Abstract
Introduction: The use of antibiotics leads to antibiotic resistance (ABR). Different methods have been used to predict and control ABR. In recent years, artificial intelligence (AI) has been explored to improve antibiotic (AB) prescribing, and thereby control and reduce ABR. This review explores whether the use of AI can improve antibiotic prescribing for human patients. Methods: Observational studies that use AI to improve antibiotic prescribing were retrieved for this review. There were no restrictions on the time, setting or language. References of the included studies were checked for additional eligible studies. Two independent authors screened the studies for inclusion and assessed the risk of bias of the included studies using the National Institute of Health (NIH) Quality Assessment Tool for observational cohort studies. Results: Out of 3692 records, fifteen studies were eligible for full-text screening. Five studies were included in this review, and a narrative synthesis was carried out to assess their findings. All of the studies used supervised machine learning (ML) models as a subfield of AI, such as logistic regression, random forest, gradient boosting decision trees, support vector machines and K-nearest neighbours. Each study showed a positive contribution of ML in improving antibiotic prescribing, either by reducing antibiotic prescriptions or predicting inappropriate prescriptions. However, none of the studies reported the engagement of AB prescribers in developing their ML models, nor their feedback on the user-friendliness and reliability of the models in different healthcare settings. Conclusion: The use of ML methods may improve antibiotic prescribing in both primary and secondary settings. None of the studies evaluated the implementation process of their models in clinical practices. Prospero Registration: (CRD42022329049).
1. Introduction
Between 2000 and 2010, global human antibiotic consumption increased by 35%, with a noticeable increase in the use of ‘last resort’ antibiotics, especially in middle-income countries [1]. Antibiotic resistance (ABR) is defined as the ability of bacteria to grow and adapt in the presence of antibiotics [2,3,4]. There is a direct association between antibiotic consumption and the emergence of antibiotic resistance (ABR) [5,6,7]. Inappropriate and excessive prescribing of antibiotics contributes to the spread of ABR [6,8,9]. ABR is associated with morbidities, hospital admissions, increased cost of healthcare, and treatment failures [10,11,12], and it has been listed as one of the top ten threats by the World Health Organization (WHO) [13]. In 2019, ABR was associated with 4.95 million deaths globally, of which 1.27 million deaths were directly attributable to ABR, with the highest number of deaths in Western Sub-Saharan Africa and the lowest number of deaths in Australasia [14]. Without concerted action, it is estimated that by 2050, worldwide, ABR will result in 300 million deaths, reduce GDP by 2.5–3% and losses of USD 60–100 trillion [15].
Different methods have been used to improve antibiotic prescribing, such as antibiotic stewardship programs (ASPs) (defined as set of interventions that promote the responsible usage of antibiotics) [16,17], and clinical decision support systems (CDSSs) (which are a source of patient-related recommendations and assessments for clinicians to help in their decision making [18]. These methods target a change in behaviour [19,20]. In addition, ASPs generally have a short-term effect [21], and need continued effort to obtain relatively small reductions in prescribing [22]. CDSSs are computer-based systems that are not always easy to integrate into the patient management systems, or in the workflow of the clinician [23].
Artificial intelligence (AI) is the ability of a machine, such as a computer, to “independently replicate intellectual processes typical of human cognition in deciding on an action in response to its perceived environment to achieve a predetermined goal” [24]. Machine learning (ML) is a subfield of AI [24,25], where machines are able to learn from data and improve their analyses by using computational algorithms [26,27]. Types of ML algorithms are supervised learning, unsupervised learning and reinforcement learning [24,28,29]. Supervised learning algorithms are those that perform prediction, and some of these algorithms perform classification based on previous data examples [28]. Examples of supervised learning algorithms are logistic regression [30], naïve bayes, support vector machine (SVM), decision trees [31], random forests [32], artificial neural networks (ANNs) [33] and gradient boosting [34]. On the other hand, unsupervised learning algorithms have the ability to explore patterns in data [28], for example, principal component analysis (PCA). Reinforcement learning algorithms are concerned with how an agent (i.e., algorithm) takes action in a space so that it can maximise a cumulative reward [31]. AI and ML, and other terms, such as data science, are often used interchangeably [35].
ML has been utilised in solving medical challenges [36], such as predicting cancer types, medical imaging, wearable sensors [37], healthcare monitoring [38], drug development, disease diagnostics, analysis of health plans, digital consultations and medical and surgical personalised treatments [39]. Recently, ML methods have been explored as means to guide the rational use of antibiotics, explore suitable antibiotic combinations or identify new antibiotic peptides (ABPs) [33]. No previous reviews were identified on the use of AI to improve antibiotic prescribing. The aim of this review was to explore the use of AI to improve antibiotic prescriptions for human patients.
2. Materials and Methods
The protocol for this review was registered in the PROSPERO database (CRD42022329049), and the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)” guideline [40] was used to design and report findings.
2.1. Eligibility Criteria
- Participants and setting: Participants were human patients without restrictions on their characteristics (i.e., gender, age, weight, morbidities). No restrictions were applied for setting (i.e., primary, secondary, or tertiary care), timing of publication or language of the studies included. The “no restriction” on participants′ inclusion, setting or language was applied to ensure that all relevant studies were retrieved;
- Study design: the studies included in this review were cohort studies or any observational study that examined the potential or actual use of AI, machine learning or data analytics to improve antibiotic prescribing or consumption in human patients;
- Outcome measures:
- ○
- The relative reduction of antibiotic prescriptions (primary outcome);
- ○
- The prediction of inappropriate antibiotics prescriptions;
- ○
- The relative reduction in re-consultations of patients, irrespective of the reason for re-consultation (infection recurrence or worsening of patient’s condition).
2.2. Data Sources and Search Strategy
- Data sources: the search strategy was applied to Scopus, OVID, ScienceDirect, EMBASE, Web of Science, IEEE Xplore and the Association for Computing Machinery (ACM);
- Search strategy: the search strategy, which can be accessed in [41], was based on three different concepts: (1) artificial intelligence, (2) prescriptions, and (3) population, and was customised to each database, depending on the special filters of these databases. The search for studies stopped in April 2022, and the snowball search (i.e., screening the references of the included studies for additional eligible studies) was conducted in August 2022.
2.3. Study Records
2.3.1. Study Selection
Two independent authors (DA, NG, AGP, SP, HV) scanned titles and abstracts of the studies to assess eligibility. Manuscripts of eligible studies were retrieved for full-text screening, and their references were checked for additional studies (i.e., snowball search). Two independent authors (DA, NG, SP) screened the full text of the retrieved studies. Whenever a conflict occurred, a third reviewer (AV) was involved in the resolution.
2.3.2. Data Extraction and Management
A customised data extraction sheet was developed, where the main sections were:
- Study information, for example, publication type, country, name of publication outlet, authors, year of publication, title, aim of study and funding source;
- Population, for example, total number of participants, cohorts, sample size calculation, methods of recruitment, age group, gender, race/ethnicity, illness, comorbidities and inclusion/exclusion criteria of patients;
- Methods and setting, for example, design of the study, data source, setting, start and end dates, machine learning methods used, training and test sets, predictors, data overfitting, valuation and validation of performance and handling of missing data;
- Outcomes, such as outcome(s) name(s) and definition and, if applicable, unit of measurement and scales;
- Results, limitations and key conclusions.
One author (DA) extracted the data, which was reviewed by a second author (NG, SP).
2.3.3. Assessment of Risk of Bias
The National Institute of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used to assess the quality of included studies [42]. The assessment was carried out by three authors (DA, NG, SP), and conflicts were resolved by discussion.
2.3.4. Data Synthesis
Due to the small number of included studies, it was not feasible to assess the heterogeneity using I2 statistics. Instead, a narrative synthesis was adopted to analyse the studies in this review.
3. Results
A total of 5223 citations were retrieved, and after removing the duplicates and irrelevant records (for example, posters, book chapters, and abstracts), 3692 records were included for screening. A total of fifteen studies were eligible for full-text screening; ten studies resulted from abstract screening, and five additional studies were identified from references screening (see Supplementary Materials Table S4 for reasons of exclusion in title and abstract screening). A final five studies [43,44,45,46,47] were included in the narrative synthesis and risk of bias assessment (see Figure 1). The characteristics of the included studies are available in Supplementary Materials Table S1, and the list of excluded studies is available in Supplementary Materials Table S2.
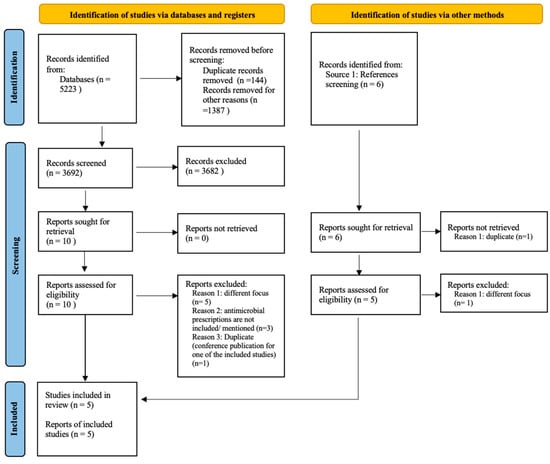
Figure 1.
Prisma flow diagram [48]: studies selection process.
3.1. Included Studies
3.1.1. Study Information and Population
Patients included were from different populations; female patients with UTIs (18–55 years old) [43], inpatients receiving an antibiotic [45], inpatients with UTIs [46] and children [44,47]. The children who were included in the Cambodian study had bloodstream infections (BSI) [47]. The population sizes ranged from 243 children with BSI [47] to 700,000 episodes of community-acquired urinary tract infections (UTIs), with 5,000,000 records of antibiotic purchases [46].
The five included studies [43,44,45,46,47] were in English and published between 2016 and 2022. The countries where the studies were conducted were the United States [43], South Korea [44], Canada [45], Israel [46] and Cambodia [47]. There was no information provided in any of the five studies [43,44,45,46,47] about sample size calculation, nor comorbidities or ethnicity.
3.1.2. Methods and Settings
- Study design and setting: four studies [43,44,46,47] were retrospective, and one [45] was prospective (see Supplementary Materials Table S1). The setting in four studies [43,44,45,47] was secondary care and primary care in one study [46]. The data sources in four studies [43,44,45,47] were the electronic health records (EHRs) of the hospitals, while in a study by Yelin et al., the data of community- and retirement home-acquired urinary tract infections (UTIs) was obtained from Maccabi Healthcare Services (MHS), the second largest healthcare maintenance organisation in Israel [46]. All five studies [43,44,45,46,47] used supervised machine learning algorithms [28];
- Machine learning models in included studies:
- ○
- Duration: the time frame of the data in the five included studies [43,44,45,46,47] ranged from 11 months to 10 years;
- ○
- Predicted (outcome) variables: the primary outcome (relative reduction in antibiotic prescriptions) was reported in one study [43]; however, it was reported as a “proportion of recommendations for second-line antibiotics” (i.e., reduction in the use of second-line antibiotics) (see Table 1 and Supplementary Materials Table S1). The second outcome (prediction of inappropriate antibiotic prescriptions) was reported in five studies [43,44,45,46,47]; however, it was defined differently in each of them (Supplementary Materials Table S1). In a study by Kanjilal et al., it was reported as a “proportion of recommendations for inappropriate antibiotic therapy” [43], while in a study by Lee et al., a prediction of “antibiotic prescription errors” [44]. In a study by Beaudoin et al., it was reported as a prediction of “inappropriate prescriptions of piperacillin–tazobactam” [45]. The outcome in the study by Yelin et al. was a prediction of “mismatched treatments” (i.e., when the sample is resistant to the prescribed antibiotic) [46], and in a study by Oonsivilai et al., the outcome was a prediction of “susceptibility to antibiotics” [47]. None of the five studies [43,44,45,46,47] reported the third outcome (i.e., the relative reduction in re-consultations of patients);
 Table 1. Characteristics of ML models in included studies.
Table 1. Characteristics of ML models in included studies. - ○
- Predictors: predictors in the studies included in this review were categorised as either “bedside” or “non-bedside” to make a distinction in the data a clinician had access to at diagnosis and prescription of the (empiric) treatment, and data that was only available after laboratory or other investigations [49]. Furthermore, predictors were divided into 10 groups: labs, antibiotics, demographics, geographical, temporal, socioeconomic conditions, gender-related, comorbidities, vital signs and medical history of patients (see Supplementary Materials Table S3). All groups of predictors used in the five studies [43,44,45,46,47] belonged to the “bedside” category, except for the lab′s group of predictors, which belonged to the “non-bedside” category. Since the clinical predictors are indicative in prescribing treatments, the inclusion in the development of ML models provides more accurate prediction compared to the clinicians who did not have this information at the time of prescribing.
Labs predictors were used in the five studies [43,44,45,46,47], while antibiotic-related predictors were used in four studies [43,44,46,47]. Demographic data were used in four studies [43,45,46,47], geographical predictors were used in three studies [43,45,46], and temporal predictors were used in two studies [46,47]. The study by Oonsivilai et al. was the only one that used socioeconomic-related predictors [47], and the study by Yelin et al. was the only one that included gender-related predictors (i.e., pregnancy) since its population was females with UTIs [46]. The comorbidities predictor was used in one study [43], as were vital signs predictors [45]. The medical history of patient-related predictors were reported in three studies [43,44,47] (Table 1). In addition, in a study by Kanjilal et al., there were two population-level predictors that belonged to both clinical and antibiotics groups of predictors [43].
- ○
- Training and test datasets: A train/test split approach is used to ensure that the performance of an ML model is validated [50]. Three studies [43,46,47] reported that a train/test split approach was used, while two studies [44,45] did not report information about a train/test split. In the study by Kanjilal et al., the train/test split consisted of a training dataset of 10,053 patients versus a test dataset of 3941 patients [43], while in the study by Yelin et al., the data was divided in a way that all data collected from 1 July 2007 to 30 June 2016 was treated as a training set, and data collected from 1 July 2016 to 30 June 2017 was treated as test set [46]. In the remaining study, Oonsivilai et al. made an 80% versus 20% train/test split [47];
- ○
- Machine learning models: All models [43,44,45,46,47] belong to the supervised machine learning models [28]. Three studies [43,46,47] used logistic regression and decision trees, while random forest models were used in two studies [43,47]. Gradient boosting decision trees (GBDTs) were used in [46,47], in addition to linear, radial and polynomial support vector machines (SVMs) and K-nearest neighbours (KNNs) in [47]. Two studies [44,45] used different models: an advanced rule-based deep neural network (ARDNN) and a supervised learning module (i.e., temporal induction of classification models (TIM));
- ○
- Method to avoid data overfitting: Data overfitting occurs when an ML model perfectly fits training data but fails to generalise to test data [51], for which regularisation can be a solution [52]. Regularisation adds a penalty to the algorithm’s smoothness or complexity to avoid overfitting and improve the generalisation of the algorithm [53]. In the studies by Kanjilal et al. [43] and Oonsivilai et al. [47], regularisation was used to avoid data overfitting. In the study by Beaudoin et al. [45], the “J-measure”, which is defined as a measure of the goodness-of-fit of a rule [54], was used to reduce overfitting, and in the study by Yelin et al., the model’s performance on the test set was contrasted with the training set to identify data overfitting [46]. The study by Lee et al. did not report on data overfitting [44];
- ○
- Handling missing data: Two studies [43,45] did not report how missing data were handled. In the study by Lee et al., 2.45% of height and weight missing data were predicted, other empty data records were deleted, and data outliers were treated as missing values [44]. In the study by Yelin et al., the missing data for antibiotic resistance was treated as “not available” and eventually dropped from the models [46]. In the study by Oonsivilai et al., missing data for binary variables were considered “negative”; however, no further explanation for these negative values was provided [47];
- ○
- Evaluation of models’ performance: The predictive ability of an algorithm is assessed by the accuracy of the predicted outcomes in the test dataset [55]. Different measures are used to evaluate the predictive performance, for example, the area under the receiver operating characteristic curve (AUROC) [56], accuracy, sensitivity (i.e., recall), specificity, precision and F1 score [57].
To evaluate the predictive performance of their models, three studies [43,46,47] reported using AUROCs. In the study by Kanjilal et al., AUROC was poor for all antibiotics predictions [43], while in the study by Yelin et al., it ranged from acceptable to excellent for predicting susceptibility to amoxicillin-CA and ciprofloxacin, respectively [46], and in the study by Oonsivilai et al., it was excellent for all antibiotics susceptibility predictions [47]. On the other hand, two studies [44,45] reported using the recall and precision measures, with both measures ranging from 73–96%, which indicates high-performing models, in addition to the F1-score measure of 77% reported in [44], and the accuracy measure of 79% reported in [45] (Table 1).
- ○
- Findings of included studies: All five studies [43,44,45,46,47] used ML models to predict their outcomes. They included both bedside and non-bedside predictors that were extracted from either EHRs, or information systems of healthcare services organisations, and thus the ML models included complete patients’ information for their development. Regarding the primary outcome, the study by Kanjilal et al. reported that their algorithm was able to make a recommendation for an antibiotic in 99% of the specimens, and chose ciprofloxacin or levofloxacin for 11% of the specimens, relative to 34% in the case of clinicians (a 67% reduction) [43].
On the other hand, the secondary outcome (prediction of inappropriate antibiotic prescriptions) was reported as the proportion of recommendations for inappropriate antibiotic therapy (i.e., second-line antibiotics) in the study by Kanjilal et al., whose algorithm’s recommendation resulted in an inappropriate antibiotic therapy (i.e., second-line antibiotics), in 10% of the specimens relative to 12% in case of clinicians (18% reduction) [43]. In the study by Lee et al., the authors reported success in predicting antibiotic prescription errors and that their algorithm was able to predict 145 prescription errors out of 179 predefined errors [44], while in the study by Beaudoin et al., pharmacists reviewed 374 prescriptions of piperacillin–tazobactam, of which 209 were defined as inappropriate [45]. The ML algorithm predicted 270 out of the 374 prescriptions as inappropriate, with a positive predictive value of 74%. Furthermore, in the study by Yelin et al., the authors reported that both algorithms used (unconstrained and constrained) were able to reduce the mismatched treatments as compared to prescribing by clinicians; the unconstrained resulted in a predicted mismatch of 5% (42% lower than the mismatch of 8.5% in case of clinicians), while the constrained resulted in a predicted mismatch of 6%, which is higher than the unconstrained models, however still lower than the 8.5% in case of clinicians [46]. In the study by Oonsivilai et al., the authors reported positive results in predicting susceptibility to antibiotics, which is used to guide antibiotic prescribing using their ML algorithms, and that was reflected by the AUROC values for the different ML models, rather than the results of the prediction models themselves [47]. In addition, the authors reported that when the performance of different ML algorithms was compared, the random forest algorithm outperformed the other ML algorithms in predicting susceptibility. (See Table 1).
3.1.3. Assessing the Risk of Bias
The assessment of the quality of the included studies in this review is shown in Table 2. Five studies [43,44,45,46,47] had a “Fair” rating, meeting 8 out of 14 criteria. Some of the criteria in this quality assessment tool [42] were not applicable to the studies included. For example, the third criterion (i.e., the participation rate for eligible persons), the fifth criterion (i.e., sample size justification), the eighth criterion (i.e., measurement of different levels or amounts of exposures), and the thirteenth criterion, (i.e., loss to follow-up). No study [43,44,45,46,47] reported any information regarding the 12th and 14th criteria (i.e., blinding of the outcome assessors and the measurement of the confounding variables).

Table 2.
Assessment of Risk of Bias.
4. Discussion
4.1. Summary of Main Findings
This review included five studies [43,44,45,46,47] to evaluate the use of artificial intelligence in improving antibiotic prescribing for human patients. All studies used supervised ML models as a means to improve prescribing, and all of them focused solely on antibiotics, and no other form of antimicrobial (i.e., antivirals or antifungals) was reported. One study [43] reported a relatively decreased second-line antibiotic (primary outcome), while all studies [43,44,45,46,47] reported the ability of their algorithms to predict inappropriate antibiotic prescriptions (secondary outcome). These findings align with what Fanelli et al. suggested, namely that ML contributes to increasing appropriate antibiotic therapies and minimising the risks of antibiotic resistance [58].
4.2. Using ML Models to Improve Antibiotic Prescribing
Developing an ML model in a healthcare context, such as the models in this review, developed to improve antibiotic prescribing, has to go through the following phases:
- Problem selection and definition;
- Data collection/curating datasets;
- ML development;
- Evaluation of ML models;
- Assessment of impacts;
- Deployment and monitoring [59].
Table 3 assesses the adherence of the studies included in this review to the prior mentioned six phases.

Table 3.
Adherence to the five phases of the development of a classifier.
Problem selection and definition: The first step in developing ML models in a healthcare context is to carefully select and define the predictive task and make sure the data needed is available [59]. In addition, careful study design is necessary to generalise the models for future clinical use [60]. Furthermore, data variation [58], which leads to better model results [61,62,63], is achieved by preparing the dataset and choosing the predictors well [64]. All five studies [43,44,45,46,47] provided clear definitions for the problems they were modelling, and reported the size of the population included in their studies, which was not necessarily large, such as in the case of the study by Oonsivilai et al. (i.e., the number of patients included were 243) [47]. Furthermore, all studies [43,44,45,46,47] reported the predictors they used in their models, which were both bedside and non-bedside predictors. However, there was no justification for how or why these predictors were chosen or if there were any associations (which may result in multicollinearity) between them.
Data collection/curating datasets: In this step, the datasets used in the model are constructed, and the development/validation sets (i.e., train/test splits) are generated [59]. None of the included studies [43,44,45,46,47] reported any information about their sample size calculation. Three studies [43,46,47] reported a train/test split for the data; however, no justification for this split was provided.
ML development: The choice of an ML model is based on several factors, such as size, type and complexity of data. Higher-performing ML models in problems relevant to ABR, such as support vector machines (SVM), random forests (RFs) and artificial neural networks (ANN), are less frequently used than simpler models, such as decision trees (DTs) and logistic regression (LR) [65]. All five studies [43,44,45,46,47] reported the ML models they used in their studies. In the study by Kanjilal et al., the authors reported using logistic regression based on interpretability and validation performance as compared to decision tree and random forest models [43]. However, they provided no justification for the three models they chose. And in the study by Lee et al., the authors reported that they collected the rules they used for their model, based on consultation with a clinician; however, no information was reported on further involvement of the clinician in the development of their model [44]. On the other hand, three studies [45,46,47] did not provide justification for their choices of the ML models they used, nor the suitability of these models for the nature or complexity of their data.
Evaluation of ML model: There are two categories for the evaluation measures of ML models, which are discrimination and calibration. Discrimination measures check the ability to distinguish or rank two classes, such as recall, precision, specificity and the area under the receiver operating characteristic curve (AUROC). On the other hand, the calibration measures evaluate how well the predicted probabilities match the actual probability, for example, the Hosmer–Lemeshow statistic. In addition, in healthcare research, subgroup analysis is used as an aspect of evaluation. Although this is not applicable in ML models, however, it can be conducted via the inclusion or exclusion of certain subgroups in an ML model [59]. All five studies [43,44,45,46,47] used discrimination measures to evaluate the performance of their models, and only two studies [43,45] reported age groups of included patients. In addition, in the study by Beaudoin et al., the authors reported that they used the results of a previous antimicrobial prescription surveillance system (APSS) used by three hospital pharmacists during the evaluation of their model’s performance [45].
Assessment of Impact: There are several challenges in using ML in a healthcare context, such as making sure a system based on an ML model would be user-friendly and reliable [59], in addition to considering automation bias [60]. None of the studies provided any information on whether their ML models were used or tested by prescribing clinicians or would be reliable tools to use in clinical practices to guide antibiotic prescribing.
Deployment and monitoring: There are several factors to consider when implementing technologies based on ML models, such as hardware and software infrastructures, reliable internet, firewalls and ethical/ privacy/regulatory/legal frameworks [59]. None of the five studies [43,44,45,46,47] provided any information on deployment or monitoring. In addition, no information was provided about any ethical, privacy or legal requirements (i.e., approvals from health departments) for the models to be deployed as tools to guide antibiotic prescriptions in different healthcare settings. This implies that there is a gap between the current studies and their application as tools to guide antibiotic prescribing in the real world.
More on ML Models and Generalisation
In Section 3.1.2, methods to avoid data overfitting and improve generalisation were discussed. Moreover, there are two more factors that have an impact on a generalisation of an ML model: bias and robustness.
- ○
- Reproducing bias in ML models: Bias can lead to the lack of generalisation in an ML model [66]. If an ML model is trained on a dataset that was generated on a biased process, the output of the model may also be biased (i.e., bias reproduction), which is a real challenge when using healthcare data sources, such as EHRs [67]. This type of bias is called “algorithmic bias” [66]. Other sources of bias in an ML model may be “data-driven” (for example, bias due to ethnicity or socioeconomic status) or “human”, in which the persons who develop the ML system reflect their personal biases [66]. Two of the included studies [43,46] reported biases in their data. In the study by Kanjilal et al. [43], the authors reported data bias, for most of their data was for Caucasian patients. And to minimise producing biased predictions, they used a national criterion adopted for uncomplicated urinary tract infections. The second bias reported was the prediction of non-susceptibility more often in environments in which the ABR prevalence is higher than what exists in the training data, and thus the authors have assessed the temporality by using longitudinal data and have confounded by indication [43]. In the study by Yelin et al. [46], the authors reported some aspects of the data they used that would introduce bias in their results, such as the later use of a purchased antibiotic (shall produce a bias towards higher odds ratio for purchases before infection), patients who used antibiotics that were not made via the information system the authors used to extract the data (shall produce a bias towards lower odds ratio for drug purchases), the UTIs that are empirically treated without culture (shall produce a bias towards measuring of more resistant samples), the elective culture testing for cultures made after failure of treatment (shall produce a bias towards measure of more resistant samples, in addition to a strong association of drug purchases and resistance), in addition to the dependence of elective cultures on demographics (shall produce associations between demographics and resistance). However, the authors did not report any means to avoid these biases, and they have reported that their models were still able to predict resistance well and for their recommendation algorithms to reduce the chances of antibiotic prescribing for a resistant infection [46]. However, this implied that there was still a chance to reproduce these biases in their predictions. On the other hand, the three remaining studies [44,45,47] did not report biases indicating the chance of bias reproducibility in their predictions;
- ○
- Robustness of ML models: The robustness of an ML model is how well the model is trained to face adversarial examples [68]. It considers the sensitivity of the model (i.e., how the model’s output is sensitive to a change in its input) [69]. In addition, robustness has been used to derive generalisation in a supervised learning ML model [70]. All five studies [43,44,45,46,47] have used different performance measures, which help in understanding the sensitivity of their models and, thus, how robust they are. They reported measures such as recall (i.e., sensitivity), F1 score, AUROC, etc., which contribute to indicating how sensitive their models were to changes in inputs. In addition, in the study by Kanjilal et al. [43], the authors reported carrying out a sensitivity analysis with several false negative rates for each antibiotic to translate the output of their models into susceptibility phenotypes. And in the study by Beaudoin et al. [45], the authors reported that they selected a distance threshold (below which a prescription for piperacillin–tazobactam was classified as inappropriate) based on previous experimentations (i.e., sensitivity analysis).
4.3. ML Models Versus AB Prescribing Clinicians
The lack of information about the engagement of clinicians or their feedback in the development process of the ML models nor their usability and applicability in clinical settings may align with what the authors in the study by Waring et al. reported regarding the lack of ML expertise among healthcare workers [71]. In addition, this lack of engagement renders the use of ML in AB prescribing a theoretical exercise, rather than a promising tool for improving AB prescribing. Furthermore, the improvements in prescribing presented by the ML models compared to clinicians could be the result of the additional laboratory information, which was not available to the clinicians at the time of prescribing.
Limitations of this review lay in the difficulty of including relevant non-peer-reviewed publications in mediums, such as arXiv and the small number of included studies that made it unsuitable to use I2 statistics to assess their heterogeneity. In addition, not all of the included studies provided enough information about the reproducibility of bias, and avoiding overfitting in their models, nor their robustness, which makes the generalisability of these models inconclusive. And the lack of implementation in clinical practices makes it hard to understand the potential problems (i.e., hardware problems, software problems, training needs, etc.) towards successful adoption of these models.
5. Conclusions
ML models may improve AB prescribing in different clinical settings; however, prescribing clinicians were not involved in the development process of the ML models nor in their evaluation of the ML models. Future research should consider a baseline ML model, developed with the same information that clinicians have at the time of prescribing, putting into consideration issues such as overfitting, bias reproduction, and robustness of their model to improve its generalisation. In addition, prescribing clinicians should be engaged in the development and deployment processes of ML models in clinical practices. Furthermore, it should explore the potential contribution of higher-performing ML models, such as support vector machines (SVMs) and artificial neural networks (ANNs), to improve AB prescribing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12081293/s1, Table S1. Characteristics of the included studies; Table S2. Excluded studies (full-text screening); Table S3. Groups of predictors; Table S4. Excluded studies reasons (title & abstract screening).
Author Contributions
Conceptualisation, D.A. and A.V.; screening, D.A., N.G.-O., A.G.P., S.P. and H.V.; risk of bias and data extraction, D.A., N.G.-O. and S.P.; data synthesis, D.A.; writing—original draft preparation, D.A.; writing—review and editing, all authors; supervision and reviewing and editing, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grant number RL-20200-03 from Research Leader Awards (RL) 2020, Health Research Board, Ireland and conducted as part of the SPHeRE Programme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010, an analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef]
- Bakhit, M.; Hoffmann, T.; Scott, A.M.; Beller, E.; Rathbone, J.; Del Mar, C. Resistance decay in individuals after antibiotic exposure in primary care: A systematic review and meta-analysis. BMC Med. 2018, 16, 126. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Surveillance of Antimicrobial Resistance in Europe 2018; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2019.
- Shrestha, P.; Cooper, B.S.; Coast, J.; Oppong, R.; Do Thi Thuy, N.; Phodha, T.; Celhay, O.; Guerin, P.J.; Werheim, H.; Lubell, Y. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob. Resist. Infect. Control 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Ten Threats to Global Health in 2019; WHO: Geneva, Switzerland, 2019; Volume 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 15 December 2022).
- Collaborators, A.R. Global burden of bacterial antimicrobial resistance in 2019, a systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- O’neill, J. Review on Antimicrobial Resistance. 2014. Available online: http://amr-review.org/ (accessed on 15 December 2022).
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Fishman, N.; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America; Pediatric Infectious Diseases Society. Policy statement on antimicrobial stewardship by the society for healthcare epidemiology of America (SHEA), the infectious diseases society of America (IDSA), and the pediatric infectious diseases society (PIDS). Infect. Control Hosp. Epidemiol. 2012, 33, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.L.; Haynes, R.B.; Hanna, S.E.; Smith, K. Effects of computer-based clinical decision support systems on physician performance and patient outcomes: A systematic review. JAMA 1998, 280, 1339–1346. [Google Scholar] [CrossRef]
- Charani, E.; Castro-Sánchez, E.; Holmes, A. The Role of Behavior Change in Antimicrobial Stewardship. Infect. Dis. Clin. N. Am. 2014, 28, 169–175. [Google Scholar] [CrossRef]
- Nabovati, E.; Jeddi, F.R.; Farrahi, R.; Anvari, S. Information technology interventions to improve antibiotic prescribing for patients with acute respiratory infection: A systematic review. Clin. Microbiol. Infect. 2021, 27, 838–845. [Google Scholar] [CrossRef]
- Skodvin, B.; Aase, K.; Charani, E.; Holmes, A.; Smith, I. An antimicrobial stewardship program initiative: A qualitative study on prescribing practices among hospital doctors. Antimicrob. Resist. Infect. Control 2015, 4, 24. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Hernandez, B.; Charani, E.; Castro-Sanchez, E.; Herrero, P.; Hayhoe, B.; Hope, W.; Georgiou, P.; Holmes, A.H. A systematic review of clinical decision support systems for antimicrobial management: Are we failing to investigate these interventions appropriately? Clin. Microbiol. Infect. 2017, 23, 524–532. [Google Scholar] [CrossRef]
- Goldenberg, S.L.; Nir, G.; Salcudean, S.E. A new era: Artificial intelligence and machine learning in prostate cancer. Nat. Rev. Urol. 2019, 16, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Koprowski, R.; Skufca, J.D. Machine learning, medical diagnosis, and biomedical engineering research–Commentary. BioMed. Eng. OnLine 2014, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Bini, S.A. Artificial intelligence, machine learning, deep learning, and cognitive computing: What do these terms mean and how will they impact health care? J. Arthroplast. 2018, 33, 2358–2361. [Google Scholar] [CrossRef]
- Naylor, C.D. On the Prospects for a (Deep) Learning Health Care System. JAMA 2018, 320, 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Hamet, P.; Tremblay, J. Artificial intelligence in medicine. Metabolism 2017, 69, S36–S40. [Google Scholar] [CrossRef]
- Panch, T.; Szolovits, P.; Atun, R. Artificial intelligence, machine learning and health systems. J. Glob. Health 2018, 8, 020303. [Google Scholar] [CrossRef]
- Jiang, T.; Gradus, J.L.; Rosellini, A.J. Supervised Machine Learning: A Brief Primer. Behav. Ther. 2020, 51, 675–687. [Google Scholar] [CrossRef]
- Mahesh, B. Machine Learning Algorithms–A Review. Int. J. Sci. Res. (IJSR) 2019, 9, 381–386. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Lv, J.; Deng, S.; Zhang, L. A review of artificial intelligence applications for antimicrobial resistance. Biosaf. Health 2021, 3, 22–31. [Google Scholar] [CrossRef]
- Friedman, J.H. Stochastic gradient boosting. Comput. Stat. Data Anal. 2002, 38, 367–378. [Google Scholar] [CrossRef]
- Rodríguez-González, A.; Zanin, M.; Menasalvas-Ruiz, E. Public Health and Epidemiology Informatics: Can Artificial Intelligence Help Future Global Challenges? An Overview of Antimicrobial Resistance and Impact of Climate Change in Disease Epidemiology. Yearb. Med. Inform. 2019, 28, 224–231. [Google Scholar] [CrossRef]
- Pogorelc, B.; Bosnić, Z.; Gams, M. Automatic recognition of gait-related health problems in the elderly using machine learning. Multimed. Tools Appl. 2012, 58, 333–354. [Google Scholar] [CrossRef]
- Shehab, M.; Abualigah, L.; Shambour, Q.; Abu-Hashem, M.; Shambour, M.K.Y.; Alsalibi, A.I.; Gandomi, A. Machine learning in medical applications: A review of state-of-the-art methods. Comput. Biol. Med. 2022, 145, 105458. [Google Scholar] [CrossRef]
- Gharaibeh, M.; Alzu’bi, D.; Abdullah, M.; Hmeidi, I.; Al Nasar, M.R.; Abualigah, L.; Gandomi, A.H. Radiology imaging scans for early diagnosis of kidney tumors: A review of data analytics-based machine learning and deep learning approaches. Big Data Cogn. Comput. 2022, 6, 29. [Google Scholar] [CrossRef]
- Malik, P.A.; Pathania, M.; Rathaur, V.K. Overview of artificial intelligence in medicine. J. Fam. Med. Prim. Care 2019, 8, 2328–2331. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Amin, D.; Garzón-Orjuela, N.; Garcia Pereira, A.; Parveen, S.; Vornhagen, H.; Vellinga, A. Search Strategy–Artificial Intelligence to Improve Antimicrobial Prescribing–A Protocol for a Systematic Review; Figshare: Iasi, Romania, 2022. [Google Scholar]
- NIH. Quality Assessment Tool for Observational Cohort and Cross-sectional Studies. 2018; Volume 2020. Available online: https://www.nhlbi.nih.gov/healthtopics/study-quality-assessment-tools (accessed on 15 December 2022).
- Kanjilal, S.; Oberst, M.; Boominathan, S.; Zhou, H.; Hooper, D.C.; Sontag, D. A decision algorithm to promote outpatient antimicrobial stewardship for uncomplicated urinary tract infection. Sci. Transl. Med. 2020, 12, eaay5067. [Google Scholar] [CrossRef]
- Lee, S.; Shin, J.; Kim, H.S.; Lee, M.J.; Yoon, J.M.; Lee, S.; Kim, Y.; Kim, J.-Y.; Lee, S. Hybrid Method Incorporating a Rule-Based Approach and Deep Learning for Prescription Error Prediction. Drug Saf. 2022, 45, 27–35. [Google Scholar] [CrossRef]
- Beaudoin, M.; Kabanza, F.; Nault, V.; Valiquette, L. Evaluation of a machine learning capability for a clinical decision support system to enhance antimicrobial stewardship programs. Artif. Intell. Med. 2016, 68, 29–36. [Google Scholar] [CrossRef]
- Yelin, I.; Snitser, O.; Novich, G.; Katz, R.; Tal, O.; Parizade, M.; Chodick, G.; Koren, G.; Shalev, V.; Kishony, R. Personal clinical history predicts antibiotic resistance of urinary tract infections. Nat. Med. 2019, 25, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Oonsivilai, M.; Mo, Y.; Luangasanatip, N.; Lubell, Y.; Miliya, T.; Tan, P.; Loeuk, L.; Turner, P.; Cooper, B.S. Using machine learning to guide targeted and locally-tailored empiric antibiotic prescribing in a children′s hospital in Cambodia. Wellcome Open Res. 2018, 3, 131. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; Forman, J.; Harrod, M.; Winter, S.; Fowler, K.E.; Krein, S.L.; Gupta, A.; Saint, S.; Singh, H.; Chopra, V. Electronic health records, communication, and data sharing: Challenges and opportunities for improving the diagnostic process. Diagnosis 2019, 6, 241–248. [Google Scholar] [CrossRef]
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine learning algorithm validation with a limited sample size. PLoS ONE 2019, 14, e0224365. [Google Scholar] [CrossRef]
- Okser, S.; Pahikkala, T.; Aittokallio, T. Genetic variants and their interactions in disease risk prediction—Machine learning and network perspectives. BioData Min. 2013, 6, 5. [Google Scholar] [CrossRef]
- Moradi, R.; Berangi, R.; Minaei, B. A survey of regularisation strategies for deep models. Artif. Intell. Rev. 2020, 53, 3947–3986. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y. A comprehensive survey on regularisation strategies in machine learning. Inf. Fusion. 2022, 80, 146–166. [Google Scholar] [CrossRef]
- Bramer, M. Using J-Pruning to Reduce Overfitting in Classification Trees. Knowl. Based Syst. KBS 2002, 15, 301–308. [Google Scholar] [CrossRef]
- Jin, H.; Ling, C.X. Using AUC and accuracy in evaluating learning algorithms. IEEE Trans. Knowl. Data Eng. 2005, 17, 299–310. [Google Scholar]
- Ferri, C.; Hernández-Orallo, J.; Modroiu, R. An experimental comparison of performance measures for classification. Pattern Recognit. Lett. 2009, 30, 27–38. [Google Scholar] [CrossRef]
- Luque, A.; Carrasco, A.; Martín, A.; de las Heras, A. The impact of class imbalance in classification performance metrics based on the binary confusion matrix. Pattern Recognit. 2019, 91, 216–231. [Google Scholar] [CrossRef]
- Fanelli, U.; Pappalardo, M.; Chinè, V.; Gismondi, P.; Neglia, C.; Argentiero, A.; Calderaro, A.; Prati, A.; Esposito, S. Role of Artificial Intelligence in Fighting Antimicrobial Resistance in Pediatrics. Antibiotics 2020, 9, 767. [Google Scholar] [CrossRef]
- Chen, P.-H.C.; Liu, Y.; Peng, L. How to develop machine learning models for healthcare. Nat. Mater. 2019, 18, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, S.G.; Beam, A.L.; van Smeden, M. Machine Learning and Statistics in Clinical Research Articles—Moving Past the False Dichotomy. JAMA Pediatr. 2023, 177, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Vuttipittayamongkol, P.; Elyan, E.; Petrovski, A. On the class overlap problem in imbalanced data classification. Knowl. Based Syst. 2021, 212, 106631. [Google Scholar] [CrossRef]
- Vuttipittayamongkol, P.; Elyan, E. Neighbourhood-based undersampling approach for handling imbalanced and overlapped data. Inf. Sci. 2020, 509, 47–70. [Google Scholar] [CrossRef]
- Elyan, E.; Moreno-Garcia, C.F.; Jayne, C. CDSMOTE: Class decomposition and synthetic minority class oversampling technique for imbalanced-data classification. Neural Comput. Appl. 2021, 33, 2839–2851. [Google Scholar] [CrossRef]
- Elyan, E.; Gaber, M.M. A fine-grained Random Forests using class decomposition: An application to medical diagnosis. Neural Comput. Appl. 2016, 27, 2279–2288. [Google Scholar] [CrossRef]
- Elyan, E.; Hussain, A.; Sheikh, A.; Elmanama, A.A.; Vuttipittayamongkol, P.; Hijazi, K. Antimicrobial Resistance and Machine Learning: Challenges and Opportunities. IEEE Access 2022, 10, 31561–31577. [Google Scholar] [CrossRef]
- Norori, N.; Hu, Q.; Aellen, F.M.; Faraci, F.D.; Tzovara, A. Addressing bias in big data and AI for health care: A call for open science. Patterns 2021, 2, 100347. [Google Scholar] [CrossRef]
- Parikh, R.B.; Teeple, S.; Navathe, A.S. Addressing Bias in Artificial Intelligence in Health Care. JAMA 2019, 322, 2377–2378. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Luo, J.; Zhao, J.; Wen, B.; Wang, Q. Recent advances in adversarial training for adversarial robustness. arXiv 2021. arXiv:210201356. [Google Scholar]
- McCarthy, A.; Ghadafi, E.; Andriotis, P.; Legg, P. Functionality-Preserving Adversarial Machine Learning for Robust Classification in Cybersecurity and Intrusion Detection Domains: A Survey. J. Cybersecur. Priv. 2022, 2, 154–190. [Google Scholar] [CrossRef]
- Bellet, A.; Habrard, A. Robustness and generalisation for metric learning. Neurocomputing 2015, 151, 259–267. [Google Scholar] [CrossRef]
- Waring, J.; Lindvall, C.; Umeton, R. Automated machine learning: Review of the state-of-the-art and opportunities for healthcare. Artif. Intell. Med. 2020, 104, 101822. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).