Characterization of the First Carbapenem-Resistant Pseudocitrobacter faecalis Harboring blaOXA-181 in China
Abstract
:1. Introduction
2. Results
2.1. Case Presentation
2.2. Characteristics of P. faecalis Strains
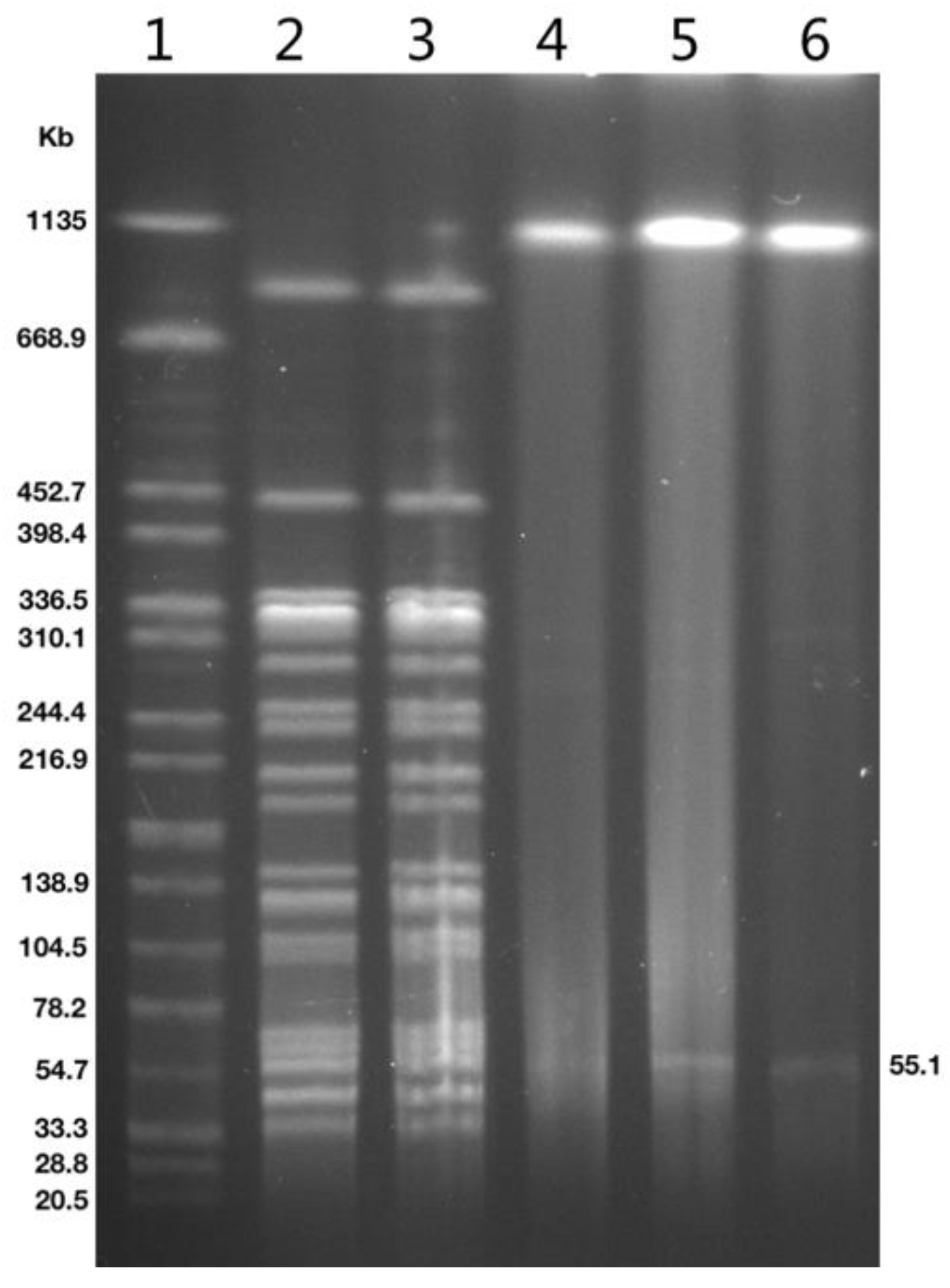
2.3. Genetic Analysis of Strains and blaOXA-181-Positive Plasmid
3. Discussion
4. Materials and Methods
4.1. Clinical Isolates and Patient Data
4.2. Antimicrobial Susceptibility Testing
4.3. Plasmid Transformation Experiments
4.4. Pulsed-Field Gel Electrophoresis (PFGE)
4.5. Whole-Genome Sequencing and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients with E. coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhanel, G.G.; Wiebe, R.; Dilay, L.; Thomson, K.; Rubinstein, E.; Hoban, D.J.; Noreddin, A.M.; Karlowsky, J.A. Comparative review of the carbapenems. Drugs 2007, 67, 1027–1052. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Tan, R.; Chen, Y.; Sun, J.; Liu, J.; Qu, H.; Wang, X. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: Factors related to the carbapenem resistance and patient mortality. Antimicrob. Resist. Infect. Control 2016, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Van Duin, D.; Arias, C.A.; Komarow, L.; Chen, L.; Hanson, B.M.; Weston, G.; Cober, E.; Garner, O.B.; Jacob, J.T.; Satlin, M.J.; et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): A prospective cohort study. Lancet Infect. Dis. 2020, 20, 731–741. [Google Scholar] [CrossRef]
- Hu, F.; Wang, M.; Zhu, D.; Wang, F. CHINET efforts to control antimicrobial resistance in China. J. Glob. Antimicrob. Resist. 2020, 21, 76–77. [Google Scholar] [CrossRef]
- Hu, F.; Zhu, D.; Wang, F.; Wang, M. Current Status and Trends of Antibacterial Resistance in China. Clin. Infect. Dis 2018, 67, S128–S134. [Google Scholar] [CrossRef]
- Tilahun, M.; Kassa, Y.; Gedefie, A.; Ashagire, M. Emerging Carbapenem-Resistant Enterobacteriaceae Infection, Its Epidemiology and Novel Treatment Options: A Review. Infect. Drug Resist. 2021, 14, 4363–4374. [Google Scholar] [CrossRef]
- Han, R.; Shi, Q.; Wu, S.; Yin, D.; Peng, M.; Dong, D.; Zheng, Y.; Guo, Y.; Zhang, R.; Hu, F. Dissemination of Carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) Among Carbapenem-Resistant Enterobacteriaceae Isolated from Adult and Children Patients in China. Front. Cell Infect. Microbiol. 2020, 10, 314. [Google Scholar] [CrossRef]
- Kieffer, N.; Nordmann, P.; Aires-de-Sousa, M.; Poirel, L. High Prevalence of Carbapenemase-Producing Enterobacteriaceae among Hospitalized Children in Luanda, Angola. Antimicrob. Agents Chemother. 2016, 60, 6189–6192. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Shen, S.; Shi, Q.; Ding, L.; Wu, S.; Han, R.; Zhou, X.; Yu, H.; Hu, F. First Report of blaIMP-4 and blaSRT-2 Coproducing Serratia marcescens Clinical Isolate in China. Front. Microbiol. 2021, 12, 743312. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P.; Glaeser, S.P.; Raza, M.W.; Abbasi, S.A.; Perry, J.D. Pseudocitrobacter gen. nov., a novel genus of the Enterobacteriaceae with two new species Pseudocitrobacter faecalis sp. nov., and Pseudocitrobacter anthropi sp. nov, isolated from fecal samples from hospitalized patients in Pakistan. Syst. Appl. Microbiol. 2014, 37, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kaempfer, P.; Fuglsang-Damgaard, D.; Overballe-Petersen, S.; Hasman, H.; Hammerum, A.M.; Fuursted, K.; Blom, J.; Glaeser, S.P.; Hansen, F. Taxonomic reassessment of the genus Pseudocitrobacter using whole genome sequencing: Pseudocitrobacter anthropi is a later heterotypic synonym of Pseudocitrobacter faecalis and description of Pseudocitrobacter vendiensis sp. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Pérez-Díaz, I.M.; Hayes, J.S.; Breidt, F. Bacteriophages Infecting Gram-Negative Bacteria in a Commercial Cucumber Fermentation. Front. Microbiol. 2020, 11, 1306. [Google Scholar] [CrossRef] [PubMed]
- Abarca, J.G.; Whitfield, S.M.; Zuniga-Chaves, I.; Alvarado, G.; Kerby, J.; Murillo-Cruz, C.; Pinto-Tomás, A.A. Genotyping and differential bacterial inhibition of Batrachochytrium dendrobatidis in threatened amphibians in Costa Rica. Microbiology 2021, 167, 001017. [Google Scholar] [CrossRef]
- Al-Kharousi, Z.S.; Guizani, N.; Al-Sadi, A.M.; Al-Bulushi, I.M.; Shaharoona, B. Hiding in Fresh Fruits and Vegetables: Opportunistic Pathogens May Cross Geographical Barriers. Int. J. Microbiol. 2016, 2016, 4292417. [Google Scholar] [CrossRef] [Green Version]
- De Andrade, L.K.; Levican, A.; Cerdeira, L.; De Morais, A.B.Z.; Braz, M.M.; Martins, E.R.; Casella, T.; Moura, Q.; Fuga, B.; Lincopan, N.; et al. Carbapenem-resistant IMP-1-producing Pseudocitrobacter vendiensis emerging in a hemodialysis unit. Braz. J. Microbiol. 2022, 53, 251–254. [Google Scholar] [CrossRef]
- Cui, J.; Li, M.; Cui, J.; Wang, J.; Qiang, X.; Liang, Z. The proportion, species distribution and dynamic trends of bloodstream infection cases in a tertiary hospital in China, 2010–2019. Infection 2021, 50, 121–130. [Google Scholar] [CrossRef]
- Zhu, S.; Kang, Y.; Wang, W.; Cai, L.; Sun, X.; Zong, Z. The clinical impacts and risk factors for non-central line-associated bloodstream infection in 5046 intensive care unit patients: An observational study based on electronic medical records. Crit. Care 2019, 23, 52. [Google Scholar] [CrossRef] [Green Version]
- Timsit, J.F.; Ruppé, E.; Barbier, F.; Tabah, A.; Bassetti, M. Bloodstream infections in critically ill patients: An expert statement. Intensive Care Med. 2020, 46, 266–284. [Google Scholar] [CrossRef]
- Widmer, A.F.; Kern, W.V.; Roth, J.A.; Dettenkofer, M.; Goetting, T.; Bertz, H.; Theilacker, C. Early versus late onset bloodstream infection during neutropenia after high-dose chemotherapy for hematologic malignancy. Infection 2019, 47, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Boev, C.; Kiss, E. Hospital-Acquired Infections: Current Trends and Prevention. Crit. Care Nurs. Clin. 2017, 29, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.W.; Boolchandani, M.; Patel, S.; Galazzo, G.; Van Hattem, J.M.; Arcilla, M.S.; Melles, D.C.; De Jong, M.D.; Schultsz, C.; Dantas, G.; et al. Destination shapes antibiotic resistance gene acquisitions, abundance increases, and diversity changes in Dutch travelers. Genome Med. 2021, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bi, Z.; Ma, S.; Chen, B.; Cai, C.; He, J.; Schwarz, S.; Sun, C.; Zhou, Y.; Yin, J.; et al. Inter-host Transmission of Carbapenemase-Producing Escherichia coli among Humans and Backyard Animals. Environ. Health Perspect. 2019, 127, 107009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Li, Y.; Yang, Y.; Shen, Z.; Cai, C.; Wang, Y.; Walsh, T.R.; Shen, J.; Wu, Y.; Wang, S. High prevalence and persistence of carbapenem and colistin resistance in livestock farm environments in China. J. Hazard Mater. 2021, 406, 124298. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Wu, Z.; Yin, W.; Schwarz, S.; Tyrrell, J.M.; Zheng, Y.; Wang, S.; Shen, Z.; et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat. Microbiol. 2017, 2, 16260. [Google Scholar] [CrossRef]
- Van Der Zwaluw, K.; Witteveen, S.; Wielders, L.; Van Santen, M.; Landman, F.; De Haan, A.; Schouls, L.M.; Bosch, T. Molecular characteristics of carbapenemase-producing Enterobacterales in the Netherlands; Results of the 2014–2018 national laboratory surveillance. Clin. Microbiol. Infect. 2020, 26, 1412.e7–1412.e12. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Fang, Y.; Zeng, Y.; Lu, J.; Sun, Q.; Zhou, H.; Shen, Z.; Chen, G. First Report of OXA-181-Producing Klebsiella pneumoniae in China. Infect. Drug Resist. 2020, 13, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Feng, Y.; Wu, W.; Xie, Y.; Wang, X.; Zhang, X.; Chen, X.; Zong, Z. First Report of OXA-181-Producing Escherichia coli in China and Characterization of the Isolate Using Whole-Genome Sequencing. Antimicrob. Agents Chemother. 2015, 59, 5022–5025. [Google Scholar] [CrossRef] [Green Version]
- Pitout, J.D.D.; Peirano, G.; Kock, M.M.; Strydom, K.A.; Matsumura, Y. The Global Ascendency of OXA-48-Type Carbapenemases. Clin. Microbiol. Rev. 2019, 33, e00102–e00119. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, A.P.A.; Landman, F.; De Haan, A.; Witteveen, S.; Van Santen-Verheuvel, M.G.; Schouls, L.M.; Dutch CPE Surveillance Study Group. Bla OXA-48-like genome architecture among carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in the Netherlands. Microb. Genom. 2021, 7, 000512. [Google Scholar] [CrossRef] [PubMed]
- Mouftah, S.F.; Pál, T.; Darwish, D.; Ghazawi, A.; Villa, L.; Carattoli, A.; Sonnevend, Á. Epidemic IncX3 plasmids spreading carbapenemase genes in the United Arab Emirates and worldwide. Infect. Drug Resist. 2019, 12, 1729–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Fang, L.; Yang, Y.; Yan, R.; Fu, Y.; Shen, P.; Zhao, D.; Chen, Y.; Hua, X.; Jiang, Y.; et al. Emergence of carbapenem-resistant Klebsiella pneumoniae harbouring blaOXA-48-like genes in China. J. Med. Microbiol. 2021, 70, 001306. [Google Scholar] [CrossRef] [PubMed]
- Redzej, A.; Ukleja, M.; Connery, S.; Trokter, M.; Felisberto-Rodrigues, C.; Cryar, A.; Thalassinos, K.; Hayward, R.D.; Orlova, E.V.; Waksman, G. Structure of a VirD4 coupling protein bound to a VirB type IV secretion machinery. EMBO J. 2017, 36, 3080–3095. [Google Scholar] [CrossRef] [Green Version]
- Wayne, P.A. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Barton, B.M.; Harding, G.P.; Zuccarelli, A.J. A general method for detecting and sizing large plasmids. Anal. Biochem. 1995, 226, 235–240. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]


| Strains | β-Lactamase Genes | Fluoroquinolone- Resistant Genes | MIC (mg/L) a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMP | MEM | CAZ | FEP | ATM | CZA | SCF | TZP | SXT | CIP | LEV | AMK | TGC | POL | |||
| P. faecalis SC48 | blaDHA-1, blaOXA-1, blaOXA-181 | qnrB4, qnrS1, aac(6’)-Ib-cr | 64 | 32 | 2 | 8 | ≤1 | 1 | 128 | >256 | 0.5 | >8 | 8 | 4 | 1 | 0.5 |
| P. faecalis SC62 | blaDHA-1, blaOXA-1, blaOXA-181 | qnrB4, qnrS1, aac(6’)-Ib-cr | 64 | 64 | 2 | 8 | ≤1 | 1 | 128 | >256 | 0.5 | >8 | 8 | 8 | 1 | 0.5 |
| E. coli DH5α- SC48-T | blaOXA-181 | qnrS1 | 1 | 0.25 | 0.5 | 0.125 | ≤1 | 0.25 | 8 | 64 | ≤0.25 | 0.25 | 0.5 | ≤1 | 0.25 | 0.25 |
| E. coli DH5α | - | - | 0.125 | ≤0.03 | 0.5 | ≤0.06 | ≤1 | 0.125 | ≤1 | 4 | ≤0.25 | ≤0.06 | ≤0.125 | ≤1 | 0.125 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Guo, Y.; Yang, Y.; Wu, S.; Han, R.; Ding, L.; Yin, D.; Hu, F. Characterization of the First Carbapenem-Resistant Pseudocitrobacter faecalis Harboring blaOXA-181 in China. Antibiotics 2022, 11, 737. https://doi.org/10.3390/antibiotics11060737
Shi Q, Guo Y, Yang Y, Wu S, Han R, Ding L, Yin D, Hu F. Characterization of the First Carbapenem-Resistant Pseudocitrobacter faecalis Harboring blaOXA-181 in China. Antibiotics. 2022; 11(6):737. https://doi.org/10.3390/antibiotics11060737
Chicago/Turabian StyleShi, Qingyu, Yan Guo, Yang Yang, Shi Wu, Renru Han, Li Ding, Dandan Yin, and Fupin Hu. 2022. "Characterization of the First Carbapenem-Resistant Pseudocitrobacter faecalis Harboring blaOXA-181 in China" Antibiotics 11, no. 6: 737. https://doi.org/10.3390/antibiotics11060737
APA StyleShi, Q., Guo, Y., Yang, Y., Wu, S., Han, R., Ding, L., Yin, D., & Hu, F. (2022). Characterization of the First Carbapenem-Resistant Pseudocitrobacter faecalis Harboring blaOXA-181 in China. Antibiotics, 11(6), 737. https://doi.org/10.3390/antibiotics11060737






