Abstract
The acquisition of hypervirulence-associated genes by carbapenemase-producing Klebsiella pneumoniae is being increasingly observed, and easy-to-use diagnostic tests are needed for the surveillance of the hypervirulent K. pneumoniae (hvKp). In this pilot study, 87 K. pneumoniae isolates from invasive infections collected in 2022 and 2023 were analysed using the LAMP-based eazyplex® Superbug CRE and hvKp assays for the simultaneous identification of carbapenemases and virulence genes (rmpA/A2, iuC, iroC, ybt, clb). Nine isolates showed a Kleborate virulence score of 4 or 5 (10.3%). The time for the results of the eazyplex® assays ranged from 6.5 to 13 min, and the total turnaround time, including sample preparation, was less than 30 min. Five isolates, three of which produced New Delhi metallo-beta lactamase (NDM), were subjected to whole-genome sequencing (WGS) analysis for further characterisation. The eazyplex® test results for beta-lactamase and virulence genes were confirmed. The eazyplex® hvKp, currently only available as a Research Use Only assay, may be a useful tool for the rapid identification of hvKp without significant additional workload when combined with the eazyplex® Superbug CRE assay for the detection of carbapenemases.
1. Introduction
As a leading cause of hospital-associated infections, Klebsiella pneumoniae has attracted particular attention, not only because of the increase in beta-lactam resistance but also because of the combination of broad-spectrum antibiotic resistance with the presence of hypervirulence-associated genes in several strains [1]. The increasing emergence of hypervirulent K. pneumoniae (hvKp) strains producing carbapenemases in Europe has led to a risk assessment by the European Centre for Disease Prevention and Control (ECDC) that carbapenem-resistant hvKp can cause difficult-to-treat or untreatable invasive infections in both immunocompromised patients and previously healthy people and that surveillance is needed to prevent further spread in healthcare settings [2].
K. pneumoniae strains are most commonly classified as hypervirulent when they possess multiple siderophores, specific capsule types, a hypermucoviscous phenotype, and are isolated from severe invasive infections [3]. The hvKp strains can belong to different sequence types (ST), however, a large cluster is of ST23, which expresses the K1 capsule [2]. Recently, outbreaks by hvKp expressing carbapenemases have been observed in regions with previously low incidence [4,5,6,7,8]. Of particular concern is the identification of strains with hybrid plasmids that harbour both beta-lactamases and virulence genes [3,9]. It is likely that hvKp is currently underdiagnosed. The ECDC risk assessment also includes a call for the early detection of such strains by testing strategies to be established in routine diagnostics [2]. In our laboratory, K. pneumoniae isolates are routinely screened for the presence of carbapenemases using the eazyplex® Superbug CRE assay (Amplex Diagnostics, Gars-Bahnhof, Germany), a rapid molecular test based on loop-mediated isothermal amplification (LAMP) [10]. The aim of this proof-of-principle study was to investigate the performance of the eazyplex® hvKP Research-Use-Only (RUO) assay (Amplex Diagnostics) targeting the hypermucoviscosity-associated genes rmpA/A2, colibactin, and the siderophores aerobactin, yersiniabactin, and salmochelin, for a combined rapid testing of carbapenemase- and virulence-associated genes in invasive K. pneumoniae isolates.
2. Results
A total of 87 K. pneumoniae isolates collected in 2022 and 2023 were analysed for this pilot evaluation study. This collection consisted of 43 isolates from blood cultures and 42 isolates from other specimens, including joint punctates, cerebrospinal fluid, abscesses, deep tissue, gallbladder fluid, urine, sputum, and bronchoalveolar lavage. Two carbapenemase-producing isolates from rectal screening swabs were also investigated. The eazyplex® superbug CRE assay identified 11 isolates producing carbapenemases OXA-48 (n = 4), NDM (n = 6), and NDM+OXA-181 (n = 1).
The Kleborate virulence score has been proposed to classify K. pneumoniae by the presence of genes encoding multiple siderophores and colibactin (see Materials and methods) [1]. The eazyplex® hvKp assay showed that 14 out of 87 isolates (16.1%) had an elevated virulence score ≥2 (Table 1). Nine of these scored 4 or 5 (10.3%). It should be noted that this rate does not reflect the true prevalence in our hospital setting, as not all K. pneumoniae isolates during the study period were included in this study. Of the 11 carbapenemase-producing K. pneumoniae, six isolates (54.5%) with the carbapenemases NDM (n = 4) and OXA-48 (n = 2) had an increased virulence score. All six isolates were also positive for the extended-spectrum beta-lactamase blaCTX-M1 group. In comparison, none of the carbapenemase-producing isolates collected between 2012 and 2021 and tested here had a Kleborate score ≥2. Only yersiniabactin, which is also common in the classical K. pneumoniae strains, was present in 66.7% of the isolates. The time for the results of the eazyplex® hvKp assay, defined by the threshold time of fluorescence intensity, ranged from 6.5 to 13 min for the specific targets (Table 1). Representative amplification curves are shown in Figure 1.

Table 1.
K. pneumoniae isolates with increased virulence scores, identified by the eazyplex hvKp assay.
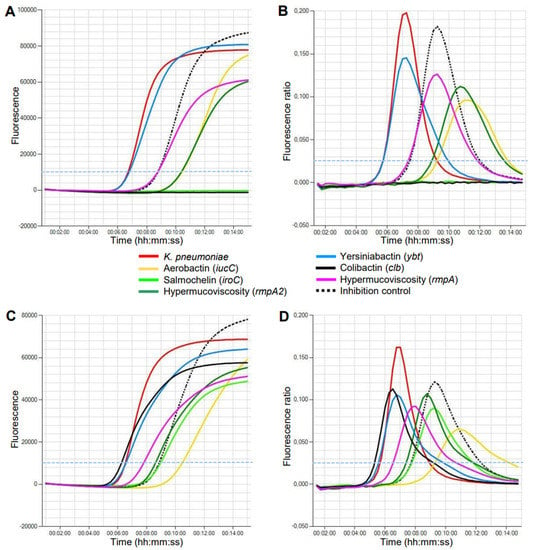
Figure 1.
Detection of K. pneumoniae virulence genes by LAMP on the Genie HT instrument within 15 min. (A,B) Isolate 1 of ST395 with a virulence score of 4. (C,D) Isolate 7 of ST23 with a virulence score of 5. The thresholds of fluorescence level (A,C) and amplification rate (B,D) are marked with a broken light blue line.
The hypermucoviscosity of the colonies was assessed by the ‘string test’, which was positive for 7 of 14 isolates. Hypermucoviscosity-associated genes rmpA/A2 were detected in nine isolates (Table 1).
The genomes of five isolates selected as representative examples based on virulence scores of 4 and 5 were sequenced. Subsequent MLST analysis revealed that isolates 1 and 2 with virulence scores of 4 belonged to ST395 and ST395-1LV, respectively. The positive eazyplex® results for the NDM and CTX-M-1 groups were verified as NDM-1 and CTX-M-15 using Kleborate, and the identification of virulence factors could be confirmed (rmpA/A2, iuc1, and ybt16). They possessed the KL2 capsule type (wzi 2 genotype). The ST395 strains have been observed in an increasing number of NDM-producing K. pneumoniae in Germany since 2022, however, the cgMLST analysis revealed no close genetic relationship between these isolates and isolates 1 and 2 in the present study [7]. Both isolates were obtained from the blood cultures of septic patients with cirrhosis. MLST analysis of the NDM-positive isolate 3, cultured from a joint aspirate from a patient with a periprosthetic infection, identified ST2096. The isolate possessed NDM-1 and the KL64 capsule type (wzi 2 genotype). The virulence score of 4, as calculated from the eazyplex® results, was confirmed by the identification of ybt14 and iuc1. The string test of this isolate was negative, although rmpA/A2 was detected. All NDM-producing isolates had a low MIC of <1/4 mg/L for aztreonam–avibactam and were susceptible to cefiderocol (Table 1).
Isolates 7 and 8, which were susceptible to third-generation cephalosporins and carbapenems but identified as hvKp with a virulence score of 5, were also further characterised by WGS. MLST demonstrated that they belonged to ST23, the archetype of hvKp [2,11]. No beta-lactamase genes could be detected, except for an intrinsic blaSHV-11 gene, but the presence of virulence-associated genes was confirmed (rmpA/A2, iuc1, ybt1, iro1, and clb2). As detected by PCR, both isolates possessed the K1 capsule type (wzi 1 genotype) and the mucoviscosity-associated gene A (magA), which contributes to K1 capsule polysaccharide synthesis. Isolate 7 was obtained from a blood culture from a patient with urosepsis. Isolate 8 was cultured from a cerebrospinal fluid sample from a patient who developed meningitis following otitis media. Interestingly, rmpA/A2 genes were confirmed using classical PCR/Sanger sequencing and the eazyplex® hvKp assay in all five genomically characterised isolates, however, the Kleborate tool failed to detect any rmpA genes in isolates 3 and 7.
3. Discussion
There is a need for rapid assays that can be integrated into daily routine diagnostics for the early detection and surveillance of hvKp [2,4]. Diagnostics should not be restricted to carbapenem-resistant isolates. Non-carbapenem-resistant hvKp can also cause severe invasive infections, and carbapenemase-producing K. pneumoniae can acquire hypervirulence-associated genes by plasmid transfer and the formation of hybrid plasmids combining both characteristics on a single transferable unit [1,3,12]. This suggests that the diagnostic focus needs to shift from the detection of antimicrobial resistance alone to a broader strategy that includes the identification of virulence factors in order to limit the spread of high-risk K. pneumoniae clones and to improve timely clinical decision-making within antibiotic stewardship programmes [13,14,15].
The results of this study demonstrate for the first time that a newly developed LAMP-based assay for the testing of several hvKp-associated virulence genes can be easily combined with the commercial eazyplex® Superbug CRE assay, which has already been evaluated as a reliable tool for the rapid identification of beta-lactamases [10].
There is currently no consensus on the definition of hvKp. Hypervirulence can generally be defined as the overexpression of capsular polysaccharides combined with the acquisition of more than one siderophore [1]. It should be noted that not only capsular serotypes K1 and K2 are associated with hvKp [1]. The ‘string test’ was not sufficient for the reliable screening of hvKp when compared to the detection of the capsular regulators rmpA/A2, which corresponds to the findings of other studies [1]. However, it has to be considered that the detection of rmpA/A2 does not prove capsule overexpression, as both genes can be truncated [1,3,6]. The Kleborate score used is only based on the detection of various siderophores and colibactin. Aerobactin is typically associated with hvKp in combination with yersiniabactin [1]. Additional suitable markers are colibactin, a genotoxin that causes interstrand DNA cross-linking and subsequent double-strand breaks, and salmochelin, which is not used in the Kleborate score [16].
Treatment options for carbapenemase-producing K. pneumoniae are very limited. Ceftazidime–avibactam is often used for OXA-48 and KPC-producing isolates and cefiderocol for infections with metallo-beta-lactamase (MBL)-producing strains [17]. The combination of aztreonam and avibactam may also be active against MBL strains, however, they are not currently available and are in phase III clinical trials [18]. Alternatively, ceftazidime–-avibactam is now often combined with aztreonam for the treatment of MBL-producing strains [19].
Whole-genome-sequencing (WGS)-based analyses are increasingly being used to identify and characterise hvKp strains on demand, and they allow phylogenetic investigations for surveillance purposes. The combined presence of several resistance and virulence determinants in several isolates in the present study is of concern, and future analyses will show whether these genes are located on hybrid plasmids, facilitating their spread. As WGS is time-consuming, expensive, and requires extensive computing, simple and rapid methods to detect hvKp in routine diagnostic workflows are of interest. The results of this proof-of-principle study show that the eazyplex® hvKp, currently only available as an RUO assay, can be a useful tool for the rapid identification of hvKp when combined with the eazyplex® Superbug CRE assay for the detection of carbapenemases. There is no significant additional work, as a single sample preparation can be used for both assays. For the surveillance of hvKp, testing of K. pneumoniae isolates from both invasive infections and carbapenemase-positive screening samples may be appropriate. LAMP is suitable for screening virulent strains, and WGS can be used for confirmation.
4. Materials and Methods
4.1. Bacterial Strains and Antimicrobial Susceptibility Testing
This study included K. pneumoniae isolates collected between January 2022 and April 2023. They were isolated from clinical samples sent to the Clinical Microbiology Laboratory of the University Hospital Jena as part of routine patient care at the Jena University Hospital and the Weimar Hospital. Out of 2140 isolates, 87 were selected for this pilot study on the basis of isolation from blood cultures, invasive infections, or the presence of carbapenemases. In addition, 25 K. pneumoniae strains collected between 2012 and 2021 and positive for OXA, KPC, NDM or VIM genes were retrospectively analysed for the presence of virulence genes. Isolates were stored by cryopreservation at −80 °C using the CRYOBANK® system (Mast Diagnostica, Reinfeld, Germany). For recultivation, one bead was immediately removed from the vial without thawing the entire sample and transferred to a solid medium. The identity of isolates in long-term storage was always verified by species identification and testing for the presence of carbapenemase genes after thawing.
Bacteria were cultured on Columbia sheep blood agar and Drigalski lactose agar (Thermo Fisher Scientific, Wesel, Germany). Single colonies were identified as K. pneumoniae using MALDI-TOF mass spectrometry (Vitek MS, bioMeriéux, Nürtingen, Germany) and examined for a hypermucoviscous phenotype using the ‘string test’, which is positive if a viscous string of >5 mm is obtained by stretching a colony on the agar plate using an inoculation loop. Antimicrobial susceptibility testing was performed by determining minimal inhibitory concentrations (MICs) using Vitek 2 (card N430, bioMérieux). For carbapenemase-producing isolates, the MICs of meropenem, ceftazidime–avibactam, and aztreonam–avibactam were determined using the MICRONAUT-S Labor Berlin MDR MIC/GN microtiter system (Merlin Diagnostika, Bornheim, Germany; distributed by Sifin Diagnostics, Berlin, Germany). The MIC of cefiderocol was determined with a gradient test strip (Liofilchem, Roseto degli Abruzzi, Italy; distributed by Bestbion, Köln, Germany). The breakpoints were interpreted according to the criteria of the European Committee on Antimicrobial Susceptibility Testing [EUCAST, v13.0, https://www.eucast.org/clinical_breakpoints (accessed on 3 January 2023)].
4.2. Eazyplex® LAMP Assays
The eazyplex® hvKp RUO assay (Amplex Diagnostics) is a ready-to-use test strip containing lyophilized master mixes with primers for K. pneumoniae, iucC, iroC, ybt, clb, rmpA, rmpA2, and an inhibition control in each well. The eazyplex® superbug CRE IVDR assay (Amplex Diagnostics) contains master mixes with primers for blaCTX-M-1 group, blaCTX-M-9 group, blaKPC, blaOXA-48, blaNDM, blaVIM, and an inhibition control. A single colony of the isolates was suspended in 500 μL of resuspension and lysis fluid (RALF buffer, Amplex Diagnostics) and boiled for 2 min. After centrifugation at 1500× g for 1 min, 25 μL of the supernatant was added to each tube of the eazyplex® test strip. Tests were run on a Genie HT machine (Amplex Diagnostics) at 65 °C for 20 min. Amplification was measured by real-time fluorescence detection using an intercalating dye contained in the lyophilized master mix of the assays. Test results were automatically calculated and reported by the integrated eazyReport™ software (Amplex Diagnostics) on the Genie HT instrument (Amplex Diagnostics). The virulence scores were assessed according to the Kleborate tool (https://github.com/katholt/Kleborate, (accessed on 3 January 2023)) as follows: 0, none present; 1, ybt only; 2, clb without iucC (regardless of ybt); 3, iucC only; 4, iucC and ybt without clb; 5, iucC, ybt, and clb. Increased virulence was defined when a score of at least 2 was reached [1].
4.3. WGS Analysis and PCR
For WGS, the isolates were grown in a Brain Heart Infusion (BHI) broth (BD, Heidelberg, Germany). The DNA was extracted from overnight cultures using a DNeasy Blood and Tissue Kit (Qiagen, Venlo, The Netherlands), according to the manufacturer’s instructions. A Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific) was used for DNA quantification. Sequencing libraries were prepared by applying a Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA, USA). Sequencing was performed on an Illumina NextSeq 550 using a v2.5 chemistry kit (2 × 150 bp), as described in the manufacturer’s protocol. The generated fastq-files were quality checked and trimmed using the Trimmomatic software with the following parameters: ILLUMINACLIP:NexteraPE-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36 [20]. De novo assembly was performed using a Unicycler v0.4.8, including the SPAdes assembler v3.13.01 [21,22]. The generated contigs were further analysed using Kleborate (https://github.com/katholt/Kleborate, (accessed on 3 January 2023)) and AMRfinder plus (https://github.com/ncbi/amr, (accessed on 3 January 2023)) [23]. Core genome multilocus sequence typing (cgMLST) was performed using SeqSphere+ v8.4.1 with the respective published MLST and cgMLST schemes [24]. Raw read data were assigned to ENA Accession No. PRJEB61660 (https://www.ebi.ac.uk/ena/browser/home, (accessed on 3 January 2023)). All isolates that were selected for genome sequencing were also tested for the hypermucoviscosity-associated genes magA and rmpA using the primers published by Yeh et al., as well as rmpA2 (primer FWD, 5′-TTATGTGCAATAAGGATGTT-3′; primer REV, 5′-CTAGGTATTTGATGTGCAC-3′) [25].
Author Contributions
Conceptualisation, J.R. and B.L.; Methodology, J.R. and Y.P.; Investigation, J.R., Y.P., M.A.F., B.E. and S.S.; Data curation and formal analysis, J.R., Y.P., M.A.F., and W.P.; Funding acquisition, B.L.; Project administration, J.R. and B.L.; Resources, B.L. Writing—Original draft, J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the German Federal Ministry of Education and Research (BMBF), grant numbers 13GW0459D (InfectoXplore) and 13GW0456A (ADA).
Institutional Review Board Statement
Ethical review and approval were not required for this study because the work was carried out using bacterial isolates and not patient samples. This included the use of cryopreserved bacterial isolates without patient reference. Information from microbiological and clinical reports was analysed anonymously.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data form sequencing analyses are openly available at the European Nucleotide Archive (ENA). Other datasets analysed in this study are available upon reasonable request from the corresponding author.
Acknowledgments
We thank Amplex Diagnostics for providing eazyplex® hvKp RUO test kits for evaluation. We thank Sibylle Müller-Bertling and Kirstin Ganske for excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lam, M.M.C.; Wick, R.R.; Watts, S.C.; Cerdeira, L.T.; Wyres, K.L.; Holt, K.E. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat. Commun. 2021, 12, 4188. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Emergence of Hypervirulent Klebsiella pneumoniae ST23 Carrying Carbapenemase Genes in EU/EEA Countries; ECDC: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Emergence-of-hypervirulent-Klebsiella-pneumoniae-ST23-carrying-carbapenemase-genes.pdf (accessed on 1 September 2021).
- Han, Y.L.; Wen, X.H.; Zhao, W.; Cao, X.S.; Wen, J.X.; Wang, J.R.; Hu, Z.D.; Zheng, W.Q. Epidemiological characteristics and molecular evolution mechanisms of carbapenem-resistant hypervirulent Klebsiella pneumoniae . Front. Microbiol. 2022, 13, 1003783. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.; DeLappe, N.; Cormican, M.; Tuohy, A.; Tobin, A.; Moran, L.; Doyle, M.; Fielding, C. A geographic cluster of healthcare-associated carbapenemase-producing hypervirulent Klebsiella pneumoniae sequence type 23. Eur. J. Clin. Microbiol. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Di Pilato, V.; Henrici De Angelis, L.; Aiezza, N.; Baccani, I.; Niccolai, C.; Parisio, E.M.; Giordano, C.; Camarlinghi, G.; Barnini, S.; Forni, S.; et al. Resistome and virulome accretion in an NDM-1-producing ST147 sublineage of Klebsiella pneumoniae associated with an outbreak in Tuscany, Italy: A genotypic and phenotypic characterisation. Lancet Microbe 2022, 3, e224–e234. [Google Scholar] [CrossRef]
- Hallal Ferreira Raro, O.; Nordmann, P.; Dominguez Pino, M.; Findlay, J.; Poirel, L. Emergence of carbapenemase-producing hypervirulent Klebsiella pneumoniae in Switzerland. Antimicrob. Agents Chemother. 2023, 67, e0142422. [Google Scholar] [CrossRef] [PubMed]
- Sandfort, M.; Hans, J.B.; Fischer, M.A.; Reichert, F.; Cremanns, M.; Eisfeld, J.; Pfeifer, Y.; Heck, A.; Eckmanns, T.; Werner, G.; et al. Increase in NDM-1 and NDM-1/OXA-48-producing Klebsiella pneumoniae in Germany associated with the war in Ukraine, 2022. Eurosurveillance 2022, 27, 2200926. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Addis, E.; Bertoncelli, A.; Mazzariol, A. Multiple detection of hypermucoviscous and hypervirulent strains of Klebsiella pneumoniae: An emergent health care threat. Acta Microbiol. Immunol. Hung. 2022, 69, 297–302. [Google Scholar]
- Hernández, M.; López-Urrutia, L.; Abad, D.; De Frutos Serna, M.; Ocampo-Sosa, A.A.; Eiros, J.M. First report of an extensively drug-resistant ST23 Klebsiella pneumoniae of capsular serotype K1 co-producing CTX-M-15, OXA-48 and ArmA in Spain. Antibiotics 2021, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Sękowska, A.; Bogiel, T.; Gospodarek-Komkowska, E. Evaluation of eazyplex®® SuperBug CRE Test for beta-lactamase genes detection in Klebsiella spp. and P. aeruginosa strains. Curr. Microbiol. 2020, 77, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Fliss, M.; van den Berg, C.H.S.B.; Kuijper, E.; Notermans, D.W.; Hendrickx, A.P.A.; Schoots, M.H.; Bathoorn, E. Brief report: Community-acquired Friedlander’s pneumonia and pulmonary metastatic Klebsiella pneumoniae infection caused by hypervirulent ST23 in the Netherlands. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1133–1138. [Google Scholar] [CrossRef]
- Bernard de Lajartre, O.; Maamar, A.; Dejoies, L.; Delamaire, F. Community-acquired hypervirulent Klebsiella pneumoniae invasive infection in critically ill patients who dramatically improved. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1275–1277. [Google Scholar] [CrossRef]
- Anantharajah, A.; Deltombe, M.; de Barsy, M.; Evrard, S.; Denis, O.; Bogaerts, P.; Hallin, M.; Miendje Deyi, V.Y.; Pierard, D.; Bruynseels, P.; et al. Characterization of hypervirulent Klebsiella pneumoniae isolates in Belgium. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 859–865. [Google Scholar] [CrossRef]
- Baron, S.A.; Pascale, L.M.; Million, M.; Briantais, A.; Durand, J.M.; Hadjadj, L.; Rolain, J.M. Whole genome sequencing to decipher the virulence phenotype of hypervirulent Klebsiella pneumoniae responsible for liver abscess, Marseille, France. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Piazza, A.; Perini, M.; Mauri, C.; Comandatore, F.; Meroni, E.; Luzzaro, F.; Principe, L. Antimicrobial susceptibility, virulence, and genomic features of a hypervirulent serotype K2, ST65 Klebsiella pneumoniae causing meningitis in Italy. Antibiotics 2022, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xia, X.; Yuan, T.; Zhu, J.; Shen, Z.; Li, M. Molecular epidemiology of antimicrobial resistance, virulence and capsular serotypes of carbapenemase-carrying Klebsiella pneumoniae in China. Antibiotics 2022, 11, 1100. [Google Scholar] [CrossRef]
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-resistant Klebsiella pneumoniae: Virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Mendes, R.E.; Carvalhaes, C.G.; Kimbrough, J.H.; Castanheira, M. Changing epidemiology of carbapenemases among carbapenem-resistant Enterobacterales from United States hospitals and the activity of aztreonam-avibactam against contemporary Enterobacterales (2019–2021). Open Forum Infect. Dis. 2023, 10, ofad046. [Google Scholar] [CrossRef]
- Falcone, M.; Daikos, G.L.; Tiseo, G.; Bassoulis, D.; Giordano, C.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Barnini, S.; Sani, S.; et al. Efficacy of ceftazidime-avibactam plus aztreonam in patients with bloodstream infections caused by metallo-β-lactamase-producing Enterobacterales. Clin. Infect. Dis. 2021, 72, 1871–1878. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Feldgarden, M.; Brover, V.; Gonzalez-Escalona, N.; Frye, J.G.; Haendiges, J.; Haft, D.H.; Hoffmann, M.; Pettengill, J.B.; Prasad, A.B.; Tillman, G.E.; et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 2021, 11, 12728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Alikhan, N.F.; Mohamed, K.; Fan, Y.; Agama Study Group; Achtman, M. The EnteroBase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Res. 2020, 30, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.M.; Kurup, A.; Siu, L.K.; Koh, Y.L.; Fung, C.P.; Lin, J.C.; Chen, T.L.; Chang, F.Y.; Koh, T.H. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J. Clin. Microbiol. 2007, 45, 466–471. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).