Abstract
Most bacteria attach to biotic or abiotic surfaces and are embedded in a complex matrix which is known as biofilm. Biofilm formation is especially worrisome in clinical settings as it hinders the treatment of infections with antibiotics due to the facilitated acquisition of antibiotic resistance genes (ARGs). Environmental settings are now considered as pivotal for driving biofilm formation, biofilm-mediated antibiotic resistance development and dissemination. Several studies have demonstrated that environmental biofilms can be hotspots for the dissemination of ARGs. These genes can be encoded on mobile genetic elements (MGEs) such as conjugative and mobilizable plasmids or integrative and conjugative elements (ICEs). ARGs can be rapidly transferred through horizontal gene transfer (HGT) which has been shown to occur more frequently in biofilms than in planktonic cultures. Biofilm models are promising tools to mimic natural biofilms to study the dissemination of ARGs via HGT. This review summarizes the state-of-the-art of biofilm studies and the techniques that visualize the three main HGT mechanisms in biofilms: transformation, transduction, and conjugation.
1. Introduction
Up to 80% of all bacteria mainly live in biofilm communities [,]. The natural habitats of biofilms include aquatic environments (rivers, hot springs, or streams), soil and rhizosphere, sediment, and gastrointestinal surfaces, or they occur as dental plaque [,,,,,]. The definition of biofilms is not restricted to microbial layers on surfaces. Biofilms form at any liquid–liquid, solid–liquid, solid–gas, liquid–gas, or air–air interfaces (”bubble biofilm”) [,,]. Furthermore, the biofilm state applies to multicellular (non-attached) microbial aggregates such as flocs, microlayers, endolithic microbial populations, pellicles, slimes, microbial mats, microorganisms in soils, sediments and aerosols, marine snow, sludge, or biofilm on leaves and plant roots [,].
Although biofilms can be beneficial for gut homeostasis and food digestion [], environmental bioremediation [] and biotechnological applications [], they are also responsible for microbial corrosion [] and biofouling []. In addition, they are associated with serious diseases and infections. The global economic impact of biofilms is estimated to cost USD 5000 billion annually []. For this reason, studying bacterial biofilm formation has gained much attention in recent decades []. Several in vivo and in vitro biofilm models have been developed to mimic natural biofilms. Testing under biofilm conditions, rather than in the planktonic state, is necessary to develop therapeutic agents for biofilm-forming bacterial pathogens. Thus, most biofilm studies have been performed to test drug susceptibility or to develop treatment strategies for chronically infected wounds, implant and device-related infections, or biofilm-associated infections [,]. Biofilms are also of primordial interest as hotspots for horizontal gene transfer (HGT) and therefore for the dissemination of antibiotic resistance genes (ARGs). Studies on environmental biofilms are not as numerous by far as those on health-related bacteria.
1.1. Methodology
This review presents the state-of-the-art of the literature on HGT in biofilms. We aimed to show HGT studies of both Gram-positive and Gram-negative bacteria in biofilms. However, research on conjugative plasmids and ICEs from Gram-positive bacteria has been highlighted, as it has not been reviewed as extensively as works from Gram-negative bacteria. Furthermore, we wanted to point out the drawbacks and restrictions of HGT studies in biofilm communities.
PubMed and Google scholar were used as electronic databases for the literature search.
The keywords used in the bibliographic research were: “abiotic factor”, “antibiofilm”, “antibiotic resistance”, “biofilm”, “biofilm formation”, “biofilm formers”, “biofilm study”, “biofilm-associated infection”, “biotic factor”, “CLSM”, “conjugation”, “conjugative plasmid”, “device-related infection”, “environment”, environmental factor”, “horizontal gene transfer”, “hospital”, “hot spot”, “ICE”, “IME”, “implant”, “in situ model”, “in vitro model”, “in vivo model”, “integrative and mobilizable elements”, “integrative conjugative elements”, “mating”, “medical device”, “membrane vesicle”, “microfluidic”, “monomicrobial”, “mono-species”, “multi-drug resistance”, “multi-species”, “nosocomial infection”, “outer membrane vesicle”, “pathogen”, “phage“, “phage therapy”, “planktonic culture”, “plasmid”, “polymicrobial”; “T4SS”, “transduction”, “transformation”, “transposon” or “wastewater treatment plant”.
The selection of articles is based on the following main criteria: (1) experimental evidence for HGT and (2) “real” biofilm studies.
1.2. Biofilm Development and Occurrence
The multistep biofilm formation process has been studied in great detail. It starts with the adherence of planktonic cells to a surface and EPS formation, followed by microcolony formation, differentiation into a mature biofilm and partial detachment and dispersion for new surface colonization [,,]. The four main stages of biofilm formation include the initial attachment of planktonic cells to a solid surface, subsequent adhesion, biofilm maturation and dispersion of planktonic cells from a mature biofilm (Figure 1).
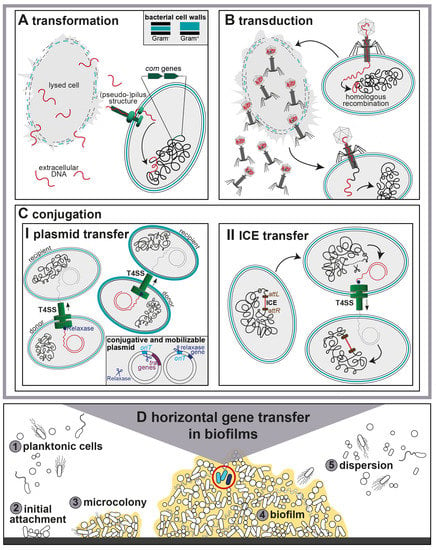
Figure 1.
Horizontal gene transfer in polymicrobial biofilms. The upper panels A–C show the three main HGT mechanisms (A) Transformation, (B) Transduction and (C) Conjugation. Transferred, transduced or conjugative DNA is shown in red, host DNA in black. (A) Transformation of competent Gram-negative bacteria. Uptake of extracellular DNA with pilus-like structures followed by incorporation into the host genome by homologous recombination. Chromosomally encoded competence (com) genes are marked in green. (B) Specialized transduction starts with the injection of phage DNA and integration into the host chromosome. Phage progeny is released during the lytic cycle. Phages can contain both viral and chromosomal DNA by erroneous encapsidation. This can be transferred to a new host cell. (C) Conjugation is illustrated in two ways: (I) Conjugative plasmid transfer for both Gram-negative and Gram-positive Type IV Secretion systems (T4SS). The relaxase (blue) bound to the single-stranded (ss) DNA after cleaving the plasmid at the origin of transfer (oriT) prior to transfer. Conjugative plasmids contain oriT and transfer (tra) genes, mobilizable plasmids only oriT and relaxase genes. (II) ICE transfer is introduced by excision of the ICE from the host chromosome at attachment (att) sites (brown). (D) Stages of polymicrobial biofilm formation from (1,2) initial attachment of planktonic cells, (3) microcolony formation to (5) dispersion of cells from a (4) mature biofilm. The light blue cells (4) show a conjugation event, the dark blue cell transformation or transduction.
Since the biofilm state applies to several biofilm architectures [], the developmental stages of biofilm formation cannot be generalized for non-surface-attached biofilms. The original concept of a biofilm life cycle is based on in vitro Pseudomonas aeruginosa biofilms and has been widely generalized to describe all biofilms []. However, the conceptual model cannot be equally applied to in vivo, in vitro and in situ conditions of biofilm growth. A recent review critically pointed out the limitations of the classical multistep biofilm model and proposed an updated, broader model of biofilm formation []. The model includes three main steps such as (1) aggregation and attachment, (2) growth and accumulation, and (3) disaggregation and detachment.
Biofilms can consist of single or multiple species, the latter being the predominant form in nature [,,]. Biofilm-forming bacteria are embedded in self-secreted extracellular polymeric substances (EPS) to protect themselves in hostile environments, in the presence of antimicrobials or to circumvent host defenses [,,]. They are primarily composed of water, enzymes, extracellular DNA (eDNA), polysaccharides, and lipids [,].
Artificial surfaces such as medical devices, wastewater pipelines, food-processing surfaces, equipment in the dairy, wine, and textile industries, and microplastics are commonly colonized by biofilms [,,,,,].
These are often formed by multiple antibiotic-resistant Gram-negative human pathogens such as Acinetobacter baumanii, Klebsiella spp., Escherichia coli, P. aeruginosa, Proteus mirabilis, Serratia marcescens, Enterobacter cloaceae and Burkholderia cepacia [,,,,]. Food-borne biofilm-forming pathogens include E. coli, Salmonella spp., Serratia spp., Listeria monocytogenes, Vibrio spp. and Campylobacter jejuni [,,,,].
Important Gram-positive biofilm producers include healthcare-associated pathogens such as Streptococcus spp., Enterococcus spp., and Staphylococcus spp. Coagulase-negative staphylococci (CoNS) and Staphylococcus aureus are the leading causes of biofilm-associated infections [,,,,].
Candida albicans also forms biofilms. The fungal pathogen often co-colonizes with bacterial pathogens and contributes to biofilm-associated infections [,,,].
Nosocomial infections such as pneumonia, urinary tract infections or device-related infections are predominantly caused by biofilm producers []. According to the National Institutes of Health (NIH) and Centers for Disease Control and Prevention (CDC), more than 60% of nosocomial infections and up to 80% of microbial infections are due to biofilms []. In the last few years, the spread of biofilms on medical implants and devices in clinical settings was the subject of many studies and has been frequently reviewed [,,,]. Medical devices can be reservoirs for multidrug-resistant, biofilm-producing strains [,,].
1.3. Biofilms as Reservoir for Antibiotic Resistance Genes
Wastewater and soil are major reservoirs for antibiotic resistomes []. The addition of antibiotic-containing manure, sewage sludge or other pollutants such as heavy metals, residuals of veterinary antibiotic compounds to agricultural soils can lead to increasing levels of antibiotic resistance and antibiotic-resistant bacteria [,,]. Organic fertilizers have been shown to contain various human pathogenic bacteria []. Some of them survive in the environment, colonize the soil and become enriched in the rhizosphere []. Subinhibitory concentrations of antibiotics can promote HGT in soil, and therefore, the selection of resistant pathogens. Soil-indwelling pathogens may recolonize the human gut when ingested by humans, leading to foodborne illnesses due to the consumption of contaminated food [,].
On the other hand, the spread of multiple ARGs among clinically relevant pathogens is a global threat that fosters mortality and poses an enormous economic burden []. The inadequate use and inappropriate prescription of antibiotics have led to a global antibiotic resistance crisis. The increase in multidrug-resistant bacterial infections and unsuccessful antibiotic treatments underlines the fact that ARGs cannot be ignored. In particular, biofilm formation promotes important features of pathogenesis such as significantly reduced susceptibility to antibiotics compared to susceptible non-biofilm producers [,,,].
Antimicrobial susceptibility tests on clinical isolates are normally performed using planktonic cultures. However, biofilms respond differently to antibiotic dosage which could lead to inadequate treatment and even treatment failure. A recent study from Lozano et al. described a simple biofilm model of human sputum to test the sensibility of P. aeruginosa strains against different antibiotics []. The development of specific growth media to better mimic in vivo biofilm formation and new biofilm antimicrobial susceptibility tests could contribute to the reduction in treatment failure [,,].
There is an urgent need to develop new antibiotics and strategies to prevent the development of antibiotic resistance []. It is not only clinical environments that support the spread of ARGs, but wastewater treatment plants (WWTPs) and agriculture are also considered hotspots for ARG propagation [,,,]. If ARGs are not completely removed by wastewater treatment, they are released into the wastewater effluent. This contributes to a vicious cycle of ARG dissemination in the environment [].
Biofilms are considered as a driving force for ARG dissemination because the proximity of bacteria in a biofilm enhances cell-to-cell contact and, therefore, the probability of genetic exchange [,].
1.4. Horizontal Gene Transfer in Biofilms
Biofilms facilitate the exchange and recycling of nucleic acids due to the proximity and long retention time of cells within the EPS matrix []. Constantly changing environments shape the genetic composition of bacteria via various mechanisms. Point mutations or recombination alter the bacterial genome and are the first steps in genetic diversification. Genetic material is transmitted via vertical transmission as well as with HGT. This mechanism can transform harmless bacteria into major human pathogens. HGT includes DNA transfer from a donor cell to a recipient cell via direct cell-to-cell contact (conjugation), uptake of extracellular naked DNA by competent cells (transformation), or bacteriophage-mediated DNA transfer (transduction).
A fourth mechanism describes the outer membrane vesicle (OMV)-mediated transfer of genetic material via cell fusion. It is less well described in the literature compared to other HGT mechanisms [,,]. Membrane vesicles originating from Gram-positive bacteria are called membrane vesicles (MVs), and those originating from Gram-negative bacteria are called OMVs []. OMVs can act as gene transfer vectors. They can contain different types of genetic material such as plasmid DNA, chromosomal DNA, phage DNA, or RNA []. The way in which OMVs contribute to HGT has recently been reviewed []. OMV-mediated transfer has been shown for several biofilm-forming organisms, such as E. coli, Acinetobacter baylyi, P. aeruginosa, A. baumanii or Porphyromonoas gingivalis. A recent study has shown that OMVs secreted by K. pneumoniae can act as cargo for ARGs []. Two different β-lactam resistance plasmids were incorporated into OMVs and transferred among phylogenetically distant bacteria such as E. coli, S. enterica, P. aeruginosa, and B. cepacia. Furthermore, A. baumannii OMVs can secrete the plasmid-borne blaOXA-24 gene [] and E. coli OMV plasmids containing blaCTX-M-55 []. Little is known about how MV-mediated HGT occurs in Gram-positive bacteria [,,].
The extent to which OMV-mediated transfer contributes to the dissemination of ARGs in biofilms is not known as most studies on OMVs have been conducted on planktonic cultures.
OMVs have been shown to promote biofilm formation in Aeromonas spp. [], P. aeruginosa [,], Pseudomonas putida [], Helicobacter pylori [], and Vibrio cholerae []. On the other hand, several studies have demonstrated that OMVs and MVs can act as antibiofilm agents, such as Burkholderia thailandensis MVs for Streptococcus mutans biofilms []. P. aeruginosa OMVs were shown to be involved in growth inhibition of S. aureus in a dual-species biofilm [].
DNA encapsulated into OMVs is protected from exonuclease degradation, inactivation via freezing, and thermodegradation []. These environmentally inert vehicles are of particular interest for OMV-mediated HGT in biofilms and harsh environments such as hot springs or the Antarctic.
Another mode of HGT is mediated by nanotubes that were discovered in B. subtilis [,]. These membranous, elongated extracellular structures enable the connection of neighboring cells in proximity. This mediates the contact-dependent exchange of molecules between the cells. Their impact and physiological relevance in shaping genetic diversity in biofilms has not yet been explored.
HGT research originated in the late 1920s. When DNA was determined to be the source for transfer, the repertoire of methods to examine gene transfer expanded. HGT occurs within the same species, but also crosses phylogenetic barriers thereby setting no limits to acquiring resistance from phylogenetically unrelated bacteria from diverse environmental habitats [,]. The driving factors of HGT are mobile genetic elements (MGEs) such as plasmids, ICEs, integrons, or transposons [,,,]. As most bacteria live in biofilms in nature, it seems reasonable that HGT occurs more frequently in biofilms than between planktonic cells [,].
1.5. Environmental Factors Influencing Biofilm Formation
Although implant materials are selected based on their optimal biocompatibility and treatment success, they unintentionally promote biofilm formation and can lead to biofilm-associated infections. The accumulation of biofilm producers and transmission of foodborne pathogens to humans via food-processing surfaces during processing, storage, production, or packaging are serious problems [].
Common materials for implants are metals such as stainless steel or titanium, ceramics, and polymers such as polyurethane or silicone. Surface parameters and material properties influence microbial adhesion [,,]. Modification of the surface topography [,] and development of antimicrobial implant coatings [,] are efficient approaches for inhibiting biofilm formation and preventing biofilm-associated infections.
Several recent reviews have described further variables that influence biofilm formation in detail. Temperature, pH, water, nutrient and oxygen availability, ion content, protozoa, salinity, or exopolysaccharide release by OMVs all affect biofilm formation [,,,].
Biofilm formation is affected not only by environmental factors, but also by the EPS composition of biofilms. A study from Blanco et al. demonstrated that EPS composition is strongly influenced by environmental parameters and plays a critical role in ecological adaptation to extreme environments [].
Biofilms undergo shear stress and different fluid dynamics in the human body (veins, catheter environments) as well as in aquatic systems [,,]. Shear dynamics further influence the composition of multispecies biofilms [,].
Sub-minimum inhibitory antibiotic concentrations (sub-MICs) are concentrations lower than MIC values. They can lead to antibiotic-induced stress and promote biofilm formation [,].
1.6. Abiotic and Biotic Factors Influence Horizontal Gene Transfer in Biofilms
Plasmid transfer rates in biofilms are affected by environmental abiotic factors such as oxygen concentration and availability [,] or stimulatory effects of subinhibitory antibiotic concentrations [,]. Both the presence of mature biofilms [,] and cell density affect horizontal transfer of MGEs in biofilms.
In addition to abiotic factors, many biotic factors have been discussed to influence the spread: co-residing (conjugative) plasmids or virulence determinants in the donor or recipient cell [,], plasmid incompatibility [], or genetic compatibility of the MGE with the host.
Moreover, the copy number of the (conjugative) plasmid can be increased during biofilm growth compared to their planktonic counterparts, independent of their replicons and replication mechanisms [,]. Other parameters include host factors such as secretion of immunoglobulins or proteases for biofilms on eukaryotic tissues [], the taxonomic relatedness between donor and recipient [], and plasmid–donor–recipient-combinations []. In absence of selection, it has been shown that conjugative plasmids were retained for longer in biofilms compared to planktonic growth, and thus acted as plasmid sinks [,].
Conjugation can be affected by the biofilm composition and the design of the mating experiment: (I) only one strain (donor or recipient) is allowed to attach to the surface, and the second strain is added to the pre-developed biofilm layer [,] or (II) a defined mixture of donor and recipient strains is inoculated from the beginning [,,,,,]. In addition, there is no standard method for determining the conjugative transfer rate. Therefore, experimental results are difficult to compare. Furthermore, terminology varies greatly in terms of how the rate of HGT in biofilms is described. This review will use the following terms to quantify the gene spread via the HGT mechanisms, transformation, transduction, and conjugation: transformation rate, transduction rate, and conjugative transfer rate.
The determination of conjugative transfer rates can be biased by several experimental conditions such as the growth rate of donor and recipient strains, initial population densities, or donor-to-recipient ratio. To bypass biased experimental results through (a)biotic variations, a computational tool was developed to allow accurate and comparable measurements of conjugative plasmid transfer rates in liquid matings []. Developing similar mathematical models for determining conjugative transfer rates in biofilms remains a challenge. Ensuring comparability across experimental conditions should account for the spatial structure of biofilms.
1.7. State of the Art of Biofilm Studies
Our understanding of HGT in clinical and environmental settings is based primarily on in vitro studies. Single-species and planktonic cultures can be better cultivated under optimized in vitro laboratory conditions in broth or on agar surfaces. This kind of homogenous cultivation might not reflect natural features and has limitations, such as the lack of a three-dimensional architecture and high cell density of biofilms []. Another key difference between in vitro and in vivo models is the absence of a host immune system in in vitro biofilms []. Although the cultivation of bacterial colonies on agar plates should be much closer to biofilm growth, several conditions such as the absence of shear stress or the method of nutrient supply can fundamentally differ from those of natural biofilms.
Other mechanisms that might influence HGT in biofilms include (I) limited interactions between donor and recipient cells in a spatially structured population creating subpopulations, (II) regulatory control of the conjugative process, (III) environmental stressors (toxins, antibiotics, and UV irradiation), (IV) nutrient supply, and (V) cell density [].
As mixed-species biofilms and polymicrobial infections are much more common in nature [,,], biofilm studies with multiple species should be performed. Clinical isolates from medical devices have shown that biofilm infections are not only due to single species but also to polymicrobial colonization including even copresence of fungi and bacteria [].
A better understanding of HGT mechanisms in biofilms would provide more clarity regarding the rapid adaptation of pathogens to changing environmental conditions and the development of multi-resistant species.
However, researchers must be aware that in vitro models do not accurately mimic the human host or the natural environment. To date, most biofilm studies have been performed in vitro, owing to the possibility of high-throughput testing, easy setup, and low cost [,]. Several in vitro and in vivo models have been extensively reviewed [,,,]. Despite the availability of numerous methods for developing biofilm models, such as microtiter plate assays and static or fluid cultivation, mimicking complex biofilms remains technically challenging. The cultivation model of choice strongly depends on the research objective and what is intended to be simulated. When selecting a biofilm model, caution is needed when interpreting test results because of in vivo/in vitro differences in biofilm biology []. Furthermore, there is a risk of oversimplifying models when using standard growth media or abiotic surfaces [].
Biofilm characterization has mainly focused on clinical isolates or anthropogenic systems such as WWTPs. Studies on biofilms from natural environments have been mostly performed with environmental isolates rather than with in situ biofilms. Research on in situ and in vivo biofilms remains time-consuming and expensive. Some studies have used non-mammalian models as alternative hosts, such as Drosophila melanogaster [,] and Caenorhabditis elegans [] to investigate biofilm infections and intestinal colonization in vivo.
1.8. Culture-Independent Approaches
Most biofilm studies have been conducted using characterized laboratory strains. A stronger focus should be laid on plasmids and strains of both environmental and clinical origin. These studies could help to mimic clinical and environmental scenarios.
Not only cultivation-based approaches are required to study the mobilization and transfer of ARGs; culture-independent techniques, such as flow cytometry, allow the detection and quantification of transconjugants (recipients which have acquired a plasmid via conjugation) within biofilms [,,] without plate counting. Phenotypically trackable markers such as fluorescently labeled plasmids can be detected with flow cytometry or fluorescence microscopy.
Quantitative PCR (qPCR) is another classical culture-independent methodology to detect ARGs, their abundance, and distribution. However, it is not suited to determine transfer rates. Metagenomic data contribute to the prediction of HGT events in microbial communities []. These analyses can also bypass cultivation and provide an overview of the distribution and occurrence of ARGs, bacteria, and MGEs in a habitat of interest. The major bottleneck is the lack of reliable assignment of ARGs to their origin in natural communities (MGEs or bacterial chromosomes) when using standard metagenomic assemblies such as shotgun sequencing [,].
CRISPR–Cas spacer acquisition is another technique enabling real-time recording of HGT events at nucleotide resolution and thus, level of DNA acquisition []. Spacer acquisition describes the incorporation of small fragments of foreign DNA, known as spacers, into CRISPR loci []. An engineered E. coli recipient (“recording”) strain contains a plasmid with the cas1-cas2 operon whereby mobile DNA entering the cell is captured and incorporated into the CRISPR array. Transient HGT events such as those lacking replication of MGEs or those with low transfer frequencies can be studied using this approach. However, the recording system is still restricted to E. coli.
The in vivo proximity-ligation method Hi-C [] enables the correct linkage between MGEs, such as plasmids and phages, to their hosts. Thus, the in situ host ranges of MGEs and the origin of ARGs can be tracked in environmental microbial communities without cultivation [,]. It would be very beneficial to understand to which hosts or MGEs ARGs belong to, and which plasmids interact with which hosts. Hi-C metagenomics broadens our knowledge of plasmid–host interactions in large, natural communities [,].
On this basis, combinations of microbial cultivation, in vivo studies with Drosophila or C. elegans, and in situ biofilm studies can contribute to a more complete understanding of HGT in biofilms.
The following chapters focus on the three main HGT mechanisms—transformation, transduction, and conjugation—and how they have been examined in biofilms. Figure 1 illustrates these three main HGT mechanisms occurring in polymicrobial biofilms.
2. Transformation
Natural transformation (also known as natural competence) was discovered in 1928 as the first mechanism of HGT which does not rely on MGEs. Transformation largely depends on the uptake and incorporation of exogenous naked DNA from the environment into the genomes of competent recipient organisms [,]. Internalized genetic material is permanently incorporated into the bacterial chromosome by homologous recombination or serves as a nutrient source, bypassing de novo biosynthesis of nucleotides []. Competence is a developmental state that occurs during specific growth phases, except for H. pylori and Neisseria spp., which are likely to be constitutively competent []. Cells can stop growing (growth arrest) when in a competent state and express proteins for DNA import [,].
In contrast to conjugation and transduction, transformation relies only on the recipient bacterium that expresses the competence machinery (Figure 1). Competence regulation is based on several conserved competence-inducing genes and quorum-sensing (QS) systems involved in exogenous DNA uptake and integration into the chromosome [,,]. Fluorescence-based and qPCR-based studies have shown that competence genes are upregulated in S. aureus [] and Streptococcus pneumoniae [,] under biofilm growth conditions compared with broth-grown bacteria.
Most transformable bacteria, both Gram-negative and Gram-positive, require type II-related secretion systems and type IV-like pilus structures for single-strand DNA (ssDNA) transport across the bacterial membrane [,].
Few transformation experiments have been conducted in biofilms compared with planktonic cultures. In the following sections, the methods by which transformation has been measured in biofilms are highlighted.
In 2017, the World Health Organization (WHO) published a list of priority pathogens for which the development of new antibiotics or other defense strategies is urgently required. The majority of these species are naturally competent [,]. Therefore, transformation can be a powerful tool for the distribution of ARGs. Natural transformation occurs in many bacteria (to date 82 species are known), including severe human pathogens such as S. pneumoniae, V. cholerae, and H. pylori []. Interestingly, naturally non-transformable pathogens, or pathogens for which natural transformation has not been demonstrated so far, can carry competence-related genes, such as streptococcal species other than S. pneumoniae [], and L. monocytogenes [,]. However, transformation conditions and inducers of competence have not yet been elucidated. Thus, it is likely that the actual number of competent species is even higher.
Common model species used to study transformation are V. cholerae, N. gonorrhoeae, Haemophilus influenzae, H. pylori, Acinetobacter spp., and Gram-positive bacteria such as B. subtilis and S. pneumoniae [].
2.1. Transformation of Gram-negative Bacteria in Biofilms
V. cholerae has long been considered as non-competent. The main niche for V. cholerae is the aquatic environment. It is often found in association with chitinous zooplankton, which induces competence in aquatic ecosystems. Therefore, this species can develop competence under special conditions when grown on chitin, as has also been shown for biofilms [,,]. In all studies, V. cholerae cells were grown on sterile crab shell fragments submerged in artificial seawater. However, the cells were detached after incubation to determine the transformants on selective agar plates.
Methodology to Assess the Transformation Rate in Biofilms
A continuous flow chamber was used to co-culture two N. gonorrhoeae strains as a biofilm, and the transformation efficiency of chromosomally-encoded ARGs was compared to that of planktonic cells []. Transformation efficiency was higher in early biofilms than in planktonic cells but decreased in late biofilms. Cell aggregates were disrupted after biofilm removal from the chamber to determine the transformants on selective agar plates.
P. aeruginosa is known for its high genetic diversity and multidrug resistance. It has been recently shown that it can take up either genomic or plasmid DNA by natural transformation [] in static broth assays and continuous flow biofilms []. Pioneering work by Hendrickx and coworkers proved natural competence in A. baylyi ADP1 (formerly named Acinetobacter sp. BD413) grown as monospecies biofilms in flow cells []. To study transformation, the gfp-carrying plasmid pGAR1 was used as the transforming DNA. In contrast to other studies, transformants were microscopically monitored in biofilms using GFP fluorescence and the nucleic acid stain Syto 60. Perumbakkan and coworkers compared the transformation of naturally competent single-species A. baylyi ADP1 biofilms with that of a soil biofilm community []. To this end, the broad-host-range cloning vector pBBR1MCS5 [] was modified to carry the GFP reporter gene gfpmut2 and an atrazine chlorohydrolase (atzA) gene for degradation of the herbicide atrazine. They examined the uptake and expression of the functional degradative gene in Acinetobacter biofilms in vivo as a potential technique for pollutant removal from bioreactors and aquifers. The biofilm was directly stained in the flow cell using Syto 60 and further examined using CLSM. Transformation frequency was determined by counting the total number of cells (fluorescence of Syto 60) and transformed cells (GFP fluorescence). The transformation frequencies obtained from both pure Acinetobacter biofilms and soil biofilms differed, depending on the biofilm sector corresponding to different biofilm heights. The authors assumed that biofilm height and shear forces influence transformation efficiency. The correlation between different biofilm metrics, such as density, cellular architecture, or EPS architecture (e.g., porosity or biovolume) and transformation frequency was demonstrated for monocultured A. baylyi BD4 and ADP1 biofilms under continuous flow conditions []. The biofilms were examined by CLSM for transformants using biofilm staining and GFP fluorescence (gfp-labeled non-conjugative pGAR38 plasmid as transforming DNA).
Santala et al. used a minimally invasive bioluminescence monitoring tool as a visualization technique for in situ transformations []. A. baylyi ADP1 was statically incubated in microtiter plates to obtain biofilm-like structures. Bioluminescence detection of transformation was performed using the bacterial luciferase operon of Photorhabdus luminescens. A replicative plasmid carrying the luxCDABE operon was used as DNA to be transformed. Homologous recombination was detected in a strain expressing luxCDE and introducing a non-replicative plasmid carrying an integrative luxAB gene cassette.
E. coli has to be artificially induced by electroporation or preparation of membranes for DNA uptake by treatment with calcium chloride or polyethylene glycol [,]. It was long thought that this species does not possess natural competence. However, several studies have shown natural competence in E. coli planktonic cells and E. coli biofilms under specific conditions [,,,,].
2.2. Transformation of Gram-positive Bacteria in Biofilms
B. subtilis is one of the best-studied Gram-positive organisms and an important model system for studying biofilms []. However, only a few studies have examined the transformation of B. subtilis in biofilms. Reduced competence has been observed in static biofilms of environmental B. subtilis strains [].
Most biofilm transformation studies have been conducted using streptococcal biofilms. The natural habitats of streptococci are multispecies biofilms on mucosal surfaces of humans and animals. Therefore, they are clinically relevant. The main colonizers of biofilms formed on dental surfaces belong to the Streptococcus mitis group, including Streptococcus oralis, Streptococcus gordonii, Streptococcus sanguinis and S. mitis, resulting in multispecies biofilms []. S. pneumoniae and S. aureus are common co-colonizers in the nasopharynx and oropharynx of humans [,,], suggesting natural transformation in these environments. The potential of gene transfer by transformation among oral bacteria was demonstrated in static biofilms, composed of naturally transformable S. gordonii with either naked plasmid DNA or the plasmid-harboring spirochete Treponema denticola as a model exchange system []. The transformation rates of S. gordonii were determined by scraping off the biofilm and selecting transformants on antibiotic-containing agar plates.
The natural transformation efficiency of pneumococcal strains has been studied during murine nasopharyngeal co-colonization by multiple strains and with static in vitro biofilm models [].
To study nasopharyngeal recombination upon ARG transformation, the human nasopharynx was simulated in a vertical diffusion chamber (bioreactor chamber) []. These chambers with pharyngeal cells were inoculated with the two pneumococcal strains to create biofilm consortia, or single-species biofilms as controls. The strains chromosomally encoded different single resistance genes. The rapid development of double antibiotic-resistant colonies was detected after 4 h of incubation within the pneumococcal biofilm consortia.
Although the natural competence of S. mutans planktonic cultures was demonstrated in 1981 [], it took 20 years to demonstrate competence development in S. mutans biofilms []. Six S. mutans strains grown in biofilms showed 10- to 600-fold higher transformation efficiencies than their planktonic counterparts grown in liquid cultures. The natural transformation of Streptococcus pyogenes biofilms grown on epithelial cells has been demonstrated in vitro and in vivo during nasopharyngeal colonization in mice [].
It was unclear for a long time whether S. aureus could develop natural competence. A decade ago, it was shown that a subpopulation expressing the secondary sigma factor SigH is capable of transformation under planktonic conditions []. However, this kind of competence requires both SigH and the SigH-dependent operons comG and comE as well as specific laboratory conditions for sigH expression. This suggests a need for highly specific environmental conditions for natural transformation. The development of natural transformation in static S. aureus biofilms was demonstrated by the transfer of the staphylococcal cassette chromosome (SCC) gene SCCmec []. Phage-dependent and conjugative transfer were excluded using phageless and heat-killed donor cells, which were unable to synthesize the conjugation apparatus.
Higher Transformation Rates in Early Biofilms Than in Planktonic Cultures
In summary, the higher transformation rates in biofilm communities, particularly in early biofilms, compared to planktonic cells, contribute to the spread of ARGs within biofilms. As biofilms spontaneously release planktonic cells during detachment, this could allow the dissemination of transformants to a broader range of habitats []. Diverse environmental and cellular cues trigger regulation of gene expression in biofilms and biofilm architecture. Multiple mechanisms and laboratory parameters (amount of exogenous DNA, choice of media, incubation time, temperature, cell density, species composition, and microbial physiological state) can induce transformation in biofilms.
Furthermore, although transformation has been studied in biofilms, in most studies, the transformation rate has not been determined in situ. Biofilms were disrupted instead (scraped or enzymatically removed) to enumerate transformants on selective agar plates [,,,,,,,,,]. The extent to which this distorted the actual transformation rate is unclear.
3. Transduction
Bacteriophages (phages) are viruses that infect bacteria. Transduction is the phage-mediated transfer of DNA to bacterial cells. As they are natural enemies of bacteria, they are found everywhere and are therefore widely present in natural environments. Furthermore, phages have a longer persistence compared to bacteria, and are not easily degradable under environmental conditions. They can tolerate acids, alkaline conditions or high temperatures [] and cannot be completely eradicated by UV irradiation or chlorination [,]. The long-term coevolution of phages and bacteria in nature has led to complex and diverse bacteria–phage interactions and mutualistic adaptations.
Phages are non-living biological entities that exploit the replication machinery of the host to produce phage progeny. Phage-mediated transduction is initiated after phage attachment and injection of phage DNA into the bacterial cytoplasm. Lytic (virulent) phages start directly with reproduction, whereas lysogenic (temperate) phages can first integrate their genome into the host chromosome (prophage) before the lytic cycle is initiated. Bacterial DNA injected by phages must be either integrated by homologous recombination into the host chromosome or maintained as a self-replicating plasmid [].
Phages and prophages also encode ARGs [,,]. To discuss ARG transfer by transduction within biofilms, three different transduction mechanisms should be considered in more detail: generalized, specialized, and lateral transduction.
3.1. Generalized Transduction
The lytic cycle degrades bacterial DNA into smaller fragments. Generalized transduction is based on erroneous packaging by pac-type phages. Phages wrongly recognize pac-site homologs (pseudo-pac sites) encoded on heterogenous DNA, resulting in the mispackaging of random segments of bacterial DNA (plasmid or chromosomal DNA) into capsids instead of phage DNA [,,]. The transfer of ARGs by generalized transducing phages has been examined in several planktonic studies, but not in biofilms [,,].
3.2. Specialized Transduction
Specialized transduction occurs when the phage genome is incorrectly excised, together with the host DNA flanking the integrated prophage []. Hence, non-phage and viral DNA are erroneously encapsidated into the phage and can be transferred to a new host cell (Figure 1).
Both transduction mechanisms provide opportunities for acquiring ARGs, but with specialized transduction the variety of bacterial genes to be excised or randomly packaged is rare and limited by genes directly adjacent to the phage integration locus. Specialized transduction is probably only a minor contributor to gene (ARG) transfer compared to generalized transduction [].
3.3. Lateral Transduction
One recently discovered mechanism is lateral transduction. The prophage is still integrated into its host chromosome, where bidirectional phage replication begins in situ. This leads to DNA encapsidation prior to excision, and replication of long fragments of adjacent bacterial DNA []. Phages are packed with both phage DNA and adjacent bacterial genes. Up to 100 kb of bacterial DNA downstream of the prophage can be encapsidated []. The immense additional excision of genes may offer potential transfer routes for resistance genes. In contrast to specialized transduction, lateral transduction is not based on incorrect excision but rather on another natural mode of phage transduction. Lateral transduction was first shown for lysogenic S. aureus phages [], but also for P. aeruginosa [], and S. enterica []. All examined strains were characterized as biofilm formers, so it can be assumed that this transduction mechanism takes place in biofilms as well. To the best of our knowledge, no biofilm studies on lateral transduction have been published so far.
3.4. Lack of Transduction Experiments in Biofilms
Transduction experiments have commonly been performed using planktonic cultures with characterized environmental, clinical, or laboratory strains [,,,]. To the best of our knowledge, only one study has demonstrated phage-mediated gene transfer through lysogenic bacteriophages in biofilms of potentially pathogenic E. coli []. In this study, recipient biofilms were formed on glass slides under static conditions. The donor strain carrying a Shiga-toxin (Stx)-encoding bacteriophage was added to the predeveloped recipient biofilm and both were incubated together. The biofilm was scraped off, disrupted, and plated onto selective agar to enumerate the transductants. The number of transductants increased over time, and donor cells were no longer detectable after six days of cultivation. The authors hypothesized that donor cells were lysed after entering the recipient biofilm. This study demonstrated that gene transfer via transduction can occur in biofilms.
3.5. Potential of Phages in ARG Dissemination
3.5.1. Occurrence of Phages in Aquatic Environments
Because phages are non-motile, bacterial host infections are mainly based on the passive diffusion of phages within biofilms [,]. The transduction of DNA, and thus ARGs, is further limited by their non-motility. The total number of phage particles on Earth is assumed to be close to 1031 [,]. Phage density is estimated to be approximately 106/mL in coastal marine waters []. Infecting bacterial hosts in ecological niches, such as aquatic environments, is feasible because of high phage concentrations. Furthermore, it has been recently shown that coliphages adsorbed onto the flagellated nonhost Bacillus cereus act as carriers for facilitated phage migration into aquatic E. coli biofilms []. Early in situ field studies have shown generalized transduction between P. aeruginosa strains in aquatic environments [,,,]. Accordingly, studies on the impact of phages on the spread of resistance genes are still of interest.
3.5.2. Phage Host Range and Its Contribution to the Spread of ARGs
Transduction events are limited by the species or even strain specificity of bacteriophages, which in turn makes the mechanism less important for the spread of ARGs than transformation and conjugation [,]. Nonetheless, phages represent vehicles for the dissemination of antibiotic resistance []. Furthermore, a few phages have been shown to infect a broad range of species []. Some phages even cross genera barriers [].
Studies on phages as ARG reservoirs are controversial, and phage-associated ARGs are assumed to be rare [,,]. On the other hand, phages recovered from various environments carry functional and novel ARGs. Therefore, they can represent reservoirs of ARGs which are disseminated via phage–host interactions [,,].
Phage-mediated transduction has been studied in detail in a wide range of genera, including Staphylococcus, Pseudomonas, Acinetobacter, Clostridium and Salmonella [,,,]. Transduction is assumed to be the driving force of HGT in S. aureus [,,,]. To the best of our knowledge, no studies on transduction events of clinically relevant Staphylococci, such as CoNS or S. aureus, in biofilms have been published to date.
3.6. Challenges of Studying Transduction in Biofilms
Biofilms are less susceptible to phage penetration than planktonic cultures because they have developed a broad spectrum of defense mechanisms against phage predation. Suitable characteristics and defense mechanisms to protect against phages include diffusion inhibition, biofilm thickness, metabolic state of bacteria (slow growth, low/no metabolic activity), biofilm maturity level, phage adsorption by the biofilm matrix, and phage-resistant/non-susceptible bacteria [].
The effects of phage-mediated transfer and phage-bacteria interactions in many ecosystems remain poorly characterized. It is assumed that transduction efficiencies in biofilms differ from those in planktonic cultures. Bacterial cultures in a well-mixed broth allow a random distribution. In biofilms, phages first adsorb to a single bacterium, and subsequently infect adjacent cells. However, it is less likely that they infect more distant bacteria []. Suitable biofilm models should be established to monitor transduction not only in planktonic cultures.
Many phage–host interactions have not been studied so far due to the non-cultivability of host bacteria and difficulties in phage isolation and detection [,]. Transduction should be detected not only by classical methods such as conventional plating using selectable genetic markers. State-of-the-art imaging techniques, such as epifluorescence microscopy or atomic force microscopy have been applied to detect and characterize uncultivated phages in environmental samples, as recently reviewed by Turzynski et al. [].
A labelling technique to quantify transduction at single-cell level is the so-called cycled primed in situ amplification-fluorescent in situ hybridization (CPRINS-FISH) [,]. This method combines the features of fluorescence in situ hybridization (FISH) and in situ PCR.
Although the range of methodologies has expanded over recent years, biofilm studies with phages have focused more on the potential of lytic bacteriophages as antibiofilm agents or as an alternative treatment of antibiotic-resistant bacterial infections [].
4. Conjugation
Conjugation is a major mechanism that facilitates bacterial adaptation by allowing the genetic exchange of ARGs, resistance to heavy metals, virulence, and other advantageous properties. It occurs at higher rates in biofilms than in planktonic cultures [,,]. In contrast to transduction and transformation, conjugation is based on direct cell-to-cell contact and mating pair formation between a donor and recipient cell established by adhesins in Gram-positive and conjugative pili in Gram-negative bacteria, as these two cell types fundamentally differ in their cell wall structure. A multiprotein complex spans the cell envelope and mediates unidirectional DNA transfer from a donor to a recipient cell. The conjugation process is mediated by MGEs such as conjugative plasmids, integrative and conjugative elements (ICEs), or transposons. Gram-negative conjugation has been studied in more detail [,,,,]. Several techniques are available to track the transfer of MGEs in microbial biofilm communities and planktonic cultures, which are addressed in the following paragraphs. Demonstrating conjugative transfer with planktonic cultures prior to biofilm studies can serve as a reference for transconjugant detection and assessment of MGE transferability. Mating assays are conventionally performed to verify the conjugative transfer of MGEs. They are mostly performed as liquid, solid, or filter mating assays. Conjugative transfer is experimentally measured as the transfer rate (transconjugants per donor or recipient cell) [,,,,,,]. Solid and filter matings seem to be the most suitable methods, resulting in higher transfer rates in comparison to liquid mating [,,,]. This could be explained by the similarity to natural conditions using solid surfaces, increased heterogeneity, and spatial structures for surface-associated microbes and, therefore, microbial communities. However, in vitro mating assays are insufficient to fully understand in vivo circumstances within natural biofilms and possess certain limitations in determining the natural transfer rate of MGEs.
Most conjugation studies have been performed with plasmids, but in recent years, other MGEs, such as ICEs and integrons, have also come into focus. The following chapter points out the current knowledge about conjugation in biofilms referring to different conjugative elements.
Integrons and genomic islands (chromosomal, resistance, or pathogenicity islands) also contribute to the distribution of ARGs via HGT [,,,]. These elements are not covered in detail here due to scarce data on biofilm studies. This chapter focuses on conjugative plasmids and ICEs instead.
4.1. Conjugative Plasmids
Plasmids autonomously replicate as extra-chromosomal elements that do not carry genes that are essential for bacterial survival. Conjugative plasmids harbor genes for a T4SS. Mobilizable (non-conjugative) plasmids do not encode the entire conjugation machinery. Instead, they require conjugative elements in the same cell to be transferred to a new host []. They carry at least an origin of transfer (oriT) sequence and a gene encoding the relaxase protein, which nicks the plasmid DNA at the oriT site, thus initiating the transport of ssDNA to the T4SS provided by a conjugative plasmid []. Plasmids carry not only transfer genes but are important vehicles for single or multiple ARGs even against “last resort antibiotics” [,,,]. Plasmids can naturally transfer between different genera or phyla [,,]. Figure 1 shows conjugative plasmid transfer for both Gram-negative and Gram-positive T4SS.
Thus, the host range of conjugative plasmids greatly contributes to the spread of ARGs. As many broad-host-range conjugative plasmids are of clinical origin [,,,], their release and distribution in the environment should be monitored.
Even though studying HGT in microbial communities can be less invasive, biofilms are often disrupted for further analyses such as staining and subsequent visualization or determination of conjugative transfer rates by plating []. The transfer of the broad-host-range plasmid RP4 was demonstrated in a mono-species biofilm of Pseudomonas strains by cultivating them in a rotating annular reactor (bioreactor) []. The conjugative transfer rate was determined with plating of disrupted biofilm samples. Another study used four different growth systems (three types of flow cells and a biofilm reactor) for cultivation and plate counting to quantify IncP-1 pB10 plasmid transfer in E. coli biofilms []. However, transfer rates in detached and disrupted biofilms are not comparable to those in intact biofilms. The location of transfer events can also not be determined. In situ biofilm formation is a promising variant to overcome many obstacles in the experimental design of environmental biofilm studies. However, it is often not used due to the experimental complexity and the use of invasive detection methods. Even when conjugation experiments occur under biofilm conditions, many researchers still use plates to enumerate transconjugants [,,,,,,,].
Methodology to Assess Conjugative Plasmid Transfer Rates in situ
Beaudoin et al. demonstrated that the conjugative transfer rate can be determined in situ []. After biofilm cultivation in a bioreactor, cryo-embedded and cryo-sectioned biofilm slides were stained to microscopically visualize donors and transconjugants expressing β-galactosidase (from a lacZ-containing mobilizable plasmid). Transconjugants were further distinguished by their relative cell sizes, with P. putida as the donor and Bacillus azotoformas as the recipient. The number of transconjugants was given per unit biofilm surface [cells/m2/h].
CLSM can be used as a non-invasive technology to directly monitor HGT in biofilms [,,,,,,,]. The milestone in monitoring conjugation in complex microbial communities was the use of a single GFP reporter as a fluorescent plasmid marker and chromosomally encoded luciferase genes (bioluminescence) for recipient labelling []. In this study, conjugative dissemination of the TOL plasmid was not investigated in biofilms, but on nutrient agar plates. In other studies, GFP expression was repressed in P. putida donor cells carrying a reporter plasmid whose fluorescence was repressed by a chromosomal repressor (lacIq). Therefore, only transconjugant cells were fluorescent [,,]. In situ determination of conjugative transfer rates in defined biofilms with GFP labelling was performed by Hausner and Wuertz []. Transfer rates were given as ratios of transconjugants to recipient cell per hour of contact time [number of transconjugants/recipient cell/h]. The conjugation rates were 1000-fold higher than those determined by solid surface mating.
An optimized technique for studying conjugation in situ is a dual-label monitoring tool that uses fluorescent reporter genes without any additional stains [,]. This technique was utilized to study environmental communities, such as wastewater and soil. The expression of two fluorescent proteins enabled tracking of the donor (chromosomal dsRed gene) and the conjugative TOL plasmid (gfp gene) for in situ monitoring of plasmid transfer in biofilm reactors [,,]. Klümper and coworkers used a donor chromosomally tagged with a constitutive red fluorescence reporter gene and a plasmid of interest encoding gfp. The GFP expression was controlled by a lacIq-repressible promoter and LacI production in the donor []. Due to the absence of a lacIq-repressible promoter in the recipients, gfp expression was restored. In this study, E. coli, P. putida and Kluyvera sp. strains were used as donors and a soil microbial community as recipients. Thus, the differentiation of donor, recipient, and transconjugant cells is highly specific.
There exist several challenges when applying the presented methods to biofilms: Bacteria within natural biofilms underlie local gradients of abiotic factors (nutrients, oxygen, pH, and waste products) []. GFP requires oxygen for maturation and activation [,]. With increasing biofilm depth or high density in biofilms, interior oxygen availability decreases and thus impairs GFP expression []. Anaerobic liquid cultures of E. coli were subjected to varying acidic pH values and simultaneously used for the anaerobic expression of fluorescent proteins mCherry and GFPmut3 []. The fluorescence of both proteins was completely recovered after exposure to oxygen; this is the so-called aerobic fluorescence recovery. At pH <5 only the fluorescence of GFPmut3 was fully recovered. Low pH values might lead to protein degradation and thus irreversible quenching of fluorescence.
A promising platform for studying HGT in biofilms is microfluidics. The technique allows on-chip mating assays for plasmid-mediated conjugation in biofilms at a microscale level. This method enables long-term cultivation, time-lapse and real-time tracking of transfer processes, transconjugant formation even at the single-cell level, and real-time imaging of in situ biofilm formation [,]. In the study of Li and coworkers, the E. coli donor strain carried the GFP-encoding IncP-1 plasmid pKJK5 with dual-label fluorescence []. The donor was cultivated together with either activated sludge community recipients or single-strain recipients on the chip. Microfluidics can mimic natural biofilms more promisingly and lead to a better understanding of the environmental influences on HGT compared to end-point measurements from solid surface matings [,,]. Conjugation of the fluorescently labeled broad-host-range IncP-1 plasmid RP4 has been monitored in real time in single-species and mixed environmental biofilms using microfluidics [,,].
Fluorescent labeling methods can be combined to specifically detect donors, recipients and transconjugants. In contrast to in vitro HGT determination, time-lapse imaging using microfluidics can further distinguish between vertical transmission and HGT. Randomly appearing GFP spots can be described as primary HGT events, the fluorescence extension from these spots is due to cell division [].
However, there are also some drawbacks of the system: potential instability of GFP-encoding plasmids in transconjugants or repression of gfp expression by the natural lac operon in E. coli recipients [] or autofluorescence of uncharacterized recipients [] cannot be excluded.
4.2. Integrative and Conjugative Elements (ICEs)
ICEs, formerly termed conjugative transposons, are MGEs that encode genes for their own excision, conjugation, and integration []. ICEs are mostly hidden in bacterial chromosomes. Thus, they are passively inherited vertically but can move horizontally by conjugation via self-encoded T4SSs between donor and recipient strains (Figure 1). Other MGEs, such as integrative and mobilizable elements (IMEs), encode genes for their excision and integration. Due to the lack of genes for conjugative transfer, IMEs must hijack the conjugative apparatus of co-residing self-transmissible elements, such as ICEs. IMEs carry ARGs and may play a major role in the dissemination of ARGs [,]. Identifying and annotating new putative ICEs and IMEs in bacterial chromosomes in silico remains challenging [,]. Nonetheless, ICEs and IMEs have been suggested to be more phylogenetically widespread than conjugative plasmids [,,].
As IMEs are rarely described, no HGT studies in biofilms exist to date.
ICEs can exist in circular extrachromosomal forms upon excision and transfer to a new host (Botelho, 2020). However, they are not stable as an extrachromosomal intermediate. They replicate only when integrated into the host chromosome via site-specific recombination between defined attachment (att) sites []. The ICE life cycle, excision, and integration have been extensively reviewed in recent years [,,]. ICEs often carry cargo genes including metabolic pathways, determinants of pathogenesis and symbiosis, and ARGs [,,].
There is little knowledge on ICEs compared to plasmids. A review from 2020 compared PubMed searches with combined keywords such as “antibiotic resistances” AND “integrative conjugative element” or AND “conjugative transposon” with that of “plasmid” AND “antibiotic resistance” []. Only 2–7% hits were found compared to a search combining the keywords “antibiotic resistance” AND “plasmid”. There are just a handful of studies that investigated ICEs in biofilms. Nevertheless, multiple studies have demonstrated ICEs as the main reservoir for several resistance genes in biofilm-producing species [,,,,].
Evolutionary dynamics and the mechanism of ICE conjugation remain largely unexplored []. Although many potential ICEs have been found in recent decades, only a few well-characterized ICE models are available to study propagation and transfer. These include B. subtilis ICEBs1 [], V. cholerae ICESXT-R391 [], E. faecalis Tn916 [], Bacteroides thetaiotaomicron CTnDOT [], Pseudomonas knackmussii ICEclc, Streptococcus thermophilus ICESt1/3 [,], and Mesorhizobium loti ICEMlSymR7A []. The genetic organization and main regulatory networks controlling ICE transfer were reviewed in detail in 2017 []. To improve their trackability, resistance markers have been inserted into ICESt1 and ICESt3 [] as well as into ICEBs1 [,].
Methodology to Assess ICE Transfer Rates in Biofilms
Conjugation studies in biofilms have only been performed with ICEBs1. They were compared with solid surface matings. The donor–recipient mixture was incubated on biofilm-stimulating growth medium to induce biofilm formation. The same experimental setting has been used in several ICEBs1 studies [,,]. In comparison to non-inducing media, a 100- to 10,000-fold increase in ICE transfer was demonstrated []. In addition, ICEBs1 transfer frequency was increased by extracellular matrix production by the recipient cells.
ICE dissemination in B. subtilis biofilms was analyzed by cryomicrotome and fluorescence microscopy []. High ICEBs1 transfer rates were almost exclusively driven by transconjugants as secondary transfer events. HGT was mainly mediated by transconjugants in close vicinity to initial donor cells, in so-called conjugative clusters, located close to the air–biofilm interface.
It is often not possible to determine exactly the impact of vertical transfer on the dissemination of MGEs within the biofilm. To this end, Bourassa et al. [] deleted an essential gene of the ICEBs1 T4SS machinery. The knockout was only complemented in trans in the donor. Thus, transconjugants cannot transfer ICEs via conjugation.
As in the conjugation experiments of Klümper et al. [], fluorescence of transconjugants was controlled by the Lac repressor. Donor–recipient mixtures were cultivated on agarose slices to facilitate mating. ICEBs1 transfer in B. subtilis cells was visualized in real time by fluorescence microscopy []. Red fluorescence was expressed in donor cells. GFP expression was repressed by fusing the gfp gene to the E. coli Lac repressor lacIq (LacI-GFP) inserted into ICEBs1. Thus, B. subtilis cells which had acquired ICEBs1 showed green fluorescence. B. subtilis cells grow naturally in chains. The authors demonstrated that rapid ICEBs1 spread among cells within the bacterial cell chain occurred via ICE conjugation.
To follow ICE transfer in real time, this fluorescent method could be applied to biofilm models, such as flow chambers or microfluidics. In comparison to plasmids, more extensive studies are needed to better validate the role of ICEs in biofilms.
5. Conclusions and Perspectives
This review aimed to provide deeper insights into the ability of pathogens to acquire ARGs and adapt to changing environments. Environmental and clinical biofilms are reservoirs of ARG-encoding bacteria. They are a serious threat to public health, as they can intervene with the treatment of infectious diseases. HGT mechanisms, such as transformation, transduction, and conjugation, shape the genetic diversity of bacteria and contribute to their rapid adaptive evolution. Mimicking biofilm conditions facilitates studying the spread of ARGs across different ecological systems. HGT experiments in biofilms are still underrepresented as biofilms are highly complex structures that are difficult to mimic under laboratory conditions. Thus, multiple approaches are required to study horizontal gene spread in biofilms. Most biofilm studies still use invasive methods to determine HGT rates. Coupling classical methods with in situ techniques such as CLSM or microfluidics would immensely enhance the knowledge of gene spread in complex environments. The use of modern and more accurate methods such as Hi-C or CRISPR–Cas spacer acquisition can help overcome the insufficient linkage of MGEs to their hosts and the lack of detection of transient HGT events. In addition, coupling biofilm studies with the application of modern HGT detection and quantification methods would help identify risk scenarios to develop intervention strategies against ARG spread.
The pool of MGEs encoding ARGs is immense. However, the transfer features of OMVs, MVs, ICEs, IMEs, integrons and other mobile gene cassettes are not as well characterized as those of conjugative plasmids. The dissemination potential of these mobile elements in heterogeneous environments such as biofilms should not be underestimated and should be studied to a greater degree in the future.
In summary, biofilms should be an important target for regular monitoring and control of the occurrence and dissemination of ARGs in clinical settings, industry, and the environment.
Author Contributions
Conceptualization, CM. and E.G.; writing, original draft preparation, C.M.; review and editing C.M. and E.G.; supervision E.G. All authors have read and agreed to the published version of the manuscript.
Funding
The Berliner Hochschule für Technik, Berlin, Germany, funded the doctoral thesis of C.M. This research was funded by the DLR (German Aerospace Center), grant number 50WB2033 to E.G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Kaushik Biswas for the initial draft of some sections of the manuscript. We thank the many scientists in the HGT field for their valuable contributions, and we apologize for omissions of published work due to constraints on page limitations.
Conflicts of Interest
We declare no conflict of interest.
References
- Rieu, A.; Aoudia, N.; Jego, G.; Chluba, J.; Yousfi, N.; Briandet, R.; Deschamps, J.; Gasquet, B.; Monedero, V.; Garrido, C.; et al. The biofilm mode of life boosts the anti-inflammatory properties of Lactobacillus. Cell Microbiol. 2014, 16, 1836–1853. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Borsodi, A.K.; Anda, D.; Makk, J.; Krett, G.; Dobosy, P.; Büki, G.; Erőss, A.; Mádl-Szőnyi, J. Biofilm forming bacteria and archaea in thermal karst springs of Gellért Hill discharge area (Hungary). J. Basic Microbiol. 2018, 58, 928–937. [Google Scholar] [CrossRef]
- Kochetkova, T.V.; Toshchakov, S.V.; Zayulina, K.S.; Elcheninov, A.G.; Zavarzina, D.G.; Lavrushin, V.Y.; Bonch-Osmolovskaya, E.A.; Kublanov, I.V. Hot in Cold: Microbial Life in the Hottest Springs in Permafrost. Microorganisms 2020, 8, 1308. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Savio, D.; Stadler, P.; Reischer, G.H.; Kirschner, A.K.T.; Demeter, K.; Linke, R.; Blaschke, A.P.; Sommer, R.; Szewzyk, U.; Wilhartitz, I.C.; et al. Opening the black box of spring water microbiology from alpine karst aquifers to support proactive drinking water resource management. WIREs Water 2018, 5, e1282. [Google Scholar] [CrossRef]
- Pandit, A.; Adholeya, A.; Cahill, D.; Brau, L.; Kochar, M. Microbial biofilms in nature: Unlocking their potential for agricultural applications. J. Appl. Microbiol. 2020, 129, 199–211. [Google Scholar] [CrossRef]
- Sjöberg, S.; Stairs, C.; Allard, B.; Hallberg, R.; Homa, F.; Martin, T.; Ettema, T.J.G.; Dupraz, C. Bubble biofilm: Bacterial colonization of air-air interface. Biofilm 2020, 2, 100030. [Google Scholar] [CrossRef]
- Paytubi, S.; Cansado, C.; Madrid, C.; Balsalobre, C. Nutrient Composition Promotes Switching between Pellicle and Bottom Biofilm in Salmonella. Front. Microbiol. 2017, 8, 2160. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Baveye, P.; Neu, T.R.; Stoodley, P.; Szewzyk, U.; Wingender, J.; Wuertz, S. Who put the film in biofilm? The migration of a term from wastewater engineering to medicine and beyond. NPJ Biofilms Microbiomes 2021, 7, 10. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Motta, J.-P.; Wallace, J.L.; Buret, A.G.; Deraison, C.; Vergnolle, N. Gastrointestinal biofilms in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 314–334. [Google Scholar] [CrossRef]
- Mishra, S.; Huang, Y.; Li, J.; Wu, X.; Zhou, Z.; Lei, Q.; Bhatt, P.; Chen, S. Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants. Chemosphere 2022, 294, 133609. [Google Scholar] [CrossRef]
- Hayta, E.N.; Ertelt, M.J.; Kretschmer, M.; Lieleg, O. Bacterial Materials: Applications of Natural and Modified Biofilms. Adv. Mater. Interfaces 2021, 8, 2101024. [Google Scholar] [CrossRef]
- Little, B.J.; Blackwood, D.J.; Hinks, J.; Lauro, F.M.; Marsili, E.; Okamoto, A.; Rice, S.A.; Wade, S.A.; Flemming, H.-C. Microbially influenced corrosion—Any progress? Corros. Sci. 2020, 170, 108641. [Google Scholar] [CrossRef]
- Curtin, A.M.; Buckley, H.L. Biofouling detection methods that are widely applicable and useful across disciplines: A mini-review. Biofouling 2021, 37, 494–505. [Google Scholar] [CrossRef]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef]
- Bahamondez-Canas, T.F.; Heersema, L.A.; Smyth, H.D.C. Current Status of In Vitro Models and Assays for Susceptibility Testing for Wound Biofilm Infections. Biomedicines 2019, 7, 34. [Google Scholar] [CrossRef]
- Vyas, H.K.N.; Xia, B.; Mai-Prochnow, A. Clinically relevant in vitro biofilm models: A need to mimic and recapitulate the host environment. Biofilm 2022, 4, 100069. [Google Scholar] [CrossRef]
- Dunny, G.M.; Hancock, L.E.; Shankar, N. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection: Enterococcal Biofilm Structure and Role in Colonization and Disease; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef]
- Sauer, K.; Camper, A.K.; Ehrlich, G.D.; Costerton, J.W.; Davies, D.G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002, 184, 1140–1154. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.K.; Periasamy, S.; Mukherjee, M.; Xie, C.; Kjelleberg, S.; Rice, S.A. Biofilm development and enhanced stress resistance of a model, mixed-species community biofilm. ISME J. 2014, 8, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Kviatkovski, I.; Mamane, H.; Lakretz, A.; Sherman, I.; Beno-Moualem, D.; Minz, D. Resistance of a multiple-isolate marine culture to ultraviolet C irradiation: Inactivation vs biofilm formation. Lett. Appl. Microbiol. 2018, 67, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Costa-Orlandi, C.B.; Bila, N.M.; Vaso, C.O.; da Silva Pires, A.C.M.; de Matos Silva, S.; Medina Alarcón, K.P.; Marcos, C.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Polymicrobial biofilms: Impact on fungal pathogenesis. In Understanding Microbial Biofilms; Elsevier: Amsterdam, The Netherlands, 2023; pp. 521–567. ISBN 9780323999779. [Google Scholar]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Mlynek, K.D.; Hettiarachchi, H.; Alamneh, Y.A.; Biggemann, L.; Zurawski, D.V.; Black, C.C.; Bane, C.E.; Kim, R.K.; Granick, M.S. Extracellular polymeric substance (EPS)-degrading enzymes reduce staphylococcal surface attachment and biocide resistance on pig skin in vivo. PLoS ONE 2018, 13, e0205526. [Google Scholar] [CrossRef]
- Hernández-Jiménez, E.; Del Campo, R.; Toledano, V.; Vallejo-Cremades, M.T.; Muñoz, A.; Largo, C.; Arnalich, F.; García-Rio, F.; Cubillos-Zapata, C.; López-Collazo, E. Biofilm vs. planktonic bacterial mode of growth: Which do human macrophages prefer? Biochem. Biophys. Res. Commun. 2013, 441, 947–952. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Davidson, D.J.; Spratt, D.; Liddle, A.D. Implant materials and prosthetic joint infection: The battle with the biofilm. EFORT Open Rev. 2019, 4, 633–639. [Google Scholar] [CrossRef]
- Khoddami, S.; Chew, B.H.; Lange, D. Problems and solutions of stent biofilm and encrustations: A review of literature. Turk. J. Urol. 2020, 46, S11–S18. [Google Scholar] [CrossRef]
- Köves, B.; Magyar, A.; Tenke, P. Spectrum and antibiotic resistance of catheter-associated urinary tract infections. GMS Infect. Dis. 2017, 5, Doc06. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J.; Mirzoyan, S.; Kerkhof, L.J.; Häggblom, M.M. Characterization of Microplastic-Associated Biofilm Development along a Freshwater-Estuarine Gradient. Environ. Sci. Technol. 2021, 55, 16402–16412. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, V. Impact of environmental biofilms: Industrial components and its remediation. J. Basic Microbiol. 2020, 60, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Scribano, D.; Sarshar, M.; Di Bonaventura, G.; Palamara, A.T.; Ambrosi, C. Gram-Negative Bacteria Holding Together in a Biofilm: The Acinetobacter baumannii Way. Microorganisms 2021, 9, 1353. [Google Scholar] [CrossRef]
- Cendra, M.D.M.; Torrents, E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol. Adv. 2021, 49, 107734. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 877995. [Google Scholar] [CrossRef] [PubMed]
- Folliero, V.; Franci, G.; Dell’Annunziata, F.; Giugliano, R.; Foglia, F.; Sperlongano, R.; de Filippis, A.; Finamore, E.; Galdiero, M. Evaluation of Antibiotic Resistance and Biofilm Production among Clinical Strain Isolated from Medical Devices. Int. J. Microbiol. 2021, 2021, 9033278. [Google Scholar] [CrossRef]
- Yaita, K.; Gotoh, K.; Nakano, R.; Iwahashi, J.; Sakai, Y.; Horita, R.; Yano, H.; Watanabe, H. Biofilm-Forming by Carbapenem Resistant Enterobacteriaceae May Contribute to the Blood Stream Infection. Int. J. Mol. Sci. 2019, 20, 5954. [Google Scholar] [CrossRef]
- Gemba, M.; Rosiak, E.; Nowak-Życzyńska, Z.; Kałęcka, P.; Łodykowska, E.; Kołożyn-Krajewska, D. Factors Influencing Biofilm Formation by Salmonella enterica sv. Typhimurium, E. cloacae, E. hormaechei, Pantoea spp., and Bacillus spp. Isolated from Human Milk Determined by PCA Analysis. Foods 2022, 11, 3862. [Google Scholar] [CrossRef]
- Ramić, D.; Ogrizek, J.; Bucar, F.; Jeršek, B.; Jeršek, M.; Možina, S.S. Campylobacter jejuni Biofilm Control with Lavandin Essential Oils and By-Products. Antibiotics 2022, 11, 854. [Google Scholar] [CrossRef]
- Chen, P.; Wang, J.J.; Hong, B.; Tan, L.; Yan, J.; Zhang, Z.; Liu, H.; Pan, Y.; Zhao, Y. Characterization of Mixed-Species Biofilm Formed by Vibrio parahaemolyticus and Listeria monocytogenes. Front. Microbiol. 2019, 10, 2543. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L. Antibiofilm effect and mechanism of protocatechuic aldehyde against Vibrio parahaemolyticus. Front. Microbiol. 2022, 13, 1060506. [Google Scholar] [CrossRef]
- De Brito, F.A.E.; de Freitas, A.P.P.; Nascimento, M.S. Multidrug-Resistant Biofilms (MDR): Main Mechanisms of Tolerance and Resistance in the Food Supply Chain. Pathogens 2022, 11, 1416. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal Biofilms. Microbiol. Spectr. 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus Biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.M.T.; Frank, K.L.; Dale, J.L.; Manias, D.A.; Powers, J.L.; Dunny, G.M. Enterococcus faecalis colonizes and forms persistent biofilm microcolonies on undamaged endothelial surfaces in a rabbit endovascular infection model. FEMS Microbes 2021, 2, xtab014. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Andam, C.P. Extensive Horizontal Gene Transfer within and between Species of Coagulase-Negative Staphylococcus. Genome Biol. Evol. 2021, 13, evab206. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Ren, B.; Zhou, X.; Zhang, L.; Xu, X. Cross-kingdom interaction between Candida albicans and oral bacteria. Front. Microbiol. 2022, 13, 911623. [Google Scholar] [CrossRef]
- Van Dyck, K.; Viela, F.; Mathelié-Guinlet, M.; Demuyser, L.; Hauben, E.; Jabra-Rizk, M.A.; Velde, G.V.; Dufrêne, Y.F.; Krom, B.P.; van Dijck, P. Adhesion of Staphylococcus aureus to Candida albicans During Co-Infection Promotes Bacterial Dissemination Through the Host Immune Response. Front. Cell. Infect. Microbiol. 2020, 10, 624839. [Google Scholar] [CrossRef] [PubMed]
- Palencia, S.L.; García, A.; Palencia, M. Multiple surface interaction mechanisms direct the anchoring, co-aggregation and formation of dual-species biofilm between Candida albicans and Helicobacter pylori. J. Adv. Res. 2022, 35, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Biofilm-forming microorganisms causing hospital-acquired infections from intravenous catheter: A systematic review. Curr. Res. Microb. Sci. 2022, 3, 100175. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Caldara, M.; Belgiovine, C.; Secchi, E.; Rusconi, R. Environmental, Microbiological, and Immunological Features of Bacterial Biofilms Associated with Implanted Medical Devices. Clin. Microbiol. Rev. 2022, 35, e0022120. [Google Scholar] [CrossRef]
- Stewart, P.S.; Bjarnsholt, T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect. 2020, 26, 1034–1038. [Google Scholar] [CrossRef]
- Pietrocola, G.; Campoccia, D.; Motta, C.; Montanaro, L.; Arciola, C.R.; Speziale, P. Colonization and Infection of Indwelling Medical Devices by Staphylococcus aureus with an Emphasis on Orthopedic Implants. Int. J. Mol. Sci. 2022, 23, 5958. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Kazek-Kęsik, A.; Brzychczy-Włoch, M.; Łos, M.J.; Ateba, C.N.; Mehrbod, P.; Ghavami, S.; Shyntum, D.Y. Recent Advances in the Control of Clinically Important Biofilms. Int. J. Mol. Sci. 2022, 23, 9526. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Yousefimashouf, R.; Arabestani, M.R.; Sedighi, I.; Alikhani, M.Y. The issue beyond resistance: Methicillin-resistant Staphylococcus epidermidis biofilm formation is induced by subinhibitory concentrations of cloxacillin, cefazolin, and clindamycin. PLoS ONE 2022, 17, e0277287. [Google Scholar] [CrossRef]
- Josephs-Spaulding, J.; Singh, O.V. Medical Device Sterilization and Reprocessing in the Era of Multidrug-Resistant (MDR) Bacteria: Issues and Regulatory Concepts. Front. Med. Technol. 2020, 2, 587352. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Alam, M.-U.; Luies, S.K.; Kamal, A.; Ferdous, S.; Lin, A.; Sharior, F.; Khan, R.; Rahman, Z.; Parvez, S.M.; et al. Contamination of Fresh Produce with Antibiotic-Resistant Bacteria and Associated Risks to Human Health: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 19, 360. [Google Scholar] [CrossRef] [PubMed]
- Blau, K.; Jacquiod, S.; Sørensen, S.J.; Su, J.-Q.; Zhu, Y.-G.; Smalla, K.; Jechalke, S. Manure and Doxycycline Affect the Bacterial Community and Its Resistome in Lettuce Rhizosphere and Bulk Soil. Front. Microbiol. 2019, 10, 725. [Google Scholar] [CrossRef]
- Kaviani Rad, A.; Astaykina, A.; Streletskii, R.; Afsharyzad, Y.; Etesami, H.; Zarei, M.; Balasundram, S.K. An Overview of Antibiotic Resistance and Abiotic Stresses Affecting Antimicrobial Resistance in Agricultural Soils. Int. J. Environ. Res. Public Health 2022, 19, 4666. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a Potential Hotspot for Antibiotic Resistance Dissemination by Horizontal Gene Transfer Events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Li, H.; Zheng, X.; Tan, L.; Shao, Z.; Cao, H.; Xu, Y. The vertical migration of antibiotic-resistant genes and pathogens in soil and vegetables after the application of different fertilizers. Environ. Res. 2022, 203, 111884. [Google Scholar] [CrossRef]
- Sharma, R.; Bisaria, V.S.; Sharma, S. Rhizosphere: A Home for Human Pathogens. In Plant Biotic Interactions; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 113–127. ISBN 978-3-030-26656-1. [Google Scholar]
- Schlech, W.F. Epidemiology and Clinical Manifestations of Listeria monocytogenes Infection. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J.J. Antibiotic resistance genes from livestock waste: Occurrence, dissemination, and treatment. Npj Clean Water 2020, 3, 4. [Google Scholar] [CrossRef]
- Mestrovic, T.; Robles Aguilar, G.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Davis Weaver, N.; Han, C.; Wool, E.E.; Gershberg Hayoon, A.; Hay, S.I.; et al. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: A cross-country systematic analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar] [CrossRef]
- Rahmoun, L.A.; Azrad, M.; Peretz, A. Antibiotic Resistance and Biofilm Production Capacity in Clostridioides difficile. Front. Cell. Infect. Microbiol. 2021, 11, 683464. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; López, M.; Rojo-Bezares, B.; Sáenz, Y. Antimicrobial Susceptibility Testing in Pseudomonas aeruginosa Biofilms: One Step Closer to a Standardized Method. Antibiotics 2020, 9, 880. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Römling, U.; Nadeem, F.; Bilal, H.M.; Zafar, M.; Jahan, H.; Ur-Rahman, A. Innovative Strategies to Overcome Antimicrobial Resistance and Tolerance. Microorganisms 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Sotto, A.; Laurent, F.; Schuldiner, S.; Vouillarmet, J.; Corvec, S.; Bemer, P.; Boutoille, D.; Dunyach-Rémy, C.; Lavigne, J.-P. Evaluation of the Use of Antibiofilmogram Technology in the Clinical Evolution of Foot Ulcers Infected by Staphylococcus aureus in Persons Living with Diabetes: A Pilot Study. J. Clin. Med. 2021, 10, 5928. [Google Scholar] [CrossRef] [PubMed]
- Tasse, J.; Croisier, D.; Badel-Berchoux, S.; Chavanet, P.; Bernardi, T.; Provot, C.; Laurent, F. Preliminary results of a new antibiotic susceptibility test against biofilm installation in device-associated infections: The Antibiofilmogram®. Pathog. Dis. 2016, 74, ftw057. [Google Scholar] [CrossRef]
- Udaondo, Z.; Matilla, M.A. Mining for novel antibiotics in the age of antimicrobial resistance. Microb. Biotechnol. 2020, 13, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-P.; Yang, Y.; Lu, D.-P.; Niu, Z.-S.; Feng, J.-N.; Chen, Y.-R.; Tou, F.-Y.; Garner, E.; Xu, J.; Liu, M.; et al. Biofilms as a sink for antibiotic resistance genes (ARGs) in the Yangtze Estuary. Water Res. 2018, 129, 277–286. [Google Scholar] [CrossRef]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J. Basic Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef]
- Stanley, D.; Batacan, R.; Bajagai, Y.S. Rapid growth of antimicrobial resistance: The role of agriculture in the problem and the solutions. Appl. Microbiol. Biotechnol. 2022, 106, 6953–6962. [Google Scholar] [CrossRef]
- Hiller, C.X.; Hübner, U.; Fajnorova, S.; Schwartz, T.; Drewes, J.E. Antibiotic microbial resistance (AMR) removal efficiencies by conventional and advanced wastewater treatment processes: A review. Sci. Total Environ. 2019, 685, 596–608. [Google Scholar] [CrossRef]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: Hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020, 96, fiaa031. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Domingues, S.; Nielsen, K.M. Membrane vesicles and horizontal gene transfer in prokaryotes. Curr. Opin. Microbiol. 2017, 38, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Grossman, A.D. Integrative and Conjugative Elements (ICEs): What They Do and How They Work. Annu. Rev. Genet. 2015, 49, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Dell’Annunziata, F.; Folliero, V.; Giugliano, R.; de Filippis, A.; Santarcangelo, C.; Izzo, V.; Daglia, M.; Galdiero, M.; Arciola, C.R.; Franci, G. Gene Transfer Potential of Outer Membrane Vesicles of Gram-Negative Bacteria. Int. J. Mol. Sci. 2021, 22, 5985. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, J.; Wang, S.; Zhou, J.; Qin, J.; Jia, Z.; Wang, Y.; Wang, Z.; Zhang, Y.; Hao, H. Research Progress on Bacterial Membrane Vesicles and Antibiotic Resistance. Int. J. Mol. Sci. 2022, 23, 11553. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Dell’Aversana, C.; Doti, N.; Donadio, G.; Dal Piaz, F.; Izzo, V.; de Filippis, A.; Galdiero, M.; Altucci, L.; Boccia, G.; et al. Outer Membrane Vesicles Derived from Klebsiella pneumoniae Are a Driving Force for Horizontal Gene Transfer. Int. J. Mol. Sci. 2021, 22, 8732. [Google Scholar] [CrossRef]
- Rumbo, C.; Fernández-Moreira, E.; Merino, M.; Poza, M.; Mendez, J.A.; Soares, N.C.; Mosquera, A.; Chaves, F.; Bou, G. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: A new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011, 55, 3084–3090. [Google Scholar] [CrossRef]
- Li, C.; Wen, R.; Mu, R.; Chen, X.; Ma, P.; Gu, K.; Huang, Z.; Ju, Z.; Lei, C.; Tang, Y.; et al. Outer Membrane Vesicles of Avian Pathogenic Escherichia coli Mediate the Horizontal Transmission of blaCTX-M-55. Pathogens 2022, 11, 481. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, H. Characterization and function of membrane vesicles in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 1795–1801. [Google Scholar] [CrossRef]
- Seike, S.; Kobayashi, H.; Ueda, M.; Takahashi, E.; Okamoto, K.; Yamanaka, H. Outer Membrane Vesicles Released From Aeromonas Strains Are Involved in the Biofilm Formation. Front. Microbiol. 2020, 11, 613650. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Miao, J.; Zhang, Z.; Ruan, J.; Xu, L.; Guo, H.; Zhang, M.; Qiao, W. Regulation of the formation and structure of biofilms by quorum sensing signal molecules packaged in outer membrane vesicles. Sci. Total Environ. 2022, 806, 151403. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.C.; Florez, C.; Dunshee, E.B.; Lieber, A.D.; Terry, M.L.; Light, C.J.; Schertzer, J.W. Pseudomonas Quinolone Signal-Induced Outer Membrane Vesicles Enhance Biofilm Dispersion in Pseudomonas aeruginosa. mSphere 2020, 5, e01109-20. [Google Scholar] [CrossRef]
- Baumgarten, T.; Sperling, S.; Seifert, J.; von Bergen, M.; Steiniger, F.; Wick, L.Y.; Heipieper, H.J. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl. Environ. Microbiol. 2012, 78, 6217–6224. [Google Scholar] [CrossRef]
- Yonezawa, H.; Osaki, T.; Kurata, S.; Fukuda, M.; Kawakami, H.; Ochiai, K.; Hanawa, T.; Kamiya, S. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 2009, 9, 197. [Google Scholar] [CrossRef]
- Altindis, E.; Fu, Y.; Mekalanos, J.J. Proteomic analysis of Vibrio cholerae outer membrane vesicles. Proc. Natl. Acad. Sci. USA 2014, 111, E1548–E1556. [Google Scholar] [CrossRef]
- Wang, Y.; Hoffmann, J.P.; Baker, S.M.; zu Bentrup, K.H.; Wimley, W.C.; Fuselier, J.A.; Bitoun, J.P.; Morici, L.A. Inhibition of Streptococcus mutans biofilms with bacterial-derived outer membrane vesicles. BMC Microbiol. 2021, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.J.; Turner, K.B.; Medintz, I.L.; Walper, S.A. Protecting enzymatic function through directed packaging into bacterial outer membrane vesicles. Sci. Rep. 2016, 6, 24866. [Google Scholar] [CrossRef] [PubMed]
- Dubey, G.P.; Ben-Yehuda, S. Intercellular nanotubes mediate bacterial communication. Cell 2011, 144, 590–600. [Google Scholar] [CrossRef]
- Dubey, G.P.; Malli Mohan, G.B.; Dubrovsky, A.; Amen, T.; Tsipshtein, S.; Rouvinski, A.; Rosenberg, A.; Kaganovich, D.; Sherman, E.; Medalia, O.; et al. Architecture and Characteristics of Bacterial Nanotubes. Dev. Cell 2016, 36, 453–461. [Google Scholar] [CrossRef]
- Von Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Glen, K.A.; Lamont, I.L. β-lactam Resistance in Pseudomonas aeruginosa: Current Status, Future Prospects. Pathogens 2021, 10, 1638. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dorosky, R.J.; Han, C.S.; Lo, C.-C.; Dichosa, A.E.K.; Chain, P.S.; Yu, J.M.; Pierson, L.S.; Pierson, E.A. Adaptation genomics of a small-colony variant in a Pseudomonas chlororaphis 30-84 biofilm. Appl. Environ. Microbiol. 2015, 81, 890–899. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Huang, Y.; Yu, K.; Chen, H.; Cui, K.; Huang, Q.; Zhang, J.; Gin, K.Y.H.; He, Y. Environmental media exert a bottleneck in driving the dynamics of antibiotic resistance genes in modern aquatic environment. Water Res. 2019, 162, 127–138. [Google Scholar] [CrossRef]
- Haudiquet, M.; de Sousa, J.M.; Touchon, M.; Rocha, E.P.C. Selfish, promiscuous and sometimes useful: How mobile genetic elements drive horizontal gene transfer in microbial populations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20210234. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Konkel, M.E.; Lu, X. Antimicrobial Resistance Gene Transfer from Campylobacter jejuni in Mono- and Dual-Species Biofilms. Appl. Environ. Microbiol. 2021, 87, e0065921. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Meesrisom, A.; Luo, Y.; Dinh, Q.N.; Lin, S.; Yang, M.; Sharma, A.; Tang, R.; Zhang, J.; Jia, Z.; et al. Listeria monocytogenes biofilm formation as affected by stainless steel surface topography and coating composition. Food Control. 2021, 130, 108275. [Google Scholar] [CrossRef]
- Xu, L.-C.; Siedlecki, C.A. Submicron topography design for controlling staphylococcal bacterial adhesion and biofilm formation. J. Biomed. Mater. Res. A 2022, 110, 1238–1250. [Google Scholar] [CrossRef]
- Krsmanovic, M.; Biswas, D.; Ali, H.; Kumar, A.; Ghosh, R.; Dickerson, A.K. Hydrodynamics and surface properties influence biofilm proliferation. Adv. Colloid Interface Sci. 2021, 288, 102336. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Lee, S.W.; Phillips, K.S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021, 268, 120595. [Google Scholar] [CrossRef]
- Ishihama, H.; Ishii, K.; Nagai, S.; Kakinuma, H.; Sasaki, A.; Yoshioka, K.; Kuramoto, T.; Shiono, Y.; Funao, H.; Isogai, N.; et al. An antibacterial coated polymer prevents biofilm formation and implant-associated infection. Sci. Rep. 2021, 11, 3602. [Google Scholar] [CrossRef]
- Kloss, M.; Moerke, C.; Woitschach, F.; Wulf, K.; Illner, S.; Schulz, S.; Pauker, V.I.; Riedel, K.; Grabow, N.; Ince, H.; et al. Novel dalbavancin-PLLA implant coating prevents hematogenous Staphylococcus aureus infection in a minimally invasive mouse tail vein model. Front. Bioeng. Biotechnol. 2022, 10, 1021827. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Pereira, J.E.; Maltez, L.; Poeta, P.; Igrejas, G. Influence of Environmental Factors on Biofilm Formation of Staphylococci Isolated from Wastewater and Surface Water. Pathogens 2022, 11, 1069. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Ji, Y. Environmental factors modulate biofilm formation by Staphylococcus aureus. Sci. Prog. 2020, 103, 36850419898659. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, S.B.; Kiridena, S.D.; Wijayaratna, U.N.; Taylor, C.; Anker, J.N.; Tzeng, T.-R.J. pH variation in medical implant biofilms: Causes, measurements, and its implications for antibiotic resistance. Front. Microbiol. 2022, 13, 1028560. [Google Scholar] [CrossRef] [PubMed]
- Blanco, Y.; Rivas, L.A.; González-Toril, E.; Ruiz-Bermejo, M.; Moreno-Paz, M.; Parro, V.; Palacín, A.; Aguilera, Á.; Puente-Sánchez, F. Environmental parameters, and not phylogeny, determine the composition of extracellular polymeric substances in microbial mats from extreme environments. Sci. Total Environ. 2019, 650, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.; Bayles, K.; Moormeier, D.; Endres, J.; Wei, T. Observations of Shear Stress Effects on Staphylococcus aureus Biofilm Formation. mSphere 2019, 4, e00372-19. [Google Scholar] [CrossRef] [PubMed]
- Tsagkari, E.; Connelly, S.; Liu, Z.; McBride, A.; Sloan, W.T. The role of shear dynamics in biofilm formation. NPJ Biofilms Microbiomes 2022, 8, 33. [Google Scholar] [CrossRef]
- Bernardi, S.; Anderson, A.; Macchiarelli, G.; Hellwig, E.; Cieplik, F.; Vach, K.; Al-Ahmad, A. Subinhibitory Antibiotic Concentrations Enhance Biofilm Formation of Clinical Enterococcus faecalis Isolates. Antibiotics 2021, 10, 874. [Google Scholar] [CrossRef]
- Pinilla-Redondo, R.; Riber, L.; Sørensen, S.J. Fluorescence Recovery Allows the Implementation of a Fluorescence Reporter Gene Platform Applicable for the Detection and Quantification of Horizontal Gene Transfer in Anoxic Environments. Appl. Environ. Microbiol. 2018, 84, e02507-17. [Google Scholar] [CrossRef]
- Skolimowski, M.; Nielsen, M.W.; Emnéus, J.; Molin, S.; Taboryski, R.; Sternberg, C.; Dufva, M.; Geschke, O. Microfluidic dissolved oxygen gradient generator biochip as a useful tool in bacterial biofilm studies. Lab Chip 2010, 10, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, R.; Sato, T.; Nomura, N.; Nakamura, T.; Senpuku, H. Potential Risk of Spreading Resistance Genes within Extracellular-DNA-Dependent Biofilms of Streptococcus mutans in Response to Cell Envelope Stress Induced by Sub-MICs of Bacitracin. Appl. Environ. Microbiol. 2020, 86, e00770-20. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.K.M.; Ghaly, T.M.; Gillings, M.R. A survey of sub-inhibitory concentrations of antibiotics in the environment. J. Environ. Sci. 2021, 99, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.B.; Sternberg, C.; Andersen, J.B.; Eberl, L.; Moller, S.; Givskov, M.; Molin, S. Establishment of new genetic traits in a microbial biofilm community. Appl. Environ. Microbiol. 1998, 64, 2247–2255. [Google Scholar] [CrossRef]
- Król, J.E.; Nguyen, H.D.; Rogers, L.M.; Beyenal, H.; Krone, S.M.; Top, E.M. Increased transfer of a multidrug resistance plasmid in Escherichia coli biofilms at the air-liquid interface. Appl. Environ. Microbiol. 2011, 77, 5079–5088. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Huisman, J.S.; Bakkeren, E.; Herter, J.A.; Stadler, T.; Ackermann, M.; Diard, M.; Egli, A.; Hall, A.R.; Hardt, W.-D.; et al. Plasmid- and strain-specific factors drive variation in ESBL-plasmid spread in vitro and in vivo. ISME J. 2021, 15, 862–878. [Google Scholar] [CrossRef]
- Haudiquet, M.; Buffet, A.; Rendueles, O.; Rocha, E.P.C. Interplay between the cell envelope and mobile genetic elements shapes gene flow in populations of the nosocomial pathogen Klebsiella pneumoniae. PLoS Biol. 2021, 19, e3001276. [Google Scholar] [CrossRef]
- Cook, L.; Chatterjee, A.; Barnes, A.; Yarwood, J.; Hu, W.-S.; Dunny, G. Biofilm growth alters regulation of conjugation by a bacterial pheromone. Mol. Microbiol. 2011, 81, 1499–1510. [Google Scholar] [CrossRef]
- Cook, L.C.; Dunny, G.M. Effects of biofilm growth on plasmid copy number and expression of antibiotic resistance genes in Enterococcus faecalis. Antimicrob. Agents Chemother. 2013, 57, 1850–1856. [Google Scholar] [CrossRef]
- Alderliesten, J.B.; Duxbury, S.J.N.; Zwart, M.P.; de Visser, J.A.G.M.; Stegeman, A.; Fischer, E.A.J. Effect of donor-recipient relatedness on the plasmid conjugation frequency: A meta-analysis. BMC Microbiol. 2020, 20, 135. [Google Scholar] [CrossRef]
- Stalder, T.; Top, E. Plasmid transfer in biofilms: A perspective on limitations and opportunities. NPJ Biofilms Microbiomes 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Røder, H.L.; Trivedi, U.; Russel, J.; Kragh, K.N.; Herschend, J.; Thalsø-Madsen, I.; Tolker-Nielsen, T.; Bjarnsholt, T.; Burmølle, M.; Madsen, J.S. Biofilms can act as plasmid reserves in the absence of plasmid specific selection. NPJ Biofilms Microbiomes 2021, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Metzger, G.A.; Ridenhour, B.J.; France, M.; Gliniewicz, K.; Millstein, J.; Settles, M.L.; Forney, L.J.; Stalder, T.; Top, E.M. Biofilms preserve the transmissibility of a multi-drug resistance plasmid. NPJ Biofilms Microbiomes 2022, 8, 95. [Google Scholar] [CrossRef]
- Beaudoin, D.L.; Bryers, J.D.; Cunningham, A.B.; Peretti, S.W. Mobilization of broad host range plasmid from Pseudomonas putida to established biofilm of Bacillus azotoformans. II. Modeling. Biotechnol. Bioeng. 1998, 57, 280–286. [Google Scholar] [CrossRef]
- Angles, M.L.; Marshall, K.C.; Goodman, A.E. Plasmid Transfer between Marine Bacteria in the Aqueous Phase and Biofilms in Reactor Microcosms. Appl. Environ. Microbiol. 1993, 59, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef]
- Tanner, W.D.; Atkinson, R.M.; Goel, R.K.; Toleman, M.A.; Benson, L.S.; Porucznik, C.A.; VanDerslice, J.A. Horizontal transfer of the blaNDM-1 gene to Pseudomonas aeruginosa and Acinetobacter baumannii in biofilms. FEMS Microbiol. Lett. 2017, 364, fnx048. [Google Scholar] [CrossRef]
- Arias-Andres, M.; Klümper, U.; Rojas-Jimenez, K.; Grossart, H.-P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 253–261. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Y.; Zhang, J.; Huang, X.; Shi, H.; Yin, H. Real-Time Study of Rapid Spread of Antibiotic Resistance Plasmid in Biofilm Using Microfluidics. Environ. Sci. Technol. 2018, 52, 11132–11141. [Google Scholar] [CrossRef]
- Huisman, J.S.; Benz, F.; Duxbury, S.J.N.; de Visser, J.A.G.M.; Hall, A.R.; Fischer, E.A.J.; Bonhoeffer, S. Estimating plasmid conjugation rates: A new computational tool and a critical comparison of methods. Plasmid 2022, 121, 102627. [Google Scholar] [CrossRef]
- Simonsen, L. Dynamics of plasmid transfer on surfaces. J. Gen. Microbiol. 1990, 136, 1001–1007. [Google Scholar] [CrossRef]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.-F.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef] [PubMed]
- Gaston, J.R.; Andersen, M.J.; Johnson, A.O.; Bair, K.L.; Sullivan, C.M.; Guterman, L.B.; White, A.N.; Brauer, A.L.; Learman, B.S.; Flores-Mireles, A.L.; et al. Enterococcus faecalis Polymicrobial Interactions Facilitate Biofilm Formation, Antibiotic Recalcitrance, and Persistent Colonization of the Catheterized Urinary Tract. Pathogens 2020, 9, 835. [Google Scholar] [CrossRef]
- Allkja, J.; Goeres, D.M.; Azevedo, A.S.; Azevedo, N.F. Interactions of microorganisms within a urinary catheter polymicrobial biofilm model. Biotechnol. Bioeng. 2023, 120, 239–249. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo Models of Bacterial Biofilm-Related Infections. Pathogens 2013, 2, 288–356. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Goeres, D.; van Bambeke, F.; Bjarnsholt, T. Should standardized susceptibility testing for microbial biofilms be introduced in clinical practice? Clin. Microbiol. Infect. 2018, 24, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, H.; Sibley, C.D.; Surette, M.G.; Lewenza, S. Drosophila melanogaster as an animal model for the study of Pseudomonas aeruginosa biofilm infections in vivo. PLoS Pathog. 2011, 7, e1002299. [Google Scholar] [CrossRef] [PubMed]
- Kamareddine, L.; Wong, A.C.N.; Vanhove, A.S.; Hang, S.; Purdy, A.E.; Kierek-Pearson, K.; Asara, J.M.; Ali, A.; Morris, J.G.; Watnick, P.I. Activation of Vibrio cholerae quorum sensing promotes survival of an arthropod host. Nat. Microbiol. 2018, 3, 243–252. [Google Scholar] [CrossRef]
- Desai, S.K.; Padmanabhan, A.; Harshe, S.; Zaidel-Bar, R.; Kenney, L.J. Salmonella biofilms program innate immunity for persistence in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2019, 116, 12462–12467. [Google Scholar] [CrossRef]
- Van Meervenne, E.; de Weirdt, R.; van Coillie, E.; Devlieghere, F.; Herman, L.; Boon, N. Biofilm models for the food industry: Hot spots for plasmid transfer? Pathog. Dis. 2014, 70, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Bryers, J.D. Non-invasive determination of conjugative transfer of plasmids bearing antibiotic-resistance genes in biofilm-bound bacteria: Effects of substrate loading and antibiotic selection. Appl. Microbiol. Biotechnol. 2013, 97, 317–328. [Google Scholar] [CrossRef]
- Yaffe, E.; Relman, D.A. Tracking microbial evolution in the human gut using Hi-C reveals extensive horizontal gene transfer, persistence and adaptation. Nat. Microbiol. 2020, 5, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.G.; Vill, A.C.; Shi, Q.; Satlin, M.J.; Brito, I.L. Widespread transfer of mobile antibiotic resistance genes within individual gut microbiomes revealed through bacterial Hi-C. Nat. Commun. 2020, 11, 4379. [Google Scholar] [CrossRef]
- Munck, C.; Sheth, R.U.; Freedberg, D.E.; Wang, H.H. Recording mobile DNA in the gut microbiota using an Escherichia coli CRISPR-Cas spacer acquisition platform. Nat. Commun. 2020, 11, 95. [Google Scholar] [CrossRef]
- McGinn, J.; Marraffini, L.A. Molecular mechanisms of CRISPR-Cas spacer acquisition. Nat. Rev. Microbiol. 2019, 17, 7–12. [Google Scholar] [CrossRef]
- Lieberman-Aiden, E.; van Berkum, N.L.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.O.; et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Press, M.O.; Sullivan, S.; Liachko, I.; Top, E.M. Linking the resistome and plasmidome to the microbiome. ISME J. 2019, 13, 2437–2446. [Google Scholar] [CrossRef]
- Baudry, L.; Foutel-Rodier, T.; Thierry, A.; Koszul, R.; Marbouty, M. MetaTOR: A Computational Pipeline to Recover High-Quality Metagenomic Bins From Mammalian Gut Proximity-Ligation (meta3C) Libraries. Front. Genet. 2019, 10, 753. [Google Scholar] [CrossRef]
- Chen, I.; Christie, P.J.; Dubnau, D. The ins and outs of DNA transfer in bacteria. Science 2005, 310, 1456–1460. [Google Scholar] [CrossRef]
- Griffith, F. The Significance of Pneumococcal Types. Epidemiol. Infect. 1928, 27, 113–159. [Google Scholar] [CrossRef] [PubMed]
- Blokesch, M. Natural competence for transformation. Curr. Biol. 2016, 26, R1126–R1130. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Martin, B.; Fichant, G.; Polard, P.; Claverys, J.-P. Bacterial transformation: Distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 2014, 12, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Losick, R.M. Bacillus subtilis: A bacterium for all seasons. Curr. Biol. 2020, 30, R1146–R1150. [Google Scholar] [CrossRef] [PubMed]
- Dubnau, D.; Blokesch, M. Mechanisms of DNA Uptake by Naturally Competent Bacteria. Annu. Rev. Genet. 2019, 53, 217–237. [Google Scholar] [CrossRef]
- Attaiech, L.; Charpentier, X. Silently transformable: The many ways bacteria conceal their built-in capacity of genetic exchange. Curr. Genet. 2017, 63, 451–455. [Google Scholar] [CrossRef] [PubMed]
- García-Curiel, L.; López-Cuellar, M.D.R.; Rodríguez-Hernández, A.I.; Chavarría-Hernández, N. Toward understanding the signals of bacteriocin production by Streptococcus spp. and their importance in current applications. World J. Microbiol. Biotechnol. 2021, 37, 15. [Google Scholar] [CrossRef]
- Maree, M.; Nguyen, T.L.T.; Ohniwa, R.L.; Higashide, M.; Msadek, T.; Morikawa, K. Natural transformation allows transfer of SCCmec-mediated methicillin resistance in Staphylococcus aureus biofilms. Nat. Commun. 2022, 13, 2477. [Google Scholar] [CrossRef]
- Marks, L.R.; Reddinger, R.M.; Hakansson, A.P. High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. mBio 2012, 3, e00200-12. [Google Scholar] [CrossRef]
- Trappetti, C.; Gualdi, L.; Di Meola, L.; Jain, P.; Korir, C.C.; Edmonds, P.; Iannelli, F.; Ricci, S.; Pozzi, G.; Oggioni, M.R. The impact of the competence quorum sensing system on Streptococcus pneumoniae biofilms varies depending on the experimental model. BMC Microbiol. 2011, 11, 75. [Google Scholar] [CrossRef]
- Averhoff, B.; Kirchner, L.; Pfefferle, K.; Yaman, D. Natural transformation in Gram-negative bacteria thriving in extreme environments: From genes and genomes to proteins, structures and regulation. Extremophiles 2021, 25, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- Mashburn-Warren, L.; Goodman, S.D.; Federle, M.J.; Prehna, G. The conserved mosaic prophage protein paratox inhibits the natural competence regulator ComR in Streptococcus. Sci. Rep. 2018, 8, 16535. [Google Scholar] [CrossRef]
- Buchrieser, C. Biodiversity of the species Listeria monocytogenes and the genus Listeria. Microbes Infect. 2007, 9, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Borezee, E.; Msadek, T.; Durant, L.; Berche, P. Identification in Listeria monocytogenes of MecA, a homologue of the Bacillus subtilis competence regulatory protein. J. Bacteriol. 2000, 182, 5931–5934. [Google Scholar] [CrossRef] [PubMed]
- Meibom, K.L.; Blokesch, M.; Dolganov, N.A.; Wu, C.-Y.; Schoolnik, G.K. Chitin induces natural competence in Vibrio cholerae. Science 2005, 310, 1824–1827. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bernardy, E.E.; Hammer, B.K.; Miyashiro, T. Competence and natural transformation in vibrios. Mol. Microbiol. 2013, 89, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Blokesch, M.; Schoolnik, G.K. Serogroup conversion of Vibrio cholerae in aquatic reservoirs. PLoS Pathog. 2007, 3, e81. [Google Scholar] [CrossRef]
- Kouzel, N.; Oldewurtel, E.R.; Maier, B. Gene Transfer Efficiency in Gonococcal Biofilms: Role of Biofilm Age, Architecture, and Pilin Antigenic Variation. J. Bacteriol. 2015, 197, 2422–2431. [Google Scholar] [CrossRef]
- Bae, J.; Oh, E.; Jeon, B. Enhanced transmission of antibiotic resistance in Campylobacter jejuni biofilms by natural transformation. Antimicrob. Agents Chemother. 2014, 58, 7573–7575. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.M.; Turnbull, L.; Katrib, M.; Osvath, S.R.; Losa, D.; Lazenby, J.J.; Whitchurch, C.B. Pseudomonas aeruginosa is capable of natural transformation in biofilms. Microbiology 2020, 166, 995–1003. [Google Scholar] [CrossRef]
- Hendrickx, L.; Hausner, M.; Wuertz, S. Natural genetic transformation in monoculture Acinetobacter sp. strain BD413 biofilms. Appl. Environ. Microbiol. 2003, 69, 1721–1727. [Google Scholar] [CrossRef]
- Perumbakkam, S.; Hess, T.F.; Crawford, R.L. A bioremediation approach using natural transformation in pure-culture and mixed-population biofilms. Biodegradation 2006, 17, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.E.; Elzer, P.H.; Hill, D.S.; Robertson, G.T.; Farris, M.A.; Roop, R.; Peterson, K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Merod, R.T.; Wuertz, S. Extracellular polymeric substance architecture influences natural genetic transformation of Acinetobacter baylyi in biofilms. Appl. Environ. Microbiol. 2014, 80, 7752–7757. [Google Scholar] [CrossRef]
- Santala, V.; Karp, M.; Santala, S. Bioluminescence-based system for rapid detection of natural transformation. FEMS Microbiol. Lett. 2016, 363, fnw125. [Google Scholar] [CrossRef]
- Baur, B.; Hanselmann, K.; Schlimme, W.; Jenni, B. Genetic transformation in freshwater: Escherichia coli is able to develop natural competence. Appl. Environ. Microbiol. 1996, 62, 3673–3678. [Google Scholar] [CrossRef]
- Etchuuya, R.; Ito, M.; Kitano, S.; Shigi, F.; Sobue, R.; Maeda, S. Cell-to-cell transformation in Escherichia coli: A novel type of natural transformation involving cell-derived DNA and a putative promoting pheromone. PLoS ONE 2011, 6, e16355. [Google Scholar] [CrossRef]
- Maeda, S.; Ito, M.; Ando, T.; Ishimoto, Y.; Fujisawa, Y.; Takahashi, H.; Matsuda, A.; Sawamura, A.; Kato, S. Horizontal transfer of nonconjugative plasmids in a colony biofilm of Escherichia coli. FEMS Microbiol. Lett. 2006, 255, 115–120. [Google Scholar] [CrossRef]
- Maeda, S.; Sawamura, A.; Matsuda, A. Transformation of colonial Escherichia coli on solid media. FEMS Microbiol. Lett. 2004, 236, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Itakura, S.; Uchii, K.; Sobue, R.; Maeda, S. Horizontal transfer of non-conjugative plasmid in colony biofilm of Escherichia coli on food-based media. World J. Microbiol. Biotechnol. 2009, 25, 1865–1869. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hasegawa, H.; Maeda, S. High temperatures promote cell-to-cell plasmid transformation in Escherichia coli. Biochem. Biophys. Res. Commun. 2019, 515, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Errington, J.; van der Aart, L.T. Microbe Profile: Bacillus subtilis: Model organism for cellular development, and industrial workhorse. Microbiology 2020, 166, 425–427. [Google Scholar] [CrossRef] [PubMed]
- She, Q.; Hunter, E.; Qin, Y.; Nicolau, S.; Zalis, E.A.; Wang, H.; Chen, Y.; Chai, Y. Negative Interplay between Biofilm Formation and Competence in the Environmental Strains of Bacillus subtilis. mSystems 2020, 5, e00539-20. [Google Scholar] [CrossRef]
- Larsen, T.; Fiehn, N.-E. Dental biofilm infections-an update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef]
- Quintero, B.; Araque, M.; van der Jongh, C.G.; Escalona, F.; Correa, M.; Morillo-Puente, S.; Vielma, S.; Hermans, P.W.M. Epidemiology of Streptococcus pneumoniae and Staphylococcus aureus colonization in healthy Venezuelan children. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 7–19. [Google Scholar] [CrossRef]
- De Lastours, V.; Malosh, R.; Ramadugu, K.; Srinivasan, U.; Dawid, S.; Ohmit, S.; Foxman, B. Co-colonization by Streptococcus pneumoniae and Staphylococcus aureus in the throat during acute respiratory illnesses. Epidemiol. Infect. 2016, 144, 3507–3519. [Google Scholar] [CrossRef]
- Sempere, J.; Llamosí, M.; Román, F.; Lago, D.; González-Camacho, F.; Pérez-García, C.; Yuste, J.; Domenech, M. Clearance of mixed biofilms of Streptococcus pneumoniae and methicillin-susceptible/resistant Staphylococcus aureus by antioxidants N-acetyl-L-cysteine and cysteamine. Sci. Rep. 2022, 12, 6668. [Google Scholar] [CrossRef]
- Wang, B.Y.; Chi, B.; Kuramitsu, H.K. Genetic exchange between Treponema denticola and Streptococcus gordonii in biofilms. Oral Microbiol. Immunol. 2002, 17, 108–112. [Google Scholar] [CrossRef]
- Lattar, S.M.; Wu, X.; Brophy, J.; Sakai, F.; Klugman, K.P.; Vidal, J.E. A Mechanism of Unidirectional Transformation, Leading to Antibiotic Resistance, Occurs within Nasopharyngeal Pneumococcal Biofilm Consortia. mBio 2018, 9, e00561-18. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.; Kuramitsu, H.K. Genetic transformation of Streptococcus mutans. Infect. Immun. 1981, 32, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Lau, P.C.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 2001, 183, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.R.; Mashburn-Warren, L.; Federle, M.J.; Hakansson, A.P. Streptococcus pyogenes biofilm growth in vitro and in vivo and its role in colonization, virulence, and genetic exchange. J. Infect. Dis. 2014, 210, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Takemura, A.J.; Inose, Y.; Tsai, M.; Le Nguyen, T.; Ohta, T.; Msadek, T. Expression of a cryptic secondary sigma factor gene unveils natural competence for DNA transformation in Staphylococcus aureus. PLoS Pathog. 2012, 8, e1003003. [Google Scholar] [CrossRef]
- Harrison, A.; Hardison, R.L.; Wallace, R.M.; Fitch, J.; Heimlich, D.R.; Bryan, M.O.; Dubois, L.; John-Williams, L.S.; Sebra, R.P.; White, P.; et al. Reprioritization of biofilm metabolism is associated with nutrient adaptation and long-term survival of Haemophilus influenzae. NPJ Biofilms Microbiomes 2019, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Håvarstein, L.S. Fratricide is essential for efficient gene transfer between pneumococci in biofilms. Appl. Environ. Microbiol. 2012, 78, 5897–5905. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Li, Y.; Lu, Y.; Xiong, K.; Zhong, Q.; Wang, J. Bacteriophage-Resistant Mutant of Enterococcus faecalis Is Impaired in Biofilm Formation. Front. Microbiol. 2022, 13, 913023. [Google Scholar] [CrossRef]
- Calero-Cáceres, W.; Muniesa, M. Persistence of naturally occurring antibiotic resistance genes in the bacteria and bacteriophage fractions of wastewater. Water Res. 2016, 95, 11–18. [Google Scholar] [CrossRef]
- Hata, A.; Kitajima, M.; Katayama, H. Occurrence and reduction of human viruses, F-specific RNA coliphage genogroups and microbial indicators at a full-scale wastewater treatment plant in Japan. J. Appl. Microbiol. 2013, 114, 545–554. [Google Scholar] [CrossRef]
- Dennehy, J.J.; Abedon, S.T. Bacteriophage Ecology. In Bacteriophages; Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 253–294. ISBN 978-3-319-41985-5. [Google Scholar]
- Du, F.; Lv, X.; Duan, D.; Wang, L.; Huang, J. Characterization of a Linezolid- and Vancomycin-Resistant Streptococcus suis Isolate That Harbors optrA and vanG Operons. Front. Microbiol. 2019, 10, 2026. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Jeon, J.H.; Kang, I.; Park, K.S.; Lee, K.; Cha, C.-J.; Lee, S.H.; Cho, J.-C. Freshwater viral metagenome reveals novel and functional phage-borne antibiotic resistance genes. Microbiome 2020, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Lekunberri, I.; Villagrasa, M.; Balcázar, J.L.; Borrego, C.M. Contribution of bacteriophage and plasmid DNA to the mobilization of antibiotic resistance genes in a river receiving treated wastewater discharges. Sci. Total Environ. 2017, 601–602, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.N.; Penadés, J.R.; Chen, J. Genetic transduction by phages and chromosomal islands: The new and noncanonical. PLoS Pathog. 2019, 15, e1007878. [Google Scholar] [CrossRef] [PubMed]
- Penadés, J.R.; Chen, J.; Quiles-Puchalt, N.; Carpena, N.; Novick, R.P. Bacteriophage-mediated spread of bacterial virulence genes. Curr. Opin. Microbiol. 2015, 23, 171–178. [Google Scholar] [CrossRef]
- Fišarová, L.; Botka, T.; Du, X.; Mašlaňová, I.; Bárdy, P.; Pantůček, R.; Benešík, M.; Roudnický, P.; Winstel, V.; Larsen, J.; et al. Staphylococcus epidermidis Phages Transduce Antimicrobial Resistance Plasmids and Mobilize Chromosomal Islands. mSphere 2021, 6, e00223-21. [Google Scholar] [CrossRef]
- Uchiyama, J.; Takemura-Uchiyama, I.; Sakaguchi, Y.; Gamoh, K.; Kato, S.; Daibata, M.; Ujihara, T.; Misawa, N.; Matsuzaki, S. Intragenus generalized transduction in Staphylococcus spp. by a novel giant phage. ISME J. 2014, 8, 1949–1952. [Google Scholar] [CrossRef]
- Leclerc, Q.J.; Wildfire, J.; Gupta, A.; Lindsay, J.A.; Knight, G.M. Growth-Dependent Predation and Generalized Transduction of Antimicrobial Resistance by Bacteriophage. mSystems 2022, 7, e0013522. [Google Scholar] [CrossRef]
- Zhang, J.; He, X.; Shen, S.; Shi, M.; Zhou, Q.; Liu, J.; Wang, M.; Sun, Y. Effects of the Newly Isolated T4-like Phage on Transmission of Plasmid-Borne Antibiotic Resistance Genes via Generalized Transduction. Viruses 2021, 13, 2070. [Google Scholar] [CrossRef]
- Borodovich, T.; Shkoporov, A.N.; Ross, R.P.; Hill, C. Phage-mediated horizontal gene transfer and its implications for the human gut microbiome. Gastroenterol. Rep. 2022, 10, goac012. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Blanco-Picazo, P.; Muniesa, M. Are Phages Parasites or Symbionts of Bacteria? In Biocommunication of Phages; Witzany, G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 143–162. ISBN 978-3-030-45884-3. [Google Scholar]
- Chen, J.; Quiles-Puchalt, N.; Chiang, Y.N.; Bacigalupe, R.; Fillol-Salom, A.; Chee, M.S.J.; Fitzgerald, J.R.; Penadés, J.R. Genome hypermobility by lateral transduction. Science 2018, 362, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Bowring, J.Z.; Su, Y.; Alsaadi, A.; Svenningsen, S.L.; Parkhill, J.; Ingmer, H. Screening for Highly Transduced Genes in Staphylococcus aureus Revealed Both Lateral and Specialized Transduction. Microbiol. Spectr. 2022, 10, e0242321. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, C.; Midoux, C.; Vergnaud, G.; Latino, L. The Basis for Natural Multiresistance to Phage in Pseudomonas aeruginosa. Antibiotics 2020, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Fillol-Salom, A.; Bacigalupe, R.; Humphrey, S.; Chiang, Y.N.; Chen, J.; Penadés, J.R. Lateral transduction is inherent to the life cycle of the archetypical Salmonella phage P22. Nat. Commun. 2021, 12, 6510. [Google Scholar] [CrossRef] [PubMed]
- Bearson, B.L.; Brunelle, B.W. Fluoroquinolone induction of phage-mediated gene transfer in multidrug-resistant Salmonella. Int. J. Antimicrob. Agents 2015, 46, 201–204. [Google Scholar] [CrossRef]
- Gunathilaka, G.U.; Tahlan, V.; Mafiz, A.I.; Polur, M.; Zhang, Y. Phages in urban wastewater have the potential to disseminate antibiotic resistance. Int. J. Antimicrob. Agents 2017, 50, 678–683. [Google Scholar] [CrossRef]
- Haaber, J.; Leisner, J.J.; Cohn, M.T.; Catalan-Moreno, A.; Nielsen, J.B.; Westh, H.; Penadés, J.R.; Ingmer, H. Bacterial viruses enable their host to acquire antibiotic resistance genes from neighbouring cells. Nat. Commun. 2016, 7, 13333. [Google Scholar] [CrossRef]
- Habets, A.; Antoine, C.; Wagemans, J.; Vermeersch, M.; Laforêt, F.; Diderich, J.; Lavigne, R.; Mainil, J.; Thiry, D. Impact of Shiga-toxin encoding gene transduction from O80:H2 Shiga toxigenic Escherichia coli (STEC) on non-STEC strains. Sci. Rep. 2022, 12, 21587. [Google Scholar] [CrossRef]
- Solheim, H.T.; Sekse, C.; Urdahl, A.M.; Wasteson, Y.; Nesse, L.L. Biofilm as an environment for dissemination of stx genes by transduction. Appl. Environ. Microbiol. 2013, 79, 896–900. [Google Scholar] [CrossRef]
- González, S.; Fernández, L.; Gutiérrez, D.; Campelo, A.B.; Rodríguez, A.; García, P. Analysis of Different Parameters Affecting Diffusion, Propagation and Survival of Staphylophages in Bacterial Biofilms. Front. Microbiol. 2018, 9, 2348. [Google Scholar] [CrossRef]
- Li, X.; Gonzalez, F.; Esteves, N.; Scharf, B.E.; Chen, J. Formation of phage lysis patterns and implications on co-propagation of phages and motile host bacteria. PLoS Comput. Biol. 2020, 16, e1007236. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, R.W.; Smith, M.C.; Burns, R.N.; Ford, M.E.; Hatfull, G.F. Evolutionary relationships among diverse bacteriophages and prophages: All the world’s a phage. Proc. Natl. Acad. Sci. USA 1999, 96, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Mushegian, A.R. Are There 1031 Virus Particles on Earth, or More, or Fewer? J. Bacteriol. 2020, 202, e00052-20. [Google Scholar] [CrossRef] [PubMed]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef]
- Yu, Z.; Schwarz, C.; Zhu, L.; Chen, L.; Shen, Y.; Yu, P. Hitchhiking Behavior in Bacteriophages Facilitates Phage Infection and Enhances Carrier Bacteria Colonization. Environ. Sci. Technol. 2021, 55, 2462–2472. [Google Scholar] [CrossRef]
- Morrison, W.D.; Miller, R.V.; Sayler, G.S. Frequency of F116-mediated transduction of Pseudomonas aeruginosa in a freshwater environment. Appl. Environ. Microbiol. 1978, 36, 724–730. [Google Scholar] [CrossRef]
- Ripp, S.; Miller, R.V. Effects of Suspended Particulates on the Frequency of Transduction among Pseudomonas aeruginosa in a Freshwater Environment. Appl. Environ. Microbiol. 1995, 61, 1214–1219. [Google Scholar] [CrossRef]
- Saye, D.J.; Ogunseitan, O.; Sayler, G.S.; Miller, R.V. Potential for transduction of plasmids in a natural freshwater environment: Effect of plasmid donor concentration and a natural microbial community on transduction in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1987, 53, 987–995. [Google Scholar] [CrossRef]
- Saye, D.J.; Ogunseitan, O.A.; Sayler, G.S.; Miller, R.V. Transduction of linked chromosomal genes between Pseudomonas aeruginosa strains during incubation in situ in a freshwater habitat. Appl. Environ. Microbiol. 1990, 56, 140–145. [Google Scholar] [CrossRef]
- Koskella, B.; Meaden, S. Understanding bacteriophage specificity in natural microbial communities. Viruses 2013, 5, 806–823. [Google Scholar] [CrossRef]
- Raj, J.R.M.; Karunasagar, I. Phages amid antimicrobial resistance. Crit. Rev. Microbiol. 2019, 45, 701–711. [Google Scholar] [CrossRef]
- De Jonge, P.A.; Nobrega, F.L.; Brouns, S.J.J.; Dutilh, B.E. Molecular and Evolutionary Determinants of Bacteriophage Host Range. Trends Microbiol. 2019, 27, 51–63. [Google Scholar] [CrossRef]
- Cazares, D.; Cazares, A.; Figueroa, W.; Guarneros, G.; Edwards, R.A.; Vinuesa, P. A Novel Group of Promiscuous Podophages Infecting Diverse Gammaproteobacteria from River Communities Exhibits Dynamic Intergenus Host Adaptation. mSystems 2021, 6, e00773-20. [Google Scholar] [CrossRef]
- Enault, F.; Briet, A.; Bouteille, L.; Roux, S.; Sullivan, M.B.; Petit, M.-A. Phages rarely encode antibiotic resistance genes: A cautionary tale for virome analyses. ISME J. 2017, 11, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Calero-Cáceres, W.; Ye, M.; Balcázar, J.L. Bacteriophages as Environmental Reservoirs of Antibiotic Resistance. Trends Microbiol. 2019, 27, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, E.; Bonnin, A.R.; Rocha, E.P. Phage-plasmids spread antibiotic resistance genes through infection and lysogenic conversion. mBio 2022, 13, e0185122. [Google Scholar] [CrossRef] [PubMed]
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages Contribute to the Spread of Antibiotic Resistance Genes among Foodborne Pathogens of the Enterobacteriaceae Family-A Review. Front. Microbiol. 2017, 8, 1108. [Google Scholar] [CrossRef]
- Brown-Jaque, M.; Rodriguez Oyarzun, L.; Cornejo-Sánchez, T.; Martín-Gómez, M.T.; Gartner, S.; de Gracia, J.; Rovira, S.; Alvarez, A.; Jofre, J.; González-López, J.J.; et al. Detection of Bacteriophage Particles Containing Antibiotic Resistance Genes in the Sputum of Cystic Fibrosis Patients. Front. Microbiol. 2018, 9, 856. [Google Scholar] [CrossRef]
- Kidambi, S.P.; Ripp, S.; Miller, R.V. Evidence for phage-mediated gene transfer among Pseudomonas aeruginosa strains on the phylloplane. Appl. Environ. Microbiol. 1994, 60, 496–500. [Google Scholar] [CrossRef]
- Goh, S.; Hussain, H.; Chang, B.J.; Emmett, W.; Riley, T.V.; Mullany, P. Phage ϕC2 mediates transduction of Tn6215, encoding erythromycin resistance, between Clostridium difficile strains. mBio 2013, 4, e00840-13. [Google Scholar] [CrossRef]
- Moon, B.Y.; Park, J.Y.; Hwang, S.Y.; Robinson, D.A.; Thomas, J.C.; Fitzgerald, J.R.; Park, Y.H.; Seo, K.S. Phage-mediated horizontal transfer of a Staphylococcus aureus virulence-associated genomic island. Sci. Rep. 2015, 5, 9784. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Wolz, C. Phages of Staphylococcus aureus and their impact on host evolution. Infect. Genet. Evol. 2014, 21, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.N.; Porse, A.; Sommer, M.O.A.; Høiby, N.; Ciofu, O. Evolution of Antibiotic Resistance in Biofilm and Planktonic Pseudomonas aeruginosa Populations Exposed to Subinhibitory Levels of Ciprofloxacin. Antimicrob. Agents Chemother. 2018, 62, e00320-18. [Google Scholar] [CrossRef]
- Stanczak-Mrozek, K.I.; Laing, K.G.; Lindsay, J.A. Resistance gene transfer: Induction of transducing phage by sub-inhibitory concentrations of antimicrobials is not correlated to induction of lytic phage. J. Antimicrob. Chemother. 2017, 72, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Wachino, J.-I.; Jin, W.; Kimura, K.; Arakawa, Y. Intercellular Transfer of Chromosomal Antimicrobial Resistance Genes between Acinetobacter baumannii Strains Mediated by Prophages. Antimicrob. Agents Chemother. 2019, 63, e00334-19. [Google Scholar] [CrossRef]
- Visnapuu, A.; van der Gucht, M.; Wagemans, J.; Lavigne, R. Deconstructing the Phage-Bacterial Biofilm Interaction as a Basis to Establish New Antibiofilm Strategies. Viruses 2022, 14, 1057. [Google Scholar] [CrossRef]
- Güemes, A.G.C.; Youle, M.; Cantú, V.A.; Felts, B.; Nulton, J.; Rohwer, F. Viruses as Winners in the Game of Life. Annu. Rev. Virol. 2016, 3, 197–214. [Google Scholar] [CrossRef]
- Martinez-Hernandez, F.; Fornas, O.; Gomez, M.L.; Bolduc, B.; de La Peña, M.J.C.; Martínez, J.M.; Anton, J.; Gasol, J.M.; Rosselli, R.; Rodriguez-Valera, F.; et al. Single-virus genomics reveals hidden cosmopolitan and abundant viruses. Nat. Commun. 2017, 8, 15892. [Google Scholar] [CrossRef]
- Turzynski, V.; Monsees, I.; Moraru, C.; Probst, A.J. Imaging Techniques for Detecting Prokaryotic Viruses in Environmental Samples. Viruses 2021, 13, 2126. [Google Scholar] [CrossRef]
- Kenzaka, T.; Tani, K.; Sakotani, A.; Yamaguchi, N.; Nasu, M. High-frequency phage-mediated gene transfer among Escherichia coli cells, determined at the single-cell level. Appl. Environ. Microbiol. 2007, 73, 3291–3299. [Google Scholar] [CrossRef]
- Kenzaka, T.; Tani, K.; Nasu, M. High-frequency phage-mediated gene transfer in freshwater environments determined at single-cell level. ISME J. 2010, 4, 648–659. [Google Scholar] [CrossRef]
- Papaianni, M.; Ricciardelli, A.; Casillo, A.; Corsaro, M.M.; Borbone, F.; Della Ventura, B.; Velotta, R.; Fulgione, A.; Woo, S.L.; Tutino, M.L.; et al. The Union Is Strength: The Synergic Action of Long Fatty Acids and a Bacteriophage against Xanthomonas campestris Biofilm. Microorganisms 2020, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, N.A.; Barnard, A.M.; Slater, H.; Simpson, N.J.; Salmond, G.P. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 2001, 25, 365–404. [Google Scholar] [CrossRef]
- Sørensen, S.J.; Bailey, M.; Hansen, L.H.; Kroer, N.; Wuertz, S. Studying plasmid horizontal transfer in situ: A critical review. Nat. Rev. Microbiol. 2005, 3, 700–710. [Google Scholar] [CrossRef]
- Christie, P.J. The Mosaic Type IV Secretion Systems. EcoSal Plus 2016, 7, 10. [Google Scholar] [CrossRef]
- Grohmann, E.; Christie, P.J.; Waksman, G.; Backert, S. Type IV secretion in Gram-negative and Gram-positive bacteria. Mol. Microbiol. 2018, 107, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Kohler, V.; Vaishampayan, A.; Grohmann, E. Broad-host-range Inc18 plasmids: Occurrence, spread and transfer mechanisms. Plasmid 2018, 99, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Tegtmeyer, N.; Stingl, K.; Backert, S. Four Chromosomal Type IV Secretion Systems in Helicobacter pylori: Composition, Structure and Function. Front. Microbiol. 2020, 11, 1592. [Google Scholar] [CrossRef]
- Shen, Z.; Tang, C.M.; Liu, G.-Y. Towards a better understanding of antimicrobial resistance dissemination: What can be learnt from studying model conjugative plasmids? Mil. Med. Res. 2022, 9, 3. [Google Scholar] [CrossRef]
- Hinnekens, P.; Koné, K.M.; Fayad, N.; Leprince, A.; Mahillon, J. pXO16, the large conjugative plasmid from Bacillus thuringiensis serovar israelensis displays an extended host spectrum. Plasmid 2019, 102, 46–50. [Google Scholar] [CrossRef]
- Fercher, C.; Probst, I.; Kohler, V.; Goessweiner-Mohr, N.; Arends, K.; Grohmann, E.; Zangger, K.; Meyer, N.H.; Keller, W. VirB8-like protein TraH is crucial for DNA transfer in Enterococcus faecalis. Sci. Rep. 2016, 6, 24643. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Spicher, C.; Haas, R.; Fischer, W. Excision and transfer of an integrating and conjugative element in a bacterial species with high recombination efficiency. Sci. Rep. 2019, 9, 8915. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.; Szabó, M.; Hegyi, A.; Douard, G.; Praud, K.; Nagy, I.; Olasz, F.; Cloeckaert, A.; Doublet, B. Identification and Characterization of oriT and Two Mobilization Genes Required for Conjugative Transfer of Salmonella Genomic Island 1. Front. Microbiol. 2019, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Redondo, R.; Olesen, A.K.; Russel, J.; de Vries, L.E.; Christensen, L.D.; Musovic, S.; Nesme, J.; Sørensen, S.J. Broad Dissemination of Plasmids across Groundwater-Fed Rapid Sand Filter Microbiomes. mBio 2021, 12, e0306821. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, L.J.; Bouwer, E.J. RP4 plasmid transfer among species of pseudomonas in a biofilm reactor. Water Sci. Technol. 1999, 39, 163–171. [Google Scholar] [CrossRef]
- Lampkowska, J.; Feld, L.; Monaghan, A.; Toomey, N.; Schjørring, S.; Jacobsen, B.; van der Voet, H.; Andersen, S.R.; Bolton, D.; Aarts, H.; et al. A standardized conjugation protocol to asses antibiotic resistance transfer between lactococcal species. Int. J. Food Microbiol. 2008, 127, 172–175. [Google Scholar] [CrossRef]
- Neil, K.; Allard, N.; Grenier, F.; Burrus, V.; Rodrigue, S. Highly efficient gene transfer in the mouse gut microbiota is enabled by the Incl2 conjugative plasmid TP114. Commun. Biol. 2020, 3, 523. [Google Scholar] [CrossRef]
- Bradley, D.E.; Taylor, D.E.; Cohen, D.R. Specification of surface mating systems among conjugative drug resistance plasmids in Escherichia coli K-12. J. Bacteriol. 1980, 143, 1466–1470. [Google Scholar] [CrossRef]
- Sabbagh, P.; Rajabnia, M.; Maali, A.; Ferdosi-Shahandashti, E. Integron and its role in antimicrobial resistance: A literature review on some bacterial pathogens. Iran. J. Basic Med. Sci. 2021, 24, 136–142. [Google Scholar] [CrossRef]
- Strugeon, E.; Tilloy, V.; Ploy, M.-C.; Da Re, S. The Stringent Response Promotes Antibiotic Resistance Dissemination by Regulating Integron Integrase Expression in Biofilms. mBio 2016, 7, e00868-16. [Google Scholar] [CrossRef]
- Pongchaikul, P.; Santanirand, P.; Antonyuk, S.; Winstanley, C.; Darby, A.C. AcGI1, a novel genomic island carrying antibiotic resistance integron In687 in multidrug resistant Achromobacter xylosoxidans in a teaching hospital in Thailand. FEMS Microbiol. Lett. 2020, 367, fnaa109. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, T.M.; Gillings, M.R.; Penesyan, A.; Qi, Q.; Rajabal, V.; Tetu, S.G. The Natural History of Integrons. Microorganisms 2021, 9, 2212. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.P.; Kwong, S.M.; Murphy, R.J.T.; Yui Eto, K.; Price, K.J.; Nguyen, Q.T.; O’Brien, F.G.; Grubb, W.B.; Coombs, G.W.; Firth, N. An updated view of plasmid conjugation and mobilization in Staphylococcus. Mob. Genet. Elements 2016, 6, e1208317. [Google Scholar] [CrossRef] [PubMed]
- Smillie, C.; Garcillán-Barcia, M.P.; Francia, M.V.; Rocha, E.P.C.; de La Cruz, F. Mobility of plasmids. Microbiol. Mol. Biol. Rev. 2010, 74, 434–452. [Google Scholar] [CrossRef]
- Binsker, U.; Käsbohrer, A.; Hammerl, J.A. Global colistin use: A review of the emergence of resistant Enterobacterales and the impact on their genetic basis. FEMS Microbiol. Rev. 2022, 46, fuab049. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mitra, S.; Dutta, S.; Basu, S. Neonatal Sepsis: The Impact of Carbapenem-Resistant and Hypervirulent Klebsiella pneumoniae. Front. Med. 2021, 8, 634349. [Google Scholar] [CrossRef]
- Fang, L.-X.; Chen, C.; Cui, C.-Y.; Li, X.-P.; Zhang, Y.; Liao, X.-P.; Sun, J.; Liu, Y.-H. Emerging High-Level Tigecycline Resistance: Novel Tetracycline Destructases Spread via the Mobile Tet(X). Bioessays 2020, 42, e2000014. [Google Scholar] [CrossRef]
- Che, Y.; Yang, Y.; Xu, X.; Břinda, K.; Polz, M.F.; Hanage, W.P.; Zhang, T. Conjugative plasmids interact with insertion sequences to shape the horizontal transfer of antimicrobial resistance genes. Proc. Natl. Acad. Sci. USA 2021, 118, e2008731118. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Y.; Zhang, J.; Liang, P.; Huang, X. Conjugative potential of antibiotic resistance plasmids to activated sludge bacteria from wastewater treatment plants. Int. Biodeterior. Biodegrad. 2019, 138, 33–40. [Google Scholar] [CrossRef]
- Li, L.; Dechesne, A.; He, Z.; Madsen, J.S.; Nesme, J.; Sørensen, S.J.; Smets, B.F. Estimating the Transfer Range of Plasmids Encoding Antimicrobial Resistance in a Wastewater Treatment Plant Microbial Community. Environ. Sci. Technol. Lett. 2018, 5, 260–265. [Google Scholar] [CrossRef]
- Valle, A.A.-D.; León-Sampedro, R.; Rodríguez-Beltrán, J.; DelaFuente, J.; Hernández-García, M.; Ruiz-Garbajosa, P.; Cantón, R.; Peña-Miller, R.; San Millán, A. Variability of plasmid fitness effects contributes to plasmid persistence in bacterial communities. Nat. Commun. 2021, 12, 2653. [Google Scholar] [CrossRef] [PubMed]
- Zaman, T.U.; Alrodayyan, M.; Albladi, M.; Aldrees, M.; Siddique, M.I.; Aljohani, S.; Balkhy, H.H. Clonal diversity and genetic profiling of antibiotic resistance among multidrug/carbapenem-resistant Klebsiella pneumoniae isolates from a tertiary care hospital in Saudi Arabia. BMC Infect. Dis. 2018, 18, 205. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, J.; Chen, F.; Yu, J.; Simner, P.; Tamma, P.; Liu, Y.; Shen, L. Emergence and establishment of KPC-2-producing ST11 Klebsiella pneumoniae in a general hospital in Shanghai, China. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Grinberg, I.; Eldar, A.; Grossman, A.D. A mobile genetic element increases bacterial host fitness by manipulating development. Elife 2021, 10, e65924. [Google Scholar] [CrossRef]
- Król, J.E.; Wojtowicz, A.J.; Rogers, L.M.; Heuer, H.; Smalla, K.; Krone, S.M.; Top, E.M. Invasion of E. coli biofilms by antibiotic resistance plasmids. Plasmid 2013, 70, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, M.; Ahmad, I.; Althubiani, A.S. Multidrug resistance and transferability of blaCTX-M among extended-spectrum β-lactamase-producing enteric bacteria in biofilm. J. Glob. Antimicrob. Resist. 2016, 6, 142–149. [Google Scholar] [CrossRef]
- Mo, S.S.; Sunde, M.; Ilag, H.K.; Langsrud, S.; Heir, E. Transfer Potential of Plasmids Conferring Extended-Spectrum-Cephalosporin Resistance in Escherichia coli from Poultry. Appl. Environ. Microbiol. 2017, 83, e00654-17. [Google Scholar] [CrossRef]
- Hausner, M.; Wuertz, S. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl. Environ. Microbiol. 1999, 65, 3710–3713. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Wattiau, P.; Wuertz, S.; Bathe, S.; Mohan, S.V.; Wilderer, P.A.; Hausner, M. Dual labeling of Pseudomonas putida with fluorescent proteins for in situ monitoring of conjugal transfer of the TOL plasmid. Appl. Environ. Microbiol. 2003, 69, 4846–4852. [Google Scholar] [CrossRef]
- Aspray, T.J.; Hansen, S.K.; Burns, R.G. A soil-based microbial biofilm exposed to 2,4-D: Bacterial community development and establishment of conjugative plasmid pJP4. FEMS Microbiol. Ecol. 2005, 54, 317–327. [Google Scholar] [CrossRef]
- Christensen, B.B.; Sternberg, C.; Molin, S. Bacterial plasmid conjugation on semi-solid surfaces monitored with the green fluorescent protein (GFP) from Aequorea victoria as a marker. Gene 1996, 173, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, C.; Bergström, M.; Hermansson, M. In Situ Detection of High Levels of Horizontal Plasmid Transfer in Marine Bacterial Communities. Appl. Environ. Microbiol. 1998, 64, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Klümper, U.; Riber, L.; Dechesne, A.; Sannazzarro, A.; Hansen, L.H.; Sørensen, S.J.; Smets, B.F. Broad host range plasmids can invade an unexpectedly diverse fraction of a soil bacterial community. ISME J. 2015, 9, 934–945. [Google Scholar] [CrossRef]
- Ma, H.; Bryers, J.D. Non-invasive method to quantify local bacterial concentrations in a mixed culture biofilm. J. Ind. Microbiol. Biotechnol. 2010, 37, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.C.; Palmer, R.J.; Udsen, C.; White, D.C.; Molin, S. Assessment of GFP fluorescence in cells of Streptococcus gordonii under conditions of low pH and low oxygen concentration. Microbiology 2001, 147, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.E.; Marsh, E.N.G.; Biteen, J.S. Extending fluorescence microscopy into anaerobic environments. Curr. Opin. Chem. Biol. 2019, 51, 98–104. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Y.; Song, Y.; Lin, H.; Yin, H. Dissecting horizontal and vertical gene transfer of antibiotic resistance plasmid in bacterial community using microfluidics. Environ. Int. 2019, 131, 105007. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, J.; Li, B.; Wen, X.; Liang, P.; Huang, X. A novel microfluidic system enables visualization and analysis of antibiotic resistance gene transfer to activated sludge bacteria in biofilm. Sci. Total Environ. 2018, 642, 582–590. [Google Scholar] [CrossRef]
- Wuertz, S.; Hendrickx, L.; Kuehn, M.; Rodenacker, K.; Hausner, M. In situ quantification of gene transfer in biofilms. Methods Enzymol. 2001, 336, 129–143. [Google Scholar] [CrossRef]
- Bellanger, X.; Payot, S.; Leblond-Bourget, N.; Guédon, G. Conjugative and mobilizable genomic islands in bacteria: Evolution and diversity. FEMS Microbiol. Rev. 2014, 38, 720–760. [Google Scholar] [CrossRef] [PubMed]
- Libante, V.; Nombre, Y.; Coluzzi, C.; Staub, J.; Guédon, G.; Gottschalk, M.; Teatero, S.; Fittipaldi, N.; Leblond-Bourget, N.; Payot, S. Chromosomal Conjugative and Mobilizable Elements in Streptococcus suis: Major Actors in the Spreading of Antimicrobial Resistance and Bacteriocin Synthesis Genes. Pathogens 2019, 9, 22. [Google Scholar] [CrossRef]
- Guédon, G.; Libante, V.; Coluzzi, C.; Payot, S.; Leblond-Bourget, N. The Obscure World of Integrative and Mobilizable Elements, Highly Widespread Elements that Pirate Bacterial Conjugative Systems. Genes 2017, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.; Lacroix, T.; Guédon, G.; Coluzzi, C.; Payot, S.; Leblond-Bourget, N.; Chiapello, H. ICEscreen: A tool to detect Firmicute ICEs and IMEs, isolated or enclosed in composite structures. NAR Genom. Bioinform. 2022, 4, lqac079. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, O.S.; de Assis, J.C.S.; Santana, M.F. Breaking the ICE: An easy workflow for identifying and analyzing integrative and conjugative elements in bacterial genomes. Funct. Integr. Genom. 2022, 22, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Guglielmini, J.; Quintais, L.; Garcillán-Barcia, M.P.; de La Cruz, F.; Rocha, E.P.C. The repertoire of ICE in prokaryotes underscores the unity, diversity, and ubiquity of conjugation. PLoS Genet. 2011, 7, e1002222. [Google Scholar] [CrossRef]
- Grindley, N.D.F.; Whiteson, K.L.; Rice, P.A. Mechanisms of site-specific recombination. Annu. Rev. Biochem. 2006, 75, 567–605. [Google Scholar] [CrossRef] [PubMed]
- Delavat, F.; Miyazaki, R.; Carraro, N.; Pradervand, N.; van der Meer, J.R. The hidden life of integrative and conjugative elements. FEMS Microbiol. Rev. 2017, 41, 512–537. [Google Scholar] [CrossRef]
- Botelho, J.; Schulenburg, H. The Role of Integrative and Conjugative Elements in Antibiotic Resistance Evolution. Trends Microbiol. 2021, 29, 8–18. [Google Scholar] [CrossRef]
- Roberts, A.P.; Mullany, P. Tn916-like genetic elements: A diverse group of modular mobile elements conferring antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 856–871. [Google Scholar] [CrossRef]
- Botelho, J.; Grosso, F.; Peixe, L. ICEs Are the Main Reservoirs of the Ciprofloxacin-Modifying crpP Gene in Pseudomonas aeruginosa. Genes 2020, 11, 889. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, H.; Anthonisen, I.L.; Zecic, N.; Hegstad, K.; Ranheim, T.E.; Skaare, D. Characterization and Fitness Cost of Tn7100, a Novel Integrative and Conjugative Element Conferring Multidrug Resistance in Haemophilus influenzae. Front. Microbiol. 2022, 13, 945411. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kuang, X.; Han, R.-J.; Zhai, Y.-J.; He, D.-D.; Zhao, J.-F.; Liu, J.-H.; Hu, G.-Z. Characterization of a Novel Linezolid Resistance Gene optrA and Bacitracin Resistance Locus-Carrying Multiple Antibiotic Resistant Integrative and Conjugative Element ICESsu1112S in Streptococccus Suis. Microbiol. Spectr. 2022, 10, e0196321. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.P.; Davis, I.J.; Seville, L.; Villedieu, A.; Mullany, P. Characterization of the ends and target site of a novel tetracycline resistance-encoding conjugative transposon from Enterococcus faecium 664.1H1. J. Bacteriol. 2006, 188, 4356–4361. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Song, Y.; Zhang, Q.; Zhang, A.; Jin, M. Characterisation of a novel integrative and conjugative element ICESsD9 carrying erm(B) and tet(O) resistance determinants in Streptococcus suis, and the distribution of ICESsD9-like elements in clinical isolates. J. Glob. Antimicrob. Resist. 2016, 7, 13–18. [Google Scholar] [CrossRef]
- Auchtung, J.M.; Lee, C.A.; Monson, R.E.; Lehman, A.P.; Grossman, A.D. Regulation of a Bacillus subtilis mobile genetic element by intercellular signaling and the global DNA damage response. Proc. Natl. Acad. Sci. USA 2005, 102, 12554–12559. [Google Scholar] [CrossRef]
- Waldor, M.K.; Tschäpe, H.; Mekalanos, J.J. A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J. Bacteriol. 1996, 178, 4157–4165. [Google Scholar] [CrossRef]
- Franke, A.E.; Clewell, D.B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J. Bacteriol. 1981, 145, 494–502. [Google Scholar] [CrossRef]
- Shoemaker, N.B.; Barber, R.D.; Salyers, A.A. Cloning and characterization of a Bacteroides conjugal tetracycline-erythromycin resistance element by using a shuttle cosmid vector. J. Bacteriol. 1989, 171, 1294–1302. [Google Scholar] [CrossRef]
- Burrus, V.; Roussel, Y.; Decaris, B.; Guédon, G. Characterization of a novel integrative element, ICESt1, in the lactic acid bacterium Streptococcus thermophilus. Appl. Environ. Microbiol. 2000, 66, 1749–1753. [Google Scholar] [CrossRef]
- Pavlovic, G.; Burrus, V.; Gintz, B.; Decaris, B.; Guédon, G. Evolution of genomic islands by deletion and tandem accretion by site-specific recombination: ICESt1-related elements from Streptococcus thermophilus. Microbiology 2004, 150, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.P.; Sullivan, J.T.; Stuart, G.S.; Lamont, I.L.; Ronson, C.W. Excision and transfer of the Mesorhizobium loti R7A symbiosis island requires an integrase IntS, a novel recombination directionality factor RdfS, and a putative relaxase RlxS. Mol. Microbiol. 2006, 62, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, X.; Roberts, A.P.; Morel, C.; Choulet, F.; Pavlovic, G.; Mullany, P.; Decaris, B.; Guédon, G. Conjugative transfer of the integrative conjugative elements ICESt1 and ICESt3 from Streptococcus thermophilus. J. Bacteriol. 2009, 191, 2764–2775. [Google Scholar] [CrossRef]
- Lécuyer, F.; Bourassa, J.-S.; Gélinas, M.; Charron-Lamoureux, V.; Burrus, V.; Beauregard, P.B. Biofilm Formation Drives Transfer of the Conjugative Element ICEBs1 in Bacillus subtilis. mSphere 2018, 3, e00473-18. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, J.-S.; Jeannotte, G.; Lebel-Beaucage, S.; Beauregard, P.B. Second-Generation Transfer Mediates Efficient Propagation of ICEBs1 in Biofilms. J. Bacteriol. 2022, 204, e0018122. [Google Scholar] [CrossRef]
- Babic, A.; Berkmen, M.B.; Lee, C.A.; Grossman, A.D. Efficient gene transfer in bacterial cell chains. mBio 2011, 2, e00027-11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).