Abstract
Background/Objective: Timely identification of bloodstream pathogens is critical for sepsis management; however, PCR inhibitors such as sodium polyanetholesulfonate (SPS) in blood culture broth compromise nucleic acid recovery and long read sequencing. We assessed whether coupling a benzyl alcohol SPS-removal step to the fully automated LabTurbo AIO extractor improves Oxford Nanopore-based pathogen detection. Methods: Thirteen positive blood culture broths were pre-treated with benzyl alcohol and divided: half volumes were purified on the LabTurbo AIO; paired aliquots underwent manual QIAamp extraction. DNA purity was evaluated by NanoDrop and Qubit. Barcoded libraries were sequenced on MinION R9.4.1 flow cells for 6 h. Results: Automated eluates showed a median A260/A280 of 1.92 and A260/A230 of 1.96, versus 1.80 and 1.48 for manual extracts. The automated workflow generated 1.69 × 106 total reads compared with 3.9 × 105 reads for manual extraction. The median N50 read length increased from 5.9 kb to 8.7 kb, and the median proportion of reads classified to species increased from 62% to 84%. The hands-on time was <5 min and the sample-to-answer turnaround was <8 h, compared with >9 h and 90 min for the manual protocol, respectively. Conclusions: Benzyl alcohol SPS removal integrated into the LabTurbo AIO extractor yielded purer, longer, and higher read counts, enhancing nanopore sequencing depth and accuracy while compressing diagnostic turnaround to a single working day. This represents a practical advance for rapid blood culture pathogen identification in critical care settings.
1. Introduction
Bloodstream infections (BSIs) are a major global health problem, accounting for an estimated 48.9 million cases and 11 million deaths in 2017 alone [1]. Every hour of delay in administering effective antibiotics after septic shock onset increases mortality, underscoring the need for rapid pathogen identification [2]. Conventional culture workflows require 24–72 h for species-level identification, with antimicrobial susceptibility testing adding at least 1 more day—driving interest in same-day molecular diagnostics.
Long-read sequencing with Oxford Nanopore Technologies (ONT) has emerged as a promising solution. Proof-of-concept studies have demonstrated that metagenomic ONT sequencing can identify bacterial pathogens and resistance genes directly from respiratory samples within 6 h [3] and positive blood culture broths in real time [4]. Prospective intensive-care trials report accurate species calls and genotype-based susceptibility predictions in <10 h [5], whereas unified clinical pipelines combining host DNA depletion, streamlined library preparation, and cloud-based analysis have reduced sample-to-answer time to ≈7 h [6]. Recent work has also highlighted the value of Nanopore sequencing for high-resolution antimicrobial-resistance (AMR) surveillance. Chung et al. generated near-complete assemblies for 98 bovine Salmonella isolates, revealing mobile AMR determinants that were invisible to short-read data [7]. Likewise, Shelenkov et al. resolved plasmid-mediated amikacin resistance in multidrug-resistant Klebsiella pneumoniae from patients with COVID-19 in the intensive care unit using a hybrid Nanopore/Illumina approach [8]. These studies underscore the capacity of ONT to deliver both taxonomic and resistome information within clinically actionable time frames.
The quality of extracted nucleic acids remains the principal bottleneck in genomic sequencing workflows. Sodium polyanetholesulfonate (SPS), an anticoagulant in blood culture bottles, co-purifies with DNA and potently inhibits PCR and other enzymatic reactions. Fredricks and Relman first showed that benzyl alcohol phase separation efficiently partitions SPS from the aqueous DNA phase and restores robust amplification [9]. Subsequent refinements incorporating a benzyl alcohol/guanidine phase step followed by silica-column purification further improved inhibitor removal [10]; however, these protocols are manual and operator-dependent.
Preserving high-molecular-weight (HMW) DNA is equally important. Harsh bead-beating markedly shortens read length, whereas enzymatic or magnetic-bead chemistries yield longer fragments suitable for ONT sequencing [11]. In urine metagenomics, an enzymatic lysis protocol has been shown to increase the median read length 2.1-fold and boost mapped reads > 11-fold over mechanical lysis [12]. A six-kit comparison across matrices identified an HMW magnetic-bead kit as being superior for long-read metagenomics [13]. Cross-biospecimen evaluations confirmed that extraction bias can skew multi-omics analyses [14], and ultra-deep ONT datasets from mock communities highlighted the fragility of long-read performance to DNA quality [15]. Beyond infectious-disease applications, metagenomic next-generation sequencing has improved the diagnosis of meningitis and encephalitis when conventional tests failed [16].
Automation offers a pragmatic path to reproducibility and scalability. Integrating automated extraction modules can cut hands-on time by >60% and deliver sequencing-ready DNA in <30 min without sacrificing yield or accuracy [17]. However, no study has combined benzyl alcohol SPS removal with a fully automated platform optimized for ONT sequencing of positive blood culture specimens.
To bridge this gap, we evaluated the LabTurbo AIO automated extraction system, which incorporates benzyl alcohol phase separation, against a reference manual silica-column protocol. We compared DNA purity and yield, ONT read-length distribution, pathogen-classification accuracy, and total turnaround time in clinical blood culture isolates. By uniting inhibitor-tolerant chemistry with walk-away automation, our study aims to deliver a practical same-day sequencing workflow for critical care bacteraemia diagnostics.
2. Results
2.1. DNA Purity After Benzyl-Alcohol Pretreatment and Automated Extraction
Spectrophotometric analysis confirmed effective SPS removal and carryover after benzyl alcohol phase separation. Excluding two low-concentration AIO extracts whose NanoDrop ratios were not reliable (clBC-09 and clBC-12, flagged “NR” in Table 1), the automated LabTurbo AIO workflow generated DNA with a median A260/A280 ratio of 1.90 (interquartile range [IQR]: 1.89–1.99) and a median A260/A230 ratio of 1.90 (IQR: 1.28–2.08). Conversely, manual QIAamp extraction produced median values of 2.17 and 2.40, respectively (p = 0.03 for A260/A230, Mann–Whitney U-test). In our series, Qubit concentration was undetectable in 2/13 AIO extracts (clBC-09 and clBC-12) but in 0/13 Qiagen extracts. Among the remaining libraries, AIO showed higher DNA concentrations (median 43.8 ng/µL; IQR 32.8–79.35) than Qiagen (9.66 ng/µL; IQR 7.77–26.9), indicating that low concentration was an infrequent event (2/13) rather than a systematic limitation of the automated workflow. As summarized in Table 1, the lower DNA yield observed in these two AIO samples, which was not seen in their manual counterparts, might be attributed to a low starting bacterial concentration in the aliquots processed. In such cases, a standardized automated protocol may be less efficient at recovering scarce nucleic acids compared to a manual procedure.

Table 1.
DNA purity and yield metrics for DNA extracted from 13 positive blood culture broths.
2.2. Automated Workflow Increases Read Count, Read Length, and Assembly Contiguity
Oxford Nanopore sequencing metrics for all 13 paired extracts are summarized in Table 2. The automated LabTurbo AIO (LabTurbo, New Taipei City, Taiwan) workflow generated a cumulative 1.73 × 106 reads (median, 91,259 per sample, IQR: 14,483–138,154), whereas the manual Qiagen workflow produced 3.9 × 105 reads in total (median 3216 per sample, IQR: 1042–36,490). Total bases followed the same trend, exceeding 1.37 × 109 for the top-yielding AIO sample compared with just over 3.4 × 108 for its manual counterpart.

Table 2.
Oxford Nanopore sequencing metrics for 13 positive blood culture specimens processed by automated LabTurbo AIO versus manual Qiagen extraction.
Read-length statistics likewise favoured the automated protocol. The median of median read lengths across AIO extracts was 1264 bp (IQR: 459–2514 bp) versus 1058 bp (IQR: 373–1174 bp) for manual extracts. The N50 value—a proxy for assembly contiguity—reached 18.3 kb in the best AIO sample. Overall, the automated workflow delivered a median N50 of 8.7 kb (IQR: 4.3–12.7 kb) compared with 5.9 kb (IQR: 4.2–7.8 kb) for the manual method. This difference, however, was not statistically significant (p = 0.1824, Mann–Whitney U-test).
Notably, the median per-read quality score was slightly lower in LabTurbo AIO samples (median Q score 10, IQR: 9–11) compared to manual samples (median Q score 11, IQR: 10–11). Per-sample quality-score distributions are shown in Supplementary Figure S1. Across 13 pairs, AIO libraries had a median Q score of 10 (IQR 9–11; 6/13 libraries ≤ 9; 4/13 ≥ 11), whereas Qiagen libraries had a median of 11 (IQR 10–11; 2/13 ≤ 9; 8/13 ≥ 11). Even with slightly lower Q scores (median 10 vs. 11), species-level classification and assembly contiguity (N50) favored AIO (Table 2); however, analyses that require single-nucleotide accuracy (e.g., SNP calling or high-resolution plasmid reconstruction) are more sensitive to per-base error and may require greater depth and/or consensus polishing. We did not benchmark variant calling in this study.
2.3. Increased Sequencing Output and Read-Length Metrics
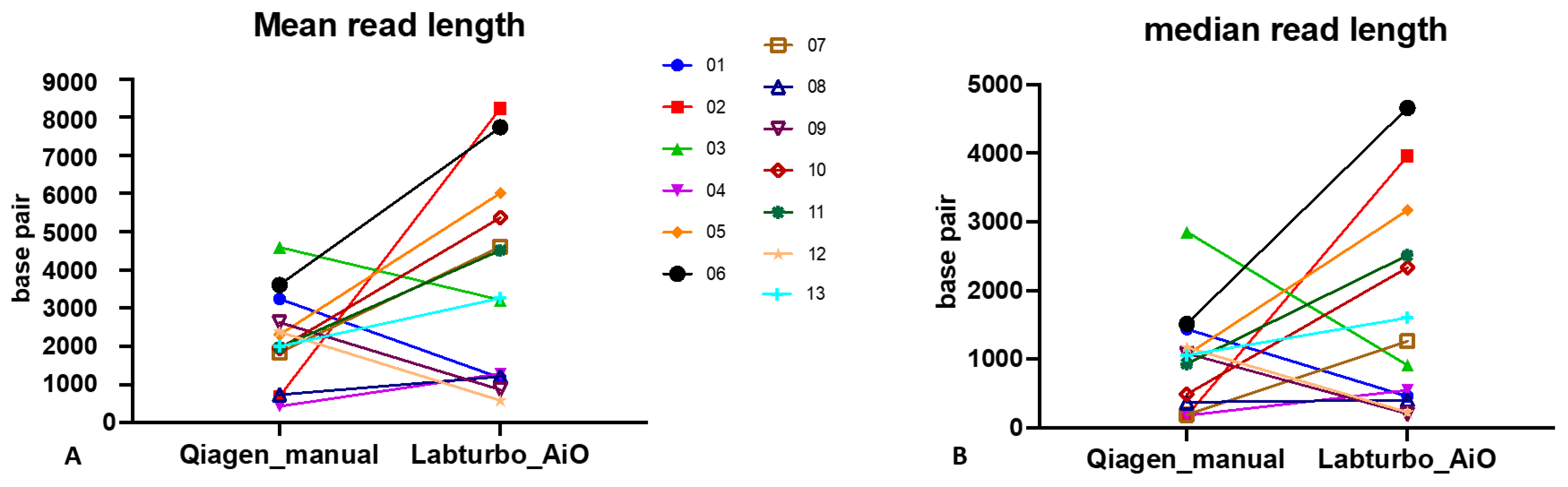
Oxford Nanopore sequencing shows a clear read-length advantage when nucleic acids are prepared with the LabTurbo AIO. Across the 13 paired extracts, median read length increased from 934 bp (IQR: 432–1439 bp) with the manual Qiagen protocol to 1264 bp (IQR: 914–3169 bp) with the automated workflow (p = 0.003, Mann–Whitney U-test). Mean read length followed the same trend, rising from 1175 bp (median across samples) to 4518 bp (Figure 1A). The mean and median read length improved in 9 of 13 AIO samples (Figure 1), confirming that the longer fragments seen in Table 2 translated into a general trend of longer reads at the single-sample level. The N50 benefit and the >4-fold increase in the total read count are detailed separately in Table 2.
Figure 1.
Automated LabTurbo AIO extraction yields longer ONT reads than manual Qiagen extraction. (A) Paired comparison of mean read length for each of the 13 positive blood culture specimens sequenced after manual Qiagen extraction or automated LabTurbo AIO extraction. (B) Paired comparison of the corresponding median read length for the same specimens.
2.4. Improved Species-Level Classification Accuracy
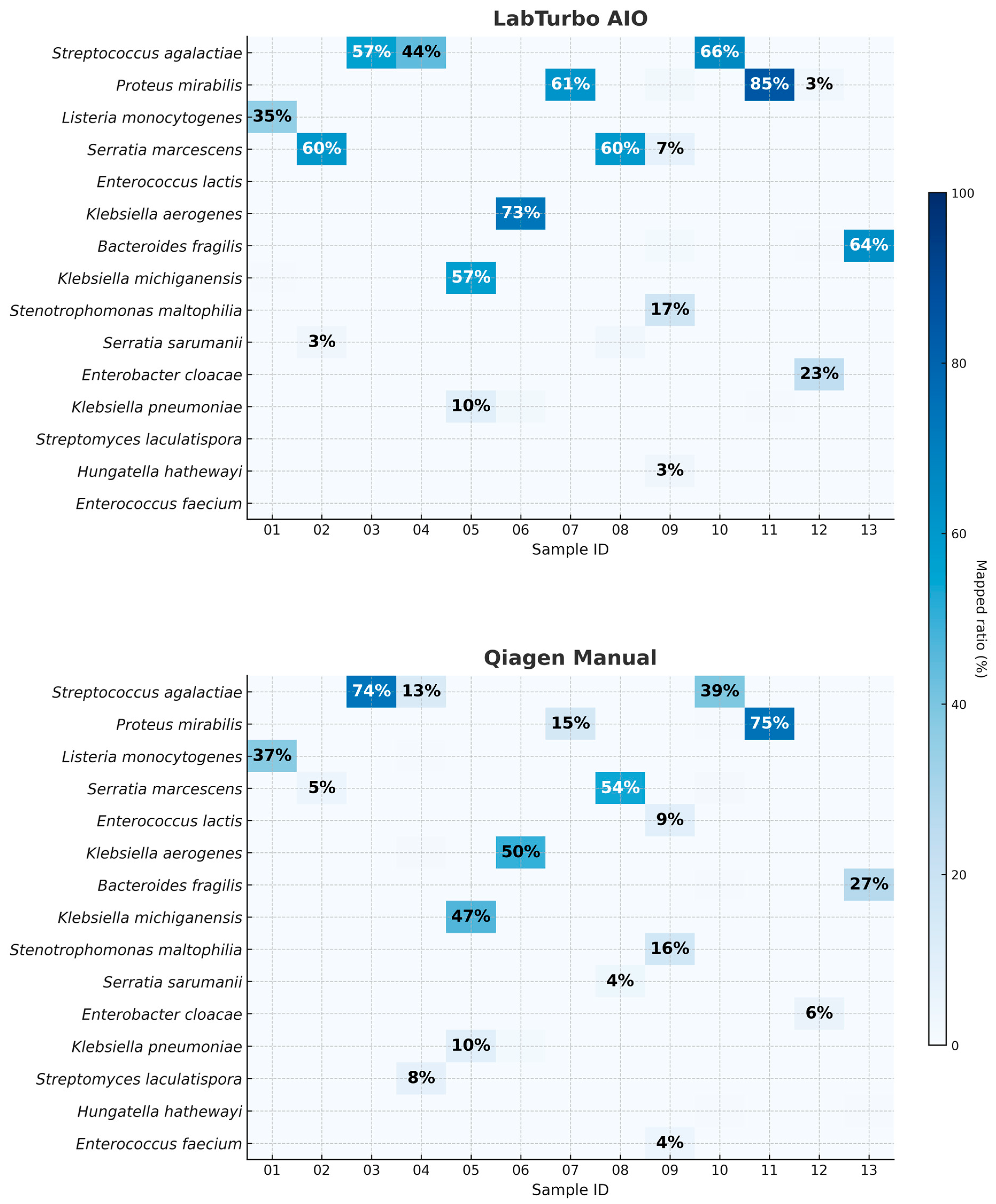
Kraken2/WIMP analysis demonstrated higher taxonomic resolution when DNA was prepared with the LabTurbo AIO. Across the 13 paired datasets, the automated workflow produced a median of 84% for reads mapped at the species level (IQR: 78–87%), compared with 62% (IQR: 52–68%) for the manual Qiagen method (p = 0.002, two-tailed Mann–Whitney U-test). As illustrated in Figure 2, one of the thirteen AIO libraries contained ≥80% reads assigned to a single dominant pathogen, whereas no libraries prepared manually reached that threshold. In some cases, such as clBC_09, the dominant species represented a relatively low proportion of reads (~17%); however, it remained the most abundant taxon and matched the culture result and was therefore considered concordant. Human background reads were ≤5% of the total in 10 out of 13 libraries processed by the LabTurbo AIO method. Detailed taxonomic assignments, including dominant species percentages, human read fractions, and concordance with culture results, are provided in Supplementary Tables S1 and S2. These data suggest that the combination of benzyl alcohol pretreatment and LabTurbo automation delivers cleaner, better-resolved metagenomic signals, thereby facilitating confident pathogen identification without the need for deep sequencing coverage.
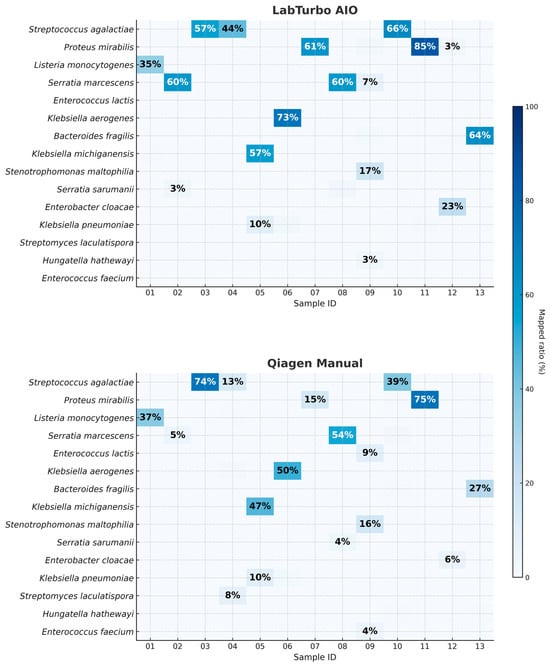
Figure 2.
Species-level read-mapping percentages for the 13 positive blood culture specimens processed by automated and manual workflows. Each cell shows the proportion of total reads (%) classified by Kraken2/WIMP as the indicated species. The upper matrix (LabTurbo AIO) and lower matrix (Qiagen manual) are aligned by specimen ID (01–13). Values in cells represent a percentage of total reads mapped to the indicated species.
2.5. End-to-End Workflow Time and Operational Efficiency
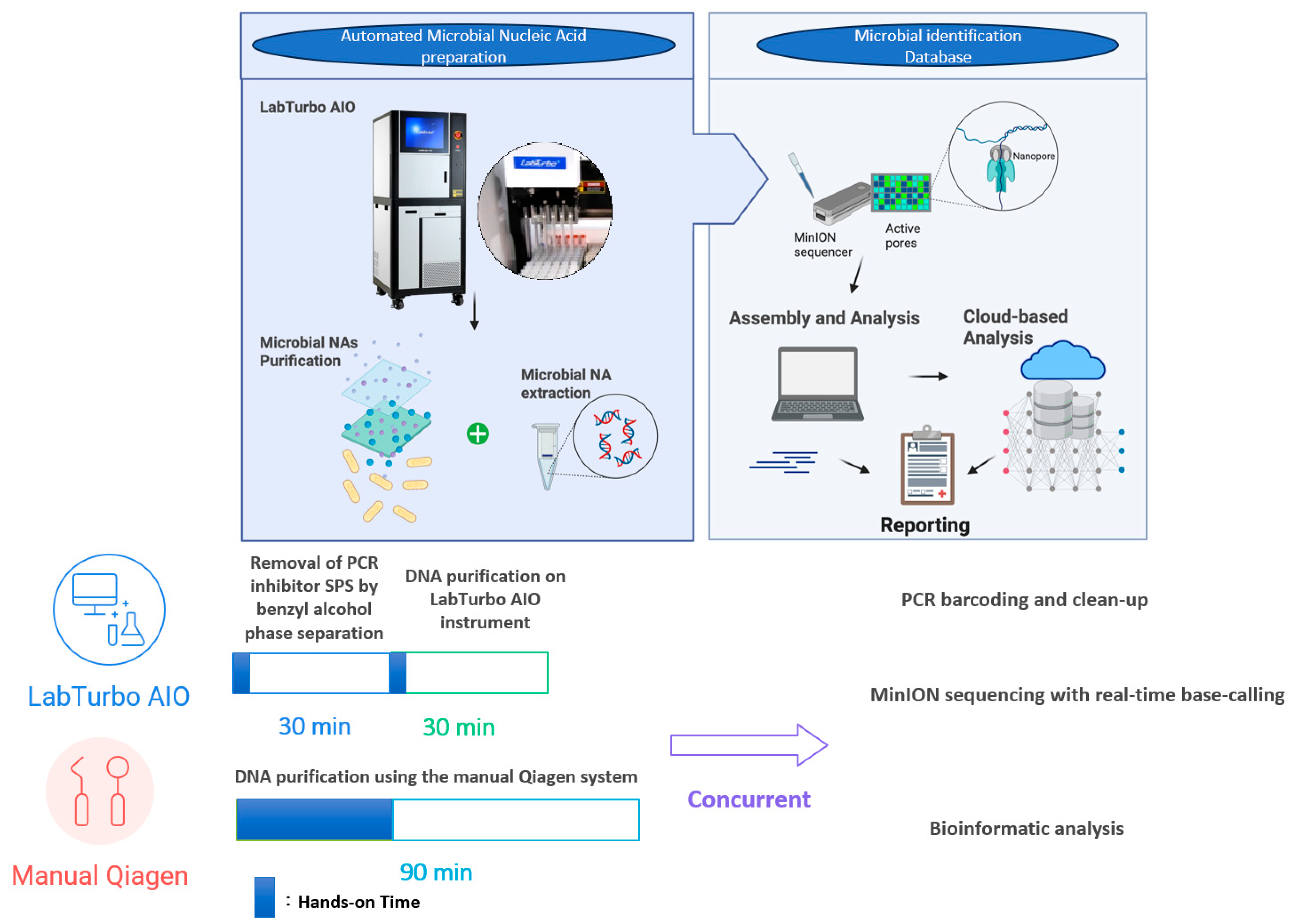
Figure 3 depicts the chronological workflow achieved with the automated protocol. Removal of PCR inhibitors (SPS) via benzyl alcohol phase separation required 30 min, with <5 min of hands-on time. DNA purification on the LabTurbo AIO instrument requires an additional 30 min of walk-away runtime, delivering eluates that proceed directly to library preparation. PCR barcoding and clean-up (≈75 min) are identical for both workflows, after which MinION sequencing runs for 6 h with real-time base-calling. Bioinformatic analysis was performed concurrently; hence, the entire sample-to-answer continuum was accomplished in under 8 h. Contrarily, the manual Qiagen method demands approximately 70 min of hands-on manipulation and 90 min total extraction time, extending the diagnostic pipeline to more than 9 h. The acceleration provided by automation was accompanied by substantive performance gains. Automated eluates display NanoDrop/Qubit (N/Q) ratios of median ≈ 1.0, indicating efficient recovery with minimal solvent carryover (Table 1). The higher molecular-weight DNA obtained yielded longer mean and median read lengths, with a median read-length N50 of 8.7 kb versus 5.9 kb for manually processed samples (Table 2). The automated workflow also generates roughly four-fold more reads, of which a larger fraction map unambiguously to the dominant pathogen (median 84% vs. 62%; Figure 2). Collectively, these improvements shorten the time to confident bacterial identification and preliminary resistance profiling to within a single working day, thereby enhancing the clinical utility of ONT-based blood culture diagnostics.
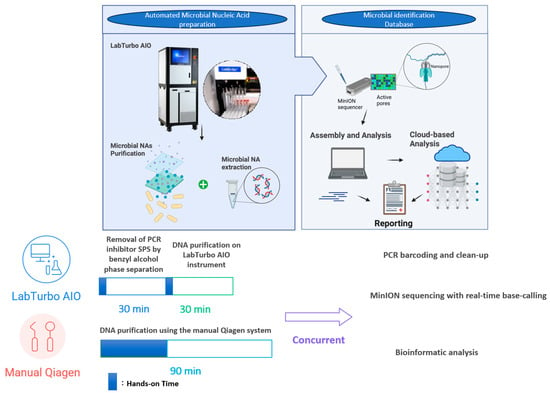
Figure 3.
Comparative Workflow Timeline for Automated and Manual DNA Extraction.
3. Discussion
The combination of benzyl alcohol phase separation with the LabTurbo AIO extractor produced a workflow that shortened the sample-to-report interval to <8 h (Figure 3). The automated workflow typically increased the read yield and read length, though some purity/quantity showed variability (Table 1); the fragment length (Figure 1 and Table 2) and species-level mapping (Figure 2) surpassed values obtained with a conventional silica-column protocol. These gains translated into deeper coverage and more confident taxonomic assignments without extending sequencing time or altering the standard PCR-barcoding kit procedure.
SPS has long been recognised as a potent inhibitor of enzymatic reactions in blood culture broths; Fredricks and Relman first showed that benzyl alcohol extraction efficiently partitions SPS into an organic phase, restoring robust amplification [9]. Here, we demonstrate that this inhibitor-tolerant chemistry can be coupled to a walk-away instrument, reducing hands-on time to <5 min per specimen and delivering sequencing-ready DNA in 30 min.
Our <8 h end-to-end timeline is comparable to the 7–9 h workflows achieved with host DNA depletion or real-time adaptive sampling [18]; however, the proposed approach uses neither depletion kits nor customised run scripts and therefore preserves sequencing yield and simplicity. The long-read performance also compares favourably with other positive-blood culture studies. Harris et al. reported a median N50 of 7.5 kb after enzymatic depletion [5], whereas the automated protocol here yielded a median N50 of 8.7 kb (Table 2). Together with a four-fold increase in the total reads, this improvement raised the median proportion of species-mapped reads from 62% (manual) to 84% (automated) (Figure 2), offering richer data for downstream resistance-gene and plasmid analyses. Per-sample Q-score distributions are shown in Supplementary Figure S1. A critical finding of our study is the trade-off between sequencing yield and data quality. While the LabTurbo AIO workflow substantially increased the total number of reads and median read length, this came at the cost of a modest reduction in per-read quality (median Q10 vs. Q11 for manual). Although this ~1-point difference did not affect species-level identification or contiguity metrics in our dataset, base-level applications (e.g., SNP calling or high-resolution plasmid reconstruction) may require additional depth and/or consensus polishing.
Table 1 shows the NanoDrop/Qubit (N/Q) ratios for AIO samples ranged from 0.76 to 4.10. Ratios near 1.0 indicate good agreement between spectrophotometric and fluorometric quantification, while higher ratios suggest NanoDrop overestimation from contaminants. Most AIO samples clustered around ~1.0 (see Table 1), with two low-concentration outliers flagged in Section 2.1, confirming efficient recovery with minimal solvent carryover. This reliability obviates repeat extractions that prolong conventional workflows and may exhaust limited specimen volume. Longer reads and higher depth further enable accurate consensus polishing and rapid pathogen identification within a single working day—an outcome likely to improve empirical-therapy optimization in sepsis management.
This investigation has a few limitations. First, the study is dependent on a positive blood culture signal, limiting its application in early sepsis. Second, only 13 single-morphotype blood culture specimens from a single centre were analysed; validation in larger, multi-centre cohorts and polymicrobial samples is warranted. Third, while sequencing was performed in real-time, library preparation remained manual and contributed ≈75 min to the timeline; forthcoming transposase-based kits may shorten this step further. Fourth, this was a purely methodological comparison without clinical outcome data. Fifth, we also note potential biological heterogeneity: extraction yields may differ between Gram-positive and Gram-negative organisms due to cell-wall architecture; further protocol refinements such as enzymatic pre-lysis may benefit Gram-positive species. Sixth, economic evaluation was not conducted; while reduced labour costs may be offset by capital investment in low-throughput settings, high-throughput laboratories may achieve cost-effectiveness through decreased processing time and accelerated reporting. Seventh, antimicrobial-resistance gene detection and plasmid reconstruction were not systematically assessed, though critical for sepsis management. Eighth, external validation in larger, multi-centre cohorts is needed to assess reproducibility.
4. Materials and Methods
4.1. Clinical Specimens
All procedures were conducted following the Declaration of Helsinki and were approved by the Institutional Review Board of Tri-Service General Hospital (TSGHIRB No.: B202405085; approved on 24 May 2024). Informed consent was obtained from all participants.
From January to March 2025, we prospectively retrieved 13 consecutive blood culture bottles that signalled positive on a BacT/ALERT Virtuo instrument (bioMérieux, Marcy-l’Étoile, France) within 1 h of the alarm. Ten bottles were aerobic and three anaerobic. Gram-stain of each broth showed a single bacterial morphotype. This positive signal served as the time-zero (T0) starting point for the subsequent < 8 h diagnostic workflow evaluated in this study.
4.2. Benzyl Alcohol Phase-Separation Pretreatment
Each positive broth (500 µL) was mixed 1:1 with lysis buffer (5 M guanidine-hydrochloride, 100 mM Tris-HCl pH 8.0; Sigma-Aldrich, St Louis, MO, USA). Benzyl alcohol (600 µL, ≥99.5%; Merck, Darmstadt, Germany) was added, the tube vortexed for 10 s, and centrifuged at 20,000× g for 5 min at 25 °C in a Model 5424 R centrifuge (Eppendorf, Hamburg, Germany). The upper aqueous phase (≈200 µL) was transferred to a new tube and processed immediately. A molecular-grade water blank underwent the same steps as a negative control.
4.3. Nucleic Acid Extraction
4.3.1. Nucleic Acid Automated Extraction
The aqueous phase was purified on the LabTurbo AIO platform (LabTurbo Biotech, New Taipei City, Taiwan) using the manufacturer’s “BC_DNA_200 µL” script. DNA was eluted in 50 µL of AE buffer pre-warmed to 56 °C. Instrument run time was 30 min and required <5 min hands-on.
4.3.2. Nucleic Acid Manual Extraction
An aliquot of the same pre-treated lysate (200 µL) was processed with the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following the “Blood and Body Fluid Spin” protocol, including a double AW2 wash and 1 min dry spin at 20,000× g. DNA was eluted in 50 µL of AE buffer.
4.4. DNA Quality Assessment
Purity ratios (260/280 and 260/230) were measured with a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Concentration was determined with a Qubit 4 fluorometer and dsDNA HS Assay Kit (Thermo Fisher Scientific). Only samples satisfying the purity criteria of A260/A280 ≈ 1.8–2.0 and A260/A230 > 1.8 proceeded to library preparation.
4.5. Library Preparation and Nanopore Sequencing
Up to 400 ng of DNA were barcoded with the PCR Barcoding Kit SQK-PBK004 (Oxford Nanopore Technologies [ONT], Oxford, UK) according to protocol PBK_9125_v109_revS_24Mar2024 [19]. Amplification used LongAmp Taq DNA polymerase (New England Biolabs, Ipswich, MA, USA) and 14 cycles of 95 °C, 30 s, 62 °C, 15 s, and 65 °C, 3 min. Barcoded libraries were pooled, loaded onto an R9.4.1 FLO-MIN106D flow cell, and sequenced for 6 h on a MinION Mk1B device. Raw signals were base-called with Guppy v6.5.7 in high-accuracy mode (Q ≥ 7), demultiplexed, and classified with EPI2ME WIMP (2025.3.2) and Kraken2 v2.1.2 against the MiniKraken2-8GB_202410 database.
4.6. Bioinformatic Metrics and Statistical Analysis
Read-length and yield statistics were generated with NanoStat v1.6.0 [20]. Purity ratios, DNA yields, total reads, and N50 read lengths from automated versus manual workflows were compared with a two-tailed Mann–Whitney U test in GraphPad Prism v10.0 (GraphPad Software, San Diego, CA, USA). Statistical significance was set at p < 0.05.
5. Conclusions
Despite these limitations, the data show that benzyl alcohol SPS removal coupled with LabTurbo AIO automation generally provides higher DNA yield and longer read fragments, facilitating same-day Oxford Nanopore–based pathogen identification with minimal operator input. Wider adoption of this workflow may shorten diagnostic time-to-therapy and improve clinical decision-making, pending outcome-linked studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics14101001/s1, Table S1: Taxonomic assignment results showing dominant species, assignment percentages, human reads, and culture agreement by specimen and method; Table S2: Complete species-level taxonomic classification of 13 positive blood culture specimens processed by LabTurbo AIO and Qiagen extraction; Figure S1: Distribution of per-read quality scores.
Author Contributions
Conceptualization, M.-J.J., C.-L.P. and P.-S.H.; Data curation, H.-Y.C. and T.-H.L.; Formal analysis, C.-K.C. and C.-S.T.; Funding acquisition, H.-S.S.; Investigation, H.-Y.C. and C.-K.C.; Methodology, H.-Y.C., T.-H.L. and C.-S.T.; Resources, M.-J.J.; Supervision, P.-S.H. and H.-S.S.; Writing—original draft, M.-J.J. and H.-S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Tri-Service General Hospital, Taipei (grant numbers TSGH-D-113105, TSGH-D-113106, TSGH-D-114101, and TSGH-D-114102).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s).
Data Availability Statement
The datasets generated and analysed in this study are not publicly available due to legal and ethical restrictions imposed by the Tri-Service General Hospital Institutional Review Board (TSGHIRB No. B202405085). De-identified data can be made available from the corresponding author upon reasonable request and with prior approval from the TSGHIRB.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AMR | Antimicrobial Resistance |
| N50 | Read length at which 50% of the total bases are contained in reads ≥ that length |
| ONT | Oxford Nanopore Technologies |
| PBS | Phosphate-Buffered Saline |
| SPS | Sodium Polyanetholesulfonate |
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Charalampous, T.; Kay, G.L.; Richardson, H.; Aydin, A.; Baldan, R.; Jeanes, C.; Rae, D.; Grundy, S.; Turner, D.J.; Wain, J.; et al. Nanopore metagenomics enables rapid clinical diagnosis of bacterial lower respiratory infection. Nat. Biotechnol. 2019, 37, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Taxt, A.M.; Avershina, E.; Frye, S.A.; Naseer, U.; Ahmad, R. Rapid identification of pathogens, antibiotic-resistance genes, and plasmids in blood cultures by nanopore sequencing. Sci. Rep. 2020, 10, 7622. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.N.A.; Bauer, M.J.; Lüftinger, L.; Egle, A.; Kaczmarek, A.; Bateson, M.; Stahlhut, J.; Schöberl, A.; Schäfer, J.; Lagler, H.; et al. Rapid nanopore sequencing and predictive susceptibility testing of positive blood cultures from intensive-care patients with sepsis. Microbiol. Spectr. 2024, 12, e0306523. [Google Scholar] [CrossRef] [PubMed]
- Alcolea-Medina, A.; Alder, C.; Snell, L.B.; Perry, C.; Williams, R.; Hakeem, L.; Frazer, Z.; Price, J.; Edgeworth, J.D.; Goldenberg, S.D. A unified metagenomic method for rapid detection of microorganisms in clinical samples. Commun. Med. 2024, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Dudley, E.; Kittana, H.; Abdo, Z.; Norman, K.; Sanchez, P.L.; Gabler, F.; Lawhon, S.D.; Andrews-Polymenis, H.L. Genomic profiling of antimicrobial resistance genes in clinical Salmonella isolates from cattle in the Texas Panhandle, USA. Antibiotics 2024, 13, 843. [Google Scholar] [CrossRef] [PubMed]
- Shelenkov, A.; Petrova, L.; Mironova, A.; Zamyatin, M.; Korobeynikov, A.; Akimkin, V.; Mikhaylova, Y.; Shcherbakova, L.; Malakhova, M. Long-read whole genome sequencing elucidates the mechanisms of amikacin resistance in multidrug-resistant Klebsiella pneumoniae isolates obtained from COVID-19 patients. Antibiotics 2022, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Fredricks, D.N.; Relman, D.A. Improved amplification of microbial DNA from blood cultures by removal of the PCR inhibitor sodium polyanetholesulfonate. J. Clin. Microbiol. 1998, 36, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Hyman, J.; Scholz, C.F.P.; Godtfredsen, S.E.; Christensen, J.J. Improved DNA extraction from blood cultures by benzyl alcohol/guanidine phase separation. PLoS ONE 2010, 5, e12095. [Google Scholar]
- Trigodet, F.; Lolans, K.; Fogarty, E.; Shaffer, M.; Glickman, C.; Zwick, M.E.; Green, R.E.; Weinstein, R.A.; Hayden, M.K.; Claassen, J.; et al. High-molecular-weight DNA extraction strategies for long-read sequencing of complex metagenomes. Mol. Ecol. Resour. 2022, 22, 1786–1802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, T.; Wang, Y.; Guo, L.; Gao, F.; Bai, H.; Li, J.; Xu, H.; Ma, L.; Zhang, R. Comparison analysis of different DNA extraction methods on suitability for long-read metagenomic nanopore sequencing. Front. Cell. Infect. Microbiol. 2022, 12, 919903. [Google Scholar] [CrossRef]
- Gand, M.; Bloemen, B.; Vanneste, K.; Heylen, K.; Coucke, W.; De Keersmaecker, S.C.J.; Roosens, N.H.C.; De Keersmaecker, S.C.J. Comparison of six DNA extraction methods for isolation of high-yield, high-molecular-weight DNA suitable for shotgun metagenomics nanopore sequencing. BMC Genomics 2023, 24, 438. [Google Scholar] [CrossRef] [PubMed]
- Rehner, J.; Schmartz, G.P.; Groeger, L.; Bunk, B.; Spröer, C.; Overmann, J.; Rupp, O.; Goesmann, A.; Sczyrba, A. Systematic cross-biospecimen evaluation of DNA extraction kits for long- and short-read multi-metagenomic sequencing studies. Genomics Proteomics Bioinform. 2022, 20, 405–417. [Google Scholar] [CrossRef]
- Nicholls, S.M.; Quick, J.C.; Tang, S.; Loman, N.J. Ultra-deep, long-read nanopore sequencing of mock microbial community standards. GigaScience 2019, 8, giz043. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Sample, H.A.; Zorn, K.C.; Arevalo, S.; Yu, G.; Neuhaus, J.; Federman, S.; Stryke, D.; Briggs, B.; Langelier, C.; et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N. Engl. J. Med. 2019, 380, 2327–2340. [Google Scholar] [CrossRef]
- Cardenas, A.M.; Smyth, D.S.; Benson, M.D.; Narayanan, M.; Deiss, T.C.; Jankeel, A.; Dobbins, S.; MacNeil, A.; Altman, D.R.; Zody, M.C.; et al. Automation of DNA Extraction Accelerates Metagenomic Sequencing for Infectious Disease Diagnostics. Clin. Chem. 2023, 69, 310–320. [Google Scholar]
- Payne, A.; Holmes, N.; Clarke, T.; Munro, R.; Debebe, B.J.; Loose, M. ReadFish enables targeted nanopore sequencing of gigabase-sized genomes by real-time mapping. Nat. Biotechnol. 2021, 39, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Oxford Nanopore Technologies. PCR Barcoding Kit SQK-PBK004: Protocol PBK_9125_v109_revS_24Mar2024; Oxford Nanopore Technologies: Oxford, UK, 2024. [Google Scholar]
- De Maayer, P.; NanoStat Developers. NanoStat v1.6.0 User Manual; Vrije Universiteit Amsterdam: Amsterdam, The Netherlands, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).