Abstract
Antimicrobial residues may pose harmful effects on the health of consumers. At the same time, an adequate quality of drinking water for animals is one of the important element to ensure animal welfare and food without antibacterials. The presented study is aimed at estimating the residue levels of antibacterial compounds, such as penicillins, cephalosporin, macrolides, tetracyclines, quinolones, sulphonamides, aminoglycosides, diaminopirymidines, pleuromutilines and lincosamides in meat and on-farm drinking water samples using liquid chromatography-tandem mass spectrometry (LC-MS/MS), as a part of a surveillance system on pig and broiler farms within the project Healthy Livestock. A total of 870 samples of muscle from pig and broiler, as well as 229 water samples were analysed for antibiotic residues. Samples were collected from farms in EU countries in two steps, before and after implementation of a tailor-made health plan. In muscle samples, the detected concentrations of doxycycline in the post-intervention step (15.9–70.8 µg/kg) were lower than concentrations in the pre-intervention step (20.6–100 µg/kg). In water samples, doxycycline in an average concentration of 119 µg/L in the pre- and 23.1 µg/L in the post-intervention step, as well as enrofloxacin at concentrations of 170 µg/L in the pre- and 1.72 µg/L in the post-intervention step were quantified. Amoxicillin was only present before intervention. The obtained results confirm the effectiveness of the intervention actions. The concentrations of antibiotics in muscles and water were lower after implementation of a health plan on the farms.
Keywords:
antimicrobials; LC-MS/MS; muscle; drinking water; reduction; pig; broiler; health; welfare 1. Introduction
Over recent decades, intensive animal husbandry systems for food production have led to a significant increase in the use of veterinary medicines. To protect the health and welfare of livestock, antimicrobial agents, especially antibacterial compounds, are used worldwide, in a variety of extensive and intensive livestock production systems.
The overuse and excessive administration of antibacterials, as well as failures to comply with the warnings on antibiotic labels and withdrawal inadequacy, may cause residue occurrence in products of animal origin. Drug residues in foods derived from animals may lead to many adverse health effects for the consumer [1,2]. The residues may result in many biological adverse effects, such as allergic reactions, increased immunological responses in susceptible individuals and intestinal microbiota disturbance in consumers [3]. According to the WHO, the spread of drug-resistant bacteria, as well as bacterial resistance acquisition, is one of the major concerns for human and animal health [4,5].
The present study has focused on two selected livestock species, pigs and broilers, which are two of the top three sources of meat [6]. The consolidation of pig and broiler production requires ensuring suitable conditions of animal maintenance along with high health conditions, consistent with the guidelines for animal welfare [7]. To ensure health and welfare on farms, a suitable intervention plan needs to be elaborated. The objective of any developed biosecurity audit, such as the one described by Van Limbergen et al., 2018, as well as the one developed as part of Healthy Livestock and described by Schreuder et al., 2022, particularly in the case of intensive broiler production systems, is to identify the weak points of farm biosecurity and arrive at targeted proposals for improvements [8,9]. At the same time, antibiotic control in animals is an important element for securing higher quality animal production, while also ensuring consumer protection. To protect consumers’ health and ensure food safety with confidence in animal production, it is crucial to control all antibiotics used in pigs and broilers.
The EU has established monitoring programmes for the control of the presence of antimicrobial residues in the food chain. Regulation (EU) No 37/2010 establishes maximum residue limits (MRL) for residues of veterinary medicinal products in animal products [10]. In EU countries, the control of veterinary medicinal product residues and other substances in animal products is carried out every year, and non-complaint results are presented in annual reports. The latest report presents the results generated in 2020 as a part of official control actions. In the latest report issued from official data, only 0.14% of the samples analysed under the Directive 96/23/EC monitoring were non-compliant with antibacterial MRL in 2020, which was similar to 2019 [11,12]. According to the data included in this report, 15 countries reported a total of 42 non-compliant samples in pigs (65 non-compliant results), representing 0.12%. For antibacterials in poultry, five countries reported a total of seven non-compliant samples and results, which is 0.04%. According to European Union Reports, the percentage of non-compliant results are relatively low, but these documents present only results with concentrations above MRL values. There are some propositions and plans to report, in the future, all samples with antibiotics presence, even much lower than MRL. The EU report reveals that the most frequently used antibiotics in the pig industry in 2020 were tetracyclines and sulphonamides, while in poultry mainly tetracyclines (doxycycline) were reported. The distribution and the use of veterinary medicines in food animals is regulated by the law and responsible agencies worldwide. In compliance with the Regulation (EU) 2019/6 on veterinary medicinal products, the use of antimicrobials such as those for growth promotion and yield increase is prohibited [13].
In the face of an emerging outbreak of disease, particularly in poultry, less frequently in pigs, antimicrobial agents are added to drinking water, which is one of the most practical and economical routes of veterinary drug administration [14,15]. It provides rapid administration of medicines to all animals in the early stages of disease, low cost of solution preparation and easy distribution and drug storage, as well as facilitating quick changes of dosage [16]. However, in such a method of drugs administration, one important factor is to guarantee adequate water quality and water hygiene [17]. The physicochemical properties of drugs should be considered, including solubility in water and adsorption in the solid phase, because some substances can form complexes with the ions present in drinking water [18,19]. Contaminated water supply systems can cause the spread of medicines to the farm environment. The physicochemical properties of some antibacterials (tetracyclines, fluoroquinolones and sulphonamides) enable them to adhere to water supply system pipes and stay in the internal surface of pipes and become fixed to the biofilm [17]. This raises some issues: drugs can be systematically eluted at the end of animal treatment, causing an unintended application of antibiotics to animals. Moreover, the biofilm can in turn break away from the inner surface of the pipes and be drunk by broilers or pigs, and further spread any resistance developed among the bacteria contained within the biofilm. Additionally, after administration of various antibacterial agents in water, many interactions may take place, which can in turn disrupt the intended therapy and consequent drug elimination from body tissues. Therefore, a regular cleaning and a system of regular sanitation procedures in water supply systems with special cleaners should be implemented on each farm where food producing animals are housed. Hence one of the most important elements to ensure both animal welfare and food without antibacterial residues is the careful control of the water supply during animal production. However, in most EU countries, no official control of antibiotics in water supply systems is carried out. Published data from one study indicate the presence of antibiotics in 52% of analysed water samples [20].
The main objective of this study was to investigate the impact of the implementation of tailor-made health plans, including biosecurity measures on the results of analyses of antimicrobial residues in muscle and water. Many classes of antibiotics and antibacterial compounds, such as β-lactams (penicillins and cephalosporins), macrolides, tetracyclines, (fluoro)quinolones, sulphonamides, aminoglycosides, lincosamides, pleuromutilins and diaminopyrimidines can be administered to food-producing animals, according to EU regulation; therefore, all these groups of substances were tested in the presented study by the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. Broiler farms from the Netherlands, Cyprus and Greece and pig farms from Italy and France were involved in this research.
2. Results
2.1. Method Validation
A full validation of the methods used in this study have been previously described [20,21]. Briefly, the method for antibiotics analysis in muscle was validated according to the criteria of Commission Decision 2002/657/EC [22]. Matrix-matched calibration was used for quantification in order to reach a high accuracy. The method is linear in a wide range, as confirmed by the correlation coefficient r > 0.99, where the lowest concentration on the calibration curve refers to the limit of quantification (LOQ). The recoveries ranged from 88% to 105% and within-laboratory reproducibility was lower than 15%. The validation results of the method for determination of antibiotics in muscle and water are reported in Table 1. The matrix-matched calibration curves for water achieved good linearity (r > 0.99). The recoveries are in the range between 84% and 109%, within-laboratory reproducibilities are below 14%, and the LOQ values are in the range of 0.02–10 µg/L, depending on analyte.

Table 1.
Recoveries, reproducibilities and LOQs achieved during validation study on spiked muscle and water.
2.2. Quantitative Analysis of Antibiotics in Muscle and Water
A total of 870 samples of muscle from pigs and broilers, as well as 229 water samples were analysed for antibiotic residues. Five hundred eighty-five muscles and 116 water samples from broiler farms were analysed. Two hundred eighty-five pig muscles and 113 water samples were tested. The main goal of this research was to demonstrate the differences in antibiotic residues before and after intervention on pig and broiler farms. No antibiotics were detected in muscle or water on pig farms, either before or after intervention. Doxycycline was detected in muscle samples from 15 broiler farms, where conventional antibiotics usage was documented and confirmed. Detected concentrations of doxycycline in the post-intervention step (15.9–70.8 µg/kg) were lower than concentrations in the pre-intervention step (20.6–100 µg/kg). Only in one muscle sample was the residue level of doxycycline equal to MRL = 100 µg/kg, before implementation of the health plan. Of all the 116 water samples analysed, antibiotics were present in 22 of them (doxycycline—15 and 13 before and after the plan, respectively; enrofloxacin—20 and 10 before and after the plan, respectively; amoxicillin—only 3 before the health plan). In water samples, doxycycline in an average concentration of 119 µg/L in the pre- and 23.1 µg/L in the post-intervention, as well as enrofloxacin in an average concentration of 170 µg/L in the pre- and 1.72 µg/L in the post-intervention step were quantified. All water samples with antibiotics were from broiler farms. The obtained results suggest the effect of the intervention actions. The concentrations of antibiotics in muscles were slightly lower after implementation of a health plan on farms. The differences in the levels of confirmed drugs are more evident in water samples. Water samples from seven farms (farm 16 to farm 22) contained 1–3 antibiotics (doxycycline, enrofloxacin or amoxicillin) before intervention, while no antibiotics in muscles from those farms were found. In these water samples, after heath plan application, only enrofloxacin with significantly lower levels was detected. At the pre-intervention step, the concentrations of amoxicillin in three water samples from three farms were relatively high (163, 2475 and 2962 µg/L), whereas antibiotics were no longer detected post-intervention. The obtained results are presented in Table 2. The statistical method used in data analysis was descriptive statistics, which summarizes data using mean concentrations and standards deviation (SD). For muscles samples (n = 10), SD was calculated, but for water samples where only two replicates were analysed, SD was not indicated. The chromatograms of LC-MS/MS analysis for water and muscle samples from broilers with detected and confirmed antibacterials are presented on Figure 1.

Table 2.
Average concentrations of antibiotics detected in muscle and water samples per broiler farm.
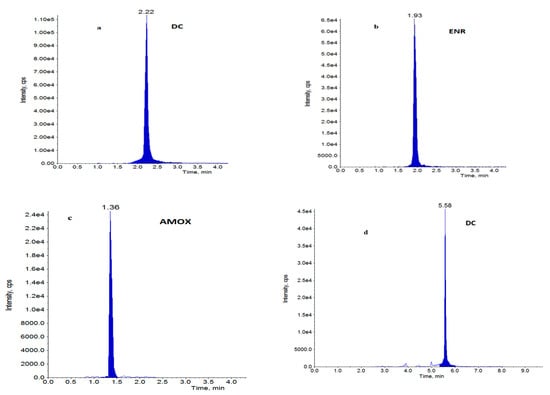
Figure 1.
Chromatograms of water samples (a–c) with: (a) doxycycline, (b) enrofloxacin, (c) amoxicillin at a concentration of 5 µg/L and muscle sample, (d) with doxycycline at a concentration of 5 µg/kg.
3. Discussion
Antibacterial spread began more than half a decade ago, when AMR was not considered a public health risk. The World Health Organization report from September 2021 declared AMR a major public health concern [23]. AMR is hastened by contemporary farming practices in which many animals are housed in overcrowded and unhygienic conditions that provide an ideal environments for the expansion and reproduction of antibiotic-resistant bacteria and resistance genes [24]. However, various measures are being taken to reverse the routine use of antibiotics in livestock.
The question arises: is it possible to raised animals without antibiotics? While antibiotic-free pork production is favourable, research by Dee et al., 2018, presented the problems of keeping livestock completely without antibiotics and declared them to be a serious disease challenge, such as in the case of porcine reproductive and respiratory syndrome virus [25]. While the total elimination of antibiotics can be challenging, the reduction and responsible use seems to be the priority in enhancing animal health and welfare [26]. Using antimicrobials in poultry producing meat and eggs, as well as in pigs producing meat for human consumption, should be carried out with special responsibility and attention. The prudent use of antimicrobials refers to the optimal choice of drug, dose and the time of antimicrobial treatment, along with limiting inappropriate administration and overuse. The Healthy Livestock research programme, under which the presented research was performed, is looking at a reduction in the risk of exposure of animals to pathogens; an early detection of health problems and specific diseases; increasing the resistance of animals to diseases; and if antimicrobials are necessary, a more prudent use or the application of alternatives. However, in order to reduce the use of antimicrobials in pig and broiler farms, it is important to implement biosecurity measures to prevent pathogens from entering the farms or avoid the spread of the pathogens within the farm premises. Both external and internal biosecurity measures contribute to this objective [27]
The analysis of antimicrobial residues in muscle and water on pig and broiler farms conducted as a part of the Healthy Livestock project aimed to compare antibiotic levels on pig and broiler farms before the implementation of health plans and after the use of some biosecurity measures to enhance animal health and welfare. Determination and implementation of measures were performed within other work packages. Each farm was visited to establish the weak points, in the level of either external or internal biosecurity, as identified using the BEAT risk assessment tool developed as part of the Healthy Livestock project [9]. Based on this analysis, tailor made health plans were designed. The biosecurity measures implemented on broiler and pig farms are presented in the Supplementary Material (Tables S1 and S2). The farm selection was an important factor in the implementation of the intervention plans and the influence on antibiotic residues detection. The broiler farms selected, particularly in Cyprus and Greece, but not so in the Netherlands, were known to be reliant on the use of antibiotic veterinary interventions in previous production cycles. Although they were not typical of the specific countries, they were specifically selected for this study so as to best test the hypothesis as to whether improvements in biosecurity would indeed improve the health status of subsequent production flocks and hence reduce the need of veterinary interventions and consequently the use of antibiotics. The BEAT system was tested on farms in Cyprus, Greece and the Netherlands to asses if the implementation of the health plan resulted in a reduction in antimicrobial use. In improving the health status of animals and reducing the use of antibiotics, thus decreasing the incidence of antibiotic residues, short-term and low-cost interventions that were mostly aimed at improving disinfection and the training of people entering the farm have proven to be most effective [9]. A detailed description of the results of implementation of health plans in broiler farms is presented in Schreuder et al., 2022 [9]. In pig farms, no residues were confirmed.
The results obtained in this research show that the percentage of muscle samples with antibacterials was quite low and reached 2.6%, considering only chicken muscles in general. In water samples, doxycycline was quantified at the pre- and post-intervention step as 13.7% and 11.2%, respectively. Even though the obtained percentage difference is slight, the concentrations of antibiotic were much lower after biosecurity actions. For enrofloxacin, both percentages of positive samples (17.2% pre- and 8.6% post-intervention) as well as concentrations were significantly lower, after biosecurity was undertaken. Samples of water with amoxicillin were found at 1.7% before interventions, while all samples were negative after the measures were introduced. On pig farms, no antibiotics were detected, both before and after implementation of the health plan.
In the presented study, doxycycline was found in both muscle and water, both pre- and post-intervention, which indicates the high stability of this antibiotic [28]. Despite the presence of enrofloxacin and amoxicillin residues in a few water samples, no residues in muscles were confirmed. Similar to the results presented in this paper, the most frequently found compound in muscles from the group of tetracyclines in the EU is doxycycline [11,12]. According to an EU Report in 2019, doxycycline was found in 24 muscle samples of pigs and 6 samples in poultry. Amoxicillin was confirmed only in pigs (six non-compliant results), while enrofloxacin was detected in one muscle sample in poultry. In a 2020 EU report, most non-compliant results in pigs concerned tetracyclines (28 samples), including 12 results with doxycycline. From (fluoro)quinolones, enrofloxacin was presented in five samples, while for penicillin, amoxicillin was confirmed in two samples together with penicillin G in three samples. In poultry, only seven non-compliant results were found, and four of these were related to doxycycline. According to the ranking of antibiotic families based on their occurrence (%) created by the World Organization of Animal Health (OIE), the most widely used group of antibacterials are tetracyclines (87.1%) and penicillins (87.1%) [29].
Roblez-Jimenez et al., 2022, reported the concentration of antibiotic residues found in the environment, livestock, animal tissues, animal products (milk and eggs), wastewater and soil, based on a very comprehensive literature review [30]. According to that study, the levels of antibiotics based on continent showed a notable differences among antimicrobials groups [30]. The antimicrobial with the highest concentration in Asia was cephalosporin, followed by fluroquinolone. The highest residual concentrations in Africa and North America involved tetracyclines, while in South America fluoroquinolones and macrolides were the most frequent. In Europe, the highest concentrations were shown by β-lactam; however, in Europe the main antibiotics sold were tetracyclines (32.8%), penicillins (25.0%) and sulphonamides (11.8%) [31]. Considering the residues of antibiotics in animal products, based on the data from various parts of the world, the largest concentration of residues was found in chicken, with the main occurrences being fluoroquinolones and tetracyclines [30].
The residue of antibiotics on farms can be present due to animal excretion, pig and poultry faeces or manure. In the literature data, the most commonly detected antibiotics in manure, faeces and slurry are tetracyclines [32]. In the research of Patyra et al., 2020, out of 70 pig and poultry faeces and manure samples, 15 were positive for doxycycline [33]. According to Rasschaer et al., 2020, the most frequently detected antibiotics were doxycycline, sulfadiazine and lincomycin, but doxycycline was found in the highest concentration, with a mean of 1476 μg/kg manure [34]. Residues of some antibiotics in poultry can also be present in feathers. Gajda et al., 2019, demonstrated high concentrations of doxycycline in broiler feathers for a long time after (22 days) post-treatment [35].
4. Materials and Methods
4.1. Sample Collection on Broiler and Pigs Farms
Muscle and water samples were collected from broiler and pig farms. Thirteen broiler houses from the Netherlands, seven from Cyprus and ten from Greece were recruited to participate in this study. Twenty pig houses in France and fifteen pig farms in Italy were also involved. All pig and broiler farms were identified and documented. The objective was to identify the biosecurity and health standards on the site and what were considered the key areas needing improvement, with the aim of decreasing the need for antimicrobial use while maintaining biological and economic performance. Such health plans included changes which could mitigate risks, could be easily implemented and could reduce the use of antimicrobials. Biological and economic data, as well as antimicrobial use, were recorded for each farm in pre-intervention cycles and post-intervention cycles. Samples for detecting any residues in muscle and water were collected pre- and post-intervention. The number of samples collected from reach country is listed in Table 3. In France, it was not possible to collect muscle samples, as no access was provided by abattoirs due to the COVID-19 pandemic.

Table 3.
Number of muscle and water samples collected for antimicrobial residue analysis.
4.1.1. Farm Selection
Broiler and pig farms were selected based on the following criteria:
- Farms had to be users of antimicrobials if any progress on this aspect was to be demonstrated before any health plan implementation. Because it was difficult to recruit farms in the Netherlands, farms with no antimicrobial usage were also included.
- Farm veterinarians and farmers had to be willing to be involved.
- Participant farms ideally had to cover a range of pig and broiler houses and practices in place, including the age of the buildings, house equipment such as feeding systems and type of bedding, as well as the labour employed.
- In addition, broiler and pig density and other commercial livestock pressures on the location of each farm had to be considered so as to have a representative range of farm locations.
Each broiler house was based on a different farm, except in Greece where some broiler houses were within the same farm area. These broiler houses had different management and antimicrobial histories and thus were handled as independent houses. For the Netherlands, only one broiler house per farm was sampled, but on some farms, the biological data of multiple houses was collected per farm. Depending on the farm (broiler or pig), the criteria for the monitoring of pre- and post-intervention flock cycle monitoring were different. Four production cycles (cycles 1–4) were followed up, of which two cycles were considered as pre-intervention and two cycles as post-intervention. Water samples were taken at the end of rounds 1 (first) and 4 (last). Meat samples were collected at the slaughterhouse after the first and last round. Between rounds 2 and 3, the intervention plan was made and, depending on the sort of intervention, it was done at round 4.
4.1.2. Pre-Intervention Flock Cycle Monitoring
It was agreed that two broiler flock cycles were first to be monitored, where the active collection of data, as well as sampling for antimicrobial residues in targeted material and selected biomarker scores, were to be recorded, before any health plan implementation (intervention) took place. A protocol to monitor risk mitigation in pig farms during a 12-month study period was developed; at least three visits were performed in each farm. Antimicrobial residues were monitored by collecting the following representative samples:
- Water at the end of the water line towards the end of the cycle when the first of the broilers were selected at thin-out. Approximately 200 mL of water were collected per occasion. These were stored a −20 °C until dispatched to the National Veterinary Research Institute in Poland for residue analysis.
- Muscle at the processing plant at or near the first thinning. For this, five birds were sampled and combined into one. These were also stored at −20 °C and then dispatched to the National Veterinary Research Institute in Poland for residues analysis. LC-MS/MS analyses were performed up to 1 week after receiving the samples.
Complete details of any antimicrobial usage, including age of birds, details of the vet prescription, the pharmaceutical product used and dosage, as well as the dates of administration were also recorded, but that was part of another work package.
4.1.3. Post-Intervention Flock Cycle Monitoring
In broilers, following the sanitary vacuum before the second pre-intervention cycle, the first post-intervention cycle of monitoring started. This was followed in all cases by a second post-intervention period. In pigs, the interval between the pre-intervention cycle and the post-intervention cycle lasted from 8 to 12 months. This interval enabled the implementation of the tailor-made health plan between the different batches.
The same monitoring and sampling process as carried out before for the pre-intervention cycles was also repeated for all post-intervention broiler flocks and pig batches. This facilitated the opportunity to compare the outcomes of the health plans which were implemented, within the time constraints imposed, with a before and after effect, with each farm site being its own control.
4.2. LC-MS/MS Analysis
4.2.1. Chemicals and Reagents
Reagents. All organic solvents were HPLCgrade and all chemicals were analytical grade. Acetonitrile, was from J.T. Baker (Deventer, the Netherlands). Trichloroacetic acid (TCA) and sodium acetate was from Sigma-Aldrich (St. Louis, MO, USA). Heptafluorobutyricacid (HFBA) was from Fluka (St. Louis, MO, USA). PVDF filters were from Restek (College, PA, USA). Strata X columns were form Phenomenex (Torrance, CA, USA). Water was deionised (>18 MΩ cm−1) in-house by the Millipore system.
Analytical standard and standard solutions. Amoxicillin (AMOX), ampicillin (AMPI), penicillin G (PEN G), penicillin V (PEN V), oxacillin (OXA), cloxacillin (CLOX), nafcillin (NAF), dicloxacillin (DICLOX), cephapirin (CFPI), ceftiofur (CFT), cefoperazone (CFPE), cephalexin (CFLE), cefquinome (CFQ), cefazolin (CFZ), cefalonium (CFLO), sulfaguanidine (SGU), sulfadiazine (SDZ), sulfathiazole (STZ), sulfamerazine (SME), sulfamethazine (SMT), sulfamethoxazole (SMA), sulfamethoxypyridazine (SMP), sulfamonomethoxine (SMM), sulfadoxine(SDX), sulfaquinoxaline (SQX), sulfadimethoxine(SDMX), tylosin (TYL), erythromycin (ERY), spiramycin (SPI), tilmicosin (TIL), josamycin (JOS), danofloxacin (DAN), difloxacin (DIF), enrofloxacin(ENR), ciprofloxacin (CIP), flumequine (FLU), sarafloxacin (SAR), marbofloxacin (MAR), norfloxacin(NOR), oxolinic acid (OXO), nalidixic acid (NAL), chlortetracycline (CTC), tetracycline (TC), doxycycline(DC), oxytetracycline (OTC), streptomycin (STRP), dihydrostrepromycin (DISTRP), gentamycin (GEN),paromomycin (PAR), spectinomycin (SPEC), kanamycin (KAN), neomycin (NEO), lincomycin (LIN) and sulfaphenazole (IS) were from Sigma-Aldrich.
4.2.2. LC-MS/MS Analysis of Muscle
Chicken or pig meat samples were minced, homogenized and stored at 0 °C until analysis. These samples were analysed for the presence of 57 antimicrobial drugs by the LC-MS/MS method, using two extraction methods previously described by Błądek et al. [21].
Briefly, the first extraction method by acetonitrile is suitable for detecting and quantifying 45 antibiotics belonging to the following classes: β-lactams, sulphonamides, macrolides, fluoroquinolones, pleuromutilins and diaminopyrimidines. The protocol of the first method was as follows. To a muscle subsample (2 g), 8 mL of acetonitrile was added, mixed thoroughly and centrifuged. Then, 6 mL of supernatant was taken and evaporated to dryness at 45 °C. The dry residue was dissolved in 0.6 mL of 0.025% heptafluorobutyric acid (HFBA) and filtered through a 0.22 μm PVDF filter into a LC vial.
The second extraction method by aqueous solution of 5% trichloroacetic acid (TCA) allows the isolation of 12 antibiotics (aminoglycosides, tetracyclines, lincosamides). Extraction with this method involved adding 6 mL of 5% TCA to 2 g of muscle subsample. The sample was vortex mixed and centrifuged. Finally, the TCA extract (1 mL) was taken and filtered by a 0.22 um PVDF filter to vial for LC-MS/MS analysis.
LC-MS/MS analysis was performed by the Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA, USA) connected to an API 4000 triple quadrupole mass spectrometer (AB Sciex Framingham, MA, USA). Separation of target compounds was performed on a Luna C18 (2) 100 A column (150 × 2.0 mm, 3 μm) using acetonitrile (A) and 0.025% HFBA (B) as mobile phases in gradient mode [21]. For quantification, two product ions were monitored to ensure specific and accurate quantification. The information on ion transitions and optimal conditions for the fragmentation of monitored antibiotics are provided in Table 4.

Table 4.
List of analytes and mass spectrometry parameters for detection of antibacterial compounds.
4.2.3. LC-MS/MS Analysis of Water
Water from breeding animal watering supply was analysed by LC-MS/MS, as previously described by Gbylik-Sikorska et al., 2015 [20]. Forty-five veterinary compounds belonging to nine different antibiotic groups, including aminoglycosides, β-lactams, diaminopyrimidines, fluoroquinolones, lincosamides, macrolides, pleuromutilins, sulphonamides and tetracyclines, were determined. The tested antibiotics are marked with an asterisk in Table 1.
Isolation of antimicrobial substances from the water samples was based on extraction with sodium acetate and the addition of ionic pairs, followed by solid phase extraction (SPE) [20]. Concisely, to 250 mL of water, 6 mL of 0.5 M sodium acetate, pH = 5.6, and 30 µL of HFBA were added, and the sample was shaken briefly for 5 min. Next, the sample was transferred to a conditioned Strata-X SPE column. The analytes were eluted from the SPE with 3 mL of a mixture of acetonitrile: 0.05 M HFBA (9:1, v/v), and the eluate was evaporated to dryness. The dry residue was dissolved in 500 ul of 0.025% HFBA and filtered through 0.22 um PVDF syringe filters into LC vials.
LC-MS/MS analysis of water was performed on the same instrument as the meat sample. However, chromatographic separation of analytes was performed on a Luna C18 (2) 100 A column (50 × 3.0 mm, 3 µm) using the same mobile phases but in a different gradient mode.
5. Conclusions
In modern livestock production systems, efforts to maintain high health standards may imply some use of antimicrobial drugs in farm animals. The mean objective of the presented study was to reduce the use of antimicrobials administrated on pig and broiler farms by implementing tailor-made health plans, including biosecurity measures, and to investigate the possible change in residues in water and meat samples. The results obtained in this study indicate a reduction in antibiotic residues in water samples on broiler farms when biosecurity measures were improved. No residues were found in the samples from pig farms. The research on antimicrobials reduction by the implementation of selected intervention actions on animal farms needs to be continued and further improved.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12020326/s1, Table S1. Biosecurity measures on broiler farms. Table S2. Biosecurity measures on pig farms
Author Contributions
Conceptualization, A.G., T.B. and M.G.-S.; methodology, A.G., T.B., M.G.-S. and E.N.-K.; software, A.G., T.B., M.G.-S. and E.N.-K.; validation, A.G., T.B., M.G.-S. and E.N.-K.; formal analysis, A.G., T.B., M.G.-S. and E.N.-K.; investigation, A.G., T.B., M.G.-S., E.N.-K., K.A., G.K., P.F., P.L., M.S., C.F., M.W.-F. and K.D.R.; resources, A.G., K.A., G.K., P.F., P.L., M.S., C.F., M.W.-F. and K.D.R.; writing, A.G. writing—review and editing, A.G., T.B. and M.G.-S.; visualization, A.G. and T.B.; supervision, A.G. and K.D.R.; project administration, K.D.R.; funding acquisition, A.G. and C.F.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was part of Healthy Livestock project. The Healthy Livestock was funded by the European Union H2020 research and innovation program under grant agreement number 773436.
Institutional Review Board Statement
Ethical review and approval were waived for this study as the analysed material originated from routine diagnostic investigations ordered by the farm owners.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article.
Acknowledgments
The authors are grateful to Magdalena Bilecka, Aleksandra Kusmierz and Iwona Szymanek-Bany for their direct laboratory and technical help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramatla, T.; Ngoma, L.; Adetunji, M.; Mwanza, M. Evaluation of Antibiotic Residues in Raw Meat Using Different Analytical Methods. Antibiotics 2017, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Riviere, J.E.; Papich, M.G. Veterinary Pharmacology and Therapeutics, 10th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-118-85588-1. [Google Scholar]
- Rana, M.S.; Lee, S.Y.; Kang, H.J.; Hurt, S.J. Reducing Veterinary Drug Residues in Animal Products: A Review. Food Sci. Anim. Resour. 2019, 39, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Acar, J.F.; Moulin, G.; Page, S.W.; Pastoret, P.-P. Antimicrobial resistance in animal and public health: Introduction and classification of antimicrobial agents. Rev. Sci. et Tech. de l’OIE 2012, 31, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- Whitton, C.; Bogueva, D.; Marinova, D.; Phillips, C.J.C. Are We Approaching Peak Meat Consumption? Analysis of Meat Consumption from 2000 to 2019 in 35 Countries and Its Relationship to Gross Domestic Product. Animals 2021, 11, 3466. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 2008/120/EC of 18 December 2008 laying down minimum standards for the protection of pigs. Off. J. Eur. Union L 2009, 47, 5–13.
- Van Limbergen, T.; Dewulf, J.; Klinkenberg, M.; Ducatelle, R.; Gelaude, P.; Méendez, J.; Heinola, K.; Papasolomontos, S.; Szeleszczuk, P.; Maes, D. Scoring biosecurity in European conventional broiler production. Poult. Sci. 2018, 97, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, J.; Simitopoulou, M.; Angastiniotis, K.; Ferrari, P.; Wolthuis-Fillerup, M.; Kefalas, G.; Papasolomontos, S. Development and implementation of a risk assessment tool for broiler farm biosecurity and a health intervention plan in the Netherlands, Greece, and Cyprus. Poult. Sci. 2023, 102, 102394. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union 2010. Commission Regulation (EU) No 37/2010. Off. J. Eur. Union 2010, L 15/1, 1–72.
- European Food Safety Authority EFSA. (2021). Report for 2019 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. Support. Publ. EFSA J. 2021, 18, EN-1997. [Google Scholar] [CrossRef]
- European Food Safety Authority EFSA. (2022). Report for 2020 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support. Publ. 2022, 19, EN-7143. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. Off. J. Eur. Union 2019, L 4/43, 43–167.
- Joosten, P.; Sarrazin, S.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.; Dewulf, J.; Graveland, H.; Schmitt, H.; et al. Quantitative and qualitative analysis of antimicrobial usage at farm and flock level on 181 broiler farms in nine European countries. J. Antimicrob. Chemother. 2019, 74, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Joosten, P.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.; Dewulf, J.; Wagenaar, J.; Graveland, H.; et al. Quantitative and qualitative analysis of antimicrobial usage patterns in 180 selected farrow-to-finish pig farms from nine European countries based on single batch and purchase data. J. Antimicrob. Chemother. 2018, 74, 807–816. [Google Scholar] [CrossRef]
- Landoni, M.; Albarellos, G. The use of antimicrobial agents in broiler chickens. Veter—J. 2015, 205, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sumano, L.H.; Gutiérrez, O.L.; Aguilera, R.; Rosiles, M.R.; Bernard, B.M.; Gracia, M.J. Influence of hard water on the bioavailability of enrofloxacin in broilers. Poult. Sci. 2004, 83, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Dorr, P.M.; Nemechek, M.S.; Scheidt, A.B.; Baynes, R.E.; Gebreyes, W.A.; Almond, G.W. Water-flow variation and pharmaco epidemiology of tetracycline hydrochloride administration via drinking water in swine finishing farms. JAVMA 2009, 235, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Sumano, L.H.; Cortés-Cuevas, A.; Rosario, C.; Gutiérrez, O.L. Assessment of key pharmacokinetic variables of bioequivalent and non-bioequivalent enrofloxacin preparations under various water management conditions. J. Poult. Sci. 2010, 47, 262–268.251. [Google Scholar] [CrossRef]
- Gbylik-Sikorska, M.; Posyniak, A.; Sniegocki, T.; Zmudzki, J. Liquid chromatography–tandem mass spectrometry multiclass method for the determination of antibiotics residues in water samples from water supply systems in food-producing animal farms. Chemosphere 2015, 119, 8–15. [Google Scholar] [CrossRef]
- Błądek, T.; Posyniak, A.; Gajda, A.; Gbylik, M.; Żmudzki, J. Multi-class procedure for analysis of antibacterial compounds in animal tissues by liquid chromatography-mass spectrometry. Bull. Vet. Inst. Pulawy 2011, 55, 741–748. [Google Scholar]
- European Communities. Commission Decision (2002/657/EC) of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, L221, 8–36. [Google Scholar]
- World Health Organization Report (2021). Comprehensive Review of the WHO Global Action Plan on Antimicrobial Resistance. September 2021. Evaluation Office, World Health Organization. Available online: https://cdn.who.int/media/docs/default-source/documents/about-us/evaluation/gap-amr-final-annexes-v2.pdf (accessed on 20 December 2022).
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health 2008, 29, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.; Guzman, J.E.; Hanson, D.; Garbes, N.; Morrison, R.; Amodie, D.; Pantoja, L.G. A randomized controlled trial to evaluate performance of pigs raised in antibiotic-free or conventional production systems following challenge with porcine reproductive and respiratory syndrome virus. PLoS ONE 2018, 13, e0208430. [Google Scholar] [CrossRef] [PubMed]
- Page, S.W.; Gautier, P. Use of antimicrobial agents in livestock. Rev. Sci. Tech. Int. Off. Epizoot. 2012, 31, 145–188. [Google Scholar] [CrossRef]
- Amass, S.F.; Clark, L.K. Biosecurity considerations for pork production units. J. Swine Health Prod. 1999, 7, 217–228. [Google Scholar]
- Kotb, S.; Ahmed, M.; Hassan, D.; Soltan, E. Stability of antibiotics in drinking water: An advanced approach towards the impacts of water quality parameters on doxycycline bioavailability. J. Adv. Veter-Anim. Res. 2019, 6, 438–444. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health (OIE) OIE List of Antimicrobials of Veterinary Importance. In Proceedings of the 75th General Session, Paris, France, 20–25 May 2007.
- Robles-Jimenez, L.E.; Aranda-Aguirre, E.; Castelan-Ortega, O.A.; Shettino-Bermudez, B.S.; Ortiz-Salinas, R.; Miranda, M.; Li, X.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; Gonzalez-Ronquillo, M. Worldwide Traceability of Antibiotic Residues from Livestock in Wastewater and Soil: A Systematic Review. Animals 2022, 12, 60. [Google Scholar] [CrossRef]
- Jane, R.; Vera, V.; Kotoji, I.; Diaz, H.L.; Brian, G.; Monnet Dominique, L.; Sarah, G.; Klaus, W. Variations in the Consumption of Antimicrobial Medicines in the European Region, 2014–2018: Findings and Implications from ESAC-Net and WHO Europe. Front. Pharmacol. 2021, 12, 727. [Google Scholar]
- Huygens, J.; Rasschaert, G.; Heyndrickx, M.; Jeroen, D.; Els Van, C.; Paul, Q.; Els, D. Ilse Becue 1Impact of fertilization with pig or calf slurry on antibiotic residues and resistance genes in the soil. Sci. Total Environ. 2022, 822, 153518. [Google Scholar] [CrossRef] [PubMed]
- Patyra, E.; Kwiatek, K.; Nebot, C.; Gavilon, R.E. Quantification of Veterinary Antibiotics in Pig and Poultry Feces and Liquid Manure as a Non-Invasive Method to Monitor Antibiotic Usage in Livestock by Liquid Chromatography Mass-Spectrometry. Molecules 2020, 25, 3265. [Google Scholar] [CrossRef] [PubMed]
- Rasschaert, G.; Van Elst, D.; Colson, L.; Herman, L.; Cardoso de Carvalho Ferreira, H.; Dewulf, J.; Decrop, J.; Meirlaen, J.; Heyndrickx, M.; Daeseleire, E. Antibiotic Residues and Antibiotic-Resistant Bacteria in Pig Slurry Used to Fertilize Agricultural Fields. Antibiotics 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Gajda, A.; Nowacka-Kozak, E.; Gbylik-Sikorska, M.; Posyniak, A. Feather analysis as a non-invasive alternative to tissue sampling for surveillance of doxycycline use on poultry farms. Poult. Sci. 2019, 98, 5971–5980. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).