Abstract
Disinfectant resistance is evolving into a serious problem due to the long-term and extensive use of disinfectants, which brings great challenges to hospital infection control. As a notorious multidrug-resistant bacterium, carbapenem-resistant Klebsiella pneumoniae (CRKP) is one of the most common and difficult pathogens of nosocomial infection. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) tests of seven kinds of disinfectants (0.1% benzalkonium bromide, 4% aqueous chlorhexidine, 75% alcohol, entoiodine II, 2% glutaraldehyde, 2000 mg/L chlorine-containing disinfectants, and 3% hydrogen peroxide) were detected by the broth dilution method. Three efflux pump genes (oqxA, oqxB, and qacE∆1-sul1) were detected by PCR. The mean MIC value of aqueous chlorhexidine from the intensive care unit (ICU) (0.0034%) was significantly higher than that from non-ICUs (0.0019%) (p < 0.05). The positive rates of three efflux pump genes oqxA, oqxB and qacE∆1-sul1 were 60.9% (39/64), 17.2% (11/64) and 71.9% (46/64) in the detected CRKP isolates, respectively. This study discovered that CRKP strains demonstrated extensive resistance to clinical disinfectants and suggest that it is necessary to perform corresponding increases in the concentration of aqueous chlorhexidine and chlorine-containing disinfectants on the basis of current standards in the healthcare industry.
1. Introduction
Since the late 1980s, with the wide use of carbapenem antibiotics in the clinical setting, carbapenem-resistant Enterobacteriaceae (CRE) has been discovered and reported all over the world [1,2]. Moreover, it has become increasingly prevalent in recent years and has been considered as an urgent threat to public health [3,4,5]. Carbapenem-resistant Klebsiella pneumoniae (CRKP) is the most popular bacterium within the CRE family, causing longer hospital stays [1], higher medical costs [6,7], and higher mortality [8,9]. These features will add to the global estimates of the burden of antimicrobial resistance [10], which inspired us on the urgency of global action against infections [11].
The European CDC suggested that environmental cleaning, equipment reprocessing, hand hygiene, and routine surveillance were core infection prevention and control measures to minimize the risk of spread of CRE, and enhanced cleaning should be performed for areas in close proximity to CRE carriers [12]. Unfortunately, many researchers have observed resistance of multi-drug resistant bacteria to disinfectants [13]; furthermore, bacteria can acquire antibiotic resistance with the induction of disinfectants according to recent research [14]. To investigate the resistance of CRKP strains to the disinfectants commonly used in clinics, we evaluated the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of seven disinfectants to CRKP isolated from clinics in order to guide the rational use of disinfectants in clinical practice [15].
The overexpression of efflux pumps plays an important role in non-specific resistance [16,17,18,19,20]. The efflux pumps can change the structure and physiological functions of the bacterial outer membrane, and protect themselves against biocides [15,21]. Some multi-drug efflux pump families are able to mediate the efflux of multiple disinfectants and antibiotics at the same time, leading to cross resistance [22,23]. To explore the correlation of efflux pump genes with the resistance of CRKP strains to disinfectants, we detected three efflux pump genes (oqxA, oqxB, and qacE∆1-sul1) [24,25,26]. OqxA and oqxB belong to the resistance-nodulation cell division (RND) family. OqxAB is encoded by a plasmid and can flux quinolones, tigecycline, and other antibacterial drugs, as well as quaternary ammonium compound (QAC) and biguanide disinfectants [24,25]. qacE∆1-sul1 belongs to the small multidrug resistance (SMR) family, and is a part of class I integrons, mediated by integrons. qacE∆1 can encode the efflux proteins to discharge QAC and biguanide disinfectants [27,28], and the sul1 gene often causes bacterial resistance to sulfonamides [26,29]. The proteins encoded by these three efflux pump genes can flux both antibiotics and disinfectants; moreover, the multidrug efflux pumps transmitted by plasmids and integrons can promote non-specific multidrug resistance via horizontal transfer [24,25,26]. This study provides molecular epidemiological evidence for strategies to control CRKP transmission and infection in hospitals.
2. Methods
2.1. Isolation and Identification of Bacterial Strains
Our experimental strains were collected from the Sun Yat-sen Memorial Hospital (SYS Memorial Hospital) from January 2015 to December 2019. The strains were isolated from clinical patients and identified to species by the VITEK-2 automatic microorganism identifying and drug sensitivity system (bioMérieux, Marcy l’ Etoile, France) according to the manufacturer’s instructions. Susceptibility testing results were interpreted under the criteria recommended by the Clinical and Laboratory Standards Institute (CLSI, 2020). A strain of Klebsiella pneumoniae (Kpn) which was resistant to either of the carbapenem antibiotics (imipenem/ertapenem/meropenem) was considered to be a CRKP strain [1,30]. Modified carbapenem inactivation method (mCIM) and EDTA-modified carbapenem inactivation method (eCIM) tests were performed to confirm phenotypic carbapenemase production [31]. The quality control (QC) strains were E. coli ATCC 25922 and Kpn ATCC 700603, which were preserved in our laboratory.
We collected and analyzed the clinical data of CRKP strains isolated from SYS Memorial Hospital from January 2015 to December 2019 in order to provide supporting data for the use of disinfectants and the basis of research on classification of disinfectant resistance.
2.2. Disinfectants and Neutralizers
In this study, seven disinfectants were used, which were low-level disinfectants: 0.1% benzalkonium bromide (SYS Memorial Hospital, Guangzhou, China) and 4% aqueous chlorhexidine (SYS Memorial Hospital, Guangzhou, China); intermediate-level disinfectants: 75% ethyl alcohol (SYS Memorial Hospital, Guangzhou, China) and entoiodine II (Li Kang Disinfection Technology Co., Ltd., Shanghai, China); and high-level disinfectants: 2.0% (w/v) glutaraldehyde (Li Kang Disinfection Technology Co., Ltd., Shanghai, China), Hagrid Suli type II chlorine containing disinfectant (An Duo Fu Disinfection Technology Co., Ltd., Shenzhen, China), and 3% hydrogen peroxide (Nan Guo Pharmaceutical Co., Ltd., Guangzhou, China). The selection of neutralizers was according to the regulation of disinfection techniques in healthcare settings (WS/T 367-2012); 75% alcohol was neutralized with common nutrient broth; chlorine-containing disinfectants, entoiodine II, and hydrogen peroxide were neutralized with 0.1% sodium thiosulfate, benzalkonium bromide and chlorhexidine were neutralized with 0.3% twain 80 and 0.3% lecithin, while glutaraldehyde was neutralized with 0.3% glycine.
2.3. Testing the MICs and MBCs of Each Disinfectant
2.3.1. The Minimum Inhibitory Concentration Test
MICs of the seven disinfectants against 64 strains of CRKP preserved in our laboratory were detected by the broth dilution method according to the guidelines of the CLSI (CLSI, 2020). Firstly, the standard bacterial concentration of 0.5 McFarland (108 CFU/mL) was applied as a bacterial suspension. Secondly, double dilution of each of the disinfectants into 2.5 mL of different concentrations was performed. Then, 2.5 mL of double concentration nutrient broth was added to each concentration of disinfectants, followed by 0.1 mL of bacterial suspension, and mixed as the test group. Nutrient broth without disinfectant was inoculated with the bacteria and used as the positive control, while nutrient broth inoculated with the same volume of deionized water was used as the negative control. All the tubes were incubated at 35 °C for 48 h before the results were obtained [32,33,34,35,36]. The MICs of the disinfectants against CRKP strains demonstrated the highest dilution for aseptic growth. Experiments were performed in triplicate, with consistent results.
2.3.2. The Minimum Bactericidal Concentration Test
The MBC test is a continuation of the MIC test. An amount of 0.5 mL of the sterile reaction was transferred into 4.5 mL of neutralizer specific for the particular disinfectant used in each test. The solution was fully mixed and interreacted for 10 min. An amount of 2.5 mL of the final reaction solution was added into 2.5 mL of the double concentration broth. The positive and negative control groups were prepared as described above in the MIC experiment, and neutralizer inoculated with the same volume of double concentration broth was used as the neutralizer control group. All the tubes were incubated at 35 °C for 24 h before the results were obtained [32]. The MBCs of the disinfectants against CRKP strains demonstrated the highest dilution for aseptic growth. Experiments were performed in triplicate, with consistent results.
2.4. PCR Detection of Efflux Pump Genes
The DNA templates of 64 CRKP strains were extracted by the boiling method. All the primers were synthesized by Guangzhou Aiji Biotechnology Co., Ltd. The primer sequences were referenced to published articles, as shown in Supplementary Materials Table S1 [26,37]. The PCR conditions were set with reference to published articles [26,37]. Amplified PCR products were analyzed on 2% agarose gel (Biowest, Nuaillé, France). The positive products of the resistance genes were confirmed using PCR followed by sequence analysis.
3. Results
3.1. Clinical Information of CRKP Strains
A total of 162 non-repetitive strains of CRKP were isolated from clinical specimens in SYS Memorial Hospital from 2015 to 2019. The average age of patients was 56.3 ± 20.7 years, among which 115 were male patients.
3.1.1. The Annual Detection Amount of CRKP Strains
From January 2015 to December 2019, the detection rate of CRKP showed an overall increasing trend year by year, and there was a rapid rising peak in 2017, as shown in Figure 1A.
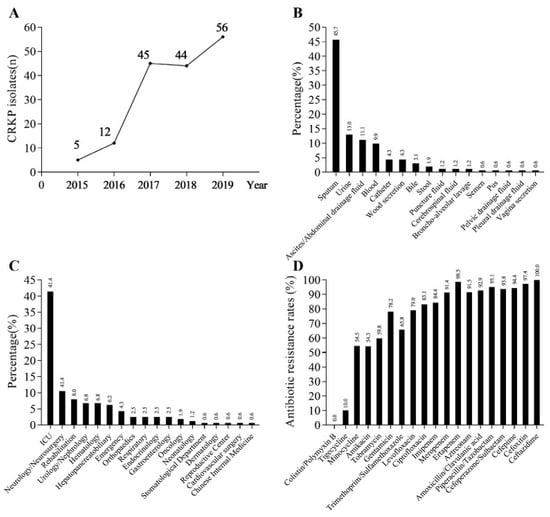
Figure 1.
Clinical information and antimicrobial sensitivity profiles of CRKP isolates. (A) The detection amount of CRKP strains in SYS Memorial Hospital from 2015 to 2019. (B) The distribution of the 162 CRKP strains in different specimen types. (C) The distribution of the 162 CRKP strains in different departments; ICU: intensive care unit. (D) Antibiotic resistance rates of CRKP strains.
3.1.2. Distribution of CRKP Strains in Different Specimen Types
The top four specimen types of the 162 strains of CRKP were sputum, urine, ascites/abdominal drainage fluid, and blood, accounting for 45.7% (74/162), 13.0% (21/162), 11.1% (18/162), and 9.9% (16/162), respectively, as shown in Figure 1B. The number of CRKP strains isolated from sputum was more than three times higher than that of urine.
3.1.3. Distribution of CRKP Strains in Different Departments
In terms of inpatient ward distribution of CRKP strains, the top three departments were the ICU, neurology/neurosurgery, and rehabilitation departments, accounting for 41.4% (67/162), 10.5% (17/162), and 8.0% (13/162), respectively, as shown in Figure 1C. The number of CRKP strains in the ICU was much higher than in other departments and was about four times higher than that in neurology/neurosurgery, the secondary department.
3.1.4. Composition of Departments That Detected CRKP Annually
From 2015 to 2019, the departments where CRKP strains were detected increased year by year. The detection number of CRKP in the ICU department was the greatest. Detailed data are shown in Table S2.
3.1.5. Distribution of Specimen Types in the Top Six Departments That Detected CRKP
We analyzed the distribution of specimen types in the top six departments, in order to identify the centralized infection sites in high-risk departments. Detailed data are shown in Table S3.
3.2. Antimicrobial Sensitivity Profile
In 162 strains of CRKP, no strains were detected to be resistant to colistin or polymyxin B, while the resistance rate of tigecycline was 10% and the resistance rate of other commonly used antibiotics were all higher than 50%, as shown in Figure 1D.
3.3. MIC and MBC Results of CRKP Strains
3.3.1. Analysis of Resistance of CRKP Strains to Seven Chemical Disinfectants Commonly Used in Clinics
The MIC and MBC results of seven clinically used chemical disinfectants against CRKP strains were expressed by diluted multiples. The higher the diluted multiple, the lower the MIC and MBC values. MIC50 and MIC90 of the CRKP strains were compared with the MIC values of the standard strains ATCC 29522 and ATCC 700603. If the diluted multiple was reduced, it indicated that the CRKP strains showed resistance to the disinfectant. The MBC data were analyzed in the same way. The results are shown in Table 1.

Table 1.
MIC and MBC results of CRKP strains against seven kinds of disinfectants (dilution multiples).
3.3.2. Recommended Concentration for CRKP Disinfection
We referred to China’s nosocomial infection control industry standards in combination with the maximum MBC values of seven disinfectants in our study to provide recommended concentrations for CRKP disinfection. Detailed results are shown in Table 2.

Table 2.
Recommended concentrations for CRKP disinfection according to health industry standards of the People’s Republic of China.
3.3.3. Comparisons of the MICs and MBCs of CRKP Strains Isolated from Different Wards and Different Specimens for Each Disinfectant
The results of MICs and MBCs of CRKP strains against seven clinical disinfectants were divided into two groups according to ICU ward (n = 23) and non-ICU wards (n = 41), and statistical analysis was performed by a Mann–Whitney U test of two independent samples (Figure 2). The MIC results of aqueous chlorhexidine were found to be statistically different between the ICU ward and non-ICU wards (p < 0.05) (Figure 2B); the MIC and MBC results of other disinfectants showed no statistical difference between the ICU ward and non-ICU wards. The results of MICs and MBCs were divided into four groups according to the different specimens, as invasive specimens (bile, catheter, ascites/abdominal drainage fluid, and blood) (n = 19), skin and soft tissue (n = 9), urine (n = 7) and sputum (n = 29), but no statistical differences between different specimen groups were found (Figure 3).
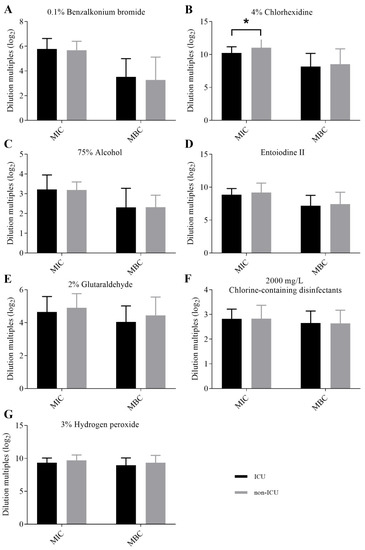
Figure 2.
Comparisons of the MICs and MBCs of CRKP strains isolated from the ICU ward and non-ICU wards for each disinfectant. (A) Comparisons of the MICs and MBCs of CRKP strains isolated from ICU ward and non-ICU wards for 0.1% benzalkonium bromide. (B) Comparisons of the MICs and MBCs of CRKP strains isolated from ICU ward and non-ICU wards for 4% aqueous chlorhexidine. * p < 0.05. The MIC results of 4% aqueous chlorhexidine were statistically different between the ICU ward and non-ICU wards (C) Comparisons of the MICs and MBCs of CRKP strains isolated from ICU ward and non-ICU wards for 75% alcohol. (D) Comparisons of the MICs and MBCs of CRKP strains isolated from ICU ward and non-ICU wards for entoiodine II. (E) Comparisons of the MICs and MBCs of CRKP strains isolated from ICU ward and non-ICU wards for 2% glutaraldehyde. (F) Comparisons of the MICs and MBCs of CRKP strains isolated from ICU ward and non-ICU wards for 2000 mg/L chlorine-containing disinfectants. (G) Comparisons of the MICs and MBCs of CRKP strains isolated from ICU ward and non-ICU wards for 3% hydrogen peroxide. MIC: minimum inhibitory concentration; MBC: minimum bactericidal concentration; ICU: intensive care unit.
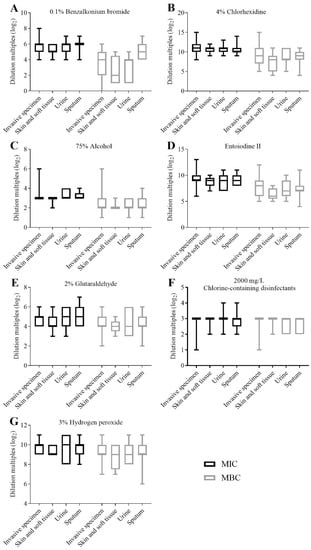
Figure 3.
Comparisons of the MICs and MBCs of CRKP strains isolated from different specimens for each disinfectant. (A) Comparisons of the MICs and MBCs of CRKP strains isolated from different specimens for 0.1% benzalkonium bromide. (B) Comparisons of the MICs and MBCs of CRKP strains isolated from different specimens for 4% aqueous chlorhexidine. (C) Comparisons of the MICs and MBCs of CRKP strains isolated from different specimens for 75% alcohol. (D) Comparisons of the MICs and MBCs of CRKP strains isolated from different specimens for entoiodine II. (E) Comparisons of the MICs and MBCs of CRKP strains isolated from different specimens for 2% glutaraldehyde. (F) Comparisons of the MICs and MBCs of CRKP strains isolated from different specimens for 2000 mg/L chlorine-containing disinfectants. (G) Comparisons of the MICs and MBCs of CRKP strains isolated from different specimens for 3% hydrogen peroxide. MIC: minimum inhibitory concentration; MBC: minimum bactericidal concentration.
3.4. Analysis of Efflux Pump Genes
3.4.1. Detection of Efflux Pump Genes
The detection rates of the three efflux pump genes (oqxA, oqxB, and qacE∆1-sul1) were 60.9% (39/64), 17.2% (11/64), and 71.9% (46/64), respectively. Partial electrophoretic results are shown in Figures S1–S3. There were five positive detection patterns among the three efflux pump genes of the 64 strains of CRKP, among which the qacE∆1-sul1 + oqxA positive pattern was the dominant one, accounting for 39.1%, followed by the single gene qacE∆1-sul1 positive pattern, accounting for 21.9%, as shown in Table 3.

Table 3.
Positive detection patterns of efflux pump genes.
3.4.2. Correlation between Efflux Pump Genes and Disinfectant Resistance
The results of MIC and MBC of the disinfectants were divided into two groups according to the negative and positive genes of efflux pump, and the t-test of two independent samples was used for statistical analysis. There was a statistical difference in the MIC values of 0.1% benzalkonium bromide between the negative and positive qacE∆1-sul1 gene groups (p < 0.05), while the MIC and MBC values of 3% hydrogen peroxide were significantly different between negative and positive oqxA gene groups (p < 0.05), and there was no statistical difference between the negative and positive efflux pump gene groups among the other disinfectants (detailed in Table 4).

Table 4.
Comparison of MIC and MBC values of disinfectants in negative and positive groups of efflux pump genes (p values).
4. Discussion
Klebsiella pneumoniae (Kpn) is one of the most common opportunistic pathogens, which can result in a variety of human infections, including lung, urinary tract, bloodstream, and surgical site infections [38,39]. With the emergence of carbapenem resistance, the management of Klebsiella pneumonia infections has been intractable and complicated [40,41]. We found that CRKP was most commonly detected in the ICU department and could be detected in all the body parts of ICU patients, mainly in sputum specimens and invasive infection specimens (ascites/abdominal drainage fluid, blood, and catheters). Hu et al. [42] and Delia et al. [43] reported that CRKP demonstrated significantly more frequent detection among ICU patients in their retrospective observational studies. This was consistent with what we observed, but in a tertiary care hospital in northern Italy, CRKP infections were concentrated in the medical ward (37.41%), geriatric ward (36.06%), and surgical ward (20.41%) [44]. Sites of infection was commonly found in the urinary tract, whereas it was the respiratory tract in our study. This indicated that CRKP infections varied greatly between different countries. In China, the isolation rate of Kpn in respiratory specimens has risen to the first place since 2017 [45]. In addition, our study found that the ICU department should be the primary focus of CRKP infection control works in our country, which may be related to the antibacterial drug treatment principle in the ICU ward. In China, “Heavy blow, comprehensive coverage” is the main principle utilized in the ICU ward, which refers to the elimination of all bacteria in the shortest time with the most powerful antibacterial drugs, with further descent down the ladder of treatment after the corresponding symptoms have been controlled. Such treatment could lead to the screening of “super drug-resistant bacteria” [46], including CRKP. We also found that the sharp increase in the detected number of CRKP strains in 2017 was mainly concentrated in the ICU, which should be closely related to the increase of ICU wards from two to three in our hospital in the beginning of 2017.
The high detection rate of CRKP brings challenges to nosocomial infection control.
Disinfectants play an important role in halting horizontal transmission within medical institutions [47]. However, more and more studies have observed resistance of bacteria to commonly used disinfectants [32,48,49]. In our study, we observed extensive resistance from the MIC and MBC results, which aroused our attention. Among the seven disinfectants in our experiment, six disinfectants (0.1% benzalkonium bromide, 4% aqueous chlorhexidine, entoiodine II, 2% glutaraldehyde, 2000 mg/L chlorine-containing disinfectants, and 3% hydrogen peroxide) were less sensitive to CRKP strains than the quality control strain. Chen et al. observed higher MICs and MBCs from 0.1% chlorhexidine and 0.1% povidone iodine (PVP-I) compared with the reference strains [48]. In their study, CRKP strains showed resistance to two of three disinfectants. Other research also reported that many of the CRKP strains exhibited resistance to many of the tested disinfectants [32]. Interestingly, three out of twenty-seven strains showed resistance to all of the tested disinfectants [32]. Bhatia et al. also discovered CRKP isolates showed lower susceptibility to sodium hypochlorite (4% available chlorine) in comparison to Kpn ATCC 700603 [49]. However, it has been speculated that the MIC and MBC levels in research were much lower than that which was used in practice, which could not accurately reflect the eliminating ability of disinfectants in real-word settings. Therefore, we compared the maximum MBC values of seven disinfectants in our study with China’s nosocomial infection control industry standards. This was because MBC is more relevant in preventing horizontal transmission of bacteria.
In clinical practice, chlorhexidine has been used as a partial preservative for over 50 years and has become the mainstay biocide in the prevention of healthcare-associated infections [50]. Jackson et al. concluded that chlorhexidine contributed to reducing the occurrence of ventilator-associated pneumonia (VAP), and recommended to implement the use of intra-oral chlorhexidine for mechanically-ventilated patients within critical care settings [51]. Chlorhexidine mouth rinse is mainly available in concentrations of 0.1%, 0.12%, or 0.2% as well as in a low concentration (≤0.06%) rinse [52]. Notably, in our study, the MBCMAX of aqueous chlorhexidine against CRKP strains was 2.5 g/L (0.25%), which meant that the current concentrations of chlorhexidine mouth rinse probably could not kill all the CRKP strains colonizing the oral cavity. Skin disinfection often utilizes 4% aqueous chlorhexidine baths [53], especially in ICU patients [54]. Multiple uses of a standardized procedure of 4% chlorhexidine baths is sufficient to inhibit or kill the colonized bacteria on the skin, reducing the risk of postoperative surgical site infections [53] and reducing microbial adherence to surfaces of implantable biomedical devices [55]. According to the data in our study, the mean MIC values of aqueous chlorhexidine in the ICU ward and non-ICU ward were 0.0034% and 0.0019%, respectively, which were much lower than the current routinely used clinical concentration (4%). Therefore, the current resistance of CRKP strains to chlorhexidine only affects mucous membrane disinfection, not skin disinfection.
Based on the above discussion and the recommended concentrations in Table 2, we propose three suggestions for nosocomial infection control: (1) aqueous chlorhexidine should be ≥2.5 g/L when rinsing or gargling mucous membranes and wounds, (2) 0.1% benzalkonium bromide should avoid dilution, and (3) chlorine-containing disinfectants should be ≥1000 mg/L to eliminate known CRKP strains. These three tips should be of good help in preventing CRKP nosocomial transmission at local medical institutions.
We further explored the correlation between efflux pump genes and disinfectant resistance. The three efflux pump genes detected in our study could both efflux QAC and biguanide disinfectants [24,25,26]. Benzalkonium bromide belongs to the QAC, and chlorhexidine belongs to the biguanide disinfectants. Our study demonstrated that over 60% of the CRKP strains carried oqxA and qacE∆1-sul1 genes. Only the MIC results of 0.1% benzalkonium bromide showed statistical difference between the negative and positive qacE∆1-sul1 gene groups, and the MIC and MBC results of 3% hydrogen peroxide showed statistical difference between the negative and positive oqxA gene groups. The results verified the role of qacE∆1-sul1 gene in the efflux of QAC disinfectants [27], and showed that the oqxA gene may be of practical significance in the efflux of hydrogen peroxide, which needs further research, but no relevant studies have been found so far. Analysis of the positive detection patterns of efflux pump genes showed that some strains carried two or three kinds of efflux pump genes at the same time, suggesting that the resistance to disinfectants may be a combination of multiple non-specific mechanisms to protect the bacteria for survival in harsh environments, which requires further investigation.
A limitation of this experiment was that the activity of efflux pumps was not detected, which was directly related with disinfectant resistance, compared with the gene carrier rates. We would like to explore the activity of the efflux pumps in the isolates with high gene carrier rates in further research.
5. Conclusions
The CRKP strains showed extensive resistance to clinically used disinfectants, with high efflux pump gene carrier rates. To eliminate CRKP in medical institutions effectively, the concentration of aqueous chlorhexidine and chlorine-containing disinfectants needs to be increased, and not used only on the basis of current standards. Importantly, efflux pump genes can be transmitted by plasmids or integrons, which can promote non-specific multidrug resistance via horizontal transfer. We suggest that disinfectant resistance should receive more attention and closer monitoring to guide the rational use of disinfectants and avoid the spread of the notorious multidrug-resistant bacterium.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11060736/s1, Figure S1. Agarose gel electrophoresis image of oqxA gene, Figure S2. Agarose gel electrophoresis image of oqxB gene, Figure S3. Agarose gel electrophoresis image of qacE∆1-sul1 gene; Table S1. Primer sequence of efflux pump genes, Table S2. Departmental distribution of isolated CRKP strains from 2015 to 2019, Table S3. The distribution of specimen types in the departments with CRKP detection.
Author Contributions
L.N. and Z.Z. were responsible for designing experiments and writing the manuscript. X.X. was responsible for writing and modifying the manuscript. R.S. and B.C. participated in the analysis of experimental results and the drawing of tables and figures. X.L. (Xiaoqiang Liu) was responsible for operating the PCR experiment. R.S. and X.W. were responsible for testing the MICs and MBCs of each disinfectant. X.L. (Xuexue Li) participated in collecting and analyzing the clinical data of CRKP strains. H.L. participated in literature searching. S.H. and X.X. were fully responsible for designing the research and gave final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China (82002203, 82071860), and the Natural Science Foundation of Guangdong Province (2021A1515010283).
Institutional Review Board Statement
This report was approved by the Clinical Research and Ethics Committee of the Sun Yat-Sen Memorial Hospital [Ethical document No. 2017 (07)]. All the bacterial isolates in this study were isolated prior to this study.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CRKP | carbapenem-resistant Klebsiella pneumoniae |
| MIC | minimal inhibitory concentration |
| MBC | minimum bactericidal concentration |
| ICU | intensive care unit |
| CRE | carbapenem-resistant Enterobacteriaceae |
| CDC | Centers for Disease Control and Prevention |
| RND | resistance-nodulation cell division |
| QAC | quaternary ammonium compound |
| SMR | small multidrug resistance family |
| SYS Memorial Hospital | Sun Yat-sen Memorial Hospital |
| CLSI | Clinical and Laboratory Standards Institute |
| Kpn | Klebsiella pneumoniae |
| mCIM | modified carbapenem inactivation method |
| eCIM | EDTA-modified carbapenem inactivation method |
| QC | quality control |
| ATCC | American type culture collection |
| PVP-I | povidone-iodine |
| VAP | ventilator-associated pneumonia |
References
- Lutgring, J.D. Carbapenem-resistant Enterobacteriaceae: An emerging bacterial threat. Semin. Diagn. Pathol. 2019, 36, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M.; Forde, B.M.; Alfaresi, M.; Alzarouni, A.; Farahat, Y.; Chong, T.M.; Yin, W.F.; Chan, K.G.; Li, J.; Schembri, M.A.; et al. Stepwise evolution of pandrug-resistance in Klebsiella pneumoniae. Sci. Rep. 2015, 5, 15082. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef]
- Potter, R.F.; D’Souza, A.W.; Dantas, G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2016, 29, 30–46. [Google Scholar] [CrossRef]
- Zhu, W.M.; Yuan, Z.; Zhou, H.Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 23. [Google Scholar] [CrossRef]
- Okeah, B.O.; Morrison, V.; Huws, J.C. Antimicrobial stewardship and infection prevention interventions targeting healthcare-associated Clostridioides difficile and carbapenem-resistant Klebsiella pneumoniae infections: A scoping review. BMJ Open 2021, 11, e051983. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Ma, X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 18. [Google Scholar] [CrossRef]
- Maraolo, A.E.; Corcione, S.; Grossi, A.; Signori, A.; Alicino, C.; Hussein, K.; Trecarichi, E.M.; Viale, P.; Timsit, J.F.; Veeraraghavan, B.; et al. The Impact of Carbapenem Resistance on Mortality in Patients with Klebsiella pneumoniae Bloodstream Infection: An Individual Patient Data Meta-Analysis of 1952 Patients. Infect. Dis. Ther. 2021, 10, 541–558. [Google Scholar] [CrossRef]
- Stewardson, A.J.; Marimuthu, K.; Sengupta, S.; Allignol, A.; El-Bouseary, M.; Carvalho, M.J.; Hassan, B.; Delgado-Ramirez, M.A.; Arora, A.; Bagga, R.; et al. Effect of carbapenem resistance on outcomes of bloodstream infection caused by Enterobacteriaceae in low-income and middle-income countries (PANORAMA): A multinational prospective cohort study. Lancet Infect. Dis. 2019, 19, 601–610. [Google Scholar] [CrossRef]
- Perez, F.; Bonomo, R.A. Carbapenem-resistant Enterobacteriaceae: Global action required. Lancet Infect. Dis. 2019, 19, 561–562. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Burns, K.; Rodríguez Baño, J.; Borg, M.; Daikos, G.; Dumpis, U.; Lucet, J.C.; Moro, M.L.; Tacconelli, E.; Simonsen, G.S.; et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control 2017, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Gnanadhas, D.P.; Marathe, S.A.; Chakravortty, D. Biocides—Resistance, cross-resistance mechanisms and assessment. Expert Opin. Investig. Drugs 2013, 22, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely Used Benzalkonium Chloride Disinfectants Can Promote Antibiotic Resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef]
- Maillard, J.Y. Resistance of Bacteria to Biocides. Microbiol. Spectr. 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Bloomfield, S.; Coelho, J.R.; Collier, P.; Cookson, B.; Fanning, S.; Hill, A.; Hartemann, P.; McBain, A.J.; Oggioni, M.; et al. Does microbicide use in consumer products promote antimicrobial resistance? A critical review and recommendations for a cohesive approach to risk assessment. Microb. Drug Resist. 2013, 19, 344–354. [Google Scholar] [CrossRef]
- Grande Burgos, M.J.; Fernández Márquez, M.L.; Pérez Pulido, R.; Gálvez, A.; Lucas López, R. Virulence factors and antimicrobial resistance in Escherichia coli strains isolated from hen egg shells. Int. J. Food Microbiol. 2016, 238, 89–95. [Google Scholar] [CrossRef]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007, 39, 162–176. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Maillard, J.Y.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Efflux pump induction by quaternary ammonium compounds and fluoroquinolone resistance in bacteria. Future Microbiol. 2016, 11, 81–92. [Google Scholar] [CrossRef]
- Nishino, K.; Yamasaki, S.; Nakashima, R.; Zwama, M.; Hayashi-Nishino, M. Function and Inhibitory Mechanisms of Multidrug Efflux Pumps. Front. Microbiol. 2021, 12, 737288. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Morante, J.; Quispe, A.M. Tolerance to disinfectants (chlorhexidine and isopropanol) and its association with antibiotic resistance in clinically-related Klebsiella pneumoniae isolates. Pathog. Glob. Health 2021, 115, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Xu, H.; Chen, D.; Zhou, H.; Hu, X.; Cheng, G. First emergence of acrAB and oqxAB mediated tigecycline resistance in clinical isolates of Klebsiella pneumoniae pre-dating the use of tigecycline in a Chinese hospital. PLoS ONE 2014, 9, e115185. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Ning, J.; Sajid, A.; Cheng, G.; Yuan, Z.; Hao, H. The nature and epidemiology of OqxAB, a multidrug efflux pump. Antimicrob. Resist. Infect. Control 2019, 8, 44. [Google Scholar] [CrossRef]
- Fan, Y.F.; Cui, S.Y.; Zhang, C.; Xu, X.M. Investigation of acquired drug-resistant genes and strains relationship in Pseudomonas aeruginosa isolated from burn patients. Zhonghua shao shang za zhi = Zhonghua shaoshang zazhi = Chin. J. Burn. 2018, 34, 83–87. [Google Scholar] [CrossRef]
- Shafaati, M.; Boroumand, M.; Nowroozi, J.; Amiri, P.; Kazemian, H. Correlation Between qacE and qacE∆1 Efflux Pump Genes, Antibiotic and Disinfectant Resistant Among Clinical Isolates of E.coli. Recent Pat. Anti-Infect. Drug Discov. 2016, 11, 189–195. [Google Scholar] [CrossRef]
- Guo, J.; Li, C. Molecular epidemiology and decreased susceptibility to disinfectants in carbapenem-resistant Acinetobacter baumannii isolated from intensive care unit patients in central China. J. Infect. Public Health 2019, 12, 890–896. [Google Scholar] [CrossRef]
- Carvalho, I.; Chenouf, N.S.; Carvalho, J.A.; Castro, A.P.; Silva, V.; Capita, R.; Alonso-Calleja, C.; Enes Dapkevicius, M.L.N. Multidrug-resistant Klebsiella pneumoniae harboring extended spectrum β-lactamase encoding genes isolated from human septicemias. PLoS ONE 2021, 16, e0250525. [Google Scholar] [CrossRef]
- Miller, S.; Humphries, R.M. Clinical laboratory detection of carbapenem-resistant and carbapenemase-producing Enterobacteriaceae. Expert Rev. Anti-Infect. Ther. 2016, 14, 705–717. [Google Scholar] [CrossRef]
- Tsai, Y.M.; Wang, S.; Chiu, H.C.; Kao, C.Y. Combination of modified carbapenem inactivation method (mCIM) and EDTA-CIM (eCIM) for phenotypic detection of carbapenemase-producing Enterobacteriaceae. BMC Microbiol. 2020, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Shan, K.; Xu, B.; Li, J. Determining the resistance of carbapenem-resistant Klebsiella pneumoniae to common disinfectants and elucidating the underlying resistance mechanisms. Pathog. Glob. Health 2015, 109, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Feßler, A.T.; Schug, A.R.; Geber, F.; Scholtzek, A.D.; Merle, R.; Brombach, J.; Hensel, V.; Meurer, M.; Michael, G.B.; Reinhardt, M.; et al. Development and evaluation of a broth macrodilution method to determine the biocide susceptibility of bacteria. Vet. Microbiol. 2018, 223, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Uzer Celik, E.; Tunac, A.T.; Ates, M.; Sen, B.H. Antimicrobial activity of different disinfectants against cariogenic microorganisms. Braz. Oral Res. 2016, 30, e125. [Google Scholar] [CrossRef][Green Version]
- Baseri, N.; Najar-Peerayeh, S.; Bakhshi, B.; Campanile, F. Phenotypic and genotypic changes of Staphylococcus aureus in the presence of the inappropriate concentration of chlorhexidine gluconate. BMC Microbiol. 2022, 22, 100. [Google Scholar] [CrossRef]
- Amini Tapouk, F.; Nabizadeh, R.; Mirzaei, N.; Hosseini Jazani, N.; Yousefi, M.; Valizade Hasanloei, M.A. Comparative efficacy of hospital disinfectants against nosocomial infection pathogens. Antimicrob. Resist. Infect. Control 2020, 9, 115. [Google Scholar] [CrossRef]
- Kim, H.B.; Wang, M.; Park, C.H.; Kim, E.C.; Jacoby, G.A.; Hooper, D.C. oqxAB encoding a multidrug efflux pump in human clinical isolates of Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 3582–3584. [Google Scholar] [CrossRef]
- Hennequin, C.; Robin, F. Correlation between antimicrobial resistance and virulence in Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2016, 35, 333–341. [Google Scholar] [CrossRef]
- Hu, Y.; Anes, J.; Devineau, S.; Fanning, S. Klebsiella pneumoniae: Prevalence, Reservoirs, Antimicrobial Resistance, Pathogenicity, and Infection: A Hitherto Unrecognized Zoonotic Bacterium. Foodborne Pathog. Dis. 2021, 18, 63–84. [Google Scholar] [CrossRef]
- Pitout, J.D.; Nordmann, P.; Poirel, L. Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrob. Agents Chemother. 2015, 59, 5873–5884. [Google Scholar] [CrossRef]
- Sękowska, A.; Gospodarek, E.; Kusza, K. The prevalence of infections and colonisation with Klebsiella pneumoniae strains isolated in ICU patients. Anaesthesiol. Intensive Ther. 2014, 46, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, C.; Shen, Z.; Zhou, H.; Cao, J.; Chen, S.; Lv, H.; Zhou, M.; Wang, Q.; Sun, L.; et al. Prevalence, risk factors and molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in patients from Zhejiang, China, 2008–2018. Emerg. Microbes Infect. 2020, 9, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Muntean, D.; Horhat, F.G. Multidrug-Resistant Gram-Negative Bacilli: A Retrospective Study of Trends in a Tertiary Healthcare Unit. Medicina 2018, 54, 92. [Google Scholar] [CrossRef] [PubMed]
- Cristina, M.L.; Sartini, M.; Ottria, G.; Schinca, E.; Cenderello, N.; Crisalli, M.P.; Fabbri, P.; Lo Pinto, G.; Usiglio, D.; Spagnolo, A.M. Epidemiology and biomolecular characterization of carbapenem-resistant Klebsiella pneumoniae in an Italian hospital. J. Prev. Med. Hyg. 2016, 57, E149–E156. [Google Scholar] [PubMed]
- CHINET. Available online: http://www.chinets.com/ (accessed on 5 February 2020).
- Chen, I.L.; Lee, C.H.; Su, L.H.; Tang, Y.F.; Chang, S.J.; Liu, J.W. Antibiotic consumption and healthcare-associated infections caused by multidrug-resistant gram-negative bacilli at a large medical center in Taiwan from 2002 to 2009: Implicating the importance of antibiotic stewardship. PLoS ONE 2013, 8, e65621. [Google Scholar] [CrossRef] [PubMed]
- Assadian, O.; Harbarth, S.; Vos, M.; Knobloch, J.K.; Asensio, A.; Widmer, A.F. Practical recommendations for routine cleaning and disinfection procedures in healthcare institutions: A narrative review. J. Hosp. Infect. 2021, 113, 104–114. [Google Scholar] [CrossRef]
- Chen, Y.; Liao, K.; Huang, Y.; Guo, P.; Huang, H.; Wu, Z.; Liu, M. Determining the susceptibility of carbapenem resistant Klebsiella pneumoniae and Escherichia coli strains against common disinfectants at a tertiary hospital in China. BMC Infect. Dis. 2020, 20, 88. [Google Scholar] [CrossRef]
- Bhatia, M.; Loomba, P.S.; Mishra, B.; Dogra, V.; Thakur, A. Reduced susceptibility of carbapenem-resistant Klebsiella pneumoniae to biocides: An emerging threat. Indian J. Med. Microbiol. 2016, 34, 355–358. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef]
- Jackson, L.; Owens, M. Does oral care with chlorhexidine reduce ventilator-associated pneumonia in mechanically ventilated adults? Br. J. Nurs. 2019, 28, 682–689. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, Cd008676. [Google Scholar] [CrossRef] [PubMed]
- Scallan, R.M.; Gerathy, S.; Price, J.; Lazarus, A.M.; Metter, E.J.; Talbot, L.A. Preoperative Chlorhexidine Gluconate Bathing on a Military Medical-Surgical Unit. Mil. Med. 2020, 185 (Suppl. S2), 15–20. [Google Scholar] [CrossRef] [PubMed]
- Strich, J.R.; Palmore, T.N. Preventing Transmission of Multidrug-Resistant Pathogens in the Intensive Care Unit. Infect. Dis. Clin. N. Am. 2017, 31, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Edmiston, C.E., Jr.; Bruden, B.; Rucinski, M.C.; Henen, C.; Graham, M.B.; Lewis, B.L. Reducing the risk of surgical site infections: Does chlorhexidine gluconate provide a risk reduction benefit? Am. J. Infect. Control 2013, 41 (Suppl. S5), S49–S55. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).