. Bacteriol. 2002, 184, 2878–2888. [Google Scholar] [CrossRef]
- Pausch, P.; Abdelshahid, M.; Steinchen, W.; Schafer, H.; Gratani, F.L.; Freibert, S.A.; Wolz, C.; Turgay, K.; Wilson, D.N.; Bange, G. Structural Basis for Regulation of the Opposing (p)ppGpp Synthetase and Hydrolase within the Stringent Response Orchestrator Rel. Cell Rep. 2020, 32, 108157. [Google Scholar] [CrossRef] [PubMed]
- Tamman, H.; Van Nerom, K.; Takada, H.; Vandenberk, N.; Scholl, D.; Polikanov, Y.; Hofkens, J.; Talavera, A.; Hauryliuk, V.; Hendrix, J.; et al. A nucleotide-switch mechanism mediates opposing catalytic activities of Rel enzymes. Nat. Chem. Biol. 2020, 16, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Bauer, C.E. Regulation of stringent factor by branched-chain amino acids. Proc. Natl. Acad. Sci. USA 2018, 115, 6446–6451. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Singal, B.; Sony Subramanian Manimekalai, M.; Wei Chen, M.; Ragunathan, P.; Gruber, G. Atomic structure of, and valine binding to the regulatory ACT domain of the Mycobacterium tuberculosis Rel protein. FEBS J. 2021, 288, 2377–2397. [Google Scholar] [CrossRef]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef]
- Singal, B.; Balakrishna, A.M.; Nartey, W.; Manimekalai, M.S.S.; Jeyakanthan, J.; Gruber, G. Crystallographic and solution structure of the N-terminal domain of the Rel protein from Mycobacterium tuberculosis. FEBS Lett. 2017, 591, 2323–2337. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef]
- Erdos, G.; Dosztanyi, Z. AIUPred: Combining energy estimation with deep learning for the enhanced prediction of protein disorder. Nucleic Acids Res. 2024, 52, W176–W181. [Google Scholar] [CrossRef]
- Erdos, G.; Deutsch, N.; Dosztanyi, Z. AIUPred—Binding: Energy Embedding to Identify Disordered Binding Regions. J. Mol. Biol. 2025, 437, 169071. [Google Scholar] [CrossRef]
- Dosztanyi, Z.; Csizmok, V.; Tompa, P.; Simon, I. The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J. Mol. Biol. 2005, 347, 827–839. [Google Scholar] [CrossRef]
- Thomas, P.D.; Dill, K.A. An iterative method for extracting energy-like quantities from protein structures. Proc. Natl. Acad. Sci. USA 1996, 93, 11628–11633. [Google Scholar] [CrossRef] [PubMed]
- Ragunathan, P.; Sielaff, H.; Sundararaman, L.; Biukovic, G.; Subramanian Manimekalai, M.S.; Singh, D.; Kundu, S.; Wohland, T.; Frasch, W.; Dick, T.; et al. The uniqueness of subunit alpha of mycobacterial F-ATP synthases: An evolutionary variant for niche adaptation. J. Biol. Chem. 2017, 292, 11262–11279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, Y.; Zhou, S.; Ran, T.; Zhang, Y.; Zhao, Z.; Feng, Z.; Yu, L.; Xu, J.; Shi, K.; et al. Inhibition of M. tuberculosis and human ATP synthase by BDQ and TBAJ-587. Nature 2024, 631, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.F.; Gruber, G. The Unique C-Terminal Extension of Mycobacterial F-ATP Synthase Subunit alpha Is the Major Contributor to Its Latent ATP Hydrolysis Activity. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Guo, H.; Courbon, G.M.; Bueler, S.A.; Mai, J.; Liu, J.; Rubinstein, J.L. Structure of mycobacterial ATP synthase bound to the tuberculosis drug bedaquiline. Nature 2021, 589, 143–147. [Google Scholar] [CrossRef]
- Wong, C.F.; Saw, W.G.; Basak, S.; Sano, M.; Ueno, H.; Kerk, H.W.; Litty, D.; Ragunathan, P.; Dick, T.; Muller, V.; et al. Structural Elements Involved in ATP Hydrolysis Inhibition and ATP Synthesis of Tuberculosis and Nontuberculous Mycobacterial F-ATP Synthase Decipher New Targets for Inhibitors. Antimicrob. Agents Chemother. 2022, 66, e0105622. [Google Scholar] [CrossRef]
- Harikishore, A.; Wong, C.F.; Ragunathan, P.; Litty, D.; Muller, V.; Gruber, G. Targeting Mycobacterial F-ATP Synthase C-Terminal alpha Subunit Interaction Motif on Rotary Subunit gamma. Antibiotics 2021, 10, 1456. [Google Scholar] [CrossRef]
- Yammine, A.; Gao, J.; Kwan, A.H. Tryptophan Fluorescence Quenching Assays for Measuring Protein-ligand Binding Affinities: Principles and a Practical Guide. Bio Protoc. 2019, 9, e3253. [Google Scholar] [CrossRef]
- Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 3.1; Schrödinger, LLC: New York, NY, USA, 2025.
- Prusa, J.; Zhu, D.X.; Stallings, C.L. The stringent response and Mycobacterium tuberculosis pathogenesis. Pathog. Dis. 2018, 76, fty054. [Google Scholar] [CrossRef]
- Mrnjavac, N.; Martin, W.F. GTP before ATP: The energy currency at the origin of genes. Biochim. Biophys. Acta Bioenerg. 2025, 1866, 149514. [Google Scholar] [CrossRef]
- Choudhary, S.; Lopus, M.; Hosur, R.V. Targeting disorders in unstructured and structured proteins in various diseases. Biophys. Chem. 2022, 281, 106742. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, B.; Toth, J.; Vertessy, B.G.; Dosztanyi, Z.; Simon, I. Proteins with complex architecture as potential targets for drug design: A case study of Mycobacterium tuberculosis. PLoS Comput. Biol. 2011, 7, e1002118. [Google Scholar] [CrossRef] [PubMed]
- Blundell, T.L.; Gupta, M.N.; Hasnain, S.E. Intrinsic disorder in proteins: Relevance to protein assemblies, drug design and host-pathogen interactions. Prog. Biophys. Mol. Biol. 2020, 156, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Anurag, M.; Dash, D. Unraveling the potential of intrinsically disordered proteins as drug targets: Application to Mycobacterium tuberculosis. Mol. Biosyst. 2009, 5, 1752–1757. [Google Scholar] [CrossRef]
- Saurabh, S.; Nadendla, K.; Purohit, S.S.; Sivakumar, P.M.; Cetinel, S. Fuzzy Drug Targets: Disordered Proteins in the Drug-Discovery Realm. ACS Omega 2023, 8, 9729–9747. [Google Scholar] [CrossRef]
- Dyson, H.J.; Wright, P.E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 2005, 6, 197–208. [Google Scholar] [CrossRef]
- Hegyi, H.; Schad, E.; Tompa, P. Structural disorder promotes assembly of protein complexes. BMC Struct. Biol. 2007, 7, 65. [Google Scholar] [CrossRef]
- Cheng, Y.; LeGall, T.; Oldfield, C.J.; Mueller, J.P.; Van, Y.Y.; Romero, P.; Cortese, M.S.; Uversky, V.N.; Dunker, A.K. Rational drug design via intrinsically disordered protein. Trends Biotechnol. 2006, 24, 435–442. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Linking folding and binding. Curr. Opin. Struct. Biol. 2009, 19, 31–38. [Google Scholar] [CrossRef]
- van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef]
- Shangary, S.; Wang, S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: A novel approach for cancer therapy. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 223–241. [Google Scholar] [CrossRef]
- Kojima, K.; Konopleva, M.; McQueen, T.; O’Brien, S.; Plunkett, W.; Andreeff, M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood 2006, 108, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, S.; Birol, M.; Rhoades, E.; Miranker, A.D.; Levine, Z.A. Targeting the Intrinsically Disordered Proteome Using Small-Molecule Ligands. Methods Enzymol. 2018, 611, 703–734. [Google Scholar] [PubMed]
- Avarbock, A.; Avarbock, D.; Teh, J.S.; Buckstein, M.; Wang, Z.M.; Rubin, H. Functional regulation of the opposing (p)ppGpp synthetase/hydrolase activities of RelMtb from Mycobacterium tuberculosis. Biochemistry 2005, 44, 9913–9923. [Google Scholar] [CrossRef] [PubMed]
- Dosztanyi, Z.; Meszaros, B.; Simon, I. ANCHOR: Web server for predicting protein binding regions in disordered proteins. Bioinformatics 2009, 25, 2745–2746. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Rovnyak, D.; Frueh, D.P.; Sastry, M.; Sun, Z.Y.; Stern, A.S.; Hoch, J.C.; Wagner, G. Accelerated acquisition of high resolution triple-resonance spectra using non-uniform sampling and maximum entropy reconstruction. J. Magn. Reson. 2004, 170, 15–21. [Google Scholar] [CrossRef]
- Joon, S.; Ragunathan, P.; Sundararaman, L.; Nartey, W.; Kundu, S.; Manimekalai, M.S.S.; Bogdanovic, N.; Dick, T.; Gruber, G. The NMR solution structure of Mycobacterium tuberculosis F-ATP synthase subunit epsilon provides new insight into energy coupling inside the rotary engine. FEBS J. 2018, 285, 1111–1128. [Google Scholar] [CrossRef]
- Lee, W.; Tonelli, M.; Markley, J.L. NMRFAM-SPARKY: Enhanced software for biomolecular NMR spectroscopy. Bioinformatics 2015, 31, 1325–1327. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fong, T.C.; Ragunathan, P.; Alag, R.; Silvester, C.; Sonthalia, S.; Mathiyazakan, V.; Grover, V.; Grüber, G. The N-Terminal Extension of the Mycobacterium avium Rel Protein Is a Dual Regulator of the Bifunctional Enzyme and Represents a Novel Target. Antibiotics 2025, 14, 964. https://doi.org/10.3390/antibiotics14100964
Fong TC, Ragunathan P, Alag R, Silvester C, Sonthalia S, Mathiyazakan V, Grover V, Grüber G. The N-Terminal Extension of the Mycobacterium avium Rel Protein Is a Dual Regulator of the Bifunctional Enzyme and Represents a Novel Target. Antibiotics. 2025; 14(10):964. https://doi.org/10.3390/antibiotics14100964
Chicago/Turabian StyleFong, Tuck Choy, Priya Ragunathan, Reema Alag, Carlos Silvester, Svarika Sonthalia, Vikneswaran Mathiyazakan, Vandana Grover, and Gerhard Grüber. 2025. "The N-Terminal Extension of the Mycobacterium avium Rel Protein Is a Dual Regulator of the Bifunctional Enzyme and Represents a Novel Target" Antibiotics 14, no. 10: 964. https://doi.org/10.3390/antibiotics14100964
APA StyleFong, T. C., Ragunathan, P., Alag, R., Silvester, C., Sonthalia, S., Mathiyazakan, V., Grover, V., & Grüber, G. (2025). The N-Terminal Extension of the Mycobacterium avium Rel Protein Is a Dual Regulator of the Bifunctional Enzyme and Represents a Novel Target. Antibiotics, 14(10), 964. https://doi.org/10.3390/antibiotics14100964






