Abstract
The present umbrella review aimed to characterize the type and regimen of antibiotics administered locally and/or systemically, alone or in combination with surgical and nonsurgical treatments, for peri-implantitis and to evaluate and compare the associated clinical, radiographic, and crevicular peri-implant outcomes. The secondary objective was to determine the most effective antibiotic type, route of administration, regimen, and protocols (antibiotics alone or in combination with other approaches) for treating peri-implantitis. The study protocol, which was developed in advance under the PRISMA statement, was registered at PROSPERO (CRD42022373957). BioMed Central, Scopus, MEDLINE/PubMed, the Cochrane Library databases, and the PROSPERO registry were searched for systematic reviews through 15 November 2022. Of the 708 records found, seven reviews were included; three were judged of a critically low and four of low quality through the AMSTAR 2 tool. Locally administered antibiotics alone or as an adjunct to surgical or nonsurgical treatments for peri-implantitis showed favorable outcomes, albeit with limited evidence. The administration of systemically-delivered antibiotics in combination with nonsurgical or surgical treatments remained questionable. Local plus systemic antibiotics have not been shown to have durable efficacy. Due to the heterogeneity of reported antibiotic types, routes, regimens, and protocols, no definitive conclusions could be drawn regarding the most effective antibiotic use in treating peri-implantitis.
1. Introduction
Dental implants have become one of the most reliable therapeutic options to replace missing teeth. As a result, a significant increase in the number of dental implants placed annually, on the one hand, and in the number of cases diagnosed with peri-implantitis, on the other hand, has been observed worldwide [1,2].
The 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions defined peri-implantitis as a pathological condition occurring in the tissues surrounding dental implants characterized by signs of inflammation of the outer tissues and progressive bone loss [3,4,5,6,7,8].
A number of therapeutic approaches have been proposed for peri-implantitis management [9,10,11,12,13,14,15,16,17,18,19], including nonsurgical, surgical, and combined treatments. Most of the peri-implantitis treatments are also performed for periodontitis because of the common etiology [3,4,5,6,7,8], and the similarities in the pathophysiology of bacterial biofilm formation on both dental implant surfaces and dental tissues [12].
Abrasive air powder, metallic or non-metallic curettes (the latter made of carbon or plastic, reinforced or not with resin), with an ultrasonic scaler with a metal or plastic tip [14,15] provide nonsurgical mechanical biofilm control. In addition, implantoplasty is performed to reduce the roughness of dental implant surfaces and, in turn, bacterial adhesion [15]. Further physical methods, such as phototherapy and laser therapy, e.g., with a continuous carbon dioxide laser, have also been proposed in peri-implantitis management [16,17].
As a counterpart, chemical biofilm control mainly relies on chlorhexidine, along with hydrogen peroxide, cotton pellets soaked in saline solution and citric acid [12].
However, nonsurgical treatment has not yet shown fully predictable results in peri-implantitis treatment [9,10], and long-term data on outcomes after surgical treatment show only a slight improvement in bone levels [10,11].
Moreover, considering that the microbial flora characterizing peri-implantitis has a broader spectrum compared to periodontitis and that the biofilm of peri-implantitis is characterized by higher counts of human cytomegalovirus and Epstein–Barr virus [7,8,13], reducing the number of pathogens and changing the bacterial biofilm composition might be even more critical in peri-implantitis management. From this point of view, the control of biofilm and the reversal of dysbiosis could benefit from the administration of probiotics [8].
Furthermore, antibiotics administration as adjuncts to peri-implantitis treatment was first proposed by Mombelli and Lang in 1992 [18]. Since then, several studies have been conducted to evaluate the beneficial effects of systemically- and locally-delivered antibiotics in combination with other treatments [19]. However, it is still controversial whether the concomitant systemic or local use of antibiotics is beneficial in treating peri-implantitis [19]. In addition, since antibiotics are among the most commonly prescribed medications by dentists, often without precise indications, thus violating antibiotic stewardship [20,21,22,23], their use should be appropriately evaluated.
Therefore, the present umbrella review aimed to characterize the type and regimen of antibiotics administered locally and/or systemically, alone or in combination with surgical and nonsurgical treatments for peri-implantitis, and to evaluate and compare the associated clinical, radiographic, and crevicular peri-implant outcomes. The secondary objective was to determine the most effective antibiotic type, route of administration, regimen, and protocols (antibiotics alone or in combination with other approaches) for treating peri-implantitis.
2. Materials and Methods
2.1. Study Protocol
Prior to the literature search, data extraction, and analysis, the study protocol was defined under the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [24], and registration was made in the PROSPERO Registry of Systematic Reviews (CRD42022373957) [25].
The questions were formulated based on the PICO model [26,27], and record search and study selection strategies were developed.
The research question [28] focused on the efficacy of type, routes of administration, and regimens of locally- and/or systemically-delivered antibiotics alone or in combination with other (surgical or nonsurgical) peri-implantitis treatments for improving peri-implant outcomes, specifically:
P-Population: subjects with at least one dental implant and implant-supported restoration(s) with peri-implantitis:
- I-Intervention: locally and/or systemically administered antibiotics alone or (all);
- C-Comparison: no intervention, placebo, between different interventions (different type, routes of administration, and regimens of locally- and/or systemically-delivered antibiotics alone or in combination with other surgical or nonsurgical peri-implantitis treatments);
- O-Outcome(s): clinical and radiographic and crevicular peri-implant parameters.
2.2. Search Strategy
Systematic reviews (with or without meta-analysis) published in English on local and/or systemic antibiotics in peri-implantitis treatment were searched electronically through the PROSPERO registry and Scopus, MEDLINE/PubMed, BioMed Central, and Cochrane Library databases by three independent reviewers (F.D.S., F.D.A., M.P.D.P.) without date restriction until 15 November 2022, using keywords and Boolean operators as follows: (“peri-implantitis” OR “peri-implant dis-ease” OR “dental implant” OR “implant loss”) AND (“local antibiotic” OR “local antibiotics” OR “general antibiotic” OR “general antibiotics” OR “antibiotic therapy” OR antibiotics OR antimicrobial OR “peri-implant mucositis”).
The following filters were applied: “review (English)” and “refine”: “review (English)” in the Scopus database; “systematic review (English)” in the MEDLINE/PubMed database; “keywords” in the Cochrane Library; no filters were used in the BioMed Central database or the PROSPERO registry.
2.3. Study Selection and Eligibility Criteria
Collected citations were recorded, duplicates were eliminated via the reference management tool EndnoteTM (Clarivate), and the remaining systematic review titles (with or without meta-analysis) were screened by the three independent reviewers (FDS, FDA, MPDP), who then screened relevant abstracts.
The full texts of these potentially eligible title-abstracts were obtained, contacting the study authors if full texts were unavailable, and were independently reviewed by the same authors (FDS, FDA, MPDP). Any differences of opinion were clarified by consultation, and in case of doubt, another author (FG) was consulted. No manual search was carried out from the reference lists of included articles.
Inclusion criteria: no restrictions were applied on publication date, the number of studies, and study design included in each systematic review; age, gender, and characteristics of the participants; the number of dental implants and type of prosthetic restorations; type and regimen of antibiotics administered locally and/or systemically, alone or in combination with other (nonsurgical or surgical) peri-implantitis treatment.
Exclusion criteria: non-English language studies and self-reported peri-implant status were excluded.
2.4. Data Extraction and Collection
Data were extracted independently and in duplicate by two authors (F.D.A., M.P.D.P.) using a standardized data extraction form developed based on the models recommended for intervention reviews of RCTs and non-RCTs [29] before data extraction; a third author (F.D.S.) was consulted in case of disagreement.
Of each systematic review (with or without meta-analysis) included in the present umbrella review, data illustrated in Table 1 were recorded.

Table 1.
Data extracted and collected from the systematic reviews included in the present umbrella review.
2.5. Data Synthesis
A narrative synthesis of the data regarding the population studied, intervention(s), and outcomes was performed.
Data from the included studies were qualitatively synthesized:
- to characterize the type and regimen of antibiotics administered locally and/or systemically alone or in combination with other (surgical or nonsurgical) peri-implantitis treatments and comparisons;
- to assess clinical, radiographic, and crevicular peri-implant outcomes according to the type and regimen of locally- and/or systemically-delivered antibiotics administered alone or in combination with other (surgical or nonsurgical) peri-implantitis provided;
- to compare clinical, radiographic, and crevicular peri-implant outcomes after administration of locally- and/or systemically-administered antibiotics alone or in combination with other (surgical or nonsurgical) peri-implantitis vs. placebo and to each other.
2.6. Quality Assessment
The quality assessment of the systematic reviews included in this umbrella review was performed using the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) 2 tool, accessed online (https://amstar.ca) on 16 November 2022, evaluating for quality the systematic reviews with or without meta-analysis [30].
3. Results
3.1. Study Selection
A total of 708 records were identified from the electronic search, specifically 64 from BioMed Central, 216 from Scopus, 252 from MEDLINE/PubMed, five from the Cochrane library databases, and 171 from the PROSPERO register.
In total, 330 duplicates were eliminated, and 378 title-abstracts were screened.
Of these 378 title-abstracts, 17 abstracts were relevant and compliant with the eligibility criteria of the present systematic review.
Full texts were screened, and 10 articles were further excluded, specifically because: (n = 4) not relevant; (n = 1) describing antibiotic use combined with antiseptic agents in implant surgery; (n = 1) antibiotics were not used in peri-implantitis treatment; (n = 2) describing prophylactic antibiotic use; (n = 2) reviews were still ongoing (Table 2).

Table 2.
Studies excluded and reasons.
A total of seven systematic reviews [41,42,43,44,45,46,47], four with meta-analysis [41,43,45,47], were finally included in the present umbrella review (Figure 1). The seven systematic reviews [41,42,43,44,45,46,47] included 42 randomized controlled trials (RCTs) [41,42,43,44,45,46,47], 10 prospective studies (PS) [41,45], seven systematic reviews (SR) [44], four case series (CS) [41,43], three case-control studies (CCS) [41], and two studies whose typology was not defined [47].
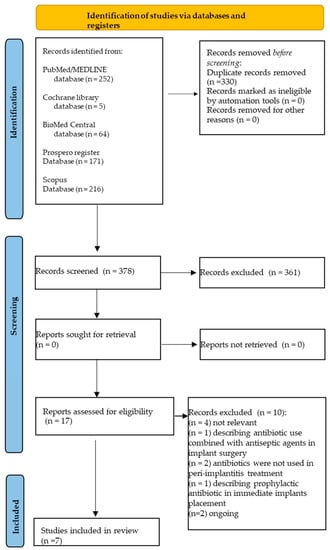
Figure 1.
Study selection flowchart.
3.2. Study Characteristics
The total number of subjects involved was 1575 with 2262 implants. However, three studies [44,46,47] did not report the sample size or the number of implants. In no case was the gender ratio or mean age of participants or the number, location, characteristics, and survival of implants affected by peri-implantitis reported.
The characteristics and outcomes from included studies are synthesized in Table 3.

Table 3.
Characteristics and outcomes from included studies. Source: first author, year, reference, journal of publication, meta-analysis, funding, and study quality (if any). Studies reported the systematic reviews included in the present umbrella review: design and number; population sample size (n.), mean age (y.o.), and gender ratio (M/F); dental implant number, position, and survival. Type, route of administration, regimen, and duration of locally- and/or systemically-delivered antibiotics alone or in combination with other (surgical or nonsurgical) peri-implantitis treatment. Clinical, radiographic, and crevicular peri-implant outcomes (statistically significant). Conclusions.
3.3. Local and Systemic Antibiotics in Peri-Implantitis Management
Locally-delivered antibiotics for treating peri-implantitis were administered alone and in combination with other nonsurgical and surgical interventions and systemic ones.
No data were available about the characteristics of systemic antibiotic therapy alone in treating peri-implantitis from included studies.
3.3.1. Locally-Delivered Antibiotics Alone and in Combination with Nonsurgical and Surgical Treatment of Peri-Implantitis
Three systematic reviews [41,42,46] described findings from studies with local antibiotic administration combined or not to other peri-implant treatments.
Local minocycline (“Arestin” in microspheres, “Periocycline” in ointment), doxycycline gel (“Atridox”, “Ligosan”), lincomycin gel, wrythromycin gel, retracycline fibers “Actisite”, and metronidazole gel “Elyzol” proved to reduce PPD and BoP [41]. However, local minocycline microspheres [42] and metronidazole gel 25% [46] did not positively affect either BoP, PPD, and PI [42], or implant failure [46], respectively.
Four systematic reviews [42,43,46,47] synthesized peri-implant outcomes following locally-delivered antibiotics combined with other interventions.
Doxycycline hyclate 8.5% + SRP, minocycline 10 mg in 0.5 g of ointment + surgical treatment, and minocycline ointment + nonsurgical treatment improved BoP, GI, PPD, and PI at 4–6 months follow-up [42].
Local tetracycline hydrochloride delivery by monolithic ethylene vinyl acetate fiber (for one time of antibiotic with a duration of 10 days) + rubber cup polishing + SRP, doxycycline “Atridox” subgingivally for one time + SRP and irrigation with 0.2% CHX, and minocycline “Periocline” subgingivally + OFD at 1, 3, and 6 months were found to positively affect PPD and BoP up to 12 months after therapy [43].
Local doxycycline hyclate 8.5% “Atridox” applied through a syringe with a blunt cannula in the peri-implant sulcus + SRP determined a more significant improvement in CAL and PPD values compared to mechanical debridement at 4 months follow-up [46].
Local minocycline gel + ultrasonic periodontal debridement, minocycline hydrochloride microspheres + SRP, and metronidazole (400 mg) + amoxicillin (500 mg) + SRP equally decreased PPD, BoP, and CAL values compared to the baseline [47].
Characteristics of antibiotic administration in peri-implantitis treatment are detailed in Table 4 for locally-delivered antibiotics administered alone (not in combination with nonsurgical and surgical treatments) and in Table 5 for locally-delivered antibiotics combined with other interventions.

Table 4.
Characteristics of locally-delivered antibiotics administered alone in treating peri-implantitis from included studies.

Table 5.
Characteristics of locally-delivered antibiotics combined with other interventions in treating peri-implantitis.
3.3.2. Systemically-Delivered Antibiotics in Combination with Nonsurgical and Surgical Treatment of Peri-Implantitis
Four systematic reviews [43,44,45,47] reported results from systemically-delivered antibiotics, which were always used in combination with other interventions in treating peri-implantitis.
Several drug regimens were recorded for amoxicillin (750 mg/12 h or 500 mg/8 h for 7 d, or 500 mg/24 h at 1 std and 250 mg/24 h for 2–4 d + mechanical implant surface debridement; 750 mg/12 h for 3 d preoperatively and 7 d postoperatively + open flap debridement + resective techniques), azithromycin (250 mg/12 h on the d of surgery + 250 mg/24 h for 4 d + open flap debridement, or 500 mg/24 h for 3 d + full mouth scaling and root planing), metronidazole (250 mg/8 h for 7 d + nonsurgical debridement 500 mg/8 h for 7 d + mechanical implant surface debridement), amoxicillin (500 mg/8 h for 7 d) + metronidazole (400 mg/8 h for 7 d) for 5 to 7 days in combination with nonsurgical treatment, open flap debridement and mechanical implant surface debridement, as well as for other antibiotic combinations (e.g., clindamycin + metronidazole + azithromycin + tetracicline for 4 weeks and metronidazole + amoxicillin + ciprofloxacin + sulfonamide + trimethroprim + metronidazole for 2 weeks), overall highlighting that systemic antibiotics should be carefully evaluated in peri-implantitis management considering the risk of antibiotic resistance [45].
Azithromycin (500 mg on 1 d and 250 mg on 2 and 4 d +/− scaling and root planing + rubber cup polishing +/− open flap debridement; 500 mg/d for 3 d preoperatively + scaling and root planing), amoxicillin (1.5 g for 3 d preoperatively and 7 d postoperatively + open flap debridement + bone recontouring + rubber cup polishing + chlorhexidine 0.2%), and amoxicillin (500 mg/8 h for 14 d) + metronidazole (400 mg/24 h for 14 d) provided benefits in clinical peri-implant outcomes for up 12 months after therapy [43]. In contrast, amoxicillin (750 mg/12 h + chlorhexidine 0.2%+ mechanical implant surface debridement) and azithromycin (250 mg/12 h for 2 d and 250/24 h for 4 d) did not show beneficial effects [44].
The association of amoxicillin and metronidazole in combination with ultrasonic debridement (500 mg/8 h + 500 mg/24 h for 7 d), scaling and root planing (375 mg/8 h for 7 d + 250 mg/8 h for 7 d; 500 mg/8 h + 400 mg/24 h for 7 d), as well as the administration of clarithromycin in combination with the antimicrobial photodynamic therapy (500 mg/24 h for 3 d) significantly reduced BoP, CAL, and PPD [47].
Table 6 synthesizes the characteristics of systemically-delivered antibiotics combined with other interventions in the treatment of peri-implantitis.

Table 6.
Characteristics of systemically-delivered antibiotics combined with other interventions in the treatment of peri-implantitis.
3.3.3. Locally- Plus Systemically-Delivered Antibiotics in Combination with Nonsurgical and Surgical Treatment of Peri-Implantitis
Two systematic reviews [39,43] reported peri-implant outcomes after local plus systemic antibiotics administration alone or combined with other interventions in treating peri-implantitis.
Local minocycline “Periocline” + amoxicillin (500 mg/8 h for 3 d) in combination with open flap debridement + scaling and root planing at 1, 3, 6 months proved some benefits in BoP and PPD values [43].
Local metronidazole 25% gel “Elyzol” associated with tetracycline hydrochloride “Ambramicine” in combination with apically repositioned flap, as well as amoxicillin (50 mg/kg/d for 8 d) in combination with SRP before surgery did not affect peri-implant outcomes at 2-year follow-up [39].
Table 7 shows the characteristics of local plus systemic antibiotics administered alone or in combination with other interventions in treating peri-implantitis.

Table 7.
Characteristics of local plus systemic antibiotics administration alone or combined with other interventions in treating peri-implantitis.
3.4. Quality Assessment
Three of the studies were judged of critically low [42,46,47] and four of low quality [41,43,44,45] through the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) 2 tool [30], as illustrated in Table 3.
4. Discussion
The treatment of peri-implantitis, which aims to reduce the microbial load, decontaminate the dental implant surface, and eliminate peri-implant mucosal inflammation, thereby preserving the peri-implant bone, can be divided into nonsurgical approaches using mechanical instrumentation, antibiotics, antiseptics, and chemical or laser decontamination of the dental implant surface and surgical approaches such as air powder abrasion, resective, or regenerative procedures [48,49,50,51,52].
Nonsurgical treatment of peri-implantitis aims to reduce inflammation by controlling biofilm [50]. Mechanical therapy includes using resin, carbon, or titanium cuvettes, specialized ultrasonic instruments, rubber bowls/polishing brushes, and air-powder devices [50]. However, nonsurgical mechanical debridement alone is ineffective for the long-term treatment of peri-implantitis [50]. Adjunctive antiseptic agents such as chlorhexidine improve clinical parameters but only for six months or less [50].
Surgical treatment of peri-implantitis is required when PPD and bone resorption have progressed or persist in removing biofilm and creating a surface that allows re-osteointegration through a full-thickness flap [50]. Accordingly, in conjunction with open-access flaps, mechanical debridement and adjuvants, such as saline, abrasive pumice, citric acid, chlorhexidine, and hydrogen peroxide, are used [50]. The open approach and the morphology of the residual bone determine the choice of surgical intervention and whether a resective or regenerative approach is opted for [53].
Given these considerations and the fact that peri-implantitis is maintained by microorganisms [48], it is legitimate to ask whether systemic and/or local antibiotics are effective in treating peri-implantitis [42]. Accordingly, concomitant antibiotics, including minocycline and doxycycline hyclate, have improved gingival inflammation and probing depths [50]. However, further studies are needed to verify their long-term efficacy.
Therefore, the present umbrella review aimed to characterize the type and regimen of antibiotics administered locally and/or systemically, alone or in combination with surgical and nonsurgical treatments for peri-implantitis, and to evaluate and compare the associated clinical, radiographic, and crevicular peri-implant outcomes. The secondary objective was to determine the most effective antibiotic type, route of administration, regimen, and protocols (antibiotics alone or in combination with other approaches) for treating peri-implantitis.
4.1. Locally-Delivered Antibiotics Alone and in Combination with Nonsurgical and Surgical Treatment of Peri-Implantitis
The efficacy of locally-delivered antibiotics alone on periodontal parameters, especially PPD, BoP, and plaque index (PI), was investigated in three studies [41,42,46] with contrasting results, probably due to the different interventions and control groups in each of the three studies [41,42,46].
Indeed, only one study [41] found an improvement in PPD and BoP after using local antibiotics compared with the control group. Toledano et al. [41] demonstrated that the local use of antibiotics in peri-implantitis patients positively affected the reduction of PPD and BoP. Specifically, the likelihood of bleeding on probing was halved when antibiotics were applied topically, and on average, a 0.30 mm reduction in PPD was observed when topically administered antibiotics were used [41]. However, peri-implant parameters in subjects under minocycline [41] were not significantly different from those using chlorhexidine [42]. Similarly, metronidazole was reported to be associated with both improvements in peri-implant conditions [41] and implant failure in the studies by Esposito et al. [46].
Four studies investigated the efficacy of local antibiotics in combination with nonsurgical and surgical treatment of peri-implantitis. Except for one study in which implant failure was recorded [46], the various combinations of tetracyclines (doxycycline or minocycline) with nonsurgical treatments (such as scaling and root planing and photodynamic therapy) or surgical flap incision always resulted in improvement of peri-implant BoP and PPD [42,43,47], even at a follow-up of 12 months [43]. Clinical attachment loss (CAL) [47] and gingival index (GI) and PI also improved in some cases [43].
Accordingly, Passarelli et al. [42] found an improvement in PPD and BoP when local antibiotics were combined with nonsurgical treatment compared with nonsurgical treatment alone [42].
Tetracyclines were the most commonly administered antibiotics [41,42], especially doxycycline and minocycline, which are also the most commonly used in treating periodontal infections (Rodrigues, 2004), followed by metronidazole, which showed a positive effect either if used alone in comparison with photodynamic therapy [42] or in combination with amoxicillin and scaling and root planing [47].
4.2. Systemically-Delivered Antibiotics in Combination with Nonsurgical and Surgical Treatment of Peri-Implantitis
Systemically-delivered antibiotics have always been evaluated in combination with other interventions, likely because antibiotic stewardship advises against prescribing antibiotics unless they are indispensable and their efficacy is supported by evidence to combat antimicrobial resistance [20,22].
A total of four studies evaluating the efficacy of systemic antibiotics in combination with nonsurgical and surgical treatment for peri-implantitis were presently retrieved.
Different associations of amoxicillin and metronidazole at variable doses and durations were administered in combination with nonsurgical (such as scaling and root planing, nonsurgical debridement) and surgical treatments with implant debridement and showed improvement in PPD and BoP in only two studies [43,47]. Conversely, Toledano-Osorio et al. [45] showed that systemically-delivered administered antibiotics did not significantly improve BoP and PPD, although improvements in CAL, suppuration, recession, and total bacterial count were described in patients with peri-implantitis [45]. Similarly, Oen et al. [44] found no improvement in PPD when systemic antibiotics were combined with surgical procedures.
Consequently, combining systemically-administered antibiotics with nonsurgical and surgical treatments for peri-implantitis remains controversial in the current state of knowledge. However, there is some evidence to support their use in recurrent and refractory peri-implantitis cases [35].
4.3. Efficacy of Locally-Plus Systemically-Delivered Antibiotics in Combination with Nonsurgical and Surgical Treatment of Peri-Implantitis
The efficacy of locally- plus systemically-delivered antibiotics in combination with nonsurgical and surgical treatment was investigated in two studies [39,43].
Wang et al. [43] concluded that using local plus systemic antibiotics in combination with other peri-implantitis treatments had a beneficial effect and significantly improved PPD values [43]. However, the authors suggested that such a positive result could also be attributable to the combination of local and systemic administration of antibiotics with open flap debridement and scaling and root planing [43].
Esposito et al. [39] also reported the concomitant use of systemic and local amoxicillin, with no significant improvement in PPD, CAL, and REC from baseline at 2-year follow-up. Therefore, despite the limited data, local plus systemic administration of amoxicillin may have no durable efficacy.
4.4. Local and Systemic Antibiotics in Peri-Implantitis Management: Clinical Considerations
Nonsurgical treatments such as mechanical debridement and oral hygiene instructions effectively managed peri-implant mucositis and reduced but did not eliminate the inflammatory signs and symptoms of peri-implantitis [42,54,55,56]. In the latter case, the surgical treatment allows direct decontamination of the dental implant surface and complete removal of the inflammatory granulation tissue [55].
Chemical biofilm control and locally- and systemically-delivered antibiotics administration have been proposed to improve nonsurgical and surgical treatment outcomes.
In particular, although supported by mild evidence, locally-delivered antibiotics used as an adjunct to nonsurgical mechanical debridement produced favorable results. In addition, local antibiotics, in combination with other surgical interventions, also showed modest clinical improvements in peri-implant parameters. Because adequate drug concentration at the site of infection is required for a sufficient time to eliminate the pathogens [57], various drug carriers have been developed to improve outcomes associated with locally-delivered antibiotics.
Conversely, systemically-delivered antibiotics had no significant effect on the treatment of peri-implantitis, either in combination with nonsurgical or surgical treatments [43,44,45,47]. As there is currently no clear indication for the use of systemically-delivered antibiotics for treating peri-implantitis, their administration should be evaluated even more cautiously, given the ever-growing phenomenon of antimicrobial resistance [45].
In fact, dentists are responsible for 10% of all antibiotic prescriptions [58], and antimicrobial resistance is mainly caused by the overuse and misuse of antibiotics [59], leading to an increased risk of complications and mortality and a more complex resolution of infectious diseases [20,22,60]. In this perspective, alternative techniques to avoid or reduce antimicrobial administration have been introduced as an adjunct to nonsurgical and surgical treatment of peri-implantitis, such as photodynamic therapy (aPDT). Specifically, Zhao et al. [47] reported promising results after aPDT comparable to those of antibiotic administration, although stating that further studies are needed to evaluate the efficacy of aPDT as an alternative to antibiotics [47].
Due to the variability of antibiotic types, routes of administration, dosages, and combinations with other nonsurgical and surgical interventions, the most effective antibiotic type, route of administration, regimen, and protocol (antibiotics alone or in combination with other approaches) for the management of peri-implantitis could not be determined from the currently retrieved data for either locally- or systemically-delivered antibiotics.
Moreover, given the conflicting results and following antibiotic stewardship to combat antimicrobial resistance [20,22,60,61,62], it may be concluded that the prescription of systemically-delivered antibiotics should be discouraged, and the use of locally-delivered ones should be cautiously evaluated based on a case- and site-specific benefit-risk assessment.
Further research on the type of antibiotic, route of administration, therapeutic regimen, and protocols (antibiotics alone or in combination with other approaches) for managing peri-implantitis is still needed to demonstrate the most effective antibiotic therapy and to limit the risks resulting from inappropriate antibiotic therapy.
Given the heterogeneous data on antibiotic type, routes of administrations, therapeutic regimens, duration, and combination with other interventions, the incomplete data on peri-implant outcomes, and those missing on dental implants’ number, location, characteristics, and survival, no meta-analysis could be conducted. Findings from the presently included systematic reviews revealed contrasting and inconclusive results concerning the effectiveness of systemically- and locally-delivered antibiotics in peri-implantitis management. As a result, the ideal antibiotic type, route of administration, regimen, and protocols (antibiotics alone or in combination with other approaches) in peri-implantitis management could not be determined.
In addition, some studies, such as those by Renvert et al. and Cha et al. [63,64], were included in multiple systematic reviews, and all included reviews were classified as of low [41,43,44,45] or critically low quality [42,46,47]. Consequently, the need for future investigations with higher evidence levels, such as randomized controlled trials, with defined antibiotic therapeutic regimens and protocols, methodically recorded peri-implant outcomes, and at least > 3 years of follow-up [44] are needed. Moreover, the need for case- and site-specific indication of antibiotic administration and benefit-risk assessment should be provided.
However, the present umbrella review may be the first to collectively evaluate the effectiveness and the proposed regimens and protocols of local and/or systemic antibiotic administration alone and in combination with other nonsurgical or surgical peri-implantitis treatments.
5. Conclusions
Seven systematic reviews were included in the present umbrella review; three were classified as critically low quality and four as low quality using the AMSTAR 2 tool.
Locally-delivered antibiotics administered alone or as an adjunct to surgical or nonsurgical treatments for peri-implantitis showed favorable results, albeit with limited evidence. The administration of systemically-delivered antibiotics in combination with nonsurgical or surgical treatments remained questionable. Local plus systemic antibiotics have not been shown to have durable efficacy.
Because of the heterogeneity of reported antibiotic types, routes, regimens, and administration protocols, no definitive conclusions could be drawn regarding the most effective antibiotic use in managing peri-implantitis. However, given the phenomenon of antimicrobial resistance, the prescription of systemically-delivered antibiotics should be discouraged, and locally-delivered ones should be used with caution based on a case- and site-specific benefit-risk assessment.
Further research is needed on antibiotics’ type, route of administration, therapeutic regimen, and protocols (antibiotics alone or in combination with other approaches) for managing peri-implantitis, pointing out the case- and site-specific indications and the most effective antibiotic therapy, concurrently limiting the risks of inappropriate antibiotic use.
Author Contributions
Conceptualization, F.D.S. and F.D.A; Methodology, F.D.S. and F.D.; Validation, M.P.D.P. and F.G.; Formal Analysis, M.A., G.B., and F.G.; Data Curation, F.D.S., F.D., and M.P.D.P.; Writing—Original Draft Preparation, F.D.S., F.D., and M.P.D.P.; Writing—Review and Editing, G.B., F.G., and M.A.; Supervision. M.A. All authors approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results can be found in the PROSPERO Registry and the Cochrane Library, BioMed Central, Scopus, and MEDLINE/PubMed databases.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of implant therapy analyzed in a Swedish population: Prevalence of peri-implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Lee, C.T.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; lo Giudice, R.; Amato, M.; di Palo, M.P.; D’Ambrosio, F.; Amato, A.; Martina, S. Inflammatory, Reactive, and Hypersensitivity Lesions Potentially Due to Metal Nanoparticles from Dental Implants and Supported Restorations: An Umbrella Review. Appl. Sci. 2022, 12, 11208. [Google Scholar] [CrossRef]
- Di Spirito, F.; Schiavo, L.; Pilone, V.; Lanza, A.; Sbordone, L.; D’Ambrosio, F. Periodontal and Periimplant Diseases and Systemically Administered Statins: A Systematic Review. Dent J 2021, 9, 100. [Google Scholar] [CrossRef]
- Schwarz, F.; Giannobile, W.V.; Jung, R.E. Groups of the 2nd Osteology Foundation Consensus Meeting. Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: Osteology Foundation Consensus Report Part 2-Effects of hard tissue augmentation procedures on the maintenance of peri-implant tissues. Clin. Oral Implant. Res. 2018, 29, 11–13. [Google Scholar]
- Di Spirito, F.; Pelella, S.; Argentino, S.; Sisalli, L.; Sbordone, L. Oral Manifestations and the Role of the Oral Healthcare Workers in COVID-19. Oral Dis. 2022, 28, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Di Spirito, F.; D’Ambrosio, F.; Boccia, G.; Moccia, G.; De Caro, F. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms 2022, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M., Jr.; Chambrone, L.; Listl, S.; Tu, Y.K. Network meta-analysis for evaluating interventions in implant dentistry: The case of peri-implantitis treatment. Clin. Implant. Dent. Relat. Res. 2013, 15, 576–588. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I.; Clafey, N. Surgical therapy for the control of peri-implantitis. Clin. Oral. Implant. Res. 2012, 23, 84–94. [Google Scholar] [CrossRef]
- Keeve, P.L.; Koo, K.T.; Ramanauskaite, A.; Romanos, G.; Schwarz, F.; Sculean, A.; Khoury, F. Surgical treatment of peri-implantitis with non-augmentative techniques. Implant. Dent. 2019, 28, 177–186. [Google Scholar] [CrossRef]
- Di Spirito, F. Oral-Systemic Health and Disorders: Latest Prospects on Oral Antisepsis. Appl. Sci. 2022, 12, 8185. [Google Scholar] [CrossRef]
- Eick, S.; Ramseier, C.A.; Rothenberger, K.; Brägger, U.; Buser, D.; Salvi, G.E. Microbiota at teeth and implants in partially edentulous patients. A 10-year retrospective study. Clin. Oral Implant. Res. 2016, 27, 218–225. [Google Scholar] [CrossRef]
- Di Spirito, F.; Toti, P.; Brevi, B.; Martuscelli, R.; Sbordone, L.; Sbordone, C. Computed Tomography Evaluation of Jaw Atrophies Before and After Surgical Bone Augmentation. Int. J. Clin. Dent. 2019, 12, 259–270. [Google Scholar]
- Valderrama, P.; Blansett, J.A.; Gonzalez, M.G.; Cantu, M.G.; Wilson, T.G. Detoxification of Implant Surfaces Affected by PeriImplant Disease: An Overview of Nonsurgical Methods. Open Dent. J. 2014, 8, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.; Ko, H.H.; Froum, S.; Tarnow, D. The use of CO(2) laser in the treatment of peri-implantitis. Photomed. Laser Surg. 2009, 27, 381–386. [Google Scholar] [CrossRef]
- Natto, Z.S.; Aladmawy, M.; Levi, P.A., Jr.; Wang, H.L. Comparison of the efficacy of different types of lasers for the treatment of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2015, 30, 338–345. [Google Scholar] [CrossRef]
- Mombelli, A.; Lang, N.P. Antimicrobial treatment of peri-implant infections. Clin. Oral Implant. Res. 1992, 3, 116–162. [Google Scholar] [CrossRef]
- Van Winkelhoff, A.J. Antibiotics in the treatment of peri-implantitis. Eur. J. Oral Implant. 2012, 5, 43–50. [Google Scholar]
- D’Ambrosio, F.; Di Spirito, F.; Amato, A.; Caggiano, M.; Lo Giudice, R.; Martina, S. Attitudes towards Antibiotic Prescription and Antimicrobial Resistance Awareness among Italian Dentists: What Are the Milestones? Healthcare 2022, 10, 1585. [Google Scholar] [CrossRef]
- Sukumar, S.; Martin, F.E.; Hughes, T.E.; Adler, C.J. Think before you prescribe: How dentistry contributes to antibiotic resistance. Aust. Dent. J. 2019, 65, 21–29. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Di Spirito, F.; De Caro, F.; Lanza, A.; Passarella, D.; Sbordone, L. Adherence to Antibiotic Prescription of Dental Patients: The Other Side of the Antimicrobial Resistance. Healthcare 2022, 10, 1636. [Google Scholar] [CrossRef] [PubMed]
- Busa, A.; Parrini, S.; Chisci, G.; Pozzi, T.; Burgassi, S.; Capuano, A. Local versus systemic antibiotics effectiveness: A comparative study of postoperative oral disability in lower third molar surgery. J. Craniofacial Surg. 2014, 25, 708–709. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocolss (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, 1000100. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACPJ Club 1995, 123, 12–13. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Chichester, UK, 2008. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, 4008. [Google Scholar] [CrossRef]
- Alenezi, A.; Chrcanovic, B. Effects of the local administration of antibiotics on bone formation on implant surface in animal models: A systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2020, 56, 177–183. [Google Scholar] [CrossRef]
- Rodríguez Sánchez, F.; Rodríguez Andrés, C.; Arteagoitia, I. Which antibiotic regimen prevents implant failure or infection after dental implant surgery? A systematic review and meta-analysis. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2018, 46, 722–736. [Google Scholar] [CrossRef]
- Grusovin, M.G.; Coulthard, P.; Worthington, H.V.; Esposito, M. Maintaining and recovering soft tissue health around dental implants: A Cochrane systematic review of randomised controlled clinical trials. Eur. J. Oral Implantol. 2008, 1, 11–22. [Google Scholar]
- Caiazzo, A.; Canullo, L.; Consensus Meeting Group; Pesce, P. Consensus Report by the Italian Academy of Osseointegration on the Use of Antibiotics and Antiseptic Agents in Implant Surgery. Int. J. Oral Maxillofac. Implant. 2021, 36, 103–105. [Google Scholar] [CrossRef]
- Khan, A.; Goyal, A.; Currell, S.D.; Sharma, D. Management of Peri-Implantitis Lesions without the Use of Systemic Antibiotics: A Systematic Review. Dent. J. 2020, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Peralvo, A.O.; Peña-Cardelles, J.F.; Kewalramani, N.; Mateos-Moreno, M.V.; Jiménez-Guerra, Á.; Velasco-Ortega, E.; Uribarri, A.; Moreno-Muñoz, J.; Ortiz-García, I.; Núñez-Márquez, E.; et al. Preventive Antibiotic Therapy in the Placement of Immediate Implants: A Systematic Review. Antibiotics 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Bizelli, V.; Bassi, A.P.; Souza, E. Analysis of the Local and Systemic Adjuvant Effect of Antibiotics in the Treatment Of Peri-Implantitis Compared to Mechanical Treatment such as Monotherapy or Associated with the Adjunctive Use of Chlorhexidine: Systematic Review and Meta-Analysis. PROSPERO 2020 CRD42020209344. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020209344 (accessed on 15 November 2022).
- Jin Lim, S.; McGowen, K.; McGowen, T.; Vithanage, A.; Ivanovski, S. Is There a Benefit to Adjunctive Antibiotics in Nonsurgical Therapy for Peri-Implantitis? A Systematic Review and Meta-Analysis of Randomised-Controlled Trials. PROSPERO 2018 CRD42018102067. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018102067 (accessed on 15 November 2022).
- Esposito, M.; Coulthard, P.; Oliver, R.; Thomsen, P.; Worthington, H.V. Antibiotics to prevent complications following dental implant treatment. Cochrane Database Syst. Rev. 2003, CD004152. [Google Scholar] [CrossRef]
- Ata-Ali, J.; Ata-Ali, F.; Ata-Ali, F. Do antibiotics decrease implant failure and postoperative infections? A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2014, 43, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Osorio, M.T.; Vallecillo-Rivas, M.; Toledano-Osorio, M.; Rodríguez-Archilla, A.; Toledano, R.; Osorio, R. Efficacy of local antibiotic therapy in the treatment of peri-implantitis: A systematic review and meta-analysis. J. Dent. 2021, 113, 103790. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, P.C.; Netti, A.; Lopez, M.A.; Giaquinto, E.F.; De Rosa, G.; Aureli, G.; Bodnarenko, A.; Papi, P.; Starzy ’nska, A.; Pompa, G.; et al. Local/Topical Antibiotics for Peri-Implantitis Treatment: A Systematic Review. Antibiotics 2021, 10, 1298. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.Y.; Stathopoulou, P.G.; Graham, L.K.; Korostoff, J.; Chen, Y.W. Efficacy of Antibiotics Used as an Adjunct in the Treatment of Peri-implant Mucositis and Peri-implantitis: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2022, 37, 235–249. [Google Scholar] [CrossRef]
- Øen, M.; Leknes, K.N.; Lund, B.; Bunæs, D.F. The efficacy of systemic antibiotics as an adjunct to surgical treatment of peri-implantitis: A systematic review. BMC Oral Health 2021, 21, 666. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Vallecillo, C.; Toledano, R.; Aguilera, F.S.; Osorio, M.T.; Muñoz-Soto, E.; García-Godoy, F.; Vallecillo-Rivas, M. A Systematic Review and Meta-Analysis of Systemic Antibiotic Therapy in the Treatment of Peri-Implantitis. Int. J. Environ. Res. Public Health 2022, 19, 6502. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Tzanetea, E.; Piattelli, A.; Worthington, H.V. Interventions for replacing missing teeth: Treatment of perimplantitis. Cochrane Database Syst. Rev. 2010, CD004970. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, R.; Qian, Y.; Shi, J.; Si, M. Antimicrobial photodynamic therapy versus antibiotics as an adjunct in the treatment of periodontitis and peri-implantitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2021, 34, 102231. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.; Oh, J.; Inglehart, M.; Aronovich, S. Etiology, Diagnosis and Treatment of Peri-Implantitis—A National Survey of AAOMS Members. J. Oral Maxillofac. Surg. 2017, 75, 355–356. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, 286–291. [Google Scholar] [CrossRef]
- Mishler, O.P.; Shiau, H.J. Management of peri-implant disease: A current appraisal. J. Evid. Based Dent. Pract. 2014, 14, 53–59. [Google Scholar] [CrossRef]
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implants Res. 2012, 23 (Suppl. 6), 67–76. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-implantitis Update: Risk Indicators, Diagnosis, and Treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef]
- Chala, M.; Anagnostaki, E.; Mylona, V.; Chalas, A.; Parker, S.; Lynch, E. Adjunctive Use of Lasers in Peri-Implant Mucositis and Peri-Implantitis Treatment: A Systematic Review. Dent. J. 2020, 8, 68. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Caggiano, M.; Schiavo, L.; Savarese, G.; Carpinelli, L.; Amato, A.; Iandolo, A. Chronic Stress and Depression in Periodontitis and Peri-Implantitis: A Narrative Review on Neurobiological, Neurobehavioral and Immune–Microbiome Interplays and Clinical Management Implications. Dent. J. 2022, 10, 49. [Google Scholar] [CrossRef]
- Figuero, E.; Graziani, F.; Sanz, I.; Herrera, D.; Sanz, M. Management of peri-implant mucositis and peri-implantitis. Periodontol. 2000 2014, 66, 255–273. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Mombelli, A. The therapy of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 325–345. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Gonçalves, C.; Souto, R.; Feres-Filho, E.J.; Uzeda, M.; Colombo, A.P. Antibiotic resistance profile of the subgingival microbiota following systemic or local tetracycline therapy. J. Clin. Periodontol. 2004, 31, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Williams, D.; Pulcini, C.; Sanderson, S.; Calfon, P.; Verma, M. Tackling Antibiotic Resistance: Why Dentistry Matters. Int. Dent. J. 2021, 71, 450–453. [Google Scholar] [CrossRef]
- Landecker, H. Antibiotic resistance and the biology of history. Body Soc. 2016, 22, 19–52. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance (who.int). Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 31 July 2022).
- Di Spirito, F.; Argentino, S.; Martuscelli, R.; Sbordone, L. Mronj Incidence After Multiple Teeth Extractions in Patients Taking Oral Bis-Phosphonates Without “Drug Holiday”: A Retrospective Chart Review. Oral Implantol. 2019, 12, 105–110. [Google Scholar]
- Di Spirito, F.; Scelza, G.; Fornara, R.; Giordano, F.; Rosa, D.; Amato, A. Post-operative endodontic pain management: An overview of systematic reviews on post-operatively administered oral medications and integrated evidence-based clinical recommendations. Healthcare 2022, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I. Treatment of pathologic peri-implant pockets. Periodontol. 2000 2018, 76, 18. [Google Scholar] [CrossRef]
- Cha, J.K.; Lee, J.S.; Kim, C.S. Surgical therapy of peri-implantitis with local minocycline: A 6-month randomized controlled clinical trial. J. Dent. Res. 2019, 98, 288–295. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).