Abstract
Wastewater (WW) is considered a source of antibiotic-resistant bacteria with clinical relevance and may, thus, be important for their dissemination into the environment, especially in countries with poor WW treatment. To obtain an overview of the occurrence and characteristics of carbapenem-resistant Gram-negative bacteria (CR-GNB) in WW of Belgrade, we investigated samples from the four main sewer outlets prior to effluent into international rivers, the Sava and the Danube. Thirty-four CR-GNB isolates were selected for antimicrobial susceptibility testing (AST) and whole-genome sequencing (WGS). AST revealed that all isolates were multidrug-resistant. WGS showed that they belonged to eight different species and 25 different sequence types (STs), seven of which were new. ST101 K. pneumoniae (blaCTX-M-15/blaOXA-48) with novel plasmid p101_srb was the most frequent isolate, detected at nearly all the sampling sites. The most frequent resistance genes to aminoglycosides, quinolones, trimethroprim-sulfamethoxazole, tetracycline and fosfomycin were aac(6′)-Ib-cr (55.9%), oqxA (32.3%), dfrA14 (47.1%), sul1 (52.9%), tet(A) (23.5%) and fosA (50%), respectively. Acquired resistance to colistin via chromosomal-mediated mechanisms was detected in K. pneumoniae (mutations in mgrB and basRS) and P. aeruginosa (mutation in basRS), while a plasmid-mediated mechanism was confirmed in the E. cloacae complex (mcr-9.1 gene). The highest number of virulence genes (>300) was recorded in P. aeruginosa isolates. Further research is needed to systematically track the occurrence and distribution of these bacteria so as to mitigate their threat.
1. Introduction
Antimicrobial resistance (AMR) has been portrayed as one of the greatest threats to public health. AMR infections result in 700,000 deaths every year, but the global AMR-associated mortality is estimated to exceed 10 million lives per year in 2050 [1]. Hence, in 2017, the World Health Organisation (WHO) released a list of critical pathogens based on their impact on human health and the urgency for developing new antibiotics to treat resistant infections [2]. The group of Gram-negative bacteria, which includes carbapenem-resistant Acinetobacter baumannii, Pseudomonas aeruginosa and carbapenem-resistant and extended-spectrum beta-lactamases (ESBL)-producing Enterobacterales, was identified as a critical priority pathogen. Thus, few treatment options are available for infections caused by these bacteria [3] and such infections are associated with high morbidity and mortality rates worldwide [4].
Variations in AMR rates and patterns in different regions of the world have usually been associated with differing rates of antimicrobial consumption through overuse and misuse of antibiotics. Still, implementation of policies restricting antibiotic prescription has not decreased AMR rates as expected. Since 2015, antibiotic consumption in Serbia decreased by 18% [5,6]; however, AMR rates of all clinically relevant bacteria have increased (i.e., in the period 2015–2020, resistance rates to carbapenems in Klebsiella pneumoniae increased from 36% to 48%, in Pseudomonas aeruginosa from 47 to 69%, and Acinetobacter spp. from 91% to 97%) [7,8], demonstrating the importance of constraining other AMR-promoting factors.
Transmission of AMR can occur between humans, animals and the environment by means of a number of different routes. Wastewater is one of the primary pathways for clinically relevant antibiotic-resistant bacteria (ARB) to reach the environment [9]. Clinical wastewater, such as wastewater from healthcare institutions and nursing homes, are hotspots for ARB [9,10]. However, more than 80% of the total antibiotic consumption in the human sector is prescribed in the community in Europe [11] and these AMR indicators are also present in municipal wastewater. Therefore, testing wastewater for the presence and diversity of ARB could provide an indication of where and to what extent clinically relevant pathogens are released into the environment [12]. After discharge into the environment, ARB may be highly prevalent in soil, plants and surface water, and may, thus, pose a risk for the colonization of humans, pets, livestock or contamination of food and food products, and further tremendously impact public health [13].
In Serbia, based on the 2017 data, less than 13% of collected municipal and clinical wastewater was treated before discharge to receiving waters (e.g., rivers) [14]. Belgrade, the capital of Serbia with 1,700,000 inhabitants, does not have any wastewater treatment facility, and although Belgrade has quite a developed sewer system, most of the sewer outlets are submerged in the final recipients, the Sava and the Danube international rivers. The Danube is the second-longest river in Europe, which connects ten European countries, running through their territories or constituting a border [15]. Its drainage basin extends into nine more countries. The Danube passes through four capital cities, more than any other river in the world, and, additionally, five more capital cities lie in the Danube’s basin. The Sava is the longest and largest tributary of the Danube by discharge. It flows through five European countries, feeding into the Danube in Belgrade.
The impact of untreated wastewater discharge on the water quality of the Danube River was previously demonstrated [16]. In the river stretch through the central part of Serbia, all midstream samples were critically polluted based on Escherichia coli numbers (main faecal indicator), and the highest level of faecal pollution was recorded downstream of Belgrade. Of note is that ARB from the urine origin, in the case of human/animal urinary tract infections, are additionally present in wastewater and its recipients. Therefore, knowing that wastewater from Belgrade significantly deteriorates the microbiological water quality of the Sava and the Danube, the need to determine the presence of ARB is of critically significance for public health at this highly impacted stretch of the international rivers was identified.
Our study aimed to evaluate the diversity of carbapenem-resistant Gram-negative bacteria of clinical significance in wastewater prior to outlet in two international rivers, the Sava and the Danube.
2. Results
2.1. Distribution of Clinically Relevant ARB Isolated from Wastewater
Overall, 16 wastewater samples were collected and investigated within this study and 34 target antibiotic-resistant Gram-negative bacteria were isolated: Klebsiella pneumoniae (n = 12), Klebsiella oxytoca (n = 3), Enterobacter cloacae complex (n = 9), Escherichia coli (n = 3), Serratia marcescens (n = 2), Citrobacter freundii (n = 1), Acinetobacter baumannii (n = 2) and Pseudomonas aeruginosa (n = 2). Distribution of isolated target ARB depending on the sampling site and sampling campaign is presented in Table 1.

Table 1.
Distribution of isolated clinically relevant antibiotic-resistant Gram-negative bacteria.
The strains of K. pneumoniae and E. cloacae complex were isolated from all sampling sites (the main sewer outlets of Belgrade) and during the whole study period. The diversity of the target ARB strains was present both in S1 and S2, which receive municipal wastewater with or without hospitals wastewater contribution, respectively.
2.2. Antimicrobial Resistance Pattern of Wastewater Isolates
An overview of the antimicrobial resistance of the investigated target bacteria is presented in Figure 1. MICs and interpretation of antimicrobial susceptibility testing for each isolate are listed in Supplementary Table S1.
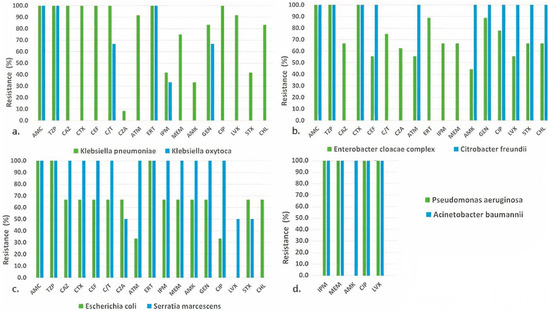
Figure 1.
Resistance to antimicrobial agents among isolates of (a) K. pneumoniae and K. oxytoca; (b) E. cloacae complex and C. freundii; (c) E. coli and S. marcescens; (d) P. aeruginosa and A. baumannii. Abbreviations for antimicrobial agents: AMC, amoxicillin-clavulanic acid; TZP, piperacillin-tazobactam; CAZ, ceftazidime; CTX, ceftriaxone; CEF, cefepime; CZA, ceftazidime-avibactam; C/T, ceftolozane-tazobactam; ERT, ertapenem; IPM, imipenem; MEM, meropenem; ATM, aztreonam; AMK, amikacin; GEN, gentamicin; CIP, ciprofloxacin; LVX, levofloxacin; CHL, chloramphenicol and STX, trimethoprim-sulfamethoxazole.
K. pneumoniae isolates were resistant to antimicrobials of different classes (Figure 1a). Interestingly, phenotypic resistance to carbapenems was not uniform, i.e., 100% to ertapenem, 41.7% to imipenem and 75% meropenem. Similar findings were found for other antimicrobial classes (amynoglycosides and fluoroquinolones) and in others isolated ARB.
In contrast to isolates of K. pneumoniae, the K. oxytoca and E. coli isolates exhibited higher susceptibility levels, while S. marcescens and C. freundii isolates higher resistance levels to almost all tested antimicrobials (Figure 1a–c). Similar to the findings for K. pneumoniae, almost all E. cloacae complex isolates showed resistance to tested antimicrobial agents (Figure 1b).
Susceptibility to the newly approved antimicrobial combination ceftazidime-avibactam was 91.7% in K. pneumoniae, 100% in K. oxytoca and C. freundii, 66.7% in E. cloacae complex and E. coli and 50% in S. marcescens. On the other hand, seven (58.3%) isolates of K. pneumoniae and one (11.1%) isolate of E. cloacae complex showed resistance to last resort antibiotic colistin (Supplementary Table S1). A. baumannii and P. aeruginosa isolates were resistant to all tested antimicrobial agents with EUCAST clinical breakpoints, except colistin and amikacin, respectively (Figure 1d, Supplementary Table S1).
Finally, antimicrobial susceptibility testing revealed that all target ARB isolates described in this study were multidrug-resistant (MDR) [17].
2.3. Characterisation of β-lactamase Genes (ESBL and Carbapenemase Genes)
Among 34 sequenced target Gram-negative isolates, a high diversity of β-lactamase genes was detected (Table 2).

Table 2.
Antibiotic resistance genes found in isolated target antibiotic-resistant bacteria.
The majority of Enterobacterales isolates (70%) harbored more than one ESBL gene, in different combinations, which included blaCTX-M-15 and various types of blaSHV, blaTEM, blaOXA and blaOXY genes. The predominant ESBL gene in all K. pneumoniae isolates was blaCTX-M-15, followed by blaOXA-1 (83.3%). Genes for the SHV-212 type of enzyme were detected exclusively in K. pneumoniae isolates that belong to ST101 (58.3%). All isolates of K. pneumoniae harbored carbapenemase blaOXA-48 gene, with one isolate co-harboring blaOXA-48 and blaNDM-1. Besides β-lactamase genes, all K. pneumoniae isolates harbored the point mutation in the ompK36 locus, which can confer resistance to carbapenems and other β-lactams through reduced permeability. Out of three K. oxytoca isolates, two isolates carried blaGES-5 carbapenemase gene in combination with different ESBLs from OXA and OXY families and one isolate carried blaOXA-48 with blaOXY ESBL gene. In E. cloacae complex, ESBL genes were found as follows: blaOXA-1 (55.5%), blaTEM-1 (44.4%), blaCTX-M-15 (22.2%), and other types of ESBLs were found in single isolates. These ESBLs were in combination with metallo-beta-lactamase, blaNDM-1 in 44.4% isolates and blaIMI-2 gene in one isolate of E. cloacae complex. All three isolates of E. coli were ESBL and carbapenemase producers. Two isolates were positive for the blaNDM-1 carbapenemase gene, and one isolate harbored the blaOXA-48 gene. C. freundii was carbapenemase negative, but ESBL positive, with the presence of blaCTX-M-162 and blaTEM-1 genes. Both S. marcescens isolates were positive for ESBL and carbapenemase genes, one with blaSHV-2a and blaGES-5, the other with blaTEM-1 and blaNDM-1 genes. In A. baumannii and P. aeruginosa isolates, different cephalosporinases from ADC and PDC families were confirmed. One isolate of P. aeruginosa harbored the blaPER-1 ESBL gene, while the other was positive for blaOXA-395 chromosomal beta-lactamase. Both A. baumannii isolates carried class D carbapenemase (oxacillinase): blaOXA-23/blaOXA-66 and blaOXA-66/blaOXA-72, respectively.
2.4. Resistome Other Than β-lactamase Genes
Antimicrobial resistance genes (ARG) besides genes conferring resistance to beta-lactams are presented in Table 2.
Aminoglycoside modifying enzymes (AME) genes were detected in almost all the target ARB isolates (97.1%). Whole-genome sequencing (WGS) analysis showed heterogeneous AME types from aac-, aad-, aph-, ant- and aba- families, including the armA gene, which confers a high level of aminoglycoside resistance. The most frequent AME genes were aac(6′)-Ib-cr, aph (6)-Id, aac (3)-IIe and aad A2.
Genes conferring resistance to fluoroquinolones, oqxA and oqxB, were detected in all K. pneumoniae isolates, including aac(6′)-Ib-cr in 83.3% isolates, which confers resistance to both aminoglycosides and fluoroquinolones. In the E. cloacae complex, the qnr gene was found in 66.7% of isolates, as well as in one E. coli isolate. Resistance to fosfomycin, mediated by the fosA gene, was detected in all K. pneumoniae and P. aeruginosa isolates, and in 88.9% of E. cloacae complex isolates.
Tetracycline-resistance gene tetD was found in 50% of K. pneumoniae and in 33.3% of E. coli isolates, tetA in 41.7% of K. pneumoniae and in 33.3% of E. cloacae complex isolates, while tetB gene was confirmed in all A. baumannii and one E. coli isolates. Genes conferring resistance to sulfonamides, sul1 and sul2, were found in 52.9% and 32.3% of target ARB isolates, respectively. Additionally, seven (20.6%) ARB isolates harbored both genes. The most common trimethoprim resistance gene was dfrA14, found in 66.7% K. pneumoniae, 66.7% of E. cloacae complexes and 33.3% of E. coli isolates. The second most common was dfrA12, detected in different species, but also in combination with dfrA14 in 22.2% of E. cloacae complex isolates. Phenicol resistance was confirmed in all species, except K. oxytoca and C. freundii, with the catB3 gene as the most common (52.9%). Finally, plasmid mediated colistin resistance was confirmed in one E. cloacae complex isolate (BS11105), which harbored the mcr-9.1 gene. In one K. pneumoniae (BS11131) and two P. aeruginosa (BS11135; BS11136) isolates, mutations in the basS/basR two-component system were detected. Additionally, six of the seven ST101 K. pneumoniae isolates had a missense mutation (TGC->AGC) in the mgrB gene, leading to substitution C28S in the MgrB regulator (Supplementary Table S2).
2.5. Plasmidome and Virulome
In-silico plasmid detection confirmed 28 different plasmids in analyzed Enterobacterales (Supplementary Table S2).
The majority of K. pneumoniae isolates (91.7%) had four or more different plasmid replicon types. The most common was Col440II (75%), followed by IncFIA(HI1) (58.3%), IncR (58.3%), IncL (50%), IncFIB (pKPHS1) (50%) and others. As expected, ST101 K. pneumoniae shared similar plasmid profiles, all harboring Col440II, IncFIA(HI1) and IncR, among other replicon types. These replicons were part of plasmid contigs in 86.7% of ST101 isolates. Plasmid p101_srb (accession number: NZ_MN218814.1) contained IncFIA(HI1) and IncR replicons, and plasmid pKp_Goe_641.3 (accession number: NZ_CP018738.1) contained the Col440II replicon. Plasmids detected in the E. cloacae complex showed the highest diversity (20 replicon types in nine isolates). In K. oxytoca, GES-5-positive isolates shared a similar plasmid profile, whereas one OXA-48 positive isolate had IncL and IncFII(Yp) replicon. Each E. coli isolate had a unique plasmid profile, with replicons from the Inc group, as well as C. freundii and S. marcescens. Additionally, inter-species detection of plasmid replicons was observed.
Virulome analysis demonstrated a plethora of virulence genes (Supplementary Table S3) in 31 strains. However, in strains of C. freundii and S. marcescens, no virulence genes were detected. The most diverse virulome showed isolates of P. aeruginosa, with more than 300 genes. The vast majority were antiphagocytosis virulence genes (alg44, alg8, algA-Z), type II secretion system—xcp secretion system (xcpP-Z), type III secretion system (pcr1-4, pcrD-V, pscB-U), type VI secretion system (tagF1-T, tse1-3, tssA1-M1), genes for iron acquisition and pigment (pvcA-D, pvdA-S) and adherence and motility genes (fimT-V, flgA-N, fliA-S). A. baumannii isolates shared the same virulome profile with 29 virulence determinants: efflux pump (adeF-H), acinetobactin (barB, basA-J, bauA-F), and csu fimbriae (csuA/B, csuB, csuD). K. pneumoniae isolates had a quite similar distribution of virulence genes, all harboring AcrAB efflux pump, adherence genes (fim, mrk), genes for iron acquisition (ent, fep, fyu, irp, ybt) and secretion system T6SS-I and T6SS-III (clpV/tssH, dotU/tssL, hcp/tssD, icmF/tssM, impA/tssA, tssF, tssG, vasE/tssK, vgrG/tssI, vipA/tssB, vipB/tssC). In E. coli, 90 virulence genes were found, with one isolate that belonged to sequence type ST131, and harbored toxins genes (hlyA-alpha, hlyB, hlyD) and invasion factors (aslA, kpsC, kpsE, kpsMII, kpsS, kpsU, ompA, ompT) among other virulence determinants.
2.6. Genomic Epidemiology and Phylogenetic Relatedness
The 12 K. pneumoniae isolates resolved into six sequence types (STs), of which one isolate represented a new ST (ST6273). The most frequent was ST101 (n = 7, 58.3%), whereas other STs represented singletons: ST15, ST16, ST29, ST437 and ST6273. Among ST101 we detected two clusters (Figure 2a), genetically close (99.55% similarity), sharing the same resistome and the same plasmid replicons but not strictly the same virulome (Supplementary Table S2). Cluster 1 grouped two ST101 strains (BS11119 and BS11134) isolated from the same sampling site (S2), but different sampling campaigns (SC1-May and SC4-August). Cluster 2 grouped three isolates (BS11125, BS11126, BS11128), two from the same sampling site and sampling campaign (S1, SC3), and a third from the same sampling campaign (SC3), but from a different sampling site (S3), placed downstream from S1. Regarding virulome differences, compared to the other isolates of cluster 1 and 2, respectively, BS11119 had an additional virulence factor (vipB/tssC) belonging to the type VI secretion system (T6SS), and in BS11125, the mrk gene cluster (mrkABCDF) and regulatory genes (mrkI, mrkJ) were missing. Compared to isolates from both clusters, other ST101 (BS11122; BS11120) were more distant (<99% similarity) and had many differences in resistome (Supplementary Table S2). A minimum spanning tree (MST) displaying the K. pneumoniae isolates is presented in Figure 3 to show the allelic differences.
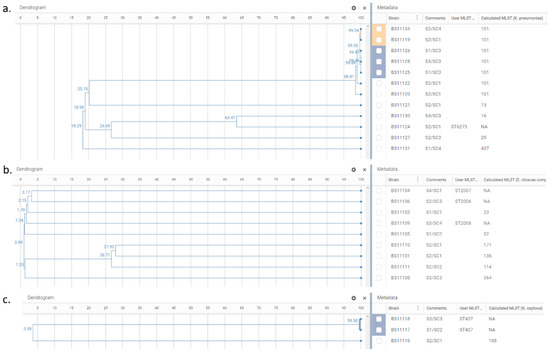
Figure 2.
Phylogenetic trees of K. pneumoniae isolates, n = 12 (a); E. cloacae complex isolates, n = 9 (b); and K. oxytoca isolates, n = 3 (c). Dendrogram panel reflects the relationships between the samples based on their wgMLST profiles and shows the similarity percentage on nodes. Metadata panel includes information relative to sample identifier (Strain), sampling site/campaign (Comments), and MLST calculated by EPISEQ® CS (Calculated MLST) or provided by the database curator when a new ST assignment was required (User MLST). The colored blocks show the clusters of closely related isolates detected by EPISEQ® CS.
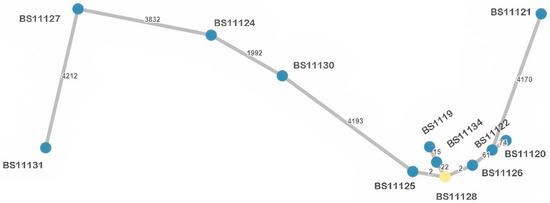
Figure 3.
Minimum spanning tree (MST) based on wgMLST allelic profiles of the analyzed K. pneumoniae isolates (n = 12). Each dot represents one isolate with its identifier. The number of allelic differences between two isolates is shown on the connecting branch between them.
The nine E. cloacae complex isolates were resolved into nine STs: ST23, ST32, ST114, ST136, ST171, ST364, and three newly assigned STs: ST2006, ST2007, ST2008. These isolates showed no clustering and were genetically unrelated (Figure 2b). An MST displaying the E. cloacae complex isolates is presented in Figure 4, showing at least 2200 allelic differences between each sample.
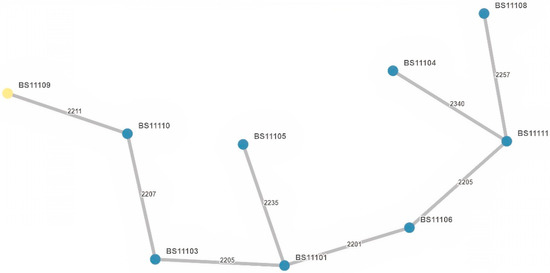
Figure 4.
Minimum spanning tree (MST) based on wgMLST allelic profiles of the analyzed E. cloacae complex isolates (n = 9). Each dot represents one isolate with its identifier. The number of allelic differences between two isolates is shown on the connecting branch between them.
One K. oxytoca isolate belonged to ST108, while the other two were a new sequence type (ST427). Both ST427 isolates, originating from different sampling sites and sampling campaigns (S1/SC2 and S3/SC3), were closely related (99.50% similarity), sharing the same resistome, but not the same virulome (traT gene only found in BS11117; no virulome feature in BS11118) (Figure 2c, Supplementary Table S2).
All isolates of E. coli, C. freundii, A. baumannii and P. aeruginosa represented singletons (Table 2). To date, no MLST scheme has been described for S. marcescens.
3. Discussion
The looming post-antibiotics era calls for urgent epidemiological measures for the containment of ARB linking all settings under the One-Health umbrella. However, the important role of wastewater and its contribution to the transmission of AMR in the environment is often not addressed or neglected [9,10,12,13]. The Belgrade sewer system consists of combined and separate sewers with a total length of over 1500 km, to which 1.7 million inhabitants are connected. Works on upgrading the sewer system and the introduction of wastewater treatment plants is ongoing; however, currently, sewage is still discharged into the rivers the Sava and the Danube without any treatment [14,18]. To our knowledge, this is the first comprehensive study to evaluate presence of ARB in the Belgrade sewer system prior to outlet to recipients and thereafter AMR pollution of two international rivers in their lower drainage basin, providing important additions to the detailed insight into the extent of the issue on a European level.
K. pneumoniae isolates from our study represents critical pathogens according to the WHO priority list, due to their MDR profile with resistance to third-generation cephalosporins and carbapenems [2]. We confirmed ESBL-positive isolates in samples from all sampling sites (S1–S4) and from all sampling campaigns. All isolates produced CTX-M-15, as well as other clinically relevant ESBLs from SHV, TEM and OXA families. CTX-Ms, usually located on epidemic resistance plasmids of incompatibility group F (IncF) [19], were detected in all K. pneumoniae isolates in our study. This contributes to the global spreading of CTX-M-15, representing the most widely distributed ESBLs, responsible for nosocomial outbreaks and community-onset infections [19,20].
An OXA enzyme with an ESBL phenotype, OXA-1, was the second most common ESBL among wastewater isolates in the study, found in 91.7% K. pneumoniae. On a global level, blaOXA-1 is in the top 15 ARGs from the environmental samples [20]. This class D enzyme is often found with other ESBLs in clinical isolates as well. Palmieri et al. detected blaCTX-M-15 and blaOXA-1 genes in the vast majority of clinical carbapenem- and colistin-resistant K. pneumoniae from Serbia [21]. Similar findings were described in the Croatian study of OXA-48 producing hospital isolates of K. pneumoniae [22]. Another specific result from our study relates to the detection of blaSHV-212 only among isolates that belonged to ST101. Overall data about this SHV variant are scarce, but it was described in a K. pneumoniae isolate from a Dutch healthcare worker [23].
The most concerning result was the detection of Ambler’s class D carbapenemase OXA-48 in all K. pneumoniae isolates. Detection of carbapenemase-positive K. pneumoniae was a consistent finding in the study, confirmed in all sampling sites and sampling campaigns. OXA-48 represents the most frequent carbapenemase from the environmental samples in Europe [20], and the most widespread carbapenemase among Enterobacterales globally [24]. The genetic environment of blaOXA-48 gene is well-described, including different mobile genetic elements, such as transposon Tn1999 and pOXA-48a-like IncL (IncL/M) plasmid [24]. The IncL plasmid was confirmed in 50% of K. pneumoniae in this study, including one ST15 and one ST101 isolate. Another 50% of isolates, all belonging to ST101, harbored plasmid p101_srb with IncFIA(HI1) and IncR replicons. p101_srb was firstly described by Palmieri et al. in an ST101 K. pneumoniae clinical isolate from Serbia, encoding OXA-48, the CTX-M-15 ESBL and several other AMR genes, and conferring an MDR phenotype [21]. This finding strongly suggests the clonal expansion of the high-risk clone ST101, and dissemination from medical settings into the environment in Serbia.
Additionally, one ST101 isolate (BS11120) harbored genes for both OXA-48 and NDM-1 enzymes. Serbia is considered endemic for NDM carbapenemase [25]; thus, the co-occurrence of blaOXA-48 and blaNDM genes could be expected, and it has been described in ST101 K. pneumoniae hospital isolates from Serbia [26].
MLST analysis revealed six STs among K. pneumoniae isolates, including a novel one—ST6273. The most prevalent was ST101, and the phylogenetic analysis of ST101 isolates showed two clusters. Cluster 1 grouped isolates (n = 2) from the same sewer outlet (S2, with no hospital input), but from different sampling campaigns (S1 and S4), indicating the persistence of this clone over time. Cluster 2 grouped isolates (n = 3) from the same sampling campaign (SC3), of which two were from sampling site S1, and one from the sewer outlet placed downstream (S3), indicated probable spreading through the ecosystem. These five isolates shared the same resistome and had the same plasmid replicons (Col440II, IncFIA(HI1), IncFIB(pKPHS1), IncR), but not strictly the same virulome. Two additional ST101 isolates were more distant compared to isolates from clusters, with differences in resistome. Nevertheless, all ST101 isolates showed the presence of virulence factors significant for hypervirulent K. pneumoniae strains, such as siderophores (enterobactin, yersiniabactin, salmochelin, aerobactin) and type 3 fimbriae, and can cause serious infections, such as liver and lung abscesses, in otherwise healthy individuals [27]. Thus, the dissemination of these ST101 in the environment in Serbia may present a worrisome public health issue. Additionally, we detected one ST437 isolate from sampling site S2, which has no hospital effluents. This clone was previously described among clinical isolates from Serbia, as a part of a single monophyletic subclade within the CG258 [21].
Multiple studies described K. oxytoca isolates from wastewater [28,29,30,31]. Among K. oxytoca isolates from our study, we confirmed carbapenemase gene blaOXA-48 in one isolate, belonging to ST108. This clone was found among hospital isolates in Australia [32], but overall data about it are scarce. The reason could be the limitation of phenotypic identification methods, which cannot distinguish members of K. oxytoca complex. In accordance with that, ST108 was described in Klebsiella michiganensis clinical isolates [33]. Interestingly, we observed phylogenetic relatedness among two other K. oxytoca wastewater isolates belonging to novel ST uncovered in this study, ST427, isolated from different sampling sites and sampling campaigns. They shared the same resistome, and formed a cluster with 99.50% similarity. One of these isolates possesses virulence gene traT, responsible for complement resistance.
E. cloacae complex was isolated from all sampling sites and sampling campaigns in our study. These isolates showed high diversity in terms of carbapenemase production, plasmid content, virulence genes and STs. Among carbapenemase-positive isolates, the blaNDM-1 gene was the most frequent. This gene is the most reported one in the environment in the past 30 years [20], whereas the hospital wastewaters are considered as the major source of blaNDM variants, especially in India, an endemic area [34]. Serbia is considered endemic for the NDM carabapenemase as well [25], but blaNDM was not confirmed in a previous study of environmental water in Belgrade, Serbia [35]. On the other hand, among clinical isolates of Enterobacter spp. from global surveillance programmes, blaNDM was predominantly found among isolates from the Balkans, India and Vietnam [36]. Among Enterobacter spp. community isolates in Belgrade, Serbia, blaNDM was the most prevalent carbapenemase gene [37]. In our study, all E. cloacae complexes belonged to different STs, and the heterogeneity of STs was demonstrated through discovery of three new STs among nine isolates. This is in accordance with the results of other studies, which have shown that spreading of blaNDM-1 was not related to a specific clone [25].
Among E. coli isolates, we detected ST131 and ST155 which are considered high-risk clones with zoonotic potential and disperse antibiotic resistance on a global scale. Extraintestinal MDR E. coli ST131 is a worldwide pandemic pathogen and a major cause of urinary tract infections, bloodstream infections and infections in companion animals and poultry [38]. Originally identified in 2008, ST131 is associated with the worldwide spread of CTX-M-15 resistance and recent reports have also identified strains that are resistant to carbapenems [39]. Although the blaCTX-M-15 gene was not present in the E. coli ST131 strain from our study, other ESBL enzyme genes (blaOXA-1, blaSHV-12, blaTEM-1) as well as the metallo-beta-lactamase gene blaNDM-1 were detected. Another ST, the E. coli ST155 strain, was also recently reported as a clonal group of animal origin that is spreading in humans and is highly drug resistant [40]. Our strain was carbapenemase-producer (OXA-48) and, in addition, resistant to aminoglycosides, tetracyclines and sulfonamides. Importantly, recent studies showed E. coli ST155 strains carrying the mcr-1 gene and novel variant mcr-1.26 [41,42]. However, our strain was susceptible to colistin in vitro and mcr genes were not detected. Moreover, some previous studies also detected E. coli ST155 strains in surface waters [43,44].
Occurrence of Acinetobacter spp. is well-described in the natural environment, with special attention to A. baumannii, as the most important species causing infections in humans [45]. Isolation of carbapenem-resistant A. baumannii (CRAB) from water environments was documented in multiple studies [46]. We detected two CRAB isolates. One isolate harboured blaOXA-66, chromosomaly located, inherited carbapenemase [47], and blaOXA-23. This isolate belonged to ST2, the most widespread clone globally [48]. ST2/blaOXA-23-positive A. baumannii was one of the most prevalent clones among CRAB hospital isolates from Serbia [49]. Furthermore, in the same study, the most frequent clone circulating in Serbia was ST492, blaOXA-66- and blaOXA-72-positive, the same as the second isolate from our research. According to Lukovic et al., a ST492/blaOXA-72-positive clone was rarely described, and only in isolates that originated from Serbian patients [49].
Finally, the most worrying findings of the study is the high level of concurrent resistance to last-resort antibiotics carbapenems and colistin in Gram-negative clinically relevant wastewater isolates in Serbia. The inappropriate use of colistin has been shown to provide selective pressure for the emergence of colistin and multidrug (including carbapenem) resistant strains. Interestingly, different mechanisms of chromosomal- and plasmid-mediated resistance to colistin were detected in isolates from the study. First, a missense mutation in the mgrB gene resulting in MgrBC28S was identified in 6 of 7 (85.7%) ST101 K. pneumoniae isolates. Although other amino acid substitutions (C28F; C28Y) have been described at the same position of the MgrB regulator in Klebsiella spp. [50], the substitution C28S was first described by Palmieri et al. in ST101 K. pneumoniae human isolates [21]. To our knowledge, this is the second study that confirms the presence of this novel mutation in the mgrB gene of K. pneumoniae in Serbian isolates. A genetic change (mutation or deletion) in almost any position of mgrB predominantly leads to functional inactivation of the MgrB peptide, which cannot act as a feedback inhibitor of the PhoP/PhoQ system; this plays a significant role in colistin-resistant K. pneumoniae strains [50]. Second, one E. cloacae complex isolate harbored plasmid-mediated mobile colistin resistance gene mcr-9.1. Enterobacter spp. and Salmonella spp. are the main hosts of mcr-9.1 gene globally [51], and can be found in different reservoirs (human, animal, food and environment) [52], making this resistance mechanism an issue under the perspective of One Health. This mcr variant was previously confirmed in a K. pneumoniae human isolate from Serbia [53], indicating potential interspecies spreading of plasmid-mediated colistin resistance. Third, mutations in the basRS genes were detected in one K. pneumoniae and two P. aeruginosa isolates. The PmrAB (also termed BasRS) two-component system plays a crucial role in mediating the modification of LPS which leads to colistin resistance in Gram-negative bacteria and mutation was previously detected in various Enterobacterales, Pseudomonas spp. and Acinetobacter spp. strains [54]. Therefore, we hypothesize that the high rates of colistin resistance observed in our study may be connected to the widespread use of colistin, particularly in hospital settings in Serbia.
The authors acknowledge the limitations of the study, mainly the reduced number of wastewater samples and bacterial isolates targeted. Future studies should also consider including samples from hospital wastewater and receiving water bodies in order to track the environmental dissemination of ARB. However, in countries with poor wastewater treatment like in Serbia, AMR findings in wastewater are mirrored in their recipients. Overall, our study implementing state-of-the-art techniques for in-depth ARB investigation can be considered a good starting point for implementing a wastewater-based molecular epidemiology survey model to reflect the behavior of carbapenem-resistant Gram-negative bacteria in the community. The study also highlighted the need to minimize the spread of ARBs within hospitals before reaching the environment. The software application EPISEQ® CS used here for WGS data analysis could also help to track, prevent, contain and stop the spread of healthcare-associated infections and AMR.
4. Materials and Methods
4.1. Study Setting and Sampling
Water samples were collected from four main sewer outlets in Belgrade prior to discharge to the Sava and the Danube rivers: site S1, the biggest combined sewer outlet “Sajam”, which accounts for almost 40% of the total of Belgrade’s wastewater discharge; site S2, combined sewer outlet “Lasta” drains the southwestern part of the central area of Belgrade; site S3, the largest separate sewer outlet “Ušće”; and site S4, “Istovarište”, the second largest combined sewer outlet in Belgrade. Figure 5 and Table 3 show locations of the sampling sites and their characteristics. Besides municipal wastewater, untreated hospital wastewater from tertiary healthcare hospitals in Belgrade are discharged in sewer outlets S1, S3 and S4 (S1 and S4 receive input from multiple hospitals, S3 from one tertiary healthcare hospital and S2 has no hospital input).
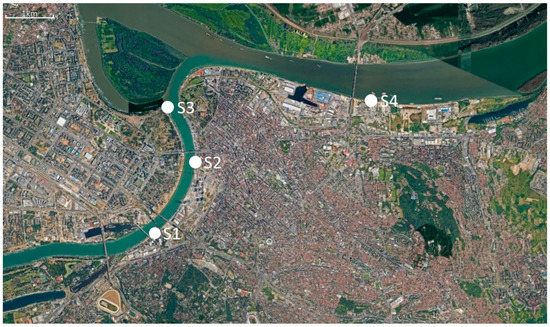
Figure 5.
Satellite image of sampling sites (Google Earth).

Table 3.
Sampling sites and characteristics of wastewater sewer outlets [18].
Sampling took place on four occasions between May and August 2018, with minimum time interval of one month between two independent sampling visits to minimize the possible overlapping of carryover of the targeted ARB. Wastewater was aseptically sampled in sterile 1L glass bottles. The samples were transported to the laboratory at a temperature of 4 °C and were processed on the day of collection.
4.2. Cultivation and Identification of Target ARB
For each water sample, 50 mL was passed through a 0.45 μm filter (Millipore, Billerica, MA, USA). Filters were incubated for 24 h at 37 °C in 5 mL of BBL fluid thioglycollate medium (Becton Dickinson, NJ, USA). Screening for target ARB was performed by streaking one loopful of enriched culture onto CHROMID® CARBA SMART (bioMérieux, Marcy-l’Étoile, France). All colonies with different morphologies were picked and subcultured onto Columbia agar with 5% sheep blood (bioMérieux) at 37 °C for 18 to 24 h. Isolates were subjected to identification by protein profiling using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF/MS) using a VITEK® MS mass spectrometer (bioMérieux) equipped with Myla software. All target ARB were purified on Columbia agar with 5% sheep blood and preserved in cryotubes at −80°C.
4.3. Antimicrobial Susceptibility Testing
The antimicrobial susceptibility profiles were performed by the VITEK® 2 automated system (bioMérieux). Panels of following antimicrobials were used: amoxicillin-clavulanic acid (AMC), piperacillin- tazobactam (TZP), ceftazidime (CAZ), ceftriaxone (CTX), cefepime (CEF), ceftazidime-avibactam (CZA), ceftolozane-tazobactam (C/T), ertapenem (ERT), imipenem (IPM), meropenem (MEM), aztreonam (ATM), amikacin (AMK), gentamicin (GEN), ciprofloxacin (CIP), levofloxacin (LVX), chloramphenicol (CHL) and trimethoprim-sulfamethoxazole (STX). Susceptibility to colistin was determined by broth microdilution method (ComASPTM Colistin, LiofilChem, Italy). Profiles were evaluated according to the EUCAST breakpoint Table 2022, with category “I” meaning “susceptible, increased exposure”. Additionally, for P. aeruginosa and A. baumannii isolates, interpretation of antimicrobial susceptibility was performed only for antimicrobial agents with EUCAST clinical breakpoint for these bacteria (https://www.eucast.org/, accessed on 9 November 2022).
4.4. DNA Extraction and Whole-Genome Sequencing
Bacterial DNA from the overnight cultures of the study isolates was extracted with a DNeasy UltraClean Microbial Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Quantitation was performed with Qubit® dsDNA BR Assay kit (Thermo Fisher Scientific, Waltham, MA, United States) on Invitrogen Qubit® Fluorometer (Thermo Fisher Scientific). DNA quality was checked using the 260/280 ratio absorbance parameter as determined by the DS-11FX spectrophotometer (DeNovix, Wilmington, DE, USA). Size measurement of genomic DNA was pefromred with HS Genomic DNA 50 kb Kit (Agilent Technologies Inc., Santa Clara, CA, USA) on Agilent Fragment Analyzer system.
Libraries were prepared using the Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA, USA) and Index Kit v2 Set A (Illumina). Library quality controls were performed with High Sensitivity DNA kit (Agilent) on Agilent 2100 Bioanalyzer system, and with Ultra Sensitivity NGS kit (Agilent) on Agilent Femto Pulse system. Library pools were prepared according to the previously described equimolar pooling strategy for multiplexing multiple bacterial species [55]. Sequencing was performed using MiSeq® Reagent kit v3 (600-cycle) on a MiSeq® Platform (Illumina) to generate 2 × 200 base paired-end reads.
The sequencing data were analyzed with the easy-to-use, fully integrated web-based software application EPISEQ® CS, version 1.2.0 (bioMérieux). The application was based on reference-free approach with automated workflow (https://www.biomerieux-episeq.com/cs-how-it-works, accessed on 9 November 2022). Sequencing data were quality checked and assembled, followed by quality control of assembled genomes. Species identity, initially selected by the user, and potential intra- and inter-species contamination were checked. Genomic strain characterization was performed through the generation of multi-locus sequence typing (MLST) results, serotyping and pathotyping results for E. coli samples, detection of virulence and antimicrobial resistance markers, and detection of plasmids. Allele calling was performed in a proprietary whole-genome MLST (wgMLST) scheme defined for each EPISEQ® CS species. Finally, based on the wgMLST allelic profiles, an epidemiological analysis was calculated and visualized in a dendrogram (% similarity) and a minimum spanning tree (number of allelic differences). For isolates (n = 7) with undefined sequence type (BS11104; BS11124) or undetermined allele variant (BS11106; BS11109; BS11113; BS11117; BS11118) in EPISEQ® CS MLST allelic profile, complementary results were obtained from WGS data (assembly FASTA file) using https://cge.food.dtu.dk/services/MLST/ (accessed on 9 November 2022) tool with a minimum depth for an allele set to only 5x. Genome sequences and allelic profiles from these isolates with unknown STs were submitted to PubMLST (E. cloacae complex and K. oxytoca) [56] or Pasteur (K. pneumoniae and E. coli) for the assignment of new STs by database curators.
4.5. Identification of Missense Mutation in mgrB Gene Resulting in MgrBC28S
For the colistin resistance mechanism via mutations in the chromosomal mgrB gene, regarding substitutions of the cysteine amino acid (AA) at position 28 in the MgrB regulator of Klebsiella spp., only C28F and C28Y were available in the EPISEQ® CS version (1.2.0) used for the study. A novel substitution (C28S) associated with ST101 Klebsiella pneumoniae was recently described [21]. To search for missense mutation in mgrB causing the substitution C28S in MgrB peptide (47 AA residues), using Geneious Prime® (2022.0.1) software the wild-type sequence of mgrB gene (with TGC codon encoding cysteine at position 28) was first extracted from annotated NCBI RefSeq chromosome assembly (Accession number: NZ_CP065838.1) of Klebsiella quasipneumoniae strain KqPF26, and then aligned against the genome assembly of all studied ST101 Klebsiella pneumoniae isolates (n = 7).
5. Conclusions
The results of this pilot study on the detection of clinically relevant strains of carbapenem-resistant Gram-negative bacteria in wastewater prior to outlet into the international rivers without previous treatment is alarming and appears an emerging future public-health issue which demands increased attention and the proposal of mitigation measures. Importantly, the plethora of determined co-existence of ARG and virulence genes in investigated ARBs highlighted the serious threats of these pathogens on human and animal health. Immediate actions as a consequence of our study results include the proper treatment of wastewater. We recommend the development of new strategies for treating hospital and municipal wastewater effluents, and the establishment of a surveillance system to monitor MDR clinically relevant bacterial species in main sewer outlets and surface waters, as it is proposed by the EU Commission for systematic surveillance of SARS-CoV-2 and its variants in EU wastewaters [57].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12020350/s1, Table S1: Antimicrobial susceptibility of analysed carbapenem-resistant Gram-negative bacteria isolated from wastewater; Table S2: Resistome and plasmidome of analysed carbapenem-resistant Gram-negative bacteria isolated from wastewater; Table S3: Virulome of analysed carbapenem-resistant Gram-negative bacteria isolated from wastewater.
Author Contributions
Conceptualization, I.C., C.M.-M., S.B.; methodology, I.C., B.H.M., P.M., V.I., C.M.-M.; validation, I.C., S.B.; investigation, I.C., B.H.M., A.J., S.B.; data curation, S.B.; writing—original draft preparation, I.C., B.H.M., A.J., S.B.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the bioMérieux company (Marcy-l’Étoile, France) for part of antibiotic susceptibility testing characterisation, for whole-genome sequencing (WGS) and WGS data analysis. This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that supports the findings of this study are available within the article and its supplementary material. Sequencing read data are deposited at the European Nucleotide Archive (https://www.ebi.ac.uk/ena/browser/home, accessed on 31 January 2023) under the project accession number PRJEB58827.
Acknowledgments
The authors are immensely grateful to Alex van Belkum for his help in establishing the collaboration, Sandra Roche for her support in using Geneious Prime software, the Institut Pasteur teams for the curation and maintenance of BIGSdb-Pasteur databases at https://bigsdb.pasteur.fr/ (accessed on 9 November 2022), curators Eva Leitner, Medical University of Graz, Austria and Tohru Miyoshi-Akiyama, National Center for Global Health and Medicine, Japan for importing novel alleles and profiles at https://pubmlst.org/ (accessed on 9 November 2022).
Conflicts of Interest
B.H.M., P.M., V.I., C.M.M. declare being employees of bioMérieux S.A.
References
- Ekwanzala, M.D.; Dewar, J.B.; Kamika, I.; Momba, M.N.B. Tracking the environmental dissemination of carbapenem-resistant Klebsiella pneumoniae using whole genome sequencing. Sci. Total Environ. 2019, 691, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Morrill, H.J.; Pogue, J.M.; Kaye, K.S.; LaPlante, K.L. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect. Dis. 2015, 2, ofv050. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655, Erratum in Lancet 2022, 400, 1102. [Google Scholar] [CrossRef] [PubMed]
- WHO Regional Office for Europe Antimicrobial Medicines Consumption (AMC) Network: AMC Data, 2014–2018; WHO Regional Office for Europe: Copenhagen, Denmark, 2021. Available online: https://www.who.int/europe/publications/i/item/9789289055567 (accessed on 9 November 2022).
- WHO Regional Office for Europe Antimicrobial Medicines Consumption (AMC) Network: AMC Data 2019; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. Available online: https://www.who.int/europe/publications/i/item/9789289058278 (accessed on 9 November 2022).
- Central Asian and Eastern European Surveillance of Antimicrobial Resistance: Annual Report 2016; WHO Regional Office for Europe: Copenhagen, Denmark, 2016. Available online: https://www.euro.who.int/__data/assets/pdf_file/0009/323568/CAESAR-Annual-report-2016.pdf (accessed on 9 November 2022).
- WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2022—2020 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Joint-WHO-ECDC-AMR-report-2022.pdf (accessed on 9 November 2022).
- Samreen, A.I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Alexander, J.; Hembach, N.; Schwartz, T. Evaluation of antibiotic resistance dissemination by wastewater treatment plant effluents with different catchment areas in Germany. Sci. Rep. 2020, 10, 8952. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Consumption Database. Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/database (accessed on 9 November 2022).
- Aarestrup, F.M.; Woolhouse, M.E.J. Using sewage for surveillance of antimicrobial resistance. Science 2020, 367, 630–632. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection Republic of Serbia, Environmental Protection Agency. Environment in Serbia 2004–2019 Extended Summary. 2019. Available online: http://www.sepa.gov.rs/download/ENG_FIN_JubilarnaPublikacija.pdf (accessed on 9 November 2022).
- Pinka, P.G.; Penčev, P.G. Danube River. Encyclopedia Britannica. 2022. Available online: https://www.britannica.com/place/Danube-River (accessed on 9 November 2022).
- Kirschner, A.K.T.; Reischer, G.H.; Jakwerth, S.; Savio, D.; Ixenmaier, S.; Toth, E.; Sommer, R.; Mach, R.; Linke, R.; Eiler, A.; et al. Multiparametric monitoring of microbial faecal pollution reveals the dominance of human contamination along the whole Danube River. Water Res. 2017, 124, 543–555. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Jaroslav Černi Institute for Development of Water Resources. Master Plan of the Belgrade Sewerage System. 2011. Available online: https://www.jcerni.rs/ (accessed on 9 November 2022). (In Serbian).
- Peirano, G.; Pitout, J.D.D. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: Update on Molecular Epidemiology and Treatment Options. Drugs 2019, 79, 1529–1541. [Google Scholar] [CrossRef]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, L.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of antibiotic resistance genes in the environment. Environ. Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef]
- Palmieri, M.; D’Andrea, M.M.; Pelegrin, A.C.; Mirande, C.; Brkic, S.; Cirkovic, I.; Goossens, H.; Rossolini, G.M.; van Belkum, A. Genomic Epidemiology of Carbapenem- and Colistin-Resistant Klebsiella pneumoniae Isolates from Serbia: Predominance of ST101 Strains Carrying a Novel OXA-48 Plasmid. Front. Microbiol. 2020, 11, 294. [Google Scholar] [CrossRef]
- Bedenić, B.; Slade, M.; Starčević, L.Ž.; Sardelić, S.; Vranić-Ladavac, M.; Benčić, A.; Zujić Atalić, V.; Bogdan, M.; Bubonja-Šonje, M.; Tomić-Paradžik, M.; et al. Epidemic spread of OXA-48 beta-lactamase in Croatia. J. Med. Microbiol. 2018, 67, 1031–1041. [Google Scholar] [CrossRef]
- Dierikx, C.M.; Meijs, A.P.; Hengeveld, P.D.; van der Klis, F.R.M.; van Vliet, J.; Gijsbers, E.F.; Rozwandowicz, M.; van Hoek, A.H.A.M.; Hendrickx, A.P.A.; Hordijk, J.; et al. Colistin-resistant Enterobacterales among veterinary healthcare workers and in the Dutch population. JAC Antimicrob. Resist. 2022, 4, dlac041. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Peirano, G.; Kock, M.M.; Strydom, K.A.; Matsumura, Y. The Global Ascendency of OXA-48-Type Carbapenemases. Clin. Microbiol. Rev. 2019, 33, e00102-19. [Google Scholar] [CrossRef]
- Dortet, L.; Poirel, L.; Nordmann, P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed. Res. Int. 2014, 2014, 249856. [Google Scholar] [CrossRef]
- David, S.; Reuter, S.; Harris, S.R.; Glasner, C.; Feltwell, T.; Argimon, S.; Abudahab, K.; Goater, R.; Giani, T.; Errico, G.; et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 2019, 4, 1919–1929. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Savin, M.; Bierbaum, G.; Mutters, N.T.; Schmithausen, R.M.; Kreyenschmidt, J.; García-Meniño, I.; Schmoger, S.; Käsbohrer, A.; Hammerl, J.A. Genetic Characterization of Carbapenem-Resistant Klebsiella spp. from Municipal and Slaughterhouse Wastewater. Antibiotics 2022, 11, 435. [Google Scholar] [CrossRef]
- King, T.L.B.; Schmidt, S.; Essack, S.Y. Antibiotic resistant Klebsiella spp. from a hospital, hospital effluents and wastewater treatment plants in the uMgungundlovu District, KwaZulu-Natal, South Africa. Sci. Total Environ. 2020, 712, 135550. [Google Scholar] [CrossRef] [PubMed]
- Rolbiecki, D.; Harnisz, M.; Korzeniewska, E.; Buta, M.; Hubeny, J.; Zieliński, W. Detection of carbapenemase-producing, hypervirulent Klebsiella spp. in wastewater and their potential transmission to river water and WWTP employees. Int. J. Hyg. Environ. Health 2021, 237, 113831. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Hellmark, B.; Ehricht, R.; Söderquist, B.; Jass, J. Related carbapenemase-producing Klebsiella isolates detected in both a hospital and associated aquatic environment in Sweden. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.; Judd, L.M.; Jenney, A.; Holt, K.E.; Wyres, K.L.; Hawkey, J. Epidemiology and genomic analysis of Klebsiella oxytoca from a single hospital network in Australia. BMC Infect. Dis. 2022, 22, 704. [Google Scholar] [CrossRef] [PubMed]
- Shibu, P.; McCuaig, F.; McCartney, A.L.; Kujawska, M.; Hall, L.J.; Hoyles, L. Improved molecular characterization of the Klebsiella oxytoca complex reveals the prevalence of the kleboxymycin biosynthetic gene cluster. Microb. Genom. 2021, 7, 000592. [Google Scholar] [CrossRef]
- Waśko, I.; Kozińska, A.; Kotlarska, E.; Baraniak, A. Clinically Relevant β-Lactam Resistance Genes in Wastewater Treatment Plants. Int. J. Environ. Res. Public Health 2022, 19, 13829. [Google Scholar] [CrossRef]
- Novovic, K.; Filipic, B.; Veljovic, K.; Begovic, J.; Mirkovic, N.; Jovcic, B. Environmental waters and blaNDM-1 in Belgrade, Serbia: Endemicity questioned. Sci. Total Environ. 2015, 511, 393–398. [Google Scholar] [CrossRef]
- Peirano, G.; Matsumura, Y.; Adams, M.D.; Bradford, P.; Motyl, M.; Chen, L.; Kreiswirth, B.N.; Pitout, J.D.D. Genomic Epidemiology of Global Carbapenemase-Producing Enterobacter spp., 2008–2014. Emerg. Infect. Dis. 2018, 24, 1010–1019. [Google Scholar] [CrossRef]
- Brkić, S.; Božić, D.; Stojanović, N.; Vitorović, T.; Topalov, D.; Jovanović, M.; Stepanović, M.; Ćirković, I. Antimicrobial Susceptibility and Molecular Characterization of Carbapenemase-Producing Enterobacter spp. Community Isolates in Belgrade, Serbia. Microb. Drug Resist. 2020, 26, 378–384. [Google Scholar] [CrossRef]
- Forde, B.M.; Roberts, L.W.; Phan, M.D.; Peters, K.M.; Fleming, B.A.; Russell, C.W.; Lenherr, S.M.; Myers, J.B.; Barker, A.P.; Fisher, M.A.; et al. Population dynamics of an Escherichia coli ST131 lineage during recurrent urinary tract infection. Nat. Commun. 2019, 10, 3643. [Google Scholar] [CrossRef]
- Kondratyeva, K.; Salmon-Divon, M.; Navon-Venezia, S. Meta-analysis of Pandemic Escherichia coli ST131 Plasmidome Proves Restricted Plasmid-clade Associations. Sci. Rep. 2020, 10, 36. [Google Scholar] [CrossRef]
- Skurnik, D.; Clermont, O.; Guillard, T.; Launay, A.; Danilchanka, O.; Pons, S.; Diancourt, L.; Lebreton, F.; Kadlec, K.; Roux, D.; et al. Emergence of Antimicrobial-Resistant Escherichia coli of Animal Origin Spreading in Humans. Mol. Biol. Evol. 2016, 33, 898–914. [Google Scholar] [CrossRef]
- Matamoros, S.; van Hattem, J.M.; Arcilla, M.S.; Willemse, N.; Melles, D.C.; Penders, J.; Vinh, T.N.; Thi Hoa, N.; Bootsma, M.C.J.; van Genderen, P.J.; et al. Global phylogenetic analysis of Escherichia coli and plasmids carrying the mcr-1 gene indicates bacterial diversity but plasmid restriction. Sci. Rep. 2017, 7, 15364, Erratum in Sci. Rep. 2020, 10, 2963. [Google Scholar] [CrossRef]
- Neumann, B.; Rackwitz, W.; Hunfeld, K.P.; Fuchs, S.; Werner, G.; Pfeifer, Y. Genome sequences of two clinical Escherichia coli isolates harboring the novel colistin-resistance gene variants mcr-1.26 and mcr-1.27. Gut Pathog. 2020, 12, 40. [Google Scholar] [CrossRef]
- Muller, A.; Stephan, R.; Nuesch-Inderbinen, M. Distribution of virulence factors in ESBL-producing Escherichia coli isolated from the environment, livestock, food and humans. Sci. Total Environ. 2016, 541, 667–672. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Shoma, S.; Bari, S.M.; Ginn, A.; Wiklendt, A.M.; Partridge, S.R.; Faruque, S.M.; Iredell, J.R. Genetic diversity and antibiotic resistance in Escherichia coli from environmental surface water in Dhaka City, Bangladesh. Diagn. Microbiol. Infect. Dis. 2013, 76, 222–226. [Google Scholar] [CrossRef]
- Adewoyin, M.A.; Okoh, A.I. The natural environment as a reservoir of pathogenic and non-pathogenic Acinetobacter species. Rev. Environ. Health 2018, 33, 265–272. [Google Scholar] [CrossRef]
- Girlich, D.; Poirel, L.; Nordmann, P. First isolation of the blaOXA-23 carbapenemase gene from an environmental Acinetobacter baumannii isolate. Antimicrob. Agents Chemother. 2010, 54, 578–579. [Google Scholar] [CrossRef]
- Zarrilli, R.; Pournaras, S.; Giannouli, M.; Tsakris, A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents 2013, 41, 11–19. [Google Scholar] [CrossRef]
- Hamidian, M.; Nigro, S.J. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb. Genom. 2019, 5, e000306. [Google Scholar] [CrossRef]
- Lukovic, B.; Gajic, I.; Dimkic, I.; Kekic, D.; Zornic, S.; Pozder, T.; Radisavljevic, S.; Opavski, N.; Kojic, M.; Ranin, L. The first nationwide multicenter study of Acinetobacter baumannii recovered in Serbia: Emergence of OXA-72, OXA-23 and NDM-1-producing isolates. Antimicrob. Resist. Infect. Control 2020, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Binsker, U.; Käsbohrer, A.; Hammerl, J.A. Global colistin use: A review of the emergence of resistant Enterobacterales and the impact on their genetic basis. FEMS Microbiol. Rev. 2022, 46, fuab049. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J.; Maningi, N.E.; Modipane, L.; Mbelle, N.M. Emergence of mcr-9.1 in Extended-Spectrum-β-Lactamase-Producing Clinical Enterobacteriaceae in Pretoria, South Africa: Global Evolutionary Phylogenomics, Resistome, and Mobilome. mSystems 2020, 5, e00148-20. [Google Scholar] [CrossRef] [PubMed]
- Manageiro, V.; Salgueiro, V.; Rosado, T.; Bandarra, N.M.; Ferreira, E.; Smith, T.; Dias, E.; Caniça, M. Genomic Analysis of a mcr-9.1-Harbouring IncHI2-ST1 Plasmid from Enterobacter ludwigii Isolated in Fish Farming. Antibiotics 2022, 11, 1232. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Hu, Y.; Zhang, G.; Zhu, B.; Gao, G.F. Detection of mobile colistin resistance gene mcr-9 in carbapenem-resistant Klebsiella pneumoniae strains of human origin in Europe. J. Infect. 2020, 80, 578–606. [Google Scholar] [CrossRef]
- El-Sayed Ahmed, M.A.E.; Zhong, L.L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef]
- Muller, B.H.; Mollon, P.; Santiago-Allexant, E.; Javerliat, F.; Kaneko, G. In-depth comparison of library pooling strategies for multiplexing bacterial species in NGS. Diagn. Microbiol. Infect. Dis. 2019, 95, 28–33. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Commission Recommendation (EU) 2021/472 of 17 March 2021 on a Common Approach to Establish a Systematic Surveillance of SARS-CoV-2 and Its Variants in Wastewaters in the EU. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=uriserv:OJ.L_.2021.098.01.0003.01.ENG (accessed on 9 November 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).