Abstract
Staphylococcus aureus are human facultative pathogenic bacteria and can be found as contaminants in the environment. The aim of our study was to determine whether methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) isolated from coastal beach and river waters, anchialine pools, sand, and wastewater on the island of Hawaiʻi, Hawaiʻi, are a potential health risk. Samples were collected from three regions on Hawaiʻi Island from July to December 2020 during the COVID-19 pandemic and were characterized using whole-genome sequencing (WGS). From WGS data, multilocus sequence typing (MLST), SCCmec type, antimicrobial resistance genes, virulence factors, and plasmids were identified. Of the 361 samples, 98.1% were positive for Staphylococcus spp. and 7.2% were S. aureus positive (n = 26); nine MRSA and 27 MSSA strains were characterized; multiple isolates were chosen from the same sample in two sand and seven coastal beach water samples. The nine MRSA isolates were multi-drug resistant (6–9 genes) sequence type (ST) 8, clonal complex (CC) 8, SCCmec type IVa (USA300 clone), and were clonally related (0–16 SNP differences), and carried 16–19 virulence factors. The 27 MSSA isolates were grouped into eight CCs and 12 STs. Seventy-eight percent of the MSSA isolates carried 1–5 different antibiotic resistance genes and carried 5–19 virulence factors. We found S. aureus in coastal beach and river waters, anchialine pools, and sand at locations with limited human activity on the island of Hawaiʻi. This may be a public health hazard.
1. Introduction
Staphylococcus aureus is a facultative pathogen that can be part of the normal flora of humans, as well as some animal species. They have been found in domestic and wild animals, as well as in a variety of environments [,,,,,]. In the United States, S. aureus has been isolated from coastal beach waters, freshwaters, and sand in California [], Florida [,,], Washington State [,,], Ohio [], the Great Lakes [], and Hawaiʻi [,,,]. Methicillin-resistant Staphylococcus aureus (MRSA)- and methicillin-susceptible S. aureus (MSSA)-related mortality rates have been estimated at 14 and 11 deaths per 100,000 hospitalizations, respectively, in the United States []. Previous studies have shown a higher prevalence of MRSA infections in the State of Hawaiʻi compared to the national average [,]. Native Hawaiians, Pacific Islanders, and children are disproportionately affected and have a higher infection rate of community-acquired MRSA infections [,,]. A steady increase in the proportion of MRSA, compared to MSSA infections, has been reported in inpatient and outpatient clinics, jails, and nursing homes in Hawaiʻi [,,,].
Marine waters and beaches have been proposed as possible sources for S. aureus infections, but only a few studies have examined the risk to beachgoers in Hawaiʻi and abroad. A correlation between swimmer density and staphylococci isolated from seawater has previously been demonstrated []. This resulted in four times higher odds of developing staphylococcal skin infections, as well as increases in respiratory and total illnesses in humans exposed to seawater compared to those who were not; however, the association could not always be confirmed [,,,].
The aim of the current study was to assess the public health hazard of S. aureus found in coastal beach and river waters, anchialine pools, sand, and wastewater on Hawaiʻi Island, Hawaiʻi, by characterizing the isolates using whole-genome sequencing (WGS). This is the first study in the State of Hawaiʻi to characterize MRSA and MSSA isolates using WGS from environmental sources. As this project started, the COVID-19 pandemic began closing tourism to the State of Hawaiʻi which severely reduced beach activities of residents and visitors through lockdowns and limitations on public gatherings. Consequently, this study may underrepresent the full extent of S. aureus contamination in coastal and aquatic Hawaiian environments.
2. Results
2.1. Prevalence of S. aureus
Ninety-eight percent (354/361) of the samples were positive for Staphylococcus spp. Overall, the prevalence of S. aureus, MRSA, and MSSA was 7.2% (26/361), 2.2% (8/361) and 5.5% (20/361), respectively, which included 44.4% (16/36) of coastal and freshwater (beach/river/anchialine pool) stations (Table 1). The prevalence of MRSA in coastal beach waters, anchialine pools, and sand was 1.8% (4/225), 10.0% (1/10), and 8.6% (3/35), respectively, and were isolated from the districts of Hilo and Puna. MRSA was not detected in waters at river/stream stations. The prevalence of MSSA in coastal beach and river/stream waters, anchialine pools, and sand was 5.3% (12/225), 3.9% (3/76), 30.0% (3/10), and 5.7% (2/35), respectively. Staphylococcus spp. MPN/100 mL counts in water varied 10-fold (Figure 1A). Staphylococcus spp. counts in sand were highest at Kehena Beach (station #31) and lowest at Hilo Bay (station #16) (Figure 1B). S. aureus isolates were obtained in sand three times, once at Lehia Beach Park (station #1), Reed’s Bay (station #11), and Honoliʻi Beach Park (station #20) (Figure 1B). Wastewater Staphylococcus spp. counts varied 10–1000-fold and were higher in influent compared to effluent and manhole samples (Figure 1C). Coastal and freshwater (beach/river/anchialine pool) S. aureus isolates were detected at 52.4% (11/21) stations in Hilo, 11.1% (1/9) stations in S. Kohala and N. Kona, and 83.3% (5/6) stations in Puna (Figure 1A). No MRSA or MSSA was detected in wastewater. A total of 36 S. aureus isolates were available for genomic analysis.

Table 1.
Distribution and prevalence of Staphylococcus aureus samples (n = 26) and isolates (n = 36) characterized from environmental sources. Methicillin-resistant S. aureus (MRSA); methicillin-susceptible S. aureus (MSSA).
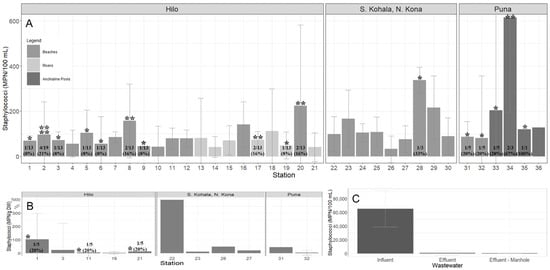
Figure 1.
Geometric mean (±SE) of staphylococci collected from the districts of Hilo (stations #1–21), S. Kohala, N. Kona (stations #22–30), and Puna (stations #31–36) from July–December 2020 on Hawaiʻi Island, Hawaiʻi. (A) Staphylococcus spp. MPN/100 mL counts from water (beach/river/stream/anchialine pool) stations; (B) Staphylococcus spp. MPN/g DW counts from beach sand stations; (C) Staphylococcus spp. MPN/100 mL counts from wastewater collected from the Hilo Wastewater Treatment Plant. Detection of S. aureus is represented by the *. The number of * is how many different isolates were identified from the same station [ranging from one to four]. The prevalence of S. aureus, reported as number of detections in the total sample pool and percent, is shown at the base of each station, if detected.
2.2. Genetic Characterization of MRSA and MSSA Isolates
Thirty-six S. aureus isolates included 12 different STs (Figure 2 and Figure 3; Table 2). All nine MRSA isolates [coastal beach water (n = 5), anchialine pool (n = 1), sand (n = 3)] were ST8 (CC8). These isolates were highly clonal based on the core genome SNP analysis (0–16 differences) (Figure 3), despite being isolated from two different districts on the island. The MRSA isolates obtained from the environment in this study were compared with the sequences of clinical MRSA isolates from hospital patients in the State of Hawaiʻi dating back from 2011 []. These isolates differed by 54–144 SNPs (Figure 4), and can thus be regarded as clonally related.
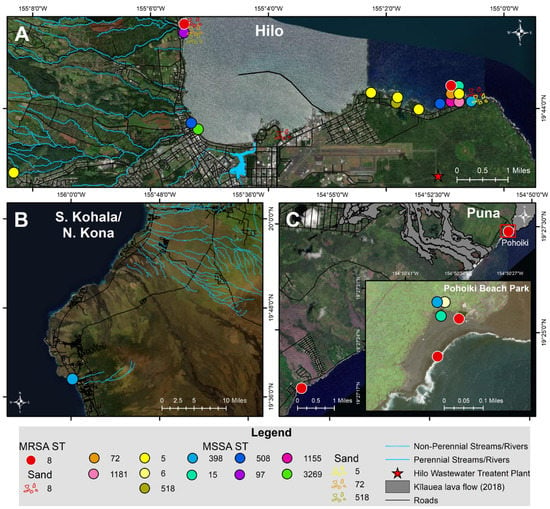
Figure 2.
Map of MRSA and MSSA sequence types (ST) isolated from coastal beach and river waters, anchialine pools, and sand from July–December 2020 in the districts of (A) Hilo, (B) S. Kohala, N. Kona, and (C) Puna on Hawaiʻi Island, Hawaiʻi.
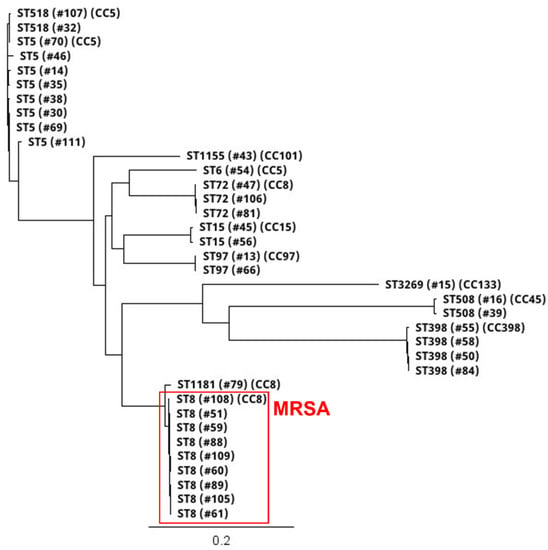
Figure 3.
Core genome single nucleotide polymorphism (SNP)-based phylogenetic tree of 12 STs and eight CCs of 36 [nine MRSA, 27 MSSA] isolates collected from coastal beach and river waters, anchialine pools, and sand on Hawaiʻi Island, Hawaiʻi, from July to December 2020. The scale bar denotes genetic distance in number of base substitutions per site. Branch tree labels indicate ST, isolate #, and CC.

Table 2.
Genomic characterization of MRSA (n = 9) and MSSA (n = 27) isolates collected from coastal beach and river waters, anchialine pools, and beach sand from July–December 2020 in the districts of Hilo, N. Kona, and Puna on Hawaiʻi Island, Hawaiʻi.
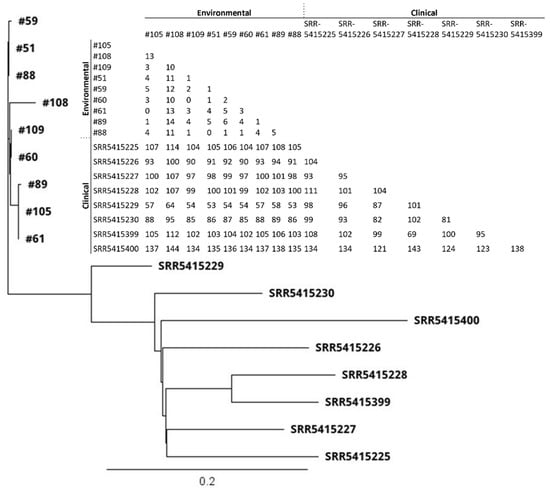
Figure 4.
Core genome SNP-based phylogenetic tree and SNP matrix of MRSA (n = 9) isolates obtained from the environment in 2020 from Hawaiʻi Island, Hawaiʻi, and clinical MRSA isolates collected from hospital patients (n = 8) in the State of Hawaiʻi in 2011. Clinical isolate data were obtained from Challagundla et al., 2018 [] under accession #PRJNA330544. The scale bar denotes genetic distance in number of base substitutions per site.
Among the 27 MSSA isolates, the most frequent lineages were ST5 (n = 8), ST398 (n = 4), ST72 (n = 3), ST15 (n = 2), ST508 (n = 2), ST97 (n = 2), ST518 (n = 2), and one isolate each of ST6, ST1155, ST3269, and ST1181 (Figure 2 and Figure 3; Table 2). The diversity of STs in each sample was lower in water (beach/river/anchialine pool) (8.7%, 2/23) compared to sand (67%, 2/3) (Table 2), although few S. aureus isolates were isolated from sand (n = 3). Within the CC8 group, the three ST72 isolates were genetically related with 5–10 SNP differences (Figure 3), while the ST1181 isolate differed with ~1000 different SNPs. Within the CC5 group, there were three STs: ST5, ST6, and ST518 (Figure 3). Two ST5 isolates were identical, with zero SNP differences, and the other CC5 isolates had 126–631 SNP differences. The two ST518 isolates had six SNP differences between them, whereas the ST6 isolate was genetically distinct from the other CC5 isolates (>10,500 SNP differences). Three of the ST398 isolates were closely related (1–2 SNP differences), whereas one ST398 isolate had 557–558 SNP differences (Figure 3). Two isolates from ST15 (CC15), the one ST508 (CC45), and the ST97 (CC97) isolate had SNP differences of 377, 246, and 7, respectively (Figure 3).
2.3. Antibiotic Resistance Genes
The MRSA isolates carried 6–9 different antibiotic resistance genes, including the mecA gene (Table 2). All isolates carried aph(3’)-III [aminoglycoside resistance], erm(C) [macrolide, lincosamide, streptogramin B resistance], mph(C) [macrolide resistance], and msr(A) [macrolide resistance] genes. Eight (88.9%) of the isolates carried the blaZ [β-lactam resistance] gene. Seven (77.8%) isolates carried ant(6)-Ia [aminoglycoside resistance] gene. The other antibiotic resistance genes were identified in fewer isolates (Table 2).
Among the MSSA, 22.2% (6/27) carried no AMR genes, 48.1% (13/27) carried a single AMR gene, 14.8% (4/27) carried two AMR genes, and 14.8% (4/27) carried 4–5 different AMR genes (Table 2). The blaZ gene was carried by 70.4% (19/27) of MSSA isolates. Less frequently carried AMR genes are listed in. Among the ST5 CC5 MSSA isolates, 62.5% (5/8) carried the blaZ gene and 25% (2/8) carried no AMR genes. All four ST398 MSSA isolates carried the erm(T) gene, whereas 75% (3/4) also carried the blaZ gene. The ST398 isolates were isolated from all three regions sampled. The ST15 (CC15) (n = 2) and ST508 (CC45) (n = 2) all carried the blaZ gene. The ST97 (n = 2), and ST3269 (n = 1) did not carry any AMR genes (Table 2).
2.4. Toxin, Exoenzyme, Host Immunity Genes
All MRSA carried the following toxin genes: hlgA, hlgB, hlgC, lukD, lukE, lukF-PV, lukS-PV, sek, and seq (Table 2). One MRSA (isolate #109) also carried the seg, sem, and sen enterotoxin genes and was isolated from sand. All MSSA isolates carried the hlgA, hlgB, and hlgC hemolysin toxin genes. The ST6 (CC8) MSSA (isolate #54) also carried the sek and seq toxin genes, as identified in the MRSA isolates, but was not found in the other MSSA isolates. The lukD and lukE toxin genes were identified in 77.8% (21/27) of the MSSA strains. Fifteen (55.6%) of the MSSA isolates contained ≥ 6 enterotoxin genes (Table 2). The most common enterotoxin genes detected were seg, sei, sem, sen, seo, and seu, which were found in 55.6% (15/27) of MSSA isolates. The tst gene was detected in both ST508 MSSA isolates and was identified in Hilo at the Wailuku River Mouth (station #17) and at Waiʻōlena (station #5). No enterotoxin genes were found for ST398, ST15, ST97, ST1155, and ST3269 isolates.
All MRSA isolates carried the following exoenzyme genes: aur, splA, splB, and splE (Table 2). The aur gene was detected in all 27 MSSA isolates. The splA gene was carried in 77.8% (21/27) of MSSA isolates. The splB gene was carried in 74.1% (20/27) of the MSSA isolates and was missing from ST1181, ST398, and ST508. The splE gene was found in 25.9% (7/27) of MSSA isolates and found in ST72, ST6, ST15, and ST1155. MRSA isolates carried the following host immunity genes: ACME, sak, and scn (Table 2). The scn gene was found in 96.3% (26/27) of MSSA isolates. The sak and scn genes were found in 74.1% (20/27) of all sequence types, except for ST398, ST15, and ST3269. ST3269 was the only MSSA isolate that did not carry any host immunity genes.
2.5. Plasmids
Among the 36 S. aureus strains, nine replicon families (rep family) were detected. The rep genes were on the same contig as the AMR and/or virulence genes (Table 3). The MRSA isolates contained 2–4 rep genes. Among the MSSA isolates, 12 contained no rep family genes, 14 contained a single rep family, and 1 contained 2 rep families (Table 3). The most dominant rep families were rep10 and rep19 among the MRSA and rep13 and rep20 among the MSSA. Among the nine MRSA, all carried the erm(C) gene on the pDLK1 plasmid with rep10, seven carried blaZ on a rep19 plasmid, two carried tet(K) on a rep17a plasmid, and one isolate each carried msr(A) on a rep19 plasmid and lnu(A) on a rep21 plasmid. Among the MSSA, all ST398 and the one ST5 carried the erm(T) gene on a plasmid with rep13. The tet(K) gene was associated with rep7a for ST1181 (isolate #79) and ST5 (isolate #111). While two MSSA isolates carried the mph(C) gene, only one had rep19. Of the six MSSA isolates that carried the blaZ gene, (3/6) were associated with rep16, (2/6) associated with rep20, and (1/6) had an unknown rep sequence. The ACME cluster on a single MRSA isolate was associated with rep7c. The aur virulence gene from three MRSA and two ST72 were associated with rep7c. Two ST5 carried the sed and sej toxin genes and were associated with rep20 and found on the SAP074A plasmid.

Table 3.
Association of antibiotic resistance genes and/or virulence genes, and replicon families (rep families) with plasmids.
3. Discussion
In this study, we isolated both MRSA and MSSA from known clonal complexes. The number of positive water samples for S. aureus in our study was lower (7.2%) than expected compared to study sites in Florida, which had a 37% positive rate for MSSA and 1% positive rate for MRSA for coastal waters []. Similarly other Flordia studies have found more MSSA vs. MRSA in their samples [,]. The lower prevalence rate of S. aureus compared to the other studies [,,] are most likely a result of the COVID-19 pandemic, whereby the beaches were much less populated with beachgoers and toruists (Hawaiʻi Tourism Authority 2020 https://www.hawaiitourismauthority.org/media/6395/december-2020-visitor-statistics-press-release-final.pdf, accessed on 8 August 2021). This is reflected by studies in Hawaiʻi that had a prevalence up to 92% for S. aureus in aquatic environments [,,]. Nevertheless, the strains isolated are of interest as they are most likely contaminated by the local population rather than tourists.
The MRSA, despite being isolated from two different districts on the island (Figure 2), were clonally related and thus, probably indigenous to the region, as they were also clonally related to strains previously isolated in Hawaiʻi State 10 years prior and from a different island []. It is clear that this MRSA clone had been circulating on the Hawaiian Islands for a long time. However, limited data are available on MRSA strains circulating in Hawaiʻi. A study conducted in 2003–2004 on the island of Oʻahu, Hawaiʻi typed 12 environmental MRSA isolates via PFGE; seven of the MRSA contained recognizable pulse field types, including: USA100 (4/12), USA300 (1/12), USA1000 (1/12), and USA1100 (1/12) []. Another study in 2004 collected 40 MRSA isolates from skin and soft tissue infections from hospital patients; 65.0% (26/40) were classified as USA300 and of those, 84.6% (22/26) were classified as USA300-0114 []. Thus, while a diversity of MRSA was found in the mid-2000′s, the dominant MRSA strain in the past decade appears to be the USA300 clone. Nevertheless, given the high infection rate with MRSA in Hawaiʻi [,], this indicates that the strain is also widespread amongst the population, as beachgoers during our sampling period were primarily local residents.
The MRSA in this study was the typical USA300 ST8 SCCmec IVa community-acquired MRSA, carrying the PVL toxin, which is a very prevalent strain in North America and the strains, though an outgroup, cluster together with the USA300 “North American Epidemic” (USA300-NAE) clone []. Moreover, an episode of acute increase of MRSA infections in a coastal community in Hawaiʻi has been described in the past []. Suggestive of a clear public health hazard associated with this strain circulating on coastal beaches. The USA300 strains have also been detected by Pulse Field Gel Electrophoresis (PFGE) analysis in the past, but only in low numbers []. This indicates that this clone has been spreading successfully on the island of Hawaiʻi. Nevertheless, future studies should include human S. aureus isolates, in connection with epidemiological data, to examine whether there is a link between recreating in S. aureus-contaminated coastal waters and the onset of skin infections. Aside from two older publications, there has been limited indication of an increased risk of developing staphylococcal skin infections as a result of recreating in staphylococci-contaminated waters and beach sand in Hawaiʻi [,]. Recreational swimmers in Hawaiʻi may be disproportionately affected by S. aureus infections, particularly on Hawaiʻi Island, due to the presence of rocky beaches and coral reefs where skin abrasions/wounds are common. These have previously been identified as an important risk factor for acquiring staphylococcal infections []. S. aureus has also been found in rivers during storms [], which attracts surfers who may be disproportionately at risk for acquiring these infections [].
Overall, this indicates that colonization of S. aureus strains may occur at coastal beaches and may, subsequently, spread in the community and cause infections. Additional genomic information of currently circulating strains infecting humans on the island of Hawaiʻi are necessary to bring more clarity to the situation.
The only former WGS data on AMR genes in MRSA from Hawaiʻi indicated that the eight USA300 strains contained 2–9 different antibiotic resistant genes []. The aac(6’)-aph(2’’), and mupA genes were not detected in the MRSA isolates in the current study. The AMR genes [erm(T), lnu(A), qacD, qacG, and tet(K)] found in the current study were not found in the clinical isolates. This suggests that during the period of 10 years, several additional resistance genes were acquired. However, additional studies are necessary to better assess the resistance genes present.
The strains in this study and the formerly isolated eight MRSA [] carried the same virulence genes, except for the ACME cluster, which was carried in only 50% (4/8) of the clinical MRSA compared to 100% (9/9) of the environmental MRSA isolates in the current study. Indeed, the current USA300-NAE strains are characterized by the presence of the ACME cluster, while the South American clones are ACME negative [].
The MSSA strains in this study were more diverse, less resistant, and carried fewer virulence genes. The MSSA isolates included closely related ST72 strains from different locations. Similarly, some of the ST5 strains were related, and others were less related to other ST5 strains. The ST518 strains were very related. Moreover, the ST398 strains were nearly the same despite being isolated from all regions sampled. This suggests that these strains may also be endemic in Hawaiʻi, but due to the lack of data, it cannot be stated for certain. The MSSA all belong to well-known clones, such as ST398 MSSA, which is human-associated unlike the MRSA ST398 [] and carries the erm(T) gene, which are not typically found in livestock-associated strains []. Furthermore, future in-depth studies should also focus on the epidemiology of MSSA, as these strains account for the majority of invasive S. aureus infections in the United States resulting in considerable morbidity and mortality, [] and may also pose a health risk for beachgoers.
The AMR genes have been shown to be located on different mobile genetic elements in MRSA and MSSA for blaZ. Indeed, blaZ is located, in general, on a transposon and may jump between different locations []. Similarly, the erm(T) gene, originally described in an MSSA ST398 [], has been found on different plasmids, though to our knowledge never on USA300 strains. Unfortunately, it is unknown what the prevalence of ST398 is in Hawaiʻi. However, we found it rather abundantly in this study, and it is a human-associated strain. It appears to be quite prevalent, and the erm(T) gene might have been transferred from ST398 to ST8, ST5, and ST518 (Table 2).
Few plasmids are common between the MRSA and MSSA, though it is also clear that the MRSA population is quite clonal and may represent a bias in the diversity. Nevertheless, it seems that the USA300 clone is also the predominant clone on the island. However, there are also clear indications of the exchanges of rep7c, rep7a and rep19 plasmids. While these plasmids have the same replicon type, they do not always carry the same resistance genes. It is clear that the MRSA are able to more easily acquire plasmids as they are overwhelmingly present in the MRSA opposed to MSSA strains.
4. Materials and Methods
4.1. Study Locations and Sampling
Water and sand samples were collected multiple times at 36 stations across the three most populous districts in Hawaiʻi County, including the most populated district, Hilo, and the less populated ones of South Kohala, North Kona, and Puna (Figure 5). Hilo stations were chosen adjacent to and within the Hilo Bay watershed, spanning ~12 km of coastline, including the Wailuku and Wailoa Rivers and Honoliʻi Stream. A majority of households in Hilo rely on decentralized wastewater management, where ~8700 cesspools leak ~21.2 million liters/day into groundwater, which contaminates nearshore coastal waters []. In Hilo, there is a wastewater treatment plant that utilizes a series of gravity-fed systems with pump stations and provides primary and secondary treatment, where effluent is discharged offshore via an ocean outfall. As a result of heavy rainfall, cesspool seepage and sewage overflows periodically occur representing a potential health hazard and thus, sewage was sampled. Twenty-one stations were selected, which included 14 State and county beaches (stations #1–12, #16, #20; 188 samples) seven river/stream stations (76 samples) along the Wailoa River (stations #13–15), Wailuku River (stations #17–19), and Honoliʻi Stream (station #21). Sand was collected at five stations in Hilo (stations #1–2, #11, #16, #20) each on five occasions (25 samples). Wastewater influent, effluent, and treated effluent collected from a manhole was collected monthly over five months (15 samples) from the Hilo Wastewater Treatment Plant (Figure 5A).
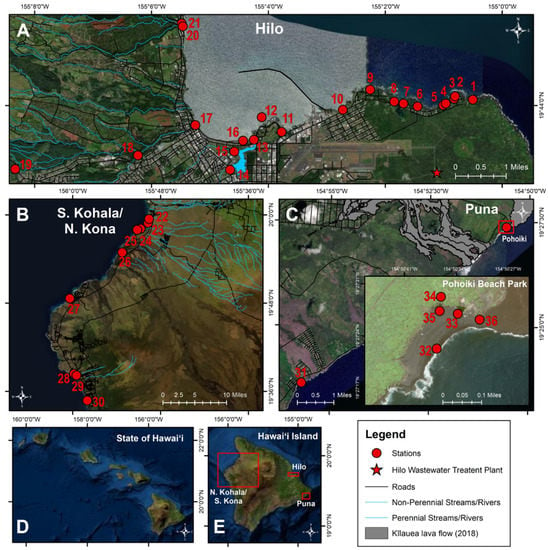
Figure 5.
Map of water sampling stations. Sampling was conducted at 36 water (beach/river/stream/anchialine pool) and sand stations in the districts of (A) Hilo (station #1–21), (B) S. Kohala, N. Kona (station #22–30), and (C) Puna (station #31–36) on (D) Hawaiʻi Island, (E) Hawaiʻi from June to December 2020.
The districts of S. Kohala and N. Kona and are located on the western side of Hawaiʻi Island, dominated by resort developments. Nine county and private beach stations were sampled (stations #22–30; 27 samples). Sand was collected once at four stations in S. Kohala and N. Kona (stations #22–23, #26–27) (Figure 5B).
Puna is a rapidly developing district located southeast of Hilo, and in 2018, a black sand beach and numerous anchialine pools formed at a popular surfing spot (Pohoiki) following a volcanic eruption []. Two county beaches (stations #31–32; 10 samples) and four anchialine pools (stations #33–36; 10 samples) were sampled. Sand was collected at two stations in Puna, each on three occasions (stations #31–32) (6 samples) (Figure 5C).
A total of 361 samples were collected and processed from coastal beach waters (n = 225), rivers/stream (n = 76), anchialine pools (n = 10), sand (n = 35), and wastewater (n = 15) between July–December 2020 (Figure 5). Liquid samples were collected in sterile, acid-washed polypropylene plastic bottles between 6 AM and noon, stored on ice, and processed within 6 h of collection. Sand samples were collected in sterile, polypropylene bottles using a metal spade 1–2” deep, within 1 m of the high tide line, transported on ice, and processed within 6 h.
4.2. Isolation and Quantification of Staphylococci
Water samples were cultured for Staphylococcus spp. using broth enrichment (1.5 X m Staphylococcus broth (Becton, Dickinson, and Company, Sparks, MD, USA), 75 µg/mL polymyxin B, and 0.01% potassium tellurite) in Quanti-Tray 2000® (IDEXX Laboratories, Westbrook, ME, USA) and incubated for 72 h at 37 °C, as previously described []. Positive wells contained a black precipitate [,]. The number of positive wells were counted and the presumptive Staphylococcus spp. MPN/100 mL was calculated using the MPN IDEXX chart and adjusted for dilution. From each Quanti-Tray 2000® sample, ≤1 mL of broth from positive wells were placed into a 1.5 mL microcentrifuge tube and vortexed for 15 s. An aliquot was inoculated onto both mannitol salt agar (Becton, Dickinson, and Company) and Oxacillin Resistance Selective Agar Base (ORSAB) with supplement (Thermo Fisher ScientificTM OxoidTM Products, Lenexa, KS, USA), and incubated at 37 °C to screen for MRSA and MSSA, respectively. Presumptive MRSA/MSSA colonies were inoculated onto blood agar (Tryptone Soya agar with sheep blood) (Thermo Fisher ScientificTM RemelTM Products), incubated at 37 °C for 18–24 h in a candle extinction jar and screened for β-hemolysis. Presumptive MRSA and MSSA isolates were identified using the StaphaurexTM latex agglutination test kit (Thermo Fisher ScientificTM RemelTM Products). Presumptive MRSA isolates were biochemically identified using the penicillin-binding protein (PBP2) latex agglutination test kit (Thermo Fisher ScientificTM RemelTM Products). A single MRSA/MSSA isolate was stored at 4 °C until further characterized. In seven (30%) of the 23 positive water (beach/river/anchialine pool) samples, two different wells contained S. aureus, which were characterized to assess diversity in the STs (Table 2).
Sand samples were processed using modified methods, as previously described []. Briefly, 10 g of wet sand from each station were shaken with 200 mL of 0.15 M NaCl for two min at 100 rpm where 25 mL of the supernatant was transferred into a sterile bottle, and sterile water added for a final volume of 50 mL. This volume was then combined with 50 mL of the enrichment broth and processed, as described above in IDEXX trays []. Up to 20 mL of wastewater was transferred; sterile water was added for a final volume of 50 mL. Samples were then combined with 50 mL of enrichment broth and placed in Quanti-Tray 2000® and processed, as previously described []. In two (67%) of the three positive sand samples, 2–3 different wells contained S. aureus, which were characterized to assess diversity in the STs (Table 2).
4.3. WGS Assembly and Analysis
From 26 S. aureus samples, nine MRSA and 27 MSSA isolates were characterized using WGS. Library preparation was performed using the Illumina DNA Prep Kit (Illumina, Inc., San Diego, CA, USA) and sequenced on a MiSeq machine (Illumina, Inc.) using a paired end 2 × 250 bp sequencing strategy, following standard Illumina protocols (Illumina, Inc., San Diego, CA, USA). Raw reads were quality filtered and trimmed using Trimmomatic (v0.39.0) [] with LEADING/TAILING 3 and MINLENGTH 36 used as default parameters. FASTQ files were imported into Geneious Prime v2021.1 and de novo assembled using the SPAdes genome assembler (v3.13.0) [] in careful mode. Sequence types were determined using the S. aureus MLST 2.0 database (https://cge.cbs.dtu.dk/services/MLST/, accessed on 1 February 2021), SCCmec type elements determined using SCCmecFinder 1.2 (https://cge.cbs.dtu.dk/services/SCCmecFinder/, accessed on 1 February 2021). ResFinder 4.1 (https://cge.cbs.dtu.dk/services/ResFinder/, accessed on 1 February 2021) and VirulenceFinder 2.0 (https://cge.cbs.dtu.dk/services/VirulenceFinder/, accessed on 1 February 2021) using identity thresholds of 90% and a minimum length of 60% for the detection of resistance and virulence genes. The presence of plasmids was assessed using MobileElementFinder, using default settings (https://cge.cbs.dtu.dk/services/MobileElementFinder/, accessed on 1 February 2021). Core genome pairwise single nucleotide polymorphism (SNP) was assessed using CSI Phylogeny 1.4 (https://cge.cbs.dtu.dk/services/CSIPhylogeny/, accessed on 1 February 2021) using default settings. The following isolates were used as reference strains for phylogenetic analyses: MRSA ST8 (CC8) (accession #SAMN18836599), MSSA ST 5 (CC5) (accession #SAMN18824826), and MSSA ST398 (CC398) (accession #SAMN18824846). Phylogenetic trees were visualized using Geneious Prime v2021.1 (Figure 2 and Figure 3). All WGS data were deposited in NCBI GenBank under project PRJNA721726 with the following accession numbers: SAMN18824809–SAMN18824812, SAMN18824826, SAMN18824828, SAMN18824831, SAMN18824834–SAMN18824835, SAMN18824839, SAMN18824841, SAMN18824843, SAMN18824846–SAMN18824847, SAMN18824850–SAMN18824852, SAMN18824854–SAMN18824857, SAMN18836577, SAMN18836580–SAMN18836581, SAMN18836590–SAMN18836592, SAMN18836595, SAMN18836599–SAMN18836600, SAMN18836616–SAMN18836620, and SAMN18836622.
4.4. Prevalence of S. aureus
Descriptive statistics (geometric mean, standard error, minimum, maximum) was carried out in R Studio (v.4.0.5).
5. Conclusions
The beaches in Hawaiʻi Island contaminated both MRSA and MSSA. The MRSA in this study were the typical USA300 strains, which are likely circulating in the community. The MRSA strains were very clonal. The MSSA strains isolated in this study were less resistant and have less virulence traits; however, there is also a lot of clonality amongst the strains. This indicates that the beaches are a potential health risk for both MRSA and MSSA infections in humans.
Author Contributions
Conceptualization, T.J.G., M.C.R. and T.N.W.; Methodology, T.J.G. and M.C.R.; Software, T.J.G.; Formal analysis, T.J.G.; Investigation, T.J.G., P.D., G.M., D.L., V.D.L.S., J.G.; Resources, T.J.G., T.N.W. and M.C.R.; Data curation, T.J.G.; Writing—original draft preparation, T.J.G., M.C.R., P.B. and T.N.W.; Writing—review and editing, T.J.G., M.C.R., P.B. and T.N.W.; Visualization, T.J.G.; Supervision, M.C.R. and T.N.W.; Project administration, T.J.G., M.C.R. and T.N.W.; Funding acquisition, T.J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Washington State Department of Health Genome Trackr Network, grant number U19FD007185, University of Washington, Stipends for Training Aspiring Researchers (STAR) program, grant number 5R25HL103180-9, National Science Foundation (NSF/GEO grant number 1565950; B. Bruno, PI), University of Washington School of Public Health, Office of the Dean’s Master fellowship 2019–2020, Graduate Opportunities and Minority Achievement Program (GO-MAP) 2019–2020, School of Public Health, Office of the Dean’s Fellowship, Castner Endowed Student Research Fund 2020, and Kamehameha Schools. Research space was provided by the University of Hawaiʻi at Hilo.
Data Availability Statement
All genomic data related to this project are available via NCBI GenBank under project PRJNA721726.
Acknowledgments
Thank you to David No and Amy Odaira for assistance in the laboratory and/or field and at the University of Hawaiʻi at Hilo, Tara Holitzi, Eric Johnson, Jonathan Awaya, and Ryan Perroy. Research space for field work was provided by the University of Hawaiʻi at Hilo.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Monecke, S.; Gavier-Widén, D.; Hotzel, H.; Peters, M.; Guenther, S.; Lazaris, A.; Loncaric, I.; Müller, E.; Reissig, A.; Ruppelt-Lorz, A.; et al. Diversity of Staphylococcus aureus isolates in European wildlife. PLoS ONE 2016, 11, e0168433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Challagundla, L.; Luo, X.; Tickler, I.A.; Didelot, X.; Coleman, D.C.; Shore, A.C.; Coombs, G.W.; Sordelli, D.O.; Brown, E.L.; Skov, R.; et al. Range expansion and the origin of USA300 North American epidemic methicillin-resistant Staphylococcus aureus. mBio 2018, 9, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Levin-Edens, E.; Meschke, J.S.; Roberts, M.C. Quantification of methicillin-resistant Staphylococcus aureus strains in marine and freshwater samples by the most-probable-number method. Appl. Environ. Microbiol. 2011, 77, 3541–3543. [Google Scholar] [CrossRef] [Green Version]
- Thapaliya, D.; Hellwig, E.J.; Kadariya, J.; Grenier, D.; Jefferson, A.J.; Dalman, M.; Kennedy, K.; DiPerna, M.; Orihill, A.; Taha, M.; et al. Prevalence and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus on public recreational beaches in Northeast Ohio. GeoHealth 2017, 1, 320–332. [Google Scholar] [CrossRef] [Green Version]
- Plano, L.R.W.; Shibata, T.; Garza, A.C.; Kish, J.; Fleisher, J.M.; Sinigalliano, C.D.; Gidley, M.L.; Withum, K.; Elmir, S.M.; Hower, S.; et al. Human-associated methicillin-resistant Staphylococcus aureus from a subtropical recreational marine beach. Microb. Ecol. 2013, 65, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.; Friese, A.; Klees, S.; Tenhagen, B.A.; Fetsch, A.; Rösler, U.; Hartung, J. Longitudinal study of the contamination of air and of soil surfaces in the vicinity of pig barns by livestock-associated methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 5666–5671. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, K.D.; McNay, M.; Cao, Y.; Ebentier, D.; Madison, M.; Griffith, J.F. A multi-beach study of Staphylococcus aureus, MRSA, and enterococci in seawater and beach sand. Water Res. 2012, 46, 4195–4207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plano, L.R.; Garza, A.C.; Shibata, T.; Elmir, S.M.; Kish, J.; Sinigalliano, C.D.; Gidley, M.L.; Miller, G.; Withum, K.; Fleming, L.E.; et al. Shedding of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus from adult and pediatric bathers in marine waters. BMC Microbiol. 2011, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Esiobu, N.; Green, M.; Echeverry, A.; Bonilla, T.D.; Stinson, C.M.; Hartz, A.; Rogerson, A.; McCorquodale, D.S. High numbers of Staphylococcus aureus at three bathing beaches in South Florida. Int. J. Environ. Health Res. 2013, 23, 46–57. [Google Scholar] [CrossRef]
- Levin-Edens, E.; Soge, O.O.; No, D.; Stiffarm, A.; Meschke, J.S.; Roberts, M.C. Methicillin-resistant Staphylococcus aureus from Northwest Marine and fresh water recreational beaches. FEMS Microbiol. Ecol. 2012, 79, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Soge, O.O.; Meschke, J.S.; No, D.B.; Roberts, M.C. Characterization of methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative Staphylococcus spp. isolated from US West coast public marine beaches. J. Antimicrob. Chemother. 2009, 64, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, L.R.; Haack, S.K.; Johnson, H.E.; Brennan, A.K.; Isaacs, N.M.; Spencer, C. Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) at ambient freshwater beaches. J. Water Health 2015, 13, 680–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estivariz, C.F.; Park, S.Y.; Hageman, J.C.; Dvorin, J.; Melish, M.M.; Arpon, R.; Coon, P.; Slavish, S.; Kim, M.; McDougal, L.K.; et al. Emergence of community-associated methicillin resistant Staphylococcus aureus in Hawaii, 2001–2003. J. Infect. 2007, 54, 349–357. [Google Scholar] [CrossRef]
- Viau, E.J.; Goodwin, K.D.; Yamahara, K.M.; Layton, B.A.; Sassoubre, L.M.; Burns, S.L.; Tong, H.-I.; Wong, S.H.C.; Lu, Y.; Boehm, A.B. Bacterial pathogens in Hawaiian coastal streams—Associations with fecal indicators, land cover, and water quality. Water Res. 2011, 45, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Economy, L.M.; Wiegner, T.N.; Strauch, A.M.; Awaya, J.D.; Gerken, T. Rainfall and streamflow effects on estuarine Staphylococcus aureus and fecal indicator bacteria concentrations. J. Environ. Qual. 2019, 48, 1711–1721. [Google Scholar] [CrossRef]
- Fowler, T. Development of Methods Using CHROMagarTM Media to Determine the Prevalence of Staphylococcus aureus and Methicillin-Resistant S. aureus (MRSA) in Hawaiian Marine Recreational Waters. Master’s Thesis, University of Hawaiʻi at Mānoa, Honolulu, HI, USA, 2005. [Google Scholar]
- Klein, E.Y.; Mojica, N.; Jiang, W.; Cosgrove, S.E.; Septimus, E.; Morgan, D.J.; Laxminarayan, R. Trends in methicillin-resistant Staphylococcus aureus hospitalizations in the United States, 2010–2014. Clin. Infect. Dis. 2017, 65, 1921–1923. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, W.R.; Schlosser, J.; Chinn, R.Y.; Tweeten, S.; Jackson, M. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at US health care facilities, 2006. Am. J. Infect. Control 2007, 35, 631–637. [Google Scholar] [CrossRef]
- Jarvis, W.R.; Jarvis, A.A.; Chinn, R.Y. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at United States health care facilities, 2010. Am. J. Infect. Control 2012, 40, 194–200. [Google Scholar] [CrossRef]
- Li, F.; Park, S.Y.; Ayers, T.L.; Miller, F.D.; MacFadden, R.; Nakata, M.; Lee, M.C.; Effler, P.V. Methicillin-resistant Staphylococcus aureus, Hawaii, 2000–2002. Emerg. Infect. Dis. 2005, 11, 1205–1210. [Google Scholar] [CrossRef]
- Len, K.A.; Bergert, L.; Patel, S.; Melish, M.; Kimata, C.; Erdem, G. Community-acquired Staphylococcus aureus pneumonia among hospitalized children in Hawaii. Pediatr. Pulmonol. 2010, 45, 898–905. [Google Scholar] [CrossRef]
- Li, F.; Miller, F.D.; Effler, P.V. Epidemiology of methicillin-resistant Staphylococcus aureus among incarcerated population in Hawaiʻi, 2000–2005. Hawaii Med. J. 2010, 69, 99–102. [Google Scholar] [PubMed]
- Li, F.; Arnsberger, P.; Miller, F.D. Profile of methicillin-resistant Staphylococcus aureus among nursing home residents in Hawaiʻi. Hawaii Med. J. 2010, 69, 126–129. [Google Scholar]
- Charoenca, N.; Fujioka, R.S. Assessment of Staphylococcus bacteria in Hawaii’s marine recreational waters. Water Sci. Technol. 1993, 27, 283–289. [Google Scholar] [CrossRef]
- Cheung, W.H.S.; Chang, K.C.K.; Hung, R.P.S.; Kleevens, J.W.L. Health effects of beach water pollution in Hong Kong. Epidemiol. Infect. 1990, 105, 139–162. [Google Scholar] [CrossRef]
- Seifred, S.E.; Tice, A.D.; Eischen, M. Diversity of community associated strains of methicillin-resistant Staphylococcus aureus in Hawaii. J. Infect. Dis. 2007, 195, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Charoenca, N.; Fujioka, R.S. Association of staphylococcal skin infections and swimming. Water Sci. Technol. 1995, 31, 11–17. [Google Scholar] [CrossRef]
- Strauß, L.; Stegger, M.; Akpaka, P.E.; Alabi, A.; Breurec, S.; Coombs, G.; Egyir, B.; Larsen, A.R.; Laurent, F.; Monecke, S.; et al. Origin, evolution, and global transmission of community-acquired Staphylococcus aureus ST8. Proc. Natl. Acad. Sci. USA 2017, 114, E10596–E10604. [Google Scholar] [CrossRef] [Green Version]
- Bothwell, N.E.; Shvidler, J.; Cable, B.B. Acute rise in methicillin-resistant Staphylococcus aureus infections in a coastal community. Otolaryngol. Neck Surg. 2007, 137, 942–946. [Google Scholar] [CrossRef]
- Early, G.J.; Seifried, S.E. Risk Factors for Community-associated Staphylococcus aureus skin infection in children of Maui. Hawaiʻi J. Med. Public Health 2012, 71, 218–223. [Google Scholar]
- Jay, J.; Rugh, M.; Burdick, B.; Diaz, M.; Hammett, M.; Merlos, F.; Molina, N.; Santana, J.; Smith, C. Prevalence of MRSA Colonization in Surfers Following Exposure in Select Southern California Coastal Waters; Institute of the Environment and Sustainability: Los Angeles, CA, USA, 2019. [Google Scholar]
- Butaye, P.; Argudín, M.A.; Smith, T.C. Livestock-associated MRSA and its current evolution. Curr. Clin. Microbiol. Rep. 2016, 3, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Chroboczek, T.; Boisset, S.; Rasigade, J.-P.; Tristan, A.; Bes, M.; Meugnier, H.; Vandenesch, F.; Etienne, J.; Laurent, F. Clonal complex 398 methicillin susceptible Staphylococcus aureus: A frequent unspecialized human pathogen with specific phenotypic and genotypic characteristics. PLoS ONE 2013, 8, e68462. [Google Scholar] [CrossRef]
- Jackson, K.A.; Gokhale, R.H.; Nadle, J.; Ray, S.M.; Dumyati, G.; Schaffner, W.; Ham, D.C.; Magill, S.S.; Lynfield, R.; See, I. Public health importance of invasive methicillin-sensitive Staphylococcus aureus Infections: Surveillance in 8 US Counties, 2016. Clin. Infect. Dis. 2020, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüger, H.; Ji, X.; Wang, Y.; Feßler, A.T.; Wang, Y.; Wu, C.; Schwarz, S. Identification of Tn553, a novel Tn554-related transposon that carries a complete blaZ-blaR1-blaI β-lactamase operon in Staphylococcus aureus. J. Antimicrob. Chemother. 2021, dkab210. [Google Scholar] [CrossRef]
- Gómez-Sanz, E.; Kadlec, K.; Feßler, A.T.; Zarazaga, M.; Torres, C.; Schwarz, S. Novel erm(T)-carrying multiresistance plasmids from porcine and human isolates of methicillin-resistant Staphylococcus aureus ST398 that also harbor cadmium and copper resistance determinants. Antimicrob. Agents Chemother. 2013, 57, 3275–3282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawaiʻi State Department of Health. Report to the Twenty-Ninth Legislature State of Hawaiʻi 2018 Regular Session Relating to Cesspools and Prioritization for Replacement; Hawaiʻi State Department of Health: Honolulu, HI, USA, 2017.
- Global Volcanism Program. Report on Kilauea (United States). Bull. Glob. Volcanism Netw. 2019, 44. [Google Scholar] [CrossRef]
- Myers, D.N.; Stoeckel, D.M.; Bushon, R.N.; Francy, D.S.; Brady, A.M.G. Chapter A7. Section 7.1. Fecal Indicator Bacteria; Techniques of Water-Resources Investigations; U.S. Geological Survey: Reston, VA, USA, 2014; p. 73. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.; Korobeynikov, A.; Lapidus, A.; Prjibelsky, A.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling genomes and mini-metagenomes from highly chimeric reads. In Proceedings of the Research in Computational Molecular Biology, Beijing, China, 7–10 April 2013; pp. 158–170. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).