Enhancing Pathogen Detection in Implant-Related Infections through Chemical Antibiofilm Strategies: A Comprehensive Review
Abstract
1. Introduction
2. Single-Stage and Two-Stage Revision Procedures in Orthopedics
3. Heart Valve Prosthesis Infections
4. Diagnostic Challenges Posed by Biofilm in Implant-Related Infections
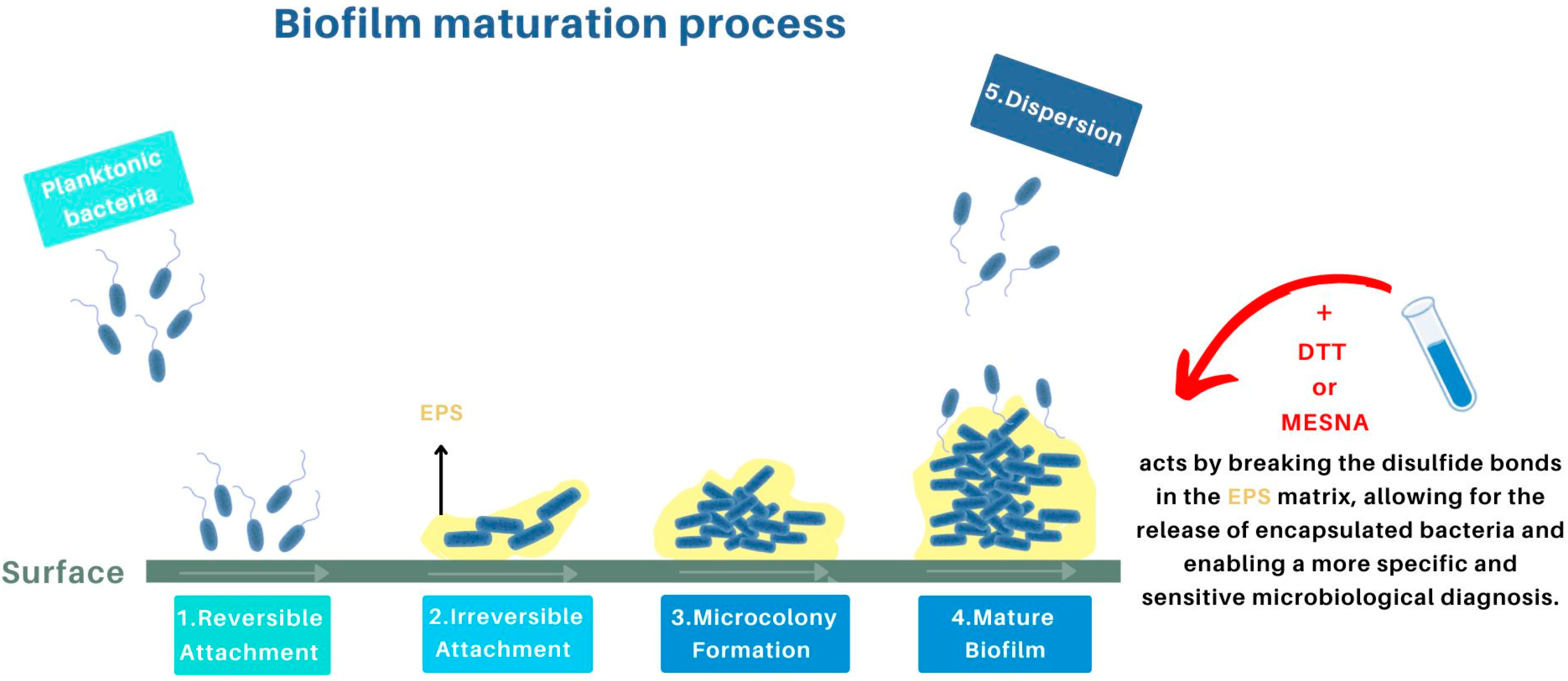
5. Recent Antibiofilm Techniques
5.1. Diagnostic Advances Due to the Use of Chemical Agents in IRIs
5.1.1. Dithiothreitol (DTT)
5.1.2. Chelating Agent Ethylenediaminetetraacetic Acid (EDTA)
5.1.3. MESNA
5.2. Innovative Treatment Approaches for Implant-Related Infections
- Antimicrobial Peptides (AMP): These peptides are used to prevent the formation of biofilms on surfaces of polymethyl methacrylate (PMMA). They exhibit antibacterial activity by breaking the cell membrane of pathogens, reducing bacterial adhesion and biofilm formation [78].
- AIEgen: These probes, employed as theranostic instruments, enhance penetration into biofilms and facilitate both the diagnosis and treatment of multi-resistant bacterial biofilm infections due to their capacity to generate localized heat [79].
- Cold Plasma Treatments: By modifying the surfaces of materials, cold plasma treatments render surfaces anti-adhesive and antibacterial, preventing adhesion and bacterial growth. This treatment is designed to modify the surface of medical implants and counteract the adhesion phase of biofilm formation, thereby improving the antibacterial activity of the materials [76].
- Silver Nanoparticles (AgNPs): Derived from Lactobacillus casei, these nanoparticles possess powerful antibiofilm properties, inhibiting the formation and growth of bacterial biofilms. These nanocubes are activated by NIR light to generate heat, which destroys bacterial biofilms and improves the effectiveness of antibiotics. They offer a promising approach to the treatment of implant-related infections due to their ability to selectively degrade biofilms [80].
6. Discussion and Conclusions
7. Future Needs to Improve the Surgical Revision Procedure
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IRIs | Implant-related infections |
| PCR | Polymerase chain reaction |
| NGS | Next-Generation Sequencing |
| DTT | Dithiothreitol |
| EDTA | Ethylenediaminetetraacetic acid |
| MESNA | Sodium 2-mercaptoethanesulfonate |
| EPS | Extracellular polymeric substance |
| AMP | Antimicrobial Peptides |
| PMMA | Polymethyl methacrylate |
| AIE | Aggregation-Induced Emission |
| AgNPs | Silver Nanoparticles |
References
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomaterials 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorlí, L.; Fresco, G.; Fernández-Sampedro, M.; Del Toro, M.D.; Guío, L.; et al. Time trends in the aetiology of prosthetic joint infections: A multicentre cohort study. Clin. Microbiol. Infect. 2016, 22, 732.e1–732.e8. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; De Feo, V. Effect of Adiantum philippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens, and characterization of bioactive metabolites: An in vitro-in silico approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Abusrewil, S.; Alshanta, O.A.; Albashaireh, K.; Alqahtani, S.; Nile, C.J.; Scott, J.A.; McLean, W. Detection, treatment and prevention of endodontic biofilm infections: What’s new in 2020? Crit. Rev. Microbiol. 2020, 46, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wen, Y.M. The role of bacterial biofilm in persistent infections and control strategies. Int. J. Oral Sci. 2011, 3, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Evelhoch, S.R. Biofilm and chronic nonhealing wound infections. Surg. Clin. 2020, 100, 727–732. [Google Scholar] [CrossRef]
- Kovaleva, J.; Peters, F.T.M.; van der Mei, H.C.; Degener, J.E. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin. Microbiol. Rev. 2013, 26, 231–254. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Trampuz, A.; Borens, O.; Bohner, M.; Ilchmann, T. Biofilm formation on bone grafts and bone graft substitutes: Comparison of different materials by a standard in vitro test and microcalorimetry. Acta Biomater. 2010, 6, 3791–3797. [Google Scholar] [CrossRef] [PubMed]
- Holinka, J.; Bauer, L.; Hirschl, A.M.; Graninger, W.; Windhager, R.; Presterl, E. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J. Orthop. Res. 2011, 29, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Cadambi, A.; Jones, R.E.; Maale, G.E. A protocol for staged revision of infected total hip and knee arthroplasties: The use of antibiotic-cement implant composites. Int. Orthop. 1995, 3, 133–145. [Google Scholar]
- Maale, G.E.; Hsu, W.K.; McLaren, A.C.; Springer, B.D. (Eds.) Debridement for orthopaedic infection. In Let’s Discuss Surgical Site Infections; American Academy of Orthopaedic Surgeons (AAOS): Rosemont, IL, USA, 2015; Chapter 5. [Google Scholar]
- Haddad, F.S.; Masri, B.A.; Campbell, D.; McGraw, R.W.; Beauchamp, C.P.; Duncan, C.P. The PROSTALAC functional spacer in two-stage revision for infected knee replacements. J. Bone Jt. Surg. Br. 2000, 82, 807–812. [Google Scholar] [CrossRef]
- Maale, G.E.; Pascoe, H.R.; Piercy, E.A. A standardized approach for the treatment of infected total joint arthroplasties by the DFW sarcoma group osteomyelitis protocol; Staged revisions at 2 weeks using antibiotic-cement-implant composites as spacers. J. Jt. Arthroplast. 1993, 8, 102. [Google Scholar] [CrossRef]
- Netti, M.P.; Long, W.J.; Della Valle, C.J. Diagnosis and treatment of infected total joint arthroplasty. World Clin. Orthoped. 2014, 1, 75–90. [Google Scholar]
- Wolf, C.F.; Gu, N.Y.; Doctor, J.N.; Manner, P.A.; Leopold, S.S. Comparison of one and two-stage revision of total hip arthroplasty complicated by infection: A markov expected-utility decision analysis. J. Bone Jt. Surg. Am. 2011, 93-A, 631–639. [Google Scholar]
- Woods, J.; Boegli, L.; Kirker, K.R.; Agostinho, A.M.; Durch, A.M.; Delancey Pulcini, E.; Stewart, P.S.; James, G.A. Development and application of a polymicrobial, in vitro, wound biofilm model. J. Appl. Microbiol. 2012, 112, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Cierny III, G.; Mader, J.T. Adult chronic osteomyelitis. Orthopedics 1984, 7, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Jamsen, E.; Stogiannidis, I.; Malmivaara, A.; Pajamaki, J.; Puolakka, T.; Konttinen, Y.T. Outcome of prosthethesis exchange for infected knee arthroplasty: The effect of treatment approach. Acta Orthop. 2009, 80, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Lonner, J.H.; Desai, P.; Dicesare, P.E.; Steiner, G.; Zuckerman, J.D. The reliability of analysis of intraoperative frozen sections for identifying active infection during revision hip or knee arthroplasty. J. Bone Jt. Surg. Am. 1996, 78, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Pangaud, C.; Ollivier, M.; Argenson, J.N. Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. EFORT Open Rev. 2019, 4, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sotelo, J.; Berry, D.J.; Hanssen, A.D. Mid-term to long-term follow-up of staged reimplantation for infected hip arthroplasty. Clin. Orthop. Relat. Res. 2009, 467, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.H.; Deakin, M.; Latham, J.M. Chronic osteomyelitis: The effect of the extent of surgical resection on infection free survival. J. Bone Jt. Surg. Br. 2001, 83, 403–407. [Google Scholar] [CrossRef]
- Uchholz, H.W.; Elson, R.A.; Engelbrecht, E.; Lodenkamper, H.; Rottger, J.; Siegel, A. Management of deep infection of total hip replacement. J. Bone Jt. Surg. Br. 1981, 63, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.M.; Tetsworth, K.D.; Calhoun, J.H.; Mader, J.T. An articulated antibiotic spacer used for infected total knee arthroplasty: A comparative in vitro elution study of Simplex and Palacos bone cements. J. Orthop. Res. 2005, 23, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Ampuero, J.; Esteban, J.; García-Cimbrelo, E.; Munuera, L.; Escobar, R. Low relapse with oral antibiotics and two-stage exchange for late arthroplasty infections in 40 patients after 2-9 years. Acta Orthop. 2007, 78, 511–519. [Google Scholar] [CrossRef]
- Thakrar, R.R.; Horriat, S.; Kayani, B.; Haddad, F.S. Indications for a single-stage exchange arthroplasty for chronic prosthetic joint infection. Bone Jt. J. 2019, 101-B (1 Suppl. A), 19–24. [Google Scholar]
- Lum, Z.C.; Holland, C.T.; Meehan, J.P. Systematic Review of Single Stage Revision for Prosthetic Joint Infection. World J. Orthoped. 2020, 11, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Donelli, G. Biofilm-Based Healthcare-Associated Infections; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1, pp. 47–63. [Google Scholar]
- Janz, V.; Wassilew, G.I.; Hasart, O.; Tohtz, S.; Perka, C. Improvement in the detection rate of PJI in total hip arthroplasty through multiple sonicate fluid cultures. J. Orthop. Res. 2013, 31, 2021–2024. [Google Scholar] [CrossRef] [PubMed]
- López, J.; Revilla, A.; Vilacosta, I.; Villacorta, E.; González-Juanatey, C.; Gómez, I.; Rollán, M.J.; San Román, J.A. Definition, clinical profile, microbiological spectrum, and prognostic factors of early-onset prosthetic valve endocarditis. Eur. Heart J. 2007, 28, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, A.; Sohail, M.R.; Steckelberg, J.M. Prosthetic valve endocarditis: State of the heart. Clin. Investig. 2012, 2, 803–817. [Google Scholar] [CrossRef]
- Hoen, B.; Duval, X. Infective Endocarditis. N. Engl. J. Med. 2013, 368, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Lamas, C. Diagnostic Strategy for Blood Culture-Negative Endocarditis. Clin. Infect. Dis. 2010, 51, 141–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sumatani, I.; Kagiyama, N.; Saito, C.; Makanae, M.; Kanetsuna, H.; Ahn, K.; Mizukami, A.; Hashimoto, Y. Infective endocarditis with negative blood culture and negative echocardiographic findings. J. Echocardiogr. 2015, 13, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections. Clin. Microbiol. Infect. 2010, 21, S1–S25. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Ghanem, E.; Sharkey, P.; Aggarwal, A.; Burnett, S.R.; Barrack, R.L. Diagnosis of infected total knee: Findings of a multicenter database. Clin. Orthop. Relat. Res. 2007, 464, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.W.; Allison, D.G.; Gilbert, P. Resistance of bacterial biofilms to antibiotics: A growth-rate related effect? J. Antimicrob. Chemother. 1988, 22, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, L. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar]
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 2006, 37, S59–S66. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Cost-Benefit Analysis of Antibiofilm Microbiological Techniques for Peri-Prosthetic Joint Infection Diagnosis. J. Clin. Med. 2020, 9, 2203. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Cadossi, M.; Giannini, S.; Pignatti, G.; Marcacci, M.; Ferrari, M.C.; Donati, D.M.; De Paolis, M. Is Treatment with Dithiothreitol More Effective than Sonication for the Diagnosis of Prosthetic Joint Infection? Clin. Orthop. Relat. Res. 2018, 476, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Fariñas-Álvarez, C.; Fernández-Roblas, R.; Esteban, J. Comparison of molecular techniques for diagnosis of prosthetic joint infection. J. Clin. Microbiol. 2009, 47, 3690–3694. [Google Scholar]
- Wade, W. Unculturable bacteria–the uncharacterized organisms that cause oral infections. J. R. Soc. Med. 2002, 95, 81–83. [Google Scholar] [PubMed]
- Sathiananthamoorthy, S.; Malone-Lee, J.; Gill, K.; Tymon, A.; Nguyen, T.K.; Gurung, S.; Collins, L.; Kupelian, A.S.; Swamy, S.; Khasriya, R.; et al. Reassessment of routine midstream culture in diagnosis of urinary tract infection. J. Clin. Microbiol. 2019, 57, e01452-18. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Sample, H.A.; Zorn, K.C.; Arevalo, S.; Yu, G.; Neuhaus, J.; Federman, S.; Stryke, D.; Briggs, B.; Langelier, C.; et al. Clinical metagenomic sequencing for diagnosis of Meningitis and Encephalitis. N. Engl. J. Med. 2019, 380, 2327–2340. [Google Scholar] [CrossRef]
- Achermann, Y.; Vogt, M.; Leunig, M.; Wust, J.; Trampuz, A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J. Clin. Microbiol. 2010, 48, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Janz, V.; Schoon, J.; Morgenstern, C.; Preininger, B.; Reinke, S.; Duda, G.; Breitbach, A.; Perka, C.F.; Geissler, S. Rapid detection of periprosthetic joint infection using a combination of 16s rDNA polymerase chain reaction and lateral flow immunoassay. Bone Jt. Res. 2018, 7, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, I.K.; Holinka, J.; Sevelda, F.; Staats, K.; Heisinger, S.; Kubista, B.; McNally, M.A.; Windhager, R. Performance of automated multiplex polymerase chain reaction (mPCR) using synovial fluid in the diagnosis of native joint septic arthritis in adults. Bone Jt. J. 2019, 101-B, 288–296. [Google Scholar]
- Fenollar, F.; Roux, V.; Stein, A.; Drancourt, M.; Raoult, D. Analysis of 525 samples to determine the usefulness of PCR amplification and sequencing of the 16S rRNA gene for diagnosis of bone and joint infections. J. Clin. Microbiol. 2006, 44, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Cazanave, C.; Greenwood-Quaintance, K.E.; Hanssen, A.D.; Karau, M.J.; Schmidt, S.M.; Gomez Urena, E.O.; Mandrekar, J.N.; Osmon, D.R.; Lough, L.E.; Pritt, B.S.; et al. Rapid molecular microbiologic diagnosis of prosthetic joint infection. J. Clin. Microbiol. 2013, 51, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.T.J.; McHugh, M.P.; Guerendiain, D.; Gwynne, P.J.; Boyd, J.; Simpson, A.H.R.W.; Walsh, T.S.; Laurenson, I.F.; Templeton, K.E. Underestimation of Staphylococcus aureus (MRSA and MSSA) carriage associated with standard culturing techniques. Bone Jt. Res. 2018, 7, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Chang, C.H.; Chiang-Ni, C.; Hsieh, P.H.; Shih, H.N.; Ueng, S.W.; Chang, Y. Rapid analysis of bacterial composition in prosthetic joint infection by 16S rRNA metagenomic sequencing. Bone Jt. Res. 2019, 8, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Tarabichi, M.; Alvand, A.; Shohat, N.; Goswami, K.; Parvizi, J. Diagnosis of Streptococcus canis periprosthetic joint infection: The utility of next-generation sequencing. Arthroplast. Today 2018, 4, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Street, T.L.; Sanderson, N.D.; Atkins, B.L.; Brent, A.J.; Cole, K.; Foster, D.; McNally, M.A.; Oakley, S.; Peto, L.; Taylor, A.; et al. Molecular diagnosis of orthopedic-device-related infection directly from sonication fluid by metagenomic sequencing. J. Clin. Microbiol. 2017, 55, 2334–2347. [Google Scholar] [CrossRef] [PubMed]
- Ruppé, E.; Lazarevic, V.; Girard, M.; Mouton, W.; Ferry, T.; Laurent, F.; Schrenzel, J. Clinical metagenomics of bone and joint infections: A proof of concept study. Sci. Rep. 2017, 7, 7718. [Google Scholar] [CrossRef] [PubMed]
- Thoendel, M.J.; Jeraldo, P.R.; Greenwood-Quaintance, K.E.; Yao, J.Z.; Chia, N.; Hanssen, A.D.; Abdel, M.P.; Patel, R. Identification of prosthetic joint infection pathogens using a shotgun metagenomics Approach. Clin. Infect. Dis. 2018, 67, 1333–1338. [Google Scholar] [CrossRef]
- Tarabichi, M.; Shohat, N.; Goswami, K.; Alvand, A.; Silibovsky, R.; Belden, K.; Parvizi, J. Diagnosis of periprosthetic joint infection: The potential of next-generation sequencing. J. Bone Jt. Surg. 2018, 100, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, C.; Li, W.; Fang, X.; Wang, Q.; Xing, L.; Li, Y.; Nie, X.; Yang, B.; Zhang, W. Metagenomic next- generation sequencing contribution in identifying prosthetic joint infection due to parvimonas micra: A case report. J. Bone Jt. Infect. 2019, 4, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yin, C.; Wang, G.; Rosenblum, J.; Krishnan, S.; Dimitrova, N.; Fallon, J.T. Optimizing a metatranscriptomic next-generation sequencing protocol for bronchoalveolar lavage diagnostics. J. Mol. Diagn. 2019, 21, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Goswami, K.; Shope, A.J.; Tokarev, V.; Wright, J.R.; Unverdorben, L.V.; Ly, T.; Chen See, J.; McLimans, C.J.; Wong, H.T.; Lock, L.; et al. Comparative meta-omics for identifying pathogens associated with prosthetic joint infection. Sci. Rep. 2021, 11, 23749. [Google Scholar] [CrossRef] [PubMed]
- Tsikopoulos, K.; Poultsides, L.; Nana, A.; Zormpala, A.; Kokkalis, Z.; Tsaganos, T.; Mavrogenis, A.F. Is Sonication Superior to Dithiothreitol in Diagnosis of Periprosthetic Joint Infections? A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 526. [Google Scholar]
- Drago, L.; Romanò, C.L. Commentary: Dithiothreitol (DTT), When Used as Biofilm Detaching Method to Diagnose Implant-Associated Infections, Does Not Affect Microorganisms’ Viability, according to the Current Literature. Front. Microbiol. 2022, 12, 814945. [Google Scholar] [CrossRef] [PubMed]
- Moris, V.; Lam, M.; Amoureux, L.; Magallon, A.; Guilloteau, A.; Maldiney, T.; Zwetyenga, N.; Falentin-Daudre, C.; Neuwirth, C. What Is the Best Technic to Dislodge Staphylococcus Epidermidis Biofilm on Medical Implants? BMC Microbiol. 2022, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Bakalakos, M.; Vlachos, C.; Ampadiotaki, M.M.; Stylianakis, A.; Sipsas, N.; Pneumaticos, S.; Vlamis, J. Role of Dithiothreitol in Detection of Orthopaedic Implant-Associated Infections. J. Pers. Med. 2024, 14, 334. [Google Scholar] [CrossRef]
- Giannetti, A.; Romano, J.; Fidanza, A.; Di Mauro, M.; Brunetti, M.; Fascione, F.; Calvisi, V. The diagnostic potential of MicroDTTect compared to conventional culture of tissue samples in orthopedic infections. Lo Scalpello J. 2022, 36, 111–115. [Google Scholar] [CrossRef]
- Karbysheva, S.; Di Luca, M.; Butini, M.E.; Winkler, T.; Schütz, M.; Trampuz, A. Comparison of Sonication with Chemical Biofilm Dislodgement Methods Using Chelating and Reducing Agents: Implications for the Microbiological Diagnosis of Implant Associated Infection. PLoS ONE 2020, 15, e0231389. [Google Scholar] [CrossRef] [PubMed]
- Hage, M.; Khelissa, S.; Akoum, H.; Chihib, N.E.; Jama, C. Cold Plasma Surface Treatments to Prevent Biofilm Formation in Food Industries and Medical Sectors. Appl. Microbiol. Biotechnol. 2022, 106, 81–100. [Google Scholar] [CrossRef] [PubMed]
- de la Torre González, C.; Huante-Guido, M.; Guadarrama, N.V.; Preciado, D.; López, G.P. Changes in Biofilm in Chronic Cholesteatomatous Otitis Media in Children Following the Application of Sodium 2-Mercaptoethanesulfonate (MESNA). Int. J. Pediatr. Otorhinolaryngol. 2018, 110, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Volejníková, A; Melicherčík, P.; Nešuta, O.; Vaňková; E.; Bednárová; L.; Rybáček, J.; Čeřovský, V. Antimicrobial Peptides Prevent Bacterial Biofilm Formation on the Surface of Polymethylmethacrylate Bone Cement. J. Med. Microbiol. 2019, 68, 961–972. [CrossRef] [PubMed]
- Liu, X.; Fan, D.; Feng, X.; Zheng, Y.; Wegner, S.V.; Liu, M.; Chen, F.; Zeng, W. Breaching Bacterial Biofilm Barriers. ACS Appl. Mater. Interfaces. 2022, 14, 41671–41683. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.J.; Patel, M.; Jahan, S.; Abdelgadir, A.; Alam, M.J.; Alshahrani, M.M.; Alturaiki, W.; Sachidanandan, M.; Khan, A.; Badraoui, R.; et al. Silver Nanoparticles Derived from Probiotic Lactobacillus Casei—A Novel Approach for Combating Bacterial Infections and Cancer. Probiotics Antimicrob. Proteins 2023. [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Board, T.; Kay, P.; Wroblewski, B.M.; Zeller, V.; Chen, S.Y.; Hsieh, P.H.; Masri, B.A.; et al. One- and two-stage surgical revision of peri-prosthetic joint infection of the hip: A pooled individual participant data analysis of 44 cohort studies. Eur. J. Epidemiol. 2018, 33, 933–946. [Google Scholar] [CrossRef] [PubMed]
| Method | Year | Method of Application | Mechanism of Action |
|---|---|---|---|
| DTT solution and formulations | 2022 [71,72,73,74] | Samples are treated with a 0.1% DTT solution at a concentration of 25 mM for 15 min. | Breaks disulfide bonds in EPS matrix, releasing encapsulated bacteria |
| EDTA | 2020 [75] | Samples are treated with an EDTA solution at a concentration of 25 mM for 15 min. | Chelates divalent cations essential for EPS stability, destabilizing biofilm structure. |
| MESNA | 2018 [77] | Samples are treated with a 4% MESNA solution for 10 min at 37 °C. | Breaks disulfide bonds in EPS matrix, releasing encapsulated bacteria |
| Method | Years | Effectiveness on the Treatment of IRIs | Mechanism of Action |
|---|---|---|---|
| Antimicrobial Peptides (AMP) | 2019 [78] | Significant reduction in bacterial adhesion and biofilm formation | Disrupt bacterial cell membranes, preventing adhesion and biofilm formation |
| AIEgen | 2022 [79] | Effective biofilm penetration and increased antibacterial efficacy against multidrug-resistant bacteria | Enhance penetration and disrupt biofilms via photothermal effect |
| Cold Plasma Treatments | 2021 [76] | Significant reduction in bacterial adhesion and biofilm formation on various surfaces | Modify surface properties to reduce bacterial adhesion and biofilm formation |
| Silver Nanoparticles (AgNPs) | 2023 [80] | Effective inhibition of biofilm formation and growth of bacterial biofilms | Interact with bacterial cell membranes to disrupt biofilm formation and bacterial growth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giarritiello, F.; Romanò, C.L.; Lob, G.; Benevenia, J.; Tsuchiya, H.; Zappia, E.; Drago, L. Enhancing Pathogen Detection in Implant-Related Infections through Chemical Antibiofilm Strategies: A Comprehensive Review. Antibiotics 2024, 13, 678. https://doi.org/10.3390/antibiotics13070678
Giarritiello F, Romanò CL, Lob G, Benevenia J, Tsuchiya H, Zappia E, Drago L. Enhancing Pathogen Detection in Implant-Related Infections through Chemical Antibiofilm Strategies: A Comprehensive Review. Antibiotics. 2024; 13(7):678. https://doi.org/10.3390/antibiotics13070678
Chicago/Turabian StyleGiarritiello, Fabiana, Carlo Luca Romanò, Guenter Lob, Joseph Benevenia, Hiroyuki Tsuchiya, Emanuele Zappia, and Lorenzo Drago. 2024. "Enhancing Pathogen Detection in Implant-Related Infections through Chemical Antibiofilm Strategies: A Comprehensive Review" Antibiotics 13, no. 7: 678. https://doi.org/10.3390/antibiotics13070678
APA StyleGiarritiello, F., Romanò, C. L., Lob, G., Benevenia, J., Tsuchiya, H., Zappia, E., & Drago, L. (2024). Enhancing Pathogen Detection in Implant-Related Infections through Chemical Antibiofilm Strategies: A Comprehensive Review. Antibiotics, 13(7), 678. https://doi.org/10.3390/antibiotics13070678







