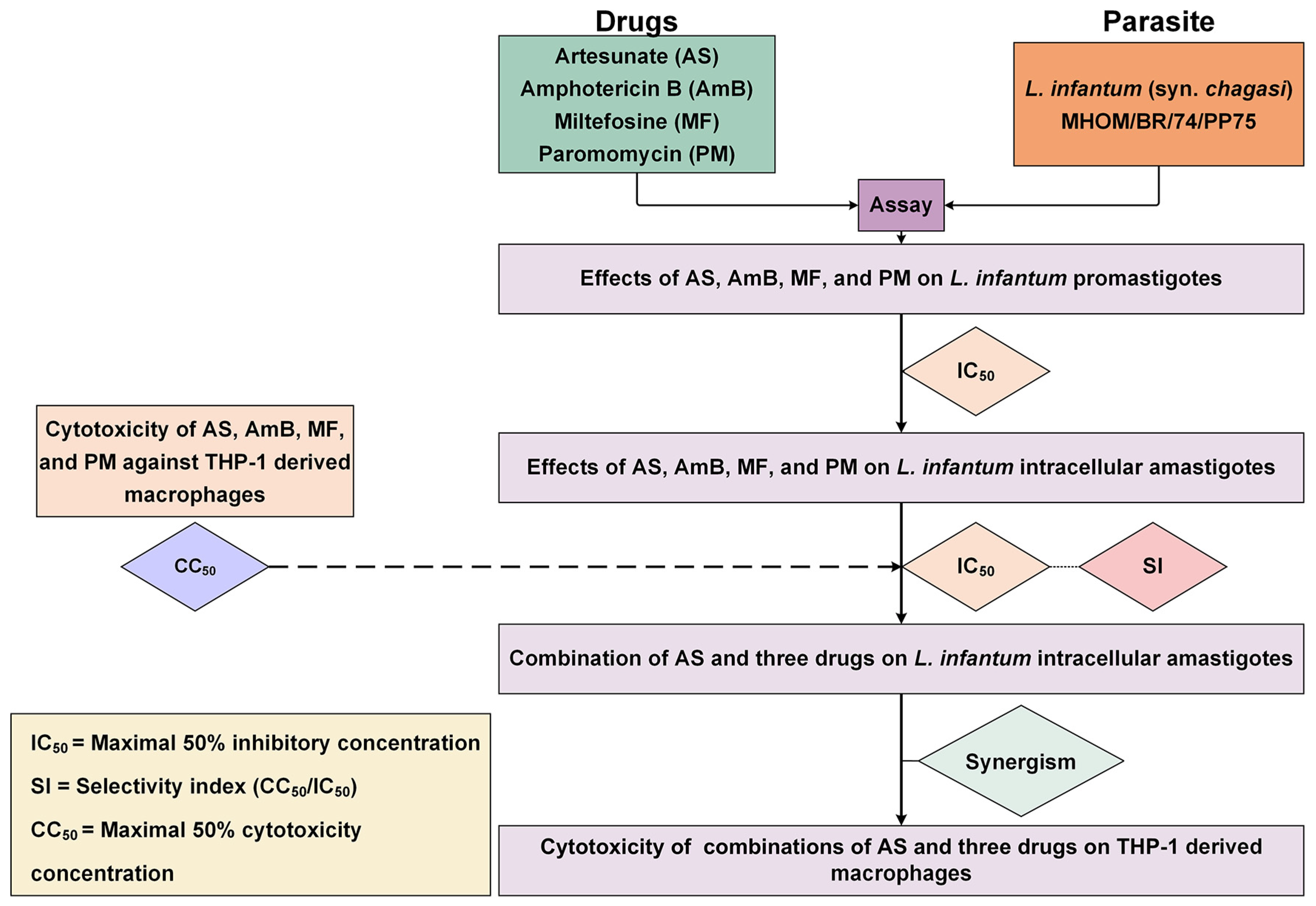
Synergistic Effects of Artesunate in Combination with Amphotericin B and Miltefosine against Leishmania infantum: Potential for Dose Reduction and Enhanced Therapeutic Strategies
Abstract
1. Introduction
2. Results
2.1. Antileishmanial Activity of AS, AmB, MF, and PM against L. infantum
2.2. Cytotoxicity of AS, AmB, MF, and PM to THP-1-Derived Macrophages
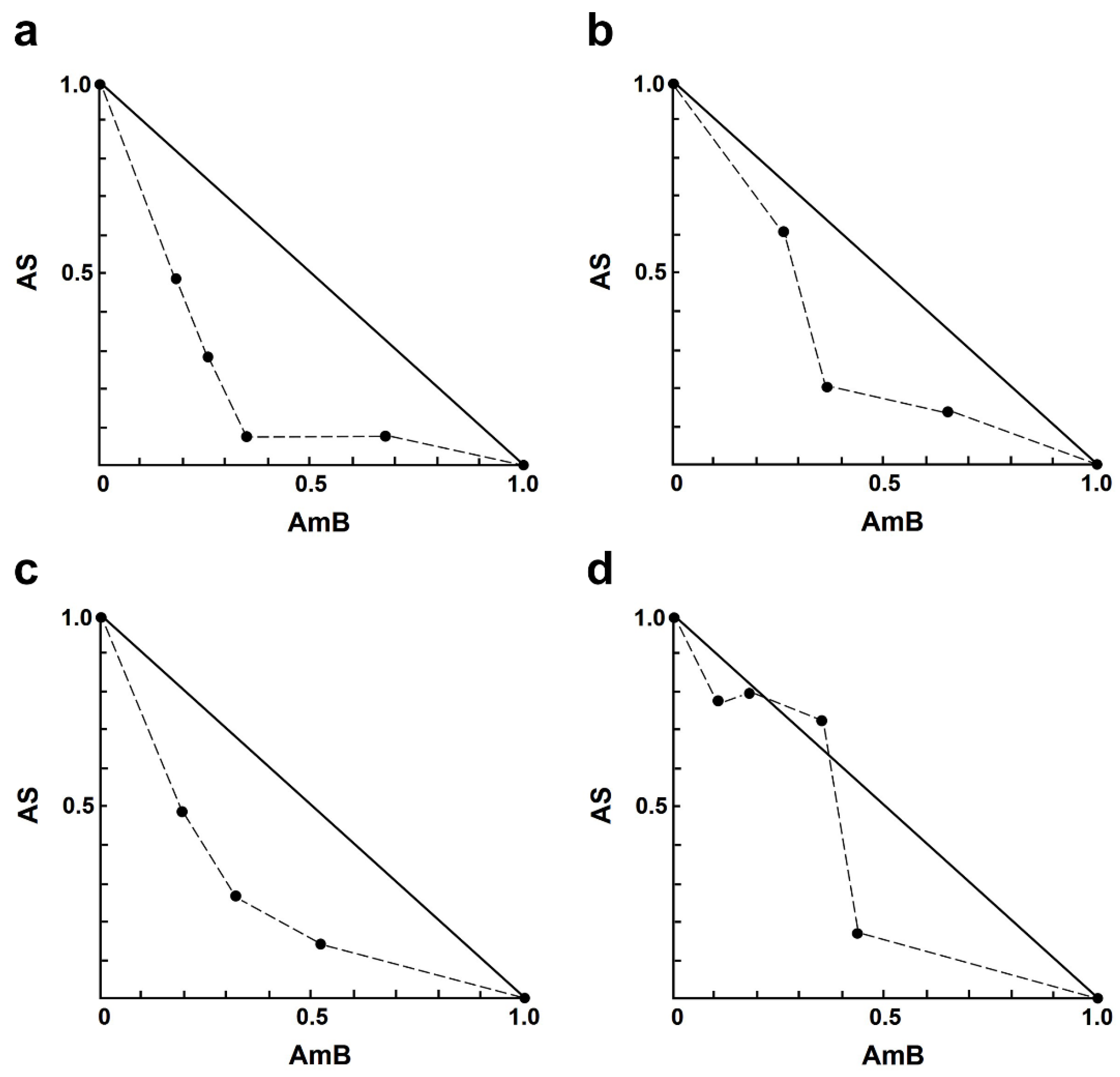
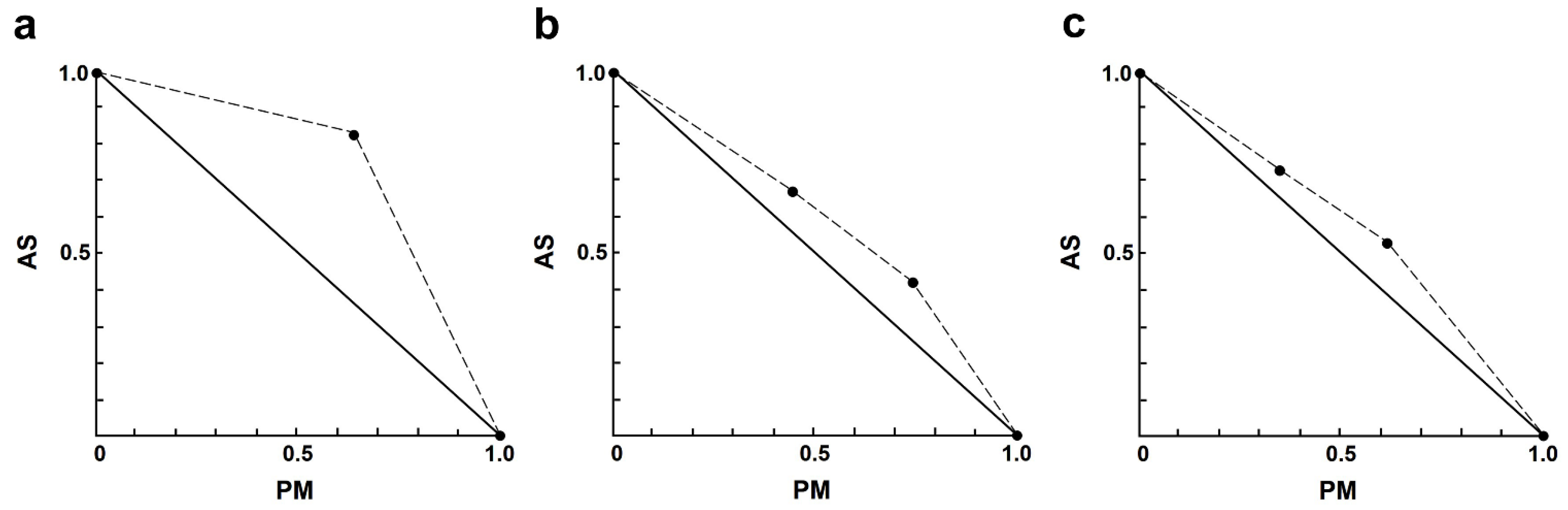
2.3. Combination Effects of Artesunate and Three Drugs against Intracellular Amastigotes
2.4. Cytotoxicity of AS–AmB, AS–MF, and AS–PM Combinations to THP-1-Derived Macrophages
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Parasite Strain
4.3. THP-1 Cell Culture
4.4. Promastigote Assay
4.5. Cytotoxicity Assay
4.6. Preparation of Promastigotes to Infect THP-1-Derived Macrophages
4.7. Intracellular Amastigote Assay and Parasite Rescue and Transformation Assay
4.8. Drug Combination Assay on Intracellular Amastigotes
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 20 June 2024).
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Maia-Elkhoury, A.N.S.; Romero, G.A.S.; Valadas, S.Y.O.B.; Sousa-Gomes, M.L.; Lindoso, J.A.L.; Cupolillo, E.; Ruiz-Postigo, J.A.; Argaw, D.; Sanchez-Vazquez, M.J. Premature deaths by visceral leishmaniasis in Brazil investigated through a cohort study: A challenging opportunity? PLoS Negl. Trop. Dis. 2019, 13, e0007841. [Google Scholar] [CrossRef]
- Varma, N.; Naseem, S. Hematologic changes in visceral leishmaniasis/kala azar. Indian. J. Hematol. Blood Transfus. 2010, 26, 78–82. [Google Scholar] [CrossRef]
- Bates, P.A. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int. J. Parasitol. 2007, 37, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Clos, J.; Grünebast, J.; Holm, M. Promastigote-to-Amastigote Conversion in Leishmania spp.-A Molecular View. Pathogens 2022, 11, 1052. [Google Scholar] [CrossRef]
- Dos Santos Marques, L.H.; IC, D.A.R.; Reis, I.A.; GM, D.A.C.; Oliveira, E.; Pfeilsticker, T.R.; VE, D.E.A.; Morais, M.H.; Rabello, A.; Carneiro, M. Leishmania infantum: Illness, transmission profile and risk factors for asymptomatic infection in an endemic metropolis in Brazil. Parasitology 2017, 144, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine Leishmaniasis: Update on Epidemiology, Diagnosis, Treatment, and Prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef]
- Singh, R.; Kashif, M.; Srivastava, P.; Manna, P.P. Recent Advances in Chemotherapeutics for Leishmaniasis: Importance of the Cellular Biochemistry of the Parasite and Its Molecular Interaction with the Host. Pathogens 2023, 12, 706. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Brustoloni, Y.M.; Fernandes, T.D.; Dorval, M.E.; Cunha, R.V.; Bóia, M.N. Severe adverse reactions to meglumine antimoniate in the treatment of visceral leishmaniasis: A report of 13 cases in the southwestern region of Brazil. Trop. Dr. 2009, 39, 180–182. [Google Scholar] [CrossRef]
- Pradhan, S.; Schwartz, R.A.; Patil, A.; Grabbe, S.; Goldust, M. Treatment options for leishmaniasis. Clin. Exp. Dermatol. 2022, 47, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert. Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Jha, T.K.; Sundar, S.; Thakur, C.P.; Bachmann, P.; Karbwang, J.; Fischer, C.; Voss, A.; Berman, J. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med. 1999, 341, 1795–1800. [Google Scholar] [CrossRef]
- Pérez-Victoria, F.J.; Sánchez-Cañete, M.P.; Seifert, K.; Croft, S.L.; Sundar, S.; Castanys, S.; Gamarro, F. Mechanisms of experimental resistance of Leishmania to miltefosine: Implications for clinical use. Drug Resist. Updat. 2006, 9, 26–39. [Google Scholar] [CrossRef]
- Andersen, E.M.; Cruz-Saldarriaga, M.; Llanos-Cuentas, A.; Luz-Cjuno, M.; Echevarria, J.; Miranda-Verastegui, C.; Colina, O.; Berman, J.D. Comparison of meglumine antimoniate and pentamidine for peruvian cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2005, 72, 133–137. [Google Scholar] [PubMed]
- J, B.; M, B.M.; Chanda, K. An Overview on the Therapeutics of Neglected Infectious Diseases-Leishmaniasis and Chagas Diseases. Front. Chem. 2021, 9, 622286. [Google Scholar] [CrossRef]
- Sinha, S.; Sundaram, S.; Kumar, V.; Tripathi, A. Antimony resistance during Visceral Leishmaniasis: A possible consequence of serial mutations in ABC transporters of Leishmania species. Bioinformation 2011, 6, 107–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sundar, S.; Jha, T.K.; Thakur, C.P.; Sinha, P.K.; Bhattacharya, S.K. Injectable paromomycin for Visceral leishmaniasis in India. N. Engl. J. Med. 2007, 356, 2571–2581. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Woon, C.Y.; Liu, C.G.; Cheng, J.T.; You, M.; Sethi, G.; Wong, A.L.; Ho, P.C.; Zhang, D.; Ong, P.; et al. Repurposing Artemisinin and its Derivatives as Anticancer Drugs: A Chance or Challenge? Front. Pharmacol. 2021, 12, 828856. [Google Scholar] [CrossRef]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef]
- Artemisinin Derivatives: Summary of Nonclinical Safety Data Introductory Remarks; World Health Organization: Geneva, Switzerland, 2016.
- Zhang, J.; Li, Y.; Wan, J.; Zhang, M.; Li, C.; Lin, J. Artesunate: A review of its therapeutic insights in respiratory diseases. Phytomedicine 2022, 104, 154259. [Google Scholar] [CrossRef]
- Islamuddin, M.; Chouhan, G.; Farooque, A.; Dwarakanath, B.S.; Sahal, D.; Afrin, F. Th1-biased immunomodulation and therapeutic potential of Artemisia annua in murine visceral leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e3321. [Google Scholar] [CrossRef]
- Sen, R.; Ganguly, S.; Saha, P.; Chatterjee, M. Efficacy of artemisinin in experimental visceral leishmaniasis. Int. J. Antimicrob. Agents 2010, 36, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Want, M.Y.; Islammudin, M.; Chouhan, G.; Ozbak, H.A.; Hemeg, H.A.; Chattopadhyay, A.P.; Afrin, F. Nanoliposomal artemisinin for the treatment of murine visceral leishmaniasis. Int. J. Nanomed. 2017, 12, 2189–2204. [Google Scholar] [CrossRef]
- Want, M.Y.; Islamuddin, M.; Chouhan, G.; Ozbak, H.A.; Hemeg, H.A.; Dasgupta, A.K.; Chattopadhyay, A.P.; Afrin, F. Therapeutic efficacy of artemisinin-loaded nanoparticles in experimental visceral leishmaniasis. Colloids Surf. B Biointerfaces 2015, 130, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.S.; Lam, N.S.; Yu, D.; Su, X.Z.; Lu, F. Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharmacol. Res. 2017, 117, 192–217. [Google Scholar] [CrossRef] [PubMed]
- Medkour, H.; Bitam, I.; Laidoudi, Y.; Lafri, I.; Lounas, A.; Hamidat, H.K.; Mekroud, A.; Varloud, M.; Davoust, B.; Mediannikov, O. Potential of Artesunate in the treatment of visceral leishmaniasis in dogs naturally infected by Leishmania infantum: Efficacy evidence from a randomized field trial. PLoS Negl. Trop. Dis. 2020, 14, e0008947. [Google Scholar] [CrossRef]
- van Griensven, J.; Balasegaram, M.; Meheus, F.; Alvar, J.; Lynen, L.; Boelaert, M. Combination therapy for visceral leishmaniasis. Lancet Infect. Dis. 2010, 10, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Don, R.; Ioset, J.R. Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitology 2014, 141, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Intakhan, N.; Siriyasatien, P.; Chanmol, W. Anti-Leishmania activity of artesunate and combination effects with amphotericin B against Leishmania (Mundinia) martiniquensis in vitro. Acta Trop. 2022, 226, 106260. [Google Scholar] [CrossRef]
- Geroldinger, G.; Tonner, M.; Quirgst, J.; Walter, M.; De Sarkar, S.; Machín, L.; Monzote, L.; Stolze, K.; Catharina Duvigneau, J.; Staniek, K.; et al. Activation of artemisinin and heme degradation in Leishmania tarentolae promastigotes: A possible link. Biochem. Pharmacol. 2020, 173, 113737. [Google Scholar] [CrossRef]
- Nemati, S.; Nahrevanian, H.; Haniloo, A.; Farahmand, M. Investigation on Nitric Oxide and C- Reactive Protein Involvement in Anti-Leishmanial Effects of Artemisinin and Glucantim on Cutaneous Leishmaniasis. Adv. Stud. Biol. 2013, 5, 27–36. [Google Scholar] [CrossRef]
- Zabala-Peñafiel, A.; Dias-Lopes, G.; Cysne-Finkelstein, L.; Conceição-Silva, F.; Miranda, L.F.C.; Fagundes, A.; Schubach, A.O.; Fernandes Pimentel, M.I.; Souza-Silva, F.; Machado, L.A.; et al. Serine proteases profiles of Leishmania (Viannia) braziliensis clinical isolates with distinct susceptibilities to antimony. Sci. Rep. 2021, 11, 14234. [Google Scholar] [CrossRef]
- Chou, T.C. Preclinical versus clinical drug combination studies. Leuk. Lymphoma 2008, 49, 2059–2080. [Google Scholar] [CrossRef]
- Rahaman, M.; Ghosh, S.; Chowdhury, L.D.; Chatterjee, M. Evaluation of anti-leishmanial activity of artemisinin combined with amphotericin B or miltefosine in Leishmania donovani promastigotes. Int. J. Basic Clin. Pharmacol. 2017, 3, 644–648. [Google Scholar]
- Zhu, C.; Liao, B.; Ye, X.; Zhou, Y.; Chen, X.; Liao, M.; Cheng, L.; Zhou, X.; Ren, B. Artemisinin elevates ergosterol levels of Candida albicans to synergise with amphotericin B against oral candidiasis. Int. J. Antimicrob. Agents 2021, 58, 106394. [Google Scholar] [CrossRef]
- Ramos, H.; Valdivieso, E.; Gamargo, M.; Dagger, F.; Cohen, B.E. Amphotericin B kills unicellular leishmanias by forming aqueous pores permeable to small cations and anions. J. Membr. Biol. 1996, 152, 65–75. [Google Scholar] [CrossRef]
- Hilgard, P.; Stekar, J.; Voegeli, R.; Engel, J.; Schumacher, W.; Eibl, H.; Unger, C.; Berger, M.R. Characterization of the antitumor activity of hexadecylphosphocholine (D 18506). Eur. J. Cancer Clin. Oncol. 1988, 24, 1457–1461. [Google Scholar] [CrossRef]
- Luque-Ortega, J.R.; Rivas, L. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1327–1332. [Google Scholar] [CrossRef]
- Paris, C.; Loiseau, P.M.; Bories, C.; Bréard, J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2004, 48, 852–859. [Google Scholar] [CrossRef]
- Pinto-Martinez, A.K.; Rodriguez-Durán, J.; Serrano-Martin, X.; Hernandez-Rodriguez, V.; Benaim, G. Mechanism of Action of Miltefosine on Leishmania donovani Involves the Impairment of Acidocalcisome Function and the Activation of the Sphingosine-Dependent Plasma Membrane Ca2+ Channel. Antimicrob. Agents Chemother. 2018, 62, e01614-17. [Google Scholar] [CrossRef]
- Rakotomanga, M.; Blanc, S.; Gaudin, K.; Chaminade, P.; Loiseau, P.M. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1425–1430. [Google Scholar] [CrossRef]
- Armitage, E.G.; Alqaisi, A.Q.I.; Godzien, J.; Peña, I.; Mbekeani, A.J.; Alonso-Herranz, V.; López-Gonzálvez, Á.; Martín, J.; Gabarro, R.; Denny, P.W.; et al. Complex Interplay between Sphingolipid and Sterol Metabolism Revealed by Perturbations to the Leishmania Metabolome Caused by Miltefosine. Antimicrob. Agents Chemother. 2018, 62, e02095-17. [Google Scholar] [CrossRef]
- Barratt, G.; Saint-Pierre-Chazalet, M.; Loiseau, P.M. Cellular transport and lipid interactions of miltefosine. Curr. Drug Metab. 2009, 10, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Rakotomanga, M.; Saint-Pierre-Chazalet, M.; Loiseau, P.M. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug-membrane interactions. Antimicrob. Agents Chemother. 2005, 49, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Chawla, B.; Jhingran, A.; Panigrahi, A.; Stuart, K.D.; Madhubala, R. Paromomycin affects translation and vesicle-mediated trafficking as revealed by proteomics of paromomycin -susceptible -resistant Leishmania donovani. PLoS ONE 2011, 6, e26660. [Google Scholar] [CrossRef]
- Jhingran, A.; Chawla, B.; Saxena, S.; Barrett, M.P.; Madhubala, R. Paromomycin: Uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 2009, 164, 111–117. [Google Scholar] [CrossRef]
- Shalev-Benami, M.; Zhang, Y.; Rozenberg, H.; Nobe, Y.; Taoka, M.; Matzov, D.; Zimmerman, E.; Bashan, A.; Isobe, T.; Jaffe, C.L.; et al. Atomic resolution snapshot of Leishmania ribosome inhibition by the aminoglycoside paromomycin. Nat. Commun. 2017, 8, 1589. [Google Scholar] [CrossRef]
- Ghaffarifar, F.; Esavand Heydari, F.; Dalimi, A.; Hassan, Z.M.; Delavari, M.; Mikaeiloo, H. Evaluation of Apoptotic and Antileishmanial Activities of Artemisinin on Promastigotes and BALB/C Mice Infected with Leishmania major. Iran. J. Parasitol. 2015, 10, 258–267. [Google Scholar]
- Yang, D.M.; Liew, F.Y. Effects of qinghaosu (artemisinin) and its derivatives on experimental cutaneous leishmaniasis. Parasitology 1993, 106 Pt 1, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Palma, E.; Peritore, A.F.; Siracusa, R.; D’Amico, R.; Fusco, R.; Licata, P.; Crupi, R. Effect of Artesunate on Leishmania Amazonesis Induced Neuroinflammation and Nociceptive Behavior in Male Balb/C Mice. Animals 2020, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Mutiso, J.M.; Macharia, J.C.; Barasa, M.; Taracha, E.; Bourdichon, A.J.; Gicheru, M.M. In vitro and in vivo antileishmanial efficacy of a combination therapy of diminazene and artesunate against Leishmania donovani in BALB/c mice. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B.L. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J. Vis. Exp. 2012, e4054. [Google Scholar] [CrossRef]
- Zakai, H.A.; Chance, M.L.; Bates, P.A. In vitro stimulation of metacyclogenesis in Leishmania braziliensis, L. donovani, L. major and L. mexicana. Parasitology 1998, 116 Pt 4, 305–309. [Google Scholar] [CrossRef]
- Rogers, M.E.; Chance, M.L.; Bates, P.A. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology 2002, 124, 495–507. [Google Scholar] [CrossRef]
- Chanmol, W.; Siriyasatien, P.; Intakhan, N. In vitro anti-Leishmania activity of 8-hydroxyquinoline and its synergistic effect with amphotericin B deoxycholate against Leishmania martiniquensis. PeerJ 2022, 10, e12813. [Google Scholar] [CrossRef]


| Drugs (µM) | IC50 5 (Promastigote) | IC50 (Amastigote) | CC50 6 | SI 7 (Amastigote) |
|---|---|---|---|---|
| AS 1 | 121.43 ± 2.05 | 5.88 ± 1.67 | 225.77 ± 21.71 | 38.40 |
| AmB 2 | 0.02 ± 0.002 | 0.14 ± 0.02 | 1.20 ± 0.27 | 8.57 |
| MF 3 | 2.95 ± 0.18 | 0.37 ± 0.11 | 51.12 ± 0.65 | 138.16 |
| PM 4 | 114.93 ± 9.46 | 64.30 ± 2.37 | >20,000 | >311 |
| Drug Combination Non-Constant Ratio (µM) 1 | Growth Inhibition (%) 2 | CI 3 | Interaction | Dose Reduction Index (DRI) 4 | |||
|---|---|---|---|---|---|---|---|
| Combinations | AS | AmB | AS | AmB | |||
| 1 | 1.00 | 0.03 | 49.1 ± 3.87 ** | 0.68 | synergism | 2.04 | 5.39 |
| 2 | 1.00 | 0.05 | 63.36 ± 2.27 **** | 0.55 | synergism | 3.45 | 3.85 |
| 3 | 1.00 | 0.10 | 86.3 ± 4.35 **** | 0.44 | synergism | 11.19 | 2.84 |
| 4 | 1.00 | 0.20 | 87.85 ± 5.14 **** | 0.76 | slight synergism | 12.65 | 1.48 |
| 5 | 2.00 | 0.03 | 5.07 ± 4.39 **** | 14.06 | very strong antagonism | 0.07 | 2.26 |
| 6 | 2.00 | 0.05 | 62.23 ± 2.76 *** | 0.87 | slight synergism | 1.65 | 3.80 |
| 7 | 2.00 | 0.10 | 84.79 ± 4.40 **** | 0.57 | synergism | 4.99 | 2.74 |
| 8 | 2.00 | 0.20 | 89.21 ± 2.27 **** | 0.79 | moderate synergism | 7.14 | 1.54 |
| 9 | 4.00 | 0.03 | 30.51 ± 3.21 **** | 4.26 | strong antagonism | 0.25 | 4.26 |
| 10 | 4.00 | 0.05 | 81.53 ± 2.91 *** | 0.69 | synergism | 2.02 | 5.10 |
| 11 | 4.00 | 0.10 | 89.87 ± 2.97 **** | 0.58 | synergism | 3.81 | 3.14 |
| 12 | 4.00 | 0.20 | 94.47 ± 3.72 **** | 0.67 | synergism | 6.90 | 1.91 |
| 13 | 8.00 | 0.03 | 85.19 ± 1.59 | 0.89 | slight synergism | 1.28 | 9.20 |
| 14 | 8.00 | 0.05 | 84.83 ± 1.30 | 0.98 | nearly additive | 1.25 | 5.84 |
| 15 | 8.00 | 0.10 | 86.14 ± 3.20 | 1.08 | nearly additive | 1.38 | 2.83 |
| 16 | 8.00 | 0.20 | 96.83 ± 1.62 *** | 0.61 | synergism | 5.85 | 2.28 |
| Drug Combination Non-Constant Ratio (µM) 1 | Growth Inhibition (%) 2 | CI 3 | Interaction | Dose Reduction Index (DRI) 4 | |||
|---|---|---|---|---|---|---|---|
| Combinations | AS | MF | AS | MF | |||
| 1 | 1.00 | 0.06 | 25.75 ± 2.64 | 1.44 | moderate antagonism | 0.96 | 2.52 |
| 2 | 1.00 | 0.13 | 51.15 ± 4.18 **** | 0.84 | moderate synergism | 2.35 | 2.42 |
| 3 | 1.00 | 0.25 | 75.99 ± 2.55 **** | 0.55 | synergism | 5.72 | 2.63 |
| 4 | 1.00 | 0.50 | 87.42 ± 2.33 **** | 0.54 | synergism | 10.77 | 2.22 |
| 5 | 2.00 | 0.06 | 57.28 ± 1.70 ** | 0.86 | moderate synergism | 1.43 | 6.19 |
| 6 | 2.00 | 0.13 | 71.17 ± 5.26 **** | 0.66 | synergism | 2.34 | 4.29 |
| 7 | 2.00 | 0.25 | 80.36 ± 5.46 **** | 0.60 | synergism | 3.52 | 3.12 |
| 8 | 2.00 | 0.50 | 96.36 ± 5.51 **** | 0.25 | strong synergism | 15.81 | 5.41 |
| 9 | 4.00 | 0.06 | 27.86 ± 3.67 **** | 4.18 | strong antagonism | 0.26 | 2.79 |
| 10 | 4.00 | 0.13 | 80.84 ± 4.83 ** | 0.72 | moderate synergism | 1.80 | 6.13 |
| 11 | 4.00 | 0.25 | 93.08 ± 4.75 **** | 0.36 | synergism | 4.58 | 6.90 |
| 12 | 4.00 | 0.50 | 96.25 ± 4.71 **** | 0.32 | synergism | 7.71 | 5.30 |
| 13 | 8.00 | 0.06 | 90.50 ± 3.06 * | 0.62 | synergism | 1.74 | 22.84 |
| 14 | 8.00 | 0.13 | 92.83 ± 3.13 ** | 0.53 | synergism | 2.22 | 12.93 |
| 15 | 8.00 | 0.25 | 98.40 ± 1.60 *** | 0.18 | strong synergism | 7.79 | 18.97 |
| 16 | 8.00 | 0.50 | 97.59 ± 3.07 *** | 0.32 | synergism | 5.57 | 7.18 |
| Drug Combination Non-Constant Ratio (µM) 1 | Growth Inhibition (%) 2 | CI 3 | Interaction | Dose Reduction Index (DRI) 4 | |||
|---|---|---|---|---|---|---|---|
| Combinations | AS | PM | AS | PM | |||
| 1 | 1.00 | 8.75 | 4.37 ± 4.79 **** | 7.70 | strong antagonism | 0.14 | 2.63 |
| 2 | 1.00 | 17.50 | 12.03 ± 3.21 *** | 3.19 | strong antagonism | 0.37 | 1.96 |
| 3 | 1.00 | 35.00 | 32.98 ± 4.09 | 1.47 | antagonism | 1.20 | 1.56 |
| 4 | 1.00 | 70.00 | 46.15 ± 3.03 ** | 1.55 | antagonism | 2.00 | 0.95 |
| 5 | 2.00 | 8.75 | 25.93 ± 5.44 ** | 2.45 | antagonism | 0.44 | 5.52 |
| 6 | 2.00 | 17.50 | 31.78 ± 4.73 * | 2.07 | antagonism | 0.57 | 3.06 |
| 7 | 2.00 | 35.00 | 57.06 ± 3.99 ** | 1.11 | slight antagonism | 1.50 | 2.24 |
| 8 | 2.00 | 70.00 | 68.63 ± 3.90 **** | 1.17 | slight antagonism | 2.36 | 1.34 |
| 9 | 4.00 | 8.75 | 24.73 ± 5.08 **** | 4.99 | strong antagonism | 0.21 | 5.39 |
| 10 | 4.00 | 17.50 | 54.13 ± 3.16 * | 1.72 | antagonism | 0.67 | 4.29 |
| 11 | 4.00 | 35.00 | 72.23 ± 3.90 * | 1.07 | nearly additive | 1.38 | 2.86 |
| 12 | 4.00 | 70.00 | 78.47 ± 1.60 *** | 1.15 | slight antagonism | 1.89 | 1.61 |
| 13 | 8.00 | 8.75 | 59.97 ± 3.84 **** | 2.50 | antagonism | 0.42 | 9.36 |
| 14 | 8.00 | 17.50 | 78.12 ± 3.54 | 1.24 | moderate antagonism | 0.93 | 6.41 |
| 15 | 8.00 | 35.00 | 75.94 ± 1.28 | 1.54 | antagonism | 0.83 | 3.07 |
| 16 | 8.00 | 70.00 | 74.88 ± 1.13 | 1.94 | antagonism | 0.78 | 1.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intakhan, N.; Saeung, A.; Rodrigues Oliveira, S.M.; Pereira, M.d.L.; Chanmol, W. Synergistic Effects of Artesunate in Combination with Amphotericin B and Miltefosine against Leishmania infantum: Potential for Dose Reduction and Enhanced Therapeutic Strategies. Antibiotics 2024, 13, 806. https://doi.org/10.3390/antibiotics13090806
Intakhan N, Saeung A, Rodrigues Oliveira SM, Pereira MdL, Chanmol W. Synergistic Effects of Artesunate in Combination with Amphotericin B and Miltefosine against Leishmania infantum: Potential for Dose Reduction and Enhanced Therapeutic Strategies. Antibiotics. 2024; 13(9):806. https://doi.org/10.3390/antibiotics13090806
Chicago/Turabian StyleIntakhan, Nuchpicha, Atiporn Saeung, Sonia M. Rodrigues Oliveira, Maria de Lourdes Pereira, and Wetpisit Chanmol. 2024. "Synergistic Effects of Artesunate in Combination with Amphotericin B and Miltefosine against Leishmania infantum: Potential for Dose Reduction and Enhanced Therapeutic Strategies" Antibiotics 13, no. 9: 806. https://doi.org/10.3390/antibiotics13090806
APA StyleIntakhan, N., Saeung, A., Rodrigues Oliveira, S. M., Pereira, M. d. L., & Chanmol, W. (2024). Synergistic Effects of Artesunate in Combination with Amphotericin B and Miltefosine against Leishmania infantum: Potential for Dose Reduction and Enhanced Therapeutic Strategies. Antibiotics, 13(9), 806. https://doi.org/10.3390/antibiotics13090806







