Abstract
Essential oils (EOs) and their vapour phase of Curcuma longa (Zingiberaceae), Cymbopogon citratus (Poaceae), Ocimum campechianum (Lamiaceae), and Zingiber officinale (Zingiberaceae) of cultivated plants grown in an Amazonian Ecuador area were chemically characterised by Gas Chromatography-Flame Ionization Detector (GC-FID), Gas Chromatography–Mass Spectrometry (GC-MS), and Head Space–Gas Chromatograph-Flame Ionization Detector–Mass Spectrometry (HS-GC-FID-MS).figure The EOs analyses led to the identification of 25 compounds for C. longa (99.46% of the total; ar-turmerone: 23.35%), 18 compounds for C. citratus (99.59% of the total; geraniol: 39.43%), 19 compounds for O. campechianum (96.24% of the total; eugenol: 50.97%), and 28 for Z. officinale (98.04% of the total; α-Zingiberene: 15.45%). The Head Space fractions (HS) revealed C. longa mainly characterised by limonene and 1,8-cineole (37.35%) and α-phellandrene (32.33%); Z. officinale and C. citratus showed camphene (50.39%) and cis-Isocitral (15.27%) as the most abundant compounds, respectively. O. campechianum EO revealed a higher amount of sesquiterpenes (10.08%), mainly characterised by E-caryophyllene (4.95%), but monoterpene fraction remained the most abundant (89.94%). The EOs were tested for antioxidant, antimicrobial, and mutagen-protective properties and compared to the Thymus vulgaris EO as a positive reference. O. campechianum EO was the most effective in all the bioactivities checked. Similar results emerged from assaying the bioactivity of the vapour phase of O. campechianum EO. The antioxidant and antimicrobial activity evaluation of O. campechianum EO were repeated through HP-TLC bioautography assay, pointing out eugenol as the lead compound for bioactivity. The mutagen-protective evaluation checked through Ames’s test properly modified evidenced a better capacity of O. campechianum EO compared with the other EOs, reducing the induced mutagenicity at 0.1 mg/plate. However, even with differences in efficacy, the overall results suggest important perspectives for the functional use of the four studied EOs.
1. Introduction
Aromatic plants have always characterised the ethnobotanical traditions and cultures of all societies in the world without distinction, finding a place as an economic botany resource in many uses including—first of all but not only—healthy preparations and therapeutic applications. Their aroma also traditionally characterises them for different and multiple uses such as cosmetics, perfuming and sanitising environments, insect repellent, and for religious and pagan propitiatory rites. The essential oils (EOs) obtained from these plants represent the maximum expression of their efficacy because steam distillation, the extraction method of choice, allows the compounds which characterise these species in terms of their aroma and multiple applications properties to be obtained in the concentrated form [1,2,3,4]. The therapeutic importance of many aromatic plants and their EOs has led to their inclusion in various pharmacopoeias which regulate their authenticity, standardisation, and application in the health and pharmaceutical fields. In addition, research into their use both as phytocomplexes and as characterising active ingredients in the treatment of various pathologies, such as infections of various aetiology, pathologies of inflammatory origin, tumours, and immune-related pathologies, is always particularly lively [5,6,7].
The evaluation of the quality and biological properties of EOs has led research to enhance their use in other areas as well, such as the veterinary sector, for the treatment, for example, of diseases characterised by decreased immunity (e.g., caused by forced weaning); that of sustainable agriculture for the identification of molecules with an insect-repellent or biopesticide action with a lower environmental impact; or that of packaging and preservatives by reducing the use of synthetic compounds [8,9,10].
Precisely, thanks to their wide use as seasoning spices, aromatic plants and their EOs have been studied—and are still being extensively investigated—for biological activities that can suggest functional applications in the food sector. In particular, modern research focuses on antimicrobial, antioxidant, and in some cases, geno-protective properties, for applications of EOs as preservatives and flavourings, protective agents against the potential carcinogenicity of substances present in foods, such as, for example, the heterocyclic amines produced by cooking, or packaging components protecting the food from microbial and chemical degradation [11,12,13].
Finally, the cultural and economic value of aromatic plants and EOs is also reflected in their inspiring role in the chemical industry of synthesis and semi-synthesis (platform chemicals) to produce compounds or new molecules of interest for various productive sectors [14].
In relation to these important premises, EOs reflect in a very effective way the physiological and ontogenetic evolution of the aromatic species of origin as well as its resilience, responding to exogenous (e.g., climate) and endogenous (e.g., hybridisation) stress factors with different qualities and quantities of secondary metabolites produced and influencing the phytochemical profile of the phytocomplex. This feature strongly affects the standardisation of the EOs produced and therefore their specific effectiveness. The objective need to use standardised EOs in terms of quality and quantity of constituents explains the need for supplies from cultivated rather than spontaneous aromatic species, to be able to control the environment and cultivation practices by minimising the variability of the composition of the phytocomplexes. Hence the need to verify the chemical composition as well as the biological properties and also to identify—with respect to specific peculiarities due to specific growth environments (e.g., latitude, climate, cultivation practices, etc.)—any new applications [15].
For several years our research group has been studying EOs from aromatic plants growing in the Amazon area, one of the largest biodiverse hot spots in the world. Starting from the ethnobotanical uses that characterise the aromatic species studied, it is of particular interest to verify the effectiveness of traditional uses from a phytochemical and bioactivity point of view, and evaluate any new applications related to the peculiarity of the chemical composition due to the specificity of the growing area, such as, for example, that Amazonian. Therefore, the present study reported the chemical composition and the biological properties of the EOs obtained from Curcuma longa L. (Zingiberaceae), Cymbopogon citratus (DC.) Stapf, (Poaceae), Ocimum campechianum Mill. (sin. Ocimum micranthum Willd.; Lamiaceae), and Zingiber officinale Roscoe (Zingiberaceae) with the object to valorise possible chemical and bioactivity characteristics due to the particularity of the southeastern region of Amazonian Ecuador where the plants were grown. The chemical composition of the EOs and the volatile fraction (headspace, HS) were determined, together with the evaluation and comparison of the antioxidant activity and antimicrobial properties related to identifying the compounds mainly responsible for the bioactivity. Finally, the mutagen-protective capacity of EOs was verified through the Ames test with the aim of suggesting a possible functional and protective role of essential oils in applicative and health-related fields such as, for example, the food sector.
2. Results and Discussion
2.1. Essential Oils (EOs) Extraction and Chemical Characterisation
The essential oils (EOs) were obtained by steam distillation from fresh plant parts collected at the balsamic period from Curcuma longa L. (Zingiberaceae; rhizome), Cymbopogon citratus (DC.) Stapf (Poaceae; aerial parts), Ocimum campechianum Mill. (Lamiaceae; aerial parts), and Zingiber officinale Roscoe (Zingiberaceae; rhizome), grown in an Amazonian Ecuador area and sourced from Fundaciòn Chankuap (Macas, Morona-Santiago province, Ecuador). The EOs yields, determined on an averaged volume/dry weight basis, and their densities are reported in Table 1. O. campechianum gave the highest EO yield, followed by Z. officinale, C. citratus, and C. longa.

Table 1.
Yields and density of Amazonian essential oils (EOs) obtained by steam distillation of Curcuma longa, Cymbopogon citratus, Ocimum campechianum, and Zingiber officinale fresh plant material.
The chemical characterisation of the EOs performed by GC-FID and GC-MS analyses (Table 2) led to the identification of 25 compounds for C. longa (99.46% of the total), 18 compounds for C. citratus (99.59% of the total), 19 compounds for O. campechianum (96.24% of the total), and 28 for Z. officinale (98.04% of the total) through a detailed interpretation of the experimental data (fragmentation and retention indices). Sesquiterpenes were the most abundant compounds in C. longa EO, mainly characterised by those oxygenated (66.29%), where ar-turmerone (23.35%), α- and β- turmerone (22.81% and 15.27% respectively) were the most representative, while sesquiterpene hydrocarbons were instead consistently lower (5.32%). The monoterpene hydrocarbons (18.74%) were mainly characterised by α-phellandrene (9.81%) and 1,8-cineole (7.85%). The chemical composition of the essential oil reflects what is generally reported by related literature [16], hence with sesquiterpenes oxygenated, typical of the genus, as the most representative chemical constituents and ar-turmerone as the leading compound, followed by α- and β-. In general, monoterpene hydrocarbons and oxygenated ones are instead quantitatively lower. All the other compounds were detected with values lower than 3.0%, or in traces. The Z. officinale EO showed a similar pattern to that of C. longa as far as monoterpenes and sesquiterpenes are concerned, but it exhibited a prevalence of hydrocarbon compounds for both chemical classes. A-zingiberene (15.45%) and camphene (14.72%) were the most abundant compounds among sesquiterpenes and monoterpenes, respectively. The chemical composition of Z. officinale EO from Amazonian Ecuador reflects interesting and important differences from analogous EOs from Z. officinale plants of different geographical origins, reporting ar-curcumene or citral or α- and β-zingiberene as the major constituents [17]. The emerging qualitative and quantitative differences stress the particularity of the Amazonian area where the plants were grown, whose environmental characteristics significantly affect the phytochemistry of the specie. This aspect is further emphasised by the chemical characteristics of O. campechianum EO, which showed a eugenol chemotype with a composition quantitatively different from the same EOs from wild plants collected in southeastern Ecuador (Morona-Santiago Region) and in the Pastaza Region of Ecuador studied in the past from our research group [18,19,20]. In fact, the EO of O. campechianum considered in this study showed a eugenol abundance of 50.97% followed by E-caryophyllene at 10.21% and 1,8-cineole at 7.36%. Other major compounds detected were β-elemene (4.85%), bicyclogermacrene (4.11%), and spathulenol (4.42%). The most abundant compounds in C. citratus were geraniol (39.43%) and citral (14.37% neral and 17.29% geranial); geranyl acetate (7.96%), citronellal (4.54%), and apiole (5.02%) were less abundant; all the other compounds were lower than 3.0%, including nerol (2.64%). This pattern is partially in contrast with those reported in literature where it is indicated a higher amount of citral (approximately 60–75%) and myrcene (approximately 4–10%), not detected in our EO sample [21,22,23].

Table 2.
Chemical composition of essential oils (EOs) from studied species through GC and GC-MS analyses: Curcuma longa; Cymbopogon citratus; Ocimum campechianum; Zingiber officinale.
The EOs were also characterised for their vapour phase fractions, also called Headspace (HS), through HS-GC-MS (Table 3). C. longa and Z. officinale vapour phases were mainly composed of monoterpene hydrocarbons and oxygenated, 98.97% and 98.74%, respectively, with an expected lower amount of sesquiterpenes due to their lower volatility (1.03% and 1.26%, respectively). In particular, the C. longa EO vapour phase was characterised by limonene and 1,8-cineole (37.35%) and α-phellandrene (32.33%) with a lower abundance of sabinene (9.76%), α-pinene (8.28%), and terpinolene (7.13%). Z. officinale showed mainly camphene (50.39%), limonene and 1,8-cineole (17.20%), and α-pinene (16.47%) with minor amounts of β-pinene (4.40%) and myrcene (4.36%). C. citratus headspace was found to contain only monoterpenes (91.44%), the majority of which were oxygenated compounds (61.52%) followed by hydrocarbon ones (29.92%). Cis-isocitral (15.27%), γ-terpineol (12.92%), citronellal (11.07%), and trans-isocitral (10.89%) were the most abundant monoterpenes while, in decreasing order of abundance, methyl-5-hepten-one (7.11%), trans-ocimene (6.64%), limonene (5.30%), cis-ocimene (4.76%), camphene (3.49%), neral (3.45%), and nerol (3.15%) were detected. Among all the EOs examined, the headspace composition of O. campechianum exhibited a higher amount of sesquiterpenes (10.08%), mainly characterised by E-caryophyllene (4.95%). However, the monoterpenes were also the most characterising fraction of the O. campechianum EO vapour phase (89.94%), mainly characterised by 1,8-cineole and limonene (30.83%), cis-ocimene (19.49%), β-pinene (10.61%), allo-ocimene (8.43%), eugenol (7.01%), and α-pinene (4.66%). To the best of our knowledge, this is the first report about the headspace composition of the EOs of Amazonian C. longa, C. citratus, O. campechianum, and Z. officinale. In fact, among the EOs studied, only some turmeric species, including C. longa, from different Asian geographical areas, have been described for the composition of the volatile fractions of their EOs. The EOs of C. longa of different Asian geographical origins evidenced a headspace composition very different in quality and amounts from that checked in our turmeric headspace samples and characterised by compounds with large concentration ranges in relation to the different harvesting areas. In particular, the most abundant compounds were turmerone (range: 11.6–41.3%) zingiberene (range: 3.4–20.7%), β-turmerone (range: 4.1–18.0%), β-sesquiphellandrene (range: 2.3–16.1%), ar-turmerone (range: 3.2–12.4%), germacrene (range: not detected-5.3%), β-bisabolene (range: not detected–3.0%) [24]. Therefore, these results represent the first report on the headspace composition of Amazonian C. longa, C. citratus, O. campechianum, and Z. officinale EOs, highlighting once more how these phytocomplexes are particularly sensitive to geographical and environmental conditions, significantly varying their composition and, consequently, their bioactive properties.

Table 3.
Headspace composition of Amazonian essential oils (EOs) through GC and GC-MS analyses: Curcuma longa; Cymbopogon citratus; Ocimum campechianum; Zingiber officinale.
2.2. Antioxidant Activity of Essential Oils: DPPH and ABTS Assays
The assessment of the biological activity of the various EOs considered the evaluation of the radical-scavenger antioxidant activity by means of spectrophotometric DPPH (1,1-diphenyl-2-picrylhydrazyl) and ABTS (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid) assays. The results were compared with those obtained from the commercial EO of Thymus vulgaris, a reference phytocomplex, and with that of Trolox®, a proven pure antioxidant compound (Table 4). The only EO that showed interesting antioxidant activity with both tests was that of O. campechianum. In particular, this EO evidenced a slightly higher IC50 in both DPPH and ABTS assays than the Trolox®. However, the most relevant result regards the comparison of the antioxidant capacity of O. campechianum EO with that of the commercial Thymus vulgaris EO. In fact, the IC50 was about 27 times better than that of thyme with the DPPH test, and more than 220 times better with the ABTS assay. These results confirmed previous evidence that emerged through several antioxidant tests performed on O. campechianum EO obtained from wild plants harvested in the same Amazonian area, pointing out that the cultivated plants reflect the same chemical and biological properties, suggesting their important applicative perspective as a source of phytocomplexes with radical-scavenging properties [18]. Z. officinale and C. longa EOs showed interesting antioxidant capacities only with the ABTS test but, in any case, their bioactivity was lower than that of the T. vulgaris OE used as the positive control. In literature, Z. officinale EO from Ecuador demonstrates interesting antioxidant capacities with different in vitro assays; thus, our results are not significantly comparable, suggesting only an interesting activity against the ABTS radical [25].

Table 4.
Antioxidant properties of EOs from studied species through DPPH and ABTS tests in comparison with that of commercial Thymus vulgaris (phytocomplex as positive control), and Trolox® (pure chemical compound as positive control).
The EO of Indian C. longa has been studied for its in vitro antioxidant properties advising its potential health benefits as a radical scavenger, but also for its significant anti-inflammatory and antinociceptive activities [26]. Similarly, a publication on the EO from Brazilian C. longa cultivated plants underlines a good antioxidant activity emerged with the same methods used for our samples using BHT (Butylated hydroxytoluene) as a positive control but detecting radical scavenging values similar to ours [27]. However, concerning our results and the positive controls, especially thyme EO, we cannot come to the same conclusions for our Ecuadorian samples. As regards the C. citratus EO, with specific reference to south American samples, the literature suggests interesting uses as a natural additive in the food industry, mainly for flavouring, against significant antioxidant results that we have not registered [28]. Therefore, given the scarce efficacy that emerged from our results, only its use as a food flavouring can be hypothesised but, for our samples, no other functional properties can be suggested, except for the EO of O. campechianum.
Considering the significant results regarding the EO of O. campechianum, HP-TLC bioautography was performed to detect the compound(s) mainly responsible for the antioxidant capacity against DPPH and ABTS radicals (Figure 1). In plates prepared and eluted as reported in [12] treated with the free radicals DPPH (Figure 1c) and ABTS (Figure 1d) at Rf 0.5 an evident and a wide bleached band appeared due to the antioxidant capacity of eugenol. In fact, at Rf 0.5 appeared a wide yellow band (Figure 1a) corresponding to eugenol, as demonstrated by the elution of the pure standard and related literature [29]. The result accounts for the important and documented antioxidant properties of eugenol, detected as the most abundant compound in our O. campechianum EO (Table 2), prodromal for numerous therapeutic properties with respect to mechanisms of action and at a pre-clinical level [30].
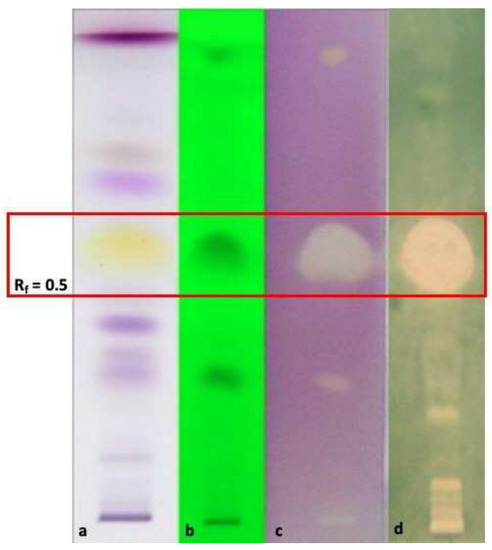
Figure 1.
HP-TLC bioautographic assay to check the antioxidant capacity of O. campechianum EO against DPPH and ABTS radicals. (a): plate eluted and treated with vanillin sulphuric acid reagent; (b): plate eluted and visualised at λ = 254 nm wavelength; (c): plate eluted ad treated with DPPH; (d): plate eluted and treated with ABTS. The band at Rf = 0.5 corresponds to eugenol, as verified eluting pure standard compound (data not shown) and comparing the result with related literature [29]. The large bleached bands at Rf = 0.5 correspond to the capacity of eugenol to contrast the oxidative reactivity of the DPPH• (3) and ABTS•+ (4) radicals.
2.3. Antimicrobial Activity: MIC of the EOs and Growth Inhibition Percentage of Their Headspace Fractions
The antimicrobial activities of the EOs from studied species were verified both employing the disk-diffusion assay and the agar vapour method (Headspace’s bioactivity) to check the efficacy of the whole essential oil and that of the most volatile fraction, respectively (Table 5 and Figure 2A–D). The bioactivity was checked against Gram-positive and Gram-negative bacteria, some non-pathogenic but potential food contaminants, others generally considered as nosocomial pathogens, especially in immunocompromised patients [31], and against the yeasts Candida albicans and Saccharomyces cerevisiae.

Table 5.
Antimicrobial activities of EOs from the studied species compared with that of commercial T. vulgaris. Chloramphenicol (CPh) and fluconazole (Flu) were used to test the sensitivity of bacterial and yeast strains respectively.
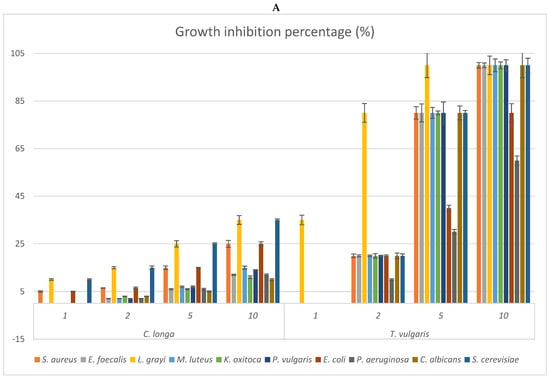
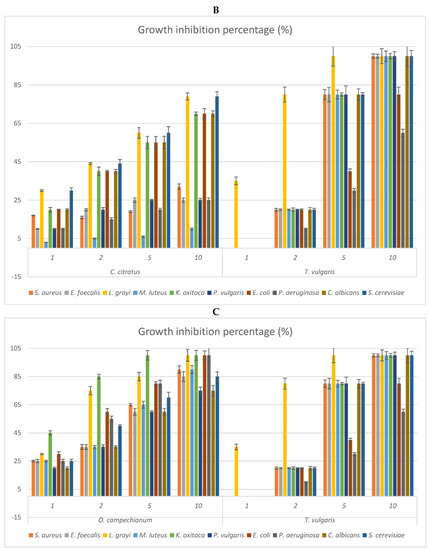
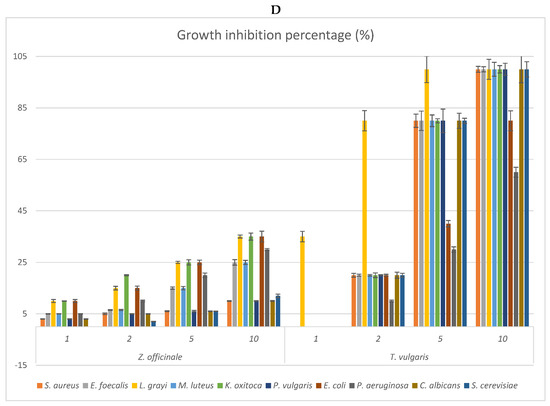
Figure 2.
(A–D) Antimicrobial activities of the vapour phase of the EOs from studied species compared to that of commercial T. vulgaris. The bioactivity is expressed as growth inhibition percentage (%) checked at the doses of 1, 2, 5, and 10 μL. The bioactivity of the vapour phase of each EO is compared to that of T. vulgaris (positive control). (A) Growth inhibition percentage of the vapour phase of Curcuma longa; (B) growth inhibition percentage of the vapour phase of Cymbopogon citratus; (C) growth inhibition percentage of the vapour phase of Ocimum campechianum; (D) growth inhibition percentage of the vapour phase of Zingiber officinale. Values are averages ± standard deviation.
In general, the MIC values of all the EOs were always higher than those shown by T. vulgaris (positive control), except for the EO of O. campechianum against the Gram-negative bacteria K. oxytoca (MIC = 0.75 mg/mL), E. coli (MIC = 1.70 mg/mL) and P. aeruginosa (MIC = 1.70 mg/mL). These results are particularly relevant because they highlight the possible use of O. campechianum EO as a new tool both to counteract infections caused by widespread and important gram-negative bacteria, generally considered more resistant to antibiotic treatments than gram-positive ones, and to meet the pressing need to contrast the increasingly emerging antibiotic resistance [32]. Moreover, the bacterial strains more sensitive to O. campechianum EO can cause intestinal (e.g., abdominal pain, vomiting, bloody diarrhoea) and extra-intestinal (e.g., urinary tract infections, peritonitis, septicaemia, pneumonia, and meningitis) diseases, up to sepsis in the worst cases. These possible consequences stress once more the importance of the antimicrobial results of O. campechianum EO [33,34,35]. Given the promising results, an HP-TLC antimicrobial bioautographic assay was performed on K. oxytoca to detect the main effective compound(s) (Figure 3). The Rf 0.5 band showed a clear zone of growth inhibition, highlighting that eugenol is largely responsible for the antibacterial activity, in line with what has been widely reported in the literature [36]. Additionally, C. citratus EO displayed an interesting antimicrobial activity, in particular against the gram-negative bacteria E. coli and P. aeruginosa in which the MIC was however comparable with what was detectable for the EO of Thymus vulgaris (positive control). However, the bioactivity results were consistently lower than those shown by O. campechianum, and partially in contrast with what was reported in the literature, where the antimicrobial activity of C. citratus (EO and extracts) was found to be noteworthy against different bacterial strains, suggesting promising application developments [37]. C. longa EO did not show any interesting antimicrobial activity evidencing the worst MIC values detected among all the EOs, in line with what was reported in the literature, in which the commercial EO displayed biological activity only in synergistic association with ascorbic acid [38]. Finally, the studied Z. officinale EO showed very weak bioactivity, resembling that of C. longa EO but in contrast to what is reported in the literature, where it is described as a phytocomplex that provides a broad antimicrobial spectrum against different microorganisms, making it an interesting alternative to synthetic antimicrobials [39].
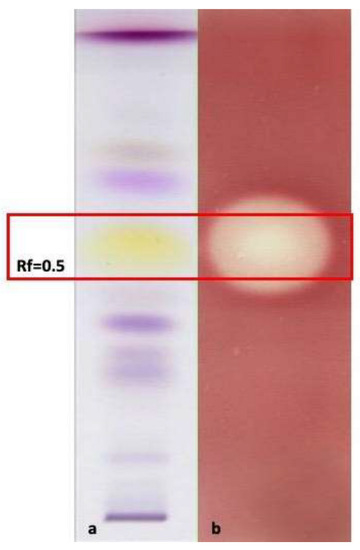
Figure 3.
HP-TLC bioautographic assay showing the antimicrobial activity of O. campechianum EO, the most interesting among the EOs tested. The bioautographic plate (b) shows the bleaching band (microbial growth inhibition zone) of the antimicrobial activity against Klebsiella oxytoca, the most sensitive bacterial strain with the lowest MIC detected (0.75 mg/mL), at Rf = 0.5, corresponding to eugenol, the compound responsible of the bioactivity for O. campechianum EO. (a) Plate eluted and treated with vanillin sulphuric acid reagent; (b) plate eluted and treated with K. oxytoca for the antimicrobial bioautographic evaluation.
The vapour phase of the EOs from the studied species was also checked to verify possible differences in the antimicrobial activities of the more volatile fractions (Figure 2). The results reflected what is evidenced by Table 5, indicating O. campechianum as the most effective, at all doses tested, of the EOs studied, particularly against the Gram-negative bacteria K. oxytoca, E. coli, and P. aeruginosa. At the dose of 5μL, the total growth inhibition of K. oxytoca stands out, as well as the 80% growth inhibition of E. coli and P. aeruginosa by the OE of O. campechianum compared to the bioactivity of T. vulgaris towards the same strains, whose growth was inhibited by 80%, 40%, and 30%, respectively. The bioactivity of the other EOs was significantly lower, with the EO of C. citratus being less active than that of O. campechianum, followed by that of Z. officinale and C. longa.
2.4. Cytotoxicity and Mutagen Protection Properties of the Amazonian EOs
To investigate the possible mutagen-protective capacity of the EOs from studied species, a properly modified Ames test was performed as reported in [12,13] using Salmonella typhimurium tester strains TA98 and TA100, with and without metabolic activation (S9 mix). 2-nitrofluorene (2 μg/plate, NF) and sodium azide (1 μg/plate, SA) were employed as mutagen inducers for TA98 and TA100 without metabolic activation, while 2-aminoanthracene (2 μg/plate, AA) was used for the same strains in presence of S9 mix. To avoid overlapping cytotoxic and antimutagenic effects, pointing out the evidence that the mutant colonies are not a result of cell-killing, a survival assay was performed for the treated bacteria to evaluate the Highest Uneffective Dose (HUD), i.e., the maximum concentration of the EOs that do not cause cytotoxicity [40] (Table 6A–D). All the EOs from the studied species evidenced significant cytotoxicity at concentrations higher than 1.00 mg/plate. This result led us to evaluate the mutagenic protection at a concentrations range of EOs between 0.00 and 1.00 mg/plate (Table 7A–D).

Table 6.
(A–D). Highest Uneffective Dose (HUD) tested with and without metabolic activation (S9 mix). HUD represents the maximum concentration of EOs DMSO diluted which does not induce cytotoxic evidence in S. typhimurium TA98 and TA100 strains cultures. The HUD data are essential to interpret the results of a significant decrease in the number of Salmonella revertants in anti-genotoxic assays. Negative controls (EO = 0.000 mg/plate) were set up with 100 mL/plate of DMSO. The results are expressed both as survival percentage ± standard deviation (s.d.) and Colony Forming Units (CFU)/plate ± standard deviation. Table 6A: Zingiber officinale EO cytotoxicity; Table 6B: Curcuma longa EO cytotoxicity; Table 6C: Ocimum campechianum EO cytotoxicity; Table 6D: Cymbopogon citratus EO cytotoxicity.

Table 7.
(A–D). Ames test (Salmonella typhimurium, strain TA98 and TA100) with and without metabolic activation (S9 mix) to assay mutagen protective activity of the four studied EOs (0.01–1.0 mg/plate concentration range) in presence of direct-acting mutagen 2-nitrofluorene (2 mg/plate; NF) and sodium azide (1 mg/plate; SA) and of the indirectly acting mutagen 2-aminoanthracene (2 mg/plate; AA). Negative controls (EOs = 0.000 mg/plate) were set up with 100 mL/plate of DMSO. The maximum concentration value (1.0 mg/plate) of the concentration range coincides with the maximum dose in which the EOs do not express cytotoxicity in HUD evaluation (Table 6A–D). Table 7A: Zingiber officinale EO mutagen-protective property; Table 7B: Curcuma longa mutagen-protective property; Table 7C: Ocimum campechianum mutagen-protective property; Table 7D: Cymbopogon citratus mutagen-protective property. The results are expressed both as revertants percentage (Rev.%) ± standard deviation (s.d.) and Colony Forming Units (CFU)/plate ± standard deviation.
Z. officinale EO showed a mutagen-protective capacity starting from the concentration of 0.20 mg/plate for TA98 strain against 2-nitrofluorene (NF) without metabolic activation. For the TA98 strain against 2-aminoantracene (AA) with metabolic activation, and TA100 strain against 2-aminoantracene and sodium azide (SA), with and without S9 respectively, the mutagen protection was instead displayed starting from the highest concentrations 0.50–1.00 mg/plate) (Table 7A). In the literature, there is no evidence of antigenotoxic properties of the EO of Z. officinale, but there are instead results about mutagen–protective properties of other kinds of extracts—e.g., aqueous ones—and their role in preventing the onset of metabolism-related pathologies such that diet-induced in pre-clinical tests [41].
C. longa EO, known to be no-genotoxic [42], instead evidenced a significant mutagen-protective capacity from 0.10 to 1.00 mg plate with TA98 against NF and TA100 against AA, without and with metabolic activation, respectively (Table 7B). This result is partly consistent with what is reported in the literature, where it is mainly reported about curcumin-rich aqueous extracts and derivatives and their protective role in nutrition, and their possible contribution to reducing the onset of tumours [43].
O. campechianum EO, already checked for its genotoxic potential through Ames tests in our previous research [20], showed a significant and very interesting mutagen-protective capacity starting from the concentration of 0.02 mg/plate for TA98 strain with and without metabolic activation. Lower but significant mutagen-protective capacity was shown for the TA100 strain starting from the concentration of 0.1 mg/plate and 0.2 mg/plate in S9 mix activated and non-activated cultures, respectively (Table 7C).
C. citratus EO is reported in the literature to have a genotoxic protective effect in pre-clinical studies [44] and our results confirmed the assumption evidencing mutagen protective effect since 0.10 mg/plate for both TA98 and TA100 strains with metabolic activation (Table 7D).
To the best of our knowledge, this is the first report about the mutagen-protection capacity of the EOs from Z. officinale, C. longa, O. campechianum, and C. citratus grown in the Amazonian area. Among the four EOs, O. campechianum in particular showed the best in vitro results with the Ames’ Salmonella strains opening important perspectives for its use, e.g., as a food additive for its protective properties against potential mutagens.
3. Materials and Methods
3.1. Plant Material and Isolation of Essential Oil
Fresh plant parts from three different sampling of Zingiber officinale (Zingiberaceae; rhizome), Curcuma longa (Zingiberaceae; rhizome), Ocimum campechianum (Lamiaceae; aerial parts), and Cymbopogon citratus (Poaceae; aerial parts), were collected in the same field at the balsamic period from plants grown in an Amazonian Ecuador area cultivated by the Fundaciòn Chankuap (Macas, Morona-Santiago province, Ecuador). Fresh plant material of each specie (approximately 7 kg) was processed by a 3 h steam distillation using a Clevenger’s type mobile essential oil distiller (Essential Oil Company, Portland, OR, USA). Three different distillations were performed for each plant species. The essential oils (EOs) were then dried over anhydrous sodium sulphate and stored in airtight glass vials with teflon-sealed caps at −18.0 ± 0.5 °C in the dark to prevent degradation before analyses [12,45].
3.2. Chemicals
All the solvents employed for chemical analyses and bioassays were chromatographic grades. Solvents and pure compounds were all purchased from Sigma–Aldrich Italy (Milano, Italy). All the microbial culture media were from Oxoid Italia (Garbagnate, Italy). Commercial essential oil of Thymus vulgaris (limonene chemotype) was purchased from Extrasynthese (Genay, France) and employed as an experimental positive reference for bioassays [21,46]. Lyophilised post-mitochondrial supernatant S9 fraction (Aroclor 1254-induced, Sprague–Dawley male rat liver in 0.154 MKCl solution), commonly used for the activation of pro-mutagens to mutagenic metabolites, was purchased from Molecular Toxicology, Inc. (Boone, NC, USA) and stored at −80 °C. The components of the S9 mix were: 8 mM MgCl2, 32.5 mM KCl, 5 mM G6P, 4 mM NADP, 0.1 m sodium phosphate buffer pH 7.4, and S9 at the concentration of 0.68 mg/mL of the mix.
3.3. Gas Chromatography—Flame Ionization Detector (FID)
EO samples were analysed and the relative peak areas for individual constituents were averaged. The relative percentages were determined using a ThermoQuest GC-Trace gas-chromatograph equipped with a FID detector and a Varian FactorFour VF-5ms poly-5% phenyl-95%-dimethyl-siloxane bonded phase column (i.d., 0.25 mm; length, 30 m; film thickness, 0.25 µm). Operating conditions were as follows: injector temperature 280 °C; FID temperature 300 °C, carrier gas (Helium) flow rate 1 mL/min and split ratio 1:50. Oven temperature was initially 55 °C and then raised to 90 °C at a rate of 1 °C/min, then raised to 250 °C at a rate of 10 °C/min and finally held at that temperature for 15 min. Each sample was dissolved in CH2Cl2 (1:100 v/v) and 1 μL was injected. The percentage composition of the EOs was computed by the normalisation method from the GC peak areas, calculated by means of three injections from each EO, without using correction factors.
3.4. Gas Chromatography—Mass Spectrometry
The analyses of the volatile compounds were performed on a GC-3800 Varian gas chromatograph coupled to a Varian MS-4000 mass spectrometer using electron impact and hooked to NIST and ABREG libraries. The constituents of the volatile oils were identified by comparing their GC retention times, LRI and the MS fragmentation pattern with those of other EOs of known composition, with pure compounds and by matching the MS fragmentation patterns and retention indices with the above-mentioned mass spectra libraries and with literature data [47]. The GC conditions were the same as reported for GC analysis (Section 3.3) and the same column was used. The MS conditions were instead as follows: ionisation voltage, 70 eV; emission current, 10 µAmp; scan rate, 1 scan/s; mass range, 40–400 Da; trap temperature, 150 °C, transfer line temperature, 300 °C. A mixture of aliphatic hydrocarbons (C8–C24) in hexane was injected under the above temperature program to calculate the arithmetic indices [12].
3.5. Headspace Gas Chromatography—Mass Spectrometry
The chemical composition of the volatile fraction of EOs was determined by static headspace analysis in GC-MS under the same conditions abovementioned (Section 3.3 and Section 3.4). Five hundred microliters of each sample were placed in an 8 mL vial sealed with a crimp top and kept at 37.0 ± 0.5 °C for 1 h. The vapour phase was drawn off with a 1 mL gas-tight syringe and injected into the gas chromatograph [46].
3.6. Biological Activities of Essential Oils (EOs)
Antioxidant, antimicrobial, and antimutagenic capacities were evaluated for all the EOs. All the bioactivities were performed by comparing all the data with those achieved with commercial T. vulgaris essential oil considered as a positive control [46,48]. Data reported for each assay are the average of three determinations of independent experiments. For the most interesting results regarding antioxidant and antimicrobial activities, the DPPH and ABTS High-Performance Thin Layer Chromatography (DPPH/ABTS-HP-TLC) bioautographic assays were performed to point out the most effective compounds and/or compound categories [12].
3.6.1. Antioxidant Properties
Radical scavenging properties were performed through 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay; 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) spectrophotometric assay. Each EO (0.5 mL/mL in Tween 40, 0.5% w/w in distilled water) was diluted 2, 5, 10, 50, 100, and 200-fold with DMSO. The antioxidant capacity of each EO was evaluated by its ability to bleach the 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid radicals (ABTS) [49]. An aliquot of 100 µL of each solution was added to 2.9 mL of 1,1-diphenyl-2-picrylhydrazyl (DPPH; 1 × 10−4 M in ethanol), shaken vigorously and kept in the dark for 30 min at room temperature. Sample absorbance was measured at 517 nm with UV–vis spectrophotometer (Thermo-Spectronic Helios γ, Cambridge, UK).
A stock solution of ABTS•+ was produced by the reaction of ABTS solution (2 mM) with 70 mM potassium persulfate (both solutions were prepared in double-distilled water) for 12–16 h, in the dark at room temperature. The stock solution was diluted in Phosphate Buffered Saline (PBS) to achieve an absorbance of 0.70 ± 0.02 at 734 nm with a UV/VIS spectrophotometer (Thermo-Spectronic Helios γ, Cambridge, UK). An aliquot of 900 µL of diluted ABTS solution was mixed with 100 µL of the sample. The absorbance at 734 nm was taken exactly 1 min after initial mixing.
For both assays, a negative control was assessed as the solution assay described above without the EOs, instead of which Tween 40 was employed. Trolox® and commercial T. vulgaris essential oil, known for their antioxidant capacity as pure compound and phytocomplex, respectively, were used as positive controls and prepared as described above for the studied EOs.
All the radical scavenging activities were reported as IC50 for each sample [46,49].
3.6.2. Antimicrobial Activity
The antimicrobial activity was checked against Gram-positive and Gram-negative bacteria (Staphylococcus aureus subsp. aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Micrococcus luteus ATCC 9622, Listeria grayi ATCC 19120, Pseudomonas aeruginosa ATCC 17934, Klebsiella oxytoca ATCC 29516, Escherichia coli ATCC 4350, Proteus vulgaris ATCC 6361), and against two yeasts (Candida albicans ATCC 48274 and Saccharomyces cerevisiae ATCC 2365) following the procedures reported in [11,20,30]. Chloramphenicol (CPh; Sigma-Aldrich) and fluconazole (Flu; Sigma-Aldrich; St. Louis, Missouri, USA) were used to test the sensitivity of bacterial and yeast strains, respectively. The EOs bioactivity was assayed employing the standard disk diffusion method: mother cultures of each micro-organism were set up 24 h before the assays to reach the stationary phase of growth. The tests were assessed by inoculating Petri dishes from the mother cultures with proper sterile media, to obtain a concentration of 105 CFU/mL for bacteria and 106 CFU/mL colony for yeasts. An aliquot of dimethyl sulfoxide (DMSO) was added to the EOs to obtain a 0.01–100 mg/mL concentration range. Serial dilutions of the DMSO/EO solution were deposited on sterile paper discs (6 mm diameter, Difco) which were then placed in the centre of the inoculated Petri dishes. Therefore, the Petri dishes were then incubated at 37 °C for 48 h for bacteria and 72 h for yeasts, and the growth inhibition zone diameter (IZD) was measured to the nearest mm. The lowest concentration of each DMSO/EO solution deposited on the sterile paper disc showing a clear zone of inhibition was taken as the minimum inhibitory concentration (MIC) [31,49].
Moreover, the biological activity of each EOs against the above-mentioned bacterial and yeast strains was performed by means of the properly modified agar vapour method to check the bioactivity of the most volatile fraction [46]. The microorganisms were grown in Petri plates (90 mm) supplemented with 15 mL/plate of appropriate agarised medium (5 × 102 CFU/mL for bacteria and yeasts; approx. 500 CFU/plate). Sterilised filter paper disks (diameter 9.0 mm) were absorbed with pure 1–10 μL of each EO and placed inside the upper lid of each plate, at about 4 mm from the strain. Plates were kept in an inverted position, tightly sealed with parafilm, and incubated for 48 h for bacteria and 72 h for yeasts at 37.0 ± 1.0 °C. Blanks served as a negative control. Three replicates were made for each treatment and the results were expressed as growth inhibition percentage based on the number of colonies counted for each plate with reference to negative control where the growth was considered as 100%. The strains were cultured in nutrient agar, tryptic soy agar, and YEPD (yeast extract, peptone, and dextrose) following the suggestions given by ATCC protocols (www.ATTC.org; accessed in 1 June 2021).
3.6.3. HP-TLC-Bioautographic Assay of the Antioxidant and Antimicrobial Most Interesting Results
To point out the main EO compounds as separate bands on TLC plates, ten microliters on a 6 mm band of 1% solutions of each EO in hexane were deposited to HP-TLC (plates silica gel 60 F254, Merck) precoated plates (90 × 10 mm), developed in a chromatographic chamber with toluene/ethyl acetate/petroleum ether 93/7/20, and dried for complete removal of solvents. Detection was achieved by UV light (254 nm) and then monitored at visible light after spraying in sequence with solutions of 10% H2SO4 ethanolic and 2% vanillin ethanolic, followed by heating at 105 °C for 10 min [29].
To perform High-Performance Thin Layer Chromatography (HP-TLC) bioautographic assay of the most relevant results emerged by radical scavenging activity spectrophotometric assays using the DPPH and the ABTS radicals, 10 μL of each EO hexane solution (1% v/v) was applied in triplicate to a 90 × 100 mm HP-TLC plate of silica gel (plates silica gel 60 F254, Merck) as 10 mm wide bands with Linomat V (Camag). Then, the same spots deposited on two distinct plates were eluted as above described. After elution, one plate was sprayed with an ethanolic solution of DPPH (2 mg/mL), while the other was sprayed with ABTS stock solution prepared as above-described (Section 3.6.1) to detect the antioxidant fractions against the two different radicals. Antiradical compounds appeared as clear white spots against a violet-coloured background for the HP-TLC-DPPH plate, while the background appeared green-coloured for the (HP)TLC-ABTS plate [12].
To perform HP-TLC bioautographic assay to point out the bioactive antimicrobial compounds and/or compound categories that emerged in the most interesting results, HP-TLC plates were prepared as described in Section 3.6.2 and treated as reported in [12]. After 24 h incubation, the results led to point out the effective fractions (bands) of the EOs, which determined the presence of localised microbial growth inhibition zones on the eluted HP-TLC plates.
Related literature consultation [29], the elution of pure compounds putative responsible for bioactivity on HP-TLC plates, and the analyses by GC-MS of the scratched-out and ethyl acetate extracted bioactive bands were the strategies adopted to identify the bioactive spots corresponding to active constituents.
3.6.4. Amazonian Essential Oils Cytotoxicity and Mutagen Protection Properties: Highest Uneffective Dose (HUD) and Ames Test Properly Modified
The inhibitory effect of the Amazonian EOs samples (concentration range 0.0–1.0 mg/plate) on the mutagenic activity of direct-acting mutagen 2-nitrofluorene (2 μg/plate) and sodium azide (1 μg/plate), was examined in plate incorporation assay, derived from mutagenicity test as described by [40] with some minor modifications, using tester strain TA98 and TA100, respectively. The inhibitory effect of the EOs on the mutagenic activity of the indirectly acting mutagen 2-aminoanthracene (2 μg/plate) was instead examined in a plate incorporation assay, using tester strain TA98 and TA100 with S9 mix. The inhibition rate for mutagenic induction was calculated according to the formula:
where A represents the revertants in the positive control and B the revertants in the EOs samples, having subtracted the spontaneous revertants. A critical point, affecting the outcome of the interaction between an antimutagen and a testing bacterial strain is the overlapping of the cytotoxic and antimutagenic dose concentrations. In other words, it is important to confirm that the dose-dependent disappearance of the mutant colonies is not a result of cell killing. For this purpose, a simple survival assay for the treated bacteria must be performed to evaluate a Highest Uneffective Dose (HUD). To verify the toxicity of the analysed samples on bacterial cells and evaluate the HUD, a toxicity test was performed [40]. A fresh 15 h culture was diluted to give a 1–2 × 104 bacteria/mL. The test samples at several concentrations (10−2, 5 × 10−2, 10−1, 5 × 10−1, 1, 5, 10 mg/plate) diluted in DMSO, mixed with 2 mL of molten top agar, were plated with 0.1 mL of the diluted culture. Histidine/biotin agar plates were enriched with 10 μmoles of L-histidine and 0.05 μmoles of biotin by incorporating these nutrients into the soft agar overlay. Triplicate plates were poured for each dose of solution. The colony-forming units (CFU) were assessed after the plates were incubated at 37 °C for 48 h and compared with that of the control, where no test samples were added. HUD for the EOs, with and without metabolic activation, was evaluated by visual estimation (colonies counting) integrated by statistical analyses.
3.7. Statistical Analysis
Relative standard deviations and statistical significance were evaluated using Student’s t-test (p < 0.05). The statistical evidence performed on the Ames test was combined with the Highest Uneffective Dose (HUD); the results were then used to interpret the results of the significant decrease in the number of Salmonella revertants. When the modulator dose concentration was statistically effective and it ranged below or coincided with the HUD, the samples were considered to present a sign of the effect (antimutagenicity). All computations were carried out using the statistical software STATISTICA 6.0 (StatSoft Italia Srl, Vigonza, Padova, Italy).
4. Conclusions
The present study reported the chemical characterisation of the essential oils (EOs) of Zingiber officinale (Zingiberaceae; rhizome), Curcuma longa (Zingiberaceae; rhizome), Ocimum campechianum (Lamiaceae; aerial parts) and Cymbopogon citratus (Poaceae; aerial parts) obtained from plants cultivated in the southeastern area of Ecuador, evidencing interesting differences in comparison with analogous literature reports—regarding both EOs from wild and cultivated plants—pointing out the peculiarity of the Ecuadorian Amazonian area in affecting the EO composition of aromatic plants. The chemical characterisation was also extended to the vapour phase fraction showing the prevalence of monoterpenes in all four EOs, but with a significant further presence of sesquiterpene hydrocarbons in the EO of O. campechianum.
The antioxidant activity of the EOs revealed O. campechianum phytocomplex as the most interesting, showing with both DPPH and ABTS assays IC50 values better than those shown by the T. vulgaris EO taken as positive reference, and comparable to those of the positive control characterised by the single compound Trolox(R). The HP-TLC bioautographic assay pointed out eugenol as the compound responsible for radical scavenging bioactivity. Assessments of antimicrobial activity performed on both the EOs and their volatile fraction showed MIC values above or comparable to those of the positive control T. vulgaris EO. O. campechianum EO showed the most interesting MIC results, particularly against the Gram-positive L. grayi and, against the Gram-negative K. oxytoca, P. aeruginosa, and E. coli, suggesting interesting developments in the field of natural antimicrobials to counter the emerging and increasingly serious problems of antibiotic resistance. The bioactivity of the other EOs was significantly lower, with the EO of C. citratus being less active than that of O. campechianum, followed by that of Z. officinale and C. longa. The same results were confirmed by the antimicrobial activity evaluated on the vapour phase fractions of the EOs. As for the antioxidant assays, the bioautographic HP-TLC assay of the most performing EO, O. campechianum, confirmed the essential role of eugenol as the bioactive compound. Regarding the assessment of the mutagenic protective capacity of the EOs evaluated with the Ames test (TA98 and TA100 Salmonella typhimurium strains towards the mutagens nitrofluorene, aminoanthracene, and sodium azide), the O. campechianum EO highlighted the interesting capacity of inhibiting the development of mutant colonies starting from a concentration of 0.02 mg/plate. The EOs of C. citratus, C. longa, and Z. officinale, even if with a proportionally lower efficacy (protective capacity starting from the concentration of 0.10–0.20 mg/plate), were found to be noteworthy. Finally, to the best of our knowledge, this is the first report about the mutagen-protection capacity of the Amazonian Z. officinale, C. longa, O. campechianum, and C. citratus and the results, even if with evident differences in efficacy, suggest important perspectives for their use, e.g., as food additives for the protective properties against potential mutagens.
Author Contributions
Conceptualisation, project administration, investigation, and writing—original draft, G.S., A.G. (Alessandra Guerrini), M.T; extraction, I.M., M.R.; chemical characterisation, A.G. (Alessandra Guerrini), M.T.; biological activities, A.G. (Alessandro Grandini), G.P., I.C.; methodology, formal analysis, statistical analysis, and data curation, G.S., A.G. (Alessandra Guerrini), M.T., A.G. (Alessandro Grandini), G.P.; revision and editing, G.S., A.G. (Alessandra Guerrini), M.T.; funding acquisition, G.S., A.G. (Alessandra Guerrini). All authors have read and agreed to the published version of the manuscript.
Funding
The research has been supported by a grant from the University of Ferrara, Italy (FAR 2021-2192449; FAR 2022-719270).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
A special thank is due to Fundación Chankuap (Macas, Ecuador) for its invaluable cooperation in the promotion of Ecuadorian Amazonian biodiversity and in the practical realisation of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, F.A.; Khan, N.M.; Ahmad, S.; Aziz, R.; Ullah, I.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A. Phytochemical profiling, antioxidant, antimicrobial and cholinesterase inhibitory effects of essential oils isolated from the leaves of Artemisia scoparia and Artemisia absinthium. Pharmaceuticals 2022, 15, 1221. [Google Scholar] [CrossRef]
- Meenua, M.; Padhanb, B.; Patel, M.; Patel, R.; Xu, B. Antibacterial activity of essential oils from different parts of plants against Salmonella and Listeria spp. Food Chem. 2023, 404, 34723. [Google Scholar] [CrossRef]
- Aqeel, U.; Aftab, T.; Khan, M.M.A.; Naeem, M. Regulation of essential oil in aromatic plants under changing environment. J. App. Res. Med. Ar. Plants 2023, 32, 100441. [Google Scholar] [CrossRef]
- Singh, I.R.; Pulikkal, A.K. Preparation, stability and biological activity of essential oil-based nano emulsions: A comprehensive review. Open Nano 2022, 8, 100066. [Google Scholar] [CrossRef]
- Sarmah, P.; Das, B.; Saikia, J.; Konwar, P.; Mudoi, K.D.; Saikia, S.P.; Banik, D. An insight on the immunomodulatory potential of wood oil of Aquilaria malaccensis Lam. with an emphasis on related phytomedicine, biomarkers, pharmacology, and toxicity. S. Afr. J. Bot. 2022, 151, 695–712. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, F.; Yu, C. Therapeutic potential of Curcuma oil and its terpenoids in gynecological cancers. Biomed. Pharmacother. 2023, 157, 114016. [Google Scholar] [CrossRef]
- Lee, M.-K.; Lim, S.; Song, J.-A.; Kim, M.-E.; Hur, M.-H. The effects of aromatherapy essential oil inhalation on stress, sleep quality and immunity in healthy adults: Randomized controlled trial. Eur. J. Integr. Med. 2017, 12, 79–86. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; Poitevin, C.G.; Bischoff, A.M.; Beger, M.; da Luz, T.S.; Mazarotto, E.J.; Benatto, A.; Martins, C.E.N.; Sales Maia, B.H.L.N.; Sari, R.; et al. Insecticidal and antifungal activities of Melaleuca rhaphiophylla essential oil against insects and seed-borne pathogens in stored products. Ind. Crop. Prod. 2022, 182, 114871. [Google Scholar] [CrossRef]
- Elumalai, K.; Krishnappa, K.; Pandiyan, J.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Barnard, D.R.; Vijayakumar, N.; Govindarajan, M. Characterization of secondary metabolites from Lamiaceae plant leaf essential oil: A novel perspective to combat medical and agricultural pests. Physiol. Mol. Plant Pathol. 2022, 117, 101752. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Zhao, L.-L.; Shao, Y.-X.; Liao, X.-D.; Zhang, L.-Y.; Luo, X.-G. Effects of dietary graded levels of cinnamon essential oil and its combination with bamboo leaf flavonoid on immune function, antioxidative ability and intestinal microbiota of broilers. J. Integr. Agr. 2019, 18, 2123–2132. [Google Scholar] [CrossRef]
- Coimbra, A.; Ferreira, S.; Duarte, A.P. Biological properties of Thymus zygis essential oil with emphasis on antimicrobial activity and food application. Food Chem. 2022, 393, 133370. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Guerrini, A.; Maietti, S.; Bruni, R.; Paganetto, G.; Poli, F.; Scalvenzi, L.; Radice, M.; Saro, K.; Sacchetti, G. Chemical fingerprinting and bioactivity of Amazonian Ecuador Croton lechleri Müll. Arg. (Euphorbiaceae) stem bark essential oil: A new functional food ingredient? Food Chem. 2011, 126, 837–848. [Google Scholar] [CrossRef]
- Rossi, D.; Guerrini, A.; Paganetto, G.; Bernacchia, G.; Conforti, F.; Statti, G.; Maietti, S.; Poppi, I.; Tacchini, M.; Sacchetti, G. Croton lechleri Müll. Arg. (Euphorbiaceae) stem bark essential oil as possible mutagen-protective food ingredient against heterocyclic amines from cooked food. Food Chem. 2013, 139, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Noshad, M.; Behbahani, B.A.; Nikfarjam, Z. Chemical composition, antibacterial activity and antioxidant activity of Citrus bergamia essential oil: Molecular docking simulations. Food Biosci. 2022, 50, 102123. [Google Scholar] [CrossRef]
- Arab, H.A.; Bahadori, F.; Mirza, M.; Badi, H.N.; Kalate-Jari, S. Variability in essential oil composition and phenolic acid profile of Thymus daenensis Celak. populations from Iran. Ind. Crop. Prod. 2022, 178, 114345. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants 2021, 10, 44. [Google Scholar] [CrossRef]
- Mahboubi, M. Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clin. Phytosci. 2019, 5, 6. [Google Scholar] [CrossRef]
- Sacchetti, G.; Medici, A.; Maietti, S.; Radice, M.; Muzzoli, M.; Manfredini, S.; Braccioli, E.; Bruni, R. Composition and Functional Properties of the Essential Oil of Amazonian Basil, Ocimum micranthum Willd., Labiatae in Comparison with Commercial Essential Oils. J. Agric. Food Chem. 2004, 52, 3486–3491. [Google Scholar] [CrossRef]
- Scalvenzi, L.; Radice, M.; Toma, L.; Severini, F.; Boccolini, D.; Bella, A.; Guerrini, A.; Tacchini, M.; Sacchetti, G.; Chiurato, M.; et al. Larvicidal activity of Ocimum campechianum, Ocotea quixos and Piper aduncum essential oils against Aedes aegypti. Parasite 2019, 26, 23. [Google Scholar] [CrossRef]
- Tacchini, M.; Guevara, M.P.E.; Grandini, A.; Maresca, I.; Radice, M.; Angiolella, L.; Guerrini, A. Ocimum campechianum Mill. from Amazonian Ecuador: Chemical Composition and Biological Activities of Extracts and Their Main Constituents (Eugenol and Rosmarinic Acid). Molecules 2021, 26, 84. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Falcao, M.A.; Fianco, A.L.B.; Lucas, A.M.; Pereira, M.A.A.; Torres, F.C.; Vargas, R.M.F.; Cassel, E. Determination of antibacterial activity of vacuum distillation fractions of lemongrass essential oil. Phytochem. Rev. 2012, 11, 405–412. [Google Scholar] [CrossRef]
- Trang, D.T.; Hoang, T.K.V.; Nguyen, T.T.M.; Cuong, P.V.; Dang, N.H.; Dang, H.D.; Quang, T.N.; Dat, N.Y. Essential Oils of Lemongrass (Cymbopogon citratus Stapf) Induces Apoptosis and Cell Cycle Arrest in A549 Lung Cancer Cells. BioMed Res. Int. 2020, 2020, 5924856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Komatsu, K.; Toume, K.; Zhu, S.; Tanaka, K.; Hayashi, S.; Anjiki, N.; Kawahara, N.; Takano, A.; Miyake, K.; et al. Essential oil composition of Curcuma species and drugs from Asia analyzed by headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry. J. Nat. Med. 2022, 77, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Höferl, M.; Stoilova, I.; Wanner, J.; Schmidt, E.; Jirovetz, L.; Trifonova, D.; Stanchev, V.; Krastanov, A. Composition and Comprehensive Antioxidant Activity of Ginger (Zingiber officinale) Essential Oil from Ecuador. Nat. Prod. Commun. 2015, 10, 1085–1090. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa L. Indian J. Pharmacol. 2011, 43, 526–531. [Google Scholar] [CrossRef]
- Avanço, G.B.; Ferreira, F.D.; Bomfim, N.S.; de Souza Rodrigues dos Santos, P.A.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; de Abreu Filho, B.A.; Mikcha, J.M.G.; Machinski, M., Jr. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2017, 73, 806–813. [Google Scholar] [CrossRef]
- Vázquez-Briones, M.C.; Hernández, L.R.; Guerrero-Beltrán, J.A. Physicochemical and Antioxidant Properties of Cymbopogon citratus Essential Oil. J. Food Res. 2015, 4, 36–45. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis; Springer-Verlag: Berlin/Heidelberg, Germany; New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A comprehensive and systematic review on potential anticancer activities of eugenol: From pre-clinical evidence to molecular mechanisms of action (a review). Phytomedicine 2022, 107, 154456. [Google Scholar] [CrossRef]
- Guerrini, A.; Rossi, D.; Paganetto, G.; Tognolini, M.; Muzzoli, M.; Romagnoli, C.; Antonioni, F.; Vertuani, S.; Medici, A.; Bruni, A.; et al. Chemical characterization (GC-MS and NMR fingerprinting) and bioactivities of South-African Pelargonium capitatum (L.) L’Herit. (Geraniaceae) essential oil. Chem. Biodivers. 2011, 8, 624–642. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.-H. Management of Highly Resistant Gram-Negative Infections in the Intensive Care Unit in the Era of Novel Antibiotics. Infect. Dis. Clin. N. Am. 2022, 36, 791–823. [Google Scholar] [CrossRef] [PubMed]
- Eva, L.; Gernot, Z.; Josefa, L.; Kathrin, H.; Shiva, P.-A.; Martin, H.; Thomas, V.; Gebhard, F.; Andrea, J.G. Contaminated Handwashing Sinks as the Source of a Clonal Outbreak of KPC-2-Producing Klebsiella oxytoca on a Hematology Ward. Antimicrob. Agents Chemother. 2015, 59, 714–716. [Google Scholar] [CrossRef]
- Gerver, S.M.; Nsonwu, O.; Thelwall, S.; Brown, C.S.; Hope, R. Trends in rates of incidence, fatality and antimicrobial resistance among isolates of Pseudomonas spp. causing bloodstream infections in England between 2009 and 2018: Results from a national voluntary surveillance scheme. J. Hosp. Infect. 2022, 120, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Balfour, J.; Barclay, M.; Danial, J.; Philip, C.; Perry, M.; Etherson, M.; Henderson, N. Risk factors for antimicrobial resistance in patients with Escherichia coli bacteraemia related to urinary tract infection. Infect. Prev. Prac. 2022, 4, 100248. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Cr. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, G.; Yew, X.Y.; Sivasamugham, L.A. Antibacterial activity of Cymbopogon citratus against clinically important bacteria. S. Afr. J. Chem. Eng. 2020, 34, 26–30. [Google Scholar] [CrossRef]
- Albino, S.; Robazza, A.W.S.; Schittler, L.; Gomes, G.A. Synergistic and antimicrobial properties of commercial turmeric (Curcuma longa) essential oil against pathogenic bacteria. Food Sci. Technol. 2012, 32, 525–530. [Google Scholar] [CrossRef]
- Beristain-Bauza, S.D.C.; Hernández-Carranza, P.; Cid-Pérez, T.S.; Ávila-Sosa, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Antimicrobial Activity of Ginger (Zingiber Officinale) and Its Application in Food Products. Food Rev. Int. 2019, 35, 407–426. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res.-Envir. Muta. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Thaís, F.L.; De Souza, C.T.; Pinho, R.A.; de Oliveira Marques, S.; Luiz, G.P.; dos Santos Tramontin, N.; da Silveira, P.C.L.; de Andrade, V.M.; Muller, A.P. Effects of Zingiber officinale extract supplementation on metabolic and genotoxic parameters in diet-induced obesity in mice. Brit. J. Nutr. 2021, 126, 970–981. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L.). Food Chem. Toxicol. 2013, 53, 52–61. [Google Scholar] [CrossRef]
- Ahmad, I.; Aqil, F.; Owais, M. Modern Phytomedicine: Turning Medicinal Plants into Drugs; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Costa, C.A.R.A.; Bidinotto, B.T.; Takahira, R.K.; Salvadori, D.M.F.; Barbisan, L.F.; Costa, M. Cholesterol reduction and lack of genotoxic or toxic effects in mice after repeated 21-day oral intake of lemongrass (Cymbopogon citratus) essential oil. Food Chem. Toxicol. 2011, 49, 2268–2272. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, Revision 3; AOAC International: Gaithersburg, MA, USA, 2010; ISBN 0935584676. [Google Scholar]
- Maietti, S.; Rossi, D.; Guerrini, A.; Useli, C.; Romagnoli, C.; Poli, F.; Bruni, R.; Sacchetti, G. Multivariate analysis approach to the study of chemical and functional properties of chemo-diverse plant derivatives: Lavender essential oils. Flav. Fragr. J. 2013, 28, 144–145. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- El Yaagoubi, M.; Mechqoq, H.; El Hamdaoui, A.; Mukku, V.J.; El Mousadik, A.; Msanda, F.; El Aouad, N. A review on Moroccan Thymus species: Traditional uses, essential oils chemical composition and biological effects. J. Ethnopharmacol. 2021, 278, 114205. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E.; Tognolini, M.; Maldonado, M.E.; Bruni, R. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) essential oils from Eastern Ecuador. Environ. Toxicol. Phar. 2009, 27, 39–48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).