Abstract
Pharmacological studies have linked a number of human health benefits with licorice due to its anticancer, anti-inflammatory, anti-oxidant, and antimicrobial properties. The aim of this study was to investigate the effects of licoricidin and glabridin, two major licorice isoflavans, on growth and virulence properties (biofilm formation, acid production, dextran production, adherence) of the cariogenic bacterium Streptococcus mutans. Moreover, the biocompatibility of these licorice compounds was assessed in an in vitro model of oral keratinocytes. We used a broth microdilution assay to show that licoricidin and glabridin exhibit a marked antibacterial activity against S. mutans. Glabridin and, to a lesser extent, licoricidin reduced the biofilm viability of S. mutans. In addition, glabridin decreased the production of dextran by S. mutans. The two licorice isoflavans attenuated the adherence of S. mutans to a saliva-coated hydroxylapatite surface, and reduced acid production from glucose. Lastly, depending on the concentrations tested, the two licorice isoflavans showed no or low toxicity toward oral keratinocytes. Within the limitations of this study, our data suggest that licoricidin and glabridin may be promising agents for controlling dental caries.
Keywords:
adherence; biofilm; cariogenic bacteria; dental caries; glabridin; licorice; licoricidin; Streptococcus mutans 1. Introduction
Dental caries is a chronic infectious disease associated with the progressive destruction of the hard tooth structures (enamel, dentin, and cementum) resulting from the metabolism of acidogenic/aciduric bacteria embedded in dental biofilms. This disease affects over 35% of people of every age group worldwide, particularly in developing countries [1]. Streptococcus mutans is considered the most important cariogenic bacterium [2]. This Gram-positive bacterium metabolizes exogenous dietary carbohydrates and produces organic acids, mainly lactic acid, that drives the dissolution of calcium and phosphate in the hydroxylapatite crystal structure of teeth [2]. S. mutans glycosyltransferases produce extracellular polysaccharides that contribute to the formation of a dense and adherent biofilm, which retains acids on the tooth surface and increases resistance to antimicrobial agents [3].
Dental caries can be prevented by regular tooth brushing and flossing. However, efficient removal of dental plaque is not always possible, especially by handicapped or elderly individuals who lack the necessary dexterity or motivation. To overcome these shortcomings, mouthwashes supplemented with chemoprophylactic agents that inhibit the growth, adhesion, and biofilm formation of cariogenic bacteria can be used as adjuncts to good oral hygiene practices [4,5]. As a number of undesirable effects, including tooth staining, unpleasant taste, and the emergence of bacterial resistance, have been associated with the chemoprophylactic agents added to mouthwashes [6,7], there is a need to identify alternatives.
In recent years, there has been growing interest in studying plant-derived molecules as effective and safe substances for the management of dental caries [8,9]. Licorice is the root of Glycyrrhiza uralensis Fisch., Glycyrrhiza glabra L., and Glycyrrhiza inflata Bat. and has been used for thousands of years as a traditional herbal remedy in China and Far Eastern countries for treating gastrointestinal disorders, chronic hepatitis, rheumatoid arthritis, and many other diseases [10,11]. Licorice and its constituents are Generally Recognized as Safe (GRAS) for use in foods and over-the-counter drugs by the United States Food and Drug Administration (FDA) (21 CFR 184.1408; 310.528; 310.544; 310.545) [12]. To date, more than 300 flavonoids have been isolated from licorice, including flavones, flavonols, flavanols, isoflavones, and isoflavans [13]. Pharmacological studies have associated several human health benefits with licorice due to its anticancer, anti-inflammatory, anti-oxidant, and antimicrobial properties [13,14,15]. Licorice compounds may also be beneficial for treating oral health conditions [16]. Although the anticariogenic properties of licorice have been touted for over 30 years, few studies on this aspect have been published. A licorice root extract (G. uralensis), which is known to kill S. mutans [17], has been incorporated into lollipops in order to reduce dental caries in children. These licorice lollipops have shown promise in reducing dental caries by decreasing S. mutans levels in saliva [18]. Glycyrrhizin, a sweet-tasting compound (50 times sweeter than sucrose), is the main triterpenoid saponin glycoside of G. glabra root and has been the subject of several investigations. Segal et al. [19] showed that while the growth of S. mutans is not affected by glycyrrhizin in the presence of sucrose, its ability to adhere to a glass surface was almost completely inhibited. Sela et al. [20] showed that glycyrrhizin dose-dependently inhibits the glucosyltransferase activity of S. mutans, which is involved in the formation of water-insoluble glucans required for biofilm formation. The aim of this study was to investigate the effects of licoricidin and glabridin, two major isoflavans of licorice, on the growth and virulence properties (biofilm formation, acid production, dextran production, and adherence) of S. mutans. The biocompatibility of these licorice compounds was also assessed using an oral keratinocyte model.
2. Results and Discussion
Licoricidin and glabridin are major isoflavans found in licorice and are characterized by the presence of a 3-phenylchromen backbone (Figure 1). The antibacterial activities of the two licorice compounds were investigated by determining their minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) against one reference (ATCC 25175) and four clinical (12A, 33A, INB, T8) strains of S. mutans in a broth microdilution assay (Table 1). On the one hand, licoricidin had a MIC of 6.25 µg/mL and a MBC varying between 6.25 and 25 µg/mL. On the other hand, glabridin showed a MIC in the range of 6.25 to 12.5 µg/mL and a MBC varying between 6.25 and 25 µg/mL. In previous studies, licoricidin has been reported to exert antibacterial activity against Porphyromonas gingivalis and Prevotella intermedia, two periodontal pathogens [21] as well as against the endodontic pathogen Enterococcus faecalis [22]. Although we did not investigate the mechanism of antibacterial action of the isoflavans tested, a previous study by Araya-Cloutier et al. [23] showed that glabridin causes membrane permeabilization as determined by the uptake of the fluorescent probe propidium iodide. Other flavonoids isolated from licorice root, including 1-methoxyficifolinol, 6,8-diprenylgenistein, 6,8-diisoprenyl-5,7,4′-trihydroxyflavone, gancaonin G, glycyrrhizol A, glycyrrhizol B, and licorisoflavan A have also been reported to exhibit antibacterial activity against S. mutans [17,24].

Figure 1.
Chemical structures of licoricidin and glabridin.

Table 1.
Minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) of licoricidin and glabridin against five strains of Streptococcus mutans. Assays were done in triplicate and a representative set of data is presented.
Mature bacterial biofilms are usually more resistant to antibacterial agents than planktonic bacteria. We determined the effect of a 2-h treatment with licoricidin and glabridin (at their MIC and 2× MIC) on the viability of S. mutans biofilms using a Filmtracer™ Live/Dead Biofilm Viability kit. A treatment with glabridin at 2× MIC significantly (p < 0.001) reduced the biofilm viability for four of the five strains of S. mutans tested (Table 2). Licoricidin, even at 2× MIC, was less effective than glabridin. Neither licoricidin nor licoricidin caused a detachment of the preformed S. mutans biofilms as determined by crystal violet staining (data not shown).

Table 2.
Effect of licoricidin and glabridin on the viability of preformed Streptococcus mutans biofilms following a 2-h treatment. The assays were carried out in triplicate, and the means ± standard deviations were calculated. *, significantly different (p < 0.001) from the control.
Dextran is formed by S. mutans from exogenous sucrose and is crucial for the establishment of cariogenic biofilms [3]. Figure 2 shows that glabridin, but not licoricidin, significantly (p < 0.001) decreased the production of dextran by S. mutans ATCC 25175. The decrease was not associated with a significant reduction in bacterial growth.

Figure 2.
Effects of licoricidin and glabridin (¼ MIC) on dextran formation by and the growth of Streptococcus mutans ATCC 25175. Dextran formation was determined using Cascade Blue-conjugated dextran dye while growth was assessed by monitoring the A660. The assays were carried out in triplicate, and the means ± standard deviations were calculated. *, significantly different (p < 0.001) from the control (no licorice compounds).
The formation of dental biofilms is initiated by the adhesion of planktonic bacteria to tooth enamel. We used a saliva-coated hydroxylapatite surface model to determine whether licoricidin and/or glabridin can impair the adhesion of S. mutans ATCC 25175. As shown in Figure 3, both glabridin and licoricidin dose-dependently and significantly (p < 0.001) reduced bacterial adherence to saliva-coated hydroxylapatite. More specifically, at a concentration of 25 µg/mL, licoricidin reduced the adherence of S. mutans by 41.6%, while glabridin reduced the adherence of S. mutans by 26.6%.

Figure 3.
Effect of licoricidin and glabridin on the adherence of fluorescein isothiocyanate (FITC)-labeled Streptococcus mutans ATCC 25175 cells to a saliva-coated hydroxylapatite surface. The number of adhered bacteria was assessed by determining the relative fluorescence units (excitation wavelength 495 nm; emission wavelength 525 nm). The assays were carried out in triplicate, and the means ± standard deviations were calculated. *, significantly different (p < 0.001) from the control (no licorice compounds).
Then, we investigated the effect of licoricidin and glabridin, at a concentration equal to 1× MIC for which these licorice compounds had no bactericidal effect, on the glycolytic pH decrease caused by S. mutans incubated in the presence of glucose. As shown in Figure 4, in the absence of the licorice compounds, 226.7 µL of 0.3 N NaOH were necessary to maintain a neutral pH of the bacterial suspension following a 70-min incubation. The presence of either licoricidin or glabridin reduced the amount of 0.3 N NaOH required to maintain a neutral pH, indicating that these compounds decreased acid production from glucose. More specifically, 68.3 µL and 104.7 µL of 0.3 N NaOH, respectively, were required to maintain a neutral pH in the presence of licoricidin and glabridin.
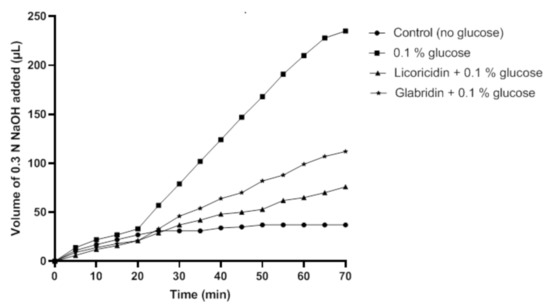
Figure 4.
Effect of licoricidin and glabridin on acid production by Streptococcus mutans ATCC 25175. Glycolysis was monitored at a constant pH (7.0) by automatic titration of the acid produced. The volume of 0.3 N NaOH required to maintain a constant pH of 7.0 was determined over a 70-min period at 37 °C. Four independent experiments were performed, and a representative set of data is presented.
Biocompatibility is an important criterion when assessing novel molecules for oral clinical applications. The toxicity of licoricidin and glabridin was tested in an oral keratinocyte model and compared to chlorhexidine. As shown in Figure 5, licoricidin did not show any cytotoxicity against keratinocytes following a 2-h exposure, while glabridin, at the highest concentration tested (50 µg/mL), reduced the viability of keratinocytes by 20.9%. In comparison, chlorhexidine exhibited greater cytotoxicity. Interestingly, the percent cell viability increased for licoricidin (all concentrations tested) and for glabridin (≤12.5 µg/mL), suggesting that they have a positive effect on cell proliferation or metabolic activity.
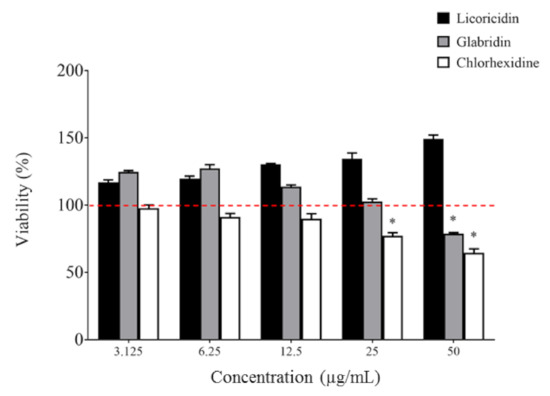
Figure 5.
Effect of licoricidin and glabridin on the viability of oral keratinocytes. Cell viability was monitored following a 2-h exposure to the compounds using a MTT (3-[4,5-diethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) colorimetric assay. The assays were carried out in triplicate, and the means ± standard deviations were calculated. *, significantly different (p < 0.01) from the control (no compounds).
In addition to the anti-caries properties identified in this study, licoricidin has been reported to possess anti-inflammatory activities that may be of interest for preventing inflammatory periodontal disease. More specifically, licoricidin inhibits the secretion of major cytokines and matrix metalloproteinases by lipopolysaccharide-stimulated human macrophages [25].
3. Materials and Methods
3.1. Compounds
Stock solutions (20 mg/mL) of glabridin (Wako Chemicals, Richmond, VA, USA) and licoricidin (EMMX Biotechnology, Lake Forest, CA, USA) were prepared in 95% ethanol and dimethyl sulfoxide, respectively. They were kept in the dark at 4 °C for up to one month. In all the assays described below, the corresponding concentrations of ethanol and dimethyl sulfoxide were used as controls and had no effect.
3.2. Bacteria and Growth Conditions
One reference strain (ATCC 25175) and four clinical strains (12A, 33A, INB, and T8) of S. mutans were used in this study. Unless indicated otherwise, the bacteria were grown aerobically at 37 °C in Brain Heart Infusion broth (BHI; BBL Microbiology Systems, Cockeysville, MD, USA) supplemented with 0.5% glucose.
3.3. Determination of Minimum Inhibitory and Minimum Bactericidal Concentrations
The minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) of licoricidin and glabridin were determined using a broth microdilution assay based on the National Committee for Clinical Laboratory Standards (NCCLS) [26]. Assays were performed in triplicate in three independent experiments, and a representative set of data is presented.
3.4. Biofilm Biomass and Viability
The ability of licoricidin and glabridin to eradicate S. mutans biofilms was investigated using a previously described protocol [27]. Briefly, the bacteria were grown in the wells of a 96-well microplate for 18 h, after which the spent medium and unattached bacteria were removed by aspiration. The biofilms were treated for 2 h at 37 °C with licoricidin or glabridin in 50 mM phosphate-buffered saline (PBS, pH 7.0) at their MIC and 2× MIC and were then washed once with distilled water. The biofilm biomass was quantified by staining with 0.01% crystal violet for 15 min. The wells were then washed twice with distilled water to remove unbound crystal violet and the plate was dried at 37 °C. One hundred µL of 75% (v/v) ethanol was added to each well, and the plate was shaken for 15 min to release the dye from the biofilms. Absorbance at 550 nm (A550) was measured using a Synergy 2 microplate reader (BioTek Instruments, Winooski, VT, USA). The ability of licoricidin and glabridin to cause the killing of S. mutans cells embedded in biofilms was also investigated using a previously described protocol with slight modifications [28]. Biofilms were formed in the wells of a 96-well, black-wall, clear-bottom microplate (Greiner Bio One, Frickenhausen, Germany) and treated with licoricidin and glabridin as described above. Bacterial viability was assessed using a Filmtracer™ Live/Dead Biofilm Viability kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The assays were carried out in triplicate, and the means ± standard deviations were calculated.
3.5. Dextran Formation
S. mutans ATCC 25175 was grown for 18 h in BHI broth supplemented with 1% sucrose. The cultures were diluted in fresh medium to obtain an optical density at 660 nm (OD660) of 0.1. Equal volumes (50 µL) of bacterial culture, licoricidin or glabridin at 1/4 MIC, and Cascade Blue-conjugated dextran dye (0.3 mg/mL; Thermo Fisher Scientific) were added to the wells of a 96-well, black-wall, clear-bottom microplate. After an 18-h incubation at 37 °C, bacterial growth was assessed by recording the OD660, while dextran formation was quantified by determining the relative fluorescence units (RFU; excitation wavelength 495 nm; emission wavelength 525 nm) using a Synergy 2 microplate reader. The assays were carried out in triplicate and the means ± standard deviations were calculated
3.6. Adherence to Hydroxylapatite
To determine the effect of licoricidin and glabridin on the adherence of S. mutans ATCC 25175 to hydroxylapatite, bacteria were first labeled with fluorescein isothiocyanate (FITC) using a previously described protocol [29]. Hydroxylapatite-coated, 96-well, black-wall, clear-bottom microplates were prepared according to the procedure described by Shahzad et al. [30]. Parafilm-stimulated saliva was collected from six non-smoking healthy volunteers with the approval of the Université Laval Ethics Committee for Research in Humans (2015-237). The volunteers were asked to sign an informed consent form. Drinking and eating were not allowed for 2 h prior to collecting the saliva. The saliva samples (≈5 mL) were pooled, centrifuged (15,000× g for 10 min), and filter-sterilized. Saliva (100 µL) was added to each well, and the plate was incubated for 30 min at room temperature. The wells were then rinsed three times with PBS. FITC-labeled S. mutans (100 µL, OD660 = 0.5) cells were added to the wells together with either licoricidin or glabridin at concentrations ranging from 6.25 to 50 µg/mL. The plate was incubated for a further 2 h at 37 °C under low agitation in the dark. Unbound bacteria were removed by aspiration, and the wells were washed twice with PBS. RFUs (excitation wavelength 495 nm; emission wavelength 525 nm) corresponding to the level of bacterial adherence were determined using a Synergy 2 microplate reader. Control wells without test compounds were used to determine 100% adherence values while wells with no bacteria were used as controls to determine basal autofluorescence. The assays were carried out in triplicate, and the means ± standard deviations were calculated.
3.7. Glycolytic pH Drop Assay
The effect of licoricidin and glabridin on S. mutans glycolysis was assessed using a glycolytic pH drop assay. S. mutans ATCC 25175 cells from an 18-h culture were harvested by centrifugation and were suspended in a saline solution (50 mM potassium chloride + 1 mM magnesium chloride; pH 7.0) to an OD660 of 2. The bacteria were incubated in one of the following solutions: (i) saline, (ii) saline + 0.1% glucose, (iii) saline + 0.1% glucose + 6.25 µg/mL of licoricidin (1× MIC), or (iv) saline + 0.1% glucose + 25 µg/mL of glabridin (1× MIC). Glycolysis was monitored at a constant pH of 7.0 with a Radiometer PHM64 Standard pH meter and TTT60 (Radiometer, Copenhagen, Denmark) by automatic titration of the acids produced by the addition of 0.3 N NaOH over a 70-min period at 37 °C. The volume of 0.3 N NaOH added to maintain a constant pH of 7.0 was determined. Four independent experiments were performed and a representative set of data was presented.
3.8. In Vitro Biocompatibility
The human oral keratinocyte cell line B11 [31], which was kindly provided by S. Groeger (Justus Liebig University Giessen, Germany), was cultured in keratinocyte serum-free medium (K-SFM; Life Technologies Inc., Burlington, ON, Canada) supplemented with growth factors (50 µg/mL of bovine pituitary extract and 5 ng/mL of human epidermal growth factor) and 100 µg/mL of penicillin G-streptomycin. The cells were seeded (1 × 105 cells in 100 µL) in the wells of a 96-well microplate (100 µL/well) and were cultured overnight at 37 °C in a 5% CO2 atmosphere until they reached confluence. The keratinocytes were treated for 2 h with either licoricidin, glabridin, or chlorhexidine (3.125 to 50 µg/mL). The wells were then immediately washed with DMEM prior to assessing cell viability using an MTT (3-[4,5-diethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay performed according to the manufacturer’s protocol (Roche Diagnostics, Mannheim, Germany). The assays were carried out in triplicate, and the means ± standard deviations were calculated.
3.9. Statistical Analysis
Statistical analysis was performed using a one-way analysis of variance with a post hoc Bonferroni multiple comparison test (GraphPad Software Inc.; La Jolla, CA, USA). Results were considered statistically significant at p < 0.01 or p < 0.001.
4. Conclusions
Compounds exhibiting dual antibacterial and anti-adherence effects may be promising therapeutic candidates for controlling biofilm-mediated diseases such as dental caries. In this study, we showed that licoricidin and glabridin exhibit antibacterial activity against biofilm-embedded and planktonic S. mutans cells. The two compounds also inhibited bacterial adherence to hydroxylapatite and acid production from glucose, and displayed low cytotoxicity for oral keratinocytes. Within the limitations of this study, including the use of a monospecies that does not mimic the complex oral microbial community, our results suggest that these licorice compounds could be added to oral hygiene products such as oral rinses, varnishes, and dentifrices in order to prevent dental caries.
Author Contributions
Conceptualization, D.G.; methodology, A.B.L., G.L., G.P. and K.V.; formal analysis, D.G.; investigation, A.B.L., G.L., G.P. and K.V.; writing—review and editing, D.G.; supervision, D.G.; project administration, D.G.; funding acquisition, D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Fonds Emile-Beaulieu.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Université Laval (2015-237).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We wish to thank S. Groeger and J. Meyle (Department of Periodontology, Justus-Liebig-University Giessen, Germany) for kindly providing the B11 keratinocyte cell line.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Koo, H.; Xiao, J.; Klein, M.I. Extracellular polysaccharides matrix—An often forgotten virulence factor in oral biofilm research. Int. J. Oral Sci. 2009, 1, 229–234. [Google Scholar] [CrossRef]
- Addy, M. Oral hygiene products: Potential for harm to oral and systemic health? Periodontol. 2000 2008, 48, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, G.M.; Kumar, S.; Fornari, C.D.; Corti, E.; Connelly, S.T. Mouthwashes in the 21st century: A narrative review about active molecules and effectiveness on the periodontal outcomes. Expert Opin. Drug Deliv. 2017, 14, 973–982. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Tadakamadla, S.K.; Connelly, S.T.; Sforza, C.; Martin, C. Adverse events associated with home use of mouthrinses: A systematic review. Ther. Adv. Drug Saf. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Zanatta, F.B.; Antoniazzi, R.P.; Rosing, C.K. Staining and calculus formation after 0.12% chlorhexidine rinses in plaque-free and plaque covered surfaces: A randomized trial. J. Appl. Oral Sci. 2010, 18, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Daliri, E.B.M.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.H. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Jeon, J.G.; Rosalen, P.L.; Falsetta, M.L.; Koo, H. Natural products in caries research: Current (limited) knowledge, challenges and future perspective. Caries Res. 2011, 45, 243–263. [Google Scholar] [CrossRef]
- Davis, E.A.; Morris, D.J. Medicinal uses of licorice through the millennia: The good and plenty of it. Mol. Cell Endocrinol. 1991, 78, 1–6. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, S.; Yang, S.; Lin, X.; He, X.; Wei, X.; Song, Q.; Li, R.; Fu, C.; Zhang, J.; et al. An “essential herbal medicine”—Licorice: A review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol. 2020, 249, 112439. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Reg. Toxicol. Pharmacol. 2006, 26, 167–192. [Google Scholar] [CrossRef]
- Yang, R.; Wang, L.Q.; Yuan, B.C.; Liu, Y. The pharmacological activities of licorice. Planta Med. 2015, 81, 1654–1669. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zhou, W.; Wang, Y.; Yang, L. Systems approaches and polypharmacology for drug discovery from herbal medicines: An example using licorice. J. Ethnopharmacol. 2013, 146, 773–793. [Google Scholar] [CrossRef]
- Nassiri Asl, M.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar]
- Messier, C.; Epifano, F.; Genovese, S.; Grenier, D. Licorice and its potential beneficial effects in common oro-dental diseases. Oral Dis. 2012, 18, 32–38. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Heber, D.; Shi, W.; Lu, Q.Y. Antibacterial compounds from Glycyrrhiza uralensis. J. Nat. Prod. 2006, 69, 121–124. [Google Scholar] [CrossRef]
- Nuvvula, S.; Nunna, M.; Almaz, M.E.; Mallineni, S.K. Efficacy of licorice lollipops in reducing dental caries in a paediatric population: A systematic review. Oral Health Prev. Dent. 2020, 18, 97–102. [Google Scholar]
- Segal, R.; Pisanty, S.; Wormser, R.; Azaz, E.; Sela, M.N. Anticariogenic activity of licorice and glycyrrhizine I: Inhibition of in vitro plaque formation by Streptococcus mutans. J. Pharm. Sci. 1985, 74, 79–81. [Google Scholar] [CrossRef]
- Sela, M.N.; Steinberg, D.; Segal, R. Inhibition of the activity of glucosyltransferase from Streptococcus mutans by glycyrrhizin. Oral Microbiol. Immunol. 1987, 2, 125–128. [Google Scholar] [CrossRef]
- Tanabe, S.; Desjardins, J.; Bergeron, C.; Gafner, S.; Villinski, J.R.; Grenier, D. Reduction of bacterial volatile sulfur compound production by licoricidin and licorisoflavan A from licorice. J. Breath Res. 2012, 6, 016006. [Google Scholar] [CrossRef] [PubMed]
- Grenier, D.; Marcoux, E.; Azelmat, J.; Ben Lagha, A.; Gauthier, P. Biocompatible combinations of nisin and licorice polyphenols exert synergistic bactericidal effects against Enterococcus faecalis and inhibit NF-κB activation in monocytes. AMB Expr. 2020, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Araya-Cloutier, C.; Vincken, J.P.; van de Schans, M.G.M.; Hageman, J.; Schaftenaar, G.; den Besten, H.M.W.; Gruppen, H. QSAR-based molecular signatures of prenylated (iso)flavonoids underlying antimicrobial potency against and membrane-disruption in Gram positive and Gram negative bacteria. Sci. Rep. 2018, 8, 9267. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Park, S.N.; Lee, Y.J.; Cho, E.J.; Lim, Y.K.; Li, X.M.; Choi, M.H.; Seo, Y.W.; Kook, J.K. In vitro antimicrobial activities of 1-methoxyficifolinol, licorisoflavan A, and 6,8-diprenylgenistein against Streptococcus mutans. Caries Res. 2015, 49, 78–89. [Google Scholar] [CrossRef]
- La, V.D.; Tanabe, S.I.; Bergeron, C.; Gafner, S.; Grenier, D. Modulation of matrix metalloproteinase and cytokine production by licorice isolates licoricidin and licorisoflavan A: Potential therapeutic approach for periodontitis. J. Periodontol. 2011, 82, 122–128. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Document M7-A6; NCCLS: Wayne, PA, USA, 2003. [Google Scholar]
- LeBel, G.; Vaillancourt, K.; Bercier, P.; Grenier, D. Antibacterial activity against porcine respiratory bacterial pathogens and in vitro biocompatibility of essential oils. Arch. Microbiol. 2019, 201, 833–840. [Google Scholar] [CrossRef]
- Drago, L.; Agrappi, S.; Bortolin, M.; Toscano, M.; Romano, C.L.; De Vecchi, E. How to study biofilms after microbial colonization of materials used in orthopaedic implants. Int. J. Mol. Sci. 2016, 17, 293. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Haas, B.; Grenier, D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci. Rep. 2017, 7, 44805. [Google Scholar] [CrossRef]
- Shahzad, M.; Millhouse, E.; Culshaw, S.; Edwards, C.A.; Ramage, G.; Comber, E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015, 6, 719–729. [Google Scholar] [CrossRef]
- Groeger, S.; Michel, J.; Meyle, J. Establishment and characterization of immortalized human gingival keratinocyte cell lines. J. Periodont. Res. 2008, 43, 604–614. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).