Meta-Analysis and Systematic Review of Phenotypic and Genotypic Antimicrobial Resistance and Virulence Factors in Vibrio parahaemolyticus Isolated from Shrimp
Abstract
1. Introduction
2. Results
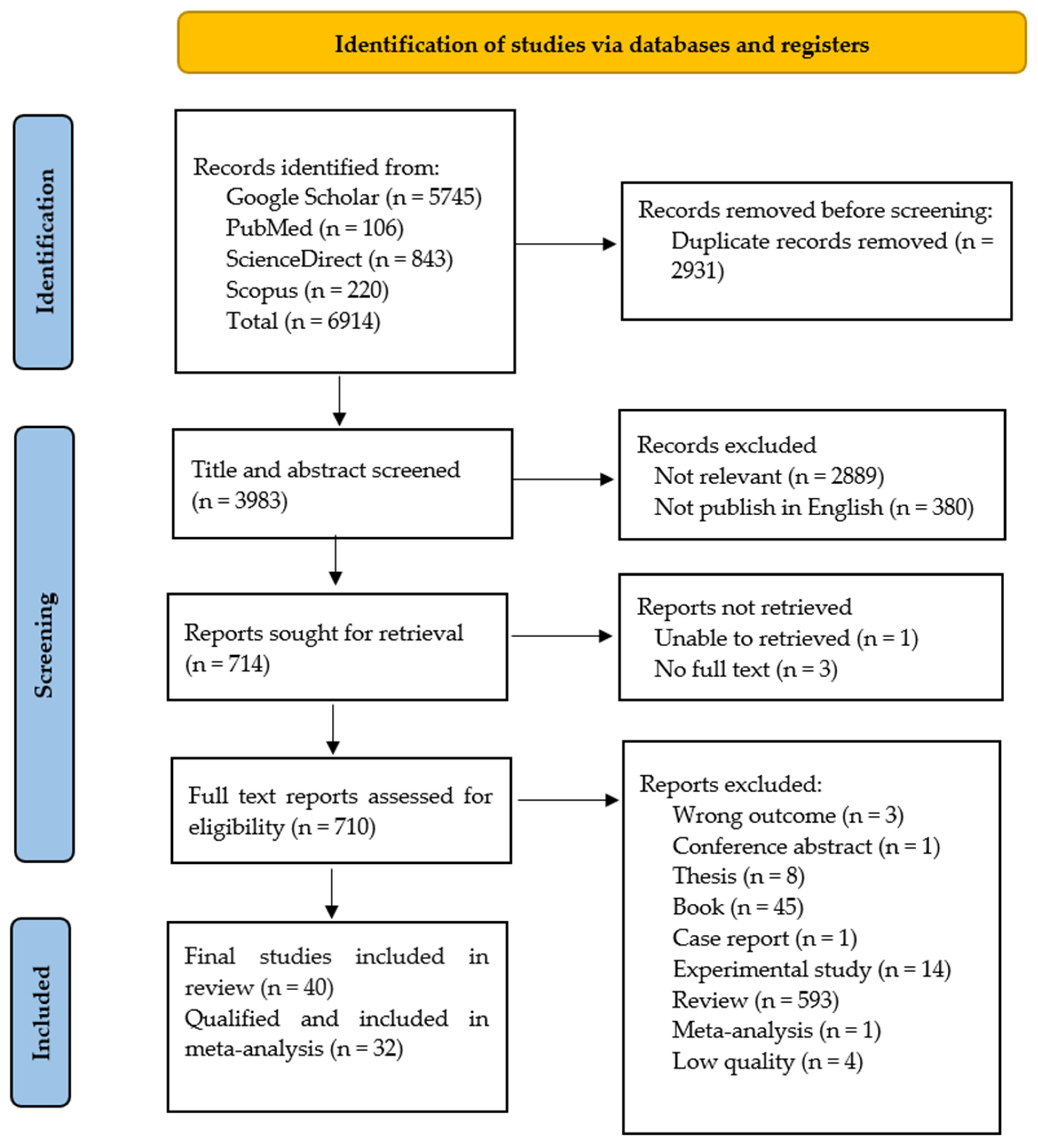
2.1. Summary of Literature Search
2.2. Bacterial Isolation and Confirmation of V. parahaemolyticus Isolates
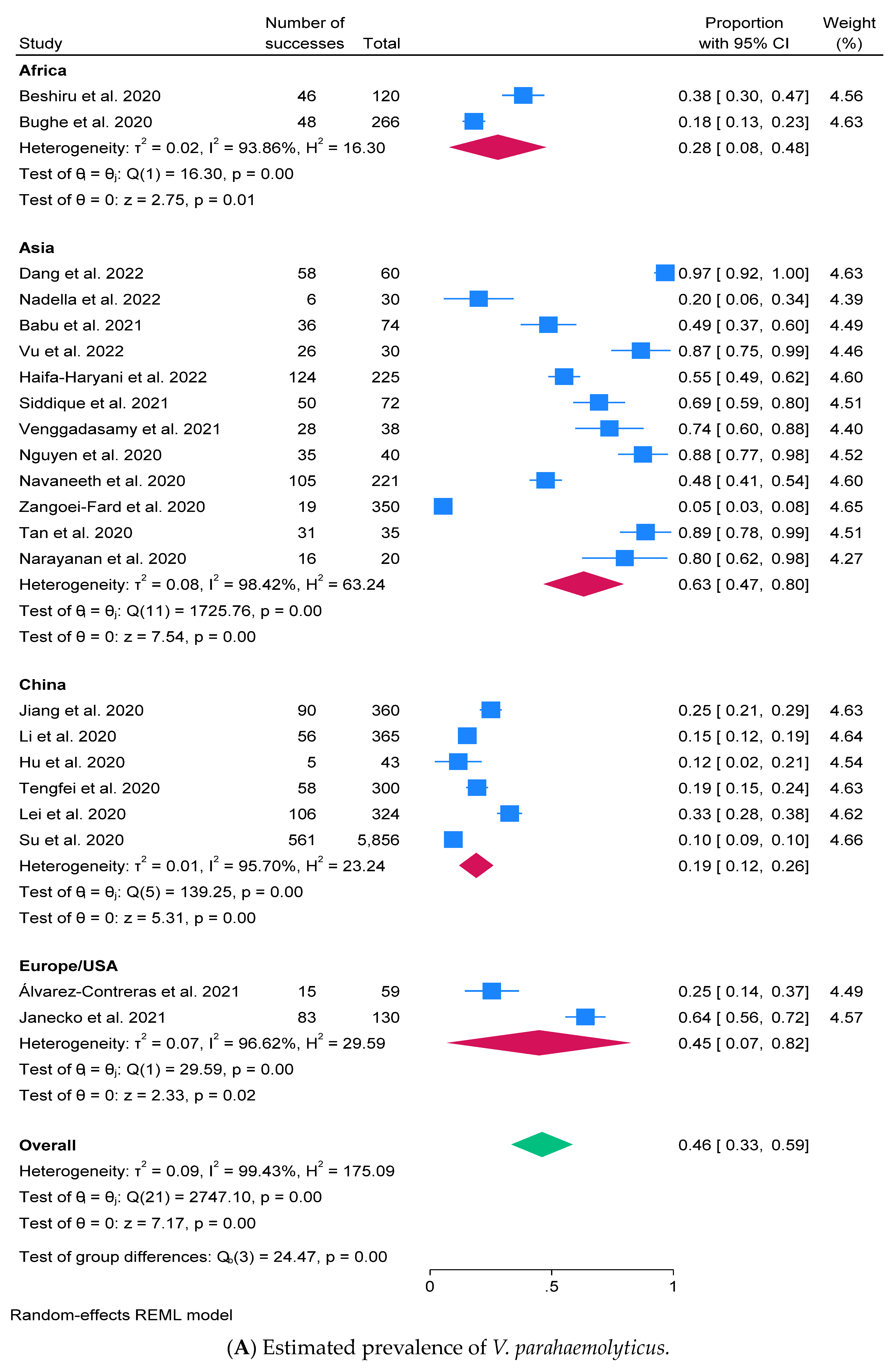
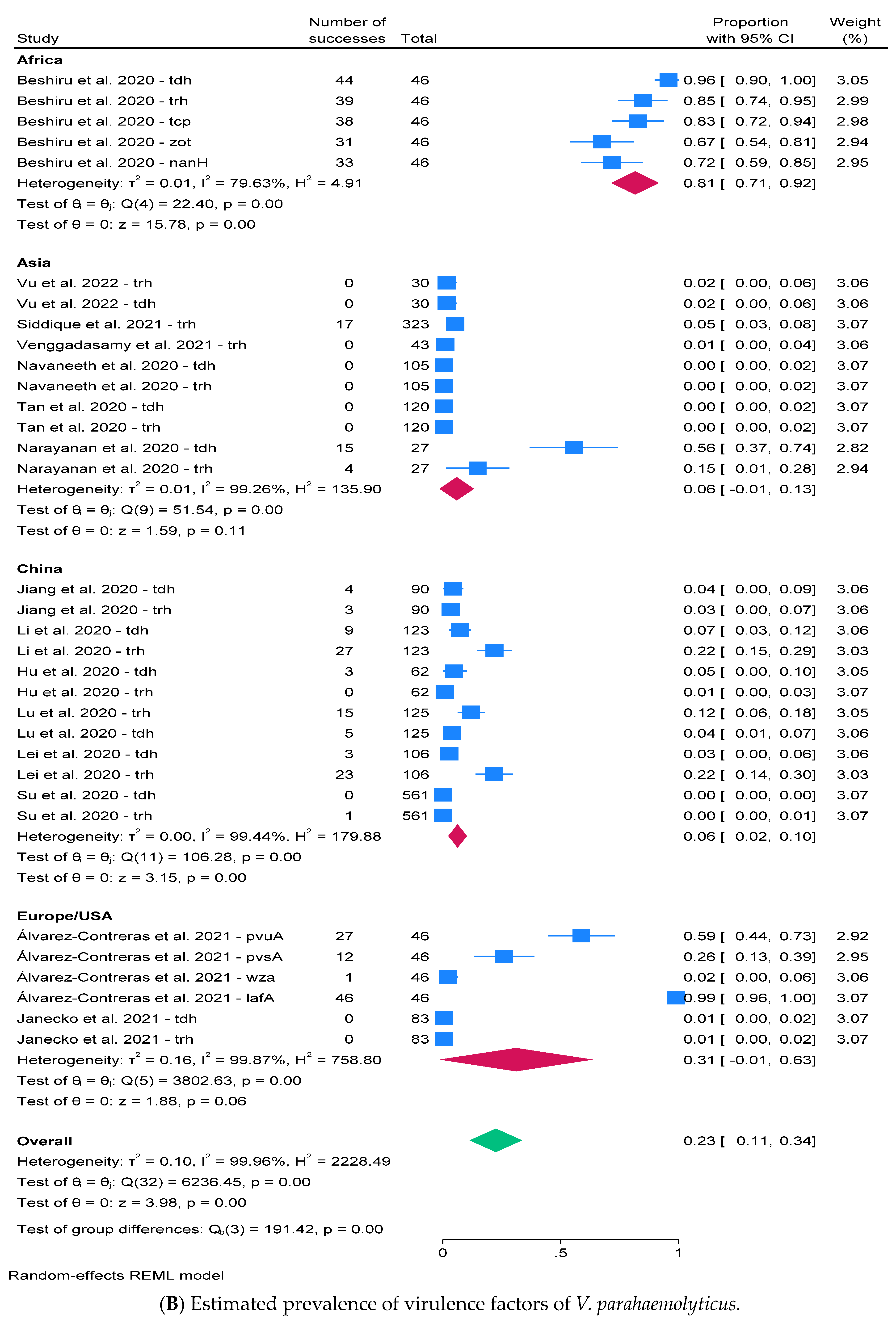
2.3. Prevalence of V. parahaemolyticus and Virulence Factors
2.4. Antimicrobial Susceptibility Testing (AST)
2.5. Prevalence of AMR and Resistant Determinants
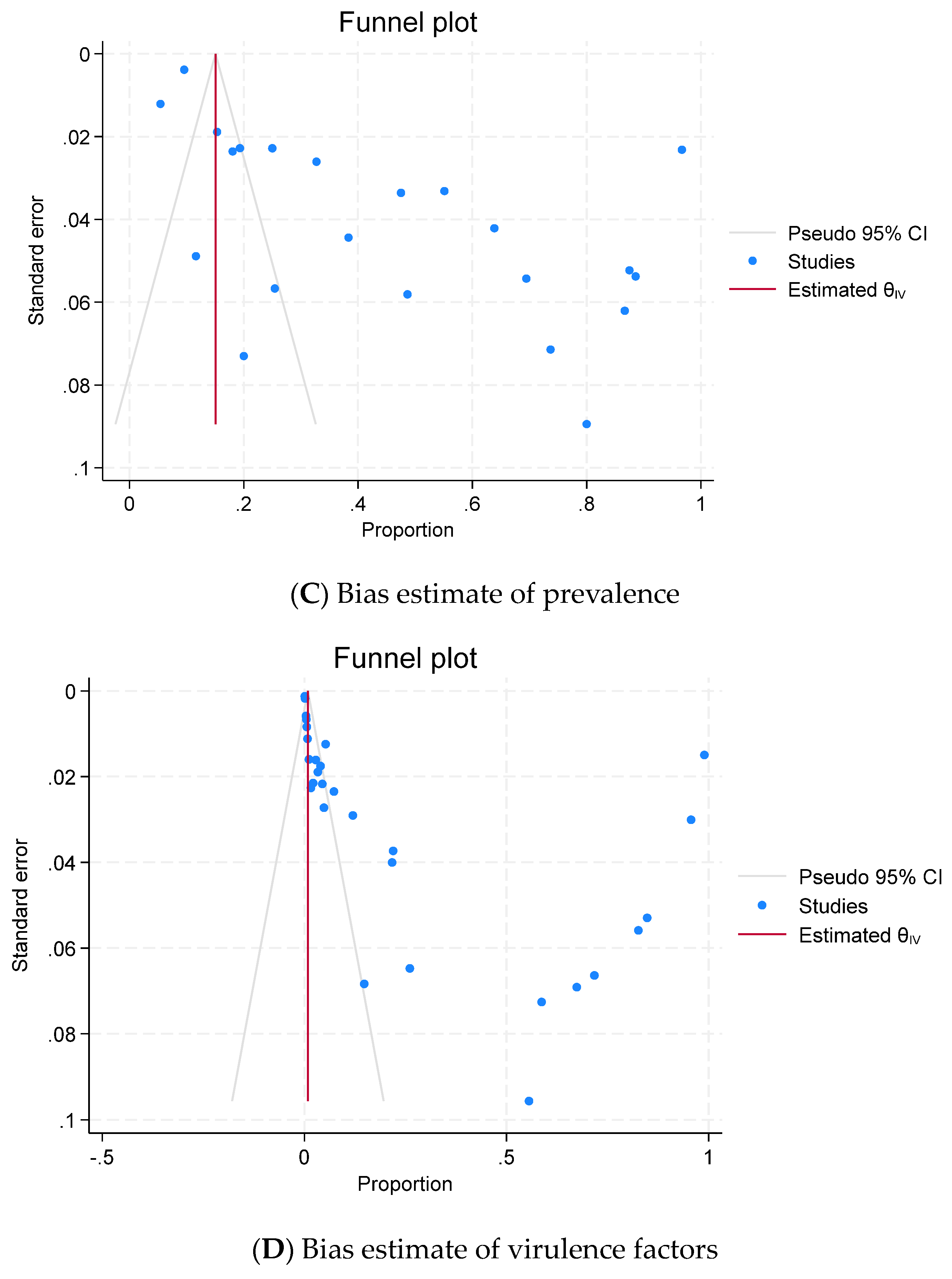
2.6. Publication Bias (Reporting Bias)
3. Discussion
4. Materials and Methods
4.1. Study Selection
4.2. Eligibility Criteria
4.3. Inclusion Criteria
4.4. Exclusion Criteria
4.5. Search Strategy
4.6. Data Collection and Assessment of Data Quality
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance. 2021. Available online: https://www.who.int/publications/i/item/9789241564748 (accessed on 5 December 2023).
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Boyd, E.F.; Cohen, A.L.; Naughton, L.M.; Ussery, D.W.; Binnewies, T.T.; Stine, O.C.; Parent, M.A. Molecular analysis of the emergence of pandemic Vibrio parahaemolyticus. BMC Microbiol. 2008, 8, 110. [Google Scholar] [CrossRef]
- Hong To, T.T.; Yanagawa, H.; Khanh Thuan, N.; Hiep, D.M.; Cuong, D.V.; Khai, L.T.L.; Taniguchi, T.; Kubo, R.; Hayashidani, H. Prevalence of Vibrio parahaemolyticus causing acute hepatopancreatic necrosis disease of shrimp in shrimp, molluscan shellfish and water samples in the Mekong Delta, Vietnam. Biology 2020, 9, 312. [Google Scholar] [CrossRef]
- Khimmakthong, U.; Sukkarun, P. The spread of Vibrio parahaemolyticus in tissues of the Pacific white shrimp Litopenaeus vannamei analyzed by PCR and histopathology. Microb. Pathog. 2017, 113, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Yilmaz, S. Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: A review. Aquaculture 2022, 551, 737951. [Google Scholar] [CrossRef]
- Fujino, T.; Okuno, Y.; Nakada, D.; Aoyama, A.; Mukai, T.; Ueho, T. On the bacteriological examination of shirasu food poisoning. Med. J. Osaka Univ. 1953, 4, 299–304. [Google Scholar]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399–411. [Google Scholar] [CrossRef]
- Janekrongtham, C.; Dejburum, P.; Sujinpram, S.; Rattanathumsakul, T.; Swaddiwudhipong, W. Outbreak of seafood-related food poisoning from undetectable Vibrio parahaemolyticus-like pathogen, Chiang Mai Province, Thailand, December 2020. Trop. Med. Int. Health 2022, 27, 92–98. [Google Scholar] [CrossRef]
- Chowdhury, A.; Ishibashi, M.; Thiem, V.D.; Tuyet, D.T.N.; Tung, T.V.; Chien, B.T.; von Seidlein, L.; Canh, D.G.; Clemens, J.; Trach, D.D.; et al. Emergence and serovar transition of Vibrio parahaemolyticus pandemic strains isolated during a diarrhea outbreak in Vietnam between 1997 and 1999. Microbiol. Immunol. 2004, 48, 319–327. [Google Scholar] [CrossRef]
- Li, Y.J.; Yang, Y.F.; Zhou, Y.J.; Zhang, R.H.; Liu, C.W.; Liu, H.; Li, X.G.; Chen, W.; Chen, Y.; Wu, Y.N. Estimating the burden of foodborne gastroenteritis due to nontyphoidal Salmonella enterica, Shigella and Vibrio parahaemolyticus in China. PLoS ONE 2022, 17, e0277203. [Google Scholar] [CrossRef]
- Martinez-Urtaza, J.; Trinanes, J.; Abanto, M.; Lozano-Leon, A.; Llovo-Taboada, J.; Garcia-Campello, M.; Pousa, A.; Powell, A.; Baker-Austin, C.; Gonzalez-Escalona, N. Epidemic dynamics of Vibrio parahaemolyticus illness in a hotspot of disease emergence, Galicia, Spain. Emerg. Infect. Dis. 2018, 24, 852–859. [Google Scholar] [CrossRef]
- Harth, E.; Matsuda, L.; Hernández, C.; Rioseco, M.L.; Romero, J.; González-Escalona, N.; Martínez-Urtaza, J.; Espejo, R.T. Epidemiology of Vibrio parahaemolyticus outbreaks, southern Chile. Emerg. Infect. Dis. 2009, 15, 163–168. [Google Scholar] [CrossRef]
- Raghunath, P. Roles of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) in Vibrio parahaemolyticus. Front. Microbiol. 2015, 5, 805. [Google Scholar] [CrossRef]
- Elmahdi, S.; DaSilva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef]
- Prabina, D.; Swaminathan, T.R.; Mohandas, S.P.; Anjana, J.C.; Manjusha, K.; Preena, P.G. Investigation of antibiotic-resistant vibrios associated with shrimp (Penaeus vannamei) farms. Arch. Microbiol. 2023, 205, 41. [Google Scholar] [CrossRef]
- Khan, M.; Paul, S.I.; Rahman, M.M.; Lively, J.A. Antimicrobial resistant bacteria in shrimp and shrimp farms of Bangladesh. Water 2022, 14, 3172. [Google Scholar] [CrossRef]
- Paria, P.; Behera, B.K.; Mohapatra, P.K.D.; Parida, P.K. Virulence factor genes and comparative pathogenicity study of tdh, trh and tlh positive Vibrio parahaemolyticus strains isolated from Whiteleg shrimp, Litopenaeus vannamei (Boone, 1931) in India. Infect. Genet. Evol. 2021, 95, 105083. [Google Scholar] [CrossRef]
- Yasin, A.; Begum, K.; Eshik, M.E.; Punom, N.J.; Ahmmed, S.; Rahman, M.S. Molecular identification and antibiotic resistance patterns of diverse bacteria associated with shrimp PL nurseries of Bangladesh: Suspecting Acinetobacter venetianus as future threat. PeerJ 2022, 10, e12808. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Chen, F.; Qi, H.; Chen, L.; Sung, Y.Y.; Huang, Y.; Lv, A.; Hu, X. Phenotypic and genomic characterization of a Vibrio parahaemolyticus strain causing disease in Penaeus vannamei provides insights into its niche adaptation and pathogenic mechanism. Microb. Genom. 2021, 7, 000549. [Google Scholar] [CrossRef]
- Yu, L.H.; The, C.S.J.; Yap, K.P.; Ung, E.H.; Thong, K.L. Comparative genomic provides insight into the virulence and genetic diversity of Vibrio parahaemolyticus associated with shrimp acute hepatopancreatic necrosis disease. Infect. Genet. Evol. 2020, 83, 104347. [Google Scholar] [CrossRef]
- Quang, H.T.; Lan, T.T.; Hai, T.T.H.; Yen, P.T.H.; Van, T.Q.K.; Tung, H.T.; Binh, M.N.; Son, N.K.H.; Linh, N.Q.; Tram, N.D.Q. Genetic diversity and toxic genes analysis of vibrio spp. isolated from white leg shrimp and marine fishes cultured in Tam G iang lagoon in Thua Thien Hue province, Vietnam. Indian J. Sci. Technol. 2020, 13, 1412–1422. [Google Scholar] [CrossRef]
- Han, J.E.; Choi, S.-K.; Han, S.-H.; Lee, S.C.; Jeon, H.J.; Lee, C.; Kim, K.Y.; Lee, Y.S.; Park, S.C.; Rhee, G.; et al. Genomic and histopathological characteristics of Vibrio parahaemolyticus isolated from an acute hepatopancreatic necrosis disease outbreak in Pacific white shrimp (Penaeus vannamei) cultured in Korea. Aquaculture 2020, 524, 735284. [Google Scholar] [CrossRef]
- Mulya, M.A.; Pasaribu, F.H.; Afiff, U.; Yuhana, M. Characterization and molecular detection of pathogenicity and antibiotic resistance genes in Vibrio parahaemolyticus isolated from pacific white shrimp. J. Akuakultur Indones. 2022, 21, 81–92. [Google Scholar] [CrossRef]
- Dang, T.H.O.; Truong, Q.P.; Nguyen, T.P. Antibacterial resistance of Vibrio parahaemolyticus isolated from shrimp farms located in east coastal region of the Mekong Delta, Vietnam. CTU J. Sci. 2022, 14, 1–7. [Google Scholar] [CrossRef]
- Nadella, R.K.; Panda, S.K.; Badireddy, M.R.; Kurcheti, P.P.; Raman, R.P.; Mothadaka, M.P. Multi-drug resistance, integron and transposon-mediated gene transfer in heterotrophic bacteria from Penaeus vannamei and its culture environment. Environ. Sci. Pollut. Res. 2022, 29, 37527–37542. [Google Scholar] [CrossRef]
- Babu, B.; Sathiyaraj, G.; Mandal, A.; Kandan, S.; Biju, N.; Palanisamy, S.; You, S.; Nisha, R.G.; Prabhu, N.M. Surveillance of disease incidence in shrimp farms located in the east coastal region of India and in vitro antibacterial efficacy of probiotics against Vibrio parahaemolyticus. J. Invertebr. Pathol. 2021, 179, 107536. [Google Scholar] [CrossRef]
- Vu, T.T.T.; Hoang, T.T.H.; Fleischmann, S.; Pham, H.N.; Lai, T.L.H.; Cam, T.T.H.; Truong, L.O.; Dac Cam Phung, V.P.L.; Alter, T. Quantification and antimicrobial resistance of Vibrio parahaemolyticus in retail seafood in Hanoi, Vietnam. J. Food Protect. 2022, 85, 786–791. [Google Scholar] [CrossRef]
- Haifa-Haryani, W.O.; Amatul-Samahah, M.A.; Azzam-Sayuti, M.; Chin, Y.K.; Zamri-Saad, M.; Natrah, I.; Amal, M.N.A.; Satyantini, W.H.; Ina-Salwany, M.Y. Prevalence, antibiotics resistance and plasmid profiling of Vibrio spp. isolated from cultured shrimp in Peninsular Malaysia. Microorganisms 2022, 10, 1851. [Google Scholar] [CrossRef]
- Kim, D.H.; Rajapaksha, L.G.T.G.; Gunasekara, C.W.R.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Kim, S.R.; Seo, B.J.; Heo, G.J.; Shin, G.W. Phylogenetic relationships and antibiotic resistance of Vibrio parahaemolyticus isolates related to acute hepatopancreatic necrosis disease in Korea. Aquaculture 2021, 545, 737253. [Google Scholar] [CrossRef]
- Mok, J.S.; Cho, S.R.; Park, Y.J.; Jo, M.R.; Ha, K.S.; Kim, P.H.; Kim, M.J. Distribution and antimicrobial resistance of Vibrio parahaemolyticus isolated from fish and shrimp aquaculture farms along the Korean coast. Mar. Pollut. Bull. 2021, 171, 112785. [Google Scholar] [CrossRef]
- Jin, J.; Zhou, Y.; Zhang, Z.; Wang, H.; Hou, W.; Wang, H.; Li, R.; Zhou, M. Characteristics of antimicrobial-resistant Vibrio parahaemolyticus strains and identification of related antimicrobial resistance gene mutations. Foodborne Pathog. Dis. 2021, 18, 873–879. [Google Scholar] [CrossRef]
- Siddique, A.B.; Moniruzzaman, M.; Ali, S.; Dewan, M.N.; Islam, M.R.; Islam, M.S.; Amin, M.B.; Mondal, D.; Parvez, A.K.; Mahmud, Z.H. Characterization of pathogenic Vibrio parahaemolyticus isolated from fish aquaculture of the southwest coastal area of Bangladesh. Front. Microbiol. 2021, 12, 635539. [Google Scholar] [CrossRef]
- Álvarez-Contreras, A.K.; Quiñones-Ramírez, E.I.; Vázquez-Salinas, C. Prevalence, detection of virulence genes and antimicrobial susceptibility of pathogen Vibrio species isolated from different types of seafood samples at “La Nueva Viga” market in Mexico City. Antonie Leeuwenhoek 2021, 114, 1417–1429. [Google Scholar] [CrossRef]
- Janecko, N.; Bloomfield, S.J.; Palau, R.; Mather, A.E. Whole genome sequencing reveals great diversity of Vibrio spp. in prawns at retail. Microb. Genom. 2021, 7, 000647. [Google Scholar] [CrossRef]
- Venggadasamy, V.; Tan, L.T.H.; Law, J.W.F.; Ser, H.L.; Letchumanan, V.; Pusparajah, P. Incidence, antibiotic susceptibility and characterization of Vibrio parahaemolyticus isolated from seafood in Selangor, Malaysia. Prog. Microbes Mol. Biol. 2021, 4, 1–34. [Google Scholar] [CrossRef]
- Nguyen, T.P.Y.; Nguyen, T.N.; Nguyen, T.B.V.; Nguyen, V.C.; Le, T.T.C.; Huynh, N.T.; Chu, V.T.; Nguyen, D.T.; Nguyen, P.H.L.; James, C.; et al. Antimicrobial residues, non-typhoidal Salmonella, Vibrio spp. and associated microbiological hazards in retail shrimps purchased in Ho Chi Minh city (Vietnam). Food Control 2020, 107, 106756. [Google Scholar]
- Navaneeth, K.A.; Bhuvaneswari, T.; Rajan, J.J.S.; Alavandi, S.V.; Vijayan, K.K.; Otta, S.K. Characterization of Vibrio parahaemolyticus isolates from shrimp farms of Southeast coast of India with special reference to Acute Hepatopancreatic Necrosis Disease (AHPND) status. Aquaculture 2020, 518, 734813. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, T.; Yang, Y.; Yu, S.; Wu, J.; Lin, R.; Li, Y.; Fang, J.; Zhu, C. Co-occurrence of antibiotic and heavy metal resistance and sequence type diversity of Vibrio parahaemolyticus isolated from Penaeus vannamei at freshwater farms, seawater farms, and markets in Zhejiang province, China. Front. Microbiol. 2020, 11, 1294. [Google Scholar] [CrossRef]
- Beshiru, A.; Okareh, O.T.; Okoh, A.I.; Igbinosa, E.O. Detection of antibiotic resistance and virulence genes of Vibrio strains isolated from ready-to-eat shrimps in Delta and Edo States, Nigeria. J. Appl. Microbiol. 2020, 129, 17–36. [Google Scholar] [CrossRef]
- Rahman, M.S.; Eshik, M.E.; Punom, N.J.; Abedin, M.; Begum, M.K. Diversity of Vibrio species and their antibiotic resistance patterns in black tiger shrimp Penaeus monodon Fabricius, 1798 cultured in South-West region of Bangladesh. Asian Fish. Sci. 2020, 33, 330–340. [Google Scholar] [CrossRef]
- Li, Y.; Xie, T.; Pang, R.; Wu, Q.; Zhang, J.; Lei, T.; Xue, L.; Wu, H.; Wang, J.; Ding, Y.; et al. Food-borne Vibrio parahaemolyticus in China: Prevalence, antibiotic susceptibility, and genetic characterization. Front. Microbiol. 2020, 16, 1670. [Google Scholar] [CrossRef] [PubMed]
- Zangoei-Fard, S.; Rahimi, E.; Shakerian, A. Incidence and phenotypic pattern of antibiotic resistance of Vibrio species isolated from seafood samples caught from the Persian Gulf. Egypt. J. Vet. Sci. 2020, 51, 337–347. [Google Scholar] [CrossRef]
- Hu, Y.; Li, F.; Zheng, Y.; Jiao, X.; Guo, L. Isolation, molecular characterization and antibiotic susceptibility pattern of Vibrio parahaemolyticus from aquatic products in the Southern Fujian coast, China. J. Microbiol. Biotechnol. 2020, 30, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, L.; Meng, J.; Zhao, Y.; Song, Y.; Zhu, Y.; Ou, J.; Pan, Y.; Liu, H. Microevolution of Vibrio parahaemolyticus isolated from clinical, acute hepatopancreatic necrosis disease infecting shrimps, and aquatic production in China. Microb. Environ. 2020, 35, ME19095. [Google Scholar] [CrossRef] [PubMed]
- Bughe, R.N.; Oben, B.O.; Nji, A.M.; Chedjou, J.P.K.; Mbange, A.E.; Ali, I.M.; Oben, P.M.; Mbacham, W.F. Occurrence and antibiotics susceptibility of Vibrio sp. From Penaeid shrimps from Kribi Coastal Water, Cameroon. Int. J. Adv. Res. Biol Sci. 2020, 7, 96–111. [Google Scholar]
- Tan, C.W.; Rukayadi, Y.; Hasan, H.; Thung, T.Y.; Lee, E.; Rollon, W.D.; Hara, H.; Kayali, A.Y.; Nishibuchi, M.; Radu, S. Prevalence and antibiotic resistance patterns of Vibrio parahaemolyticus isolated from different types of seafood in Selangor, Malaysia. Saudi J. Biol. Sci. 2020, 27, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Tengfei, X.; Quan, Y.; Xiong, T.; Jing, Z.; Xujun, H. Prevalence, antibiotic susceptibility and characterization of Vibrio parahaemolyticus isolates in China. FEMS Microbiol. Lett. 2020, 367, fnaa136. [Google Scholar]
- Lei, T.; Jiang, F.; He, M.; Zhang, J.; Zeng, H.; Chen, M.; Pang, R.; Wu, S.; Wei, L.; Wang, J.; et al. Prevalence, virulence, antimicrobial resistance, and molecular characterization of fluoroquinolone resistance of Vibrio parahaemolyticus from different types of food samples in China. Int. J. Food Microbiol. 2020, 317, 108461. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.A.; Shipa, S.A.; Naser, N.; Miah, F. Surveillance of Vibrio spp. in Penaeus monodon collected from shrimp pond of Satkhira, Bangladesh. J. Zoo. Res. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Narayanan, S.V.; Joseph, T.C.; Peeralil, S.; Koombankallil, R.; Vaiyapuri, M.; Mothadaka, M.P.; Lalitha, K.V. Tropical shrimp aquaculture farms harbour pathogenic Vibrio parahaemolyticus with high genetic diversity and carbapenem resistance. Mar. Pollut. Bull. 2020, 160, 111551. [Google Scholar] [CrossRef]
- Amatul-Samahah, M.A.; Muthukrishnan, S.; Omar, W.H.H.W.; Ikhsan, N.F.M.; Ina-Salwany, M.Y. Vibrio sp. Associated with acute hepatopancreatic necrosis disease (AHPND) found in penaeid shrimp pond from east cost of peninsular Malaysia. J. Environ. Biol. 2020, 41, 1160–1170. [Google Scholar] [CrossRef]
- Su, C.; Chen, L. Virulence, resistance, and genetic diversity of Vibrio parahaemolyticus recovered from commonly consumed aquatic products in Shanghai, China. Mar. Pollut. Bull. 2020, 160, 111554. [Google Scholar] [CrossRef] [PubMed]
- ISO 6222: 1999; Water Quality—Enumeration of Culturable Micro-Organisms-Colony Count by Inoculation in a Nutrient Agar Culture Medium, 2nd ed. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 4833–2:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Surface Plating Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 21872–1:2017; Microbiology of the Food Chain—Horizontal Method for the Determination of Vibrio spp.—Part 1: Detection of Potentially Enteropathogenic Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus. International Organization for Standardization: Geneva, Switzerland, 2017.
- GB 4789.7-2013; National Food Safety Standard—Food Microbiological Examination—Vibrio parahaemolyticus. National Health and Family Planning Commission of China: Beijing, China, 2013.
- SNI 01-2332.5-2006; Cara uji Mikrobiologi—Bagian 5: Penentuan Vibrio parahaemolitycus Pada Produk Perikanan. Badan Standar Nasional: Jakarta, Indonesia, 2006.
- Oanh, D.T.H.; Nguyen, T.N.; Tran, V.T.; Bondad-Reantaso, M.G. Identification and characterization of Vibrio bacteria isolated from shrimp infected with early mortality syndrome/acute hepatopancreatic necrosis syndrome (EMS/AHPNS) in Vietnam. Asian Fish. Sci. 2018, 31S, 283–292. [Google Scholar] [CrossRef]
- Blanco-Abad, V.; Ansede-Bermejo, J.; Rodriguez-Castro, A.; Martinez-Urtaza, J. Evaluation of different procedures for the optimized detection of Vibrio parahaemolyticus in mussels and environmental samples. Int. J. Food Microbiol. 2009, 129, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Barrow, G.I.; Feltham, R.K.A. Cowan and Steel’s Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: London, UK, 1993. [Google Scholar]
- Bergey, D.H.; Holt, J.G. Bergey’s Manual of Systemic Bacteriology, Volume 2: The Proteobacteria, Part B: The Gammaproteobacteria; Springer: New York, NY, USA, 2005. [Google Scholar]
- Raissy, M.; Moumeni, M.; Ansari, M.; Rahimi, E. Occurrence of Vibrio spp. in lobster and crab from the Persian Gulf. J Food Saf. 2012, 32, 198–203. [Google Scholar] [CrossRef]
- OIE. List of Antimicrobials of Veterinary Importance. Available online: https://www.oie.int/fileadmin/Home/eng/Internationa_Standard_Setting/docs/pdf/OIE_list_antimicrobials.pdf (accessed on 5 December 2023).
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Broth Dilution and Disk Diffusion Susceptibility Testing of Bacteria Isolated from Aquatic Animals, 2nd ed.; CLSI Guideline VET03; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Guideline M100-S30; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Albini, E.; Orso, M.; Cozzolino, F.; Sacchini, L.; Leoni, F.; Magistrali, C.F. A systematic review and meta-analysis on antimicrobial resistance in marine bivalves. Front. Microbiol. 2022, 13, 1040568. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Wang, M.; Chen, L.; Xiao, Y. Sources, distribution and dynamics of antibiotics in Litopenaeus vannamei farming environment. Aquaculture 2021, 545, 737200. [Google Scholar] [CrossRef]
- Luu, Q.H.; Nguyen, T.B.T.; Nguyen, T.L.A.; Do, T.T.T.; Dao, T.H.T.; Padungtod, P. Antibiotics use in fish and shrimp farms in Vietnam. Aquac. Rep. 2021, 20, 100711. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Zhao, Z.; Cao, Y.; Li, B. Hospital wastewater as a reservoir for antibiotic resistance genes: A meta-analysis. Front. Public Health 2020, 8, 574968. [Google Scholar] [CrossRef]
- Clinical Laboratory Standard Institute (CLSI). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed.; CLSI document M45; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Singhal, M.; Agrawal, M.; Bhavna, K.; Gondkar, K.S.; Sethiya, N.K.; Joshi, K.; Kumar, R.; Rai, U.; Bhargava, S.; Rana, V.S. Macrolide antibiotics. In Antibiotics—Therapeutic Spectrum and Limitations, 1st ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 167–181. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Bangar, Y.C.; Singh, B.; Dohare, A.K.; Verma, M.R. A systematic review and meta-analysis of prevalence of subclinical mastitis in dairy cows in India. Trop. Anim. Health Prod. 2014, 47, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
| ID | Author and Published Year | Country | Study Period | Bacteria | Virulence | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TS | PS | P (%) | Gene | TS | PS | P (%) | ||||
| 1 | Mulya et al. (2022) [24] | Indonesia | NA | NA | 12 | NA | tdh trh | 12 12 | 12 0 | 100.0 0 |
| 2 | Dang et al. (2022) [25] | Vietnam | NA | 60 | 58 | 96.7 | NA | NA | NA | NA |
| 3 | Nadella et al. (2022) [26] | India | January 2017–December 2018 | 30 | 6 | 20.0 | NA | NA | NA | NA |
| 4 | Babu et al. (2021) [27] | India | February 2014–July 2015 | 74 | 36 | 48.6 * | NA | NA | NA | NA |
| 5 | Vu et al. (2022) [28] | Vietnam | May 2020–October 2020 | 30 | 26 | 86.7 | tdh trh | 30 30 | 0 0 | 0 0 |
| 6 | Haifa-Haryani et al. (2022) [29] | Malaysia | March 2019–March 2021 | 225 | 124 | 55.1 | NA | NA | NA | NA |
| 7 | Kim et al. (2021) [30] | South Korea | Autumn 2016 | NA | 48 | NA | NA | NA | NA | NA |
| 8 | Mok et al. (2021) [31] | South Korea | April 2018–November 2018 | 11 | 6 | 54.5 ** | NA | NA | NA | NA |
| 9 | Jin et al. (2021) [32] | China | 2015–2016 | 180 | NA | NA | NA | NA | NA | NA |
| 10 | Siddique et al. (2021) [33] | Bangladesh | May 2017–April 2018 | 72 | 50 | 69.4 | trh | 323 | 17 | 5.3 |
| 11 | Álvarez-Contreras et al. (2021) [34] | Mexico | August 2017–February 2018 | 59 | 15 | 25.4 | pvuA pvsA wza lafA | 46 46 46 46 | 27 12 1 46 | 58.7 26.1 2.2 100.0 |
| 12 | Janecko et al. (2021) [35] | United Kingdom | May 2018–April 2019 | 130 | 83 | 63.8 * | tdh trh | 83 83 | 0 0 | 0 0 |
| 13 | Venggadasamy et al. (2021) [36] | Malaysia | NA | 38 | 28 | 73.7 | trh | 43 | 0 | 0 |
| 14 | Yasin et al. (2021) [19] | Bangladesh | NA | 13 | 1 | 7.7 | NA | NA | NA | NA |
| 15 | Nguyen et al. (2020) [37] | Vietnam | March 2018–June 2018 | 40 | 35 | 87.5 | NA | NA | NA | NA |
| 16 | Navaneeth et al. (2020) [38] | India | April 2013–March 2017 | 221 | 105 | 47.5 | tdh trh | 105 105 | 0 0 | 0 0 |
| 17 | Jiang et al., 2020 [39] | China | 2017–2019 | 360 | 90 | 25.0 | tdh trh | 90 90 | 4 3 | 4.4 3.3 |
| 18 | Beshiru et al., 2020 [40] | Nigeria | November 2016–December 2017 | 120 | 46 | 38.3 | tdh trh tcp zot nanH | 46 46 46 46 46 | 44 39 38 31 33 | 95.7 84.8 82.6 67.4 71.7 |
| 19 | Rahman et al., 2020 [41] | Bangladesh | NA | 16 | 4 | 25.0 | NA | NA | NA | NA |
| 20 | Li et al., 2020 [42] | China | September 2015–March 2016 | 365 | 56 | 15.3 | tdh trh | 123 123 | 9 27 | 7.3 22.0 |
| 21 | Zangoei-Fard et al., 2020 [43] | Iran | October 2017–October 2018 | 350 | 19 | 5.4 | NA | NA | NA | NA |
| 22 | Hu et al., 2020 [44] | China | June 2018–October 2018 | 43 | 5 | 11.6 | tdh trh | 62 62 | 3 0 | 4.8 0 |
| 23 | Lu et al., 2020 [45] | China | April 2014–December 2015 | NA | NA | NA | tdh trh | 125 125 | 15 5 | 12.0 4.0 |
| 24 | Bughe et al., 2020 [46] | Cameroon | May 2014–April 2015 | 266 | 48 | 18.1 | NA | NA | NA | NA |
| 25 | Tan et al., 2020 [47] | Malaysia | Jane 2018–June 2018 | 35 | 31 | 88.6 | tdh trh | 120 120 | 0 0 | 0 0 |
| 26 | Hong To et al., 2020 [4] | Vietnam | 2015–2017 | NA | NA | NA | pirA pirB | 12 12 | 10 12 | 83.3 100 |
| 27 | Tengfei et al., 2020 [48] | China | January 2017–December 2019 | 300 | 58 | 19.3 | NA | NA | NA | NA |
| 28 | Lei et al., 2020 [49] | China | June 2014–June 2015 | 324 | 106 | 32.7 | tdh trh | 106 106 | 3 23 | 2.8 21.7 |
| 29 | Amin et al., 2020 [50] | Bangladesh | NA | NA | NA | NA | NA | NA | NA | NA |
| 30 | Narayanan et al., 2020 [51] | India | NA | 20 | 16 | 80 | tdh trh | 27 27 | 15 4 | 55.6 14.8 |
| 31 | Amatul-Samahah et al., 2020 [52] | Malaysia | May 2017 | NA | NA | NA | tdh trh pirA pirB | 2 2 2 2 | 0 0 2 2 | 0 0 100 100 |
| 32 | Su et al., 2020 [53] | China | July 2017–August 2017 | 5856 | 561 | 9.6 | tdh trh | 561 561 | 0 1 | 0 0.2 |
| Antimicrobial Class | Antimicrobials | Total Tested Isolates | Pooled Prevalence (%) | 95% C.I. | tau2 | I2 (%) | H2 (%) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Aminocyclitol * | Spectinomycin | 561 | NA | |||||
| Aminoglycosides | Amikacin; gentamicin; kanamycin; piperacillin; streptomycin; netilmicin; neomycin; tobramycin | 5049 | 21.7 | 14.0–29.3 | 0.070 | 99.57 | 230.44 | <0.0001 |
| Africa | 264 | 1.7 | 0.2–3.2 | 0.0 | 0.11 | 1.00 | 0.017 | |
| Asia | 1537 | 15.1 | 5.5–24.7 | 0.052 | 99.51 | 204.6 | <0.0001 | |
| China | 3110 | 33.7 | 18.3–49.1 | 0.098 | 99.63 | 272.35 | <0.0001 | |
| Europe/USA | 138 | 38.0 | 20.3–55.7 | 0.020 | 80.67 | 5.17 | 0.004 | |
| Beta-lactams | Amoxicillin; ampicillin; ampicillin-sulbactam; carbenicillin; penicillin; amoxicillin/clavulanic acid; carboxybenzicillin; oxacillin; piperacillin; piperacillin/tazobactam; ticarcillin | 3842 | 56.0 | 44.6–67.5 | 0.145 | 99.77 | 442.14 | <0.0001 |
| Africa | 270 | 50.6 | 21.5–79.7 | 0.129 | 97.87 | 46.91 | <0.0001 | |
| Asia | 1861 | 52.2 | 34.4–70.1 | 0.182 | 99.87 | 755.17 | <0.0001 | |
| China | 1619 | 63.7 | 46.1–81.3 | 0.103 | 99.53 | 213.54 | <0.0001 | |
| Europe/USA | 92 | 63.9 | −5.4–133.2 | 0.247 | 99.07 | 107.85 | <0.0001 | |
| Carbapenems | Imipenem; meropenem; tebipenem | 824 | 0.7 | 0.01–1.3 | 0 | 0.05 | 1.00 | 0.001 |
| Africa | 92 | 15.0 | 7.70–22.2 | 0 | 0.03 | 1.00 | 0.561 | |
| Asia | 642 | 0.6 | 0–1.2 | 0 | 0.01 | 1.00 | 0.059 | |
| China * | 90 | 0.5 | −1.0–2.1 | 0 | NA | NA | - | |
| Cephalosporins | Cefamandole; cefazolin; cefepime; cefixime; cefoperazone; cefotaxime; cefoxitin; cefradine; ceftazidime; ceftiofur; ceftizoxime; ceftriaxone; cefuroxime; cephalexin; cephalothin; cephazolin | 4481 | 23.8 | 16.2–31.3 | 0.083 | 99.7 | 336.89 | 0.210 |
| Africa | 132 | 15.4 | −1.5–32.2 | 0.020 | 90.43 | 10.45 | <0.0001 | |
| Asia | 2552 | 25.0 | 14.7–35.2 | 0.089 | 99.71 | 340.82 | <0.0001 | |
| China | 1659 | 16.2 | 6.1–26.3 | 0.047 | 99.55 | 221.02 | <0.0001 | |
| Europe/USA | 138 | 63.5 | 12.7–114.3 | 0.199 | 99.18 | 122.35 | <0.0001 | |
| Folate pathway inhibitors/sulfonamides | Sulfadiazine; sulfamethoxazole; sulfisoxazole; trimethoprim; trimethoprim-sulfamethoxazole | 2356 | 26.1 | 13.9–38.3 | 0.075 | 99.55 | 224.17 | <0.0001 |
| Africa * | 92 | 36.7 | 26.9–46.6 | 0.0 | 0.0 | 1.0 | 0.386 | |
| Asia | 652 | 24.1 | 4.8–43.4 | 0.104 | 99.72 | 359.28 | <0.0001 | |
| China | 1566 | 29.4 | 9.0–49.8 | 0.064 | 99.16 | 124.03 | <0.0001 | |
| Europe/USA * | 46 | 8.7 | 0.6–16.8 | 0.0 | - | - | - | |
| Glycopeptides | Vancomycin; novobiocin | 219 | 60.7 | 19.6–101 | 0.129 | 98.12 | 53.21 | <0.0001 |
| Asia | 157 | 60.9 | −10.2–132 | 0.260 | 98.89 | 90.44 | <0.0001 | |
| China * | 62 | 59.7 | 19.6–71.9 | 0.0 | - | - | - | |
| Lincosamides * | Clindamycin | 62 | NA | |||||
| Macrolides | Azithromycin; erythromycin; medemycin | 857 | 22.1 | 5.0–39.3 | 0.082 | 99.47 | 189.31 | <0.0001 |
| Africa * | 46 | 15.2 | 4.8–25.6 | 0.0 | - | - | - | |
| Asia | 283 | 41.3 | 9.3–73.3 | 0.129 | 98.84 | 86.40 | <0.0001 | |
| China | 528 | 4.9 | 0.5–9.4 | 0.002 | 89.61 | 9.63 | <0.0001 | |
| Nitrofurans | Nitrofurantoin | 260 | 58.8 | 17.8–99.8 | 0.127 | 98.03 | 50.76 | <0.0001 |
| Asia * | 124 | 87.9 | 79.5–96.3 | 0.0 | - | - | - | |
| China * | 90 | 19.4 | 12.4–26.3 | 0.0 | - | - | - | |
| Europe/USA * | 46 | 70.0 | 49.9–90.1 | 0.0 | - | - | - | |
| Oxazolidines | Furazolidone | 62 | NA | |||||
| Phenicols | Chloramphenicol; florfenicol | 2062 | 14.5 | 3.9–25.1 | 0.063 | 99.76 | 415.43 | <0.0001 |
| Africa | 86 | 10.6 | −9.4–30.7 | 0.019 | 90.52 | 10.55 | 0.001 | |
| Asia | 699 | 1.1 | 0.3–1.9 | 0.0 | 5.40 | 1.06 | 0.007 | |
| China | 1185 | 32.8 | 4.8–60.8 | 0.142 | 99.84 | 634.79 | <0.0001 | |
| Europe/USA | 92 | 5.0 | −4.4–14.4 | 0.042 | 75.77 | 4.13 | 0.004 | |
| Polypeptides | Bacitracin; colistin; polymyxin B | 381 | 31.2 | −5.7–68.2 | 0.213 | 99.91 | 1058.5 | <0.0001 |
| Africa | 92 | 1.7 | −0.9–4.3 | 0.0 | 0.03 | 1.00 | 0.328 | |
| Asia | 137 | 90.9 | 74.3–107.5 | 0.013 | 93.52 | 15.44 | <0.0001 | |
| China | 152 | 0.6 | −0.6–1.9 | 0.0 | 0.35 | 1.00 | 0.858 | |
| Quinolones | Ciprofloxacin; enrofloxacin; levofloxacin; nalidixic acid; norfloxacin; ofloxacin; pefloxacin | 3395 | 4.4 | 2.3–6.5 | 0.004 | 96.02 | 25.15 | <0.0001 |
| Africa | 80 | 1.2 | −1.2–3.6 | 0.0 | 0.0 | 1.00 | 1.000 | |
| Asia | 1819 | 3.1 | 1.7–4.5 | 0.001 | 81.06 | 5.28 | <0.0001 | |
| China | 1496 | 4.9 | 0–9.7 | 0.009 | 98.21 | 55.79 | <0.0001 | |
| Rifampicins * | Rifampicin | 561 | NA | |||||
| Tetracyclines | Doxycycline; minocycline; oxytetracycline; tetracycline | 2625 | 6.0 | 2.0–10.0 | 0.011 | 98.71 | 77.68 | <0.0001 |
| Asia | 1188 | 8.2 | 1.1–15.4 | 0.023 | 99.24 | 131.64 | <0.0001 | |
| China | 1437 | 1.6 | 0.6–2.5 | 0.0 | 40.70 | 1.69 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thaotumpitak, V.; Odoi, J.O.; Anuntawirun, S.; Jeamsripong, S. Meta-Analysis and Systematic Review of Phenotypic and Genotypic Antimicrobial Resistance and Virulence Factors in Vibrio parahaemolyticus Isolated from Shrimp. Antibiotics 2024, 13, 370. https://doi.org/10.3390/antibiotics13040370
Thaotumpitak V, Odoi JO, Anuntawirun S, Jeamsripong S. Meta-Analysis and Systematic Review of Phenotypic and Genotypic Antimicrobial Resistance and Virulence Factors in Vibrio parahaemolyticus Isolated from Shrimp. Antibiotics. 2024; 13(4):370. https://doi.org/10.3390/antibiotics13040370
Chicago/Turabian StyleThaotumpitak, Varangkana, Justice Opare Odoi, Saran Anuntawirun, and Saharuetai Jeamsripong. 2024. "Meta-Analysis and Systematic Review of Phenotypic and Genotypic Antimicrobial Resistance and Virulence Factors in Vibrio parahaemolyticus Isolated from Shrimp" Antibiotics 13, no. 4: 370. https://doi.org/10.3390/antibiotics13040370
APA StyleThaotumpitak, V., Odoi, J. O., Anuntawirun, S., & Jeamsripong, S. (2024). Meta-Analysis and Systematic Review of Phenotypic and Genotypic Antimicrobial Resistance and Virulence Factors in Vibrio parahaemolyticus Isolated from Shrimp. Antibiotics, 13(4), 370. https://doi.org/10.3390/antibiotics13040370






