Abstract
Clinical practice guidelines (CPGs) and computerized clinical decision support programs are effective antimicrobial stewardship strategies. The DigitalAMS™, a mobile-based application for choosing empirical antimicrobial therapy under the hospital’s CPGs, was implemented at Siriraj Hospital and evaluated. From January to June 2018, a cross-sectional study was conducted among 401 hospitalized adults who received ≥1 dose of antimicrobials and had ≥1 documented site-specific infection. The antimicrobial regimen prescribed by the ward physician (WARD regimen), recommended by the DigitalAMS™ (APP regimen), and recommended by two independent infectious disease (ID) physicians before (Emp-ID regimen) and after (Def-ID regimen) the final microbiological results became available were compared in a pairwise fashion. The percent agreement of antimicrobial prescribing between the APP and Emp-ID regimens was 85.7% in the bacteremia group, 59.1% in the pneumonia group, 78.6% in the UTI group, and 85.2% in the SSTI group. The percent agreement between the APP and Emp-ID regimens was significantly higher than that between the WARD and Emp-ID regimens in three site-specific infection groups: the bacteremia group (85.7% vs. 47.9%, p < 0.001), the UTI group (78.6% vs. 37.8%, p < 0.001), and the SSTI group (85.2% vs. 40.2%, p < 0.001). Furthermore, the percent agreement between the APP and Def-ID regimens was similar to that between the Emp-ID and Def-ID regimens in all sites of infection. In conclusions, the implementation of DigitalAMS™ seems useful but needs some revisions. The dissemination of this ready-to-use application with customized clinical practice guidelines to other hospital settings may be beneficial.
1. Introduction
The emergence of antimicrobial resistance is a growing problem worldwide [,]. Antimicrobial resistance results in higher mortality and morbidity, a longer hospital length of stay, and increased healthcare costs []. Many previous studies have revealed an association between the emergence of antimicrobial resistance and antimicrobial exposure [,].
Appropriate antimicrobial therapy is considered a key component in preventing and containing the emergence of antimicrobial resistance []. Many studies have confirmed that various antimicrobial stewardship (AMS) strategies, such as implementing clinical practice guidelines (CPGs), using computerized clinical decision support programs, providing individual feedback, and performing bedside infectious disease (ID) consultations are effective in reducing inappropriate antimicrobial therapy [,,,,,,]. Although developing CPGs is not a sophisticated task, implementing and maintaining CPG compliance can be challenging [,,].
The 2016 Infectious Diseases Society of America and Society for Healthcare Epidemiology of America guidelines for implementing an AMS program recommend the development of facility-specific CPGs accompanied by a dissemination and implementation strategy []. The guidelines also suggest incorporating computerized clinical decision support at the time of prescribing antimicrobials into the AMS program [].
In 2018, the Division of Infectious Diseases and Tropical Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, developed and implemented the DigitalAMS™ application. DigitalAMS™ is a ready-to-use offline mobile-based application for Siriraj Hospital healthcare personnel. This application is used to choose an empirical antimicrobial regimen for four common site-specific infections based on the existing CPGs for relevant healthcare personnel at Siriraj Hospital. In addition to the hospital CPG, some AMS interventions such as education programs, customized antibiograms, drug use evaluation programs, and antibiotic authorization were successfully implemented at our hospital before the DigitalAMS™ project.
We conducted the present cross-sectional study to evaluate the use of the DigitalAMS™ application. Our primary objective was to determine the results of the implementation of DigitalAMS™ in choosing empirical antimicrobial therapy for four common site-specific infections (bacteremia, pneumonia, urinary tract infection (UTI), and skin and soft tissue infection (SSTI)) by using the ID physician’s decision as the gold standard. Our secondary objective was to determine the limitations of using the application and accordingly improve its implementation.
2. Methods
2.1. Study Design and Population
We conducted a cross-sectional study at Siriraj Hospital from January to June 2018. Siriraj Hospital is a 2200-bed university hospital located in Bangkok, Thailand. The study was approved by the Siriraj Institutional Review Board, and the requirement for informed consent was waived (certification of approval number: Si 700/2017).
Hospitalized patients aged ≥18 years who met the following inclusion criteria were eligible for participation: (1) They had received at least one dose of an antimicrobial agent during hospitalization, and (2) they had at least one of the following site-specific infections: bacteremia, pneumonia, UTI, or SSTI. We used Centers for Disease Control and Prevention/National Healthcare Safety Network surveillance definitions for bacteremia, pneumonia, UTI, and SSTI []. If an eligible patient had more than one site of infection within the same period, each site of infection was assessed separately. In patients with multiple sites of infection, it was sometimes difficult to distinguish which antibiotic was prescribed for treatment at which site. Therefore, all antibiotics prescribed during such infection episodes were considered for treatment of the index infection. If an eligible patient had more than one episode of infection during hospitalization, only the first episode of infection was included.
2.2. Development of Hospital CPGs and DigitalAMS™ Application
DigitalAMS™ is an offline mobile application specifically designed to help relevant healthcare personnel choose appropriate empirical antimicrobial regimens. A user needs to enter only three important patient-related factors: (1) the patient’s risk stratification (admission to the general ward or intensive care unit), (2) the risk of acquisition of multidrug-resistant pathogens, and (3) the suspected site of infection. After the user has entered these three factors, the application will suggest the first-line regimen and an alternative regimen of empiric antimicrobial therapy. To access the application, a first-time user must register with a designated username and an individual password before downloading the application via the internet. Once the application has been successfully downloaded and installed on the user’s mobile phone, the user can access the application anywhere without an internet connection. The application is a health initiative owned, operated, and provided by MSD Pharmaceuticals Private Limited (MSD), although MSD does not have liability relating to the output of the application. The application is provided to Siriraj Hospital at no cost. Figure 1 shows the interface of the DigitalAMS™ application.

Figure 1.
The DigitalAMSTM application interfaces.
The Siriraj CPGs of the empirical antimicrobial regimen for each targeted site of infection were customized based on the pharmacokinetic/pharmacodynamics of the antibiotics, the hospital antibiogram, and previous studies conducted at Siriraj Hospital [,,]. The antimicrobial regimens were finally approved by the Division of Infectious Diseases and Tropical Medicine, Department of Medicine, Faculty of Medicine Siriraj Hospital. Supplementary Table S1 shows the details of the antimicrobial regimens according to the patients’ risk stratification, risk of acquisition of multidrug-resistant pathogens, suspected site of infection, and all related definitions. These hospital CPGs were distributed in a published version to all clinical departments before the implementation of the application. Additionally, the online version of the CPGs was available to all hospital healthcare personnel.
2.3. Data Collection
Potential participants in this study were identified through the hospital’s electronic database. A medical record review was performed on all hospitalizations with at least one International Classification of Disease (ICD) code of the index infection and with at least one prescription of an antimicrobial agent to determine the patient’s eligibility. Of all the potential participants, only those who met the study inclusion criteria were randomly enrolled by using a computer program.
An in-depth medical record review was subsequently performed to obtain all the necessary information, including demographics, baseline clinical characteristics, details of the index infection, laboratory and microbiological results, antimicrobial therapy, and treatment outcomes. Demographic data included age, sex, and weight (if available). Clinical data included a history of previous hospitalization, previous antimicrobial therapy, and underlying diseases. Details of the index infection were recorded in a narrative format and were accompanied by all necessary laboratory and microbiological results. Data on antimicrobial therapy, including type, dose, and duration, were also recorded.
Antimicrobial regimens were retrieved from four sources: (1) the antimicrobial regimen that the eligible patient received upon hospitalization (WARD regimen), (2) the antimicrobial regimen recommended by using the DigitalAMS™ application as empirical antimicrobial therapy (APP regimen), (3) the antimicrobial regimen recommended by the ID physician as empirical therapy before the microbiological results became available (Emp-ID regimen), and (4) the antimicrobial regimen recommended by the ID physician as the definitive therapy after the microbiological results became available (Def-ID regimen).
The WARD regimen was directly obtained by performing a medical record review, whereas the APP regimen was obtained by entering three important parameters into the DigitalAMS™ application. For the Emp-ID and the Def-ID regimens, two independent ID physicians reviewed the patient’s case record form as well as all the initial laboratory results with and without the final microbiological results. The ID physicians were masked to the WARD regimen and the patient’s treatment outcome. If disagreement occurred between the recommendations of two ID physicians, the recommendation of a third ID physician was considered final. The Def-ID regimen was available only among patients with identified causative pathogens.
2.4. Statistical Analysis
The agreement between each pair of recommendations was estimated to be approximately 50% with an allowable error of 10%, and the expected sample size was 97 patients per site-specific infection. After adjusting for a 10% loss to follow-up, the final sample size was 107 patients per site-specific infection or 438 patients for 4 site-specific infections.
An agreement of antimicrobial regimens was defined as the matching of two antimicrobial regimens (same drugs or different drugs with a similar spectrum). A too-broad or too-narrow antimicrobial regimen was considered mismatched. Examples of matching regimens include amoxicillin/clavulanic acid and ampicillin/sulbactam, ceftriaxone and cefotaxime, and meropenem and imipenem/cilastatin. Examples of mismatching regimens include ertapenem and meropenem, meropenem and colistin, and vancomycin and linezolid.
Categorical variables are reported as frequency and percentage, and continuous variables are shown as mean ± standard deviation. We calculated the percent agreement by performing a pairwise comparison of each site-specific infection: (1) the WARD regimen and APP regimen, (2) the APP regimen and Emp-ID regimen, (3) the APP regimen and Def-ID regimen, and (4) the WARD regimen and Emp-ID regimen. The percent agreement of the APP regimen and Emp-ID regimen was considered to be the concordance of the application, which was the primary outcome. The percent agreement was reported with its 95% confidence interval. All analyses were performed with Stata/IC version 14.0 (StataCorp, College Station, TX, USA).
3. Results
During the 6-month study period, there were 7995 hospitalizations with at least 1 antimicrobial prescription and at least 1 ICD-10 code of target site-specific infections. Of these 7995 hospitalizations, we performed a medical record review and randomly enrolled a total of 401 episodes of the index infection (314 unique patients): 98 patients with bacteremia, 98 with pneumonia, 103 with UTI, and 102 with SSTI. The study flowchart is shown in Figure 2.
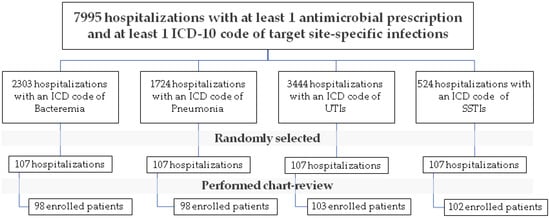
Figure 2.
Study flowchart.
Table 1 shows the patients’ baseline characteristics, clinical characteristics, and treatment outcomes stratified by the site of infection. The mean age of patients with bacteremia, pneumonia, UTI, and SSTI was 69.05 ± 15.20, 72.35 ± 16.82, 71.81 ± 14.42, and 62.59 ± 19.90 years, respectively (p = 0.006). Underlying diseases were comparable between the four groups. However, the proportion of cerebrovascular disease was slightly higher in the pneumonia group (37.7%) and the UTI group (37.8%) when compared with the bacteremia group (27.5%) and the SSTI group (16.6%, p = 0.002). Furthermore, the SSTI group was more likely to have community-acquired infections (50.0%), and the UTI group was more likely to have hospital-acquired infections (61.1%, p < 0.001) than the other groups. The proportion of ventilator dependency was highest in the pneumonia group (36.7%, p < 0.001).

Table 1.
Baseline characteristics, clinical features, and treatment outcomes, stratified by the site-specific infection.
The leading causative pathogen was Escherichia coli in the bacteremia group (53.0%), Pseudomonas aeruginosa in the pneumonia group (12.2%), E. coli in the UTI group (40.7%), and methicillin-susceptible Staphylococcus aureus in the SSTI group (10.7%). Piperacillin/tazobactam was the most commonly prescribed antimicrobial agent in the bacteremia group (46.9%), the pneumonia group (63.2%), and the UTI group (53.4%), whereas amoxicillin/clavulanic acid was the most commonly prescribed antimicrobial agent in the SSTI group (38.2%).
With respect to treatment outcomes, the 28-day mortality rate was highest in the pneumonia group (30.6%), followed by the bacteremia group (22.4%), the UTI group (12.6%), and the SSTI group (6.8%) (p < 0.001). The 28-day infection-related mortality rate, the overall hospital mortality rate, and the length of stay were also highest in the pneumonia group.
Table 2 shows the percent agreement of each comparison group stratified by the infection site. The percent agreement between the APP regimen and the Emp-ID regimen was 85.7% in the bacteremia group, 59.1% in the pneumonia group, 78.6% in the UTI group, and 85.2% in the SSTI group. The percent agreement between the WARD regimen and the Emp-ID regimen was only 47.9% in the bacteremia group, 55.1% in the pneumonia group, 37.8% in the UTI group, and 40.2% in the SSTI group. The percent agreement between the APP regimen and the Emp-ID regimen was significantly higher than that between the WARD regimen and the Emp-ID regimen in three site-specific infection groups: the bacteremia group (85.7% vs. 47.9%, p < 0.001), the UTI group (78.6% vs. 37.8%, p < 0.001), and the SSTI group (85.2% vs. 40.2%, p < 0.001). The percent agreement between the APP regimen and the Def-ID regimen was 54.1% in the bacteremia group, 50.0% in the pneumonia group, 46.2% in the UTI group, and 64.6% in the SSTI group. The percent agreement between the Emp-ID regimen and the Def-ID regimen was 55.1% in the bacteremia group, 68.2% in the pneumonia group, 42.9% in the UTI group, and 68.8% in the SSTI group. The percent agreement between the APP regimen and the Def-ID regimen and that between the Emp-ID regimen and the Def-ID regimen was comparable at all sites of infection (all p > 0.05).

Table 2.
Percent agreement of each comparison group, stratified by the infection site.
4. Discussion
Our study results revealed that DigitalAMS™ provided an acceptable percent agreement in nearly all the sites of the index infection except for pneumonia when using the ID physician’s recommendation as the gold standard. Although the percent agreement between the APP regimen and the Emp-ID regimen did not reach 100%, the percent agreement was significantly higher than that of the WARD regimen and the Emp-ID regimen. Additionally, the percent agreement between the APP regimen and the Def-ID regimen was similar to that between the Emp-ID regimen and the Def-ID regimen at all sites of infections. This suggests that the APP regimen may provide benefits comparable to those obtained by an ID specialist’s recommendation.
We also investigated the underlying causes of the lower percent agreement between the APP regimen and the Emp-ID regimen within the pneumonia group (59.1%). The disagreement commonly occurred among patients with a history of aspiration and those who may require an anti-anaerobic agent. Given that the 2018 version of the hospital’s CPG did not include a regimen for aspiration pneumonia, more than one-third of the APP regimen did not match the Emp-ID regimen. These findings resulted in a subsequent revision of the hospital’s CPGs.
The results of our study are similar to those of previous studies [,]. The DigitalAMS™ application helps to improve antimicrobial use and thus optimize patient care. Priorities can be identified based on the patient location (general ward vs. intensive care unit) and the antimicrobial spectrum, antimicrobial cost, duration of therapy, and the number of different antimicrobials prescribed to a single patient. Such mobile applications must be designed and tested by members of the AMS program, and new applications and updates are needed.
Our study had two main strengths. First, to our knowledge, this is the first study in Thailand that was specifically designed to evaluate the implementation of the DigitalAMS™ application in choosing empirical antimicrobial therapy for four common site-specific infections. Second, in addition to using the ID physician’s recommendation as the gold standard, we also obtained a recommendation for definitive antimicrobial therapy from DigitalAMS™ for patients with identified causative pathogens.
Our study also had three main limitations. First, the retrospective nature of the study may have resulted in information bias. The antimicrobial regimen recommended by the DigitalAMS™ and the antimicrobial regimen recommended by the ID physician relied on information from the medical record review. Therefore, some information may have been missing, negatively influencing the ID physician’s decision. Second, the study did not have enough power to determine the association between the percent agreement of the WARD and APP regimens and the treatment outcomes because this study was designed to determine the agreement of the APP and the Emp-ID regimens as the primary outcome, and the sample size was computed to detect the primary outcome. Third, this study was conducted at a university hospital in Thailand. Therefore, its results may not be applicable to other hospital settings.
In conclusion, the implementation of DigitalAMS™ for choosing an appropriate antimicrobial regimen for empirical treatment of four common site-specific infections in hospitalized patients at Siriraj Hospital seems to be useful. However, both this mobile application and the hospital CPGs need some revisions to improve the concordance between them. The dissemination of this ready-to-use application with the customized CPGs to other hospital settings may be beneficial. A future multi-center study is necessary to fully explore the benefits of the application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12010113/s1, Table S1: Suggested Empiric Antibiotics for Suspected Common Infections in Hospitalized Adult Patients at Siriraj Hospital.
Author Contributions
P.R. and V.T. conceptualized the study design. K.C., W.W. and P.K. performed the data collection, cleaning, and analysis. All authors participated in drafting the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research development grant (R016231001) from the Faculty of Medicine Siriraj Hospital, Mahidol University (Thailand), and the Health Systems Research Institute (Thailand). The funding body was not involved in the design or execution of the study, the analysis of the study results, or the preparation of the manuscript.
Institutional Review Board Statement
The Institutional Review Board of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (certificate of approval number: Si700/2017) approved the study protocol.
Informed Consent Statement
The requirement for informed consent was waived because the study was retrospectively performed.
Data Availability Statement
The study dataset is available from the corresponding author upon reasonable request.
Acknowledgments
The authors gratefully acknowledge Yadawadee Wongthanasuporn for the study coordination and data management. The authors also thank Angela Morben, DVM, ELS, from Edanz for editing a draft of this manuscript.
Conflicts of Interest
All authors declare no personal or professional conflicts of interest relating to any aspect of this study.
References
- World Health Organization (WHO). Draft Global Action Plan on Antimicrobial Resistance; World Health Organization (WHO): Geneva, Switzerland, 2015. [Google Scholar]
- Thamlikitkul, V.; Rattanaumpawan, P.; Boonyasiri, A.; Pumsuwan, V.; Judaeng, T.; Tiengrim, S.; Paveenkittiporn, W.; Rojanasthien, S.; Jaroenpoj, S.; Issaracharnvanich, S. Thailand Antimicrobial Resistance Containment and Prevention Program. J. Glob. Antimicrob. Resist. 2015, 3, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Pumart, P.P.T.; Thamlikitkul, V.; Riewpaiboon, A.; Prakongsai, P.; Limwattananon, S. Health and economic impacts of antimicrobial resistant infections in Thailand: A preliminary study. J. Health Syst. Res. 2012, 6, 352–360. [Google Scholar]
- Bhavnani, S.M.; Callen, W.A.; Forrest, A.; Gilliland, K.K.; Collins, D.A.; Paladino, J.A.; Schentag, J.J. Effect of fluoroquinolone expenditures on susceptibility of Pseudomonas aeruginosa to ciprofloxacin in U.S. hospitals. Am. J. Health Pharm. 2003, 60, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Soontaros, S.; Leelakanok, N. Association between carbapenem-resistant Enterobacteriaceae and death: A systematic review and meta-analysis. Am. J. Infect. Control 2019, 47, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Brown, E.; Fenelon, L.; Finch, R.; Gould, I.; Holmes, A.; Ramsay, C.; Taylor, E.; Wiffen, P.; Wilcox, M. Systematic review of antimicrobial drug prescribing in hospitals. Emerg. Infect. Dis. 2006, 12, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Rattanaumpawan, P.; Sutha, P.; Thamlikitkul, V. Effectiveness of drug use evaluation and antibiotic authorization on patients’ clinical outcomes, antibiotic consumption, and antibiotic expenditures. Am. J. Infect. Control 2010, 38, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Schuts, E.C.; Hulscher, M.E.J.L.; Mouton, J.W.; Verduin, C.M.; Stuart, J.W.T.C.; Overdiek, H.W.P.M.; van der Linden, P.D.; Natsch, S.; Hertogh, C.M.P.M.; Wolfs, T.F.W.; et al. Current evidence on hospital antimicrobial stewardship objectives: A systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Rattanaumpawan, P.; Boonyasiri, A.; Vong, S.; Thamlikitkul, V. Systematic review of electronic surveillance of infectious diseases with emphasis on antimicrobial resistance surveillance in resource-limited settings. Am. J. Infect. Control 2018, 46, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rattanaumpawan, P.; Chuenchom, N.; Thamlikitkul, V.; Siriraj Antimicrobial Stewardship Program. Individual feedback to reduce inappropriate antimicrobial prescriptions for treating acute upper respiratory infections in an outpatient setting of a Thai university hospital. J. Glob. Antimicrob. Resist. 2018, 12, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Wangchinda, W.; Srisompong, J.; Chayangsu, S.; Ruangkriengsin, D.; Thamlikitkul, V.; Koomanachai, P.; Sirijatuphat, R.; Rattanaumpawan, P. Impact of Antibiotic Authorisation at Three Provincial Hospitals in Thailand: Results from a Quasi-Experimental Study. Antibiotics 2022, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Koomanachai, P.; Srisompong, J.; Chayangsu, S.; Ruangkriengsin, D.; Thamlikitkul, V.; Wangchinda, W.; Sirijatuphat, R.; Rattanaumpawan, P. Implementation of Clinical Practice Guidelines for Empirical Antibiotic Therapy of Bacteremia, Urinary Tract Infection, and Pneumonia: A Multi-Center Quasi-Experimental Study. Antibiotics 2022, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Cabana, M.D.; Rand, C.S.; Powe, N.R.; Wu, A.W.; Wilson, M.H.; Abboud, P.-A.C.; Rubin, H.R. Why Don’t Physicians Follow Clinical Practice Guidelines? A Framework for Improvement. Pediatr. Res. 1999, 45, 121A. [Google Scholar] [CrossRef]
- Dryden, M.S.; Cooke, J.; Davey, P. Antibiotic stewardship—More education and regulation not more availability? J. Antimicrob. Chemother. 2009, 64, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef] [PubMed]
- National Healthcare Safety Network 2022, Patient Safety Component Manual. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf (accessed on 1 February 2022).
- Thamlikitkul, V.; Danchaivijitr, S.; Kongpattanakul, S.; Ckokloikaew, S. Impact of an educational program on antibiotic use in a tertiary care hospital in a developing country. J. Clin. Epidemiol. 1998, 51, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Sirijatuphat, R.; Sripanidkulchai, K.; Boonyasiri, A.; Rattanaumpawan, P.; Supapueng, O.; Kiratisin, P.; Thamlikitkul, V. Implementation of global antimicrobial resistance surveillance system (GLASS) in patients with bacteremia. PLoS ONE 2018, 13, e0190132. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.; Stenehjem, E.; Buckel, W.; Thorell, E.; Howe, S.; Wu, X.; Jones, P.; Lloyd, J.; Evans, R. Use of Computer Decision Support in an Antimicrobial Stewardship Program (ASP). Appl. Clin. Informatics 2015, 6, 120–135. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).